Abstract
This review analyzes in detail the topic of supercapacitors based on biochar technologies, including their advantages, disadvantages, and development potential. The main topic is the formation of precursors in the process of pyrolysis and activation, and the possibility of the application of biochar itself in various fields is brought closer. The structure, division, and principle of operation of supercondensates are discussed, where their good and bad sides are pointed out. The current state of the scientific and legal knowledge on the topic of biocarbon and its applications is verified, and the results of many authors are compared to examine the current level of the research on supercapacitors based on biochar electrodes created from lignocellulosic biomass. Current application sites for supercapacitors in transportation, electronics, and power generation (conventional and unconventional) are also examined, as is the potential for further development of the technology under discussion.
1. Introduction
The dynamic development of the economic and industrial sectors, as well as the development of civilization, which coincides with the era of consumerism in which we live, comes down to increased consumption of electricity and heat. This is associated with ever-increasing demand, which the energy sector must meet [1]. As conventional fuels are being depleted, and with ever newer standards and regulations for reducing emissions of carbon dioxide and other dangerous oxides, along with legislation regulating their acquisition, other sources of energy are being sought to diversify domestic and global energy production, to improve energy security, and to reduce emissions of pollutants [2,3]. Renewable sources of energy from wind, solar, and water, unfortunately, have a huge disadvantage, which is instability in their supply of energy. This is caused by many factors related to the specifics of the renewable resource; for example, energy from the photovoltaic process is limited according to the time of day and year and degree of cloud cover, so energy is not produced continuously. Similar problems occur at wind power stations, where production depends on the strength of the wind [4,5,6]. In the case of hydropower, only one type of power plant, specifically pumped-storage power stations, has the ability to produce energy in a relatively stable and continuous manner, by creating storage in case of a lack of flow. This storage is in the form of a water reservoir, called an upper reservoir, in which the collected water represents the potential energy which, when released, turns into kinetic energy that drives the power plant. This is one of the few effective and efficient ways to store energy [7]. This is forcing the community to research the development of new ways to store electricity that would reduce the costs associated with its transmission. Modern energy storage facilities would also allow for the maximum potential of currently installed renewable energy sources to be used. A promising technological solution may be the use of storage devices such as, for example, supercapacitors, fuel cells, and lithium batteries [8,9]. This is thanks to their properties, such as high power and energy density [10]. In recent years, there has been a growing demand for their production and research to develop this energy storage technology [11]. Despite the wide range of applications in power generation and industry, ultracapacitors are not used because of their price. It is worth noting that the main cost during production is the cost of the membrane, accounting for up to 60% of production costs, and the cost of producing the cathode, accounting for about 38% of production overheads [11]. These costs can be minimized by using alternative materials, such as biochar from agricultural crops or plant or animal waste. Biochar, after additional refinement processes, improves its conductive properties and has a hierarchical pore structure, making it a material for possible use in supercapacitors [12].
2. Biochar
2.1. Production and Use
Biochar is a product considered renewable; confirmation of this thesis can be found in the European Union Regulations 2019/2164, 2019/1009 and 2021/1165, where its statute and quality requirements are clearly defined [13,14,15]. It is a product of thermal processing of biomass under aerobic conditions at temperatures between 350 and 900 °C [16,17,18]. Depending on the temperature used and how long the material is held at the final temperature, biochar materials with different physical and chemical compositions are obtained [19]. Substrates used during the process are decomposed into smaller compounds or elements of lower molecular weight; solid (biochar), liquid (pyrolysis oil), and gaseous (biogas) materials are distinguished [20,21]. The process consists of a series of complex sequential and parallel reactions [17]. Research confirmed that the pyrolysis process of lignocellulosic biomass can be carried out in a way that maximizes the obtaining of the desired final product, through the separate thermal decomposition of individual components, such as lignin, cellulose, and hemicellulose [22,23]. The decomposition of individual components takes place at different temperatures and produces different compounds (hemicellulose: 200–260 °C; cellulose: 240–350 °C; lignin: 280–500 °C). Thus, from hemicellulose and cellulose, volatile compounds are formed, while the product of lignin is a solid residue that is also referred to as a natural polymer [24,25,26]. In biochar production, the most desirable substrates are those rich in lignin [23]. Many researchers classify pyrolysis methods based on the temperatures at which they are carried out. The most common are the three main pyrolysis process methods used [27,28,29,30,31], which are presented in Table 1. The final product, which is biochar, i.e., its quality and quantity, is actually made up of many factors, starting with the type of biomass, its chemical composition, moisture content, and the processes to which it was subjected in the technological sequence. This is critical when evaluating the final product, which affects the range of applications and effectiveness of the product [32,33].

Table 1.
Pyrolysis methods with the most commonly used times and temperatures.
Apart from pyrolysis, there are other modern techniques for biochar production. Unfortunately, they require expensive and specialized equipment, and due to their high energy consumption, they are not cost-effective for large-scale use. These include gasification (partial combustion at temperatures of 600–1200 °C) [34,35], torrefaction (a process close to pyrolysis carried out at 200–300 °C) [35,36], hydrothermal carbonization (a process that takes place at high water pressure at 180–260 °C) [34,37], microwave and plasma pyrolysis (a process that uses microwave energy and plasma energy in the ranges of 300–800 °C) [34,38,39].
Biochar materials are successfully used in the energy and metallurgical (iron and steel) sectors as a highly efficient fuel, with low emissions of harmful substances into the atmosphere [40]. Biochar can also be used as a valuable fertilizer to improve soil structure by changing the physicochemical properties of the soil and can affect the reduction of nitrogen emissions into the atmosphere, thereby improving its utilization by plants [41,42,43]. Its physical and chemical properties make it a great alternative as a solid fuel in opposition to conventional fuels, and it also has potential as a sorbent for water and soil contaminants. Another advantage is that it is possible to obtain substrates for its production from various types of organic waste [44,45]. Biochar used in agriculture can not only reduce the negative impact of pesticides but also increase crop yields and improve the soil by creating favorable conditions for soil life [46]. Apart from agriculture, researchers are also looking for the use of biochar in construction, e.g., as an addition to asphalt and various types of binders to increase durability [47,48]. Biochar is also used in all kinds of filters for purifying and retaining heavy metals in air and water [31,49]. More application sites for pure, activated, and modified biochar are presented in Figure 1.
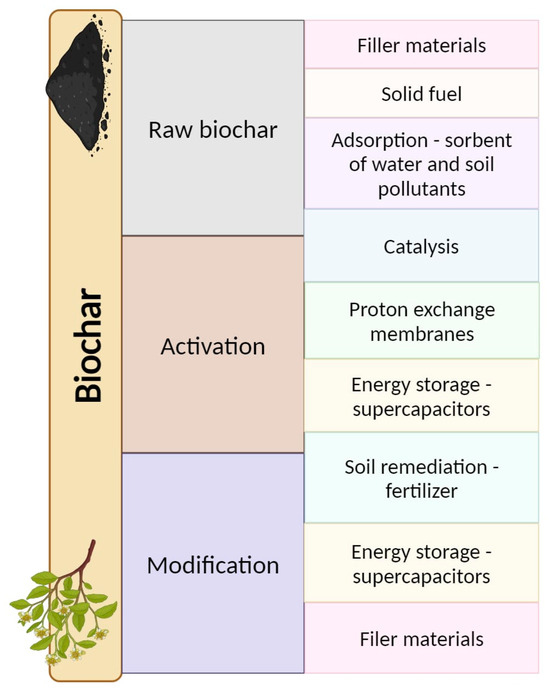
Figure 1.
Alternative application areas for biochar derived from lignocellulosic biomass. Own elaboration.
2.2. Biochar in Supercapacitors
In recent years, biochar has gained a new application in the energy industry as a material used in the manufacture of supercapacitors as a material for making electrodes, as well as a binding material used in the preparation of membranes for proton exchange [11]. However, the expansion of various economic sectors is promoting the generation of huge amounts of wastewater with organic content, forcing the development of pollutant removal technologies. An example of advanced oxidation processes that produce reactive oxygen species involve the electron transfer pathway, which is often pointed to as an effective pollutant removal technology. In this method, biocarbon is appreciated for its excellent physicochemical properties, such as high conductivity, huge surface area, active functional groups, and porous structure, all of which make biocarbon well suited as a catalyst for this method and as an electrode in EDLC-type supercapacitors [50,51,52]. The choice of precursors is influenced by factors such as cost, availability, and their environmental impact; examples of frequently used lignocellulosic biomass are shown in Table 2. Apart from plant residues, it is possible to use animal residues to produce highly porous biochar; an example is bones, such as pig bones [53,54]. Other materials that have been investigated for use in supercapacitors include litter from rearing chickens [55] and marine biomass waste [56].

Table 2.
Examples of the most commonly used lignocellulosic biomass for biochar production.
Depending on the intended use, for some purposes, the biochar does not need to undergo activation, which reduces costs and the number of production processes. Meanwhile, when the highest possible purity, stability, and efficiency are desired, using only carbonized precursors may not be sufficient. Therefore, for the most demanding applications, biochar activation techniques are additionally used [65,66]. The current state of knowledge on the topic of biochar activation distinguishes two classes of activation—conventional and advanced. Conventional methods include chemical and physical methods or a combination thereof, while advanced activation methods include chemical-assisted microwave radiation and low-temperature hydrothermal carbonization. It is also important for researchers to combine the above methods and use additional substances to increase the efficiency of activated carbon [67,68,69,70,71,72]. Biochar activated with KHCO3 has a high oxygen content on the surface and a high proximity of defective structures, which will affect its applicability as a supercapacitor material (Figure 2).
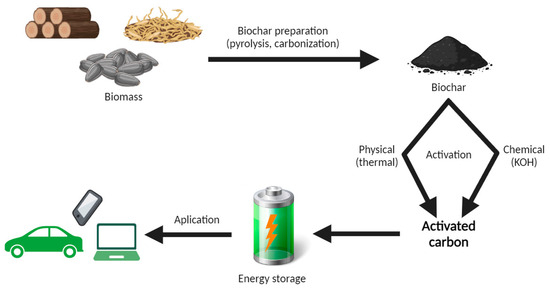
Figure 2.
An example of the process of making and using biochar in supercapacitors. Own elaboration.
Tests conducted on biochar made from corn stalks confirmed the great potential of biochar material as a supercapacitor product because its maximum capacity was 317.01 F g−1, and 10,000 charge cycles could be carried out on it [73]. An interesting waste material from the agricultural sector is tobacco waste; the authors examined the stems and leaves for their suitability for supercapacitors. The results were promising, with a capacity result of 328.07 F g−1 for the stems and 278.48 F g−1 for the leaves at a density of 1 A g−1 [74]. Other authors utilized used coffee grounds, subjected them to a pyrolysis process to obtain biochar with a purity of 96%, and then used it in its raw form in a supercapacitor, which reached a capacity of about of 200 F g−1 [75]. Both tobacco waste and used coffee grounds represent great examples of waste recycling. More examples of the use of biochar biomass in the supercapacitors are shown in Table 3.

Table 3.
Comparison of biochar electrodes made from different lignocellulosic biomasses, along with the electrolytes used, energy densities, and their capacities.
The electrochemical properties of biocarbon depend on the characteristics of its features, which include the atomic arrangement of carbon, but also its morphological texture, including porosity and surface chemistry [84]. In addition to the type of substrate used to produce the biochar, the activation process itself for these materials and the electrolyte used have a significant impact on the final performance of the supercapacitor. For example, in the case of white birch as a substrate for biochar production, differences were noted between physical activation (CO2) and chemical activation (KOH); this was significant in terms of the porosity of the materials. In addition, the type of electrolyte used also significantly affects the capacity of the supercapacitor [78]. Physical activation is a simple method that takes place in the presence of oxidizing gases, such as steam, air, and carbon dioxide. Importantly, the process occurs at a high temperature of 700–1100 °C [85,86]. In the case of chemical activation, it is a one-step process that most often uses chemical compounds, such as the alkaline compounds KOH and NaOH, but also acids HNO3, H2SO4, and H3PO4 [87,88]. There is also the use as an activator of hydrogen peroxide (H2O2), which is an oxidant [89]. In addition to the above-mentioned substances, a sulfonating agent, such as SO3H, can also be used for chemical activation, as can ZnCl2 [90]. The choice of the appropriate activator depends on the subsequent use of the biocarbon. The application of a chemical activator increases the porosity of carbon, and at the same time, its specific surface area, through reactions such as degradation, dehydration, and complexation [88]. The parameters important for electrolytes are primarily high conductivity, which facilitates the transport of ions to the electrode. The arrangement of electrolytes used from the one with the lowest conductivity to the highest is as follows: Na2SO4 < KCl < KOH < H2SO4 [91]. The use of acidic or basic electrolytes may involve the occurrence of other reactions on the surface of carbon materials. When an acidic electrolyte (H2SO4) is used, the complex chemical bonds present in the carbon structure are broken. Bonds between H-C-O, C=O, and C-OH groups undergo reversible electrochemical reactions, breaking and forming new bonds between C-O-C and C-H groups. Acidic electrolytes can also cause redox reactions, resulting in the appearance of pseudocapacity. The use of an alkaline electrolyte (KOH), on the other hand, is associated with redox processes to which carbonyl groups are subjected [92]. The advantage of using H2SO4 as an electrolyte is its high ionic conductivity at a temperature of 25 °C; however, this acid can cause corrosion risks. It is possible to use H2SO4 together with KNO3 to prevent corrosion [91,93,94].
3. Division of Supercapacitors and Their Construction
The first capacitors were patented in 1957 by the General Electric corporation and in 1966, they were improved by Becker and named supercapacitors [95]. The biggest breakthrough in the field of supercapacitors came in 1982 and 1994, when supercapacitors were used for military purposes. Current research is focused on improving the current supercapacitors by using cheaper materials for manufacturing while allowing for increased performance [96,97].
The development of energy technologies correlates with the development of energy storage technologies. We are faced with the need for energy storage that will be cheap, capacious, and viable, yet easy to dispose of. Of the well-known energy storage devices, such standard electrolytic capacitors, batteries, and fuel cells, it is supercapacitors that stand out due to their high power and energy density, fast charging capability, and repeatable duty cycles [98,99]. The specific power density for supercapacitors ranges from 10 to 10,000 W/kg, while maintaining low resistance. The flexibility of this technology is presented in Figure 3. Applications for supercapacitors can be found precisely in renewable energy, as well as in electronic devices with high requirements and vehicles based on electric motors [100,101,102].
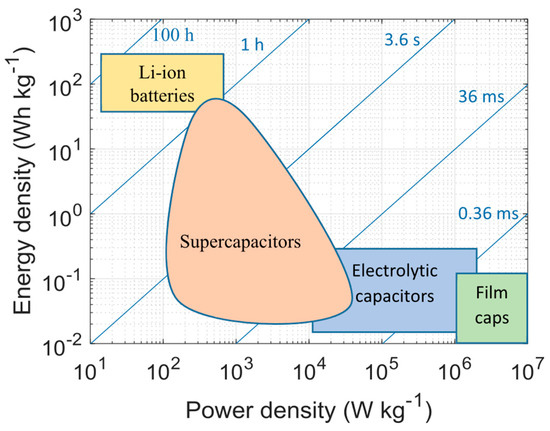
Figure 3.
Diagram showing the efficiency breakdown of various energy storage technologies [103].
Unlike traditional capacitors whose construction is based on two conductive electrodes isolated by dielectric material, supercapacitors consist of an electrolyte and a separator isolating the two electrodes (Figure 4). Supercapacitors are divided into three types depending on how they store electricity: electrochemical double-layer, pseudocapacitors, and hybrid supercapacitors [104,105].
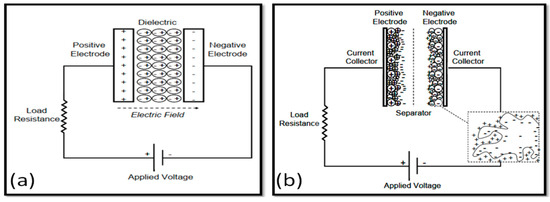
Figure 4.
Diagram showing the operation of a traditional capacitor (a) and a supercapacitor (b) [104].
For the production of supercapacitors, electrodes and electrolytes created from different materials are used; this affects the ways in which energy is stored in them. The building blocks of a supercapacitor are two mostly porous electrodes that act as an anode and cathode, electrolyte, and separator [106] (Figure 5). The entire operation of such a supercapacitor is based on its charging and discharging. During charging, electrons travel from the negative electrode to the positive one, causing cations to accumulate from the electrolyte as close to the negative electrode as possible, and anions to accumulate on the positive electrode, thus forming an electrical double layer, or initiating Faradaic redox reactions to compensate when the charges are out of balance. On discharge, electrons travel in the opposite direction, causing ion mixing or reverse redox reactions [103,106].
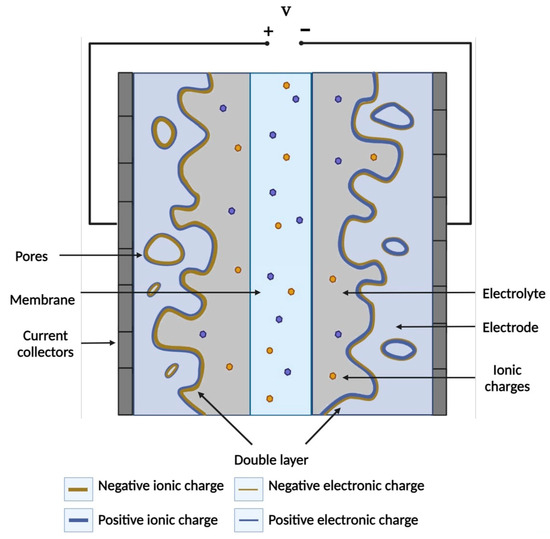
Figure 5.
Diagram and method of operation of the supercapacitor. Compiled from [103].
Depending on the materials used, the mechanism of energy storage changes, so there are three main types of supercapacitors, and these include: electrical double-layer supercapacitors (EDLCs), pseudocapacitors, and also hybrid supercapacitors (Figure 6).
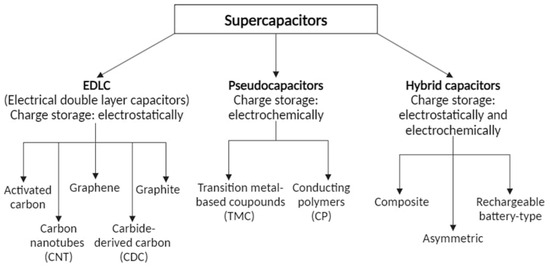
Figure 6.
Division of supercapacitors.
Double-layer supercapacitors base their operation on the accumulation of charges at the electrode–electrolyte interface, under the influence of electrostatic force, and in this way, they store energy [80]. The electrochemical reactions occurring in this type of supercapacitor are exclusively adsorption and desorption reactions of ions on the available surface of the electrodes [103]. The most commonly used electrodes are carbon-based nanoporous materials, which are characterized by easy and inexpensive production. The electrodes used are shown in Figure 6 and the parameters of the carbon-based electrodes are shown in Table 4. The key factor for electrodes is precisely the porous structure that translates into their capacity and properties; a higher pore density increases the surface area, and thus, the number of places to store charges [107,108,109].

Table 4.
Other carbon-based materials used in EDLC-type supercapacitors and the most important parameters.
Pseudocapacitors base their operation and name on the known pseudocapacitance reaction that occurs in traditional batteries, that is, rapid redox reactions that occur only at and on the electrode [124]. This is related to the storage of additional charge in faradaic processes, resulting from the presence of various functional groups on the surface of carbon electrodes (groups containing nitrogen, oxygen, sulfur, boron, and phosphorus); this translates into their increased capacity, which is often cited as an advantage [124,125,126,127]. Among the electroactive materials that exhibit pseudocapacitive tendencies in opposition to metal oxides and to metal carbides, conductive polymers, to which more attention has also been paid in recent years, are also used [128,129]. Disadvantages of pseudocapacitors include a short life cycle, which is associated with redox reactions that mechanically degrade the electrodes, and lower energy density, which is associated with reaction dynamics [124,130,131].
Hybrid capacitors actually combine the action of the previous two supercapacitors by having two electrodes. They are divided into three groups called asymmetric, battery-type, and composite hybrids. The idea behind their creation was to overcome the disadvantages of double-layer supercapacitors and pseudocapacitors, resulting in capacitors with efficiencies of up to three times higher [132,133,134].
Lifetime, Aging, and Recycling of Supercapacitors
The lifespan of supercapacitors is also an important parameter. Reliability is based on stressors such as impurities in the electrode, overvoltage, excessive temperatures, and the number of cycles. In the research on this topic, authors have proposed various modeling systems that allow for predicting the lifetimes and potential causes of failure of supercapacitors [135]. The end of a supercapacitor’s life is considered to be when its capacitance drops by 20%. Usability in its original purpose, which requires high energy density, will no longer be fulfilled, but it can still be used in devices with lower requirements. This was also proposed in the studies of Saha et al. [136]. Many authors investigating new biochar materials for the production of supercapacitors also examine their stability after a certain number of cycles, as shown in Table 3.
When it is not possible to reuse a supercapacitor, it should be recycled in accordance with European Union Directive 2012/19/EU [137]. The recycling of supercapacitors is mainly based on shredding and thermal treatment. The authors have investigated various methods of cheap and effective recovery of materials from supercapacitors. This supports the idea of sustainable development where energy storage devices are easy to produce and recycle [113,138,139,140].
4. Opportunities and Threats
Among many modern technologies, supercapacitors are becoming a key element for energy storage. The research and development of supercapacitors is influencing their cost-effectiveness and their increasing use in new and advanced devices [141]. Due to their high power density and fast charging and power dissipation, they are increasingly used in many fields and sectors, such as transportation (cars, buses, and railroads), power generation (frequency control and load balancing), industry, electronics, heavy industrial equipment, wind and solar power plants, and energy storage for the UPS and off-grid installations [103]. In the case of powering electric and hybrid cars, supercapacitors act mainly as temporary stores of energy recovered during braking; in the industry of heavy vehicles and machinery where diesel and electric engines are used; they have been applied in the recovery of energy from braking or lowering their components [103,142,143]. In addition, supercapacitors have great potential for use in the IT industry as components used in mass storage [143]. Supercapacitors are much more environmentally friendly than traditional energy storage systems using traditional batteries due to their properties and long life spans [144,145]. Supercapacitors are also used as semiconductor devices to support energy storage systems using batteries [142]. Supercapacitors have also found use as components in hybrid photovoltaic farm energy storage systems [133,145,146,147]. The development of renewable energy sources and the energy industry has contributed significantly to the growth of the global supercapacitor market. In 2023, it was valued at USD 520 billion. Economic forecasts for 2024–2031 estimate that it will be worth USD 1504.29 billion by 2031 [148]. The market for supercapacitors is growing steadily, as can be confirmed by the use of ultracapacitors with voltages up to 48V created specifically for construction equipment and hybrid buses [149]. The development of this sector also encourages the development of new technologies, such as flexible and ultra-thin supercapacitors [150,151]. The most significant advantages and disadvantages are presented in Table 5.

Table 5.
Advantages and disadvantages of supercapacitors.
Since the patenting of the first supercapacitors, further development of this technology has been slowed considerably by the high cost of production and the low need for them. Currently, it is possible to speak of increased interest in this subject. In recent years, there has been increased work by researchers in the field of supercapacitors, which has been particularly abundant in terms of the number of scientific articles on the use of biochar in supercapacitors [132,146] (Figure 7).
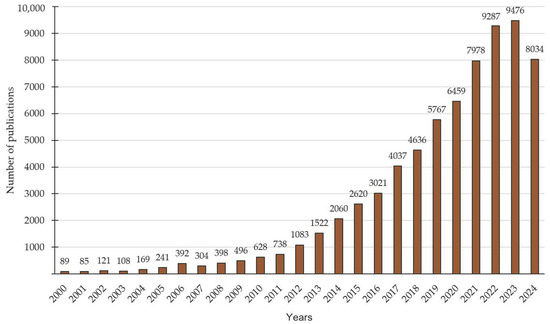
Figure 7.
Timeline of the number of articles published on the topic of supercapacitors. Compiled from [156].
The European Union is actively introducing new directives that promote and push European Member States to reduce emissions and promote sustainable development, aiming to create a climate-neutral continent by 2050. The ambitious goals of the “European Green Deal” adopted in 2019 call for a 55% reduction in emissions by 2030 relative to 1990, a 32% share of RES relative to global production, and the achievement of energy efficiency of 32.5% [157]. In view of the above, the use of biochar seems attractive and most justifiable considering its market, which is booming, and the increasingly higher prices in the EU CO2 tax system, which are even more inclined toward biochar, which is considered ecological [158,159,160]. The authors of many publications, in their research, have paid attention to the ecological and economic aspects of biochar-based supercapacitors; the intensification of research in this field significantly contributes to the growth of the most relevant parameters, which are capacity, power, and service life. They also highlight the potential of biochar as a material with a wide range of applications but also as a porous material for supercapacitor electrodes [40,83,161,162,163]. A summary of the most important features of biochar-based supercapacitors is shown in Table 6.

Table 6.
Advantages and disadvantages of biochar-based supercapacitors.
The opportunity for the use of biochar in supercapacitors is the high price of traditional supercapacitors, so the use of a source such as low-cost agricultural waste to produce biochar materials may seem attractive and environmentally neutral, which is also directly related to the idea of a sustainable society and a closed-loop economy [173]. Supercapacitors themselves are already widely used, and their spectrum of applications is wide. They help to save energy and reduce emissions in every field where they are used, which is why manufacturers and researchers are focusing on the search for solutions that can reduce costs and increase their efficiency, which only confirms the validity of the use and search for new biochar materials [154,175].
Factors that could be a barrier to the use of biochar in supercapacitors include the use of carbon materials with higher parameters than biochar and the lack of batch-to-batch reproducibility, along with uncontrolled redox reactions. Small changes in the source material or biochar preparation process can lead to significant changes in the parameters of supercapacitors [118,172].
5. Conclusions
This review confirms the still lively interest in supercapacitors, especially those based on biochar electrodes. Scientists are exploring new materials, techniques, and mechanisms to create ever more efficient biochar supercapacitors, constantly increasing their surface area and energy density. A number of activated carbon precursors have already been analyzed, a large number of which appears promising for use in this industry sector. This topic is vast and has many interesting issues that still require more tests, as well as a more detailed understanding of the mechanisms that govern them. Many new avenues of technology development, like ultra-thin and flexible supercapacitors, are also open, providing room for new research. The market and manufacturers are also showing interest and demand for modern supercapacitors, which reinforces the belief that the topic will be further developed. The idea of modern supercapacitors based on biochar coincides with European aspirations for carbon-free energy and climate neutrality, so the development of this branch could be crucial from an environmental and political point of view.
Author Contributions
Conceptualization, B.S., R.K. and M.F.; methodology, B.S., M.F. and R.K.; formal analysis, B.S., M.F. and R.K.; data curation, B.S., M.F. and R.K.; writing—original draft preparation, B.S., M.F. and R.K.; writing—review and editing, B.S.; supervision, B.S.; project administration, B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gajdzik, B.; Wolniak, R.; Nagaj, R.; Žuromskaitė-Nagaj, B.; Grebski, W.W. The Influence of the Global Energy Crisis on Energy Efficiency: A Comprehensive Analysis. Energies 2024, 17, 947. [Google Scholar] [CrossRef]
- Triguero-Ruiz, F.; Avila-Cano, A.; Trujillo Aranda, F. Measuring the diversification of energy sources: The energy mix. Renew. Energy 2023, 216, 119096. [Google Scholar] [CrossRef]
- Kołatek, R. Unijne regulacje prawne w zakresie odnawialnych źródeł energii (OZE) jako narzędzie transformacji energetycznej państw Unii Europejskiej. Kwart. Prawa Publicznego 2024, 21, 7–23. [Google Scholar] [CrossRef]
- Ahmed, B.; Al Mubarak, M.; Khouj, M. Renewable Technologies: Solar Power and Wind Power Energy Utilization—Advantages and Disadvantages. In Technological Sustainability and Business Competitive Advantage; Internet of Things; Al Mubarak, M., Hamdan, A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 507–519. ISBN 978-3-031-35524-0. [Google Scholar] [CrossRef]
- Deshmukh, M.K.G.; Sameeroddin, M.; Abdul, D.; Abdul Sattar, M. Renewable energy in the 21st century: A review. Mater. Today Proc. 2023, 80, 1756–1759. [Google Scholar] [CrossRef]
- Yang, Y. Research on the proportion of solar energy replacing conventional energy under the development trend of green building. E3S Web Conf. 2024, 490, 01010. [Google Scholar] [CrossRef]
- Blakers, A.; Stocks, M.; Lu, B.; Cheng, C. A review of pumped hydro energy storage. Prog. Energy 2021, 3, 022003. [Google Scholar] [CrossRef]
- Chen, F.; Ji, Y.; Deng, Y.; Ren, F.; Tan, S.; Wang, Z. Ultrasonic-assisted fabrication of porous carbon materials derived from agricultural waste for solid-state supercapacitors. J. Mater. Sci. 2020, 55, 11512–11523. [Google Scholar] [CrossRef]
- Senthil, C.; Lee, C.W. Biomass-derived biochar materials as sustainable energy sources for electrochemical energy storage devices. Renew. Sustain. Energy Rev. 2021, 137, 110464. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Liu, B.; Su, Z. A critical review on the application and recent developments of post-modified biochar in supercapacitors. J. Clean. Prod. 2021, 310, 127428. [Google Scholar] [CrossRef]
- Ahuja, V.; Palai, A.K.; Kumar, A.; Patel, A.K.; Farooque, A.A.; Yang, Y.-H.; Bhatia, S.K. Biochar: Empowering the future of energy production and storage. J. Anal. Appl. Pyrolysis 2024, 177, 106370. [Google Scholar] [CrossRef]
- Li, C.; He, D.; Huang, Z.-H.; Wang, M.-X. Hierarchical Micro-/Mesoporous Carbon Derived from Rice Husk by Hydrothermal Pre-Treatment for High Performance Supercapacitor. J. Electrochem. Soc. 2018, 165, A3334–A3341. [Google Scholar] [CrossRef]
- European Commission. Commission Delegated Regulation (EU) 2021/2088 of 7 July 2021 Amending Annexes II, III and IV to Regulation (EU) 2019/1009 of the European Parliament and of the Council for the Purpose of Adding Pyrolysis and Gasification Materials as a Component Material Category in EU Fertilising Products (Text with EEA Relevance); European Commission: Brussels, Belgium, 2021; Volume 427. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2021/1165 of 15 July 2021 Authorising Certain Products and Substances for Use in Organic Production and Establishing Their Lists (Text with EEA Relevance); European Commission: Brussels, Belgium, 2021; Volume 253. [Google Scholar]
- European Commission. Commission Delegated Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003 (Text with EEA Relevance); European Commission: Brussels, Belgium, 2019; Volume 170. [Google Scholar]
- Fisher, T.; Hajaligol, M.; Waymack, B.; Kellogg, D. Pyrolysis behavior and kinetics of biomass derived materials. J. Anal. Appl. Pyrolysis 2002, 62, 331–349. [Google Scholar] [CrossRef]
- Jahirul, M.; Rasul, M.; Chowdhury, A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Saffari, N.; Hajabbasi, M.A.; Shirani, H.; Mosaddeghi, M.R.; Mamedov, A.I. Biochar type and pyrolysis temperature effects on soil quality indicators and structural stability. J. Environ. Manag. 2020, 261, 110190. [Google Scholar] [CrossRef]
- Uslu, A.; Faaij, A.P.C.; Bergman, P.C.A. Pre-treatment technologies, and their effect on international bioenergy supply chain logistics. Techno-economic evaluation of torrefaction, fast pyrolysis and pelletisation. Energy 2008, 33, 1206–1223. [Google Scholar] [CrossRef]
- Arfan, M.; Eriksson, O.; Wang, Z.; Soam, S. Life cycle assessment and life cycle costing of hydrogen production from biowaste and biomass in Sweden. Energy Convers. Manag. 2023, 291, 117262. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Mofijur, M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An Overview of Recent Developments in Biomass Pyrolysis Technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Zou, J.; Hu, H.; Xue, Y.; Li, C.; Li, Y.; Yellezuome, D.; He, F.; Zhang, X.; Maksudur Rahman, M.; Cai, J. Exploring kinetic mechanisms of biomass pyrolysis using generalized logistic mixture model. Energy Convers. Manag. 2022, 258, 115522. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Gouws, S.M.; Carrier, M.; Bunt, J.R.; Neomagus, H.W.J.P. Lumped chemical kinetic modelling of raw and torrefied biomass under pressurized pyrolysis. Energy Convers. Manag. 2022, 253, 115199. [Google Scholar] [CrossRef]
- Phuakpunk, K.; Chalermsinsuwan, B.; Assabumrungrat, S. Pyrolysis kinetic parameters investigation of single and tri-component biomass: Models fitting via comparative model-free methods. Renew. Energy 2022, 182, 494–507. [Google Scholar] [CrossRef]
- Wang, K.; Remón, J.; Jiang, Z.; Ding, W. Recent Advances in the Preparation and Application of Biochar Derived from Lignocellulosic Biomass: A Mini Review. Polymers 2024, 16, 851. [Google Scholar] [CrossRef]
- Holtzer, M.; Kmita, A.; Roczniak, A. Processes of pyrolysis and their effect on cast quality and working conditions. Trans. Foundry Res. Inst. 2016, 93, 175–192. [Google Scholar] [CrossRef]
- Kufka, D.; Igo, P.-I.; Wrocławski, U. Adaptacja modułowego reaktora ciśnieniowego do testów pirolitycznej konwersji biomasy. Górnictwo Odkryw. 2015, 56, 43–46. [Google Scholar]
- Li, L.; Rowbotham, J.S.; Christopher Greenwell, H.; Dyer, P.W. An Introduction to Pyrolysis and Catalytic Pyrolysis: Versatile Techniques for Biomass Conversion. In New and Future Developments in Catalysis; Elsevier: Amsterdam, The Netherlands, 2013; pp. 173–208. ISBN 978-0-444-53878-9. [Google Scholar] [CrossRef]
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass pyrolysis: Past, present, and future. Environ. Dev. Sustain. 2020, 22, 17–32. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Kalina, M.; Sovova, S.; Hajzler, J.; Kubikova, L.; Trudicova, M.; Smilek, J.; Enev, V. Biochar Texture—A Parameter Influencing Physicochemical Properties, Morphology, and Agronomical Potential. Agronomy 2022, 12, 1768. [Google Scholar] [CrossRef]
- Saletnik, B.; Saletnik, A.; Zaguła, G.; Bajcar, M.; Puchalski, C. The Use of Wood Pellets in the Production of High Quality Biocarbon Materials. Materials 2022, 15, 4404. [Google Scholar] [CrossRef]
- Safarian, S. Performance analysis of sustainable technologies for biochar production: A comprehensive review. Energy Rep. 2023, 9, 4574–4593. [Google Scholar] [CrossRef]
- Čespiva, J.; Niedzwiecki, L.; Wnukowski, M.; Krochmalny, K.; Mularski, J.; Ochodek, T.; Pawlak-Kruczek, H. Torrefaction and gasification of biomass for polygeneration: Production of biochar and producer gas at low load conditions. Energy Rep. 2022, 8, 134–144. [Google Scholar] [CrossRef]
- Bajcar, M.; Saletnik, B.; Zaguła, G.; Puchalski, C. Analysis of the Effect of the Biomass Torrefaction Process on Selected Parameters of Dust Explosivity. Molecules 2020, 25, 3525. [Google Scholar] [CrossRef] [PubMed]
- Erses Yay, A.S.; Birinci, B.; Açıkalın, S.; Yay, K. Hydrothermal carbonization of olive pomace and determining the environmental impacts of post-process products. J. Clean. Prod. 2021, 315, 128087. [Google Scholar] [CrossRef]
- Dermawan, D.; Febrianti, A.N.; Setyawati, E.E.P.; Pham, M.-T.; Jiang, J.-J.; You, S.-J.; Wang, Y.-F. The potential of transforming rice straw (Oryza sativa) and golden shower (Cassia fistula) seed waste into high-efficiency biochar by atmospheric pressure microwave plasma. Ind. Crops Prod. 2022, 185, 115122. [Google Scholar] [CrossRef]
- Potnuri, R.; Surya, D.V.; Rao, C.S.; Yadav, A.; Sridevi, V.; Remya, N. A review on analysis of biochar produced from microwave-assisted pyrolysis of agricultural waste biomass. J. Anal. Appl. Pyrolysis 2023, 173, 106094. [Google Scholar] [CrossRef]
- Ibitoye, S.E.; Loha, C.; Mahamood, R.M.; Jen, T.-C.; Alam, M.; Sarkar, I.; Das, P.; Akinlabi, E.T. An overview of biochar production techniques and application in iron and steel industries. Bioresour. Bioprocess. 2024, 11, 65. [Google Scholar] [CrossRef]
- DeLuca, T.H.; MacKenzie, M.D.; Gundale, M.J.; Holben, W.E. Wildfire-Produced Charcoal Directly Influences Nitrogen Cycling in Ponderosa Pine Forests. Soil Sci. Soc. Am. J. 2006, 70, 448–453. [Google Scholar] [CrossRef]
- Yanai, Y.; Toyota, K.; Okazaki, M. Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiments. Soil Sci. Plant Nutr. 2007, 53, 181–188. [Google Scholar] [CrossRef]
- Wang, S.; Chai, Y.; Wang, Y.; Luo, G.; An, S. Review on the Application and Development of Biochar in Ironmaking Production. Metals 2023, 13, 1844. [Google Scholar] [CrossRef]
- Saletnik, B.; Fiedur, M.; Kwarciany, R.; Zaguła, G.; Bajcar, M. Pyrolysis as a Method for Processing of Waste from Production of Cultivated Tobacco (Nicotiana tabacum L.). Sustainability 2024, 16, 2749. [Google Scholar] [CrossRef]
- Saletnik, B.; Zaguła, G.; Bajcar, M.; Tarapatskyy, M.; Bobula, G.; Puchalski, C. Biochar as a Multifunctional Component of the Environment—A Review. Appl. Sci. 2019, 9, 1139. [Google Scholar] [CrossRef]
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 2010, 333, 117–128. [Google Scholar] [CrossRef]
- Singhal, S. Biochar as a cost-effective and eco-friendly substitute for binder in concrete: A review. Eur. J. Environ. Civ. Eng. 2023, 27, 984–1009. [Google Scholar] [CrossRef]
- Pravina Kamini, G.; Tee, K.F.; Gimbun, J.; Chin, S.C. Biochar in cementitious material—A review on physical, chemical, mechanical, and durability properties. AIMS Mater. Sci. 2023, 10, 405–425. [Google Scholar] [CrossRef]
- Amen, R.; Yaseen, M.; Mukhtar, A.; Klemeš, J.J.; Saqib, S.; Ullah, S.; Al-Sehemi, A.G.; Rafiq, S.; Babar, M.; Fatt, C.L.; et al. Lead and cadmium removal from wastewater using eco-friendly biochar adsorbent derived from rice husk, wheat straw, and corncob. Clean. Eng. Technol. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Ren, S.; Song, N.; Zhang, Y.; Wang, C.; Lu, X. Controllable fabrication of cobalt molybdate nanofibers with oxygen vacancies induced by calcinable polymer for peroxymonosulfate activation towards water treatment. Appl. Surf. Sci. 2024, 674, 160965. [Google Scholar] [CrossRef]
- Yan, H.; Lai, C.; Liu, S.; Wang, D.; Zhou, X.; Zhang, M.; Li, L.; Ma, D.; Xu, F.; Huo, X.; et al. Insight into the selective oxidation behavior of organic pollutants via Ni-N4-C mediated electron transfer pathway. Chem. Eng. J. 2023, 473, 145253. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gopinath, A.; Ranjith, N.; Praveen Akre, A.; Sreedharan, V.; Suresh Kumar, M. Potential role of biochar in advanced oxidation processes: A sustainable approach. Chem. Eng. J. 2021, 405, 126582. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, H.; Huang, Y.; Wang, W.; Wei, S. Hierarchical porous carbon obtained from animal bone and evaluation in electric double-layer capacitors. Carbon 2011, 49, 838–843. [Google Scholar] [CrossRef]
- Zhou, X.; Zeng, Z.; Zeng, G.; Lai, C.; Xiao, R.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. Persulfate activation by swine bone char-derived hierarchical porous carbon: Multiple mechanism system for organic pollutant degradation in aqueous media. Chem. Eng. J. 2020, 383, 123091. [Google Scholar] [CrossRef]
- Pontiroli, D.; Scaravonati, S.; Magnani, G.; Fornasini, L.; Bersani, D.; Bertoni, G.; Milanese, C.; Girella, A.; Ridi, F.; Verucchi, R.; et al. Super-activated biochar from poultry litter for high-performance supercapacitors. Microporous Mesoporous Mater. 2019, 285, 161–169. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, R.; Li, A.; Ji, G. In-situ self-activation strategy toward highly porous biochar for supercapacitors: Direct carbonization of marine algae. J. Electroanal. Chem. 2021, 882, 114986. [Google Scholar] [CrossRef]
- Jiang, C.; Yakaboylu, G.A.; Yumak, T.; Zondlo, J.W.; Sabolsky, E.M.; Wang, J. Activated carbons prepared by indirect and direct CO2 activation of lignocellulosic biomass for supercapacitor electrodes. Renew. Energy 2020, 155, 38–52. [Google Scholar] [CrossRef]
- Yuan, R.; Yu, S.; Shen, Y. Pyrolysis and combustion kinetics of lignocellulosic biomass pellets with calcium-rich wastes from agro-forestry residues. Waste Manag. 2019, 87, 86–96. [Google Scholar] [CrossRef]
- Charoensook, K.; Huang, C.-L.; Tai, H.-C.; Lanjapalli, V.V.K.; Chiang, L.-M.; Hosseini, S.; Lin, Y.-T.; Li, Y.-Y. Preparation of porous nitrogen-doped activated carbon derived from rice straw for high-performance supercapacitor application. J. Taiwan Inst. Chem. Eng. 2021, 120, 246–256. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, S.-C.; Sun, G.-T.; Kang, K.; Zhu, M.-Q.; Geng, Z.-C. Comparison of activated carbons prepared by one-step and two-step chemical activation process based on cotton stalk for supercapacitors application. Energy 2021, 215, 119144. [Google Scholar] [CrossRef]
- Ozpinar, P.; Dogan, C.; Demiral, H.; Morali, U.; Erol, S.; Samdan, C.; Yildiz, D.; Demiral, I. Activated carbons prepared from hazelnut shell waste by phosphoric acid activation for supercapacitor electrode applications and comprehensive electrochemical analysis. Renew. Energy 2022, 189, 535–548. [Google Scholar] [CrossRef]
- Gurten Inal, I.I.; Aktas, Z. Enhancing the performance of activated carbon based scalable supercapacitors by heat treatment. Appl. Surf. Sci. 2020, 514, 145895. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo De Medeiros, G.; Do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Akar, S.T.; Yilmazer, D.; Celik, S.; Balk, Y.Y.; Akar, T. Effective biodecolorization potential of surface modified lignocellulosic industrial waste biomass. Chem. Eng. J. 2015, 259, 286–292. [Google Scholar] [CrossRef]
- Duan, D.; Chen, D.; Huang, L.; Zhang, Y.; Zhang, Y.; Wang, Q.; Xiao, G.; Zhang, W.; Lei, H.; Ruan, R. Activated carbon from lignocellulosic biomass as catalyst: A review of the applications in fast pyrolysis process. J. Anal. Appl. Pyrolysis 2021, 158, 105246. [Google Scholar] [CrossRef]
- Pallarés, J.; González-Cencerrado, A.; Arauzo, I. Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenergy 2018, 115, 64–73. [Google Scholar] [CrossRef]
- Luo, M.; Chen, J.; Li, Q.; Wang, Y. Cotton-Based Activated Carbon Fiber with High Specific Surface Area Prepared by Low-Temperature Hydrothermal Carbonization with Urea Enhancement. Ind. Eng. Chem. Res. 2023, 62, 8744–8753. [Google Scholar] [CrossRef]
- Cruz, O.F., Jr.; Serafin, J.; Azar, F.-Z.; Casco, M.E.; Silvestre-Albero, J.; Hotza, D.; Rambo, C.R. Microwave-Assisted hydrothermal carbonization and characterization of Amazonian biomass as an activated carbon for methane adsorption. Fuel 2024, 358, 130329. [Google Scholar] [CrossRef]
- Azeez, M.O.; Tanimu, A.; Alhooshani, K.; Ganiyu, S.A. Synergistic effect of nitrogen and molybdenum on activated carbon matrix for selective adsorptive Desulfurization: Insights into surface chemistry modification. Arab. J. Chem. 2022, 15, 103454. [Google Scholar] [CrossRef]
- Tibor, S.T.; Grande, C.A. Industrial production of activated carbon using circular bioeconomy principles: Case study from a Romanian company. Clean. Eng. Technol. 2022, 7, 100443. [Google Scholar] [CrossRef]
- Awitdrus; Siregar, G.M.G.; Agustino; Saktioto; Iwantono; Syahputra, R.F.; Farma, R. KOH Activation with Microwave Irradiation and its Effect on the Physical Properties of Orange Peel Activated Carbon. J. Phys. Conf. Ser. 2021, 2049, 012025. [Google Scholar] [CrossRef]
- Taher, T.; Maulana, S.; Mawaddah, N.; Munandar, A.; Rianjanu, A.; Lesbani, A. Low-temperature hydrothermal carbonization of activated carbon microsphere derived from microcrystalline cellulose as carbon dioxide (CO2) adsorbent. Mater. Today Sustain. 2023, 23, 100464. [Google Scholar] [CrossRef]
- Li, C.; Feng, Y.; Zhong, F.; Deng, J.; Yu, T.; Cao, H.; Niu, W. Optimization of microwave-assisted hydrothermal carbonization and potassium bicarbonate activation on the structure and electrochemical characteristics of crop straw-derived biochar. J. Energy Storage 2022, 55, 105838. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, B.; Liu, L.; Cao, L.; Yuan, Q.; Tian, J.; Huang, Z.; Zong, Z.; Zhang, P.; Lin, Z.; et al. Tobacco Waste Biomass for Electrochemical Energy Storage Application. J. Phys. Conf. Ser. 2022, 2160, 012052. [Google Scholar] [CrossRef]
- Andrade, T.S.; Vakros, J.; Mantzavinos, D.; Lianos, P. Biochar obtained by carbonization of spent coffee grounds and its application in the construction of an energy storage device. Chem. Eng. J. Adv. 2020, 4, 100061. [Google Scholar] [CrossRef]
- Husain, Z.; Shakeelur Raheman, A.R.; Ansari, K.B.; Pandit, A.B.; Khan, M.S.; Qyyum, M.A.; Lam, S.S. Nano-sized mesoporous biochar derived from biomass pyrolysis as electrochemical energy storage supercapacitor. Mater. Sci. Energy Technol. 2022, 5, 99–109. [Google Scholar] [CrossRef]
- Wang, K.; Xu, M.; Gu, Z.; Ahrenkiel, P.; Lee, J.; Gibbons, W.; Croat, J.; Fan, Q. Pyrrole modified biomass derived hierarchical porous carbon as high performance symmetrical supercapacitor electrodes. Int. J. Hydrogen Energy 2016, 41, 13109–13115. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Cuña, A.; Da Silva, E.L.; Amaral-Labat, G.; Lenz E Silva, G.F.B.; Bouafif, H.; Koubaa, A. The conversion of wood residues, using pilot-scale technologies, into porous activated biochars for supercapacitors. J. Porous Mater. 2020, 27, 537–548. [Google Scholar] [CrossRef]
- Wang, X.; Yun, S.; Fang, W.; Zhang, C.; Liang, X.; Lei, Z.; Liu, Z. Layer-Stacking Activated Carbon Derived from Sunflower Stalk as Electrode Materials for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 11397–11407. [Google Scholar] [CrossRef]
- Shu, Y.; Bai, Q.; Fu, G.; Xiong, Q.; Li, C.; Ding, H.; Shen, Y.; Uyama, H. Hierarchical porous carbons from polysaccharides carboxymethyl cellulose, bacterial cellulose, and citric acid for supercapacitor. Carbohydr. Polym. 2020, 227, 115346. [Google Scholar] [CrossRef]
- EL-Ouahabi, H.; Khaddor, M.; Lillo-Rodenas, M.A.; Roman Martinez, M.D.C.; Elmouwahidi, A.; Pérez-Cadenas, A.F.; Ouzzine, M. Bio-Waste Argan Nut Shell-Derived Porous Activated Carbon for High Performance Supercapacitor Electrode Material. ECS Meet. Abstr. 2023, MA2023-01, 1098. [Google Scholar] [CrossRef]
- Bui, T.A.N.; Huynh, T.V.; Tran, H.L.; Doong, R. Erbium-Doped GQD-Embedded Coffee-Ground-Derived Porous Biochar for Highly Efficient Asymmetric Supercapacitor. Nanomaterials 2022, 12, 1939. [Google Scholar] [CrossRef]
- Rawat, S.; Boobalan, T.; Sathish, M.; Hotha, S.; Thallada, B. Utilization of CO2 activated litchi seed biochar for the fabrication of supercapacitor electrodes. Biomass Bioenergy 2023, 171, 106747. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Azargohar, R.; Dalai, A.K. Steam and KOH activation of biochar: Experimental and modeling studies. Microporous Mesoporous Mater. 2008, 110, 413–421. [Google Scholar] [CrossRef]
- Hoseinzadeh Hesas, R.; Arami-Niya, A.; Wan Daud, W.M.A.; Sahu, J.N. Microwave-assisted production of activated carbons from oil palm shell in the presence of CO2 or N2 for CO2 adsorption. J. Ind. Eng. Chem. 2015, 24, 196–205. [Google Scholar] [CrossRef]
- Anto, S.; Sudhakar, M.P.; Shan Ahamed, T.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Activation strategies for biochar to use as an efficient catalyst in various applications. Fuel 2021, 285, 119205. [Google Scholar] [CrossRef]
- Sumangala Devi, N.; Hariram, M.; Vivekanandhan, S. Modification techniques to improve the capacitive performance of biocarbon materials. J. Energy Storage 2021, 33, 101870. [Google Scholar] [CrossRef]
- Gámiz, B.; Hall, K.; Spokas, K.A.; Cox, L. Understanding Activation Effects on Low-Temperature Biochar for Optimization of Herbicide Sorption. Agronomy 2019, 9, 588. [Google Scholar] [CrossRef]
- Tan, X.; Liu, S.; Liu, Y.; Gu, Y.; Zeng, G.; Hu, X.; Wang, X.; Liu, S.; Jiang, L. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Mendhe, A.; Panda, H.S. A review on electrolytes for supercapacitor device. Discov. Mater. 2023, 3, 29. [Google Scholar] [CrossRef]
- Ma, C.; Tang, L.; Cheng, H.; Li, Z.; Li, W.; He, G. Biochar for supercapacitor electrodes: Mechanisms in aqueous electrolytes. Battery Energy 2024, 3, 20230058. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Ramavath, J.N.; Raja, M.; Kumar, S.; Kothandaraman, R. Mild acidic mixed electrolyte for high-performance electrical double layer capacitor. Appl. Surf. Sci. 2019, 489, 867–874. [Google Scholar] [CrossRef]
- Ho, J.; Jow, T.R.; Boggs, S. Historical introduction to capacitor technology. IEEE Electr. Insul. Mag. 2010, 26, 20–25. [Google Scholar] [CrossRef]
- Naoi, K.; Simon, P. New Materials and New Configurations for Advanced Electrochemical Capacitors. Electrochem. Soc. Interface 2008, 17, 34–37. [Google Scholar] [CrossRef]
- Zhao, J.; Burke, A.F. Review on supercapacitors: Technologies and performance evaluation. J. Energy Chem. 2021, 59, 276–291. [Google Scholar] [CrossRef]
- Thiagarajan, K.; Theerthagiri, J.; Senthil, R.A.; Madhavan, J. Simple and low cost electrode material based on Ca2V2O7/PANI nanoplatelets for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2017, 28, 17354–17362. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Masrur Ahmed, A.A.; Bailek, N.; Abualigah, L.; Bouchouicha, K.; Kuriqi, A.; Sharifi, A.; Sareh, P.; Al Khatib, A.M.G.; Mishra, P.; Colak, I.; et al. Global control of electrical supply: A variational mode decomposition-aided deep learning model for energy consumption prediction. Energy Rep. 2023, 10, 2152–2165. [Google Scholar] [CrossRef]
- Song, Z.; Hou, J.; Hofmann, H.; Li, J.; Ouyang, M. Sliding-mode and Lyapunov function-based control for battery/supercapacitor hybrid energy storage system used in electric vehicles. Energy 2017, 122, 601–612. [Google Scholar] [CrossRef]
- Saikia, B.K.; Benoy, S.M.; Bora, M.; Tamuly, J.; Pandey, M.; Bhattacharya, D. A brief review on supercapacitor energy storage devices and utilization of natural carbon resources as their electrode materials. Fuel 2020, 282, 118796. [Google Scholar] [CrossRef]
- Berrueta, A.; Ursua, A.; Martin, I.S.; Eftekhari, A.; Sanchis, P. Supercapacitors: Electrical Characteristics, Modeling, Applications, and Future Trends. IEEE Access 2019, 7, 50869–50896. [Google Scholar] [CrossRef]
- Jalal, N.I.; Ibrahim, R.I.; Oudah, M.K. A review on Supercapacitors: Types and components. J. Phys. Conf. Ser. 2021, 1973, 012015. [Google Scholar] [CrossRef]
- Halper, M.S.; Ellenbogen, J.C. Supercapacitors: A Brief Overview; The MITRE Corporation: McLean, VA, USA, 2006. [Google Scholar]
- Czagany, M.; Hompoth, S.; Keshri, A.K.; Pandit, N.; Galambos, I.; Gacsi, Z.; Baumli, P. Supercapacitors: An Efficient Way for Energy Storage Application. Materials 2024, 17, 702. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, C.; Cho, S.; Moon, G.D.; Kim, B.; Chang, H.; Jang, H.D. High capacitance and energy density supercapacitor based on biomass-derived activated carbons with reduced graphene oxide binder. Carbon 2018, 132, 16–24. [Google Scholar] [CrossRef]
- Bello, A.; Barzegar, F.; Momodu, D.; Dangbegnon, J.; Taghizadeh, F.; Manyala, N. Symmetric supercapacitors based on porous 3D interconnected carbon framework. Electrochim. Acta 2015, 151, 386–392. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520. [Google Scholar] [CrossRef]
- Phiri, J.; Dou, J.; Vuorinen, T.; Gane, P.A.C.; Maloney, T.C. Highly Porous Willow Wood-Derived Activated Carbon for High-Performance Supercapacitor Electrodes. ACS Omega 2019, 4, 18108–18117. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Vermisoglou, E.C.; Giannouri, M.; Todorova, N.; Giannakopoulou, T.; Lekakou, C.; Trapalis, C. Recycling of typical supercapacitor materials. Waste Manag. Res. J. Sustain. Circ. Econ. 2016, 34, 337–344. [Google Scholar] [CrossRef]
- Seo, D.H.; Han, Z.J.; Kumar, S.; Ostrikov, K. (Ken) Structure-Controlled, Vertical Graphene-Based, Binder-Free Electrodes from Plasma-Reformed Butter Enhance Supercapacitor Performance. Adv. Energy Mater. 2013, 3, 1316–1323. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor Devices Based on Graphene Materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Krüner, B.; Odenwald, C.; Tolosa, A.; Schreiber, A.; Aslan, M.; Kickelbick, G.; Presser, V. Carbide-derived carbon beads with tunable nanopores from continuously produced polysilsesquioxanes for supercapacitor electrodes. Sustain. Energy Fuels 2017, 1, 1588–1600. [Google Scholar] [CrossRef]
- Zhong, W.; Sun, H.; Pan, J.; Zhang, Y.; Yan, X.; Guan, Y.; Shen, W.; Cheng, X. Hierarchical porous TiO2/carbide-derived carbon for asymmetric supercapacitor with enhanced electrochemical performance. Mater. Sci. Semicond. Process. 2021, 127, 105715. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Gao, P.-C.; Tsai, W.-Y.; Daffos, B.; Taberna, P.-L.; Pérez, C.R.; Gogotsi, Y.; Simon, P.; Favier, F. Graphene-like carbide derived carbon for high-power supercapacitors. Nano Energy 2015, 12, 197–206. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, G.; Lu, L.; Wang, T.; Xu, H.; Yu, C.; Li, H.; Tian, W. Improving the electrochemical performances of active carbon-based supercapacitors through the combination of introducing functional groups and using redox additive electrolyte. J. Saudi Chem. Soc. 2018, 22, 908–918. [Google Scholar] [CrossRef]
- Bai, X.; Hu, X.; Zhou, S.; Yan, J.; Sun, C.; Chen, P.; Li, L. In situ polymerization and characterization of grafted poly (3,4-ethylenedioxythiophene)/multiwalled carbon nanotubes composite with high electrochemical performances. Electrochim. Acta 2013, 87, 394–400. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, B.; Song, H.; Chen, X. Preparation and electrochemical performance of polyaniline-based carbon nanotubes as electrode material for supercapacitor. Electrochim. Acta 2010, 55, 7021–7027. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhang, L.; Du, T.; Ren, B.; Xu, Y.; Wang, S.; Miao, J.; Liu, Z. A review of carbon materials for supercapacitors. Mater. Des. 2022, 221, 111017. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To Be or Not To Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839. [Google Scholar] [CrossRef]
- Majumdar, D.; Maiyalagan, T.; Jiang, Z. Recent Progress in Ruthenium Oxide-Based Composites for Supercapacitor Applications. ChemElectroChem 2019, 6, 4343–4372. [Google Scholar] [CrossRef]
- Lou, S.; Cheng, X.; Wang, L.; Gao, J.; Li, Q.; Ma, Y.; Gao, Y.; Zuo, P.; Du, C.; Yin, G. High-rate capability of three-dimensionally ordered macroporous T-Nb2O5 through Li+ intercalation pseudocapacitance. J. Power Sources 2017, 361, 80–86. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Lin, C.; Yang, Y.; Xu, L.; Du, X.; Xie, J.; Lin, J.; Sun, J. Achieving High Pseudocapacitance of 2D Titanium Carbide (MXene) by Cation Intercalation and Surface Modification. Adv. Energy Mater. 2017, 7, 1602725. [Google Scholar] [CrossRef]
- Kurra, N.; Jiang, Q.; Syed, A.; Xia, C.; Alshareef, H.N. Micro-Pseudocapacitors with Electroactive Polymer Electrodes: Toward AC-Line Filtering Applications. ACS Appl. Mater. Interfaces 2016, 8, 12748–12755. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Q.; Liu, L.; Manasa, P.; Kang, L.; Ran, F. Vanadium nitride for aqueous supercapacitors: A topic review. J. Mater. Chem. A 2020, 8, 8218–8233. [Google Scholar] [CrossRef]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef] [PubMed]
- Afif, A.; Rahman, S.M.; Tasfiah Azad, A.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage—A review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Khan, M.A.; Zeb, K.; Sathishkumar, P.; Ali, M.U.; Uddin, W.; Hussain, S.; Ishfaq, M.; Khan, I.; Cho, H.-G.; Kim, H.-J. A Novel Supercapacitor/Lithium-Ion Hybrid Energy System with a Fuzzy Logic-Controlled Fast Charging and Intelligent Energy Management System. Electronics 2018, 7, 63. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Wang, H. Review on reliability of supercapacitors in energy storage applications. Appl. Energy 2020, 278, 115436. [Google Scholar] [CrossRef]
- Saha, P.; Dey, S.; Khanra, M. Second-life applications of supercapacitors: Effective capacitance prognosis and aging. J. Power Sources 2021, 496, 229824. [Google Scholar] [CrossRef]
- European Commission. Directive 2012/19/EU of the European Parliament and of the Council of 4 July 2012 on Waste Electrical and Electronic Equipment (WEEE) (Recast); Text with EEA Relevance; European Commission: Brussels, Belgium, 2012; Volume 197. [Google Scholar]
- Jiang, G.; Pickering, S.J. Recycling supercapacitors based on shredding and mild thermal treatment. Waste Manag. 2016, 48, 465–470. [Google Scholar] [CrossRef]
- Jiang, G.; Pickering, S.J. Recycling Graphene from Supercapacitor Electrodes as Reinforcing Filler for Epoxy Resins. Waste Biomass Valorization 2019, 10, 215–221. [Google Scholar] [CrossRef]
- Yanshyna, O.; Weissman, H.; Rybtchinski, B. Recyclable electrochemical supercapacitors based on carbon nanotubes and organic nanocrystals. Nanoscale 2020, 12, 8909–8914. [Google Scholar] [CrossRef] [PubMed]
- Deep, A.; Gupta, A.; Singh, S.; Khatoon, N.; Gupta, G. Overview of Supercapacitors: A Comprehensive Review. Preprints 2024. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, V. Current Technology of Supercapacitors: A Review. J. Electron. Mater. 2020, 49, 3520–3532. [Google Scholar] [CrossRef]
- Jing, W.; Hung Lai, C.; Wong, S.H.W.; Wong, M.L.D. Battery-supercapacitor hybrid energy storage system in standalone DC microgrids: Areview. IET Renew. Power Gener. 2017, 11, 461–469. [Google Scholar] [CrossRef]
- Şahin, M.E.; Blaabjerg, F. A Hybrid PV-Battery/Supercapacitor System and a Basic Active Power Control Proposal in MATLAB/Simulink. Electronics 2020, 9, 129. [Google Scholar] [CrossRef]
- Glavin, M.E.; Hurley, W.G. Optimisation of a photovoltaic battery ultracapacitor hybrid energy storage system. Sol. Energy 2012, 86, 3009–3020. [Google Scholar] [CrossRef]
- Manandhar, U.; Ukil, A.; Gooi, H.B.; Tummuru, N.R.; Kollimalla, S.K.; Wang, B.; Chaudhari, K. Energy Management and Control for Grid Connected Hybrid Energy Storage System Under Different Operating Modes. IEEE Trans. Smart Grid 2019, 10, 1626–1636. [Google Scholar] [CrossRef]
- Logerais, P.-O.; Riou, O.; Camara, M.A.; Durastanti, J.-F. Study of Photovoltaic Energy Storage by Supercapacitors through Both Experimental and Modelling Approaches. J. Sol. Energy 2013, 2013, 659014. [Google Scholar] [CrossRef]
- Supercapacitor Market Analysis, Size, Share & Trends|2031. Available online: https://www.skyquestt.com/report/supercapacitor-market (accessed on 19 June 2024).
- Maxwell Technologies Products—Mouser Poland. Available online: https://www.mouser.pl/c/?m=Maxwell%20Technologies (accessed on 19 June 2024).
- Wang, Y.; Wu, X.; Han, Y.; Li, T. Flexible supercapacitor: Overview and outlooks. J. Energy Storage 2021, 42, 103053. [Google Scholar] [CrossRef]
- Zhao, Z.; Xia, K.; Shao, W.; Wang, X.; Zhang, Q.; Hou, Y.; Ye, Z.; Zheng, Z.; Lu, J. Ultrathin Tape-Supercapacitor with High Capacitance and Durable Flexibility via Repeated In-situ Polymerizations of Polyaniline. Chem. Eng. J. 2023, 471, 144721. [Google Scholar] [CrossRef]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C.-M. Biomass-derived porous carbon materials with different dimensions for supercapacitor electrodes: A review. J. Mater. Chem. A 2019, 7, 16028–16045. [Google Scholar] [CrossRef]
- Şahin, M.; Blaabjerg, F.; Sangwongwanich, A. A Comprehensive Review on Supercapacitor Applications and Developments. Energies 2022, 15, 674. [Google Scholar] [CrossRef]
- Kumar, N.; Kim, S.-B.; Lee, S.-Y.; Park, S.-J. Recent Advanced Supercapacitor: A Review of Storage Mechanisms, Electrode Materials, Modification, and Perspectives. Nanomaterials 2022, 12, 3708. [Google Scholar] [CrossRef]
- Shang, W.; Yu, W.; Xiao, X.; Ma, Y.; He, Y.; Zhao, Z.; Tan, P. Insight into the self-discharge suppression of electrochemical capacitors: Progress and challenges. Adv. Powder Mater. 2023, 2, 100075. [Google Scholar] [CrossRef]
- ScienceDirect.com|Science, Health and Medical Journals, Full Text Articles and Books. Available online: https://www.sciencedirect.com/ (accessed on 16 July 2024).
- European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions; The European Green Deal; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Ånensen, K.R. Biochar—A Market Analysis and Predictions for the Future. Master Thesis, University of Stavanger, Stavanger, Norway, 2023. [Google Scholar]
- Biochar Market Size Is Expanding around USD 633.31 Mn by 2032. Available online: https://www.precedenceresearch.com/biochar-market (accessed on 22 July 2024).
- European Commission. Directive 2003/87/EC of the European Parliament and of the Council of 13 October 2003 Establishing a Scheme for Greenhouse Gas Emission Allowance Trading within the Community and Amending Council Directive 96/61/EC (Text with EEA Relevance); European Commission: Brussels, Belgium, 2003; Volume 275. [Google Scholar]
- Lin, Y.; Li, F.; Zhang, Q.; Liu, G.; Xue, C. Controllable preparation of green biochar based high-performance supercapacitors. Ionics 2022, 28, 2525–2561. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Cai, M.; Zhou, Y.; Guo, C.; Han, Y.; Zhang, L. Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives. Polymers 2023, 15, 2741. [Google Scholar] [CrossRef] [PubMed]
- Mian, M.M.; Kamana, I.M.L.; An, X.; Abbas, S.C.; Ahommed, M.S.; He, Z.; Ni, Y. Cellulose nanofibers as effective binders for activated biochar-derived high-performance supercapacitors. Carbohydr. Polym. 2023, 301, 120353. [Google Scholar] [CrossRef]
- Ma, Z.-W.; Liu, H.-Q.; Lü, Q.-F. Porous biochar derived from tea saponin for supercapacitor electrode: Effect of preparation technique. J. Energy Storage 2021, 40, 102773. [Google Scholar] [CrossRef]
- Gao, M.; Wang, W.-K.; Zheng, Y.-M.; Zhao, Q.-B.; Yu, H.-Q. Hierarchically porous biochar for supercapacitor and electrochemical H2O2 production. Chem. Eng. J. 2020, 402, 126171. [Google Scholar] [CrossRef]
- Lima, R.M.A.P.; Dos Reis, G.S.; Thyrel, M.; Alcaraz-Espinoza, J.J.; Larsson, S.H.; De Oliveira, H.P. Facile Synthesis of Sustainable Biomass-Derived Porous Biochars as Promising Electrode Materials for High-Performance Supercapacitor Applications. Nanomaterials 2022, 12, 866. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, R.; Khoja, A.H.; Naqvi, S.R.; Gao, N.; Amin, N.A.S. A Review on Production and Surface Modifications of Biochar Materials via Biomass Pyrolysis Process for Supercapacitor Applications. Catalysts 2022, 12, 798. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, P.; Jia, J.; Liu, Z.; Huo, L.; Zhao, L.; Zhao, Y.; Niu, W.; Yao, Z. Machine learning in clarifying complex relationships: Biochar preparation procedures and capacitance characteristics. Chem. Eng. J. 2024, 485, 149975. [Google Scholar] [CrossRef]
- Khedulkar, A.P.; Dang, V.D.; Thamilselvan, A.; Doong, R.; Pandit, B. Sustainable high-energy supercapacitors: Metal oxide-agricultural waste biochar composites paving the way for a greener future. J. Energy Storage 2024, 77, 109723. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Ma, M.-G.; Ji, X.-X. Preparation of Lignin-Based Carbon Materials and Its Application as a Sorbent. Materials 2019, 12, 1111. [Google Scholar] [CrossRef]
- Zhang, M.; Duan, Y.; Chen, T.; Qi, J.; Xu, T.; Du, H.; Si, C. Lignocellulosic materials for energy storage devices. Ind. Crops Prod. 2023, 203, 117174. [Google Scholar] [CrossRef]
- Rawat, S.; Wang, C.-T.; Lay, C.-H.; Hotha, S.; Bhaskar, T. Sustainable biochar for advanced electrochemical/energy storage applications. J. Energy Storage 2023, 63, 107115. [Google Scholar] [CrossRef]
- Khedulkar, A.P.; Pandit, B.; Dang, V.D.; Doong, R. Agricultural waste to real worth biochar as a sustainable material for supercapacitor. Sci. Total Environ. 2023, 869, 161441. [Google Scholar] [CrossRef]
- Liu, H.; Liu, S.; Liu, L. Biomass-Derived Porous Carbon: Synthesis and Application for Energy Conversion and Storage. Adv. Mater. Sci. Technol. 2023, 5, 11809. [Google Scholar] [CrossRef]
- Lu, W.; Si, Y.; Zhao, C.; Chen, T.; Li, C.; Zhang, C.; Wang, K. Biomass-derived carbon applications in the field of supercapacitors: Progress and prospects. Chem. Eng. J. 2024, 495, 153311. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).