Comparative Analysis of Primary and Secondary Emission Mitigation Measures for Small-Scale Wood Chip Combustion
Abstract
1. Introduction
2. Material and Methods
2.1. Wood Chips
2.2. Additives
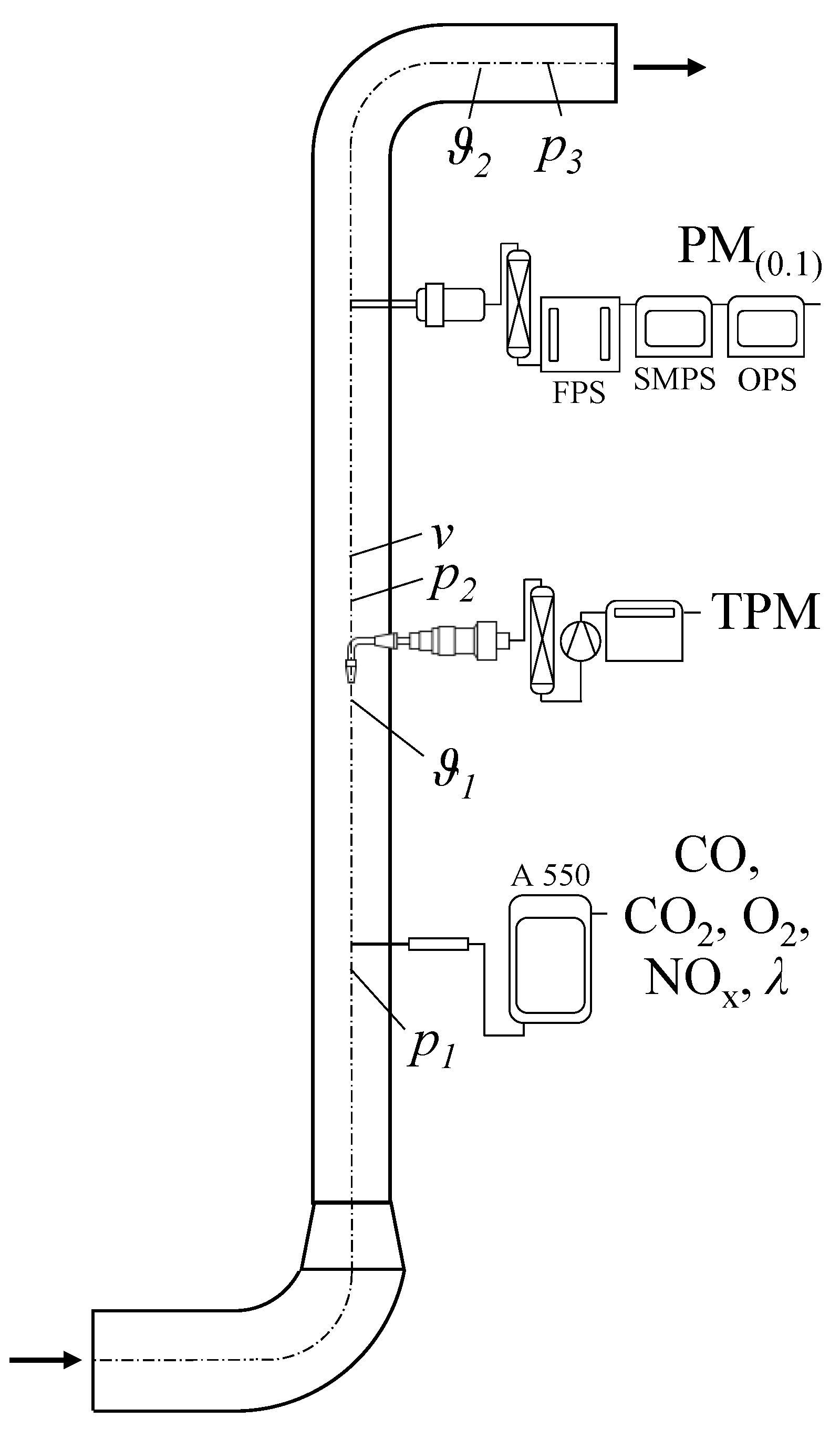
2.3. Combustion Plant
2.4. Total Particulate Matter (TPM) Emissions
2.4.1. Gravimetric Determination
2.4.2. Chemical Composition
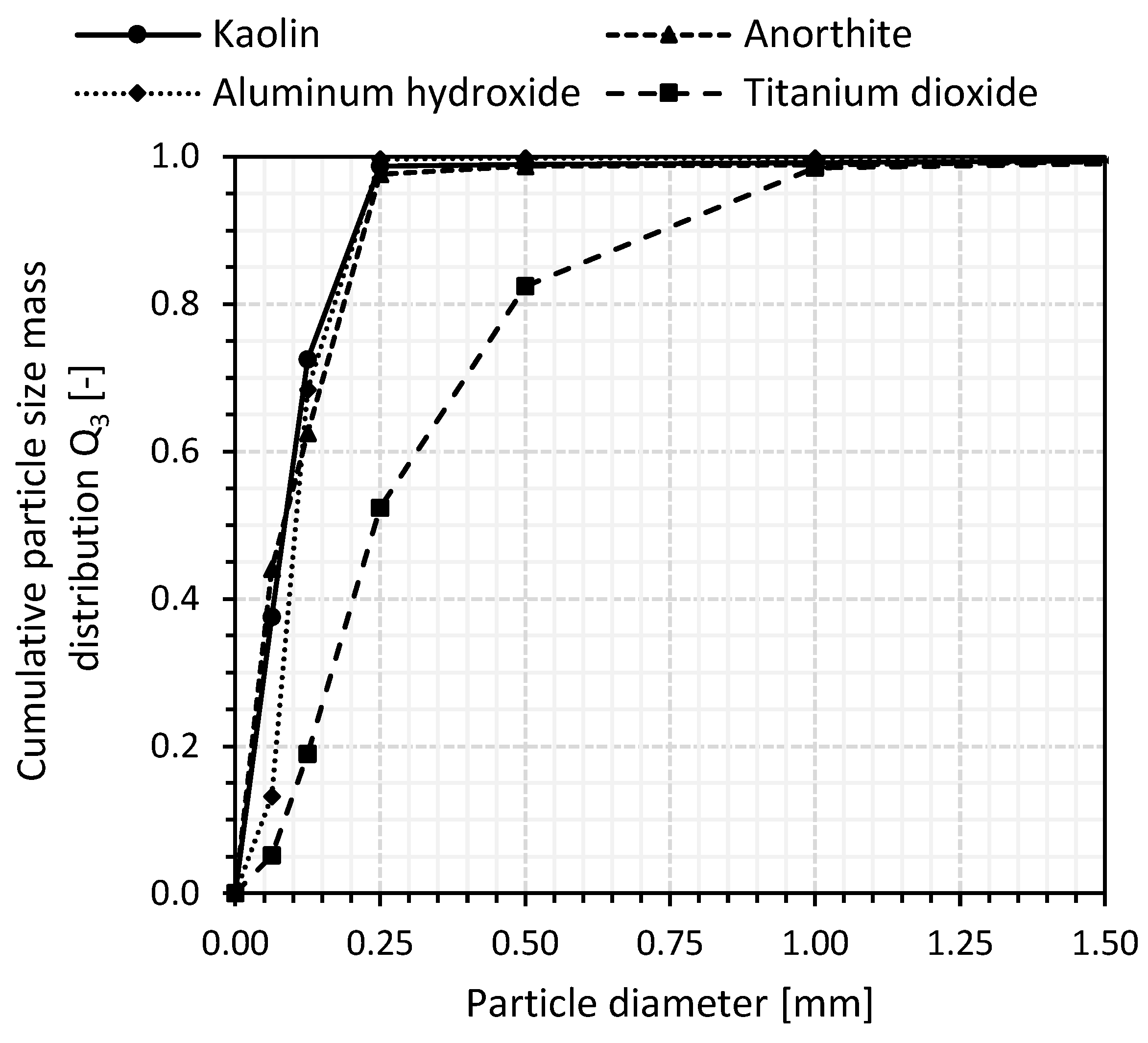
2.4.3. Particle Size Number Distribution
2.5. Carbon Monoxide (CO) Emissions
2.6. Ashes
2.6.1. Chemical Composition
2.6.2. Crystalline Phases
3. Results and Discussion
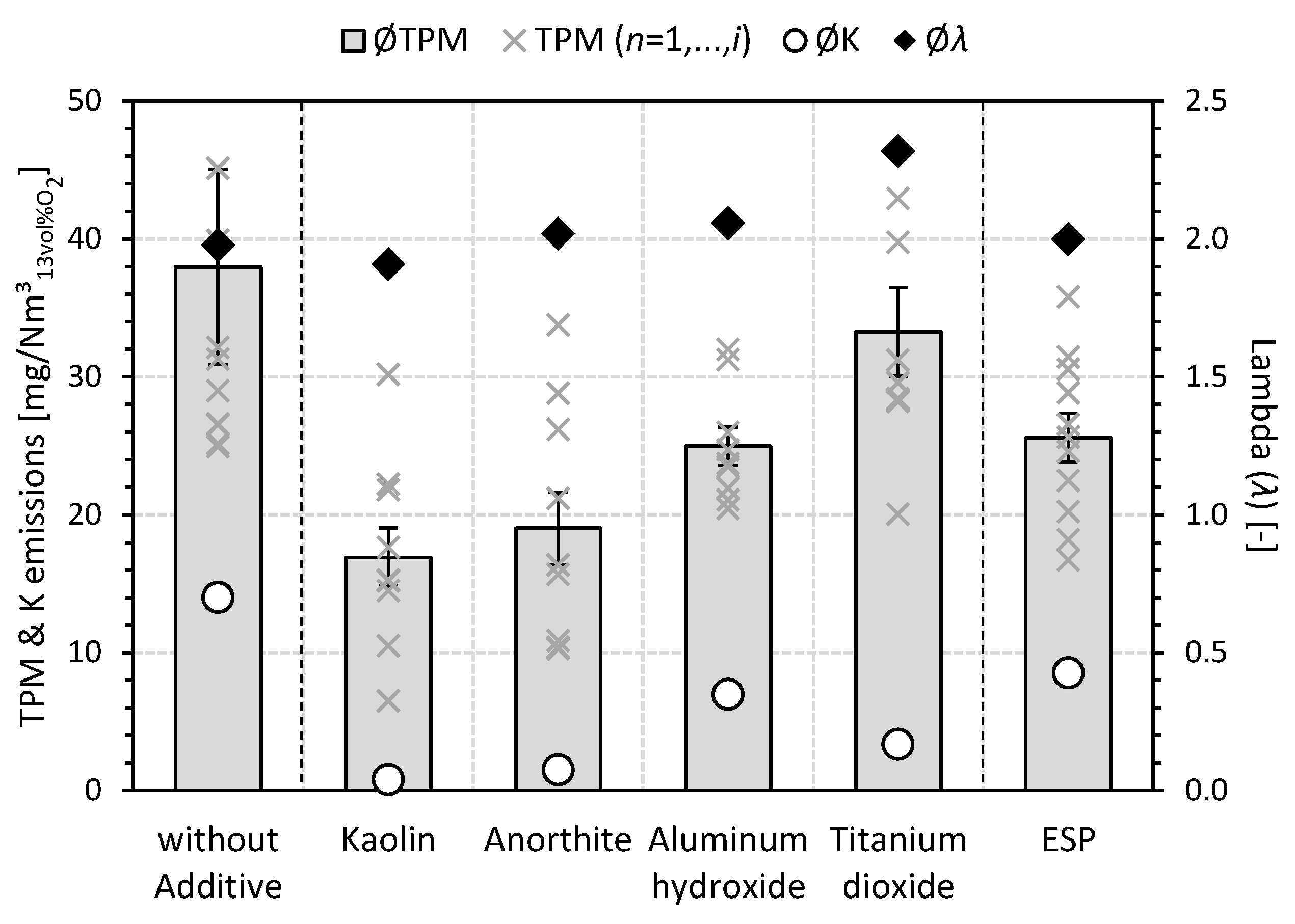
3.1. Total Particulate Matter (TPM) Emissions
3.1.1. Gravimetric Determination
3.1.2. Chemical Composition
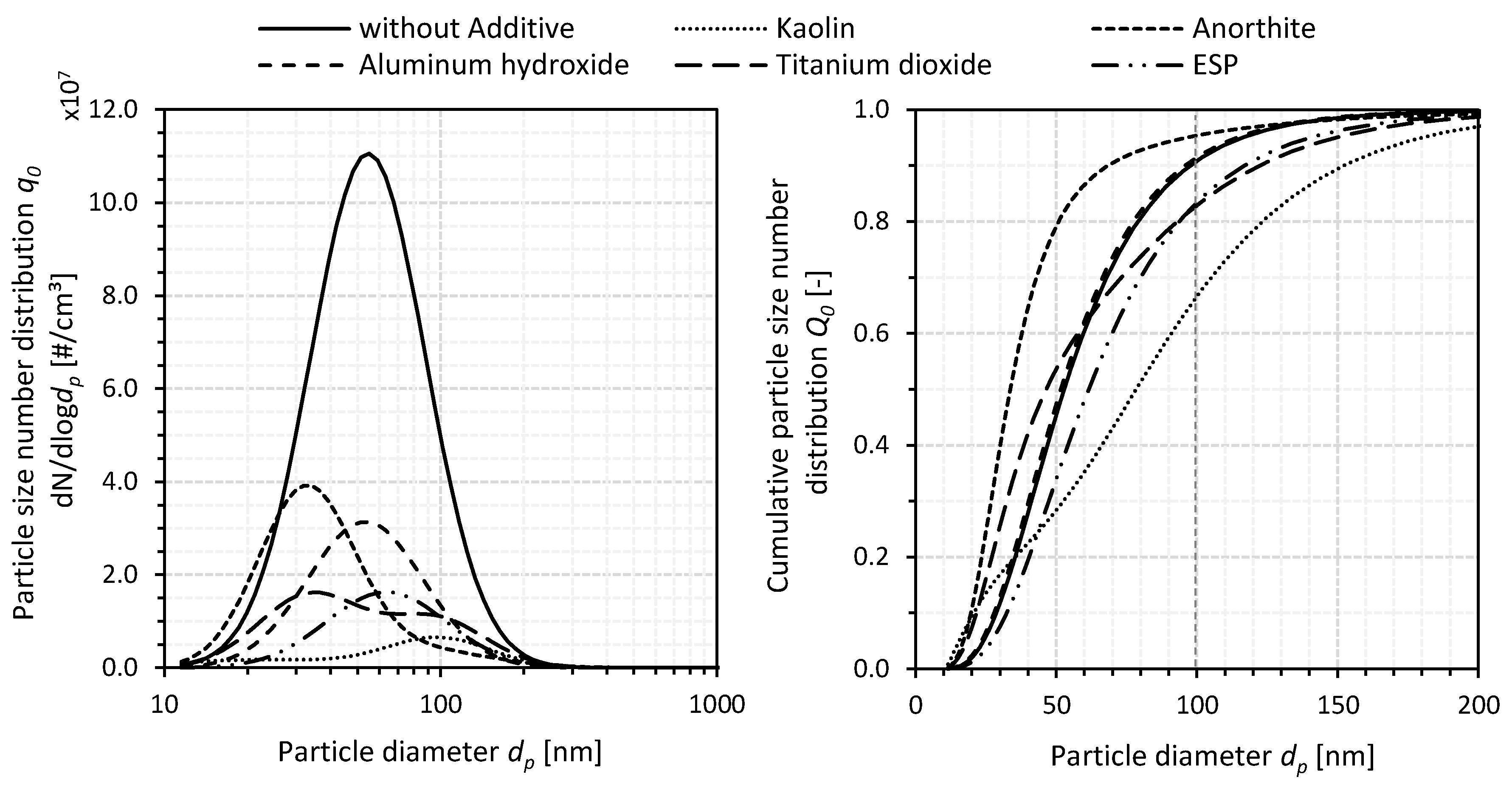
3.1.3. Particle Size Number Distribution
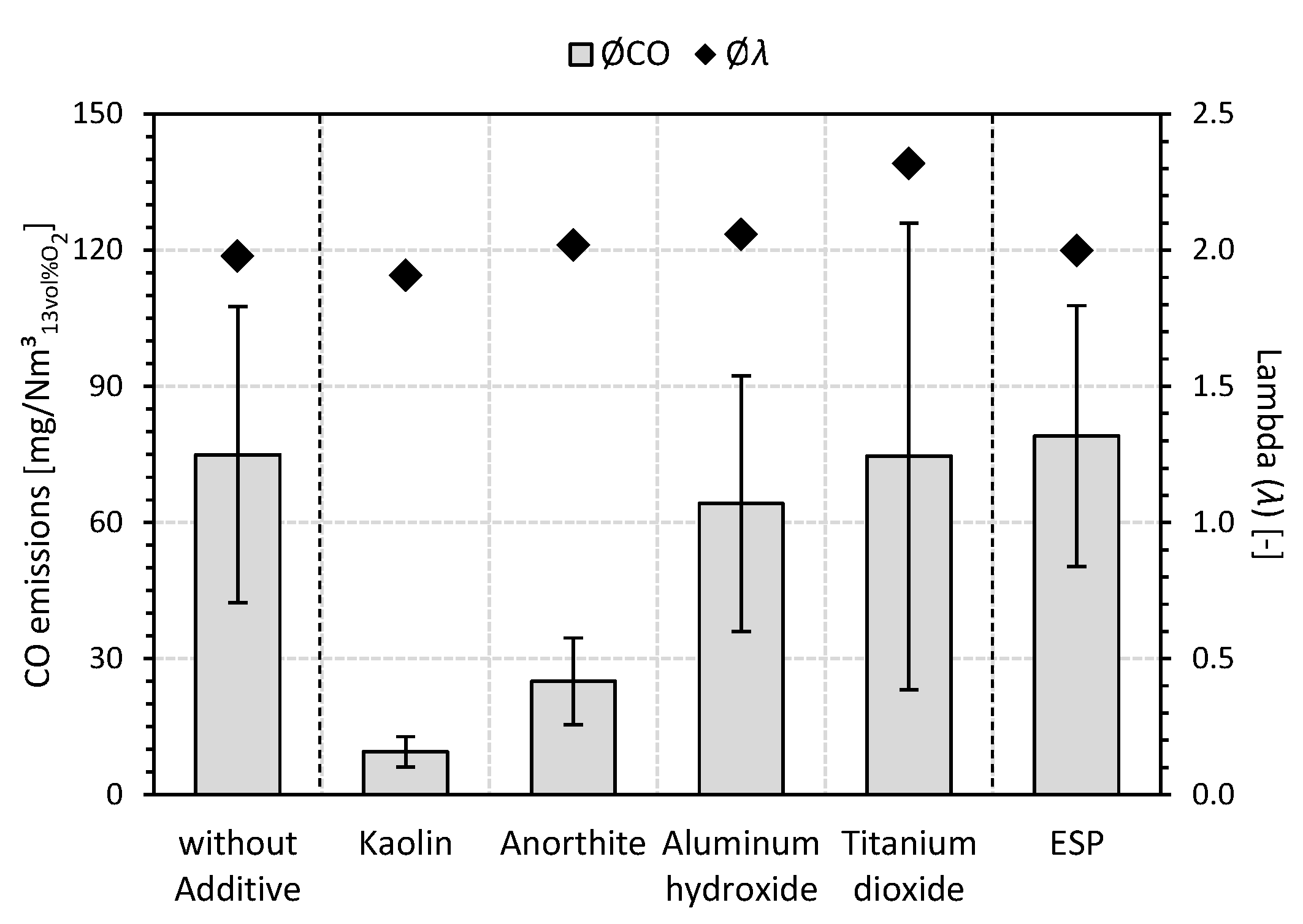
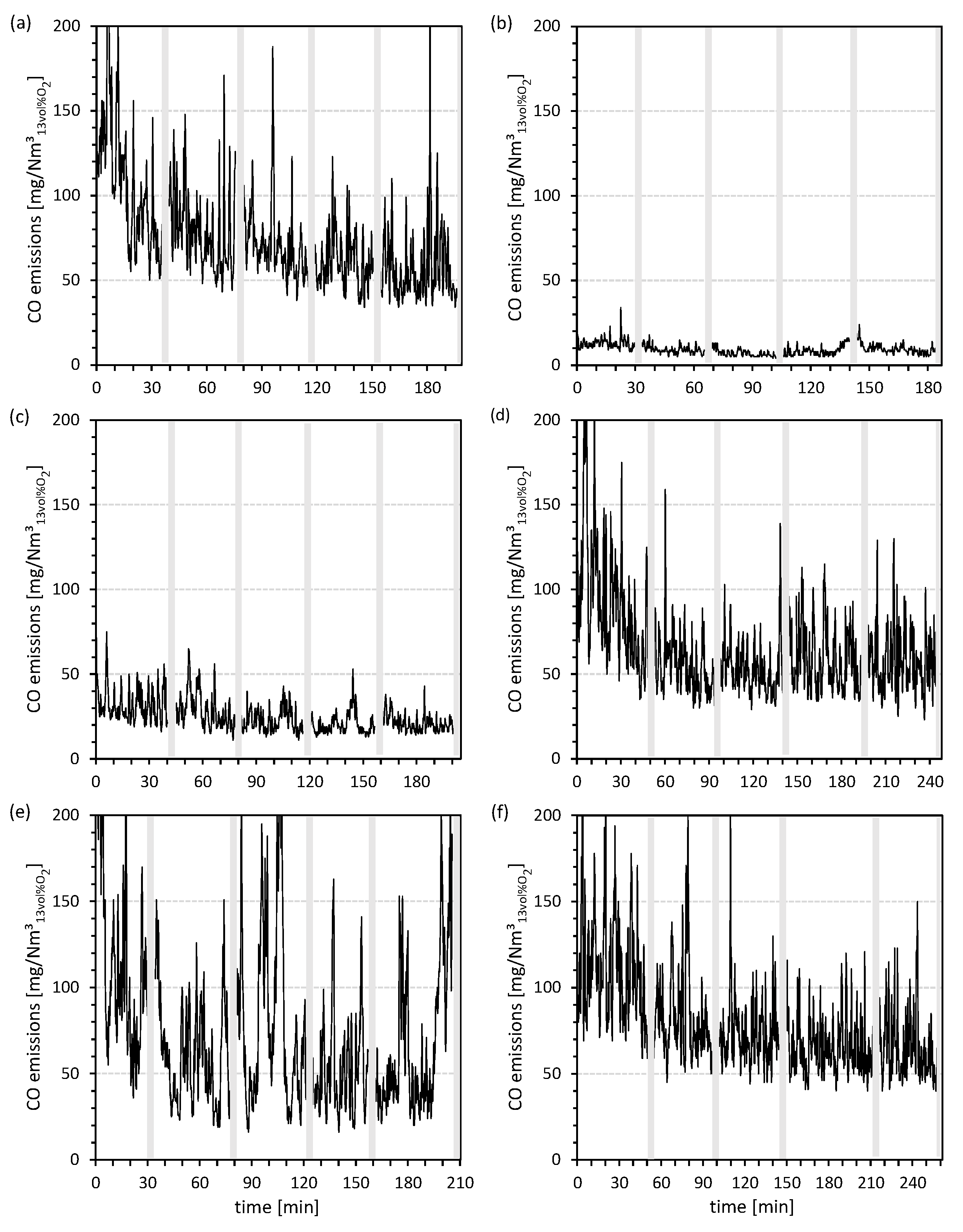
3.2. Carbon Monoxide (CO) Emissions
3.3. Ashes
3.3.1. Chemical Composition
3.3.2. Crystalline Phases
4. Conclusions
- ▪
- The transferability of (fuel) additivation of wood chips for not exclusively aluminum-silicate-based additives (e.g., kaolin or kaolinite [49,53]) could be demonstrated for a conventional small-scale combustion plant (33 kW). For all investigated additives (i.e., kaolin, anorthite, aluminum hydroxide, and titanium dioxide), a reduction of the mean TPM emissions could be observed during the combustion of additivated wood chips compared to the reference case without additives and without ESP.
- ▪
- In agreement with a preliminary laboratory study [52], titanium dioxide showed the lowest reduction in TPM emissions (−12%) among the additives considered. The formation of high-temperature stable K titanates (e.g., K2TiO3 or K2Ti6O13) determined during the laboratory investigation [52] can also be qualitatively confirmed for the practical scale and used as a reason for the mitigation of K emissions (−76%).
- ▪
- The reduction of the absolute mean TPM emissions by the use of aluminum hydroxide (−34%) is slightly behind the achievable effects by kaolin (−55%) and anorthite (−50%); however, compared to the non-additivated reference case, it leads to a comparable emission level as the ESP (26 mg/Nm3).
- ▪
- With reductions in mean K emissions of 94% and 89% compared to the reference case without additives and without ESP, respectively, the (fuel) additivation of the wood chips with 1.0 wt%a.r. kaolin or anorthite achieved the strongest effects. This is due to the formation of high-temperature stable K aluminum silicates (e.g., kalsilite (KAlSiO4), leucite (KAlSi2O6), and orthoclase/microcline (KAlSi3O8)) in the associated ashes with mean emission levels of 17 mg/Nm3 (kaolin) and 19 mg/Nm3 (anorthite). Thus, for example, the German legal TPM emission limit of 20 mg/Nm3 [51] can be met. Thus, aluminum-silicate-based or calcium- and aluminum-silicate-based (fuel) additivation not only exceeds the reduction effect of the secondary mitigation measure in the form of the ESP but can also enable a plant operation that, in principle, does not require the use of a secondary mitigation measure. Nevertheless, the combined use of additivation as a primary fuel-related TPM mitigation measure and ESP as a secondary TPM mitigation measure appears to be very desirable in order to be able to exploit additional reduction potentials. In addition, the statement [52] can be underlined that a sole focus on kaolin or kaolinite as a promising additive does not necessarily appear to be justifiable against the background of the experimental results from the small-scale combustion plant considered with regard to anorthite.
- ▪
- The (fuel) additivation of the wood chips with kaolin and the use of the ESP resulted in a shift of the particle size number distribution to larger particle diameters compared to the non-additivated reference case without ESP. For both mitigation measures, a reduction of the share of the ultrafine particle fraction (PM0.1) in the associated PM emissions was also detectable. Hence, the wood chips additivated with 1.0 wt%a.r. kaolin showed the largest simultaneous reduction of TPM, K, and PM0.1 emissions for the investigated mitigation measures (i.e., (fuel) additivation depending on the additive type as well as ESP).
- ▪
- In addition to the aluminum-silicate-based additivation of the wood chips with kaolin [49,53], the use of the calcium- and aluminum-silicate-based additive anorthite also leads to a significant decrease in the mean CO emissions (i.e., kaolin (−87%) and anorthite (−67%)) compared to the combustion of non-additivated wood chips as well as compared to the (fuel) additivation with titanium dioxide. With regard to the chemical composition of the additives used (e.g., kaolin, anorthite, and aluminum hydroxide), it can be assumed that the presence and reactive availability of aluminum and/or silicon as components of the additives could lead to a decrease in CO emissions (e.g., by influencing the (moist) CO oxidation due to the incorporation of the gaseous K species KOH and KCl into the ash). Despite the relatively strong reduction of K emissions (−76%) due to the use of titanium dioxide, neither the TPM nor the CO emissions can be significantly reduced by this additive, which highlights the phenomenologically deviating behavior of titanium dioxide compared to the other additives.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vicente, E.D.; Alves, C.A. An overview of particulate emissions from residential biomass combustion. Atmos. Res. 2018, 199, 159–185. [Google Scholar] [CrossRef]
- Obaidullah, M.; Bram, S.; Verma, V.K.; de Ruyck, J. A Review on Particle Emissions from Small Scale Biomass Combustion. Int. J. Renew. Energy Res. (IJRER) 2012, 2, 147–159. [Google Scholar]
- Tissari, J.; Sippula, O.; Kouki, J.; Vuorio, K.; Jokiniemi, J. Fine Particle and Gas Emissions from the Combustion of Agricultural Fuels Fired in a 20 kW Burner. Energy Fuels 2008, 22, 2033–2042. [Google Scholar] [CrossRef]
- Schmidl, C.; Luisser, M.; Padouvas, E.; Lasselsberger, L.; Rzaca, M.; Ramirez-Santa Cruz, C.; Handler, M.; Peng, G.; Bauer, H.; Puxbaum, H. Particulate and gaseous emissions from manually and automatically fired small scale combustion systems. Atmos. Environ. 2011, 45, 7443–7454. [Google Scholar] [CrossRef]
- McDonald, J.D.; Zielinska, B.; Fujita, E.M.; Sagebiel, J.C.; Chow, J.C.; Watson, J.G. Fine Particle and Gaseous Emission Rates from Residential Wood Combustion. Environ. Sci. Technol. 2000, 34, 2080–2091. [Google Scholar] [CrossRef]
- Höfer, I.; Kaltschmitt, M.; Beckendorff, A. Emissions from solid biofuel combustion, Pollutant formation and control options. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Naeher, L.P.; Brauer, M.; Lipsett, M.; Zelikoff, J.T.; Simpson, C.D.; Koenig, J.Q.; Smith, K.R. Woodsmoke health effects: A review. Inhal. Toxicol. 2007, 19, 67–106. [Google Scholar] [CrossRef]
- Singh, R.; Shukla, A. A review on methods of flue gas cleaning from combustion of biomass. Renew. Sustain. Energy Rev. 2014, 29, 854–864. [Google Scholar] [CrossRef]
- Lim, M.T.; Phan, A.; Roddy, D.; Harvey, A. Technologies for measurement and mitigation of particulate emissions from domesic combustion of biomass: A review. Renew. Sustain. Energy Rev. 2015, 49, 574–584. [Google Scholar] [CrossRef]
- Obernberger, I.; Brunner, T.; Mandl, C.; Kerschbaum, M.; Svetlik, T. Strategies and technologies towards zero emission biomass combustion by primary measures. Energy Procedia 2017, 120, 681–688. [Google Scholar] [CrossRef]
- Lamberg, H.; Sippula, O.; Tissari, J.; Jokiniemi, J. Effects of Air Staging and Load on Fine-Particle and Gaseous Emissions from a Small-Scale Pellet Boiler. Energy Fuels 2011, 25, 4952–4960. [Google Scholar] [CrossRef]
- Gehrig, M.; Jaeger, D.; Pelz, S.K.; Weissinger, A.; Groll, A.; Thorwarth, H.; Haslinger, W. Influence of firebed temperature on inorganic particle emissions in a residential wood pellet boiler. Atmos. Environ. 2016, 136, 61–67. [Google Scholar] [CrossRef]
- Gollmer, C.; Höfer, I.; Kaltschmitt, M. Additives as a fuel-oriented measure to mitigate inorganic particulate matter (PM) emissions during small-scale combustion of solid biofuels. Biomass Convers. Biorefinery 2019, 9, 3–20. [Google Scholar] [CrossRef]
- Míguez, J.L.; Porteiro, J.; Behrendt, F.; Blanco, D.; Patiño, D.; Dieguez-Alonso, A. Review of the use of additives to mitigate operational problems associated with the combustion of biomass with high content in ash-forming species. Renew. Sustain. Energy Rev. 2021, 141, 110502. [Google Scholar] [CrossRef]
- Wang, L.; Hustad, J.E.; Skreiberg, Ø.; Skjevrak, G.; Grønli, M. A Critical Review on Additives to Reduce Ash Related Operation Problems in Biomass Combustion Applications. Energy Procedia 2012, 20, 20–29. [Google Scholar] [CrossRef]
- Pollex, A.; Zeng, T.; Khalsa, J.; Erler, U.; Schmersahl, R.; Schön, C.; Kuptz, D.; Lenz, V.; Nelles, M. Content of potassium and other aerosol forming elements in commercially available wood pellet batches. Fuel 2018, 232, 384–394. [Google Scholar] [CrossRef]
- Sippula, O.; Hytönen, K.; Tissari, J.; Raunemaa, T.; Jokiniemi, J. Effect of Wood Fuel on the Emissions from a Top-Feed Pellet Stove. Energy Fuels 2007, 21, 1151–1160. [Google Scholar] [CrossRef]
- Lamberg, H.; Tissari, J.; Jokiniemi, J.; Sippula, O. Fine Particle and Gaseous Emissions from a Small-Scale Boiler Fueled by Pellets of Various Raw Materials. Energy Fuels 2013, 27, 7044–7053. [Google Scholar] [CrossRef]
- van Lith, S.C.; Jensen, P.A.; Frandsen, F.J.; Glarborg, P. Release to the Gas Phase of Inorganic Elements during Wood Combustion. Part 2: Influence of Fuel Composition. Energy Fuels 2008, 22, 1598–1609. [Google Scholar] [CrossRef]
- Gehrig, M.; Wöhler, M.; Pelz, S.; Steinbrink, J.; Thorwarth, H. Kaolin as additive in wood pellet combustion with several mixtures of spruce and short-rotation-coppice willow and its influence on emissions and ashes. Fuel 2019, 235, 610–616. [Google Scholar] [CrossRef]
- van Lith, S.C.; Alonso-Ramírez, V.; Jensen, P.A.; Frandsen, F.J.; Glarborg, P. Release to the Gas Phase of Inorganic Elements during Wood Combustion. Part 1: Development and Evaluation of Quantification Methods. Energy Fuels 2006, 20, 964–978. [Google Scholar] [CrossRef]
- Boman, C.; Nordin, A.; Boström, D.; Öhman, M. Characterization of Inorganic Particulate Matter from Residential Combustion of Pelletized Biomass Fuels. Energy Fuels 2004, 18, 338–348. [Google Scholar] [CrossRef]
- Knudsen, J.N.; Jensen, P.A.; Dam-Johansen, K. Transformation and Release to the Gas Phase of Cl, K, and S during Combustion of Annual Biomass. Energy Fuels 2004, 18, 1385–1399. [Google Scholar] [CrossRef]
- Wang, G.; Jensen, P.A.; Wu, H.; Frandsen, F.J.; Sander, B.; Glarborg, P. Potassium Capture by Kaolin, Part 1: KOH. Energy Fuels 2018, 32, 1851–1862. [Google Scholar] [CrossRef]
- Wang, G.; Jensen, P.A.; Wu, H.; Frandsen, F.J.; Sander, B.; Glarborg, P. Potassium Capture by Kaolin, Part 2: K2CO3 KCl, and K2SO4. Energy Fuels 2018, 32, 3566–3578. [Google Scholar] [CrossRef]
- Johansson, L.S.; Tullin, C.; Leckner, B.; Sjövall, P. Particle emissions from biomass combustion in small combustors. Biomass Bioenergy 2003, 25, 435–446. [Google Scholar] [CrossRef]
- Steenari, B.-M.; Lundberg, A.; Pettersson, H.; Wilewska-Bien, M.; Andersson, D. Investigation of Ash Sintering during Combustion of Agricultural Residues and the Effect of Additives. Energy Fuels 2009, 23, 5655–5662. [Google Scholar] [CrossRef]
- Punjak, W.A.; Uberoi, M.; Shadman, F. High-temperature adsorption of alkali vapors on solid sorbents. Am. Inst. Chem. Eng. J. 1989, 35, 1186–1194. [Google Scholar] [CrossRef]
- Marinkovic, J.; Seemann, M.; Schwebel, G.L.; Thunman, H. Impact of Biomass Ash–Bauxite Bed Interactions on an Indirect Biomass Gasifier. Energy Fuels 2016, 30, 4044–4052. [Google Scholar] [CrossRef]
- Öhman, M.; Boström, D.; Nordin, A.; Hedman, H. Effect of Kaolin and Limestone Addition on Slag Formation during Combustion of Wood Fuels. Energy Fuels 2004, 18, 1370–1376. [Google Scholar] [CrossRef]
- Effect of Fuel Additive Sorbents (Kaolin and Calcite) on Aerosol Particle Emission and Characteristics during Combustion of Pelletized Woody Biomass; Boman, C., Boström, D., Öhman, M., Eds.; J. Schmid: Florence, Italy, 2008. [Google Scholar]
- Steenari, B.-M.; Lindqvist, O. High-temperature reactions of straw ash and the anti-sintering additives Kaolin and Dolomite. Biomass Bioenergy 1998, 14, 67–76. [Google Scholar] [CrossRef]
- Höfer, I.; Huelsmann, T.; Kaltschmitt, M. Influence of Ca- and Al-additives on the pollutant emissions from blends of wood and straw in small-scale combustion. Biomass Bioenergy 2021, 150, 106135. [Google Scholar] [CrossRef]
- Lindström, E.; Sandström, M.; Boström, D.; Öhman, M. Slagging Characteristics during Combustion of Cereal Grains Rich in Phosphorus. Energy Fuels 2007, 21, 710–717. [Google Scholar] [CrossRef]
- Wu, H.; Castro, M.; Jensen, P.A.; Frandsen, F.J.; Glarborg, P.; Dam-Johansen, K.; Røkke, M.; Lundtorp, K. Release and Transformation of Inorganic Elements in Combustion of a High-Phosphorus Fuel. Energy Fuels 2011, 25, 2874–2886. [Google Scholar] [CrossRef]
- De Fusco, L.; Boucquey, A.; Blondeau, J.; Jeanmart, H.; Contino, F. Fouling propensity of high-phosphorus solid fuels: Predictive criteria and ash deposits characterisation of sunflower hulls with P/Ca-additives in a drop tube furnace. Fuel 2016, 170, 16–26. [Google Scholar] [CrossRef]
- Novaković, A.; van Lith, S.C.; Frandsen, F.J.; Jensen, P.A.; Holgersen, L.B. Release of Potassium from the Systems K−Ca−Si and K−Ca−P. Energy Fuels 2009, 23, 3423–3428. [Google Scholar] [CrossRef]
- Höfer, I.; Kaltschmitt, M. Assessment of additives avoiding the release of problematic species into the gas phase during biomass combustion—Development of a fast screening method based on TGA. Biomass Convers. Biorefinery 2019, 9, 21–33. [Google Scholar] [CrossRef]
- Corcoran, A.; Marinkovic, J.; Lind, F.; Thunman, H.; Knutsson, P.; Seemann, M. Ash Properties of Ilmenite Used as Bed Material for Combustion of Biomass in a Circulating Fluidized Bed Boiler. Energy Fuels 2014, 28, 7672–7679. [Google Scholar] [CrossRef]
- Dragutinovic, N.; Höfer, I.; Kaltschmitt, M. Effect of additives on thermochemical conversion of solid biofuel blends from wheat straw, corn stover, and corn cob. Biomass Convers. Biorefinery 2019, 9, 35–54. [Google Scholar] [CrossRef]
- Wiinikka, H.; Grönberg, C.; Öhrman, O.; Boström, D. Influence of TiO2 Additive on Vaporization of Potassium during Straw Combustion. Energy Fuels 2009, 23, 5367–5374. [Google Scholar] [CrossRef]
- Aho, M.; Paakkinen, K.; Taipale, R. Destruction of alkali chlorides using sulphur and ferric sulphate during grate combustion of corn stover and wood chip blends. Fuel 2013, 103, 562–569. [Google Scholar] [CrossRef]
- Kassman, H.; Bäfver, L.; Åmand, L.-E. The importance of SO2 and SO3 for sulphation of gaseous KCl—An experimental investigation in a biomass fired CFB boiler. Combust. Flame 2010, 157, 1649–1657. [Google Scholar] [CrossRef]
- Kassman, H.; Pettersson, J.; Steenari, B.-M.; Åmand, L.-E. Two strategies to reduce gaseous KCl and chlorine in deposits during biomass combustion—Injection of ammonium sulphate and co-combustion with peat. Fuel Process. Technol. 2013, 105, 170–180. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, X.; Ruan, R.; Li, S.; Bai, S.; Zhang, J.; Tan, H. Effect of SO2 Addition on PM Formation from Bio-mass Combustion in an Entrained Flow Reactor. Energy Fuels 2018, 32, 11030–11037. [Google Scholar] [CrossRef]
- Wu, H.; Pedersen, M.N.; Jespersen, J.B.; Aho, M.; Roppo, J.; Frandsen, F.J.; Glarborg, P. Modeling the Use of Sulfate Additives for Potassium Chloride Destruction in Biomass Combustion. Energy Fuels 2014, 28, 199–207. [Google Scholar] [CrossRef]
- Huelsmann, T.; Mack, R.; Kaltschmitt, M.; Hartmann, H. Influence of kaolinite on the PM emissions from small-scale combustion. Biomass Convers. Biorefinery 2019, 9, 55–70. [Google Scholar] [CrossRef]
- Khalil, R.A.; Todorovic, D.; Skreiberg, O.; Becidan, M.; Bac—kman, R.; Goile, F.; Skreiberg, A.; Sørum, L. The effect of kaolin on the combustion of demolition wood under well-controlled conditions. Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. ISWA 2012, 30, 672–680. [Google Scholar] [CrossRef]
- Mack, R.; Kuptz, D.; Schön, C.; Hartmann, H. Combustion behavior and slagging tendencies of kaolin additivated agricultural pellets and of wood-straw pellet blends in a small-scale boiler. Biomass Bioenergy 2019, 125, 50–62. [Google Scholar] [CrossRef]
- Geisen, B.; Givers, F.; Kuptz, D.; Peetz, D.; Schmidt-Baum, T.; Schön, C.; Schreiber, K.; Schulmeyer, F.; Thudium, T.; Zelinski, V.; et al. Handbuch zum Qualitätsmanagement von Holzhackschnitzeln; Fachagentur Nachwachsende Rohstoffe e. V. (FNR) and Bundesverband Bioenergie e. V. (BBE): Gülzow-Prüzen, Germany, 2017. [Google Scholar]
- Bundesrepublik Deutschland, Bundesministerium für Justiz, Bundesamt für Justiz. Erste Verordnung zur Durchführung des Bundes-Immissionsschutzgesetzes (Verordnung über kleine und mittlere Feuerungsanlagen—1. BImSchV); Bundesministerium für Justiz: Berlin, Germany; Bundesamt für Justiz: Bonn, Germany, 2010. [Google Scholar]
- Gollmer, C.; Höfer, I.; Harms, D.; Kaltschmitt, M. Potential additives for small-scale wood chip combustion—La-boratory-scale estimation of the possible inorganic particulate matter reduction potential. Fuel 2019, 254, 115695. [Google Scholar] [CrossRef]
- Gollmer, C.; Weigel, V.; Kaltschmitt, M. Emission Mitigation by Aluminum-Silicate-Based Fuel Additivation of Wood Chips with Kaolin and Kaolinite. Energies 2023, 16, 3095. [Google Scholar] [CrossRef]
- Kaltschmitt, M.; Höfer, I.; Gollmer, C. Entwicklung, Erprobung und Untersuchung eines innovativen Verfahrens zur Additivierung von Hackschnitzeln zwecks Reduzierung der Emissionen aus Holzfeuerungen; Deutsche Bundesstiftung Umwelt (DBU): Osnabrück, Germany, 2022. [Google Scholar]
- Diedrich, H.; Stahl, A. NCHS-Elementaranalyse. M02.001. 02; Technische Universität Hamburg, Zentrallabor Chemische Analytik: Hamburg, Germany, 2021. [Google Scholar]
- Fütterer, C.; Stahl, A. Elementbestimmung mit ICP-OES. M02.015. 03; Technische Universität Hamburg, Zentrallabor Chemische Analytik: Hamburg, Germany, 2021. [Google Scholar]
- Cöllen, H.; Frerichs, H.; Stahl, A. Elementbestimmung per ICP-MS. M02.013. 01; Technische Universität Hamburg, Zentrallabor Chemische Analytik: Hamburg, Germany, 2021. [Google Scholar]
- Deutsches Institut für Normung e. V. DIN EN ISO 17225-1; Biogene Festbrennstoffe—Brennstoffspezifikationen und -klassen—Teil 1: Allgemeine Anforderungen. Beuth Verlag GmbH: Berlin, Germany, 2014.
- Deutsches Institut für Normung e. V. DIN EN ISO 17225-4; Biogene Festbrennstoffe—Brennstoffspezifikationen und -klassen—Teil 4: Klassifizierung von Holzhackschnitzeln. Beuth Verlag GmbH: Berlin, Germany, 2014.
- Nordin, A. Chemical elemental characteristics of biomass fuels. Biomass Bioenergy 1994, 6, 339–347. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; et al. FactSage thermo-chemical software and databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Gebrüder Dorfner GmbH & Co. Kaolin- und Kristallquarzsand-Werke KG: Sicherheitsdatenblatt—Kaolin Gemahlen; Gebrüder Dorfner GmbH & Co.: Hirschau, Germany, 2018. [Google Scholar]
- Andrea Wolbring GmbH & Co. KG: Anorthit, Anzing. Available online: https://shop.keramikbedarf-online.de/Anorthit/24001 (accessed on 28 August 2024).
- Andrea Wolbring GmbH & Co. KG: Tonerdehydrat, Anzing. Available online: https://shop.keramikbedarf-online.de/Tonerdehydrat/2440 (accessed on 28 August 2024).
- Merck KGaA. Sicherheitsdatenblatt—Titan(IV)-oxid; Merck KGaA: Darmstadt, Germany, 2022. [Google Scholar]
- Heizomat—Gerätebau + Energiesysteme GmbH. Technische Daten RHK-AK 30; Heizomat-Gerätebau + Energiesysteme GmbH: Gunzenhausen, Germany, 2021. [Google Scholar]
- Deutsches Institut für Normung e. V. DIN EN 15259; Luftbeschaffenheit—Messung von Emissionen aus Stationären Quellen—Anforderungen an Messstrecken und Messplätze und an die Messaufgabe, den Messplan und den Messbericht. Beuth Verlag GmbH: Berlin, Germany, 2008.
- Deutsches Institut für Normung e. V. DIN EN 13284-1; Emissionen aus Stationären Quellen—Ermittlung der Staubmassenkonzentration bei Geringen Staubkonzentrationen—Teil 1: Manuelles Gravimetrisches Verfahren. Beuth Verlag GmbH: Berlin, Germany, 2018.
- Verein Deutscher Ingenieure e. V. VDI 2066 Blatt 1; Messen von Partikeln—Staubmessung in Strömenden Gasen—Gravimetrische Bestimmung der Staubbeladung. Beuth Verlag GmbH: Berlin, Germany, 2019.
- Deutsches Institut für Normung e. V. DIN 22022-1; Feste Brennstoffe—Bestimmung der Gehalte an Spurenelementen—Teil 1: Allgemeine Regeln, Probenahme und Probenvorbereitung—Vorbereitung der Analysenprobe für die Bestimmung (Aufschlussverfahren). Beuth Verlag GmbH: Berlin, Germany, 2014.
- Deutsches Institut für Normung e. V. DIN 22022-3; Feste Brennstoffe—Bestimmung der Gehalte an Spurenelementen—Teil 3: AAS-Flammentechnik. Beuth Verlag GmbH: Berlin, Germany, 2001.
- Deutsches Institut für Normung e. V. DIN 38406 Teil 13; Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung—Kationen (Gruppe E)—Bestimmung von Kalium mittels Atomabsorptionsspektrometrie (AAS) in der Luft-Acetylen-Flamme (E 13). Beuth Verlag GmbH: Berlin, Germany, 1992.
- Deutsches Institut für Normung e. V. DIN 38406 Teil 14; Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung—Kationen (Gruppe E)—Bestimmung von Natrium Mittels Atomabsorptionsspektrometrie (AAS) in der Luft-Acetylen-Flamme (E14). Beuth Verlag GmbH: Berlin, Germany, 1992.
- Deutsches Institut für Normung e. V. DIN EN ISO 7980; Wasserbeschaffenheit—Bestimmung von Calcium und Magnesium—Verfahren mittels Absorptionsspektrometrie. Beuth Verlag GmbH: Berlin, Germany, 2000.
- Deutsches Institut für Normung e. V. DIN EN ISO 10304-1; Wasserbeschaffenheit—Bestimmung von Gelösten Anionen Mittels Flüssigkeits-Ionenchromatographie—Teil 1: Bestimmung von Bromid, Chlorid, Fluorid, Nitrat, Nitrit, Phosphat und Sulfat. Beuth Verlag GmbH: Berlin, Germany, 2009.
- Tritscher, T.; Koched, A.; Han, H.-S.; Filimundi, E.; Johnson, T.; Elzey, S.; Avenido, A.; Kykal, C.; Bischof, O.F. Multi-Instrument Manager Tool for Data Acquisition and Merging of Optical and Electrical Mobility Size Distribu-tions. J. Phys. Conf. Ser. 2014, 617, 012013. [Google Scholar] [CrossRef]
- Deutsches Institut für Normung e. V. DIN EN ISO 16967; Biogene Festbrennstoffe—Bestimmung von Hauptelementen—Al, Ca, Fe, Mg, P, K, Si, Na und Ti. Beuth Verlag GmbH: Berlin, Germany, 2015.
- Deutsches Institut für Normung e. V. DIN EN ISO 16968; Biogene Festbrennstoffe—Bestimmung von Spurenelementen. Beuth Verlag GmbH: Berlin, Germany, 2015.
- Deutsches Institut für Normung e. V. DIN EN 13925-1; Zerstörungsfreie Prüfung—Röntgendiffraktometrie von Polykristallinen und Amorphen Materialien—Teil 1: Allgemeine Grundlagen. Beuth Verlag GmbH: Berlin, Germany, 2003.
- Deutsches Institut für Normung e. V. DIN EN 13925-2; Zerstörungsfreie Prüfung—Röntgendiffraktometrie von Polykristallinen und Amorphen Materialien—Teil 2: Verfahrensabläufe. Beuth Verlag GmbH: Berlin, Germany, 2003.
- Gollmer, C.; Höfer, I.; Kaltschmitt, M. Laboratory-scale additive content assessment for aluminum-silicate-based wood chip additivation. Renew. Energy 2021, 164, 1471–1484. [Google Scholar] [CrossRef]
- Obernberger, I.; Brunner, T.; Baernthaler, G. Fine particulate emissions from modern Austrian small-scale biomass combustion plants. In Proceedings of the 15th European Biomass Conference and Exhibition for Research to Market Deployment: For Research to Market Deployment, Berlin, Germany, 11 May 2007. [Google Scholar]
- Ebert, F. Particle separation for biomass combustion. In Aerosols from Biomass Combustion; Nussbaumer, T., Ed.; Verenum: Zürich, Switzerland, 2001. [Google Scholar]
- Obernberger, I.; Brunner, T.; Jöller, M. Characterisation and formation of aerosols and fly-ashes from fixed-bed biomass combustion. In Aerosols from Biomass Combustion; Nussbaumer, T., Ed.; Verenum: Zürich, Switzerland, 2001. [Google Scholar]
- Hueglin, C.; Gaegauf, C.; Künzel, S.; Burtscher, H. Characterization of Wood Combustion Particles: Morphology, Mobility, and Photoelectric Activity. Environ. Sci. Technol. 1997, 31, 3439–3447. [Google Scholar] [CrossRef]
- Leskinen, J.; Tissari, J.; Uski, O.; Virén, A.; Torvela, T.; Kaivosoja, T.; Lamberg, H.; Nuutinen, I.; Kettunen, T.; Joutsensaari, J.; et al. Fine particle emissions in three different combustion conditions of a wood chip-fired appliance—Particulate physicochemical properties and induced cell death. Atmos. Environ. 2014, 86, 129–139. [Google Scholar] [CrossRef]
- Nussbaumer, T. Aerosols from Biomasse Combustion—Technical Report on Behalf of the IEA Bioenergy Task 32; IEA Bioenergy: Zurich, Germany, 2017. [Google Scholar]
- Tissari, J.; Lyyränen, J.; Hytönen, K.; Sippula, O.; Tapper, U.; Frey, A.; Saarnio, K.; Pennanen, A.S.; Hillamo, R.; Salonen, R.O.; et al. Fine particle and gaseous emissions from normal and smouldering wood combustion in a conventional masonry heater. Atmos. Environ. 2008, 42, 7862–7873. [Google Scholar] [CrossRef]
- Bäfver, L.S.; Rönnbäck, M.; Leckner, B.; Claesson, F.; Tullin, C. Particle emission from combustion of oat grain and its potential reduction by addition of limestone or kaolin. Fuel Process. Technol. 2009, 90, 353–359. [Google Scholar] [CrossRef]
- Schmidt, G.; Trouvé, G.; Leyssens, G.; Schönnenbeck, C.; Genevray, P.; Cazier, F.; Dewaele, D.; Vandenbilcke, C.; Faivre, E.; Denance, Y.; et al. Wood washing: Influence on gaseous and particulate emissions during wood combustion in a domestic pellet stove. Fuel Process. Technol. 2018, 174, 104–117. [Google Scholar] [CrossRef]
- Chanpirak, A.; Hashemi, H.; Frandsen, F.J.; Wu, H.; Glarborg, P.; Marshall, P. The chemical coupling between moist CO oxidation and gas-phase potassium sulfation. Fuel 2023, 336, 127127. [Google Scholar] [CrossRef]
- Glarborg, P. Hidden interactions—Trace species governing combustion and emissions. Proc. Combust. Inst. 2007, 31, 77–98. [Google Scholar] [CrossRef]
- Hindiyarti, L.; Frandsen, F.; Livbjerg, H.; Glarborg, P. Influence of potassium chloride on moist CO oxidation un-der reducing conditions: Experimental and kinetic modeling study. Fuel 2006, 85, 978–988. [Google Scholar] [CrossRef]
- Chanpirak, A.; Wu, H.; Glarborg, P.; Marshall, P. An experimental and chemical kinetic modeling study of the role of potassium in the moist oxidation of CO. Fuel 2023, 335, 127075. [Google Scholar] [CrossRef]
- Siegmund, T.; Gollmer, C.; Scherzinger, M.; Kaltschmitt, M. A review of CO emissions during solid biofuel combustion—Formation mechanisms and fuel-related reduction measures. J. Energy Inst. 2024, 116, 101762. [Google Scholar] [CrossRef]
- Siegmund, T.; Gollmer, C.; Horstmann, N.; Kaltschmitt, M. Carbon monoxide (CO) and particulate matter (PM) emissions during the combustion of wood pellets in a small-scale combustion unit—Influence of aluminum-(silicate-)based fuel additivation. Fuel Process. Technol. 2024, 262, 108111. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behaviour of biomass during combustion: Part I. Phase-mineral transformations of organic and inorganic matter. Fuel 2013, 112, 391–449. [Google Scholar] [CrossRef]
| Sample Name | Biomass | Mitigation Measure |
|---|---|---|
| Without Additive | Pine wood chips | – |
| Kaolin | Pine wood chips | 1 wt%a.r. Kaolin |
| Anorthite | Pine wood chips | 1 wt%a.r. Anorthite |
| Aluminum hydroxide | Pine wood chips | 1 wt%a.r. Aluminum hydroxide |
| Titanium dioxide | Pine wood chips | 1 wt%a.r. Titanium dioxide |
| ESP | Pine wood chips | Electrostatic precipitator |
| Parameter | Unit | Wood Chips |
|---|---|---|
| Moisture content | wt%a.r. | 8.1 |
| Ash content | wt%d.b. | 0.3 |
| Carbon (C) | wt%d.b. | 49.0 |
| Hydrogen (H) | wt%d.b. | 6.7 |
| Oxygen (O) * | wt%d.b. | 43.4 |
| Nitrogen (N) | wt%d.b. | <0.1 |
| Sulphur (S) | wt%d.b. | <0.2 |
| Potassium (K) | mg/kgd.b. | 290 |
| Sodium (Na) | mg/kgd.b. | <5 |
| Calcium (Ca) | mg/kgd.b. | 709 |
| Magnesium (Mg) | mg/kgd.b. | 155 |
| Silicon (Si) | mg/kgd.b. | n.d. |
| Manganese (Mn) | mg/kgd.b. | 29 |
| Phosphorus (P) | mg/kgd.b. | n.d. |
| Aluminum (Al) | mg/kgd.b. | 18 |
| Iron (Fe) | mg/kgd.b. | 12 |
| Copper (Cu) | mg/kgd.b. | 7 |
| Zinc (Zn) | mg/kgd.b. | 6 |
| Lead (Pb) | mg/kgd.b. | <1 |
| Parameter | Unit | Kaolin | Anorthite | Aluminum Hydroxide | Titanium Dioxide |
|---|---|---|---|---|---|
| SiO2 | wt%a.r. | 50.2 | 43.2 | – | – |
| Al2O3 | wt%a.r. | 34.4 | 36.6 | 65.4 | – |
| H2O | wt%a.r. | 12.0 | 20.2 | 34.6 | – |
| CaO | wt%a.r. | <0.1 | – | – | – |
| TiO2 | wt%a.r. | 0.4 | – | – | 100.0 |
| Fe2O3 | wt%a.r. | 0.5 | – | – | – |
| K2O | wt%a.r. | 2.1 | – | – | – |
| Na2O | wt%a.r. | 0.2 | – | – | – |
| MgO | wt%a.r. | <0.1 | – | – | – |
| P2O5 | wt%a.r. | 0.2 | – | – | – |
| Parameter | Unit | Without Additive | Kaolin | Anorthite | Aluminum Hydroxide | Titanium Dioxide | ESP |
|---|---|---|---|---|---|---|---|
| Potassium (K) | wt%d.b. | 36.9 | 4.9 | 8.0 | 28.1 | 10.1 | 33.4 |
| Sodium (Na) | wt%d.b. | 1.3 | 0.8 | 3.0 | 0.8 | 0.9 | 0.7 |
| Calcium (Ca) | wt%d.b. | 4.8 | 12.4 | 10.0 | 7.2 | 7.8 | 7.6 |
| Magnesium (Mg) | wt%d.b. | <0.1 | <0.1 | 0.2 | 0.3 | 0.2 | 0.2 |
| Aluminum (Al) | wt%d.b. | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Iron (Fe) | wt%d.b. | 2.7 | 5.8 | 5.0 | 3.3 | 3.4 | 4.9 |
| Zinc (Zn) | wt%d.b. | 1.4 | 2.1 | 1.8 | 1.6 | 1.5 | 1.1 |
| Sulfate (SO42−) | wt%d.b. | 41.7 | 7.7 | 21.2 | 42.7 | 10.0 | 30.1 |
| Chloride (Cl−) | wt%d.b. | 6.3 | 3.9 | 14.4 | 8.5 | 6.3 | 5.0 |
| Not identified | wt%d.b. | 4.9 | 62.3 | 36.5 | 7.5 | 59.7 | 17.0 |
| Parameter | Unit | Without Additive | Kaolin | Anorthite | Aluminum Hydroxide | Titanium Dioxide | ESP |
|---|---|---|---|---|---|---|---|
| Potassium (K) | wt%d.b. | 13.2 | 3.7 | 3.5 | 4.6 | 3.7 | 10.4 |
| Sodium (Na) | wt%d.b. | 0.1 | 0.1 | 2.4 | 0.3 | 0.3 | 0.3 |
| Calcium (Ca) | wt%d.b. | 23.3 | 5.6 | 8.5 | 8.3 | 6.4 | 25.6 |
| Magnesium (Mg) | wt%d.b. | 5.4 | 1.6 | 1.6 | 1.8 | 1.3 | 5.9 |
| Aluminum (Al) | wt%d.b. | 2.5 | 11.7 | 8.8 | 28.2 | 1.4 | 4.2 |
| Iron (Fe) | wt%d.b. | 0.6 | 0.7 | 1.2 | 0.4 | 0.5 | 1.1 |
| Zinc (Zn) | wt%d.b. | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Sulfate (SO42−) | wt%d.b. | 1.5 | 0.8 | 0.6 | 0.7 | 0.6 | 1.5 |
| Chloride (Cl−) | wt%d.b. | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Not identified | wt%d.b. | 53.3 | 75.8 | 73.4 | 55.6 | 85.9 | 51.0 |
| Parameter | Without Additive | Kaolin | Anorthite | Aluminum Hydroxide | Titanium Dioxide | ESP |
|---|---|---|---|---|---|---|
| K2SO4 | x | x | ||||
| K2Ca(CO3)2 | x | x | ||||
| KAlSiO4 | x | |||||
| KAlSi2O6 | x | |||||
| KAlSi3O8 | x | x | x | |||
| KTi8O16 | x | |||||
| K2TiO3 | x | |||||
| K2Ti6O13 | x | |||||
| K2TiSi6O15 | x | |||||
| SiO2 | x | x | ||||
| Al2O3 | x | |||||
| CaO | x | x | ||||
| CaCO3 | x | x | x | x | x | |
| MgO | x | x | x | |||
| TiO2 | x | |||||
| Al6Si2O13 | x | |||||
| CaAl2Si2O8 | x | |||||
| Ca3Al2O6 | x | |||||
| CaTiO3 | x |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gollmer, C.; Siegmund, T.; Weigel, V.; Kaltschmitt, M. Comparative Analysis of Primary and Secondary Emission Mitigation Measures for Small-Scale Wood Chip Combustion. Energies 2024, 17, 4403. https://doi.org/10.3390/en17174403
Gollmer C, Siegmund T, Weigel V, Kaltschmitt M. Comparative Analysis of Primary and Secondary Emission Mitigation Measures for Small-Scale Wood Chip Combustion. Energies. 2024; 17(17):4403. https://doi.org/10.3390/en17174403
Chicago/Turabian StyleGollmer, Christian, Theresa Siegmund, Vanessa Weigel, and Martin Kaltschmitt. 2024. "Comparative Analysis of Primary and Secondary Emission Mitigation Measures for Small-Scale Wood Chip Combustion" Energies 17, no. 17: 4403. https://doi.org/10.3390/en17174403
APA StyleGollmer, C., Siegmund, T., Weigel, V., & Kaltschmitt, M. (2024). Comparative Analysis of Primary and Secondary Emission Mitigation Measures for Small-Scale Wood Chip Combustion. Energies, 17(17), 4403. https://doi.org/10.3390/en17174403






