Abstract
Anaerobic digestion (AD) of food waste (FW) is considered an environmentally sustainable process that can divert the disposal of FW to landfill and prevent greenhouse gas (GHG) emissions in managing the FW. Although several studies have attempted to demonstrate the AD of FW, low methane yields and a high incidence of process instability have been reported due to the rapid generation and accumulation of volatile fatty acids (VFAs). This paper reviews the recent research and development with high variation in FW composition, such as the carbon-to-nitrogen (C/N) ratio and, consequently, the effect of its physicochemical composition on process performance and methane yields. The paper highlights the significance of optimizing the anaerobic co-digestion (AcoD) of FW with carbon-rich substrates such as garden waste (GW) and/or the addition of trace elements as strategies that can improve the process performance and methane yields from FW. This review focuses on the factors effecting the feasibility of food organics and garden organics (FOGO) as a substrate for methane production. The review also critically analyses the prospects of enhancement of biomethane yield by optimizations of the impactful parameters. The progress in research related to these methods and identifying existing limitations to efficient AD of FOGO are the key findings of this review. This review also assesses the impact of nanotechnology on the process performance of the digester. The integration of FO and GO in AD processes has demonstrated enhanced biogas yields, improved process stability, and better waste management outcomes compared to the digestion of either substrate alone. Despite these advantages, challenges such as feedstock variability, process optimization, and the need for advanced pretreatment methods remain. Addressing these issues through continued research and technological innovations will be crucial for maximizing the efficiency and scalability of AD systems. Moreover, the economic feasibility and policy frameworks supporting AD need further development to promote broader adoption.
1. Introduction
The management of organic waste (OW) has emerged as a significant worldwide issue and has garnered particular attention from the European Commission as it strives to promote a circular economy [1]. We discard up to 60% of our domestic garbage each week in the form of food and garden organics (FOGO). Nonetheless, depending on the food we consume, the goods we purchase, whether we have a garden or not, and if we compost at home, the composition of everyone’s bin varies slightly [2]. Methane (CH4) is a major GHG with a global warming potential substantially higher than carbon dioxide (CO2), and it is produced when organic material decomposes in the anaerobic conditions of a landfill [3]. GHG emissions are minimised when landfill gas is recovered and either flared or used to produce electricity. However, not all landfill gas can be collected due to intrinsic collection system inefficiencies. Lifetime capture efficiency estimates can vary greatly but typically range from 40 to 75 percent. AD provides a synergistic solution to the issues of waste disposal and clean renewable energy generation [4,5,6]. About 65–75% of the biogas produced by anaerobic digestion is composed of CH4, and about 35–45% of it is CO2 [7] that can be converted for fuel application. Numerous naturally occurring, facultative, purely anaerobic bacteria that are present in the environment carry out the process of digestion. Biogas utilisation has gained a lot of momentum recently in daily life as a more environmentally friendly alternative energy supply for the creation of power, such as for heating and cooking needs (Table 1).

Table 1.
Environmental benefits of biogas.
Generally, it is anticipated that 100 m3 of biogas with a 6 kWh/m3 energy content (CH4 content of 60%) will be produced from 1 tonne of mixed garden and food organics (biowaste). Figure 1 [13] shows the breakdown of electricity generated by biogas in Australia by state. It not only shows the overall increase but also the growth that each region has experienced over time. This provides scientists and investors with a promising incentive to pursue contemporary biogas production solutions by utilising all available technologies.
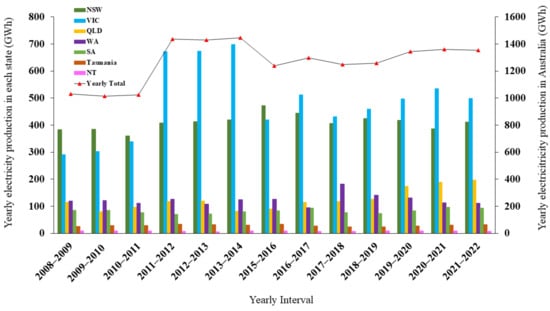
Figure 1.
Regional breakdown in yearly electricity production from biogas in Australia by state: New South Wales (NSW), Victoria (VIC), Queensland (QLD), Western Australia (WA), Tasmania, NT (Northern Territory), and Yearly total (data source [13]).
In a direct interspecies electron transfer (DIET) system like anaerobic digestion, synergistic microbial consortia cooperate and coexist. Anaerobic digestion occurs in four distinct stages, namely hydrolysis, acidogenesis, acetogenesis, and methanogenesis, which are all carried out by a variety of micro-organisms [14,15]. The most essential process in AD, known as methanogenesis, is undertaken by methanogens, which are members of the Archaea domain. These micro-organisms rely heavily on the presence of trace metals [16] in addition to the key elements [17] like C, N, H, S, and P to grow, survive, and execute numerous metabolic activities. Thus, it is vital to have an AD system that has the optimal amount of trace elements (TE) that boosts biogas production [18,19]. The generation of biogas is aided by metals such as zinc (Zn), iron (Fe), copper (Cu), nickel (Ni), and cadmium (Cd), but their concentration must be kept under control, i.e., in traces, since a large amount of them can stifle microbial activity, make the reaction unstable and cause reactor toxicity, impairing biogas output [20]. Food waste (FW) digesters are frequently prone to high levels of VFAs and ammonia (NH3) concentrations, which have potential inhibitory effects, especially on the acetoclastic methanogens, and may eventually cause digester breakdown over time due to the high levels of VFAs produced and relatively low levels of hydrogen released (and used by hydrogenotrophic methanogens) [21,22]. NH3 stripping, selective trace element (TE) dosage, and, more recently, the addition of biochar have all been studied as ways to increase digester stability and biomethane generation from FW [23,24,25]. The anaerobic co-digestion mechanism may be significantly impacted by the co-substrates’ characteristics. Additionally, the synergistic effect brought on by the right co-substance mixing ratio results in a larger CH4 yield and boosts system effectiveness [26]. The ideal C/N ratio for the anaerobic digestion process falls between 20 and 35 [27]. The C/N ratio of GO has a wide range, usually falling between 20 and 50. Because GO has a high concentration of lignocellulose, which is harder to decompose, it produces less CH4 and takes longer to digest [28]. FO has a significant amount of organic material that can degrade easily, resulting in high CH4 yields. Nevertheless, pertaining to the accumulation of VFAs and the decline in pH caused by swift hydrolysis, the AD system is susceptible to acidification [29]. The C/N ratio of FW of is relatively low (2–30) and significantly differs by countries and regions due to diverse eating habits.
In recent years, there has been much focus on the co-digestion of FW and yard waste (YW) as it reduces the impact of excess VFA accumulation and NH3 inhibition on the digestors [30]. Researchers have optimized the food-to-micro-organism (F/M) ratio during anaerobic co-digestion of FW and YW to enhance biogas production [31]. In another study by Siying et al. [32], co-digestion resulted in 84.46% organic matter removal for FW + KW (kitchen waste) and 65.64% for FW + GW (garden waste). Co-digestion led to an increase in the presence of hydrolytic bacteria Defluviitoga and Hydrogenispora, as well as the methanogen Methanoculleus. Additionally, co-digestion enhanced glycoside hydrolases (GHs), which are essential carbohydrate-active enzymes (CAZymes). One research demonstrated that replacing 20% of the FW organic loading rate (OLR) with YW resulted in enhanced biogas production and increased specific CH4 yield. Furthermore, the efficiency of converting organic compounds, specifically volatile solids (VS), achieved a remarkable 83% [30]. Moreover, it was observed that with the application of thermal and extrusion pretreatment rather than conventional digestion, there was an average 0.25-fold increase in biogas production [33].
In spite of all the investigations regarding CH4 yield from FW and co-digestion of FW with a variety of other substrates, there has not been a comprehensive study that collectively presents all the aspects that need to be considered for conducting research on FOGO. Figure 2 suggests that although there has been significant research on diverting OW from landfill, there are only 14 publications that have a close relation with treating food waste with lignocellulosic biomasses, out of which only 7 have studied the effect of FW, KW, YW, and GW co-digestion. In general, prior research indicated that the AD process might be enhanced by the addition of TE in the form of NPs, although the processes have received less attention. There is still much to learn about how metal oxide NPs affect the several AD process phases.
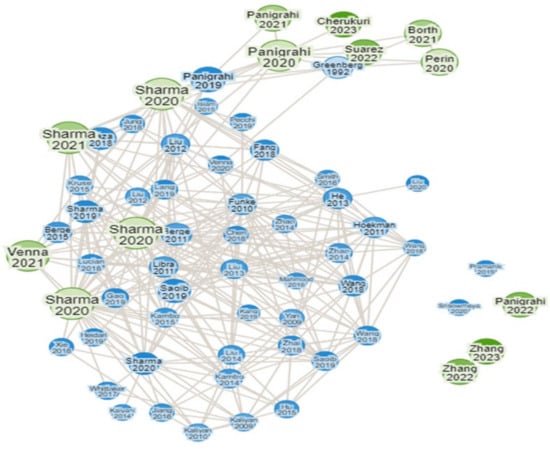
Figure 2.
Publication related to AD process of FW, YW, GW, and KW (green publications have direct relation with FOGO).
Thus, this review will focus on the overall process and impact of various factors and nanomaterials on the yield and enhancement of biomethane from FOGO. The objective of this article is to provide an overview of the most significant challenges associated with producing biogas or methane from food waste and other lignocellulosic wastes using the most recent findings and expertise in the field. The study highlights the potential for anaerobic digestion to develop as a means for processing FOGO. By addressing different digester configurations and operating conditions, the paper aims to provide a thorough analysis of current practices and future directions in the optimisation of the anaerobic digestion process of FOGO.
2. Foundational Concepts in Anaerobic Digestion of Organic Waste
2.1. Anaerobic Digestion
Anaerobic digestion (AD) is a complex anoxic biochemical process that transforms biomass or organic waste into biogas and nutrient-rich digestate in the absence of oxygen. The nutrient-rich digestate is also known as effluent. Based on the main metabolic groups of micro-organisms involved, the AD process can be divided into four stages (Figure 3): hydrolysis, fermentation or acidogensis, acetogenesis, and methanogenesis [34]. Among all biodegradable waste disposal technologies, AD is one of the most cost-effective applications when taking into account its energy input and recovery, as well as its effects on the environment [35,36,37].
2.1.1. Hydrolysis
Several lignocellulytic enzymes are released by the micro-organisms as they interact with the substrates throughout the hydrolysis process, helping to break down the complex molecules into more easily soluble ones. For instance, the lipids, proteins, and carbohydrates are reduced to volatile fatty acids (VFAs), reducing sugars and amino acids when they interact with enzymes like lipases, cellulases, and proteases that are generated by microbes. The pH range for the AD process is 6.5 to 7.5. If there is a significant buildup of VFAs during the hydrolysis step, the pH drops, limiting the AD process. Similar to this, greater NH3 buildup causes pH to rise over 7.9. Another important component of the AD process is the carbon-to-nitrogen (C/N) ratio, which is typically maintained at 25 to 30 [38,39]. A higher nitrogen content causes a greater buildup of NH3, which directly impacts reducing CH4 production [40]. As a result, the rate-limiting stage of the entire anaerobic digestion process is considered to be hydrolysis [41].
2.1.2. Acidogenesis
The hydrolysis products are transformed into short-chain VFAs (acetic acid, propionic acid, butyric acid, valeric acid, and caproic acid), alcohols, NH3, water, CO2, and other compounds during the acidogenesis stage [42]. Sometimes, using too much substrate might cause VFAs to build up and cause the pH of the system to drop [38]. The methanogenesis process is halted when the pH of the system drops to an exceptionally low level because low pH inhibits the formation of methanogens. In order to prevent an overabundance of intermediate chemicals, such as VFAs and NH3, which could cause pH fluctuations, it is crucial to give the ideal ratio of inoculum to substrate (ISR).
2.1.3. Acetogenesis
The acetogenic bacteria in this process turn the volatile fatty acids, fermenting sugars, and amino acids into acetic acid, hydrogen, and CO2. Methanogens may be inhibited if the hydrogen molecules created in this phase are not digested. Acidogenesis and acetogenesis products both serve as a substrate for the methanogenesis process [43].
2.1.4. Methanogenesis
Acetic acid, CO2, and hydrogen are transformed into CH4 and CO2 during the methanogenesis process [44]. CH4 concentrations for lignocellulosic substrates typically range from 55 to 60 percent, while CO2 concentrations are in the 25 to 30 percent range. The pH must be 6.5 to 7.5 during this process since the microbes are sensitive to pH ranges that are too high or too low [45]. Accumulation of NH3 and nitrate compounds in the system causes high pH ranges. High pH can interfere with the function of key enzymes involved in methane production, such as methyl-coenzyme M reductase, reducing their overall activity [46]. Alkaline conditions can alter the cellular membrane potential and proton gradients that are crucial for maintaining cell integrity and function. This disruption can affect nutrient uptake, waste removal, and energy production [46]. Extremely high pH can favour the growth of other micro-organisms that are more tolerant to alkaline conditions, potentially out-competing methanogens and leading to shifts in microbial community structure. This could further impact the overall methane production process in the environment [47]. When beyond the tolerant levels, pH can cause stress to methanogenic cells, leading to damage to cellular structures and increased susceptibility to other environmental stresses, resulting in cell death or reduced viability of methanogens. On the other hand, acidic conditions prevent ATP (adenosine triphosphate) synthesis and its depletion, disrupting energy equilibrium. Therefore, low levels of ATP may cause damage or malfunction in specific metabolic processes, such microbial disintegration and decay, since high levels of ATP are needed to pump extra protons out of cells and keep them at a neutral pH [48]. Under low pH environment, the inhibition of methanogenesis may be explained by discrepancies in the intra- and extracellular permeability of undissociated acetate. When certain conditions are met, the highly lipophilic extracellular undissociated acetate diffuses passively across membranes and into the cells. The vast intracellular acetates then tend to dissociate and produce an excess of protons, which causes an imbalance in the pH levels inside the cell and decreases cell stability [49].
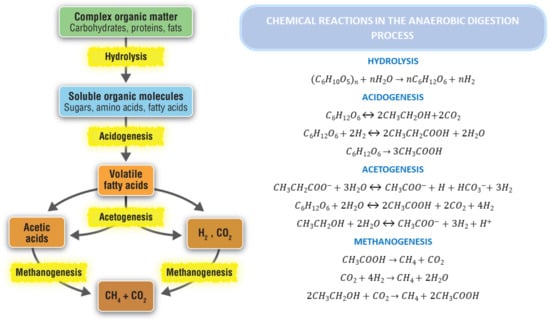
Figure 3.
Overview of steps and reactions involved in AD process (adapted from [50]).
2.2. Factors Affecting Anaerobic Digestion
The AD process is a beneficial method for producing renewable energy. The energy consumed during the process is determined by CH4 recovery, which can be used as fuel or power generation. To improve crop recovery, anaerobic sludge can be utilised as a biofertilizer [51]. All the bacteria must synergise with one another for a steady AD process to prevent an excessive buildup of intermediate products [52]. A stable anaerobic digestion under mesophilic conditions requires a pH range of 6.5 to 7.5 [53]. According to the investigation performed by [54] on the production of biogas from wheat bran, pH ranges between 6.9 and 7.1 resulted in the highest yields of biogas, which were 68%/32% CH4/CO2 mixtures. Lower CH4 yields may be the result of any process that takes place outside of this range. Similarly, excessive levels of VFAs, NH3, and TE, like Na+ and K+, have a significant impact on the methanogenesis process [55]. The following is a summary of some of the main variables that obstruct the process of AD.
2.2.1. Chemical Composition of the Substrate
Understanding the mechanics of anaerobic digestion and figuring out the process parameters needed for the best efficiency depending on the chemical composition of the substrates. For AD, FW is an unconventional substrate with high protein, lipid, and carbohydrates [56]. According to composition, the majority of pre-consumer food waste comes from the production of fruit and vegetable wastes, roots and tubers, and cereals, together with their harvest residues and processing by-products such as husks, peels, and pomace. These waste streams contain significant amounts of starch, protein, sugars, lipids, dietary fibre, mineral acids, inorganic compounds (such as silica), or phytochemicals and are best processed into animal feed. Alternatively, they can be used to extract or synthesise highly sought-after chemicals for the food industry, pharmaceutical industry, and cosmetic industry [57]. Due to its high protein and fat content, it generates a high biochemical CH4 production (BMP) of 467–529 mL/gVSadded [58,59]. Furthermore, according to previous studies, lipid-rich FW has a greater BMP than FWs that are abundant in carbohydrates and proteins [60]. According to a certain hypothesis, AD from slaughterhouse wastes with high lipid and protein contents positively impacted the CH4 yield, resulting in a 43% higher yield than predicted and a better microbial environment balance [61]. The CH4 production rose from 25 to 50 m3 biogas/m3 when fish lipids (total concentration of 5%) were added to a cattle waste anaerobic reactor [5]. The methanogenic activity of lipid-rich FW, however, may be inhibited by long-chain fatty acids (LCFAs) during the breakdown of lipid substrates, according to some research [62,63]. Usually, the inhibitory effects are partially caused by the LCFAs, which are absorbed by the microbial biomass surface and cause problems with mass transfer [64,65]. Acute inhibition was reported by Cirne et al. [64] to be caused by a lipid content of 31–47% (w/w, COD basis). A substantial inhibition was also found by Sun et al. [63] upon reaching 65% of the lipid content (w/w, VS basis). Carbohydrate- and protein-rich FW have a number of disadvantages in addition to lipid-rich FW. Because of its imbalanced carbon/nitrogen (C/N) ratio and low nutritional content, FW that is carbohydrate-rich produces more hydrogen and accelerates acidification [66,67]. In general, the composition and scarcity of TE in FW were easily correlated with the performance of FW for AD [68,69,70,71].
The substrates’ dry matter content, also known as TS, has a direct impact on the hydrolysis procedure [72]. The VS hydrolyzes into short-chain fatty acids, reducing sugars and amino acids as part of the AD process. A larger concentration of substrate VS causes a higher buildup of VFAs, which may cause the systems’ pH to decrease. Likewise, too much nitrogen causes too much NH3 production, leading to a rise in alkalinity and pH. However, if the same procedure needs to be changed to produce both H2 and VFAs, a higher substrate VS concentration must be maintained to ensure maximum VFA generation [73]. To calculate the best ISR ratio for batch studies as well as the OLR for reactor research, VS concentration is a crucial parameter.
2.2.2. Inoculum-to-Substrate Ratio (ISR)
For batch-scale applications, the biochemical CH4 potential (BMP) testing of any substrate depends critically on the inoculum-to-substrate (ISR) ratio [74]. The higher buildup of VFAs is caused by lower ISR ratios. The batch digesting system’s pH tends to drop when VFA accumulation occurs [75]. The production of CH4 by methanogenic microbes is inhibited by pH reduction. Low pH also causes the AD process to take longer to complete its initial stages. Higher inoculum concentration encouraged higher CH4 concentration in biogas production, according to a study on AD [76]. However, for the first few days, CH4 generation accelerates quickly at extremely high inoculum concentrations. The microbes eventually consume all the substrates, which causes the generation of CH4 to gradually decline.
ISR 2–ISR 4 has the capacity to create the largest CH4 concentration, according to several research works on the effect of inoculum-to-substrate ratios. ISR 4 was shown to be the ideal ratio in a specific study on the co-digestion of slaughterhouse wastes with sewage sludge because it generated the highest biogas production. Additionally, the system’s pH did not alter much compared to the previous ISR, indicating a consistent anaerobic digesting process [77]. Choosing the best ISR also involves evaluating the TS% needed for each substrate. NH3 inhibition and acidification can both be boosted by raising the TS of the substrate [78]. In order to maintain a controlled VFA production and an optimal TS%, it is crucial to choose an ISR.
2.2.3. C/N Ratio
In addition to other parameters, the AD process is stable at an ideal carbon/nitrogen (C/N) ratio in the range of 20 to 30, which is enough to meet foreseeable energy needs [79,80,81]. Only those substrates that have an ideal C/N ratio can meet the bacteria’s possible nutritional needs. Thus, it is clear that C/N is a key component in improving the effectiveness of the AD process of producing biogas when choosing substrates for AD or anaerobic co-digestion.
Table 2 lists the C/N ratios of several substrates. It is challenging to pinpoint the ideal ratio, though, as it can be influenced by a number of variables, including the type of substrate, the presence of TE, chemical makeup, and biodegradability. The system fails, there is instability, and the amount of biogas produced is reduced when the C/N ratio deviates from its ideal level in either direction. Due to their high C/N ratio, the co-substrates have a limited ability to act as a buffer and produce a considerable amount of volatile fatty acids during fermentation. In contrast, co-substrates with low C/N ratios have significant buffer capacities, and as fermentation progressed, the NH3 concentration rose and hampered the development of micro-organisms in the system. Furthermore, the effluent’s N2O emissions increase with a lower C/N ratio and vice versa [82].

Table 2.
C/N ratio of different substrates used in AD (source: [79,83,84,85,86,87]).
2.2.4. Operation pH
One of the key operational elements that significantly influences the digestive process is the pH level. Despite some species, the majority of microbes favour a pH range that is neutral. There are numerous organisms involved in the biogas production process, each of which has a different ideal pH for growth. The pH range that allows for the most biogas production in AD is 6.8 to 7.2 [88]. Methanogenesis bacteria in the AD process prefer a pH of about 7.0 and are quite sensitive to pH changes. Acidogenesis bacteria are tolerated in the pH range of 4.0–8.5 and are substantially less sensitive to pH. However, between 5.5 and 6.5 is the ideal pH for acidogenesis and hydrolysis [83,89]. One primary justification for dividing some digesters into two phases, the acidogenic phase and the methanogenesis phase, is the ideal pH value [83,90]. The pH value is also an important factor because it affects the ratio of ionized and non-ionized forms, and since excessive concentrations of hydrogen sulphide, fatty acids, and NH3 are hazardous in their non-ionized forms [91]. The pH number normally indicates that the digester’s micro-organisms have a favourable environment [92,93,94,95]. Co-digestion helps maintain stable pH levels by limiting the circumstances that lead to excessive acidification. When compared to the digestion of a single substrate, the pH value change of combined raw materials in co-digestion is more stable and easier to sustain in a suitable pH range [96].
2.2.5. Operating Temperature
One of the most important aspects of micro-organism survival during the AD process is temperature. The primary method for differentiating between different digesting processes is temperature range. Anaerobic digesters can operate in three temperature ranges: psychrophilic (25 °C), mesophilic (about 35 °C), and thermophilic (around 55 °C) [83]. Mesophilic and thermophilic temperature ranges are ideal for the growth of micro-organisms. In general, raising the temperature benefits micro-organism metabolism and speeds up the digestive processes, but the thermophilic process is more difficult to control and requires more energy to keep the reactor’s temperature constant. The generation of biogas will be significantly reduced as a result of the temperature variation, which can have an impact on microbial growth [79,83]. A high NH3 content in the AD process makes it unstable at thermophilic temperatures [97]. Since there are fewer types of micro-organisms that are available and active in thermophilic ranges, mesophilic processes typically involve a diversity of micro-organisms and are more stable than thermophilic processes [88]. Unlike thermophilic bacteria, which can survive in mesophilic temperatures but develop slowly, mesophilic bacteria cannot survive in thermophilic temperatures [98]. Temperature is a crucial factor in allowing micro-organisms to develop according to their ideal needs and to increase the production of biogas.
2.2.6. Trace Elements
For methanogenic bacteria to grow in an AD reactor, trace metals are necessary [99]. It has been discovered that metal nutrients like Fe, Co, and Ni, have a substantial impact on the AD process [100,101]. The performance of AD depends on optimal concentrations of micronutrients (such as Ni, Fe, Mo, Co, and Se) and macronutrients (such as N, P, and S), which are respectively necessary cofactors in a variety of enzymatic processes involved in methanogenesis and fundamental components of micro-organisms. For instance, the enzyme formate dehydrogenase in methanogens needs TE tungsten (W), selenium (Se), and molybdenum (Mo) to oxidise formate [40]. While Co can slow down microbial metabolism, chromium aids in the metabolism of glucose. Bacterial enzymes are activated by manganese, and CH4-producing micro-organisms are stabilised [102]. Zinc can increase the toxicity of other metals, accelerate cell growth, and impair metabolism. The activity of fundamental enzymes in these organism types appears to be particularly dependent on the trace element concentrations of Fe, Co, and Ni. Consider the CO-dehydrogenase, which is necessary for syntrophic acetogenesis and requires Fe and Ni to function [103]. Co is found in cyanocobalamin (B12 family) and is involved in several reactions in both hydrogenotrophic and acetoclastic CH4 formation; Fe is found in several enzymes, including ferredoxin-containing dehydrogenases; and Ni is required for factor F430, which is involved in the methyl reductase complex, which carries out the final step of methanogenesis [104]. To reach profitable loading rates, it is frequently necessary to supply Fe along with TE in ionic form because the breakdown of protein-rich materials produces high quantities of sulphides, which precipitate TE.
2.2.7. Organic Loading Rate (OLR)
The daily COD or VS supplied to each digester unit volume is measured by OLR (Equation (1)) [105]. The OLR is a critical parameter affecting stability, performance, and the cost of the AD process.
The OLR is a crucial parameter that affects AD process stability, performance, and cost. The process indicators are related to OLR mathematically. The methane production rate is directly influenced by the OLR, the amount of volatile solids removed, and the retention time (Equation (2)) [106]. Higher OLR can lead to increased VS input, affecting the overall reduction efficiency (Equation (3)). Equation (4) indicates how efficiently the organic matter is converted into methane, with OLR being a key factor. OLR impacts the flow rate and, consequently, the HRT, which is crucial for maintaining process stability (Equation (5)) [106,107]. The production of VFAs (proton source) is significantly affected by OLR, which can influence the pH of the system (Equation (6)) [108]. High OLR can lead to VFA accumulation, which may inhibit methanogenesis if not properly managed (Equation (7)) [109].
- OLR = Organic loading rate (kg VSin/L/day);
- Q = Flow rate of the feed (L/day);
- VSin = Volatile solids concentration in the feed (kg/L);
- VSremoval = Volatile solids concentration in the digestate (kg/L);
- VSout = Volatile solids concentration in the digestate (kg/L);
- V = Volume of the digester (L);
- VSR = Volatile solid removal (%);
- MPR = Methane production rate (NL-CH4/day);
- SMY = Specific methane yields (NL-CH4/kg VSin);
- VFAaccumulation = Rate of VFA accumulation;
- VFAproduction = Rate of VFA production;
- VFAconsumed = Rate of VFA consumed.
Using a pilot-scale digester, Liu et al. (2012) [110] examined the impact of OLR on the co-digestion of MSW. They found that the process was stable between OLRs of 1.2 and 8.0 kg VS/m3d, with the greatest CH4 production rate reported at the OLR of 8.0 kg VS/m3d. Similar to this, a rise in the OLR from 1.8 to 5.0 kg VS/m3d resulted in a 479% increase in the amount of CH4 produced per litre of food waste-fed digester [111]. The microbial community participating in the process and, as a result, the biogas yield are both influenced by the OLR. An increase in OLR may boost biogas production to a certain extent, but beyond that, imbalances between the four stages of AD are likely to arise, leading to process inhibition due to VFA accumulation. Process failure and irreversible acidification could eventually result from too-high OLRs. Effluent recirculation is a method for addressing the issues brought on by overloading. In general, compared to mesophilic circumstances, thermophilic environments exhibit more efficient and steady behaviour when producing CH4 from food waste at high OLR [112,113,114]. A mesophilic AD’s CH4 yield, however, reported by Guo et al. [115], demonstrated greater stability than a thermophilic AD’s in response to an OLR rise of 1.0 to 2.5 g VS/L d. When compared to thermophilic digesters, they found that mesophilic digesters had higher levels of microbial variety and abundance.
2.2.8. Toxicity
NH3, sulphide, light metal ions, heavy metals, and organics are among the inhibitors that are frequently found in anaerobic digesters. The literature results on inhibition produced by particular toxicants vary greatly since anaerobic inocula, waste composition, experimental methodologies, and circumstances vary. The pH has an impact on both the composition of TAN and the proliferation of micro-organisms when treating waste with high levels of total NH3 nitrogen (TAN). Many industrial wastewaters commonly contain sulphate-reducing bacteria that convert sulphate to sulphide in anaerobic reactors (SRB) [97]. A sulphate decrease results in two levels of inhibition. The primary inhibitor, which reduces CH4 synthesis, is brought on by SRB’s competition with common organic and inorganic substrates. The toxicity of sulphide to several bacterial groups causes secondary inhibition. Sulphide in the form of H2S is harmful because it can diffuse into cell membranes. H2S may be inhibitory after it enters the cytoplasm by interfering with the different coenzyme sulphide connections, denaturing native proteins through the creation of sulphide and disulphide cross-links between polypeptide chains, and interfering with the assimilatory metabolism of sulphur [116]. According to some reports, the mechanism of aluminium suppression is caused by its competition with Fe and manganese or by its attachment to the membrane or wall of the microbial cell, which may have an impact on microbial growth [117]. Heavy metals like chromium, Co, cadmium, and nickel can have a significant toxic effect on AD because they can disrupt enzyme structure and function by binding to thiol and other groups on protein molecules or by substituting synthetic metals for naturally occurring metals in the prosthetic groups of enzymes. The methanogenic efficiency can be greatly increased by co-digesting waste with other waste, allowing micro-organisms to adapt to inhibitory chemicals, and using techniques to remove or neutralise toxicants before anaerobic digestion.
2.2.9. Hydraulic Retention Time (HRT)
For organic resources to be converted into biogas, the bacteria need a long enough retention period. Most likely, the most significant process variable impacting the yield and pace of CH4 production is the length of the retention time [118]. Higher retention rates are the result of a longer reduction of volatile solids, an increase in digester volume needed, and improved tolerance to hazardous chemicals and pH changes. However, shorter retention periods need a smaller digester volume and a lower investment cost while producing biogas of the same quality and quantity [119]. The ideal retention period is influenced by feed composition, OLR, and temperature. In order to prevent biomass wash-out, such digesters must have a minimum retention time of 10 to 15 days. In mesophilic anaerobic digesters, the typical retention duration ranges from 15 to 30 days [53]. Anaerobic digesters should take up to three months to reach maximum capacity during the start-up phase in order to supply the required concentration of biomass [119]. HRT and solid retention time (SRT) are the two distinct measures used to express retention time. The average length of time the TS remains in the digester is known as SRT, and the digester volume-to-substrate flow rate ratio is known as HRT.
AD is a delicate balancing act between intricate microbial communities and physiological bioreactor parameters, and it is quickly compromised by changes in the temperature, slurry concentration, pH, composition of the substrate, liquid dynamics, modes of operation, and reactor designs [120]. Additionally, because they contain hazardous and recalcitrant elements that could harm AD’s effectiveness, some organic wastes may require specific handling. According to Thanh et al. [121], excessive residual organic matter, NH3 (used to preserve natural latex), and latex floating particles make it challenging to accomplish the AD of raw natural rubber processing effluent. According to Jiménez et al. [122], phenolic chemicals found in molasses interfere with the activity of methanogens, prevent the clearance of organic materials, and lengthen the HRT. The high salinity of distillery slops or vinasses may be problematic for the bacteria’ osmotic pressure. According to Rodriguez-Abalde et al. [123], materials with high COD, BOD, TKN, and salt levels frequently cause processes to fail, particularly in reactors with high HRTs where biomass washout is unavoidable (Table 3). In order to address these issues, a number of materials have found use in anaerobic digesters for everything from biogas production to purification. However, nanomaterials have found the best uses in the AD process, where their presence has a substantial impact on the stability of the process, microbial growth, and reactor productivity, allowing for a significant improvement in the efficiency of waste to bioenergy conversion. There is still much that needs to be learned about the fundamental processes involved in electron transport, ion exchange, nutrient release, absorption, and catalytic characteristics of nanomaterials. Our ability to develop specialised materials that can enhance the anaerobic digestion technology of diverse substrate types in various reactors can be enhanced by our understanding of their roles in the anaerobic digestion process.

Table 3.
Inhibiting factors of substrates affecting AD process.
3. Feasibility of FOGO as a Promising Substrate for Biomethane Production
3.1. Characteristics of FOGO
FW decomposes quickly and contains a large amount of organic matter. On a dry mass basis, the percentage of volatile solids ranges from 70% to 90% [130]. These characteristics are crucial because they enable the conversion of FW into useful products, such as energy recovery. However, other traits of FW, such as low pH levels, high nitrogen content, and low C/N ratios, are known to be detrimental to AD processes [131]. Table 4 lists some of the traits of food waste that have been documented in previous findings.

Table 4.
Characteristics of FW reported in previous studies.
Due to the possibility of increasing the system’s C/N ratio and creating the conditions for the growth of anaerobic bacteria, which would subsequently increase the production of biogas, AcoD with garden leftovers has been the subject of much research in this respect [136]. Due to its structural characteristics, lignocellulosic material may be challenging to recover energy from. However, its application in AcoD is continually growing, especially because there is so much content available [137]. Only a few research works have examined the effects of adding lignocellulosic substrates, including garden waste, to the treatment of food waste in the literature [138].
AcoD has the ability to considerably boost the biogas yield. Agricultural waste with a high lignocellulosic content or animal manure serves as the primary substrate for AcoD with FW. Table 5 lists some of the studies that used FW, YW, or GW as co-substrates. Lignocellulosic waste improved the system by slowing down the rapid acidity caused by the quick hydrolysis of labile organic matter because of its slower biodegradability. The output of biogas has increased from as low as 19% [139] to as high as 55.2% [140], with a CH4 percentage of roughly 60%, depending on the type of substrate added to the co-digestion with FW. By lowering the pH of the hydrolysis mixture and raising the concentration of VFAs during the hydrolysis and acidification stage, FW supplementation may make GW pre-acidification easier (acting as simulative chemical pretreatment). NH3 is cited in older scientific papers as the main cause of digester inhibition because it enters bacterial cells and disrupts proton balance, changes intercellular pH, and inhibits certain enzyme reactions. In order to balance the C/N ratio in the digester and prevent the revival of NH3, co-digestion with other waste is an effective strategy. Food waste and organic garden waste make up a sizable portion of landfill waste (Figure 4). However, the utilisation of food organics and garden organics jointly handled as a single substrate has only been the subject of a small number of prior research works. Despite the excellent potential of food and garden organics, there is still a lack of knowledge about the diversity of microbial communities, which makes it challenging to use FOGO in a full-scale biogas plant [36].
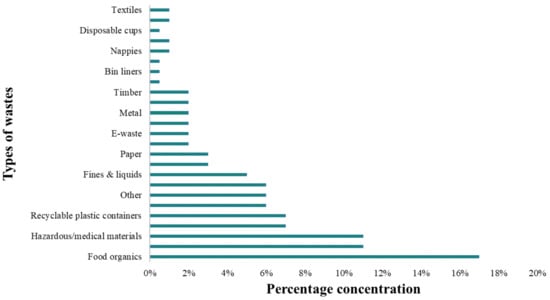
Figure 4.
Typical landfill composition in Australia (source [141]).

Table 5.
Biogas yield and operational parameters for the AcoD of FW in relation to other organic wastes.
Table 5.
Biogas yield and operational parameters for the AcoD of FW in relation to other organic wastes.
| Substrate | Co-Substrate | Mixing Ratio | Mode of AD | Operating Conditions | Biogas Yield | Comments | Reference |
|---|---|---|---|---|---|---|---|
| FW | Pig Manure (PM) | PM:KW = 1:0, 4:1, 3:2, 2:3, 1:4, 0:1. | Batch | OLR—5.2 g VS, Temp—37 °C HRT—8 days | 0.521 m3/kg VS | Ideal mixing ratio = 1:4, no VFA or NH3 inhibition | [142] |
| FW | De-oiled grease trap waste | _ | CSTR: −3 system: single-stage, two-stage | HRT—30 days | 0.60 m3/kg VS | 19% increase in biogas yield, lipid–lipid/TS breakdown at 40% | [139] |
| FW: canteen waste | Straw from maize, sorgos, and wheat | 5:1 | Batch | HRT—8 days, OLR—5 g VS/L, Temp—35 °C | 0.392 m3 CH4/kg VS | Increased CH4 yields by 39.5% and 149.7%, respectively. | [143] |
| KW: canteen waste | CM | 1:1 | Batch | HRT—45 days, OLR—TS 8%, Temp—35 °C | 0.859 m3/kg VS | The effects of the initial pH were investigated, with 6.0 causing digester failure and 7.5 recommended. | [93] |
| FW: canteen waste | Rice husk | Combined to provide a 28 C/N ratio | Plug flow pilot plant single-stage, | OLR—6 kg VS/m3d, HRT—25 days Temp—37 °C | 0.446 m3 biogas/kg VS | With an increase in OLR and a drop in HRT, VS removal efficiency reduced; instability caused by VFA and high alkalinity (0.94) at OLR of 9 kg VS/m3d. | [144] |
| Yard waste (YW) | FW | 100% YW; YW:FW = 9:1; YW:FW = 4:1 | Batch | OLR—0.0–166 g/L, Temp—36 ± 1 °C HRT—30 days | 8.6 Lmethane/Lwork | When FW increased from 0% to 10%, the CH4 yield climbed by two times, at 20%, induced VFA inhibition, and CH4 output fell by 9.7 times. | [145] |
| FW | Microwave-treated YW | 1.5 | Batch | Temp—30 °C HRT—30 days | 431 mL/gVSadded | Max CH4 yield at an F/M ratio of 1.5. Highest nett energy gain of 6.5 kJ/gVS at F/M ratio of 1.0, suggesting the possibility of field application at 1.0 instead of 1.5. | [146] |
| YW | Sewage Sludge (SS) and FW | YW:FW:SS = 9:3:4; 6:6:4; 3:9:4 | Batch | Temp—37 °C HRT—60 days | 314.9 ± 17.1 mL/gVS | AcoD of SS (25%, VS basis) with YW boosted the CH4 yield by 2.04 times | [147] |
| OFMSW (FW, GW) | _ | _ | Batch | Temp—37 °C HRT—21 days | 126 mL CH4/(gVSadded) | S/I > 1.0 based on TS, accumulation of inhibitory intermediates can result in system failure due to mass transfer constraint under low moisture conditions. | [148] |
| FW | GW | 4:1 | Pilot scale reactors (500 L) | OLR—0.24 kgVS m−3d−1, Temp—40 °C HRT—40 days | 0.47 LCH4 gVS−1 | Garden waste promoted growth of micro-organisms as a sustaining medium for biofilms, delaying the reactor’s acidification | [149] |
| FW | GW, WAS (waste-activated sludge) | FW/YW/WAS = 0.8:1.7:0.5FW/YW/WAS = 1:1:1 | 4.7 L semi-continuous biodigester | HRT—28 days, Temp—35 °C | 186 mL/gVS | Compared to mixes with FW/YW/WAS = 1:1:1, mixtures with FW/YW/WAS = 0.8:1.7:0.5 had greater CH4 yields (134 ± 15 mL CH4/gVS) but took longer (10 days) to recover from volatile fatty acid inhibition. | [150] |
| FW: university canteen | CM | _ | Batch | Temp—35 °C HRT—28 days | 0.388 m3 CH4/kg VS | AcoD increased CH4 generation by 41.1% in batch mode and by 55.2% in semi-continuous mode at optimal mixing at 2. | [140] |
3.2. Methods of Biomethane Production from FOGO
Food waste undergoes anaerobic digestion and is categorised based on reactor feeding type, temperature, and moisture content during the treatment phase [151,152,153,154]. Stage techniques (single, double, or multi-stage) also have an impact on the anaerobic digestion process’s performance.
3.2.1. Comparison between Single-Stage and Multi-Stage Process
The four metabolic steps of anaerobic digestion are acetogenesis, methanogenesis, hydrolysis, and acidogenesis [155]. A single-stage process is a system in which all these processes occur in a single reactor. Due to their low cost and simplicity of operation, single-stage digesting systems are frequently used. Lower CH4 generation, a pH range of 6 to 7, a lesser organic loading rate, longer hydraulic retention time, and lower economic investment are the primary characteristics of this kind of operation. The fundamental disadvantage of the single-stage reactor is that when the circumstances necessary for methanogenesis are not satisfied, acidogenic micro-organisms thrive due to the rapid acidification of food waste, compete with methanogenic microbes, and upset the desirable microbial flora concentration. However, this problem can be resolved by incorporating a buffer and changing the feeding rate [156,157,158]. When compared to non-buffered systems, buffers such as sodium bicarbonate minimise the volatile solids and shorten the time required for degradation. In a 2015 study, the effectiveness of anaerobic digestion of food waste was assessed in relation to four distinct alkalinity sources, including eggshells, calcium carbonate, sodium bicarbonate, and lime clay from the paper industry. According to reports, adding a buffer during the feeding phase increased both the process stability and CH4 production. Single-stage reactors are still extensively utilised today owing to these advantages.
For each of the four steps, the double- or multi-stage process occurs in a separate chamber. In the first reactor, the processes of hydrolysis, acidogenesis, and acetogenesis occur [159,160]. Methanogenesis is typically employed in the second reactor. According to studies, the acidification stage takes place when the hydraulic retention period is 2–3 days, and the pH is 5.5 to 6.6, but the slow-growing methanogenic bacteria need a longer retention time of 20–30 days and an alkaline pH of 7–8 [161]. Two-stage or multi-stage reactors have a number of advantages, including increased CH4 generation, a higher organic loading rate, improved processing stability, better pathogen control, and increased effectiveness in removing volatile solids. The complicated operation, greater maintenance costs, and higher installation capital expenses are the key negatives, nevertheless. Both procedures produced comparable results when compared at a retention period of 30 days, but the double-step process produced more biogas and degraded materials more effectively. It was also noticed that the multiple stages produced greater net energy at a lower cost and with a simpler post-remediation dewatering process.
A compact, three-stage anaerobic digester has been developed by researchers. It consists of three successive chambers that perform hydrolysis, acidification, and wet CH4 generation. When compared to the previously used one-stage or double-stage anaerobic digester, they found that this arrangement produced 25–50% more CH4 with an organic loading rate of 10 g volatile solid per litre [4,162,163]. The removal of volatile solids by this three-stage method likewise showed an efficiency rate of 83.5%. In order to control the microbial load and growth during anaerobic digestion, pH is a key element. For optimal performance, each type of microbe responds to a certain pH range. Researchers found that after 3 weeks of anaerobic digestion of mixed kitchen waste, primarily consisting of 15 kg of fruits and vegetables, with a 15-week hydraulic retention period, there was a noticeable reduction in pH. This reduction was attributed to the conversion of the waste to a high concentration of fatty acids. While methanogenic bacteria can tolerate a pH of up to 8, acidogenic bacteria can only survive in a pH range of 5 to 7 [164,165]. The pH now drops even lower as the work of the acidogenic bacteria to create organic acid begins. After week nine, the methanogenic phase starts, during which the methanogens eat the organic acids and produce CH4 as a byproduct. Therefore, maintaining pH by the addition of buffers is essential for a higher output of biogas. Figure 5 illustrates the comparison between single-stage and multi-stage AD.
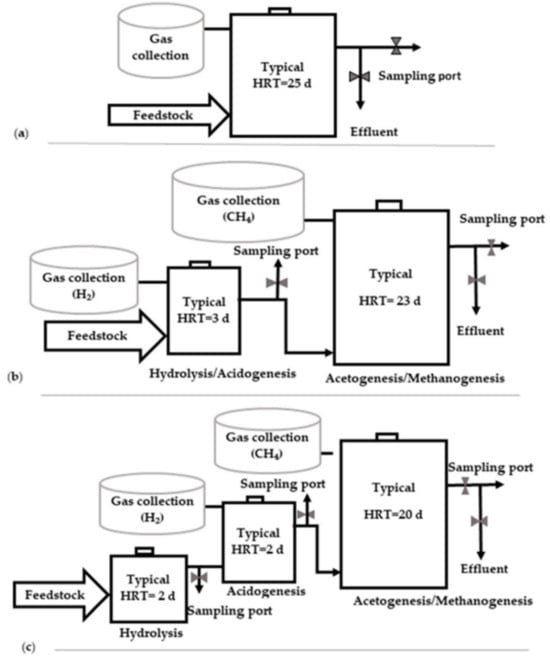
Figure 5.
Types of digester configuration: (a) single-stage; (b) two-stage; and (c) three-stage digester (source Rabii et al. [166]).
3.2.2. Reactor Configuration
Reactors used for fermentation can be broadly categorised as batch, semicontinuous, or continuous, depending on how the process is carried out.
Batch Reactors
Batch reactors are the most basic reactors and are typically used in laboratories for initial research on choosing the best conditions for dark fermentation and anaerobic digestion. The reactors’ architecture is straightforward and affordable, and they allow for convenient control of fermentation parameters, particularly pH and temperature. Figure 6 illustrates a typical batch reactor used for the AD process for producing H2/CH4. Irrespective of the type of substrate, the batch process provides a vivid image of the AD process for that substrate.

Figure 6.
A typical batch reactor for AD. This picture has been adapted from Angelidaki et al. [167].
Continuously Stirred-Tank Reactor
Continuously stirred-tank reactors (CSTR) are the ones that are most frequently used for continuous fermentation. The reactors have a mechanical stirring system and are shaped like cylinders. In a continuous or sequential mode, the feedstock is supplied into the reactor and the effluent is extracted from it. For agitation, biogas can be recirculated, mechanical stirrers can be used as well, and inoculum can also be added continuously [168,169]. Due to its shorter hydraulic retention durations, this type of reactor’s dimensions are significantly less than those of traditional fermenters used to produce CH4. The CSTR reactors are widely used because of their straightforward design, ease of adjusting operating parameters, and stirring that helps to maintain uniform medium conditions and promotes good interaction between micro-organisms and substrates. Stirring also offers efficient temperature and pH control inside the reactor. The performance of the anaerobic digestion feeding system stayed constant when diluted food waste was continuously fed, according to the researchers’ analysis of several feeding techniques. This continuous intake of diluted food waste leads to a consistent output of biogas. In the meantime, there were differences in the batch-fed reactor’s performance. In the batch reactor, the CH4 production was 1.51 L/day with an organic loading rate of 6.1 kg COD/m3, compared to 2.78 L/day at an organic loading rate of 8.6 kg COD/m3 [170].
The industrial production of hydrogen and CH4 requires the use of continuous reactors due to higher predicted process efficiency. When initiating continuous reactors, batch mode is often utilised to ensure proper inoculum preparation and pre-treatment. The start-up technique has a big impact on whether the switch to continuous mode operation goes smoothly. Continuous stirred-tank reactors (CSTR) (Figure 7), upflow anaerobic sludge blanket reactors (UASB) (Figure 8), anaerobic fluidised bed reactors (AFBR) (Figure 9), and membrane bioreactors (MBR) (Figure 10) are the configurations of bioreactors that are most frequently utilised.
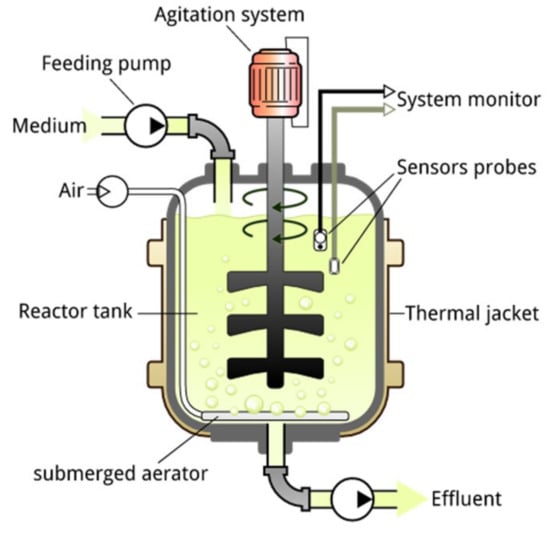
Figure 7.
A typical CSTR for AD process (source: Maria et al. [171]).
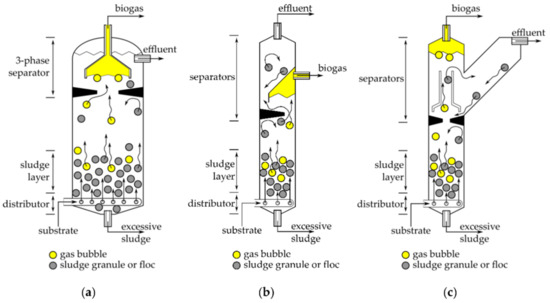
Figure 8.
Typical operational infrastructure of an up-flow anaerobic sludge blanket (UASB) reactor: (a) traditional; (b) modified gas collector; and (c) Y-shaped (source: Pererva et al. [172]).
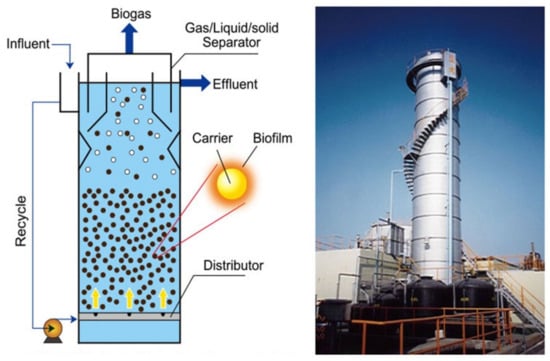
Figure 9.
Schematic diagram and full-scale anaerobic fluidized bed reactor (source: Technology [173]).
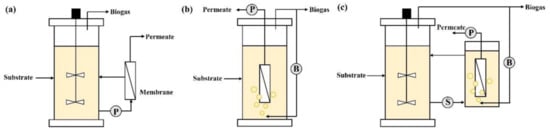
Figure 10.
Basic configurations of anaerobic MBR: (a) external MBR, (b) submerged MBR, and (c) external submerged MBR (source: Li et al. [174]).
A comparison of reactor characteristics and biogas generation with respect to the FOGO is shown in Table 6.

Table 6.
A comparative analysis of reactor specifications and biogas yield respective to the FOGO.
4. Application of Nanotechnology on Enhancement of AD
4.1. Factors Involved in Enhancement of AD Performance
As shown in Figure 11, different methods can be utilised to optimise the metabolic phases of the AD process for resource recovery from the organic fraction of municipal waste (OFMSW), of which FOGO makes up the majority of the composition. According to the literature that is currently accessible, mechanical pre-treatment has been utilised frequently to speed up the hydrolysis process. Simple mechanical pre-treatment techniques, including maceration, sonication, and the use of a high-pressure homogenizer, can improve the solubilization of OFMSW [186]. Although lignocellulosic-based OFMSW benefits from quick digestion when subjected to mechanical pre-treatment, this method has significant energy needs and runs the risk of contamination from impurities during the procedure. Although they are not frequently utilised in industrial applications, chemical, microbiological, and enzymatic pretreatment, i.e., glutamylase, amylase, cellulase, and xylanase, pre-treatments can work well with other pretreatment procedures during hydrolysis [187]. Future research ought to similarly concentrate on assessing the viability of integrating pre-treatment technologies and looking into novel pre-treatment technologies. The use of TE and granular activated carbon (GAC) to optimise the acidogenesis and acetogenesis phases of AD has been beneficial [188]. The advantages of using TE and GAC include boosting enzyme activities, stimulating the formation of methanogens, and counteracting VFA inhibition to improve process stability. However, running biogas plants with GAC is challenging and demands advanced techniques [189].
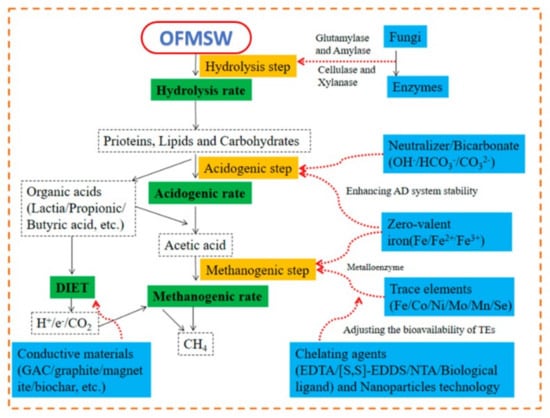
Figure 11.
Strategies to improve metabolic stages of the anaerobic digestion process of organic fraction municipal solid wastes (source: Li et al. [190]).
The performance of AD depends on optimal concentrations of micronutrients (such as Ni, Fe, Mo, Co, and Se) and macronutrients (such as N, P, and S), which are, respectively, necessary cofactors in a variety of enzymatic processes involved in methanogenesis and fundamental components of micro-organisms. According to Garcia-Peña et al., the inclusion of a protein-rich source can boost biogas production by as much as 50% and increase the buffering ability against acid buildup [191]. However, research also indicated that the total Kjedahl nitrogen (TKN) content had an inhibitory effect and reduced biogas generation by 30–50%. High-NH3-concentration systems have been reported to benefit from TE supplementation. To oxidise formate, the methanogen enzyme formate dehydrogenase requires important TEs such as tungsten (W), molybdenum (Mo), and selenium (Se) [192]. In Table 7, we can see the role of certain trace metals on AD.
The most efficient TE to overcome NH3 toxicity is currently unknown. It has also been found that TE can overcome VFA inhibition. In a comparable manner, studies on which TE works best against which VFA are still in their early stages. Although propionate has been found to be the main VFA in the dysfunctional AD system [193], lactate has been found to be the main inhibitory VFA in some investigations [194]. Recently, trace elements such as salts, pure metals, and metal oxides in the nanoscale range have been added to the feedstock to boost the production of biogas [195]. Anaerobic digestion may be impacted negatively or favourably by nanoscale material additions. Nanotechnology has been used in biological, physical, chemical, medical, and other applications due to the thermal, mechanical, and structural characteristics of nanoscale materials.
Since NPs have unique properties, scientists are investigating how NPs impact the AD process to generate green energy [196]. The surface-to-volume ratio increases as particle size decreases, and the physical and chemical properties of materials at the nanoscale appear to be radically different from those of their equivalent bulk sizes [197].

Table 7.
Role of metal trace elements on AD.
Table 7.
Role of metal trace elements on AD.
| Trace Elements | Metabolic Activity | Threshold for Enhancement of CH4 Conc. (mg/L) | Threshold for Inhibition of CH4 Conc. (mg/L) | References |
|---|---|---|---|---|
| Cu | Microbial community, methanogenic activity, cellulase activity, and volatile fatty acid concentration | 5 | 130 | [198] |
| Fe | Cellulase activity | 0–1000 | 20,010 | [199] |
| Ni | Methanogenic activity, cellulase activity | 0–20 | 32 | [200] |
| Zn | Methanogenic activity | 0–100 | _ | [201] |
| Molybdenum | CO2 reduction in methylotrophic pathways | 0.1–0.3 | 1 | [202] |
4.2. Impact of Nanomaterials on Biological Performances
During AD, a cooperative symbiosis between fermentative bacteria and the methanogen, called syntrophic methanogenesis, takes place. IET is the main driver of the syntrophic methanogenic process, which aids in the anaerobic digestion process’s energy utilisation. There are three modes of IET:
- (i)
- Transfer of hydrogen between species: Hydrogen carries electrons from VFAs [203].
- (ii)
- Formate transfer between species: Formate is an electron carrier [203].
- (iii)
- DIET: DIET is a different channel for the transfer of electrons between oxidising bacteria and the methanogen during AD.
IET, which employs formate and hydrogen as electron carriers, is the primary technique for transferring electrons between methanogens and fermenter bacteria [204]. The most sought-after field of research for the various IET modes is DIET. Indirect interspecies electron transfer is defined as interspecies hydrogen transfer and interspecies formate transfer (IIET). DIET (Figure 12) [195] is favoured over IIET [203] for the production of CH4 for the following reasons [205]:
- (i)
- Reduced energy consumption;
- (ii)
- No requirement for redox mediators to promote electron exchange;
- (iii)
- The avoidance of hazardous volatile fatty acid accumulation in the event of a process standstill;
- (iv)
- Redox mediators are produced, consumed, and diffused without the need for laborious enzymatic procedures, which have thermodynamic benefits.
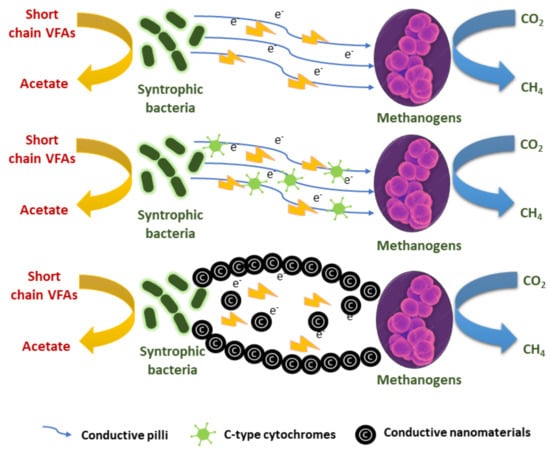
Figure 12.
Distinct DIET mechanisms existing between methanogens and fermenting micro-organisms.
According to Cheng and Call [206], DIET can improve methanogenic bacteria’s performance since it happens more quickly and is more effective than IET using hydrogen and formate.
Methanogenic activity is enhanced by the abiotic material-mediated cell-to-cell electron route between acidogenic bacteria and DIET, a methanogen that increases the production of CH4 [205]. Because of their physiochemical characteristics, which include size, surface area, solubility, surface structure, and catalytic nature, nanomaterials are valuable in a range of applications [207]. The mobility of nanomaterials in an anaerobic environment alters DIET and, in turn, affects biogas production. Because they can cross cell membranes, nanomaterials are useful in many biological applications [208,209]. Additionally, the inclusion of NPs accelerates CH4 production and shortens the lag phase [210,211].
4.3. Types of Nanomaterials and Their Applications on AD of FOGO
Fe oxide NPs have been used in a great deal more studies over the past several years because of their high surface-to-volume ratios, unique electrical properties, supermagnetic characteristics, and catalytic activities. Fe oxides’ low oxidation-reduction potential and electron-donating characteristics speed up the methanation process. A detailed summary of metal oxide nanoparticles (NPs) and their impact on biogas production may be found in Table 8. First, Kato et al. [212] demonstrated that DIET is an abiotic conductive material using magnetite (Fe3O4) at a concentration of 20 mM and noted an increase in CH4 production. From a feedstock treated with 0.01 to 0.1 g/L of Fe3O4, the CH4 production rate increased by 44% [213]. Similar outcomes were reached by Baek et al. [214], who discovered a threefold increase in CH4 production rate. In addition to conductivity, Fe3O4 also demonstrates a magnetic feature that, from a magnetism perspective, improves DIET and CH4 production [215]. Although conductivity is a crucial factor in promoting DIET, the inclusion of conductive materials also significantly speeds up the processes of hydrolysis and acidification. Sludge treated with 10 g/L of Fe3O4 demonstrated a 13.9% reduction in the lag phase and produced 15.4% more CH4 [216].
Fe3O4 particles ranging in size from 7 nm to 24 nm were introduced to the anaerobic digestion process in one study [217] to investigate their effects on biogas production. The authors claim that biogas production stopped on day 21, when there were no NPs present, and continued until day 40, when there were NPs present. This demonstrates the advantageous benefits of adding Fe3O4 NPs: biogas synthesis was increased by 93% over a control sample when a substrate treated with 7 nm Fe3O4 NPs was added, whereas biogas generation was unaffected when a feedstock treated with 24 nm NPs was added.
Anaerobic digestion is influenced by nanoparticle concentration in the substrate in addition to nanoparticle size. The substrate was supplemented with 0.75 and 1.5 g of nanoscale Fe3O4, respectively, by Suanon et al. [218]. The production of CH4 increased by 26% in the former sample as compared to the control. A high concentration of Fe3O4 (1.5 g) was found to suppress CH4 generation, with a decrease of 11.5% when compared to the control condition. Similar findings were obtained by Yang et al. [219], who claimed that the cytotoxic effect on anaerobic digestion decreases CH4 production. In a similar vein, the influence of Fe3O4 NPs on the formation of CH4 at various concentrations (50, 75, 100, and 125 mg/L) was investigated [220]. A substrate treated with 75 mg/L Feoxide NPs produced the maximum CH4 yield, which was 53.3% greater than the control condition. The scientists also observed a decrease in CH4 generation as the concentration of oxide nanoparticles increased. The enzymatic absorption activity and rapid hydrolysis may be the cause of this.
Juntupally et al. [221] conducted a comparative study of Fe3O4, Co3O4, NiO, and MoO3 micronutrients and NPs with CM slurry in the single- and bi-phasic AD at 37 ± 2 °C for 20 days. Fe3O4 NPs produced 0.16 L/(g VS. decreased) biogas during a single-phase AD. In the single-phase process, an increase in biogas production with improved CH4 is recorded (70–80%); however, in the bi-phase, AD Fe2O3 and its related NPs show an increase of 76%. Compared to Co3O4 and MoO3 NPs, NiO NPs had a peak biogas output of 0.3 L/(g VS. decreased) in the biphasic AD. NiO and Co3O4 NPs produced 0.15 L/g of biogas during single-phasic AD (gVS. reduced).
Studying the impact of NPs on biogas production was another contribution made by Abdelsalam et al. [222]. Biogas and CH4 production were increased by 1.66 and 1.96 times, respectively, by adding merely 20 mg/L Fe3O4 NPs, using varied concentrations on CM slurry, a mixing temperature of 37 ± 0.3 °C at a rpm of 90, and an HRT of 50 days. By adding Fe3O4 NPs (100 ppm) to organic waste for almost two months, Casals et al. [217] also carried out an anaerobic experiment in mesophilic conditions. This set of circumstances predicts an increase in CH4 and biogas output of 234% and 18%, respectively. Additionally, Fe2+ was found to be the primary contributing aspect since it helps to brilliantly break down trash under anaerobic conditions. It has been one of the largest increases in biogas and CH4 output that one can discover in the literature currently. Ali et al. [223] examined the impact of biocompatible Fe3O4 NPs, and the results showed that lower doses of 50 and 75 mg/L were more successful in increasing CH4 generation than higher concentrations of 100 and 125 mg/L. This is in contrast to what Abdelsalam et al. [222] found.
In the study conducted by Zhang et al. [224], an inverse relationship between VFA generation and ZnO NP concentrations was established. ZnO NPs prevented the hydrolysis–acidification of sewage sludge, namely protein. The effect of ZnO NPs on protein hydrolysis reduced the production of biogas and slowed the formation of VFA during AD. This process, which was found to be the primary cause of the decrease in biogas, also altered the composition of the bacterial community.
According to one study [225], the cumulative CH4 generation from substrates treated with 1, 10, and 100 mg/L Fe4NiO4Zn decreased by 5.6, 65.6, and 98.4%, respectively, when compared to the control. CH4 yields from substrates containing 1 and 10 mg/L Fe2NiO4 NPs were equal to controls, whereas a substrate containing 100 mg/L Fe2NiO4 NPs produced 30% more CH4 than the control. Nickel ferrite NPs, thus, can increase CH4 generation by acting as a potential adsorbent. The impact of nickel ferrite and Ni-Co ferrite NPs on the production of biogas from anaerobic digestion was also reported by Abdallah et al. [226]. Nickel ferrite and Ni-Co ferrite nanoparticle concentrations were utilised at the same levels in the study by the authors. The inclusion of NPs made of nickel improved biogas production. The substrate treated with 130 mg/L Ni-Co NPs produced 33.0% more biogas than a control reactor. However, for smaller concentrations (20 mg/L) of nickel ferrite NPs, the biogas output increased in comparison to higher concentrations (70 and 130 mg/L). The scientists discovered that the substrate treated with 20 mg/L nickel ferrite NPs produced 30.8% more biogas than a control condition.
One research investigated the effect of adding nickel graphene (Ni-Gr) nano-composite to an AD of industrial effluent containing MEG to increase biohydrogen generation [227]. The unique properties of Ni-based NPs served as Ni ion suppliers, while graphene was employed by the scientists as a support material. This is the first work that used Ni-Gr nanocomposite in the AD procedure. The findings demonstrated that at dosages of 60 mg/L, hydrogen generation from other concentrations rose by 105%. With a dose of 60 mg/L, Ni-Gr-NC was able to produce the most specific hydrogen at a rate of 294.24 ± 12.06 mL/L. Ni ions affected the hydrogenase enzyme activity in the presence of graphene, leading to an increased hydrogen production. Ni-Co-Ferrite was investigated for its impact on biogas production by Mansour et al. [228], who found that it increases biogas output by roughly 30%. While zero-valent iron (ZVI) solely increases methanogenesis, Fe3O4 can also improve acidification and hydrolysis processes. In order to encourage hydrolysis–acidification, Fe3O4 (10 g/L) has been added to the first phase of AD, whereas ZVI (10 g/L) has been added to the second phase to encourage methanogenesis and prevent Fe3O4 inhibition. When ZVI or Fe3O4 alone was added, the output of CH4 was increased by 10.2% or 18.1%, respectively [229]. In comparison to the control, in reactors with zinc ferrite substitute, CH4 output increased by 185.3% [230]. Additionally, adding Fe3O4 particles to a methanogenic sludge increased the amount of CH4 that could be produced from propionate by up to 33% [231]. The most amount of biogas produced during the anaerobic incubation of cellulose was enhanced by the application of Fe3O4 NPs. DIET between bacteria and methanogens can be effectively facilitated by Fe2O3 NPs. In comparison to the control, the application of Fe2O3 NPs (100 mg/g) and ZVI NPs (10 mg/g) increased biogas yield by 117% and 120%, respectively, and had a favourable effect on the activity of methanogenic archaea. According to one study, the application of 20 mg/L ZVI NPs and 20 mg/L Fe3O4 magnetic NPs increased the production of biogas by 45% and 66%, respectively [232]. Fe(OH)3 (0.3%) is the least often used Fe-based addition for increasing biogas yield, with nano ZVI (21.4%), Fe3O4 NPs (18.8%), and ZVI (15.2%) being the most frequently used [233]. In the future, combining nanomaterials with Fe and its oxides will likely be a big development trend. The steady annual increase in Fe-based additive research points to a global hunt for efficient additives to enhance AD.

Table 8.
Impact of different nanomaterials on biogas and CH4 yield from various substrates.
Table 8.
Impact of different nanomaterials on biogas and CH4 yield from various substrates.
| NP Type | Size | Substrate | Temp | HRT | Dosage | Effect | References |
|---|---|---|---|---|---|---|---|
| Fe oxide | – | Seed sludge | 36 °C | 96 h | 750 mg/L | 38.2% enhancement in CH4 production | [234] |
| ZVI | 9 nm | Cattle dung slurry | 37 °C | 50 days | 5–20 mg/L | 43.75–45.37% increase in biogas production | [235] |
| Fe3O4 | 7 nm | 5–20 mg/L | 63.03–65.62% enhancement in biogas yield | ||||
| Fe3O4 | 7 nm24 nm | Municipal waste | 37 °C | 60 days | 100 ppm | 93.24% increase in biogas formation No enhancement | [217] |
| ZVI | 50 nm | Dewatered sludge | 37 °C | 12 days | 0.75 g 1.5 g | 45.77% increase in CH4 production 29.66% decrease in CH4 production | [218] |
| Fe3O4 | 20 nm | 0.75 g 1.5 g | 25.61% increase in CH4 production 11.51% decrease in CH4 production | ||||
| Fe3O4 | 10–35 nm | Municipal solid waste | 37 °C | 60 days | 50 mg/L 75 mg/L | 65.3–72.09% enhancement in CH4 yield | [221] |
| 100 mg/L | 44.22% increase in CH4 yield | ||||||
| 125 mg/L | 42.54% increase in CH4 yield | ||||||
| ZVI | 45 nm | Waste-activated sludge | 37 °C | 14 days | 1000 mg/L | Highest CH4 content is 88% | [236] |
| Zeolite | 7.13 µm | 4 g/L | No effect | ||||
| Mixture of zeolite and nZVI | – | 4 g/L and 500 mg/L | The production of biogas first grew before abruptly declining | ||||
| nZVI coated with zeolite | 24.1 µm | 500 mg/L 1000 mg/L | Biogas output decreased until the eighth day when it abruptly rose | ||||
| Fe | 200 nm | Activated sludge | 37 °C | 14 days | 50–3000 mg/L | 19–105% increase in biogas generation | [237] |
| Bimetallic Cu-Fe nanoparticle | 100 nm | 50–3000 mg/L | Increase in biogas production by 47.16–108.29% | ||||
| Fe3O4 | 20–30 nm | Waste sludge | 37 °C | 12 days | 20–200 mg/L | CH4 content increased from 58.5% to 65.5% But at 200 mg/L, ethane content reduced to 52.3% | [238] |
| ZVI | 60 nm | Anaerobic activated sludge | 37 °C | 14 days | 50 mg/L | 13.54% decline in biogas yield | [239] |
| ZVI | – | Sludge from municipal wastewater treatment | 37 °C | 30 days | 0.10% | 3.1% increase in CH4 content | [240] |
| Fe powder | – | Slaughterhouse sludge | 35 °C | 30 days | 1.60% | 11.6% increase in CH4 content | [241] |
| Fe powder | – | Slaughterhouse sludge | 35 °C | 30 days | 1.60% | 11.6% increase in CH4 content | [241] |
| Biosynthesized Fe | 20–40 nm | 3–9 mg/L | 32.9–33.3% rise in biogas yield | ||||
| Fe2O3 | 30–60 nm | Waste activated sludge | 37 °C | 48 days | 15–500 mg/g | 4–28.9% decline in CH4 yield | [242] |
| ZVI Fe oxide | 60 nm 20–40 nm | Anaerobic activated sludgeDairy manure | 37 °C 38 °C | 72 h 30 days | 5–1000 mg/g | 1.4–8.7% enhancement in biogas generation | [239] [243] |
| 100–1000 mg/L | 18.14–56.89% increase in CH4 yield | ||||||
| ZVI | 50 nm 20 nm | Dewatered sludge | 35 °C | 100 days | 0.5–4 g/L | 21.65% increase in methane yield | [244] |
| Fe3O4 | – | Slaughterhouse sludge | 35 °C | 30 days | 1.60% | 11.6% increase in CH4 content | [241] |
| Biosynthesized Fe | 20–40 nm | 0.5–4 g/L | 24.4% increase in methane yield | ||||
| Fe2NiO4 Fe4NiO4Zn | <50 nm <100 nm | Anaerobic sludge | 30 °C | 9 days | 1–100 mg/L | 30% enhancement in CH4 generation | [245] |
| 72 h | 1–100 mg/L | 65.6% decline in CH4 generation | |||||
| NiFe | 0.31 nm | Cow manure | 37 °C | 35 days | 20–130 mg/L | 30.8% increase in biogas production | [226] |
| NiFe | 0.57 nm 28 nm 17 nm | Dewatered sludge Livestock manure | 35 °C37 °C | 100 days 50 days | 20–130 mg/L 0.5–2 mg/L | 32.9% rise in biogas yield 64.12% rise in biogas yield | [244] [222] |
| CoNickel | |||||||
| 0.5–2 mg/L | 74.26% rise in biogas yield | ||||||
| Co | 100 nm | Microalgae Poultry litter | 37 °C 35 °C | 160 h 66 days | 0.4 mg/g | 9% rise in biogas production | [101] [246] |
| Co | 30–50 nm | 0.6 mg/g | 29.7% rise in biogas production | ||||
| Nickel | <100 nm | Green Algae | 37 °C | 264 h | 1.34 mg/g VS | 31.73% increase in biogas production | [247] |
| Titanium di oxide | 7.5 nm (spherical) | 0.84 mg/mL | Zero or low toxicity | ||||
| Gold | 20 nm (spherical) | Livestock manure | 37 °C | 50 days | 0.075 mg/mL | Zero or low toxicity | [222] |
| Silver | 30 nm (spherical) | 0.13 mg/mL | 33% inhibition on methanogenesis | ||||
| Fe2O3 | 20–40 nm | Cattle manure | 38 °C | 30 days | 20 mg/L 100 mg/L | 10.5% increase in CH4 production 19.1% increase in CH4 production | [248] |
| Titanium di oxide | 25 nm | 100 mg/L 500 mg/L | 9.7% increase in CH4 production | ||||
| 21.3% increase in CH4 production | |||||||
| Fe2O3 + TiO2 | – | (20–100) mg/L + 500 mg/L | 13.3% rise in CH4 production15% increase in CH4 production | ||||
| Titanium di oxide | 4–8 nm | Anaerobic sludge | 35 °C | 28 days | 500–2000 mg/L | 14.9% rise in CH4 production | [249] |
| Al2O3 | 40–50 nm | Waste activated sludge | 37 °C | 48 days | 50–500 mg/g | 14.8% enhancement in CH4 generation | [242] |
| Graphene | – | Waste sludge | 35 °C | 12 days | 0.5–2 g/L | 25% enhancement in CH4 generation | [250] |
| Nanosized biochar | 100–600 nm | FW and SS | 55 °C | 81 days | 7.5 g/L | 117% increase in CH4 yield | [251] |
| Fe3O4 NPs | 15 to 21 nm | FW | (37 ± 0.5 °C) | 60 days | 50, 75, 100, 125 mg/L | Improved DIET, reduced lag phase, higher CH4 yields | [252] |
| TiO2 NPs | – | Food and green waste co-digestion | 37 °C | 45 days | 0.5 to 2.5 mg/L | No significant effect | [67] |
| Biochar supported nZVI | – | FW | 35 ± 1 ℃ | 18 days | 1, 2, and 3 g/L | Compared with the control group, the abundance of acetoclastic methanogenesis increased by 1.92% in the BC-nZVI reactors | [253] |
5. Conclusions
This review has identified the primary attributes of FOGO as a reference point for establishing suitable operating conditions for AD. Subsequently, a compilation and discussion of numerous popular and efficacious enhancement strategies have been conducted. It can be concluded that the research related to using FW and GW or YW for AD had been scanty with no such publications considering using FOGO directly derived from domestic bins as is. Several significant findings and related suggestions for further research are as follows:
- (i)
- Investigating the impact of varying feedstock ratios (e.g., food waste to kitchen waste or garden waste) derived from realistic kerbside FOGO bins on biogas production can provide valuable insights.
- (ii)
- Optimizing AD process parameters (e.g., temperature, pH, retention time) is essential. Research should explore how variations in these parameters impact biogas production from FOGO. Reactor studies should be conducted to examine the factors and conditions leading to inhibition and poisoning.
- (iii)
- Significant research is important to overcome reactor failures and enhancement of biogas yield from FOGO.
- (iv)
- To improve and synchronise the reaction rates of the multi-step AD process, two- and three-stage AD bioreactors can be studied. It is anticipated that multi-stage anaerobic bioreactors, which are becoming more and more significant in research, will soon be available for commercialization.
- (v)
- Nanotechnology offers promising techniques for incorporating additives into the feedstock. In anaerobic digesters for FOGO, nanomaterials can serve as effective immobilisation matrices for DIET and aid in determining the predominant metabolic routes of microbial communities. Therefore, future studies ought to concentrate on investigations that aid in determining which compounds are best for augmenting FOGO’s AD process.
- (vi)
- Integrating and harmonising different improvement methods is a difficult but necessary undertaking to maximise the efficiency of anaerobic digesters and ensure the continuous conversion of FOGO into renewable energy. This should also include clarification of the implications and potential for expanding large-scale AD strategies in an industrial context.
Author Contributions
Conceptualization, S.M. and P.K.; methodology, P.K.; software, S.M.; validation, S.M. and P.K.; formal analysis, P.K.; investigation, S.M.; resources, P.K.; data curation, S.M.; writing—original draft preparation, S.M.; writing—review and editing, S.M. and P.K.; visualization, S.M. and P.K.; supervision, P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Shweta’s Ph.D. studies are supported by Griffith University, School of Engineering and Built Environment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brusselaers, J.; Van Der Linden, A. Bio-Waste in Europe—Turning Challenges into Opportunities; European Environment Agency: Luxembourg, 2020. [Google Scholar]
- Department of Sustainability, Environment, Water, Population and Communities. Population and Communities Food And Garden Organics. Best Practice Collection Manual. Available online: https://www.dcceew.gov.au/sites/default/files/documents/collection-manual.pdf (accessed on 1 May 2024).
- Change, I.C. Mitigation of climate change. Contrib. Work. Group III Fifth Assess. Rep. Intergov. Panel Clim. Change 2014, 1454, 147. [Google Scholar]
- Awasthi, M.K.; Sarsaiya, S.; Patel, A.; Juneja, A.; Singh, R.P.; Yan, B.; Awasthi, S.K.; Jain, A.; Liu, T.; Duan, Y.; et al. Refining biomass residues for sustainable energy and bio-products: An assessment of technology, its importance, and strategic applications in circular bio-economy. Renew. Sustain. Energy Rev. 2020, 127, 109876. [Google Scholar] [CrossRef]
- Awasthi, S.K.; Sarsaiya, S.; Awasthi, M.K.; Liu, T.; Zhao, J.; Kumar, S.; Zhang, Z. Changes in global trends in food waste composting: Research challenges and opportunities. Bioresour. Technol. 2020, 299, 122555. [Google Scholar] [CrossRef]
- Wu, L.; Wei, W.; Song, L.; Woźniak-Karczewska, M.; Chrzanowski, Ł.; Ni, B.-J. Upgrading biogas produced in anaerobic digestion: Biological removal and bioconversion of CO2 in biogas. Renew. Sustain. Energy Rev. 2021, 150, 111448. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, H.; He, S.; Zhao, Q.; Wei, L. A review of biochar in anaerobic digestion to improve biogas production: Performances, mechanisms and economic assessments. Bioresour. Technol. 2021, 341, 125797. [Google Scholar] [CrossRef]
- Rehl, T.; Müller, J. Life cycle assessment of biogas digestate processing technologies. Resour. Conserv. Recycl. 2011, 56, 92–104. [Google Scholar] [CrossRef]
- Cuéllar, A.D.; Webber, M.E. Cow power: The energy and emissions benefits of converting manure to biogas. Environ. Res. Lett. 2008, 3, 034002. [Google Scholar] [CrossRef]
- Tambone, F.; Scaglia, B.; D’Imporzano, G.; Schievano, A.; Orzi, V.; Salati, S.; Adani, F. Assessing amendment and fertilizing properties of digestates from anaerobic digestion through a comparative study with digested sludge and compost. Chemosphere 2010, 81, 577–583. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, S.; Wang, Y.; Wang, R. Advantages of the integrated pig-biogas-vegetable greenhouse system in North China. Ecol. Eng. 2005, 24, 175–183. [Google Scholar] [CrossRef]
- Jiang, X.; Sommer, S.G.; Christensen, K.V. A review of the biogas industry in China. Energy Policy 2011, 39, 6073–6081. [Google Scholar] [CrossRef]
- Department of Industry, Science, Energy, and Resources. Table O, Australian Energy Statistics; Australian Bureau of Statistics: Canberra, Australia, 2022. [Google Scholar]
- Awasthi, S.K.; Joshi, R.; Dhar, H.; Verma, S.; Awasthi, M.K.; Varjani, S.; Sarsaiya, S.; Zhang, Z.; Kumar, S. Improving methane yield and quality via co-digestion of cow dung mixed with food waste. Bioresour. Technol. 2018, 251, 259–263. [Google Scholar] [CrossRef]
- Kasinath, A.; Fudala-Ksiazek, S.; Szopinska, M.; Bylinski, H.; Artichowicz, W.; Remiszewska-Skwarek, A.; Luczkiewicz, A. Biomass in biogas production: Pretreatment and codigestion. Renew. Sustain. Energy Rev. 2021, 150, 111509. [Google Scholar] [CrossRef]
- Myszograj, S.; Stadnik, A.; Płuciennik-Koropczuk, E. The influence of trace elements on anaerobic digestion process. Civ. Environ. Eng. Rep. 2018, 28, 105–115. [Google Scholar] [CrossRef]
- Veraart, A.J.; Steenbergh, A.K.; Ho, A.; Kim, S.Y.; Bodelier, P.L. Beyond nitrogen: The importance of phosphorus for CH4 oxidation in soils and sediments. Geoderma 2015, 259–260, 337–346. [Google Scholar] [CrossRef]
- Liu, H.; Kumar, V.; Jia, L.; Sarsaiya, S.; Kumar, D.; Juneja, A.; Zhang, Z.; Sindhu, R.; Binod, P.; Bhatia, S.K.; et al. Biopolymer poly-hydroxyalkanoates (PHA) production from apple industrial waste residues: A review. Chemosphere 2021, 284, 131427. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, M.; Liang, C.; Chui, C.; Wang, N.; Shi, J.; Liu, L. Multivariate insights into enhanced biogas production in thermophilic dry anaerobic co-digestion of food waste with kitchen waste or garden waste: Process properties, microbial communities and metagenomic analyses. Bioresour. Technol. 2022, 361, 127684. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, K.; Devanand, J.; Kumar, P.S.; Soundarajan, K.; Sivasubramanian, V.; Sindhu, J.; Vo, D.-V.N. A review on nano-catalysts and biochar-based catalysts for biofuel production. Fuel 2021, 306, 121632. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Wang, X. Effects of alkalinity sources on the stability of anaerobic digestion from food waste. Waste Manag. Res. 2015, 33, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Serna-Maza, A.; Heaven, S.; Banks, C. Biogas stripping of ammonia from fresh digestate from a food waste digester. Bioresour. Technol. 2015, 190, 66–75. [Google Scholar] [CrossRef]
- De la Rubia, M.Á.; Walker, M.; Heaven, S.; Banks, C.J.; Borja, R. Preliminary trials of in situ ammonia stripping from source segregated domestic food waste digestate using biogas: Effect of temperature and flow rate. Bioresour. Technol. 2010, 101, 9486–9492. [Google Scholar] [CrossRef]
- Serna-Maza, A.; Heaven, S.; Banks, C.J. Ammonia removal in food waste anaerobic digestion using a side-stream stripping process. Bioresour. Technol. 2014, 152, 307–315. [Google Scholar] [CrossRef]
- Walker, M.; Iyer, K.; Heaven, S.; Banks, C. Ammonia removal in anaerobic digestion by biogas stripping: An evaluation of process alternatives using a first order rate model based on experimental findings. Chem. Eng. J. 2011, 178, 138–145. [Google Scholar] [CrossRef]
- Siddique, M.N.I.; Wahid, Z.A. Achievements and perspectives of anaerobic co-digestion: A review. J. Clean. Prod. 2018, 194, 359–371. [Google Scholar] [CrossRef]
- Zamri, M.; Hasmady, S.; Akhiar, A.; Ideris, F.; Shamsuddin, A.; Mofijur, M.; Fattah, I.R.; Mahlia, T. A comprehensive review on anaerobic digestion of organic fraction of municipal solid waste. Renew. Sustain. Energy Rev. 2021, 137, 110637. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, L.; Wang, W.; Wang, Q.; Bai, R.; Zhuang, X.; Guo, Y.; Qi, W.; Yuan, Z. Impact of blending on hydrolysis and ethanol fermentation of garden wastes. J. Clean. Prod. 2018, 190, 36–43. [Google Scholar] [CrossRef]
- Jayanth, T.; Mamindlapelli, N.K.; Begum, S.; Arelli, V.; Juntupally, S.; Ahuja, S.; Dugyala, S.K.; Anupoju, G.R. Anaerobic mono and co-digestion of organic fraction of municipal solid waste and landfill leachate at industrial scale: Impact of volatile organic loading rate on reaction kinetics, biogas yield and microbial diversity. Sci. Total. Environ. 2020, 748, 142462. [Google Scholar]
- Borth, P.L.B.; Perin, J.K.H.; Torrecilhas, A.R.; Pan, N.C.; Kuroda, E.K.; Fernandes, F. Biochemical methane potential of food and garden waste co-digestion with variation in solid content and inoculum: Substrate ratio. J. Mater. Cycles Waste Manag. 2021, 23, 1974–1983. [Google Scholar] [CrossRef]
- Panigrahi, S.; Sharma, H.B.; Dubey, B.K. Optimization of F/M Ratio During Anaerobic Codigestion of Yard Waste with Food Waste: Biogas Production and System Stability. In Treatment and Disposal of Solid and Hazardous Wastes; Sengupta, D., Dubey, B.K., Goel, S., Eds.; Springer International Publishing: Cham, Germany, 2022; pp. 185–192. [Google Scholar]
- Zhang, S.; Liang, C.; Xiao, M.; Chui, C.; Wang, N.; Ji, Y.; Wang, Z.; Shi, J.; Liu, L. Metagenomic characterization of the enhanced performance of multicomponent synergistic thermophilic anaerobic co-digestion of food waste utilizing kitchen waste or garden waste as co-substrate. Water Res. 2023, 244, 120457. [Google Scholar] [CrossRef]
- Nithin Raja, C.; Purushothaman, P. Impact of sequential hybrid pretreatment in anaerobic digestion of food waste and garden waste co-digestion on waste characteristics and biogas production. J. Mater. Cycles Waste Manag. 2023, 25, 2937–2950. [Google Scholar]
- Arif, S.; Liaquat, R.; Adil, M. Applications of materials as additives in anaerobic digestion technology. Renew. Sustain. Energy Rev. 2018, 97, 354–366. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The anaerobic digestion of solid organic waste. Waste Manag. 2011, 31, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.-Y. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: Current advances, full-scale application and future perspectives. Renew. Sustain. Energy Rev. 2017, 69, 559–577. [Google Scholar] [CrossRef]
- Lu, D.; Liu, X.; Apul, O.G.; Zhang, L.; Ryan, D.K.; Zhang, X. Optimization of biomethane production from anaerobic Co-digestion of microalgae and septic tank sludge. Biomass Bioenergy 2019, 127, 105266. [Google Scholar] [CrossRef]
- Khanal, S.K.; Tirta Nindhia, T.G.; Nitayavardhana, S. Chapter 11—Biogas From Wastes: Processes and Applications. In Sustainable Resource Recovery and Zero Waste Approaches; Taherzadeh, M.J., Bolton, K., Wong, J., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–174. [Google Scholar]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.K.; Suja, F.B.; Zain, S.M.; Pramanik, B.K. The anaerobic digestion process of biogas production from food waste: Prospects and constraints. Bioresour. Technol. Rep. 2019, 8, 100310. [Google Scholar] [CrossRef]
- Dahiya, S.; Sarkar, O.; Swamy, Y.; Mohan, S.V. Acidogenic fermentation of food waste for volatile fatty acid production with co-generation of biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar] [CrossRef]
- Drake, H.L. Acetogenesis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Fenchel, T.; King, G.M.; Blackburn, T.H. Chapter 1—Bacterial Metabolism. In Bacterial Biogeochemistry, 3rd ed.; Fenchel, T., King, G.M., Blackburn, T.H., Eds.; Academic Press: Boston, MA, USA, 2012; pp. 1–34. [Google Scholar]
- Wu, C.; Huang, Q.; Yu, M.; Ren, Y.; Wang, Q.; Sakai, K. Effects of digestate recirculation on a two-stage anaerobic digestion system, particularly focusing on metabolite correlation analysis. Bioresour. Technol. 2018, 251, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Thauer, R.K. Methyl (alkyl)-coenzyme M reductases: Nickel F-430-containing enzymes involved in anaerobic methane formation and in anaerobic oxidation of methane or of short chain alkanes. Biochemistry 2019, 58, 5198–5220. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, X.; Xia, W.; Li, Z.; Wang, L.; Chen, Z.; Ge, S. Effect of extreme pH conditions on methanogenesis: Methanogen metabolism and community structure. Sci. Total Environ. 2023, 877, 162702. [Google Scholar] [CrossRef]
- Sun, M.; Liu, B.; Yanagawa, K.; Ha, N.T.; Goel, R.; Terashima, M.; Yasui, H. Effects of low pH conditions on decay of methanogenic biomass. Water Res. 2020, 179, 115883. [Google Scholar] [CrossRef]
- Ni, R.; Xu, C.; Shi, X.; Yang, S.; Li, L.; Peng, X.; Song, L. Acetoclastic methanogenesis pathway stability despite the high microbial taxonomic variability in the transition from acidogenesis to methanogenesis during food waste anaerobic digestion. J. Clean. Prod. 2022, 372, 133758. [Google Scholar] [CrossRef]
- Greene, P. Managing Digester Feedstocks. Available online: https://www.biocycle.net/managing-digester-feedstocks/ (accessed on 1 May 2024).
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef]
- Santos, C.A.; Reis, A. Microalgal symbiosis in biotechnology. Appl. Microbiol. Biotechnol. 2014, 98, 5839–5846. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Cioabla, A.E.; Ionel, I.; Dumitrel, G.-A.; Popescu, F. Comparative study on factors affecting anaerobic digestion of agricultural vegetal residues. Biotechnol. Biofuels 2012, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Wintsche, B.; Glaser, K.; Sträuber, H.; Centler, F.; Liebetrau, J.; Harms, H.; Kleinsteuber, S. Trace Elements Induce Predominance among Methanogenic Activity in Anaerobic Digestion. Front. Microbiol. 2016, 7, 2034. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Li, A. Enhanced anaerobic digestion of food waste by trace metal elements supplementation and reduced metals dosage by green chelating agent [S, S]-EDDS via improving metals bioavailability. Water Res. 2015, 84, 266–277. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Browne, J.D.; Murphy, J.D. The impact of increasing organic loading in two phase digestion of food waste. Renew. Energy 2014, 71, 69–76. [Google Scholar] [CrossRef]
- Pagés-Díaz, J.; Pereda-Reyes, I.; Taherzadeh, M.J.; Sárvári-Horváth, I.; Lundin, M. Anaerobic co-digestion of solid slaughterhouse wastes with agro-residues: Synergistic and antagonistic interactions determined in batch digestion assays. Chem. Eng. J. 2014, 245, 89–98. [Google Scholar] [CrossRef]
- Ohemeng-Ntiamoah, J.; Datta, T. Evaluating analytical methods for the characterization of lipids, proteins and carbohydrates in organic substrates for anaerobic co-digestion. Bioresour. Technol. 2018, 247, 697–704. [Google Scholar] [CrossRef]
- Mao, C.; Wang, X.; Xi, J.; Feng, Y.; Ren, G. Linkage of kinetic parameters with process parameters and operational conditions during anaerobic digestion. Energy 2017, 135, 352–360. [Google Scholar] [CrossRef]
- Li, N.; He, J.; Yan, H.; Chen, S.; Dai, X. Pathways in bacterial and archaeal communities dictated by ammonium stress in a high solid anaerobic digester with dewatered sludge. Bioresour. Technol. 2017, 241, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, D.; Yan, J.; Qiao, W.; Wang, W.; Zhu, T. Effects of lipid concentration on anaerobic co-digestion of municipal biomass wastes. Waste Manag. 2014, 34, 1025–1034. [Google Scholar] [CrossRef]
- Cirne, D.G.; Paloumet, X.; Björnsson, L.; Alves, M.M.; Mattiasson, B. Anaerobic digestion of lipid-rich waste—Effects of lipid concentration. Renew. Energy 2007, 32, 965–975. [Google Scholar] [CrossRef]
- Wagner, A.O.; Lins, P.; Malin, C.; Reitschuler, C.; Illmer, P. Impact of protein-, lipid- and cellulose-containing complex substrates on biogas production and microbial communities in batch experiments. Sci. Total. Environ. 2013, 458–460, 256–266. [Google Scholar]
- Cook, S.M.; Skerlos, S.J.; Raskin, L.; Love, N.G. A stability assessment tool for anaerobic codigestion. Water Res. 2017, 112, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Paritosh, K.; Mathur, S.; Pareek, N.; Vivekanand, V. Feasibility study of waste (d) potential: Co-digestion of organic wastes, synergistic effect and kinetics of biogas production. Int. J. Environ. Sci. Technol. 2018, 15, 1009–1018. [Google Scholar] [CrossRef]
- Méndez-Acosta, H.O.; Campos-Rodríguez, A.; González-Álvarez, V.; García-Sandoval, J.P.; Snell-Castro, R.; Latrille, E. A hybrid cascade control scheme for the VFA and COD regulation in two-stage anaerobic digestion processes. Bioresour. Technol. 2016, 218, 1195–1202. [Google Scholar] [CrossRef]
- Jiang, Y.; Dennehy, C.; Lawlor, P.G.; Hu, Z.; Zhan, X.; Gardiner, G.E. Inactivation of enteric indicator bacteria and system stability during dry co-digestion of food waste and pig manure. Sci. Total. Environ. 2018, 612, 293–302. [Google Scholar] [CrossRef]
- Chinellato, G.; Cavinato, C.; Bolzonella, D.; Heaven, S.; Banks, C.J. Biohydrogen production from food waste in batch and semi-continuous conditions: Evaluation of a two-phase approach with digestate recirculation for pH control. Int. J. Hydrogen Energy 2013, 38, 4351–4360. [Google Scholar] [CrossRef]
- Ebner, J.H.; Labatut, R.A.; Lodge, J.S.; Williamson, A.A.; Trabold, T.A. Anaerobic co-digestion of commercial food waste and dairy manure: Characterizing biochemical parameters and synergistic effects. Waste Manag. 2016, 52, 286–294. [Google Scholar] [CrossRef]
- Hübner, M.; Oechsner, H.; Koch, S.; Seggl, A.; Hrenn, H.; Schmiedchen, B.; Wilde, P.; Miedaner, T. Impact of genotype, harvest time and chemical composition on the methane yield of winter rye for biogas production. Biomass Bioenergy 2011, 35, 4316–4323. [Google Scholar] [CrossRef]
- Bouzas, A.; Gabaldón, C.; Marzal, P.; Penya-roja, J.M.; Seco, A. Fermentation of Municipal Primary Sludge: Effect of Srt and Solids Concentration on Volatile Fatty Acid Production. Environ. Technol. 2002, 23, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Cremonez, P.A.; Sampaio, S.C.; Teleken, J.G.; Meier, T.W.; Dieter, J.; Teleken, J. Influence of inoculum to substrate ratio on the anaerobic digestion of a cassava starch polymer. Ind. Crops Prod. 2019, 141, 111709. [Google Scholar] [CrossRef]
- Zhang, W.; Li, L.; Xing, W.; Chen, B.; Zhang, L.; Li, A.; Li, R.; Yang, T. Dynamic behaviors of batch anaerobic systems of food waste for methane production under different organic loads, substrate to inoculum ratios and initial pH. J. Biosci. Bioeng. 2019, 128, 733–743. [Google Scholar] [CrossRef]
- Cordoba, V.; Fernandez, M.; Santalla, E. The effect of substrate/inoculum ratio on the kinetics of methane production in swine wastewater anaerobic digestion. Environ. Sci. Pollut. Res. 2018, 25, 21308–21317. [Google Scholar] [CrossRef] [PubMed]
- Latifi, P.; Karrabi, M.; Danesh, S. Anaerobic co-digestion of poultry slaughterhouse wastes with sewage sludge in batch-mode bioreactors (effect of inoculum-substrate ratio and total solids). Renew. Sustain. Energy Rev. 2019, 107, 288–296. [Google Scholar] [CrossRef]
- Pastor-Poquet, V.; Papirio, S.; Trably, E.; Rintala, J.; Escudié, R.; Esposito, G. High-solids anaerobic digestion requires a trade-off between total solids, inoculum-to-substrate ratio and ammonia inhibition. Int. J. Environ. Sci. Technol. 2019, 16, 7011–7024. [Google Scholar] [CrossRef]
- Divya, D.; Gopinath, L.; Christy, P.M. A review on current aspects and diverse prospects for enhancing biogas production in sustainable means. Renew. Sustain. Energy Rev. 2015, 42, 690–699. [Google Scholar] [CrossRef]
- Naik, L.; Gebreegziabher, Z.; Tumwesige, V.; Balana, B.B.; Mwirigi, J.; Austin, G. Factors determining the stability and productivity of small scale anaerobic digesters. Biomass Bioenergy 2014, 70, 51–57. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Visvanathan, C. Effect of C/N ratio and ammonia-N accumulation in a pilot-scale thermophilic dry anaerobic digester. Bioresour. Technol. 2012, 113, 294–302. [Google Scholar]
- Wang, J. Decentralized biogas technology of anaerobic digestion and farm ecosystem: Opportunities and challenges. Front. Energy Res. 2014, 2, 10. [Google Scholar] [CrossRef]
- Kwietniewska, E.; Tys, J. Process characteristics, inhibition factors and methane yields of anaerobic digestion process, with particular focus on microalgal biomass fermentation. Renew. Sustain. Energy Rev. 2014, 34, 491–500. [Google Scholar] [CrossRef]
- Nyoman, W.I.; Seno, J. The kinetic of biogas production rate from cattle manure in batch mode. Int. J. Chem. Biol. Eng. 2010, 3, 39–45. [Google Scholar]
- Zhang, T.; Liu, L.; Song, Z.; Ren, G.; Feng, Y.; Han, X.; Yang, G. Biogas production by co-digestion of goat manure with three crop residues. PLoS ONE 2013, 8, e66845. [Google Scholar] [CrossRef]
- Mnkeni, P.; Austin, L. Fertiliser value of human manure from pilot urine-diversion toilets. Water SA 2009, 35, 133–138. [Google Scholar] [CrossRef][Green Version]
- Muzenda, E. Bio-methane generation from organic waste. In Proceedings of the World Congress on Engineering and Computer Science, San Francisco, CA, USA, 22–24 October 2014. [Google Scholar]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Kallistova, A.Y.; Goel, G.; Nozhevnikova, A. Microbial diversity of methanogenic communities in the systems for anaerobic treatment of organic waste. Microbiology 2014, 83, 462–483. [Google Scholar] [CrossRef]
- Arslan, C.; Sattar, A.; Changying, J.; Nasir, A.; Ali Mari, I.; Zia Bakht, M. Impact of pH management interval on biohydrogen production from organic fraction of municipal solid wastes by mesophilic thermophilic anaerobic codigestion. BioMed Res. Int. 2015, 2015, 590753. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]
- Dai, B.; Xu, J.; He, Y.; Xiong, P.; Wang, X.; Deng, Y.; Wang, Y.; Yin, Z. Acid inhibition during anaerobic digestion of biodegradable kitchen waste. J. Renew. Sustain. Energy 2015, 7, 023118. [Google Scholar] [CrossRef]
- Zhai, N.; Zhang, T.; Yin, D.; Yang, G.; Wang, X.; Ren, G.; Feng, Y. Effect of initial pH on anaerobic co-digestion of kitchen waste and cow manure. Waste Manag. 2015, 38, 126–131. [Google Scholar] [CrossRef]
- Normak, A.; Suurpere, J.; Suitso, I.; Jogi, E.; Kokin, E.; Pitk, P. Improving ADM1 model to simulate anaerobic digestion start-up with inhibition phase based on cattle slurry. Biomass Bioenergy 2015, 80, 260–266. [Google Scholar] [CrossRef]
- Souza, T.S.; Carvajal, A.; Donoso-Bravo, A.; Peña, M.; Fdz-Polanco, F. ADM1 calibration using BMP tests for modeling the effect of autohydrolysis pretreatment on the performance of continuous sludge digesters. Water Res. 2013, 47, 3244–3254. [Google Scholar] [CrossRef] [PubMed]
- Monou, M.; Pafitis, N.; Kythreotou, N.; Smith, S.R.; Mantzavinos, D.; Kassinos, D. Anaerobic co-digestion of potato processing wastewater with pig slurry and abattoir wastewater. J. Chem. Technol. Biotechnol. 2008, 83, 1658–1663. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Schnurer, A.; Jarvis, A. Microbiological handbook for biogas plants. Swed. Waste Manag. U 2010, 2009, 1–74. [Google Scholar]
- Qiang, H.; Niu, Q.; Chi, Y.; Li, Y. Trace metals requirements for continuous thermophilic methane fermentation of high-solid food waste. Chem. Eng. J. 2013, 222, 330–336. [Google Scholar] [CrossRef]
- Kelly, C.R.; Switzenbaum, M.S. Anaerobic treatment: Temperature and nutrient effects. Agric. Wastes 1984, 10, 135–154. [Google Scholar] [CrossRef]
- Zaidi, A.A.; RuiZhe, F.; Shi, Y.; Khan, S.Z.; Mushtaq, K. Nanoparticles augmentation on biogas yield from microalgal biomass anaerobic digestion. Int. J. Hydrogen Energy 2018, 43, 14202–14213. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenergy 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Müller, B.; Sun, L.; Schnürer, A. First insights into the syntrophic acetate-oxidizing bacteria—A genetic study. MicrobiologyOpen 2013, 2, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Birošová, L.; Mackuľak, T.; Bodík, I.; Ryba, J.; Škubák, J.; Grabic, R. Pilot study of seasonal occurrence and distribution of antibiotics and drug resistant bacteria in wastewater treatment plants in Slovakia. Sci. Total. Environ. 2014, 490, 440–444. [Google Scholar] [CrossRef]
- Dhar, H.; Kumar, P.; Kumar, S.; Mukherjee, S.; Vaidya, A.N. Effect of organic loading rate during anaerobic digestion of municipal solid waste. Bioresour. Technol. 2016, 217, 56–61. [Google Scholar] [CrossRef]
- Singh, P.K.; Mohanty, P.; Mishra, S.; Adhya, T.K. Food waste valorisation for biogas-based bioenergy production in circular bioeconomy: Opportunities, challenges, and future developments. Front. Energy Res. 2022, 10, 903775. [Google Scholar] [CrossRef]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A review of the role of critical parameters in the design and operation of biogas production plants. Appl. Sci. 2019, 9, 1915. [Google Scholar] [CrossRef]
- Bajpai, P.; Bajpai, P. Process parameters affecting anaerobic digestion. In Anaerobic Technology in Pulp and Paper Industry; Springer: Berlin/Heidelberg, Germany, 2017; pp. 13–27. [Google Scholar]
- Rocha-Meneses, L.; Zannerni, R.; Inayat, A.; Abdallah, M.; Shanableh, A.; Ghenai, C.; Kamil, M.; Kikas, T. Current progress in anaerobic digestion reactors and parameters optimization. Biomass Convers. Biorefinery 2022, 1–24. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Shi, Y.; Zheng, L.; Gao, X.; Qiao, W.; Zhou, Y. Pilot-scale anaerobic co-digestion of municipal biomass waste and waste activated sludge in China: Effect of organic loading rate. Waste Manag. 2012, 32, 2056–2060. [Google Scholar] [CrossRef]
- Morken, J.; Gjetmundsen, M.; Fjørtoft, K. Determination of kinetic constants from the co-digestion of dairy cow slurry and municipal food waste at increasing organic loading rates. Renew. Energy 2018, 117, 46–51. [Google Scholar] [CrossRef]
- Gou, C.; Yang, Z.; Huang, J.; Wang, H.; Xu, H.; Wang, L. Effects of temperature and organic loading rate on the performance and microbial community of anaerobic co-digestion of waste activated sludge and food waste. Chemosphere 2014, 105, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kobayashi, T.; Qi, W.; Oshibe, H.; Xu, K.-Q. Effect of temperature and organic loading rate on siphon-driven self-agitated anaerobic digestion performance for food waste treatment. Waste Manag. 2018, 74, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, W.; Anwar, N.; Ma, Z.; Liu, G.; Zhang, R. Effect of Organic Loading Rate on Anaerobic Digestion of Food Waste under Mesophilic and Thermophilic Conditions. Energy Fuels 2017, 31, 2976–2984. [Google Scholar] [CrossRef]
- Guo, X.; Wang, C.; Sun, F.; Zhu, W.; Wu, W. A comparison of microbial characteristics between the thermophilic and mesophilic anaerobic digesters exposed to elevated food waste loadings. Bioresour. Technol. 2014, 152, 420–428. [Google Scholar] [CrossRef]
- Conn, E.; Stumpf, P. Outlines of Biochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Cabirol, N.; Barragán, E.; Durán, A.; Noyola, A. Effect of aluminium and sulphate on anaerobic digestion of sludge from wastewater enhanced primary treatment. Water Sci. Technol. 2003, 48, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Singh, S.P.; Prerna, P. Review of recent advances in anaerobic packed-bed biogas reactors. Renew. Sustain. Energy Rev. 2009, 13, 1569–1575. [Google Scholar] [CrossRef]
- Thanh, N.T.; Watari, T.; Thao, T.P.; Hatamoto, M.; Tanikawa, D.; Syutsubo, K.; Fukuda, M.; Tan, N.M.; Anh, T.K.; Yamaguchi, T.; et al. Impact of aluminum chloride on process performance and microbial community structure of granular sludge in an upflow anaerobic sludge blanket reactor for natural rubber processing wastewater treatment. Water Sci. Technol. 2016, 74, 500–507. [Google Scholar] [CrossRef]
- Jiménez, A.M.; Borja, R.; Martín, A. A comparative kinetic evaluation of the anaerobic digestion of untreated molasses and molasses previously fermented with Penicillium decumbens in batch reactors. Biochem. Eng. J. 2004, 18, 121–132. [Google Scholar] [CrossRef]
- Rodríguez-Abalde, Á.; Flotats, X.; Fernández, B. Optimization of the anaerobic co-digestion of pasteurized slaughterhouse waste, pig slurry and glycerine. Waste Manag. 2017, 61, 521–528. [Google Scholar] [CrossRef]
- Raynal, J.; Delgenès, J.P.; Moletta, R. Two-phase anaerobic digestion of solid wastes by a multiple liquefaction reactors process. Bioresour. Technol. 1998, 65, 97–103. [Google Scholar] [CrossRef]
- Bouallagui, H.; Touhami, Y.; Ben Cheikh, R.; Hamdi, M. Bioreactor performance in anaerobic digestion of fruit and vegetable wastes. Process. Biochem. 2005, 40, 989–995. [Google Scholar] [CrossRef]
- Peng, S.; Colosi, L.M. Anaerobic digestion of algae biomass to produce energy during wastewater treatment. Water Environ. Res. 2016, 88, 29–39. [Google Scholar] [CrossRef]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Zollitsch, W.; Mayer, K.; Gruber, L. Biogas production from maize and dairy cattle manure—Influence of biomass composition on the methane yield. Agric. Ecosyst. Environ. 2007, 118, 173–182. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Belostotskiy, D.E.; Ilinskaya, O.N.; Boulygina, E.A.; Grigoryeva, T.V.; Ziganshin, A.M. Effect of the Organic Loading Rate Increase and the Presence of Zeolite on Microbial Community Composition and Process Stability During Anaerobic Digestion of Chicken Wastes. Microb. Ecol. 2015, 70, 948–960. [Google Scholar] [CrossRef]
- Durán-Barrantes, M.M.; Álvarez-Mateos, P.; Jiménez-Rodriguez, A.; Romero-Guzmán, F.; Fiestas-Ros De Ursinos, J.A. Anaerobic treatment of swine wastewater in semicontinuous clayey support reactors. Chem. Biochem. Eng. Q. 2009, 23, 385–391. [Google Scholar]
- Jiang, Y.; Heaven, S.; Banks, C. Strategies for stable anaerobic digestion of vegetable waste. Renew. Energy 2012, 44, 206–214. [Google Scholar] [CrossRef]
- Woon, K.S.; Lo, I.M. A proposed framework of food waste collection and recycling for renewable biogas fuel production in Hong Kong. Waste Manag. 2016, 47, 3–10. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, Y.-W.; Jahng, D. Anaerobic co-digestion of food waste and piggery wastewater: Focusing on the role of trace elements. Bioresour. Technol. 2011, 102, 5048–5059. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Tan, T. Batch and semi-continuous anaerobic digestion of food waste in a dual solid–liquid system. Bioresour. Technol. 2013, 145, 10–16. [Google Scholar] [CrossRef]
- Zhang, R.; El-Mashad, H.M.; Hartman, K.; Wang, F.; Liu, G.; Choate, C.; Gamble, P. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ge, Y.; Wang, K.; Li, X.; Pang, Y. Characteristics and anaerobic digestion performances of kitchen wastes. Kezaisheng Nengyuan/Renew. Energy Resour. 2010, 28, 76–80. [Google Scholar]
- Oleszek, M.; Król, A.; Tys, J.; Matyka, M.; Kulik, M. Comparison of biogas production from wild and cultivated varieties of reed canary grass. Bioresour. Technol. 2014, 156, 303–306. [Google Scholar] [CrossRef]
- Zou, H.; Chen, Y.; Shi, J.; Zhao, T.; Yu, Q.; Yu, S.; Shi, D.; Chai, H.; Gu, L.; He, Q.; et al. Mesophilic anaerobic co-digestion of residual sludge with different lignocellulosic wastes in the batch digester. Bioresour. Technol. 2018, 268, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Rowan, M.; Umenweke, G.C.; Epelle, E.I.; Afolabi, I.C.; Okoye, P.U.; Gunes, B.; Okolie, J.A. Anaerobic co-digestion of food waste and agricultural residues: An overview of feedstock properties and the impact of biochar addition. Digit. Chem. Eng. 2022, 4, 100046. [Google Scholar] [CrossRef]
- Wu, L.-J.; Kobayashi, T.; Kuramochi, H.; Li, Y.-Y.; Xu, K.-Q. Improved biogas production from food waste by co-digestion with de-oiled grease trap waste. Bioresour. Technol. 2016, 201, 237–244. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, G.; Peng, L.; Su, H.; Tan, T. The anaerobic co-digestion of food waste and cattle manure. Bioresour. Technol. 2013, 129, 170–176. [Google Scholar] [CrossRef]
- Melbourne and Taupo. 2019 Parkville Waste Audit. Available online: https://sustainablecampus.unimelb.edu.au/reduce-reuse-recycle/waste-audits (accessed on 1 May 2024).
- Dennehy, C.; Lawlor, P.G.; Croize, T.; Jiang, Y.; Morrison, L.; Gardiner, G.E.; Zhan, X. Synergism and effect of high initial volatile fatty acid concentrations during food waste and pig manure anaerobic co-digestion. Waste Manag. 2016, 56, 173–180. [Google Scholar] [CrossRef]
- Yong, Z.; Dong, Y.; Zhang, X.; Tan, T. Anaerobic co-digestion of food waste and straw for biogas production. Renew. Energy 2015, 78, 527–530. [Google Scholar] [CrossRef]
- Jabeen, M.; Yousaf, S.; Haider, M.R.; Malik, R.N. High-solids anaerobic co-digestion of food waste and rice husk at different organic loading rates. Int. Biodeterior. Biodegrad. 2015, 102, 149–153. [Google Scholar] [CrossRef]
- Brown, D.; Li, Y. Solid state anaerobic co-digestion of yard waste and food waste for biogas production. Bioresour. Technol. 2013, 127, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.; Sharma, H.B.; Dubey, B.K. Anaerobic co-digestion of food waste with pretreated yard waste: A comparative study of methane production, kinetic modeling and energy balance. J. Clean. Prod. 2020, 243, 118480. [Google Scholar] [CrossRef]
- Mu, L.; Zhang, L.; Zhu, K.; Ma, J.; Ifran, M.; Li, A. Anaerobic co-digestion of sewage sludge, food waste and yard waste: Synergistic enhancement on process stability and biogas production. Sci. Total Environ. 2020, 704, 135429. [Google Scholar] [CrossRef]
- Dixon, P.J.; Ergas, S.J.; Mihelcic, J.R.; Hobbs, S.R. Effect of substrate to inoculum ratio on bioenergy recovery from food waste, yard waste, and biosolids by high solids anaerobic digestion. Environ. Eng. Sci. 2019, 36, 1459–1465. [Google Scholar] [CrossRef]
- Borth, P.L.B.; Perin, J.K.H.; Torrecilhas, A.R.; Lopes, D.D.; Santos, S.C.; Kuroda, E.K.; Fernandes, F. Pilot-scale anaerobic co-digestion of food and garden waste: Methane potential, performance and microbial analysis. Biomass Bioenergy 2022, 157, 106331. [Google Scholar] [CrossRef]
- Lee, E.; Bittencourt, P.; Casimir, L.; Jimenez, E.; Wang, M.; Zhang, Q.; Ergas, S.J. Biogas production from high solids anaerobic co-digestion of food waste, yard waste and waste activated sludge. Waste Manag. 2019, 95, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Wainaina, S.; Lukitawesa Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Wang, K.; Yun, S.; Xing, T.; Li, B.; Abbas, Y.; Liu, X. Binary and ternary trace elements to enhance anaerobic digestion of cattle manure: Focusing on kinetic models for biogas production and digestate utilization. Bioresour. Technol. 2021, 323, 124571. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, X.; Chen, X.; Ren, L. Effects of bentonite on antibiotic resistance genes in biogas slurry and residue from thermophilic and mesophilic anaerobic digestion of food waste. Bioresour. Technol. 2021, 336, 125322. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, Y.; Lu, C.; Kongjan, P.; Wang, J.; Awasthi, M.K.; Tahir, N.; Zhang, Q. A syntrophic co-fermentation model for bio-hydrogen production. J. Clean. Prod. 2021, 317, 128288. [Google Scholar] [CrossRef]
- Yadav, M.; Joshi, C.; Paritosh, K.; Thakur, J.; Pareek, N.; Masakapalli, S.K.; Vivekanand, V. Organic waste conversion through anaerobic digestion: A critical insight into the metabolic pathways and microbial interactions. Metab. Eng. 2021, 69, 323–337. [Google Scholar] [CrossRef]
- Aziz, M.M.A.; Kassim, K.A.; ElSergany, M.; Anuar, S.; Jorat, M.E.; Yaacob, H.; Ahsan, A.; Imteaz, M.A. Recent advances on palm oil mill effluent (POME) pretreatment and anaerobic reactor for sustainable biogas production. Renew. Sustain. Energy Rev. 2020, 119, 109603. [Google Scholar] [CrossRef]
- Qin, S.; Wainaina, S.; Liu, H.; Soufiani, A.M.; Pandey, A.; Zhang, Z.; Awasthi, M.K.; Taherzadeh, M.J. Microbial dynamics during anaerobic digestion of sewage sludge combined with food waste at high organic loading rates in immersed membrane bioreactors. Fuel 2021, 303, 121276. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Kumar, V.; Yadav, V.; Sarsaiya, S.; Awasthi, S.K.; Sindhu, R.; Binod, P.; Kumar, V.; Pandey, A.; Zhang, Z. Current state of the art biotechnological strategies for conversion of watermelon wastes residues to biopolymers production: A review. Chemosphere 2021, 290, 133310. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, C.; He, Z.; Qin, Y.; Li, Y.-Y. Enhanced biomethanation of lipids by high-solid co-digestion with food waste: Biogas production and lipids degradation demonstrated by long-term continuous operation. Bioresour. Technol. 2022, 348, 126750. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Lukitawesa, L.; Duan, Y.; Taherzadeh, M.J.; Zhang, Z. Bacterial dynamics during the anaerobic digestion of toxic citrus fruit waste and semi-continues volatile fatty acids production in membrane bioreactors. Fuel 2022, 319, 123812. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Lee, C. A review of the effects of iron compounds on methanogenesis in anaerobic environments. Renew. Sustain. Energy Rev. 2019, 113, 109282. [Google Scholar] [CrossRef]
- De Vrieze, J.; Smet, D.; Klok, J.; Colsen, J.; Angenent, L.T.; Vlaeminck, S.E. Thermophilic sludge digestion improves energy balance and nutrient recovery potential in full-scale municipal wastewater treatment plants. Bioresour. Technol. 2016, 218, 1237–1245. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sarsaiya, S.; Wainaina, S.; Rajendran, K.; Kumar, S.; Quan, W.; Duan, Y.; Awasthi, S.K.; Chen, H.; Pandey, A.; et al. A critical review of organic manure biorefinery models toward sustainable circular bioeconomy: Technological challenges, advancements, innovations, and future perspectives. Renew. Sustain. Energy Rev. 2019, 111, 115–131. [Google Scholar] [CrossRef]
- Ndayisenga, F.; Yu, Z.; Zheng, J.; Wang, B.; Liang, H.; Phulpoto, I.A.; Habiyakare, T.; Zhou, D. Microbial electrohydrogenesis cell and dark fermentation integrated system enhances biohydrogen production from lignocellulosic agricultural wastes: Substrate pretreatment towards optimization. Renew. Sustain. Energy Rev. 2021, 145, 111078. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sindhu, R.; Sirohi, R.; Kumar, V.; Ahluwalia, V.; Binod, P.; Juneja, A.; Kumar, D.; Yan, B.; Sarsaiya, S.; et al. Agricultural waste biorefinery development towards circular bioeconomy. Renew. Sustain. Energy Rev. 2022, 158, 112122. [Google Scholar] [CrossRef]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.; Guwy, A.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Madhavan, A.; Sindhu, R.; Pugazhendhi, A.; Binod, P.; Sirohi, R.; Awasthi, M.K.; Tarafdar, A.; Pandey, A. Advanced biomaterials for sustainable applications in the food industry: Updates and challenges. Environ. Pollut. 2021, 283, 117071. [Google Scholar]
- Awasthi, M.K.; Wainaina, S.; Mahboubi, A.; Zhang, Z.; Taherzadeh, M.J. Methanogen and nitrifying genes dynamics in immersed membrane bioreactors during anaerobic co-digestion of different organic loading rates food waste. Bioresour. Technol. 2021, 342, 125920. [Google Scholar]
- Qin, S.; Giri, B.S.; Patel, A.K.; Sar, T.; Liu, H.; Chen, H.; Juneja, A.; Kumar, D.; Zhang, Z.; Awasthi, M.K.; et al. Resource recovery and biorefinery potential of apple orchard waste in the circular bioeconomy. Bioresour. Technol. 2021, 321, 124496. [Google Scholar] [CrossRef]
- Maria, G.; Şcoban, A.G. Optimal Operating Policy of a Fluidized Bed Bioreactor used for Mercury Uptake from Wastewaters by Using Immobilized, P. Putida Cells. Curr. Trends Biomed. Eng. Biosci. 2017, 2, 66–72. [Google Scholar] [CrossRef]
- Pererva, Y.; Miller, C.D.; Sims, R.C. Approaches in Design of Laboratory-Scale UASB Reactors. Processes 2020, 8, 734. [Google Scholar] [CrossRef]
- Service and R&D of Innovative Water Technology. Anaerobic Fluidized Bed (AFB) Process. Available online: https://www.itriwater.org.tw/Eng/technology/More?id=106 (accessed on 2 April 2024).
- Li, Y.; Ren, Y.; Ji, J.; Li, Y.-Y.; Kobayashi, T. Anaerobic Membrane Bioreactors for Municipal Wastewater Treatment, Sewage Sludge Digestion and Biogas Upgrading: A Review. Sustainability 2023, 15, 15129. [Google Scholar] [CrossRef]
- Borowski, S.; Boniecki, P.; Kubacki, P.; Czyżowska, A. Food waste co-digestion with slaughterhouse waste and sewage sludge: Digestate conditioning and supernatant quality. Waste Manag. 2018, 74, 158–167. [Google Scholar] [CrossRef]
- Guven, H.; Ersahin, M.E.; Dereli, R.K.; Ozgun, H.; Isik, I.; Ozturk, I. Energy recovery potential of anaerobic digestion of excess sludge from high-rate activated sludge systems co-treating municipal wastewater and food waste. Energy 2019, 172, 1027–1036. [Google Scholar] [CrossRef]
- Orellana, E.; Guerrero, L.D.; Davies-Sala, C.; Altina, M.; Pontiggia, R.M.; Erijman, L. Extracellular hydrolytic potential drives microbiome shifts during anaerobic co-digestion of sewage sludge and food waste. Bioresour. Technol. 2022, 343, 126102. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.-S.; Han, Y.; Cao, S.; Wang, X.C. Effects of long-term acclimatization on the optimum substrate mixture ratio and substrate to inoculum ratio in anaerobic codigestion of food waste and cow manure. Bioresour. Technol. 2020, 317, 123994. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, Y.; Zhang, M.; Wu, S.; Zhou, L. A collaborative strategy for enhanced anaerobic co-digestion of food waste and waste activated sludge by using zero valent iron and ferrous sulfide. Bioresour. Technol. 2022, 347, 126420. [Google Scholar] [CrossRef]
- Chuenchart, W.; Logan, M.; Leelayouthayotin, C.; Visvanathan, C. Enhancement of food waste thermophilic anaerobic digestion through synergistic effect with chicken manure. Biomass Bioenergy 2020, 136, 105541. [Google Scholar] [CrossRef]
- Helenas Perin, J.K.; Biesdorf Borth, P.L.; Torrecilhas, A.R.; Santana da Cunha, L.; Kuroda, E.K.; Fernandes, F. Optimization of methane production parameters during anaerobic co-digestion of food waste and garden waste. J. Clean. Prod. 2020, 272, 123130. [Google Scholar] [CrossRef]
- Li, W.; Loh, K.-C.; Zhang, J.; Tong, Y.W.; Dai, Y. Two-stage anaerobic digestion of food waste and horticultural waste in high-solid system. Appl. Energy 2018, 209, 400–408. [Google Scholar] [CrossRef]
- Qin, Y.; Li, L.; Wu, J.; Xiao, B.; Hojo, T.; Kubota, K.; Cheng, J.; Li, Y.-Y. Co-production of biohydrogen and biomethane from food waste and paper waste via recirculated two-phase anaerobic digestion process: Bioenergy yields and metabolic distribution. Bioresour. Technol. 2019, 276, 325–334. [Google Scholar] [CrossRef]
- Xu, F.; Okopi, S.I.; Jiang, Y.; Chen, Z.; Meng, L.; Li, Y.; Sun, W.; Li, C. Multi-criteria assessment of food waste and waste paper anaerobic co-digestion: Effects of inoculation ratio, total solids content, and feedstock composition. Renew. Energy 2022, 194, 40–50. [Google Scholar] [CrossRef]
- Casallas-Ojeda, M.; Soto-Paz, J.; Alfonso-Morales, W.; Oviedo-Ocaña, E.R.; Komilis, D. Optimization of Operational Parameters during Anaerobic Co-digestion of Food and Garden Waste. Environ. Process. 2021, 8, 769–791. [Google Scholar] [CrossRef]
- Panigrahi, S.; Dubey, B.K. A critical review on operating parameters and strategies to improve the biogas yield from anaerobic digestion of organic fraction of municipal solid waste. Renew. Energy 2019, 143, 779–797. [Google Scholar] [CrossRef]
- Taheri, M.E.; Salimi, E.; Saragas, K.; Novakovic, J.; Barampouti, E.M.; Mai, S.; Malamis, D.; Moustakas, K.; Loizidou, M. Effect of pretreatment techniques on enzymatic hydrolysis of food waste. Biomass Convers. Biorefinery 2021, 11, 219–226. [Google Scholar] [CrossRef]
- Canul Bacab, F.; España Gamboa, E.; Ruiz Espinoza, J.E.; Leal-Bautista, R.M.; Tapia Tussell, R.; Domínguez Maldonado, J.; Canto Canché, B.; Alzate-Gaviria, L. Two phase anaerobic digestion system of municipal solid waste by utilizing microaeration and granular activated carbon. Energies 2020, 13, 933. [Google Scholar] [CrossRef]
- Mahmood, Z.; Cheng, H.; Tian, M. A critical review on advanced anaerobic membrane bioreactors (AnMBRs) for wastewater treatment: Advanced membrane materials and energy demand. Environ. Sci. Water Res. Technol. 2022, 8, 2126–2144. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; He, Y.; Wang, Y.; Wang, S.; Zheng, Z.; Wang, S.; Xu, J.; Cai, Y.; Ying, H. A Comprehensive Review of the Strategies to Improve Anaerobic Digestion: Their Mechanism and Digestion Performance. Methane 2024, 3, 227–256. [Google Scholar] [CrossRef]
- Garcia-Peña, E.I.; Parameswaran, P.; Kang, D.; Canul-Chan, M.; Krajmalnik-Brown, R. Anaerobic digestion and co-digestion processes of vegetable and fruit residues: Process and microbial ecology. Bioresour. Technol. 2011, 102, 9447–9455. [Google Scholar] [CrossRef] [PubMed]
- Bardi, M.J.; Vinardell, S.; Astals, S.; Koch, K. Opportunities and challenges of micronutrients supplementation and its bioavailability in anaerobic digestion: A critical review. Renew. Sustain. Energy Rev. 2023, 186, 113689. [Google Scholar] [CrossRef]
- Rahimieh, A.; Nosrati, M. A review on biochemistry, microbiology and thermodynamic aspects of propionate: The key intermediate in the anaerobic digestion and wastewater treatment. Desalination Water Treat. 2024, 317, 100191. [Google Scholar] [CrossRef]
- Nozhevnikova, A.; Russkova, Y.I.; Litti, Y.V.; Parshina, S.; Zhuravleva, E.; Nikitina, A. Syntrophy and interspecies electron transfer in methanogenic microbial communities. Microbiology 2020, 89, 129–147. [Google Scholar] [CrossRef]
- Ajay, C.M.; Mohan, S.; Dinesha, P.; Rosen, M.A. Review of impact of nanoparticle additives on anaerobic digestion and methane generation. Fuel 2020, 277, 118234. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Tabatabaei, M.; Aghbashlo, M.; Panahi, H.K.S.; Nizami, A.-S. A state-of-the-art review on the application of nanomaterials for enhancing biogas production. J. Environ. Manag. 2019, 251, 109597. [Google Scholar] [CrossRef]
- Eduok, S.; Ferguson, R.; Jefferson, B.; Villa, R.; Coulon, F. Aged-engineered nanoparticles effect on sludge anaerobic digestion performance and associated microbial communities. Sci. Total. Environ. 2017, 609, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tian, Z.; Lv, Z.; Chen, Z.; Liu, Y.; Yong, X.; Zhou, J.; Xie, X.; Jia, H.; Wei, P. Effects of copper salts on performance, antibiotic resistance genes, and microbial community during thermophilic anaerobic digestion of swine manure. Bioresour. Technol. 2020, 300, 122728. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Niu, J.; Zhang, W.; Liu, J.; Yuan, J.; Li, H.; Yue, X. Mini art review for zero valent iron application in anaerobic digestion and technical bottlenecks. Sci. Total. Environ. 2021, 791, 148415. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Awais, M.; Javed, H.M.A.; Mustafa, M.S.; Tlili, I.; Khan, S.U.; Safdari Shadloo, M. Photo-catalytic pretreatment of biomass for anaerobic digestion using visible light and Nickle oxide (NiOx) nanoparticles prepared by sol gel method. Renew. Energy 2020, 154, 128–135. [Google Scholar] [CrossRef]
- Pang, L.; Xu, K.; Qi, L.; Chatzisymeon, E.; Liu, X.; Yang, P. Response behavior of antibiotic resistance genes to zinc oxide nanoparticles in cattle manure thermophilic anaerobic digestion process: A metagenomic analysis. Bioresour. Technol. 2022, 347, 126709. [Google Scholar] [CrossRef]
- Thanh, P.M.; Ketheesan, B.; Yan, Z.; Stuckey, D. Trace metal speciation and bioavailability in anaerobic digestion: A review. Biotechnol. Adv. 2016, 34, 122–136. [Google Scholar] [CrossRef]
- Zhang, M.; Zang, L. A Review of Interspecies Electron Transfer in Anaerobic Digestion; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; p. 042026. [Google Scholar]
- Park, J.-H.; Kang, H.-J.; Park, K.-H.; Park, H.-D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and potential of direct interspecies electron transfer in anaerobic digestion. Energies 2018, 11, 107. [Google Scholar] [CrossRef]
- Cheng, Q.; Call, D.F. Hardwiring microbes via direct interspecies electron transfer: Mechanisms and applications. Environ. Sci. Process. Impacts 2016, 18, 968–980. [Google Scholar] [CrossRef]
- Hussein, A.K. Applications of nanotechnology in renewable energies—A comprehensive overview and understanding. Renew. Sustain. Energy Rev. 2015, 42, 460–476. [Google Scholar] [CrossRef]
- Lu, X.; Wang, H.; Ma, F.; Zhao, G.; Wang, S. Enhanced Anaerobic Digestion of Cow Manure and Rice Straw by the Supplementation of an Iron Oxide–Zeolite System. Energy Fuels 2017, 31, 599–606. [Google Scholar] [CrossRef]
- Huangfu, X.; Xu, Y.; Liu, C.; He, Q.; Ma, J.; Ma, C.; Huang, R. A review on the interactions between engineered nanoparticles with extracellular and intracellular polymeric substances from wastewater treatment aggregates. Chemosphere 2019, 219, 766–783. [Google Scholar] [CrossRef]
- Qiang, H.; Lang, D.-L.; Li, Y.-Y. High-solid mesophilic methane fermentation of food waste with an emphasis on Iron, Cobalt, and Nickel requirements. Bioresour. Technol. 2012, 103, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Z.; Lu, T.; Liu, J.; Wang, Y.; Shen, P.; Wei, Y. Response and mechanisms of the performance and fate of antibiotic resistance genes to nano-magnetite during anaerobic digestion of swine manure. J. Hazard. Mater. 2019, 366, 192–201. [Google Scholar] [CrossRef]
- Kato, S.; Hashimoto, K.; Watanabe, K. Methanogenesis facilitated by electric syntrophy via (semi) conductive iron-oxide minerals. Environ. Microbiol. 2012, 14, 1646–1654. [Google Scholar] [CrossRef]
- Jing, Y.; Wan, J.; Angelidaki, I.; Zhang, S.; Luo, G. iTRAQ quantitative proteomic analysis reveals the pathways for methanation of propionate facilitated by magnetite. Water Res. 2017, 108, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Kim, J.; Cho, K.; Bae, H.; Lee, C. The biostimulation of anaerobic digestion with (semi) conductive ferric oxides: Their potential for enhanced biomethanation. Appl. Microbiol. Biotechnol. 2015, 99, 10355–10366. [Google Scholar] [CrossRef]
- Baek, G.; Jung, H.; Kim, J.; Lee, C. A long-term study on the effect of magnetite supplementation in continuous anaerobic digestion of dairy effluent–magnetic separation and recycling of magnetite. Bioresour. Technol. 2017, 241, 830–840. [Google Scholar] [CrossRef]
- Yin, Q.; Miao, J.; Li, B.; Wu, G. Enhancing electron transfer by ferroferric oxide during the anaerobic treatment of synthetic wastewater with mixed organic carbon. Int. Biodeterior. Biodegrad. 2017, 119, 104–110. [Google Scholar] [CrossRef]
- Casals, E.; Barrena, R.; García, A.; González, E.; Delgado, L.; Busquets-Fité, M.; Font, X.; Arbiol, J.; Glatzel, P.; Kvashnina, K. Programmed iron oxide nanoparticles disintegration in anaerobic digesters boosts biogas production. Small 2014, 10, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Suanon, F.; Sun, Q.; Mama, D.; Li, J.; Dimon, B.; Yu, C.-P. Effect of nanoscale zero-valent iron and magnetite (Fe3O4) on the fate of metals during anaerobic digestion of sludge. Water Res. 2016, 88, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, J.; Hu, Z. Impact of nano zero valent iron (NZVI) on methanogenic activity and population dynamics in anaerobic digestion. Water Res. 2013, 47, 6790–6800. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Shah, T.A.; Afzal, A.; Tabassum, R. Exploring lignocellulosic biomass for bio-methane potential by anaerobic digestion and its economic feasibility. Energy Environ. 2018, 29, 742–751. [Google Scholar] [CrossRef]
- Juntupally, S.; Begum, S.; Allu, S.K.; Nakkasunchi, S.; Madugula, M.; Anupoju, G.R. Relative evaluation of micronutrients (MN) and its respective nanoparticles (NPs) as additives for the enhanced methane generation. Bioresour. Technol. 2017, 238, 290–295. [Google Scholar] [CrossRef]
- Abdelsalam, E.; Samer, M.; Attia, Y.; Abdel-Hadi, M.; Hassan, H.; Badr, Y. Effects of Co and Ni nanoparticles on biogas and methane production from anaerobic digestion of slurry. Energy Convers. Manag. 2017, 141, 108–119. [Google Scholar] [CrossRef]
- Ali, A.; Mahar, R.B.; Soomro, R.A.; Sherazi, S.T.H. Fe3O4 nanoparticles facilitated anaerobic digestion of organic fraction of municipal solid waste for enhancement of methane production. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 1815–1822. [Google Scholar] [CrossRef]
- Zhang, L.; He, X.; Zhang, Z.; Cang, D.; Nwe, K.A.; Zheng, L.; Li, Z.; Cheng, S. Evaluating the influences of ZnO engineering nanomaterials on VFA accumulation in sludge anaerobic digestion. Biochem. Eng. J. 2017, 125, 206–211. [Google Scholar] [CrossRef]
- Chen, J.L.; Steele, T.W.; Stuckey, D.C. The effect of Fe2NiO4 and Fe4NiO4Zn magnetic nanoparticles on anaerobic digestion activity. Sci. Total Environ. 2018, 642, 276–284. [Google Scholar] [CrossRef]
- Abdallah, M.S.; Hassaneen, F.Y.; Faisal, Y.; Mansour, M.S.; Ibrahim, A.; Abo-Elfadl, S.; Salem, H.; Allam, N.K. Effect of Ni-Ferrite and Ni-Co-Ferrite nanostructures on biogas production from anaerobic digestion. Fuel 2019, 254, 115673. [Google Scholar] [CrossRef]
- Elreedy, A.; Ibrahim, E.; Hassan, N.; El-Dissouky, A.; Fujii, M.; Yoshimura, C.; Tawfik, A. Nickel-graphene nanocomposite as a novel supplement for enhancement of biohydrogen production from industrial wastewater containing mono-ethylene glycol. Energy Convers. Manag. 2017, 140, 133–144. [Google Scholar] [CrossRef]
- Mansour, M.S.; Abdallah, M.S.; Allam, N.K.; Ibrahim, A.; Khedr, A.M.; Al-Bulqini, H.M.; Zayed, M.F. Biogas production enhancement using nanocomposites and its combustion characteristics in a concentric flow slot burner. Exp. Therm. Fluid Sci. 2020, 113, 110014. [Google Scholar] [CrossRef]
- Zhang, G.; Shi, Y.; Zhao, Z.; Wang, X.; Dou, M. Enhanced two-phase anaerobic digestion of waste-activated sludge by combining magnetite and zero-valent iron. Bioresour. Technol. 2020, 306, 123122. [Google Scholar] [CrossRef]
- Hassaneen, F.Y.; Abdallah, M.S.; Ahmed, N.; Taha, M.M.; Abd ElAziz, S.M.M.; El-Mokhtar, M.A.; Badary, M.S.; Allam, N.K. Innovative nanocomposite formulations for enhancing biogas and biofertilizers production from anaerobic digestion of organic waste. Bioresour. Technol. 2020, 309, 123350. [Google Scholar] [CrossRef]
- Chen, J.; Yun, S.; Shi, J.; Wang, Z.; Abbas, Y.; Wang, K.; Han, F.; Jia, B.; Xu, H.; Xing, T.; et al. Role of biomass-derived carbon-based composite accelerants in enhanced anaerobic digestion: Focusing on biogas yield, fertilizer utilization, and density functional theory calculations. Bioresour. Technol. 2020, 307, 123204. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.M.; Thorat, N.D.; Shete, P.B.; Bedge, P.A.; Gavde, S.; Joshi, M.G.; Tofail, S.A.; Bohara, R.A. Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticles. Biochem. Biophys. Rep. 2018, 13, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, S.; Biscoff, R.; Enweremadu, C. A meta-analysis of iron-based additives on enhancements of biogas yields during anaerobic digestion of organic wastes. J. Clean. Prod. 2020, 269, 122449. [Google Scholar] [CrossRef]
- Ambuchi, J.J.; Zhang, Z.; Feng, Y. Biogas enhancement using iron oxide nanoparticles and multi-wall carbon nanotubes. Int. J. Chem. Mol. Nucl. Mat. Metall. Eng 2016, 10, 1239–1246. [Google Scholar]
- Abdelsalam, E.; Samer, M.; Attia, Y.; Abdel-Hadi, M.; Hassan, H.; Badr, Y. Influence of zero valent iron nanoparticles and magnetic iron oxide nanoparticles on biogas and methane production from anaerobic digestion of manure. Energy 2017, 120, 842–853. [Google Scholar] [CrossRef]
- Amen, T.W.; Eljamal, O.; Khalil, A.M.; Matsunaga, N. Biochemical methane potential enhancement of domestic sludge digestion by adding pristine iron nanoparticles and iron nanoparticles coated zeolite compositions. J. Environ. Chem. Eng. 2017, 5, 5002–5013. [Google Scholar] [CrossRef]
- Amen, T.W.M.; Eljamal, O.; Khalil, A.M.E.; Sugihara, Y.; Matsunaga, N. Methane yield enhancement by the addition of new novel of iron and copper-iron bimetallic nanoparticles. Chem. Eng. Process.-Process. Intensif. 2018, 130, 253–261. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, L.; Wang, Y.; Zhao, Y.; She, Z.; Gao, M.; Guo, Y. Application of iron oxide (Fe3O4) nanoparticles during the two-stage anaerobic digestion with waste sludge: Impact on the biogas production and the substrate metabolism. Renew. Energy 2020, 146, 2724–2735. [Google Scholar]
- Amen, T.W.; Eljamal, O.; Khalil, A.M.; Matsunaga, N. Evaluation of nano zero Valent Iron Effects on Fermentation of Municipal Anaerobic Sludge and Inducing Biogas Production; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017; p. 012004. [Google Scholar]
- Suanon, F.; Sun, Q.; Li, M.; Cai, X.; Zhang, Y.; Yan, Y.; Yu, C.-P. Application of nanoscale zero valent iron and iron powder during sludge anaerobic digestion: Impact on methane yield and pharmaceutical and personal care products degradation. J. Hazard. Mater. 2017, 321, 47–53. [Google Scholar] [CrossRef]
- Yazdani, M.; Ebrahimi-Nik, M.; Heidari, A.; Abbaspour-Fard, M.H. Improvement of biogas production from slaughterhouse wastewater using biosynthesized iron nanoparticles from water treatment sludge. Renew. Energy 2019, 135, 496–501. [Google Scholar] [CrossRef]
- Ünşar, E.K.; Perendeci, N.A. What kind of effects do Fe2O3 and Al2O3 nanoparticles have on anaerobic digestion, inhibition or enhancement? Chemosphere 2018, 211, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Farghali, M.; Andriamanohiarisoamanana, F.J.; Ahmed, M.M.; Kotb, S.; Yamamoto, Y.; Iwasaki, M.; Yamashiro, T.; Umetsu, K. Prospects for biogas production and H2S control from the anaerobic digestion of cattle manure: The influence of microscale waste iron powder and iron oxide nanoparticles. Waste Manag. 2020, 101, 141–149. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, Z.; Zhang, Y.; Xu, R.; Zheng, Y.; Hu, J.; Li, X.; Jia, M.; Xiong, W.; Cao, J. Influence of nanoscale zero-valent iron and magnetite nanoparticles on anaerobic digestion performance and macrolide, aminoglycoside, β-lactam resistance genes reduction. Bioresour. Technol. 2019, 294, 122139. [Google Scholar] [CrossRef]
- Chen, R.; Konishi, Y.; Nomura, T. Enhancement of methane production by Methanosarcina barkeri using Fe3O4 nanoparticles as iron sustained release agent. Adv. Powder Technol. 2018, 29, 2429–2433. [Google Scholar] [CrossRef]
- Hassanein, A.; Lansing, S.; Tikekar, R. Impact of metal nanoparticles on biogas production from poultry litter. Bioresour. Technol. 2019, 275, 200–206. [Google Scholar] [CrossRef]
- Zaidi, A.A.; RuiZhe, F.; Malik, A.; Khan, S.Z.; Bhutta, A.J.; Shi, Y.; Mushtaq, K. Conjoint effect of microwave irradiation and metal nanoparticles on biogas augmentation from anaerobic digestion of green algae. Int. J. Hydrogen Energy 2019, 44, 14661–14670. [Google Scholar] [CrossRef]
- Farghali, M.; Andriamanohiarisoamanana, F.J.; Ahmed, M.M.; Kotb, S.; Yamashiro, T.; Iwasaki, M.; Umetsu, K. Impacts of iron oxide and titanium dioxide nanoparticles on biogas production: Hydrogen sulfide mitigation, process stability, and prospective challenges. J. Environ. Manag. 2019, 240, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Avilés, P.; Ida, J.; Toda, T.; Cuevas-Rodríguez, G. Effects and fate of TiO2 nanoparticles in the anaerobic treatment of wastewater and waste sludge. J. Environ. Manag. 2018, 222, 227–233. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Zhang, J.; Zhou, J.; Cen, K.; Murphy, J.D. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 239, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, F.; Tsui, T.-H.; Yoh, K.; Sun, J.; Loh, K.-C.; Wang, C.-H.; Dai, Y.; Tong, Y.W. Microbial succession analysis reveals the significance of restoring functional microorganisms during rescue of failed anaerobic digesters by bioaugmentation of nano-biochar-amended digestate. Bioresour. Technol. 2022, 352, 127102. [Google Scholar] [CrossRef]
- Ali, A.; Mahar, R.B.; Abdelsalam, E.M.; Sherazi, S.T.H. Kinetic Modeling for Bioaugmented Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste by Using Fe3O4 Nanoparticles. Waste Biomass Valorization 2019, 10, 3213–3224. [Google Scholar] [CrossRef]
- Li, Y.; Wang, P.; Zhao, L.; Yang, X.; Ren, L. Microbial mechanism underlying the effect of biochar supported nano zero-valent iron on the anaerobic digestion of food waste. J. Environ. Chem. Eng. 2023, 11, 111286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).