Direct Sonochemical Leaching of Li, Co, Ni, and Mn from Mixed Li-Ion Batteries with Organic Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods—Leaching Procedure
- (i)
- mechanical stirring leaching—in closed cylindrical reaction vessels with a thermostatic jacket and mechanical stirrer (300 rpm) (Lenz with Heidolph SN stirrer and thermostat Grant LT Ecocool 150 SN, Royston, UK),
- (ii)
- sonochemical leaching—in closed vessels with immersed titanium probe conductive ultrasound produced by an ultrasonic generator at 20 kHz frequency, and amplitude range 65% (SONOPhULS HD 4200, Bandelin, Berlin, Germany). Under the assumed operating parameters of the generator, the sonotrode generated a temperature of 60 °C in the solution, which was maintained as a constant value in the sonochemical leaching experiments.
3. Results and Discussion
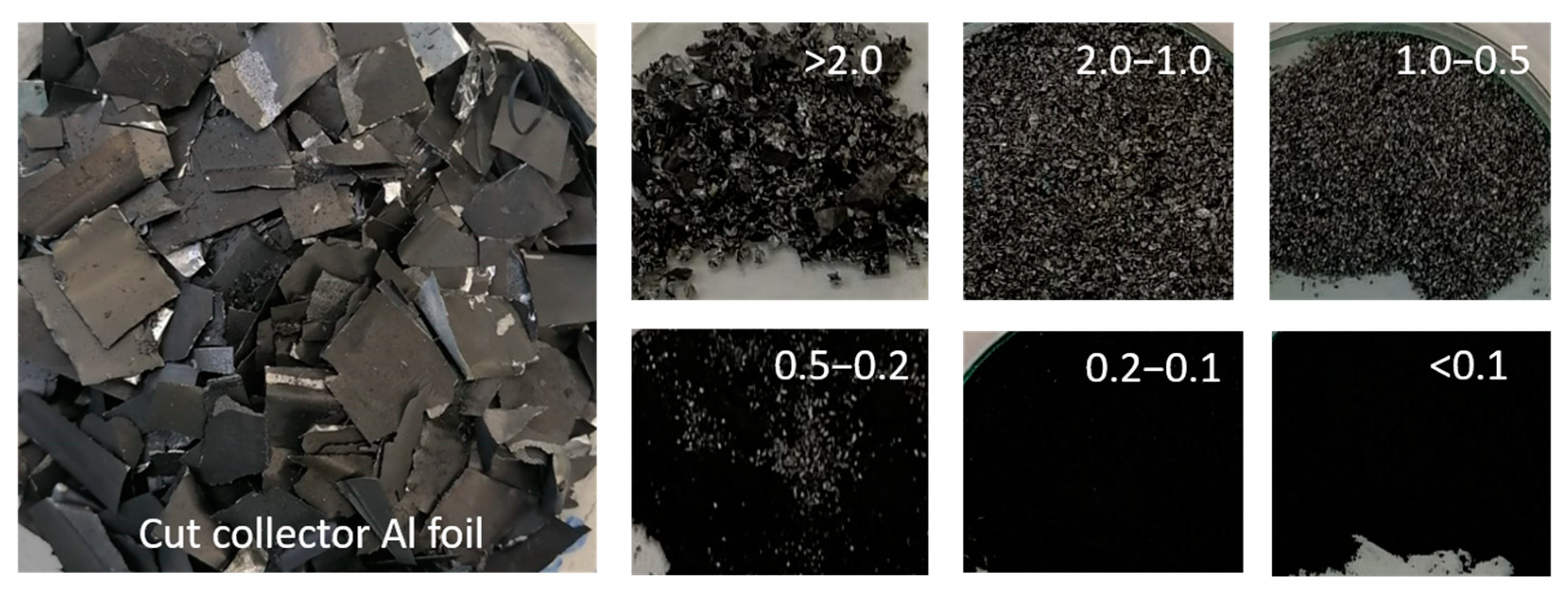
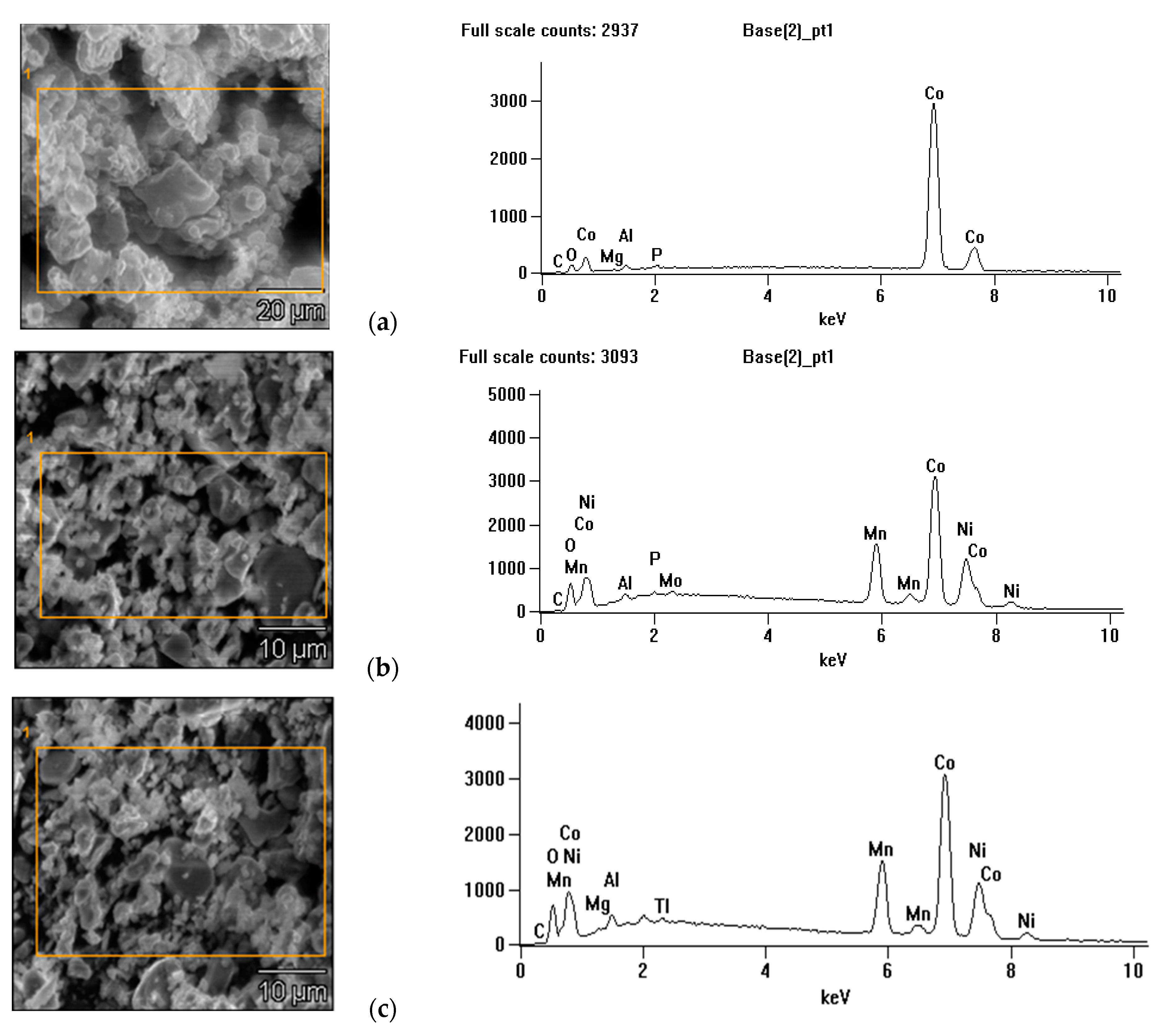
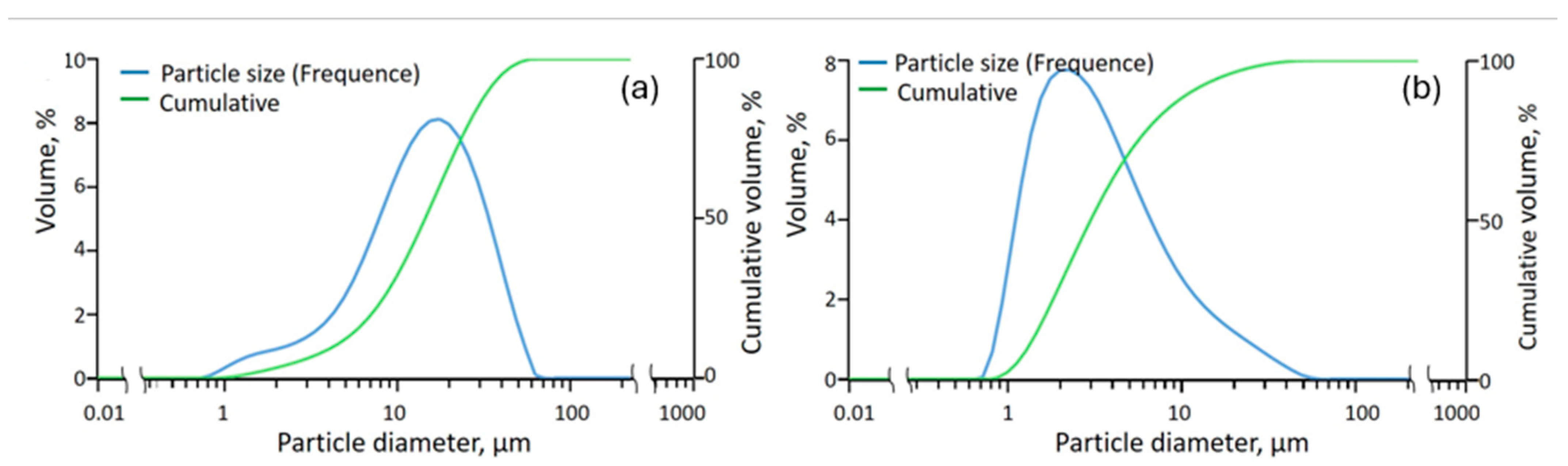
3.1. Cathode Active Material Characterization
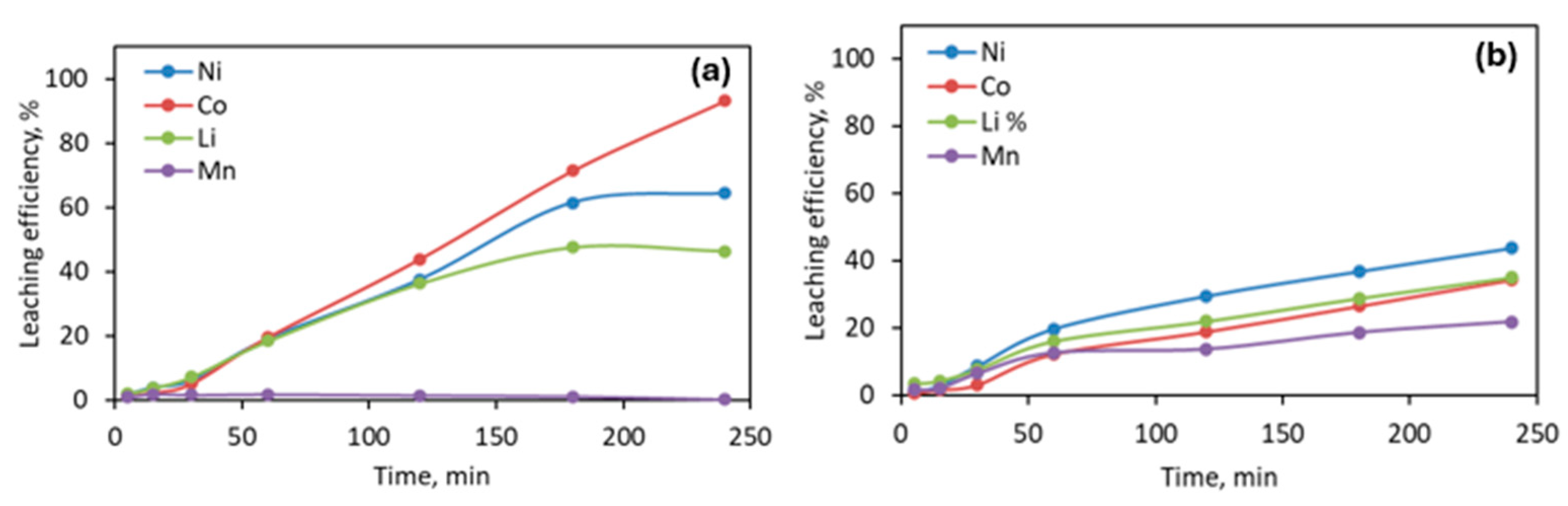
3.2. Effect of Time on Leaching Efficiency
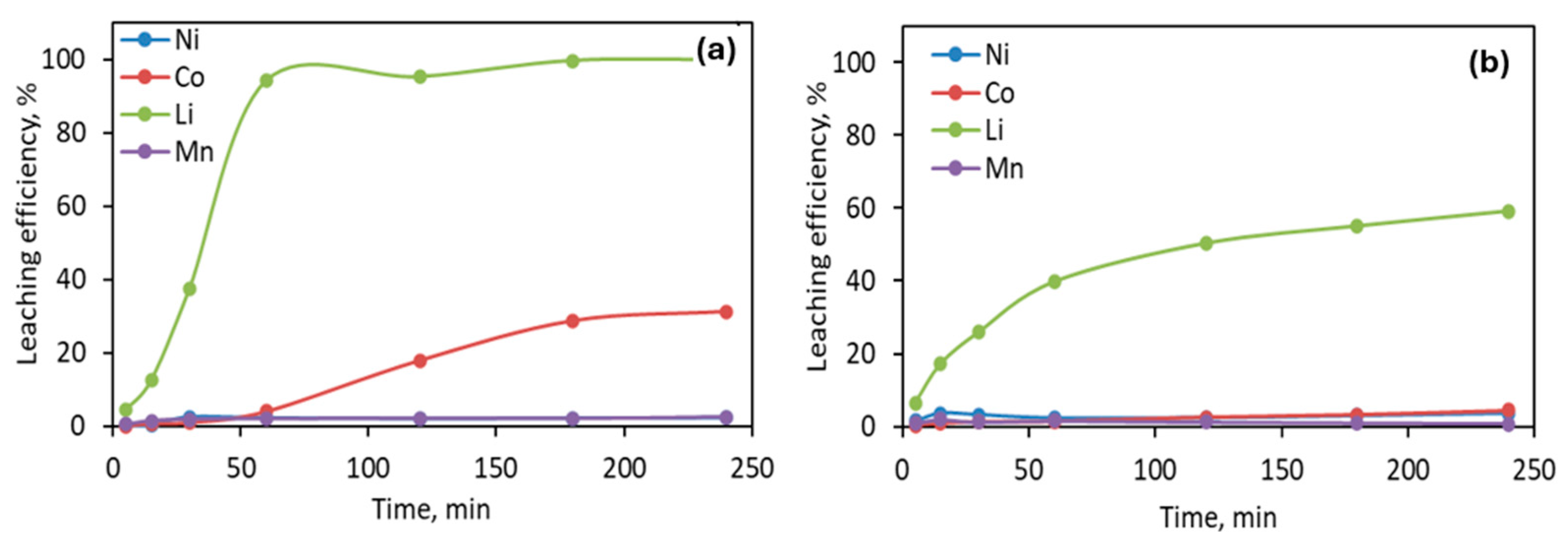
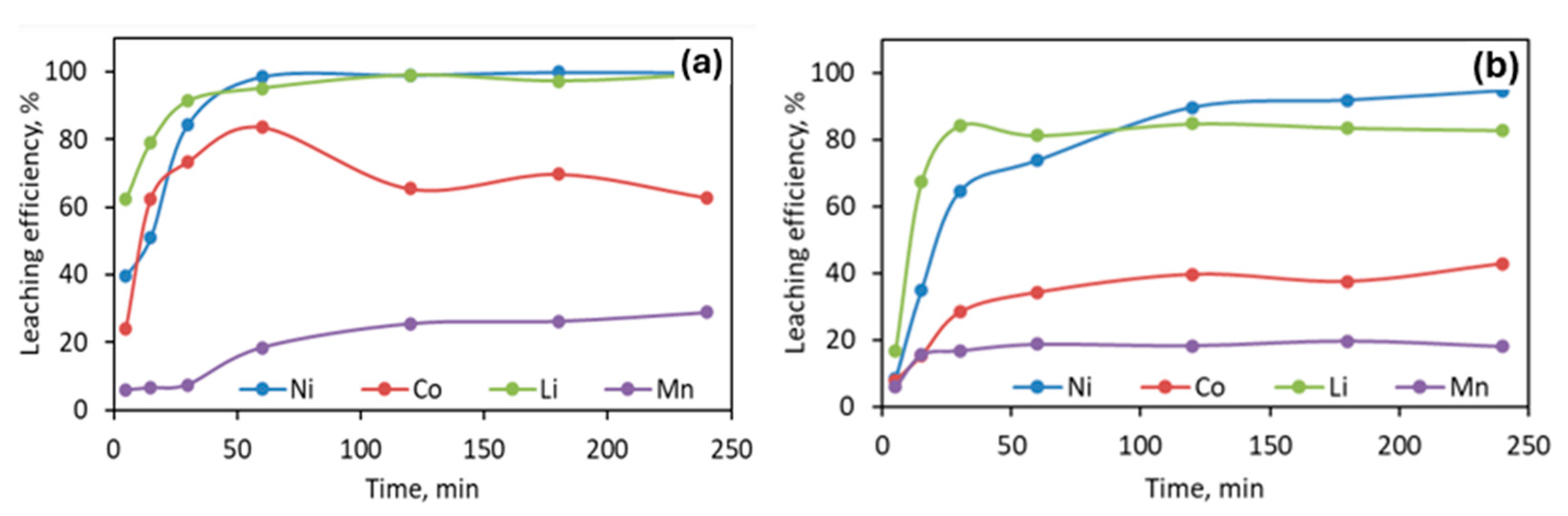
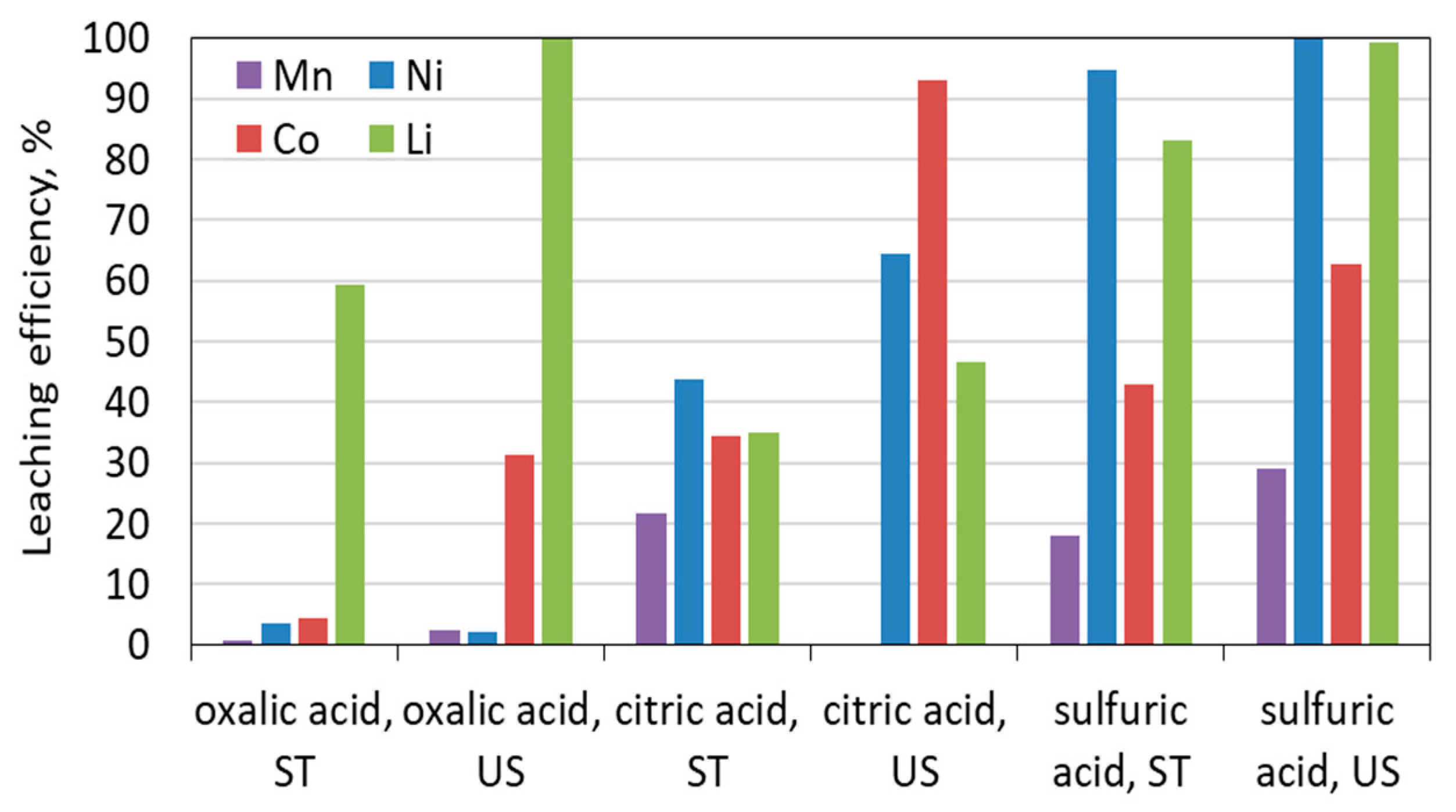
3.2.1. Leaching with Oxalic Acid
3.2.2. Leaching with Sulfuric Acid
3.2.3. Leaching with Citric Acid
3.3. Effect of Leaching Temperature on Li Efficiency with Oxalic Acid
3.4. Leaching under Sonic Conditions vs. a Mechanical Stirring System
3.5. Cathode Active Material after Ultrasound-Assisted Leaching
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blengini, G.A.; El Latunussa, C.; Eynard, U.; Torres De Matos, C.; Wittmer, D.; Georgitzikis, K.; Pavel, C.; Carrara, S.; Mancini, L.; Unguru, M.; et al. Study on the EU’s List of Critical Raw Materials; Final Report 31.01.2020; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Gaines, L.; Richa, K.; Spangenberger, J. Key issues for Li-ion battery recycling. MRS Energy Sustain. 2018, 5, 12. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Chmielarz, A.; Kopyto, D.; Ochmański, M.; Benke, G.; Palmowski, A.; Sobianowska-Turek, A.; Łoś, P.; Fornalczyk, A.; Zygmunt, M.; et al. An Innovative Method of Leaching of Battery Masses Produced in the Processing of Li-Ion Battery Scrap. Appl. Sci. 2024, 14, 397. [Google Scholar] [CrossRef]
- Yu, W.; Guo, Y.; Xu, S.; Yang, Y.; Zhao, Y.; Zhang, J. Comprehensive recycling of lithium-ion batteries: Fundamentals, pretreatment, and perspectives. Energy Storage Mater. 2023, 5, 172–220. [Google Scholar] [CrossRef]
- Available online: https://www.nsenergybusiness.com/news/industry-news/global-lithium-demand-2024/ (accessed on 10 June 2024).
- Available online: https://rmis.jrc.ec.europa.eu/?page=crms-in-strategic-sectors-and-technologies-e8c632 (accessed on 10 June 2024).
- Fourth List of CRMs—In 2020-Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Critical Raw Materials Resilience: Charting a Path towards greater Security and Sustainability; COM(2020) 474 final; European Commission: Brussels, Belgium, 2020.
- Halleux, V. New EU Regulatory Framework for Batteries Setting Sustainability Requirements; BRIEFING EU Legislation in Progress PE 689.337; European Parliamentary Research Service: Brussels, Belgium, 2021. [Google Scholar]
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium-Ion Batteries, Part II: Laboratory-Scale Research Developments in Mechanical, Thermal, and Leaching Treatments. J. Sustain. Metall. 2020, 6, 142–160. [Google Scholar] [CrossRef]
- Or, T.; Gourley, S.W.D.; Kaliyappan, K.; Yu, A.; Chen, Z. Recycling of mixed cathode lithium-ion batteries for electric vehicles, Current status and future outlook. Carbon Energy 2020, 2, 6–43. [Google Scholar] [CrossRef]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Sobianowska-Turek, A.; Urbanska, W.; Janicka, A.; Zawiślak, M.; Matla, J. The Necessity of Recycling of Waste Li-Ion Batteries Used in Electric Vehicles as Objects Posing a Threat to Human Health and the Environment. Recycling 2021, 6, 35. [Google Scholar] [CrossRef]
- Guimarães, L.F.; Botelho Junior, A.B.; Espinosa, D.C.R. Sulfuric acid leaching of metals from waste Li-ion batteries without using reducing agent. Miner. Eng. 2022, 183, 107597. [Google Scholar] [CrossRef]
- Shin, S.M.; Kim, N.H.; Sohn, J.S.; Yang, D.H.; Kim, Y.H. Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 2005, 79, 172–181. [Google Scholar] [CrossRef]
- Balázs Illés, I.; Kékesi, T. Extraction of pure Co, Ni, Mn, and Fe compounds from spent Li-ion batteries by reductive leaching and combined oxidative precipitation in chloride media. Miner. Eng. 2023, 201, 108169. [Google Scholar] [CrossRef]
- Sadia, I.; Rajiv Ranjan, S.; Vinay, K.S.; Ruan, C.; Hyunjung, K. Recovery of critical metals from spent Li-ion batteries: Sequential leaching, precipitation, and cobalt–nickel separation using Cyphos IL104. Waste Manag. 2022, 154, 175–186. [Google Scholar] [CrossRef]
- Qian, H.; Hong, Z.; Zhanfang, C. High-efficiency leaching of Li and Ni from spent lithium-ion batteries based on sodium persulfate. Sep. Purif. Technol. 2023, 325, 124653. [Google Scholar] [CrossRef]
- Song, D.; Wang, T.; Liu, Z.; Zhao, S.; Quan, J.; Li, G.; Zhu, H.; Huang, J.; He, W. Characteristic comparison of leaching valuable metals from spent power Li-ion batteries for vehicles using the inorganic and organic acid system. J. Environ. Chem. Eng. 2022, 10, 107102. [Google Scholar] [CrossRef]
- Sedlakova-Kadukova, A.; Marcincakova, R.; Mrazikova, A.; Willner, J.; Fornalczyk, A. Closing the loop: Key role of iron in metal-bearing waste recycling. Arch. Metall. Mater. 2017, 62, 1459–1466. [Google Scholar] [CrossRef]
- Arya, S.; Kumar, S. Bioleaching: Urban mining option to curb the menace of E-waste challenge. Bioengineered 2020, 11, 640–660. [Google Scholar] [CrossRef] [PubMed]
- Golmohammadzadeh, R.; Rashchi, F.; Vahidi, E. Recovery of lithium and cobalt from spent lithium-ion batteries using organic acids: Process optimization and kinetic aspects. Waste Manag. 2017, 64, 244–254. [Google Scholar] [CrossRef]
- Meshram, P.; Mishra, A.; Sahu, R. Environmental impact of spent lithium ion batteries and green recycling perspectives by organic acids—A review. Chemosphere 2020, 242, 125291. [Google Scholar] [CrossRef]
- Li, D.; Yin, Y.; Jiao, B.; Song, L.; Wang, Y.; Qiao, J.; Xu, Z. Chemical dissolving of citric acid on bioleaching of copper mine tailings by Acidithiobacillus ferrooxidans ATCC 23270. Res. J. Biotechnol. 2013, 8, 12. [Google Scholar]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating organic acids as alternative leaching reagents for metal recovery from lithium ion batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of lithium and cobalt from spent lithium ion batteries (LIBs) using organic acids as leaching reagents: A review. Resour Conserv Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Ren, Y.; Zhang, X.X.; Chen, R.J.; Wu, F.; Amine, K. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries. J. Power Sources 2012, 218, 21–27. [Google Scholar] [CrossRef]
- Li, L.; Zhai, L.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Recovery of valuable metals from spent lithium-ion batteries by ultrasonic-assisted leaching process. J. Power Sources 2014, 262, 380–385. [Google Scholar] [CrossRef]
- Park, Y.; Lim, H.; Moon, J.-H.; Lee, H.-N.; Son, S.H.; Kim, H.; Kim, H.-J. High-yield one-pot recovery and characterization of nanostructured cobalt oxalate from spent lithium-ion batteries and successive re-synthesis of LiCoO2. Metals 2017, 7, 303. [Google Scholar] [CrossRef]
- Menga, F.; Liua, Q.; Kim, R.; Wang, J.; Liu, G.; Ghahreman, A. Selective recovery of valuable metals from industrial waste lithium-ion batteries using citric acid under reductive conditions: Leaching optimization and kinetic analysis. Hydrometallurgy 2020, 191, 105160. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J. Hazard. Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Shen, B. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid. J. Hazard Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, X.; Zheng, X.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Lithium carbonate recovery from cathode scrap of spent lithium-ion battery: A closed loop process. Environ. Sci. Technol. 2017, 51, 1662–1669. [Google Scholar] [CrossRef]
- Li, L.; Qu, W.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Succinic acid-based leaching system: A sustainable process for recovery of valuable metals from spent Li-ion batteries. J. Power Sources 2015, 282, 544–551. [Google Scholar] [CrossRef]
- Urbanska, W.; Osial, M. Investigation of the Physico-Chemical Properties of the Products Obtained after Mixed Organic-Inorganic Leaching of Spent Li-Ion Batteries. Energies 2020, 13, 6732. [Google Scholar] [CrossRef]
- Rix, A.; Lederle, W.; Theek, B.; Lammers, T.; Moonen, C.; Schmitz, G.; Kiessling, F. Advanced Ultrasound Technologies for Diagnosis and Therapy. J. Nucl. Med. 2018, 59, 740–746. [Google Scholar] [CrossRef]
- Xu, T.; Xu, L.P.; Zhang, X. Ultrasound propulsion of micro-/nanomotors. Appl. Mater. Today 2017, 9, 493–503. [Google Scholar] [CrossRef]
- Li, J.; Li, T.; Xu, T.; Kiristi, M.; Liu, W.; Wu, Z.; Wang, J. Magneto–Acoustic Hybrid Nanomotor. Nano Lett. 2015, 15, 4814–4821. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Y.; Xie, F.C.; Ma, Y. Ultrasonic recovery of copper and iron through the simultaneous utilization of Printed Circuit Boards (PCB) spent acid etching solution and PCB waste sludge. J. Hazard Mater. 2011, 185, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Toache-Pérez, A.D.; Bolarín-Miró, A.M.; Sánchez-De Jesús, F.; Lavine, G.T. Facile method for the selective recover of Gd and Pr from LCD screen wastes using ultrasound-assisted leaching. Sustain. Environ. Res. 2020, 30, 20. [Google Scholar] [CrossRef]
- Oza, R.; Shah, N.; Patel, S. Recovery of nickel from spent catalysts using ultrasonication-assisted leaching. J. Chem. Technol. Biotechnol. 2011, 86, 1276–1281. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, E.; Yang, C.; Zhou, J.; Peng, J.; Zhang, L.; Srinivasakannan, C. Kinetics of ultrasound-assisted silver leaching from sintering dust using thiourea. Green Process. Synth. 2016, 5, 31–40. [Google Scholar] [CrossRef]
- Yin, S.; Pei, J.; Jiang, F.; Li, S.; Peng, J.; Zhang, L.; Ju, S.; Srinivasakannan, C. Ultrasound-assisted leaching of rare earths from the weathered crust elution deposited ore using magnesium sulfate without ammonia-nitrogen pollution. Ultrason. Sonochem. 2020, 41, 156–162. [Google Scholar] [CrossRef]
- Sobianowska-Turek, A.; Grudniewska, K.; Maciejewski, P.; Gawlik-Kobylińska, M. Removal of Zn(II) and Mn(II) by Ion Flotation from Aqueous Solutions Derived from Zn-C and Zn-Mn(II) Batteries Leaching. Energies 2021, 14, 1335. [Google Scholar] [CrossRef]
- Li, H.; Li, S.; Peng, J.; Srinivasakannan, C.; Zhang, L.; Yin, S. Ultrasound augmented leaching of nickel sulfate in sulfuric acid and hydrogen peroxide media. Ultrason. Sonochem. 2018, 40, 1021–1030. [Google Scholar] [CrossRef]
- Esmaeili, M.; Rastegar, S.O.; Beigzadeh, R.; Gu, T. Ultrasound-assisted leaching of spent lithium ion batteries by natural organic acids and H2O2. Chemosphere 2020, 254, 126670. [Google Scholar] [CrossRef]
- Vyas, S.; Ting, Y.P. A Review of the Application of Ultrasound in Bioleaching and Insights from Sonication in (Bio)Chemical Processes. Resources 2018, 7, 3. [Google Scholar] [CrossRef]
- Swamy, K.M.; Sarveswara, K.; Narayana, K.L.; Murty, J.S.; Ray, H.S. Application of Ultrasound in Leaching. Miner. Process. Extr. Metall. Rev. 1995, 14, 179–192. [Google Scholar] [CrossRef]
- Tzanakis, I.; Lebon, G.S.B.; Eskin, D.G.; Pericleous, K.A. Characterizing the cavitation development and acoustic spectrum in various liquids. Ultrason. Sonochem. 2017, 34, 651–662. [Google Scholar] [CrossRef]
- Available online: https://www.reuters.com/article/us-catl-batteries-idUSKCN25B0BA (accessed on 18 January 2024).
- Jiang, F.; Chen, Y.; Ju, S.; Zhu, Q.; Zhang, L.; Peng, J.; Wang, X.; Miller, J.D. Ultrasound-assisted leaching of cobalt and lithium from spent lithium-ion batteries. Ultrason. Sonochem. 2018, 48, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Nshizirungu, T.; Rana, M.; Jo, Y.T.; Uwiragiye, E.; Kim, J.; Park, J.-H. Ultrasound-assisted sustainable recycling of valuable metals from spent Li-ion batteries via optimisation using response surface methodology. J. Environ. Chem. Eng. 2024, 12, 2371. [Google Scholar] [CrossRef]
- Yan, S.; Sun, C.; Zhou, T.; Gao, R.; Xie, H. Ultrasonic-assisted leaching of valuable metals from spent lithium-ion batteries using organic additives. Sep. Purif. Technol. 2021, 257, 117930. [Google Scholar] [CrossRef]
- Okonkwo, E.G.; Wheatley, G.; Liu, Y.; He, Y. A cavitation enabled green leaching of metals from spent lithium-ion batteries. Chem. Eng. Process.-Process. Intensif. 2024, 202, 109850. [Google Scholar] [CrossRef]
- Ning, P.; Meng, Q.; Dong, P.; Duan, J.; Xu, M.; Lin, Y.; Zhang, Y. Recycling of cathode material from spent lithium ion batteries using an ultrasound-assisted DL-malic acid leaching system. Waste Manag. 2020, 103, 52–60. [Google Scholar] [CrossRef]
- Urbańska, W. Recovery of Co, Li, and Ni from Spent Li-Ion Batteries by the Inorganic and/or Organic Reducer Assisted Leaching Method. Minerals 2020, 10, 555. [Google Scholar] [CrossRef]
- Morrison, R.T.; Boyd, R.N. Chap. 11. In Organic Chemistry, 6th ed.; Prentice Hall International, Inc.: London, UK, 1992. [Google Scholar]
- Krishnamurty, K.V.; Harris, G.M. The Chemistry of the Metal Oxalato Complexes. Chem. Rev. 1961, 61, 213–246. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Y.; Xu, S.; Fan, E.; Xue, Q.; Guan, Y.; Wu, F.; Li, L.; Chen, R. Innovative Application of Acid Leaching to Regenerate Li(Ni1/3Co1/3Mn1/3)O2 Cathodes from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 5959–5968. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, K. Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag. 2021, 32, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Rumble, J.R.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Verma, A.; Kore, R.; Corbin, D.R.; Shiflett, M.B. Metal Recovery Using Oxalate Chemistry: A Technical Review. Ind. Eng. Chem. Res. 2019, 58, 15381–15393. [Google Scholar] [CrossRef]
- Wang, H.; Friedrich, B. Development of a highly efficient hydrometallurgical recycling process for automotive Li–ion batteries. J. Sustain. Met. 2015, 1, 168–178. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Recovery of valuable metals from cathodic active material of spent lithium ionbatteries: Leaching and kinetic aspects. Waste Manag. 2015, 45, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Xu, Z.; Yao, Z.; Cheng, W.; Gao, H.; Zhao, Q.; Li, J.; Zhou, A. Oxalate co-precipitation synthesis of LiNi0.6Co0.2Mn0.2O2 for low-cost and high-energy lithium-ion batteries. Mater. Today Commun. 2019, 19, 262–270. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, T. Hydrometallurgical process for the recovery of metal values from spent lithium-ion batteries in citric acid media. Waste Manag. Res. 2014, 32, 1083–1093. [Google Scholar] [CrossRef]
- Yoon, W.S.; Nam, K.-W.; Jang, D.; Chung, K.Y.; Hanson, J.; Chen, J.-M.; Yang, X.-Q. Structural study of the coating effect on the thermal stability of charged MgO-coated LiNi0.8Co0.2O2 cathodes investigated by in situ XRD. J. Power Sources 2012, 217, 128–134. [Google Scholar] [CrossRef]
- Sohn, J.-S.; Shin, S.-M.; Yang, D.-H.; Kim, S.-K.; Lee, C.-K. Comparison of Two Acidic Leaching Processes for Selecting the Effective Recycle Process of Spent Lithium ion Battery. Geosyst. Eng. 2006, 9, 1−6. [Google Scholar] [CrossRef]
- Eskin, G.I. Cavitation mechanism of ultrasonic melt degassing. Ultrason. Sonochem. 1995, 2, S137–S141. [Google Scholar] [CrossRef]
- Zhang, K.; Li, B.; Wu, Y.; Wang, W.; Li, R.; Zhang, Y.N.; Zuo, T. Recycling of indium from waste LCD: A promising non-crushing leaching with the aid of ultrasonic wave. Waste Manag. 2017, 64, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Riesz, P.; Berdahl, D.; Christman, C.L. Free radical generation by ultrasound in aqueous and nonaqueous solutions. Environ. Health Perspect. 1985, 64, 233–252. [Google Scholar] [CrossRef]
- Thompson, L.H.; Doraiswamy, L.K. Sonochemistry: Science and engineering. Ind. Eng. Chem. Res. 1999, 38, 1215–1249. [Google Scholar] [CrossRef]
- Ziembowicz, S.; Kida, M.; Koszelnik, P. Sonochemical Formation of Hydrogen Peroxide. Proceedings 2018, 2, 188. [Google Scholar]
- Jha, M.K.; Kumari, A.; Jha, A.K.; Kumar, V.; Hait, J.; Pandey, B.D. Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Waste Manag. 2013, 33, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Rhee, K.I. Preparation of LiCoO2 from spent lithium-ion batteries. J. Power Sources 2002, 109, 17–21. [Google Scholar] [CrossRef]
- Sattar, R.; Ilyas, S.; Bhatti, H.N.; Ghaffar, A. Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries. Sep. Purif. Technol. 2019, 209, 725–733. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Machado, C.M.; Silva, M.L.; Calgaro, C.O.; Dotto, G.L.; Tanabe, E.H. Recovery of cobalt from spent lithium-ion batteries using supercritical carbon dioxide extraction. Waste Manag. 2016, 51, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, H.; Luo, C.; Zhou, T. Recovery of valuable metals from waste cathode materials of spent lithium-ion batteries using mild phosphoric acid. J. Hazard. Mater. 2017, 326, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Son, Y.; Khim, J. The effects of hydrogen peroxide on the sonochemical degradation of phenol and bisphenol A. Ultrason. Sonochem. 2014, 21, 1976–1981. [Google Scholar] [CrossRef]
- Pétrier, C. 31: The use of power ultrasound for water treatment. In Power Ultrasonics; Woodhead Publishing: Oxford, UK, 2015; pp. 939–972. [Google Scholar]
- Franco, F.; Pérez-Maqueda, L.A.; Pérez-Rodríguez, J.L. The effect of ultrasound on the particle size and structural disorder of a well-ordered kaolinite. J. Colloid Interface Sci. 2004, 274, 107–117. [Google Scholar] [CrossRef]
- Pilli, S.; Bhunia, P.; Yan, S.; LeBlanc, R.; Tyagi, R.; Surampalli, R. Ultrasonic pretreatment of sludge: A review. Ultrason. Sonochem. 2011, 18, 1–18. [Google Scholar] [CrossRef]
- Mao, Y.; Xie, G.; Liang, L.; Xia, W.; Peng, Y. Effects of ultrasonic treatment on the particle size, shape and ash content of fine coal. Physicochem. Probl. Miner. Process. 2019, 55, 679–688. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, E.-D.; Zhang, L.-B.; Peng, J.-H.; Zhou, J.-W.; Srinivasakannan, C.; Yang, C.-J. A comparison of ultrasound-augmented and conventional leaching of silver from sintering dust using acidic thiourea. Ultrason. Sonochem. 2017, 34, 222–231. [Google Scholar] [CrossRef] [PubMed]


| Type of Material | Leaching Agent | Type of Sonic Source/Power | Leaching Conditions | Leaching Effectiveness | Ref. |
|---|---|---|---|---|---|
| LiCoO2 | 2 M H2SO4 + 5% H2O2 | direct/360 W | 0.5 h, 30 °C, 100 g/L | Co: 91.6% Li: 92.7% | [50] |
| LiCoO2 | citric acid + H2O2, | indirect | 0.5 h, 40 °C | Li: 100% Co: 100% Ni: 100% | [45] |
| LiCoO2 | 0.5 M C6H8O7 + 0.55 M H2O2, | indirect/90 W | 5 h, 60 °C, 25 g/L | Co: 96.1% Li: 98.4% | [27] |
| LiCoO2 | 2 M H2SO4 + 0.55 M H2O2 | indirect/90 W | 5 h, 40 °C, 25 g/L | Co: 45.7% Li: 97.0% | |
| LiCoO2 | 2 M HCl | indirect/90 W | 5 h, 60 °C, 25 g/L | Co: 76.4% Li: 98.2% | |
| LiCoO2 | 0.3 M C6H12O7 | indirect/300 W | 1 h, 180 °C, 3 g/200 mL | Co: 96.0% Li: 98.0% | [51] |
| LiCoO2 | 0.5 M CH3COOH + 0.2 M C6H8O6 + bagasse pith | direct/450 W | 40 min, 50 °C, 20 g/L | Co: 98.6% Li: 99.6% | [52] |
| LiCoO2 | CH4O3S +sugarcane molasses | direct/400 W | 1 h, 90 °C, 20 g/L | Co: 97.0% Li: 97.0% | [53] |
| LiCoO2 | 1 M DL C4H6O5 + H2O2 | indirect/90 W | 0.5 h, 80 °C, 5 g/L | Co: 97.3% Li: 98.0% | [54] |
| Ingredient | Content, % |
|---|---|
| Aluminum Foil | 2–10 |
| Metal Oxide (proprietary) | 20–50 |
| Polyvinylidene Fluoride (PVDF) | <5 |
| Copper Foil | 2–10 |
| Carbon (proprietary) | 10–30 |
| Electrolyte (proprietary | 10–20 |
| Stainless steel, Nickel and inert materials | residue |
| Type of Acid | Leaching System | Temperature, °C | Initial pH of Leach Liquor |
|---|---|---|---|
| C2H2O4 | Mechanical stirrer | 22 | 0.68 |
| 40 | |||
| 60 | |||
| Ultrasound | 60 | ||
| C6H8O7 | Mechanical stirrer | 60 | 0.94 |
| Ultrasound | 60 | ||
| H2SO4 | Mechanical stirrer | 60 | −0.25 |
| Ultrasound | 60 |
| Element | Co | Ni | Mn | Li | Al |
|---|---|---|---|---|---|
| wt% | 19.59 | 5.02 | 8.90 | 3.12 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willner, J.; Fornalczyk, A.; Gajda, B.; Figlus, T.; Swieboda, A.; Wegrzyński, D.; Mlonka, A.; Perenc, B.; Kander, M. Direct Sonochemical Leaching of Li, Co, Ni, and Mn from Mixed Li-Ion Batteries with Organic Acids. Energies 2024, 17, 4055. https://doi.org/10.3390/en17164055
Willner J, Fornalczyk A, Gajda B, Figlus T, Swieboda A, Wegrzyński D, Mlonka A, Perenc B, Kander M. Direct Sonochemical Leaching of Li, Co, Ni, and Mn from Mixed Li-Ion Batteries with Organic Acids. Energies. 2024; 17(16):4055. https://doi.org/10.3390/en17164055
Chicago/Turabian StyleWillner, Joanna, Agnieszka Fornalczyk, Bernadeta Gajda, Tomasz Figlus, Adam Swieboda, Dawid Wegrzyński, Aleksander Mlonka, Bartosz Perenc, and Michał Kander. 2024. "Direct Sonochemical Leaching of Li, Co, Ni, and Mn from Mixed Li-Ion Batteries with Organic Acids" Energies 17, no. 16: 4055. https://doi.org/10.3390/en17164055
APA StyleWillner, J., Fornalczyk, A., Gajda, B., Figlus, T., Swieboda, A., Wegrzyński, D., Mlonka, A., Perenc, B., & Kander, M. (2024). Direct Sonochemical Leaching of Li, Co, Ni, and Mn from Mixed Li-Ion Batteries with Organic Acids. Energies, 17(16), 4055. https://doi.org/10.3390/en17164055








