Abstract
Molten salt electrodeposition is the process of producing impressively dense deposits of refractory metals using the electrolysis of molten salts. However, predicting which electrochemical parameters and setup will best control different kinds of deposition (density, homogeneity, etc.) is an ongoing challenge, due to our limited understanding of the properties and mechanisms that drive molten salt electrodeposition. Because these advancements have been made rapidly and in different arenas, it is worth taking the time to stop and assess the progress of the field as a whole. These advancements have increasing relevance for the energy sector, the development of space materials and engineering applications. In this review, we assess four critical facets of this field: (1) how the current understanding of process variables enhances the electrodeposition of various molten salts and the quality of the resulting product; (2) how the electrochemical setup and the process parameters (e.g., cell reactions) are known to impact the electrodeposition of different metal coatings and refractory-metal coatings; (3) the benefits and drawbacks of non-aqueous molten salt electrodeposition, and (4) promising future avenues of research. The aim of this work is to enhance our understanding of the many procedures and variables that have been developed to date. The expectation is that this review will act as a stimulant, motivating scientists to delve further into the investigation of refractory-metal alloys by utilizing molten salt electrodeposition.
1. Introduction
In colloquial usage, “salt” is sodium chloride (NaCl), but according to the most basic chemistry definition, a “salt” is any chemical compound with a positively charged cation and a negatively charged anion. At room temperature and pressure, all salts are solid, crystalline, brittle, rigid, and ionic. Although “molten salts” are also solid at room temperature, they melt into liquid form at higher temperatures (~473–1273 K), at which the molten salts maintain a single-phase liquid state. It also demonstrates a range of thermodynamic properties that have proven to be remarkably valuable for energy technologies and a wide range of other extreme-environment applications, including a high boiling point (~723–863 K), low viscosity (0.71 × 10−6–0.96 × 10−6 m2 s−1 at 1013 K), superior electrical conductivity, low vapor pressure, large volumetric heat capacities, and capacity to serve as a solvent (e.g., for molten salt electrodeposition) [1]. While there are organic molten salts of interest [2,3], the majority of molten salt systems are comprised of inorganic salts, such as nitrates [4,5,6,7], chlorides [8,9,10], fluorides [11,12], bromides [13,14], hydroxides [15,16], and carbonates [17,18], each of which was developed and optimized for unique applications.
Molten salts came to prominence for energy-related advancement technologies several decades ago and have since been adapted for energy generation, storage, and transfer including solar energy harvesting [19,20,21], molten salt batteries [22,23], nuclear energy reactor fuels [1,24,25], and thermal energy storage systems [20,26]. Because molten salts are readily compatible with various energy sources and production techniques, they also allow for the creation of high-efficiency combined-energy production systems, making them a prime resource for the development of clean, effective, sustainable, and environmentally friendly energy [27]. In these contexts, molten salts are typically used in combinations that have been chosen for the unique physical, chemical, and thermodynamic characteristics that best suit the application and the process conditions (i.e., temperature and the fluid property requirements) [1]. Combined molten salts may incorporate multiple salts from the same class or salts selected from a range of classes. For example, nitrate and nitrite molten salts are widely utilized as high-efficiency heat storage materials in concentrated solar power plants and thermal energy storage systems [4,5,6,7,21]; chloride-based molten salts are used in molten salt reactors and for different alloy heat treatments [8,9,10,19]; and fluoride molten salts are being optimized for nuclear reactor applications [11,12].
In addition to these basic applications for energy technologies, molten salts are also used in the (usually high-temperature) electrodeposition of metal coatings, for which an additional choice of molten salt combination is crucial because the candidates’ physical, thermodynamic, and chemical parameters determine the coating’s final product and properties. In particular, molten salt electrodeposition is one of the most practical methods for creating a coating of refractory metals with higher melting points (~473–1273 K) that are unsuitable for coating in aqueous media at ambient temperatures. In these cases, molten salts broaden the electrochemical window, prevent the release of hydrogen molecules during electrolysis, minimize solvent evaporation, and have desirable thermal stability [28]. These properties are particularly valuable at present because molten salt electrodeposition (including electroplating, electroforming, electrowinning, and electrorefining) techniques offer a relatively inexpensive alternative for creating scalable, industrial-quality metal coatings for various types of alkaline, alkaline earth, transition, and refractory metals [29], enabling the production of coatings with highly specific qualities (e.g., homogeneous, dense, compact, and strongly adherent to the substrate) for a wide variety of alloys and substances [30].
However, there are drawbacks to this promising technology, including low current efficiency, dendritic depositions, inadequate heat balancing within the cell, and corrosion of the electrochemical setup components [28]. In a green energy context, the fact that the electrodeposition’s overpotential cannot cover the window of working potential for molten salt electrodeposition remains an ongoing challenge [31]. To better comprehend and manage the electrochemical deposition, we urgently need a more comprehensive understanding of the deposition mechanisms informed by in-depth research that considers all of the system characteristics [32]. Therefore, this review paper was designed to provide a thorough analysis of the most promising molten salt chemistry materials and molten salt electrodeposition techniques and to more broadly assess the field’s strengths, challenges, and weaknesses and different metal coatings and emergent refractory-metal electrodeposition and its property characterization. We also discuss best practices for addressing safety concerns in laboratories, particularly regarding high-temperature electrochemical systems and, additionally, the progress of the well-established field of molten salt electrodeposition and the molten salt electrodeposition of the non-refractory metals and refractory metals.
2. Molten Salt Overview
Molten salts are important for numerous areas of material science, engineering, and chemistry, and each domain (separately and in conjunction) has developed methods for synthesis, targeting compositions with field-specific parameters and utilities (e.g., electrochemistry, heat transmission, energy applications, nuclear reactors, and chemical oxidation and reduction baths) that take advantage of the categories of properties that make molten salts so desirable:
- Wide thermal stability range and often low vapor pressure allows for high-temperature chemistry with rapid reaction rates.
- High dissolution capacity for a broad range of inorganic compounds such as oxides, carbides, nitrides, and other salts ensure that molten salts make perfect solvents for electrometallurgy, metal coating, by-product treatment, and energy conversion.
- Large potential window between decomposition limits enables the electrowinning of extremely electropositive elements or the synthesis of very electronegative elements [33].
Common mixtures of molten salts (e.g., halides and oxysalts like nitrates, sulfates, silicates, borates, and organic salts) are used in many arenas and, from an engineering standpoint, many molten salt synthesis processes have proven to be dependable, reproducible, durable, and financially viable. However, many new methods are constantly being invented to tune the molten salt’s physical properties (structure, size of the particle, crystallinity, porosity, morphology, texture, and surface area of the material) for emergent needs [34]. Due to the molten salt’s role as a fluid and solvent, its capacity to conduct electricity, its ability to operate at a wider range of temperatures, and its ability to have larger heat transfer capability, all those fields are merged and connected to the conventional function of molten salt [35].
2.1. Properties of Molten Salts
The ionic arrangement of the molten salt is related to its properties, (e.g., melting temperatures, ionicity, density, viscosity) which are largely determined by long-ranged Coulombic interactions [36]. In general, combining two different pure salts with higher melting points can lower the overall melting point. In the phase diagram, a mixture of these two salts creates a eutectic point, which represents the lowest temperature on the liquidus curve in the binary salt system. Typically, the melting temperature of a mixture of two components is lower than that of either pure component, a phenomenon known as melting point depression. Furthermore, the liquidus curve drops as the concentration of impurities increases relative to the pure salt, with each salt acting as an impurity to the other, thus lowering the melting point. For instance, potassium nitrate has a melting point of 607 K and sodium nitrate has a melting temperature of 579 K, but the melting point of their salt mixture is 495 K [37]. The huge number of possible salt combinations allows for massive breadth and tunability of molten salt temperature ranges for many applications.
Molten salts are composed of ions held together by Coulombic interactions. The primary factor determining the ionicity of molten salts is the Coulombic force between the ionic species of their components. A molten salt solution will contain ions in several valence states and (because water does not serve as a solvent), the ions will be in direct contact, held together by Coulombic attractive forces between the cations and anions in the liquid medium. Both the radius of the ion and the ion’s valence state play a significant role in these attractive forces. The presence of powerful attractive forces enhances the ionic strength of the salt [38]. The most fundamental, crucial, and distinctive thermophysical characteristic of a molten salt is its density (i.e., mass of a material per unit volume); however, many variables can impact the density of molten salt, including temperature, type of material, the pressure, the mass, and the volume. Thus, to optimize the rational design of molten salts for commercial applications (where mass and volume are essential variables) and to theoretically calculate molten salt structures (and the correlation and derivation of different thermophysical and thermodynamic properties), we must be able to both measure and precisely control the molten salt’s density [36].
In industrial applications, molten salt viscosity directly impacts pump and distribution-system costs; thus, engineering design calculations for industrial molten salt applications require very precise knowledge of each molten salt’s viscosity. In broad terms, any fluid’s viscosity is a measure of how much the fluid resists deformation at a certain velocity and is likely to have a direct correlation with intermolecular forces and pressure, an inverse correlation with temperature, and a variable correlation with molecular structure.
2.2. Molten Salt Applications
Molten salts are extensively employed in various industrial applications, due to their exceptional properties. These molten substances are widely used, due to their high thermal stability and low vapor pressure, making them perfect for high-temperature chemistry and rapid reaction rates. Additionally, molten salts are capable of dissolving a range of inorganic compounds, including nitrides, oxides, carbides, and other salts, making them useful solvents in metal coating, electrometallurgy, energy conversion and by-product treatment [33]. Molten salts are also essential in the development of energy resources, particularly in the reprocessing of nuclear wastes, which has been a priority for many nations using nuclear energy. To this end, various pyrochemical devices have been investigated that employ molten salt solvents. Moreover, molten salts are seen as a promising pathway towards the development of safer nuclear energy, including nuclear reactors of Generation IV. Recently, thorium-based nuclear reactors have gained attention for their potential to enable continuous waste molten salt processing and enhance safety [33]. Figure 1 illustrates the various applications of molten salts across different fields.
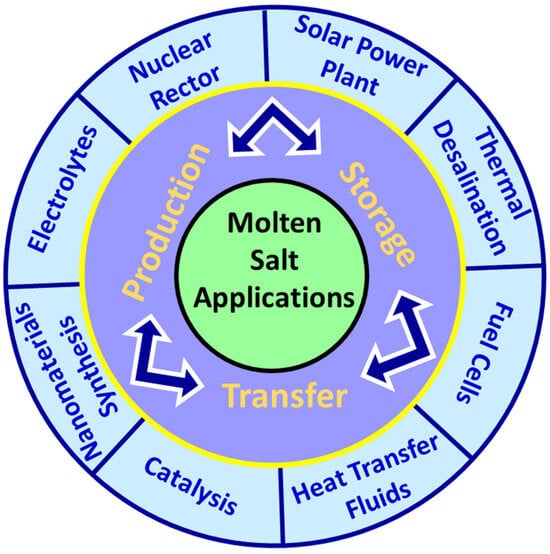
Figure 1.
The schematic diagram of molten salt applications.
Laboratory experiments using fused salt are leading to significant opportunities in various fields. By using fused-salt electrolysis, materials for energy storage devices such as the lithium–hydrogen energy cycle, lithium-and sodium-metal batteries, and silicon electrorefining can be effectively prepared. High-temperature molten salt batteries are also being studied for their potential to store large amounts of energy. Molten salt batteries make use of molten salts as an electrolyte and are suitable for applications that require high power density [39]. These batteries can be a feasible choice for powering electric cars, due to their advantageous characteristics. However, the operating temperatures of up to 973 K can pose significant challenges [39]. Molten salts, similar to molten metals, are utilized in solar energy systems for heat transfer and storage because of their excellent thermal conductivity and heat capacity. Energy storage in solar collectors often involves the use of a mixture of sodium and potassium nitrates. In addition, multicomponent alkali nitrate/nitrite melts are useful for heat transport and storage in solar plants. Molten salts have been widely used in various industries, especially in the surface treatment of tool steels such as boriding, nitrocarburizing, nitriding, and other steel surface-hardening methods. They are also known for their efficient use in heat-treating various metals from ductile iron to high-speed tool steel, as well as non-metals such as rubber plastics, and glass. These applications provide numerous benefits, and as a result, there is a renewed interest in the study of high-temperature ionic liquids’ unique properties [33].
The extraction of aluminum involves dissolving alumina in cryolite, while reactive metals like titanium, magnesium, and sodium are extracted using molten salts, usually chlorides. Molten carbonate fuel cells, which are being developed for various applications, including natural gas-and coal-based powerplants, military, and industrial uses, do not need an external reformer to produce hydrogen, since the fuel is converted into hydrogen within the cell. Molten salts are commonly used in the heat-treating industry for various processes such as annealing, case-hardening, quenching, stress-relieving, tempering, and cleaning. The rate of corrosion in molten salts is typically a type of oxidation, and attacks are usually intensified at the interface between salt and air. The chemical industry utilizes molten salts for processes such as organic and gas synthesis, plastic curing and vulcanizing, in the pyrolysis of hazardous and scrap materials and cracking and catalysis [39]. Also, molten salts have multiple uses as a medium for various chemical reactions including halogenation, semiconductor preparation, and inorganic powders [40]. Innovative molten salt technologies for recovering metals from secondary resources and reserving metals from primary natural sources should be developed. Among these methods, molten salt electrolysis is a cost-effective and environmentally beneficial way of extracting metals from waste. Furthermore, their broad potential window between decomposition limits allows for the electrowinning of highly electropositive elements and the preparation of highly electronegative elements. Surface functional-coating materials made of reactive metals and refractory metals, alloys, and compounds can also be electrodeposited with molten salt.
Molten salt also has exciting applications beyond renewable energy; for example, Gupta has outlined some critical parameters for salts to be regarded as acceptable for the effective synthesis of nanomaterials utilizing molten salts [34]. These requirements include a low melting point, the ability to blend well with reactants, high water solubility so that it can be easily removed after synthesis through washing, good chemical and thermal stability, being non-toxic, inexpensive, and readily available, and having a low vapor pressure to prevent loss during the process. Different salts or salt combinations can be utilized, depending on the individual synthesis circumstances and compatibility [34].
Molten salt also has applications as solvents for etching and electrodeposition for the laboratory [41]. In the research field, especially molten salt electrodeposition, the molten salt system is frequently chosen based on specific research goals. It is important to choose supportive elements that work well with the salt. Salts are kept in crucibles on a modest scale. Stainless steel, graphite, quartz, alumina, glassy carbon, nickel, tungsten, zirconium, tantalum (Ta) and magnesia are a few common materials found in crucibles [41]. The crucible, which serves as the counter electrode in the molten salt electrodeposition, is comprised of electrically conductive materials like glassy carbon, metal or graphite. It is crucial that the crucible material be as chemically inert to the molten salt as is practical. It is imperative that the crucible materials do not end up being the sources of impurities in the salt, and the significance of this criterion cannot be emphasized. There are situations when conical crucibles with tapered walls make it easier to discharge hardened salt than in cylindrical crucibles with straight walls. Salts that have hardened well can be released from crucibles composed of glassy carbon [41].
3. Molten Salt Electrodeposition
During molten salt electrodeposition, high-temperature molten salts are used to deposit a range of metallic coatings, alloys, and compounds. Electrodeposition is generally valued because it can easily be used to coat large objects with complex shapes, using simple and cost-effective equipment. Moreover, the electrodeposition conditions (e.g., current, potential, electrolyte, time) can be adjusted to ensure desired coating composition, structure, and thickness [30]. Nevertheless, molten salts provide two extra benefits when compared to other electrolytes such as aqueous, ionic, and organic ones. First, molten salts can be used at much higher temperatures (423 to 1323 K) [42], enabling the deposition of a greater variety of materials, including the following: reactive metals (e.g., Al, Mg, Ti), refractory metals (e.g., W, Mo and Nb) and compounds (e.g., borides and carbides) [43], transition-metal alloys, light-metal alloys, rare-earth-containing alloys, and metal-containing compounds—all of which can be challenging to electrodeposit using aqueous solutions [30], Secondly, molten salt electrodeposition functions through diffusion, allowing metallurgical bonding between the coating and the substrate, which results in highly adherent coatings [42]. In industry, molten salt electrodeposition is being widely used to produce material coatings that are protective against corrosion [44], high-temperature oxidation [45] and wear [46], or to produce materials with enhanced functional coatings produced through the process of molten salt electrodeposition. These coatings can improve the surface properties of substrates for protection purposes, including corrosion resistance [44], high-temperature oxidation resistance [45] and wear resistance [46] and, additionally, coatings with desirable physical and chemical properties(e.g., optical, ferromagnetic, and catalytic) properties [30]. Several factors need to be considered before setting up the molten salt electrodeposition process, and Figure 2 illustrates the variables involved in this process.
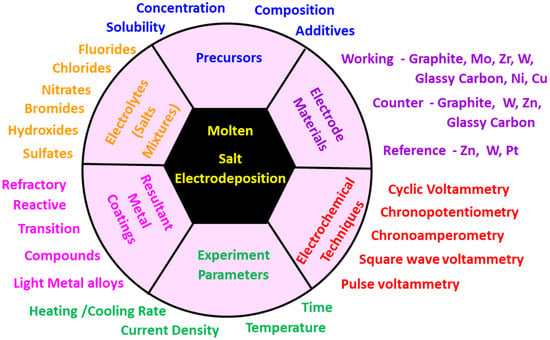
Figure 2.
The schematic diagram of variables in the molten salt electrodeposition.
3.1. Electrochemical Cells
The electrochemical cell is primarily useful for measuring the electrochemical performance of materials and synthesizing material coatings. In an electrochemical cell, chemical reactions potentially initiated by an electrical power source are utilized to convert chemical energy into electrical energy, or the other way around. The efficiency of such devices relies heavily on low Ohmic resistance, minimal interference from surface-active metals, high-rate charge-transfer reactions, and secondary chemical reactions. For instance, the three-electrode cell is a widely used electrochemical-measurement setup that can utilize aqueous, organic, ionic, or molten salt electrolytes. While the electrochemical measurements in molten salt are similar to those in aqueous solutions, taking accurate measurements can be challenging due to the highly corrosive, high-temperature environment; consequently, there are inconsistencies in the published findings that directly result from variations in high-temperature electrochemical measurements. Process control can be used to manipulate crucial parameters (e.g., reversible potentials and diffusion coefficients), in large part because mass-transfer conditions govern the chemical reactions that occur in molten salts. Examining electrodeposition from molten salts through electrochemical methods offers valuable understanding of the anodic and cathodic reactions occurring in the process. This knowledge can aid in the maintenance and control of electrochemical cells, leading to increased process efficiency.
Molten salt electrochemical cells are comprised of two or three electrodes submerged in a molten salt electrolyte. As the cell cycle proceeds, the target material (pure metal, alloy, or compound) is electrodeposited on the electrode surfaces. However, the specific components of the electrochemical cell and the number of electrodes may vary from two (working electrode (WE) and counter electrode (CE)) to three (WE, CE, and reference electrode (RE)), depending on the electrodeposition method (e.g., electroplating, electrowinning, electrorefining, and electroforming) and the desired outcome (e.g., properties, parameters, conditions, and availability). The potentiostat then controls and records the electrochemical measurements. Experimental parameters and conditions can be adjusted to obtain the desired coating thickness, and the electrochemical cell can be subjected to constant or varying current and potential, according to requirements. Several electrochemical-measurement techniques that are frequently used are linear sweep voltammetry, cyclic voltammetry, normal pulse voltammetry, square wave voltammetry, chronopotentiometry, chronoamperometry, differential pulse voltammetry, open-circuit potential and electrochemical impedance spectroscopy. Figure 3 shows the schematic diagram of the electrochemical cell used in the molten salt electrodeposition process.
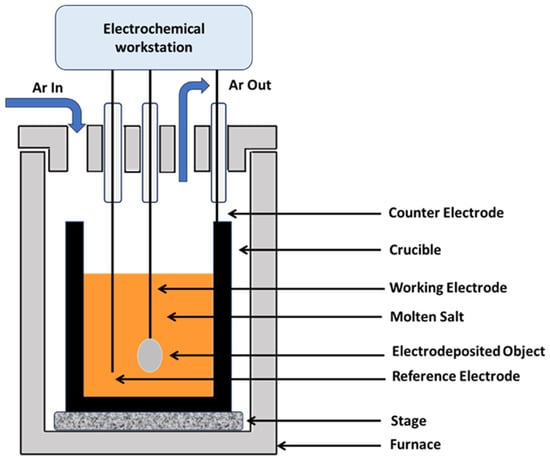
Figure 3.
The schematic diagram of the electrochemical cell.
In order to avoid difficulties in electrochemical experiments and the misreading of data during the process of molten salt electrodeposition, a strong grasp of these principles becomes essential due to the high temperatures at which molten salt systems function. These guidelines encompass various subjects, such as the significance and prerequisites of reference electrodes, measurements of voltage and current, arrangement of electrochemical cells, correct utilization of potentiostats, interpretation of electrochemical data, laboratory protocols, and safety considerations.
3.1.1. Electrodes
Potentiostats typically come with five leads: RE, CE, WE, working sense (WS), and counter sense (CS). However, as the experimental design requires, WS and CS leads can also be used with separate electrodes. The number of electrodes depends on the type of electrodeposition being performed: the three-electrode configuration is sufficient for most electrochemical measurements in molten salts; however, some higher-temperature molten salt electrodeposition techniques can be accomplished with a two-electrode system. In the full five-lead configuration, the three electrodes are separately immersed in the molten salt, while the WE lead is connected to the WS, and the CE lead is connected to the CS outside the furnace.
In recent years, several refinements to the WE have improved the performance of electrochemical measurements in molten salts. For example, because the salt-wetted surface area of the WE is often limited in molten salt electrochemical measurements, a small-diameter wire can be connected to the object that needs to be coated, and then submerged in molten salt. Likewise, although the height of the WE in the salt can be easily adjusted, the selection of the WE material (e.g., graphite, glassy carbon, molybdenum, zirconium, and tungsten) is critical for accurate electrochemical measurements of the specific object being coated. Ideally, the WE material should be inert to the interfacial chemistry because any chemical interactions can shift the potential at which reactions occur. For example, in the case of cathodic potential, it is crucial that the reduced metal and the WE do not form an alloy, which can alter redox potentials. To avoid this problem, binary phase diagrams of candidate metals can be used to determine the possibility of alloy formation. At the same time, the development of a chemically stable, compatible CE that is chemically stable and compatible is still necessary.
Although the RE is not used in all measurements, it can serve a unique purpose. The absolute electrical potential of a point is not meaningful unless it is compared to a reference point (e.g., earth ground or an RE), and the voltage is generally understood to be the difference in electrical potential between those two points. Earth ground has zero potential, and is therefore generally used to stabilize electrical signals and protect electronic equipment in experimental setups; in contrast, RE is generally designed and positioned to minimize potential errors in the voltage calculations, and one advantage of using an RE is that it establishes a stable and unchanging potential on the electrode’s surface, so the circuit does not carry significant current. At present, standard REs have been developed and assigned a potential of zero in both aqueous electrochemistry (the standard hydrogen electrode) and chloride molten salt electrochemistry (the standard chlorine electrode). However, given the complexity of any individual experimental setup, standard REs may be unsuitable, in which case, customized REs must be created. Fortunately, the outputs can be made comparable; for example, if the half-cell reaction at a customized RE is fully thermodynamically characterized, the resulting electrochemical measurements can be converted to values that would have been obtained if a standard RE had been used instead of the customized RE. This kind of conversion is often required when the aim of the experiment is to measure the thermodynamic properties of the half-cell reaction at the WE [47]. Glassy carbon can serve as a pseudo RE which has been demonstrated to provide dependable voltage measurements for short-term measurements (a few hours to two days); unfortunately, over longer durations, the pseudo RE will exhibit dusting and subsequent potential measurement drifts.
Great attention is required when selecting any electrode material (WE, CE, or RE) for gas-evolving molten salt applications, particularly those which include gases that originate from halide salts or dissolved oxides, such as Cl2(g), F2(g), Br2(g), O2(g), CO(g), and CO2(g). The gases produced at the electrode can cause severe corrosion to both the electrode materials and the construction materials of the experimental system, so the preferred materials allow for the construction of “inert anodes”, which minimize corrosion in standard gas-evolving molten salt systems and are consumed (at either acceptable or unacceptable rates) in high-temperature gas-evolving molten salt systems [47].
3.1.2. Electrolyte
For molten salt electrodeposition, the electrolyte necessarily consists of a mixture of salts, although the specific salts in use will vary depending on the desired application, experimental conditions, parameters, and system compatibility. Interestingly, while the fundamental physical and chemical properties of the salts (e.g., density, viscosity, surface tension, heat capacity, and electrical conductivity) may be important for certain engineering applications, for electrochemical measurements they are less important that the optimal characteristics of the electrolyte: high purity, electrical conductivity, and decomposition voltage; low vapor pressure, melting point, and viscosity; and minimal corrosive properties, pollution, and cost. Despite the broad variety of salts and salt mixtures currently available, very few mixtures possess all the desired properties, and many of the desired properties come at a trade-off. For example, the electrochemical measurements for high-purity salts are more accurate and reproducible but will also substantially drive up the cost.
Part of the challenge is maintaining the precursor salts in optimal conditions before they are used for molten salt deposition. For example, at room temperature, highly hygroscopic salts (e.g., alkaline chlorides) will readily react with moisture and oxygen in the air, forming saltwater. Exothermic salts also readily dissolve in water and require extra care during dissolution for molten salt electrodeposition. If moisture or other impurities are introduced, some remediation strategies can be employed to re-purify the precursor salts. For example, salt drying is achieved with a simple application of heat under the purge of an inert gas (e.g., argon), which will dry some salts back to their chloride or fluoride forms. However, drying more-reactive chloride or fluoride salts (e.g., MgCl2 or NaF) will require the addition of a chemical potential (chlorine or fluorine) to prevent the formation of undesirable byproducts (oxychlorides or oxyfluorides). Generally, metals that are more stable as chlorides than as oxides or oxychlorides (e.g., Na as NaCl) can be dried without the need for a chlorine potential. Alternatively, a vacuum-drying approach works for certain precursor salts, particularly alkaline earth metal halides that contain chemically bound water molecules. For example, the application of a moderate dynamic vacuum at a very slow heating rate can effectively remove trace moisture from nominally anhydrous CaCl2 salt. At elevated room temperatures, some salts will readily react with moisture and undergo hydrolysis; chloride salts will hydrolyze to form metal oxides, oxychlorides, and HCl(g); and fluoride salts will hydrolyze to form metal oxides, oxyfluorides, and HF(g) [47].
Some salts can be further purified in a molten state by sparging gas through the molten material; for example, chloride salts can be purified by sparging HCl(g) and Cl2(g), whereas fluoride salts can be purified by sparging HF(g) and F2(g). Unfortunately, this process is slow, taking several hours even for small quantities of salt. Alternatively, reactive metals can be added to the molten salt mixture to reduce the residual content of moisture, oxychlorides, or oxyfluorides. The most appropriate method of salt purification depends on the specific application.
3.1.3. Resulting Electrolytes–After Heat is Applied
During high-temperature electrodeposition processes, complex chemical reactions necessarily occur as the precursor salts melt, blend, and interact with the other materials involved in the electrodeposition process (the electrodes, the object being coated, etc.). However, undesirable chemical reactions can negatively impact the final coating outcome. For example, the loss of feed (due to volatilization, disproportionation, or polymerization) may result in the formation of cluster compounds, and the creation of insoluble oxides, oxyhalides, and stable oxy-cations within the high-temperature molten salt.
- Loss of Materials through Volatilization: the fluoride solvents are much less vulnerable to loss by volatilization (i.e., the unwanted formation of fluoride electrolytes) because fluoride ions will strongly complex the refractory-metal ions of the highest oxidation numbers and, consequently, fluoride ions in the bulk melts are likely to displace chloride ions from the coordination shells of high-oxidation-number refractory-metal ions. For example, it is possible to measure the potential of a Ta electrode as a function of the number of moles of F− ions added; however, interpreting the complex-ion formation is problematic because the Ta(IV) chloride disproportionately reacts into Ta(V) fluoride, causing the Ta(V) to exhibit dynamic equilibria amongst the following species of chlorides and fluorides [48]. The partial pressure in solutions of refractory-metal chlorides suggests that the effects of both the solvent’s anions and its cationic characteristics can be very significant and, at times, even critical. For tantalum chlorides, binary-phase data offer some insights into the relative stability of tantalum chlorides in molten alkali chlorides [49]. These findings suggest that in the case of lithium chloride, the reaction tends to favor the right, leading to unstable solutions where covalency is predominant. In the case of stable Ta(V) solutions in CsCl, the ligand field effect plays a significant role. However, the presence of the Cs+ cation complicates matters by increasing the likelihood of lower oxidation states.
- Disproportionation Reactions: all the refractory metals may exist in a variety of oxidation states. For example, Ta exhibits oxidation states ranging from +5 to −1 [50]. It is also known that tantalum chlorides in the pure state can undergo disproportionation reactions at relatively low temperatures. The influence of solvation on these processes when the pure halide is dissolved in a molten alkali halide is not well established. Two types of disproportionation equilibria are possible in chlorides, for example, using Ta as our example, namely, a homogeneous equilibrium or a heterogeneous equilibrium [50].
- Formation of Clusters: cluster-compound formation or polymerization is a characteristic of the lower states of oxidation of the denser refractory metals [49]. Metal-atom cluster compounds have been manufactured employing molten alkali metal chloride melts [51]; for instance, the Ta cluster complex Ta6Cl184− is generated through a comproportionation reaction in which M = Li, Na, K, Rb, Cs. Temperatures range from 773 K to 1073 K based on M, with the reaction taking place in a closed system to prevent TaCl5 loss. It was obvious that the suitable choice of M+ will influence the preparation’s success [51]. M2RCl6, M3R2Cl9, MR4Cl11 and M4R5Cl18 are all potential polymerized cluster compounds in the molten solution. When R is Nb, a feasible candidate for M is Na+ or K+ [51]. The chemical production of such ions that are complex is likely to be hampered kinetically, while being favored thermodynamically. Because TaCl5 is readily formed on Ta anodes at high potential, it appears that the electrochemical production of clusters is similarly hampered. This could have significant implications for electrodeposition. Because the reaction is inhibited, the synthesis of Ta from TaCl5(g) can be carried out, and progresses at a relatively high current efficiency of 0.85–0.88 [52]. However, Nb production is only possible with a low current efficiency of 0.30, possibly due to the preferred cluster formation processes in the abundance of NaCl in the melt.
- Development of Compound-Based Oxide: oxyhalides, insoluble oxides, and stable oxycations can develop at high temperatures in a molten solution [53]. These oxides are exceedingly tough compounds of refractory materials, and it is critical that they are completely dissolved before electrolysis occurs. Impure materials like alkali-and alkaline-earth metal oxides, on the other hand, might have more subtle impacts. These impurities, for example, can react with lower-valency ions formed during electrodeposition in all-chloride or all-fluoride melts, leading to the production of insoluble coatings on the electrode surfaces. These unanticipated films, which can be oxypolynuclear anions or insoluble oxides, can have negative implications [49]. Metal oxide deposition from stable oxycations may be intrinsic in molten salt electrolysis.
3.2. Strategies for Tuning Electrodeposition
Tuning the molten salt electrodeposition process requires simultaneously accounting for three critical factors: thermodynamics, kinetics, and electrolyte conductivity requirements (i.e., the deposition on the anode). The amount of energy required by an electrochemical reaction depends on various factors, including the cell’s geometry [54], electrode kinetics [55], and the thermodynamics of the overall cell reaction.
3.2.1. Cell Geometry
The aim is to minimize the cell potential for the specific process at hand; however, because the cell potential is affected by the type of cation and solvent used, it must be calculated for each unique case. Crucially, the most efficient mechanisms for cell-potential optimization do not work for all desirable electrodeposited materials. For example, while cell potential is optimized with electrolytes in their lowest oxidation states (e.g., chloride- or oxide-based electrolytes, such as mixed borate phosphate halide), low current operation may not be possible for refractory metals [54,56] (e.g., W and Mo), because the interaction with the solvent tends to result in the undesirable formation of ion and polynuclear complexes. With additional steps, these complexes can be removed, but it may be preferable to use electrolytes with a higher oxidation state [57] (e.g., fluoride-based electrolyte), which have higher oxide solubility, produce coherent plates, and inhibit the formation of polynuclear and ion complexes.
3.2.2. Electrode Kinetics
At elevated temperatures, kinetic limits may appear to be minor; however, anode reactions that involve oxide species and graphite anodes result in complex heterogeneous chemical reactions (depending on the surface features of the electrode) and, thus, a decrease in anodic reaction rate [55]. In contrast, cathodic processes are driven by three key factors: electrolyte mass transport, electron transfer, and crystal nucleation and growth. In molten salt systems at extreme temperatures, electron transmission between metal ions at the surfaces has a fast standard exchange-current density (≥1 Acm−2) [58]. However, the precise mechanism is contested. One theory is that the fast current density is driven by a previous chemical reaction, rather than a delayed electron transfer [59], but a second theory implies that when Mn+ + ne → M is irreversible [60], there may be a related chemical reaction. Importantly, if the high oxidation state of a metal ion complexed to metal decreases, the anionic partners will be shed and atoms will pass from the diffuse multilayer onto the metal electrode, allowing additional metal atoms to incorporate into the diffuse multilayer and the development of a crystal lattice.
Nucleation overvoltage was previously thought to be absent in molten salt processes, but recent observations and discoveries suggest that nucleation overvoltage can be triggered by the structure of the electrical double-layer and specific anionic effects [61]. These double-layer effects may also be responsible for the strong influence of oxide and chloride ions on coherent metal deposition (compared to fluoride melts). Maximum conductivity is seen in fluorides of the lighter alkali and alkaline earth metals (or combinations thereof), which may be preferable for Ohmic overvoltage.
3.2.3. Thermodynamics of Cell Reactions
To accommodate specific reactor designs, the source of thermal power must be optimized. For fused-salt electrolytic reactors with internal Joule heating, the solvents’ strong thermal conductivities and fairly high heat capacities ensure that heat transmission within the reactor can be easily regulated by selecting appropriate electrolyte mass, surface-area-to-reactor-volume ratio, reactor insulation, cell current, electrode geometry, and interelectrode spacing. In contrast, conventional reactors have poor specific areas and are influenced by the proximity of anode reaction products, resulting in lower cathodic-current efficiency.
In molten salt electrolysis, the type of deposition that forms (powder, dendrite, etc.) may be uniquely challenging, for three reasons: first, the solvents used in molten salt electrolysis do not stay in a liquid state when the cathodes are taken out of the melts after electrolysis (i.e., deposits formed in dendritic shapes will block the solidified melt and require a major leaching process to separate the metal from the melt); second, dendritic deposits can destabilize the size of the cathodes, lowering current efficiency and, in severe cases, cell shorting; and third, powdery deposits that result from secondary electrode processes require separation operations, which can introduce impurities due to the high metal-surface-to-volume ratios.
3.3. Advantages and Disadvantages of Molten Salt Synthesis Processes
Molten salts (e.g., carbonates, halides, and sulfates) are selected to optimize the atomic-level reactions that drive macroscale behavior at working temperatures (e.g., fluidity and diffusion rate), which must be optimized for electrodeposition with a potentially wide range of materials, each with unique properties. Compared to traditional material synthesis processes (e.g., hydrothermal, co-precipitation, wet chemical transfer, solid-phase, sol–gel), molten salts offer several advantages: lower synthesis temperature, shorter reaction times, more consistent regulation (of composition, morphology, and powder properties), short heat-preservation times, low cost, environmentally friendly components, and relative ease of use [40]. This section compares molten salt processes with traditional processes used to produce the same materials.
3.3.1. Molten Salt Synthesis (MSS)
Unlike traditional solid-state processes, MSS is liquid and therefore has both high mobility and massive areas of contact between the molecules that make up the liquid molten salt, resulting in faster reaction rates, lower product-formation temperatures, and minimal risk of agglomeration [62]. Moreover, because flux-aided crystal growth occurs in the absence of a thermal gradient, the final products tend to have defect-free highly crystalline structures with well-defined facets, and homogeneous particle sizes and shapes. In practice, MSS can also stabilize incongruent melting and highly nonstoichiometric mixtures.
Given that MSS is also scalable, cost-effective, and easy to conduct, it is unsurprising that MSS has also been extensively utilized in the manufacture of functional materials and, more recently, atomic catalysts (although the technology has yet to advance from gram-scale production and promising laboratory results to full-scale practical application). During MSS, molten salts go through a slow liquefication–ionization–solidification procedure, during which the space confinement effect is essential. Liquid molten salts generate a large dispersion of ionized anions and cations, which act as ion precursors that prevent metal-ion aggregation and, thus, the formation of molecular metal sites on scaffolds. At elevated temperatures, the combination of anions and cations creates a powerful polarization force, destabilizing covalent, ionic, and metallic bonds (achieving high polarities not possible with organic solvents). This directly affects the surface structure of the final material and can be adjusted through facet and defect engineering and atomic catalyst coordination [63]. The exceptional dissolving capacity of molten salts broadens the spectrum of precursors, and the molten salts’ homogenous fluid environment encourages diffusion and convention, allowing for quick mass transfer, decreasing the required reaction temperature, and increasing reactivity, resulting in a short response time. Further, because molten salts congeal into well-homogenized crystals, the MSS process prevents the formation of porous structures that might eventually cause material shrinking or collapse (particularly in carbonaceous materials).
3.3.2. MSS Challenges and Solutions
- Crystal Quality: one of the potential concerns with the MSS is that the molten salts occasionally interact with the reaction vessels. For example, because of their high oxo-basicity, salts like alkali metal hydroxides and PbO are frequently utilized in MSS but tend to corrode Al and Pt reaction containers [64]. The resulting erosion of the surfaces of the Al and Pt reaction vessels could pollute the final material with traces of these elements. Similarly, traces of the molten salt materials may also be included into the final material. For example, flux/salt ions may result in flux inclusions within the material’s crystals (resulting in lower crystal quality) or alter the final crystal structure (resulting in crystals with undesirable performance). To circumvent these problems, the analogue of the common ion effect was investigated by utilizing salts with common ions present in the final desired output.
- Material Costs: most of the salts (e.g., such as KCl and NaCl) commonly used in MSS processes are plentiful and affordable, but the enormous volume of utilized salts is driving up the cost of large-scale nanomaterial manufacturing. Some of the alkali metals (e.g., Li) used in salt compounds are also individually expensive; however, a clever workaround has recently been demonstrated. Because alkali metals are water-soluble, when the metal salts are water-washed off of the material’s surface, they can be recycled. The development of optimized salt-recycling techniques has become increasingly critical for industrialization.
- Safety Hazards: some of the salts commonly used for MSS (e.g., metal fluorides) are human-toxic, and direct exposure may cause skin/eye irritation and damage to human tissue. As a result, caution should be exercised when using fluoride-based molten salts. Some salts, such as AlCl3, are either acidic or alkaline by nature and can induce corrosion. Heavy metal salts, including BaCl2 or BaF2, are known to be toxic to humans. They must be handled with caution and under all safeguards. Unless the reactions are carried out in closed systems, it is best to avoid employing salts with significant vapor pressures that consist of BaCl2, CsI, and ZnCl2. Furthermore, harmful NOx and SOx gases would be created if the salt mixture were heated close to the melting point of the salt. If breathed, they are exceedingly dangerous. As a result, it is preferable to perform the MSS in well-ventilated fume hoods. Personnel executing the reactions should additionally employ masks, goggles, gloves, and other safety equipment to protect themselves from corrosive and hazardous gases.
3.3.3. Molten Salt Electrodeposition
While many molten salts have characteristics that facilitate efficient electrolytic processing, alkali and alkaline earth halide salts mixed with aluminum fluoride have good ionic conductivity, which means that controlled Joule heating can be used to heat the cell contents; however, several critical challenges remain. 1. These solvents have very negative free energies of formation (i.e., the solvent requires a high voltage to enable electrochemical decomposition), which must be reduced by lowering the activity of the deposited solvent metal (e.g., by tuning the solvent cations, the electrode substrate, or both). 2. These solvents typically incur a small cathodic polarization overvoltage, but because mass transfer-controlled processes often result in undesirable dendritic deposits, it is often more effective to expend greater energy to produce a massive and continuous deposit. 3. These solvents are also highly effective at dissolving inorganic materials, but this property can make it challenging to select appropriate electrical and thermal insulating materials for the cell components, and the rate of Joule heat dissipation and the maximum operating temperature required by the electrolyte properties limit the cell current. 4. Although there are generally no chemical reactions between the metal ions and the solvents, given the various oxidation states of the refractory metals, the interactions between the deposited metals and the electrolyte ions must be controlled.
3.3.4. Molten Salt Electrodeposition Challenges and Solutions
- One disadvantage of using molten salts as solvents in electrolytic operations is that the metal deposits produced during electrolysis can be dendritic and/or powdery. The special precautions of the significant leaching process to separate the metal from the melt or avoid the secondary depositions from the melt can be taken.
- Recovering the deposit from the solidified solvent is a process similar to the beneficiation of crude ores, and requires energy-intensive and wasteful leaching, grinding, and flotation operations, followed by suitable consolidation processes such as arc melting or electron-beam melting.
- The hydrolysis of many hygroscopic salts used as electrolytes and solvents can cause problems when used on a large scale, requiring significant peripheral handling facilities.
- The high volatility of many high-valency refractory-metal halides, especially chlorides, and the tendency of their lower-valency halides, especially chlorides, to form insoluble cluster-type compounds. The disproportionation of intermediate-valency-state compounds can also cause issues. Additionally, in the case of the concomitant anodic reaction, energy considerations often conflict with cathode product purity due to the nature of refractory metals and the small scale of operations. Practical limitations can arise from factors such as the corrosion of cell and electrode materials. Material problems can also occur due to the oxidation of lower-valency-state compounds by anode products, such as chlorine, necessitating the use of porous diaphragms, which can be more easily corroded by the melts than the bulk materials due to the large surface areas involved if they are inorganic.
4. Electrodeposition of Metal Coatings
Molten salt electrodeposition is a viable method for obtaining reactive metals, light metals, transition metals, refractory metals, and rare earth metals, as well as their alloys and carbides and borides, which are difficult to deposit from aqueous solutions. Additionally, electrodeposition at high temperatures from molten salts helps create strongly adherent coatings, as the diffusion process allows for metallurgical bonding between the resultant coating and the substrate. Consequently, a diverse range of metals and alloys has been successfully prepared using molten salt electrodeposition. Table 1 provides a summary of the various types of metal coatings obtained through molten salt electrodeposition.
A refractory metal is typically defined as heat- and wear-resistant, with a “high” melting point, but there is currently no consensus on the exact quantification of “high”. However, it does generally indicate that refractory-metal electrodeposition is more feasible in molten salt environments rather than aqueous environments. For example, coherent thick refractory-metal coatings with a dense, fine grain can be formed by dissolving refractory-metal fluoride in alkali metal fluoride combinations. The basic molten salt electrodeposition technique is well understood and has been commercially successful (for Ta electrodeposition) since 20th century. In most cases, a refractive-metal halide or oxide is reduced by an active metal, carbon, or hydrogen (e.g., Ta2O5 reduction by carbon, or WO3 reduction by hydrogen), yielding the electrodeposited material in particulate or partially agglomerated form (e.g., roundels (Ta) or fine powders (Mo, W)). However, one of the ongoing challenges has been to ensure desirable purity for a range of final materials and material structures (powders, dendrites, crystals, etc.), which has driven research into new techniques for refractory-metal electrodeposition, including electrolytic extraction and refining technologies. New techniques are also being developed from a deep fundamental understanding of electrochemistry. For example, during the electroreduction of complex metal ions that have multiple valence states (e.g., refractory metals in molten electrolytes), the simulation of electrode processes can enable several steps (e.g., chloride to chloride-fluoride and oxofluoride) without incurring any additional or unwanted reactions. Kuznetsov’s recent study found that, as the melt’s basicity increases, the complex electroreduction processes transition from reversible to irreversible discharge processes. Thus, increasing the melt’s basicity decreases the number of stages required for the electroreduction process to form metal, and increases the number of electrons transferred in a single electrochemical stage [65]. Additionally, changing the anion composition of the first coordination sphere and, in some cases, the cation composition of the second coordination sphere can decrease the number of electrode processes required. Understanding the electrode and chemical reactions involved in the electroreduction of refractory metals and the processes of alloy formation can lead to the development of new compounds and functional materials.

Table 1.
Different types of metal coatings from molten salt electrodeposition.
Table 1.
Different types of metal coatings from molten salt electrodeposition.
| Metal/Alloy Deposition | Molten Salt Mixture | Metal Precursor | Temp./K | Electrodes | Refs. | |||
|---|---|---|---|---|---|---|---|---|
| WE | CE | RE | ||||||
| Refractory Metals and Alloys | Mo | EMPyrCl-ZnCl2 | MoCl3, MoCl5 | 473, 423 | Ni | Zn | Zn | [66] |
| W | ZnCl2-NaCl-KCl-KF | WO3 | 523 | Ni | Glassy carbon | Zn | [67] | |
| Mo-W | Me2WO4− Na2MoO4 (Me = Li, Na, K, Rb) | Me2WO4- Na2MoO4 | 1173–1323 | Ni/Steel | Graphite | O2 (Pt)|Na2WO4 −20 mol % WO3 | [68] | |
| Light Metals and Alloys | Al | AlCl3-NaCl-KCl | AlCl3 | 448 | Mild steel | - | - | [69] |
| Mg-Li | LiCl-KCl | Mg | 723 | Mo/Mg | graphite rod | - | [70] | |
| Mg-Li-Zn | LiCl-KCl | MgCl2-ZnCl2 | 943 | Mo | graphite rod | Ag wire|AgCl in LiCl-KCl melts | [71] | |
| Al-Cr-Ni | AlCl3-NaCl-KCl | CrCl2-NiCl2 | 423 | glassy carbon | pure-aluminum plate | Pure-Al rod|AlCl3-NaCl-KCl melt | [72] | |
| Mg-Zn-Li-Ca | LiCl-KCl-MgCl2-ZnCl2-CaCl2 | LiCl-MgCl2-ZnCl2-CaCl2 | 943 | Mo | graphite rod | LiCl-KCl-AgCl (1%) | [73] | |
| Transition Metals and Alloys | Ti | KF-KCl | K3TiF6 | 923 | Ni | Pt | - | [74] |
| Si | KF-KCl | K2SiF6-SiCl4 | 923–1023 | Graphite | Graphite | - | [74] | |
| Ni | LiF-NaF-KF | Nickel-based superalloy Inconel 718 | 923 | Pt | Mo | Pt/graphite rod | [75] | |
| Fe-Si | NaCl-KCl-NaF | SiO2 | - | Steel | - | - | [76] | |
| Co/Zn-Co | zinc chloride-1-ethyl-3-methylimidazolium chloride | ZnCl2-CoCl2 | 353 | W/Cu/Ni/glassy carbon | zinc spiral|ZnCl2-EMIC melt | Zn wire|ZnCl2-EMIC melt | [77] | |
| Rare earth metals and Alloys | Al-Li-Y | LiCl-KCl | AlCl3-Y2O3 | 753 | Mo wire | pure-graphite rod | Ag wire|AgCl in LiCl-KCl melts | [78] |
| Al-Yb | LiCl-KCl | AlCl3- Yb2O3 | 750 | W/Al | pure-graphite rod | Ag wire|AgCl in LiCl-KCl melts | [79] | |
| LaNi5/Ni-S | Na3AlF6-La2O3-Al2O3 | - | 1273 | Ni wire | graphite rod | - | [80] | |
| Sm-Ni | LiCl-KCl | SmCl3 | 723 | Mo/Ni | glassy carbon/Al plate | Ag wire|LiCl-KCl | [81] | |
| Mg-Yb | LiCl-KCl | YbCl3 | 773 | Mg rod | pure-graphite rod | Ag wire|AgCl in LiCl-KCl melts | [82] | |
| Metals containing ceramics | TiB2 | LiF-NaF-KF | K2TiF6-KBF4 | 973 | Mo | - | - | [83] |
| TiB2 | KCl-KF | K2TiF6-KBF4 | 1083 | graphite | - | - | [84] | |
| MgB2 | MgCl2-KCl-NaCl | MgB2O4 | 873 | graphite | graphite rod | - | [85] | |
| Ta2C | LiF-NaF-KF | KeTaF7, K2CO3 | 1023–1050 | Ta/C | Ni | - | [86] | |
| Mo₂C | LiF-NaF-KF | Li2MoO4, K2CO3 | 873 | Pt wire | pyrolytic graphite | - | [87] | |
4.1. Emerging Refractory-Metal Depositions
Many researchers have used high-temperature molten salt electrodeposition to electrodeposit pure refractive metals (e.g., Nb, Mo, W, Ta, and Re) or refractive metal alloys. To give a few representative examples:
4.1.1. Mo Metal Depositions
- Nitta et al. tested a novel molten salt system, N-ethyl-N-methyl-pyrrolidinium chloride (EMPyrCl)-ZnCl2, for Mo electrodeposition at intermediate temperatures. The phase diagram for the EMPyrCl-ZnCl2 system reveals the lowest melting point (318 K) for an equimolar mixture demonstrated, to date; however the actual electrodeposition of a smooth metallic Mo layer on a nickel substrate occurred at higher temperatures, both in an equimolar melt containing MoCl5 and KF (423 K) and in an equimolar melt containing MoCl3 and KF (473 K) [66].
4.1.2. W Metal Depositions
- Nakajima and colleagues demonstrate that WO3 does not dissolve well in a ZnCl2–NaCl–KCl melt at 523 K. However, when KF is added to the melt, the solubility of WO3 increases, enabling the electrodeposition of a dense metallic tungsten film. A potentiostatic electrolysis at 0.06 V versus Zn(II)/Zn produced a smooth, fine (2.5 µm) film of metallic tungsten on a nickel substrate after 6 h of electrolysis, offering a new mechanism for low-temperature W electrodeposition [67].
- Nitta and colleagues attempted to enable thicker W electrodeposition in a ZnCl2-NaCl-KCl-KF-WO3 melt at 523 K. After 6 h in the standard electrolysis procedure [0.08 V vs. Zn(II)/Zn], the current density was reduced from 1.2 mAcm−2 to 0.3 mAcm−2, resulting in a thin W layer (2.1 µm) with a predicted current efficiency of 93%. An ICP-AES and XRD analysis of the melt’s supernatant and bottom salts revealed that the soluble W entities (the WO3F anions) had slowly turned into insoluble entities (ZnWO4 and K2WO2F4). Consequently, adjusting the electrodeposition process to include an additional dose of WO3 every 2 h preserved the original current density and produced a denser (4.2 µm) tungsten film. The intermittent addition of WO3 has also been shown to be beneficial in producing a denser tungsten film [88].
- Nitta’s team also investigated Li2WO4-Na2WO4-K2WO4 and its derived melts at 873K, with the goal of developing a stable method for high-quality W electrodeposition. For the Li2WO4-Na2WO4-K2WO4 melt, galvanostatic electrolysis at 25 mAcm−2 produced a W and tungsten oxide deposit; in the same conditions, the Li2WO4-Na2WO4-K2WO4 -LiCl-NaCl-KCl melt produced a dendritic W, presumably due to the solution’s decreased viscosity. In s repeated attempt to prevent dendrite growth, they first vibrated the electrode during electrodeposition (which produced a deposit coated in big, angular crystal grains) and then added a small amount of KF (which produced shorter crystal grains and a more-uniform surface due to the inhibition of α-W crystal development, allowing for the formation of a combination of α-W and β-W). They also produced a remarkably thick (9.7 µm) W sheet, following 1 h of electrolysis at 25 mAcm−2 [89].
- Jiang and colleagues discovered that they could generate a smooth and compact W coating on a Mo substrate by electrodepositing a molten salt of Na2WO4-WO3 at 1173 K in the presence of air. The W covering had a columnar structure with a growth orientation that was primarily toward (2 0 0). In the Mo substrate, a 2 μm-thick W diffusion layer was created, and the W coating adhered effectively to the substrate. Mutual diffusion between the coating and the substrate was promoted at high temperatures. The adhesion of the W coatings to the Mo substrates was robust. As the cathodic current density increased, the thickness of the W coatings also increased, but the grain size decreased [45].
- Jiang and colleagues were able to deposit pure-W covering with a body-centered cubic structure from molten Na2WO4-WO3-NaPO3 salt in an air atmosphere at 1153K. With a thin W diffusion layer in the Cu substrate, the coatings had an inner layer of tooth-like grains and an exterior layer of columnar grains. The researchers looked at the impact of current density and electrodeposition duration on the morphology and microstructure of the coatings. As the current density increased, the grain size of the W coating also increased. Simultaneously, the coating’s thickness initially increased and then decreased with the rising current density. Furthermore, the preferred orientation of the coatings shifted from (2 2 0) to (2 1 1). Interestingly, this preference for the (2 1 1) orientation remained constant, even as the duration was extended. Direct-current electrodeposition is not suited for thick-W-coating electroplating due to the low current efficiency for lengthy electrodeposition durations [90].
4.1.3. Mo and W Alloys
- Malyshev et al. carried out research to identify the best techniques for Mo-W alloy electrodeposition. They evaluated the impact of molybdenum(VI) oxide content and melt cationic composition on the nature and properties of the cathode product. Experiments were carried out at 1173 K with the cathode the density of current of 5 Adm−2 using melts of Me2WO4-Na2MoO4 (Me = Li, Na, K, and Rb). The researchers discovered that a high MoO3 concentration in the melt caused the formation of molybdenum(VI) oxide, rather than metallic Mo. As the alkali metal cation radius rose in the sequence Li2WO4 < Na2WO4 < K2WO4 < Rb2WO4, the minimum concentration of MoO3 that produced deposition changed from 2.0 to 8.5 mol%. When the MoO3 concentration was less than 1.0 mol.%, Mo-W alloys with 1–5 at.% W contents were deposited at the cathode, regardless of the cationic composition. Metallic Mo was deposited at higher MoO3 concentrations. They discovered that the alloy’s Mo content was raised with MoO3 concentration and diminished with WO3 concentration and cathodic current density [68].
4.1.4. Ta Metal Depositions
- Mehmood and colleagues successfully deposited a solid coating of pure-Ta on W using a LiF-NaF-CaF2 melt that contained 2% K2TaF7. Galvanostatic polarization was used to deposit the Ta. When a high current density of 1.5 kAm−2 was applied, the deposited coating contained some porosity, due to dendritic growth. At very low current densities of 0.15–0.2 kAm−2, nucleation of the deposit was difficult because of a comproportionation reaction, resulting in the lack of deposit on most of the surface. However, the reaction product was soluble and diffused away from the electrode, preventing contamination of the deposit [91].
4.1.5. Ta and W Alloys
- Lee and colleagues investigated coatings of Ta-W (Ta-7.31 W, Ta-4.12 W, and Ta-1.92 W), utilizing a multi-anode reactive-alloy coating technique in molten salt (LiF-NaF-K2TaF7) at various cathode–anode distances. The Ta-4.12 W coating had a corrosion rate in hydriodic acid of less than 0.0087 mm/year at 433 K and a thickness uniformity of 30 ± 0.7 µm. The resultant coating layer was also found to be up to 12.9% harder than a pure-Ta plating layer. When compared to pure Ta, these alloy coating films considerably improved corrosion resistance and mechanical qualities [92].
4.1.6. Re Metal Depositions
Re is considered an excellent high-temperature coating material, and the electrodeposition technology has gained interest in recent years due to its low cost and superior mechanical properties.
- Chernyshev et al. investigated the behavior of Re ions in a molten KF-KBF4-B2O3 salt using a three-electrode electrochemical cell at 773 K. Their research aimed to obtain highly pure metallic rhenium (99.98%) with a filamentous structure at the cathode. The study utilized cyclic voltammetry, stationary galvanostatic, and polarization curve analyses, to determine the reduction and diffusion mechanisms of Re ions within the molten salt. The results showed that the electrodeposition of Re at a constant potential was a one-step reaction from Re(VII) to Re, occurring from two types of complex rhenium ions, KReO4 and K3ReO5 [93].
- Wang et al. conducted a study on the characteristics of Re coatings deposited in ternary melts of NaCl-KCl-CsCl. The coatings were electrodeposited at current densities ranging from 20 to 250 mAcm−2 and temperatures from 973 to 1150 K. The study found that the growth mechanism of the coatings changed from lateral growth with a preferred crystal plane of (002) to outward growth with a preferred crystal plane of (110) as current density increased or temperature decreased. The morphologies of the coatings included faceted grains, dendritic powder, and surface structures resembling pyramids, starfish, and intertwined roots. The researchers identified the optimal deposition-process parameters for producing dense and smooth Re coatings to be a temperature range of 1073–1123 K and a current density of 50–100 mAcm−2 [94].
4.1.7. Nb Metal Depositions
- Chernyshev and colleagues proposed the deposition of high-quality, thick, dense, and adhesive Nb coatings using a CsBr-KBr-NbBr3 melt as an electrolytic solution. When compared to the regularly used fluoride and chloride electrolytic solution, this melt was shown to be less harsh and provide greater rate of deposition of dense Nb layers. The researchers looked at how temperature and cathode current density affected the structure and morphology of the Nb coatings during electrodeposition. The researchers utilized cyclic voltammetry to investigate the cathode process in CsBr-KBr-NbBr3 melts with varying Nb concentrations at temperatures ranging from 893 to 1013 K. A two-stage mechanism involving Nb(III) and Nb(II) ions was hypothesized, and the efficient coefficient of diffusion of Nb cations in bromide melt was determined using the results of cyclic voltammetry and numerical simulation data [95].
- Cheek and colleagues investigated the electrochemical processes of Nb(V) in acidic AlCl3: 1-ethyl-3-methylimidazolium chloride (EMIC) melts in depth. They found that reduction occurs in several one-electron stages that are largely irreversible. The existence of insoluble Nb(IV) species complicates the voltammetry. They discovered that the lowest-valent species that experiences reduction before the commencement of aluminum reduction is Nb(III). When Nb powder is introduced to the acidic AlCl3: EMIC melts, a low-valent species, possibly Nb(III), develops slowly in the melt. Electrodeposition from Nb(V)-containing solutions, on the other hand, is less successful. Earlier reduction of NbCl5 through the addition of Nb powder to acidic AlCl3: EMIC melts, on the other hand, enables the deposition of stable and homogeneous films containing up to 15% Nb [96].
4.2. Characterization of Molten Salt and Refractory Metals
Characterization studies are vital to help researchers gain practical insights into specific materials and establish a relationship between the material’s structure and properties. Many mixtures of solid ionic salts (with various stoichiometries) can be easily characterized at room-temperature traditional techniques (e.g., X-ray diffraction). However, given the fragility of the characterization instruments, structural identification becomes significantly more difficult when the salts are molten. When a molten salt is quenched, the quenching process may modify the liquid structure, so the resulting solid is unlikely to fairly represent the organization of atoms and compounds in the molten salt. Given the translucency of many molten salts, refractory-metal coatings that utilize non-translucent fillers (e.g., cordierite, talc, zircon, and mullite) can be visible during electrodeposition. Important information can also be gleaned after electrodeposition; for example, by discovering how the composition of a deposited material (with regulated rheological properties) is altered when different suspending agents and fillers are used. At that stage, SEM can be used to examine the morphology and microstructure of the refractory metals, and X-ray diffraction can be employed to determine and monitor changes in the metals’ phase composition.
Unfortunately, it is still extremely difficult to conduct in situ characterization to better reveal the structure and dynamics of molten salts. Remarkably, in situ characterization has been demonstrated for fluoride molten salt electrolytes; however, the electrode materials must be able to resist extremely high temperatures and molten salt corrosiveness, while still enabling robust signal detection. This combination of traits is rare and has prompted researchers to start with less-corrosive (e.g., chloride) molten salts.
Due to the many challenges of spectroscopically characterizing molten salts, only a small number of previously demonstrated techniques have been modified for this purpose, primarily Raman, nuclear magnetic resonance (NMR), and extended X-ray absorption fine structure (EXAFS) spectroscopy. However, each of these methods has its own unique restrictions, characteristics, and demands when applied to molten salts. For example, to detect the initial solvation shells of the ionic species, which correspond to the local structures, Raman spectroscopy identifies the vibration modes of molecular ionic species with specified symmetry, NMR detects changes in a nucleus’ magnetic field caused by its neighbors, and EXAFS detects photoelectron diffraction resulting from the surrounding nuclei. As a result, these methodologies complement one another, such that integrating their results may provide a more-precise description of the molten salt’s local structure. The many types of ions that compose the solution determine the spectra of the molten salt, which dramatically complicates the process of identifying the structural characteristics of the molten salt via spectroscopic and diffraction measurements; for example, to specify the local structure of a molten metal halide, it is necessary to specify everything from the first adjacent shell to nanometer-scale correlations. Fortunately, given the prevalence of Coulombic interactions and the establishment of intermediate-range ordering, molten salts display significant organization over extremely long distances. Thus, to analyze the local structure of molten salts, most methodologies offer an average view over a given time span; it is possible to measure the radial distribution function, coordination number, and the production of ionic complexes with a picosecond lifespan; however, the local structures cannot be described without considering longer-ranged correlations.
For many years, Raman spectroscopy was the only technology that could show the local order of molten ionic compounds at extreme temperatures. The vibrational modes of the molecular ionic species that exist within in the melt can be correlated with distinct bands in the Raman spectra, which demonstrate that the melt is, in fact, a mixture of various species, whose ratios will change according to the molten salt’s composition and temperature. The anion dispersion is determined using the band intensities and scattering coefficients that are specific to each type. Conveniently, alkali elements in molten mixtures significantly impact the coordination number distribution, which makes them readily visible in Raman spectra. However, at elevated temperatures, the frequency mode dispersion and disorder cause spectral widening and, thus, substantial overlapping, which makes interpretation challenging. Furthermore, temperature has an enormous impact on band intensities, but it is challenging to maintain the exact consistency of molten-drop size and shape in windowless cells, so investigations are typically carried out at steady temperatures, which limits the outcomes to relative intensity measurements.
In recent years, NMR has proven to be another powerful molten salt technique for studying the local structure surrounding a specific nucleus, whether it is a cation or an anion, without being bound by the disorder prevalent in fluids. The latest developments in NMR at extreme temperatures allow for the examination of a wide number of molten complexes and the proposal of a more comprehensive account of the microscopic structure, including species identification, average coordination, and first-neighbor identification. However, given the quick exchange of distinct bonding arrangements, the NMR spectrum in a melt tends to follow a solitary, narrow, Lorentzian-shaped line, whose placement reflects the weighted average of each contribution. Selective NMR monitoring of the different nuclei in the network provides unique local data that can substantially aid in structure comprehension.
The combination of fine-structure X-ray scattering and extended X-ray absorption diffraction spectroscopy can produce pair distribution functions that explain the local coordination geometry surrounding each ion. These functions have a charge-ordering tendency that remains even at huge separations; however, the data do not imply that these functions should be described in terms of specific molecular structures. Due to the challenges of manipulating the temperature, most high-temperature X-ray absorption spectroscopic experiments on molten salts have been undertaken on chlorides and bromides, with only a handful on fluorides. The difficulty is finding a suitable mixing-weight ratio sample that will not leave the matrix or allow the matrix powder to alter the local structure. Conditioning is ineffective for protecting the sample from reactions with oxides or moisture, and vaporization can cause alterations to the composition in fluoride mixes. However, it has been recently demonstrated that an extra barrier can be added to the cell for radioactive materials to improve airtightness and prevent leaking without impacting signal quality.
It is possible to characterize the deposition of refractory-metal coatings in a molten salt bath using electrochemical techniques (e.g., chronoamperogram or chronopotentiogram of potentiostatic electrolysis, stationary polarization curves, and cyclic voltammogram) or analytical techniques for material characterizations [e.g., scanning electron microscopy (SEM), energy-dispersive X-ray analysis (EDAX), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), and optical emission spectrometry with inductively coupled plasma (ICP-OES)]. SEM can be used to examine the morphology and microstructure of the refractory metal. EDAX can examine metallic materials to reveal the crystalline structures, homogeneous metal distribution, and any additional metal and element inclusions (including percentages and distribution through the sample). XRD aids in the identification of mineral and metal compounds inside a solid material, the determination of deposition purity, the verification of a material composition, and the presence of any other phase components or contaminants. XPS identifies and measures the chemical differences between optically different spots on the metal. ICP-OES detects a wide range of elements in metals and determines which are present in each sample. TEM forms images by passing an electron beam through an extremely thin-metal material and can thus be used to determine the size, shape, and distribution of metal grains.
5. Propensity for Advancement
Molten salts are highly advantageous for heat transfer and energy storage applications (e.g., building heating, and wind power dissipation), due to their ability to operate at higher temperatures, specific heat capacity, thermal stability, low viscosity, saturated vapor pressure, convective heat transfer coefficient, and low cost. However, to realize large-scale preparation and application of molten salt materials, further research is needed to develop multi-component molten salt formulations that can simultaneously encompass multiple desired properties: a wide operating-temperature range, low melting point, stability, thermal conductivity, and low cost.
Over the last few decades, significant advancements have been made in the molten salt electrodeposition of dense pure-metal coatings, light-metal alloys (e.g., Al and Mg), rare earth alloys, transition metal alloys, refractory alloys, and compounds that are exceedingly difficult or impossible to electroplate from aqueous electrolytic media. However, many challenges remain.
- To optimize the quality of materials for increasingly demanding and specific applications, we urgently need more fundamental research into the mechanics of high-temperature molten salt deposition, which necessitates deeper investigation into the interaction between the molten salt coatings’ fabrication variables, characteristics, structures, associated electrochemical processes, and growth mechanisms. The versatility of molten salt electrodeposition is tied to its greater operating-temperature range and wider electrochemical window, but we have much to learn about the specific impacts of the variables, including the molten salt components, operation temperature, electrochemical parameters (e.g., potential, current, time, and electrodeposition mode), and electrochemical techniques (e.g., pulse current, pulse potential, constant current, and constant potential), which all impact the nucleation and growth process, and, thus, the morphology, microstructure, and composition of the final product (e.g., surface quality, depth gradient, imperfections, and stresses). Fundamental investigation of the physical and chemical mechanisms of molten salt electrodeposition will provide the insight required to develop better metal-doping alloys to meet specific application needs, and even provide fresh inspiration for new applications.
- The development of in situ characterization for molten salt electrodeposition is projected to provide a significant contribution to the basic comprehension of electrochemical reactions, processes, and side reactions. This characterization may allow us to expand the existing repertoire of molten salt compounds (metal chlorides, fluorides, nitrates, sulphates, and oxides) with complex (binary, ternary, quaternary, etc.) compositions. whose component ratios are optimized for specific deposition materials and application requirements. Further investigation into the mechanisms of the electrochemical process (including side reactions, unanticipated formations, and inorganic molecular processes) will also enhance our ability to reliably produce high-purity coatings and avoid unwanted reactions.
- Molten salt processes are often volatile and corrosive (especially at the highest temperatures), which can be environmentally damaging and may have a negative effect on electrodes, cell materials, and coatings. We need more research into the corrosion mechanisms of structural materials during molten salt electrodeposition; priority should be given to the investigation of corrosion-resistant materials, recyclable molten salts, and new options for minimal-temperature molten salt deposition.
6. Conclusions
The knowledge and expertise gained in the evolution of molten salt electrodeposition over recent decades will lay a robust groundwork for future advancements in molten salt energy technology and its related applications. Ongoing sustainable research and development in molten salt electrolysis should primarily concentrate on enhancing existing process operations and optimizing the design of electrolytic cells, encompassing electrolytes, electrodes, and cell materials. The aim is to decrease energy consumption, greenhouse gas emissions, and overall costs. Molten salt electrodeposition is still in the experimental stages at the laboratory level, and significant endeavors are necessary before it can be effectively incorporated into practical applications. There is considerable room for improvement, particularly in the development of electrodes and electrolytes in the near future.
Funding
This work was supported by NASA’s Space Technology Mission Directorate (STMD) through the Space Nuclear Propulsion (SNP) project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roper, R.; Harkema, M.; Sabharwall, P.; Riddle, C.; Chisholm, B.; Day, B.; Marotta, P. Molten salt for advanced energy applications: A review. Ann. Nucl. Energy 2022, 169, 108924. [Google Scholar] [CrossRef]
- Smith, G.P.; Pagni, R.M. Homogeneous organic reactions in molten salts. Selected topics. In Molten Salt Chemistry: An Introduction and Selected Applications Book; Springer: Dordrecht, The Netherlands, 1987; pp. 383–404. [Google Scholar]
- Adamson, M.G.; Hsu, P.C.; Hipple, D.L.; Foster, K.G.; Hopper, R.W.; Ford, T.D. Organic waste processing using molten salt oxidation. High Temp. Mater. Process. 1998, 2, 559–580. [Google Scholar]
- D’Aguanno, B.; Karthik, M.; Grace, A.N.; Floris, A. Thermostatic properties of nitrate molten salts and their solar and eutectic mixtures. Sci. Rep. 2018, 8, 10485. [Google Scholar] [CrossRef] [PubMed]
- Sötz, V.A.; Bonk, A.; Forstner, J.; Bauer, T. Molten salt chemistry in nitrate salt storage systems: Linking experiments and modeling. Energy Procedia 2018, 155, 503–513. [Google Scholar] [CrossRef]
- Bradshaw, R.W.; Siegel, N.P. Molten nitrate salt development for thermal energy storage in parabolic trough solar power systems. Energy Sustain. 2008, 43208, 631–637. [Google Scholar]
- Reddy, R.G.; Wang, T.; Mantha, D. Thermodynamic properties of potassium nitrate–magnesium nitrate compound [2KNO3·Mg(NO3)2]. Thermochim. Acta 2012, 531, 6–11. [Google Scholar] [CrossRef]
- Zhao, Y. Molten Chloride Thermophysical Properties, Chemical Optimization, and Purification (No. NREL/TP-5500-78047); National Renewable Energy Lab. (NREL): Golden, CO, USA, 2020.
- Ding, W.; Bonk, A.; Bauer, T. Molten chloride salts for next generation CSP plants: Selection of promising chloride salts & study on corrosion of alloys in molten chloride salts. AIP Conf. Proc. 2019, 2126, 200014. [Google Scholar]
- Williams, T.; Shum, R.; Rappleye, D. Concentration Measurements In Molten Chloride Salts Using Electrochemical Methods. J. Electrochem. Soc. 2021, 168, 123510. [Google Scholar] [CrossRef]
- Sprouster, D.; Zheng, G.; Lee, S.-C.; Olds, D.; Agca, C.; McFarlane, J.; Z, Y.; Khaykovich, B. Molecular Structure and Phase Equilibria of Molten Fluoride Salt with and without Dissolved Cesium: FLiNaK–CsF (5 mol %). ACS Appl. Energy Mater. 2022, 5, 8067–8074. [Google Scholar] [CrossRef]
- Romatoski, R.R.; Hu, L.W. Fluoride salt coolant properties for nuclear reactor applications: A review. Ann. Nucl. Energy 2017, 109, 635–647. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Z.; Wu, Y.; Xu, P.; Ding, Y.; Chang, C.; Ma, C. Performance enhancement of bromide salt by nano-particle dispersion for high-temperature heat pipes in concentrated solar power plants. Appl. Energy 2019, 237, 171–179. [Google Scholar] [CrossRef]
- Percival, S.J.; Lee, R.Y.; Gross, M.M.; Peretti, A.S.; Small, L.J.; Spoerke, E.D. Electrochemistry of the NaI-AlBr3 Molten Salt System: A Redox-Active, Low-Temperature Molten Salt Electrolyte. J. Electrochem. Soc. 2021, 168, 036510. [Google Scholar] [CrossRef]
- Yang, J.; Du, K.; Hu, L.; Wang, D. Scalable Fabrication of Carbon Nanomaterials by Electrochemical Dual-Electrode Exfoliation of Graphite in Hydroxide Molten Salt. Ind. Eng. Chem. Res. 2020, 59, 10010–10017. [Google Scholar] [CrossRef]
- Li, X.; Hao, X.; Zhao, M.; Wu, Y.; Yang, J.; Tian, Y.; Qian, G. Exfoliation of hexagonal boron nitride by molten hydroxides. Adv. Mater. 2013, 25, 2200–2204. [Google Scholar] [CrossRef] [PubMed]
- Prieto, C.; Fereres, S.; Ruiz-Cabañas, F.J.; Rodriguez-Sanchez, A.; Montero, C. Carbonate molten salt solar thermal pilot facility: Plant design, commissioning and operation up to 700 °C. Renew. Energy 2020, 151, 528–541. [Google Scholar] [CrossRef]
- Fereres, S.; Prieto, C.; Giménez-Gavarrell, P.; Rodríguez, A.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Molten carbonate salts for advanced solar thermal energy power plants: Cover gas effect on fluid thermal stability. Sol. Energy Mater. Sol. Cells 2018, 188, 119–126. [Google Scholar] [CrossRef]
- Vignarooban, K.; Xu, X.; Wang, K.; Molina, E.E.; Li, P.; Gervasio, D.; Kannan, A.M. Vapor pressure and corrosivity of ternary metal-chloride molten salt based heat transfer fluids for use in concentrating solar power systems. Appl. Energy 2015, 159, 206–213. [Google Scholar] [CrossRef]
- Bauer, T.; Odenthal, C.; Bonk, A. Molten salt storage for power generation. Chem. Ing. Tech. 2021, 93, 534–546. [Google Scholar] [CrossRef]
- Fernández, A.G.; Cortes, M.; Fuentealba, E.; Pérez, F.J. Corrosion properties of a ternary nitrate/nitrite molten salt in concentrated solar technology. Renew. Energy 2015, 80, 177–183. [Google Scholar] [CrossRef]
- Garcia, B.; Lavallée, S.; Perron, G.; Michot, C.; Armand, M. Room temperature molten salts as lithium battery electrolyte. Electrochim. Acta 2004, 49, 4583–4588. [Google Scholar] [CrossRef]
- Masset, P.; Guidotti, R.A. Thermal activated (thermal) battery technology: Part II. Molten salt electrolytes. J. Power Sources 2007, 164, 397–414. [Google Scholar] [CrossRef]
- Le Brun, C. Molten salts and nuclear energy production. J. Nucl. Mater. 2007, 360, 1–5. [Google Scholar] [CrossRef]
- Dai, Z. Chapter 17—Thorium molten salt reactor nuclear energy system (TMSR). In Molten Salt Reactors and Thorium Energy; Dolan, T.J., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 531–540. [Google Scholar] [CrossRef]
- Song, X.; Zhang, G.; Tan, H.; Chang, L.; Cai, L.; Xu, G.; Deng, Z.; Han, Z. Review on thermophysical properties and corrosion performance of molten salt in high temperature thermal energy storage. IOP Conf. Ser. Earth Environ. Sci. 2020, 474, 052071. [Google Scholar] [CrossRef]
- Sharma, S.; Ivanov, A.S.; Margulis, C.J. A Brief Guide to the Structure of High-Temperature Molten Salts and Key Aspects Making Them Different from Their Low-Temperature Relatives, the Ionic Liquids. J. Phys. Chem. B 2021, 125, 6359–6372. [Google Scholar] [CrossRef] [PubMed]
- Simka, W.; Puszczyk, D.; Nawrat, G. Electrodeposition of metals from non-aqueous solutions. Electrochim. Acta 2009, 54, 5307–5319. [Google Scholar] [CrossRef]
- Popov, K.I.; Djokić, S.S.; Grgur, B.N. Electrodeposition of metals From molten salts. In Fundamental Aspects of Electrometallurgy; Springer: Boston, MA, USA, 2002; pp. 271–289. [Google Scholar]
- Gu, Y.; Liu, J.; Qu, S.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Electrodeposition of alloys and compounds from high-temperature molten salts. J. Alloys Compd. 2017, 690, 228–238. [Google Scholar] [CrossRef]
- Bae, S.-E.; Park, Y.J.; Min, S.K.; Cho, Y.H.; Song, K. Aluminum assisted electrodeposition of europium in LiCl–KCl molten salt. Electrochim. Acta 2010, 55, 3022–3025. [Google Scholar] [CrossRef]
- Martínez, A.M.; Haarberg, G.M.; Castrillejo, Y.; Børresen, B.; Tunold, R. Electrodeposition of Metals from Molten Salts. ECS Proc. Vol. 1999, 1, 624. [Google Scholar] [CrossRef]
- Gaune-Escard, M.; Haarberg, G.M. Molten Salts Chemistry and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Gupta, S.K.; Mao, Y. Recent Developments on Molten Salt Synthesis of Inorganic Nanomaterials: A Review. J. Phys. Chem. C 2021, 125, 6508–6533. [Google Scholar] [CrossRef]
- Gadzuric, S.; Suh, C.; Gaune-Escard, M.; Rajan, K. Extracting information from the molten salt database. Metall. Mater. Trans. A 2006, 37, 3411–3414. [Google Scholar] [CrossRef]
- Iwadate, Y. Chapter 260—Structures and Properties of Rare-Earth Molten Salts. In Handbook on the Physics and Chemistry of Rare Earths; Bünzli, J.-C.G., Pecharsky, V.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 44, pp. 87–168. [Google Scholar]
- Bauer, T.; Laing, D.; Tamme, R. Recent Progress in Alkali Nitrate/Nitrite Developments for Solar Thermal Power Applications. In Molten Salts Chemistry and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 543–553. [Google Scholar] [CrossRef]
- Sato, Y. (Invited) Physical Properties of High Temperature Molten Salts. ECS Trans. 2010, 33, 145. [Google Scholar] [CrossRef]
- Baker, B.A. 1.16—Types of Environments. In Shreir’s Corrosion; Cottis, B., Graham, M., Lindsay, R., Lyon, S., Richardson, T., Scantlebury, D., Stott, H., Eds.; Elsevier: Oxford, UK, 2010; pp. 399–406. [Google Scholar] [CrossRef]
- Xi, X.-L.; Feng, M.; Zhang, L.-W.; Nie, Z.-R. Applications of molten salt and progress of molten salt electrolysis in secondary metal resource recovery. Int. J. Miner. Metall. Mater. 2020, 27, 1599–1617. [Google Scholar] [CrossRef]
- Greene, S.R.; Gehin, J.C.; Holcomb, D.E.; Carbajo, J.J.; Ilas, D.; Cisneros, A.T.; Varma, V.K.; Corwin, W.R.; Wilson, D.F.; Yoder, G.L., Jr.; et al. Pre-Conceptual Design of a Fluoride-Salt-Cooled Small Modular Advanced High-Temperature Reactor (SmAHTR); ORNL/TM-2010/199; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2010.
- Zhao, Y.; VanderNoot, T.J. Electrodeposition of aluminium from nonaqueous organic electrolytic systems and room temperature molten salts. Electrochim. Acta 1997, 42, 3–13. [Google Scholar] [CrossRef]
- Song, J.; Wang, Q.; Zhu, X.; Hou, J.; Jiao, S.; Zhu, H. The influence of fluoride anion on the equilibrium between titanium ions and electrodeposition of titanium in molten fluoride–chloride salt. Mater. Trans. 2014, 55, 1299–1303. [Google Scholar] [CrossRef]
- Ueda, M.; Teshima, T.; Matsushima, H.; Ohtsuka, T. Electroplating of Al-Zr alloys in AlCl3-NaCl-KCl molten salts to improve corrosion resistance of Al. J. Solid State Electrochem. 2015, 19, 3485–3489. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, Y.; Sun, N.; Leng, J. Tungsten coating prepared on molybdenum substrate by electrodeposition from molten salt in air atmosphere. Appl. Surf. Sci. 2015, 327, 432–436. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Xia, W.; Jiang, X.; Gui, C. Microstructure and wear resistance performance of Cu–Ni–Mn alloy based hardfacing coatings reinforced by WC particles. J. Alloys Compd. 2016, 654, 63–70. [Google Scholar] [CrossRef]
- Fredrickson, G.L.; Cao, G.; Tripathy, P.K.; Shaltry, M.R.; Herrmann, S.D.; Yoo, T.-S.; Karlsson, T.; Horváth, D.; Gakhar, R.; Williams, A.N.; et al. Review—Electrochemical Measurements in Molten Salt Systems: A Guide and Perspective. J. Electrochem. Soc. 2019, 166, D645–D659. [Google Scholar] [CrossRef]
- Warren, G.F.; White, S.H.; Inman, D. The Electrochemistry of Tantalum in Fused Chlorides. ECS Proc. Vol. 1976, 1976, 218. [Google Scholar] [CrossRef]
- Warren, G.F. Electrochemistry of Tantalum in Fused Alkali Halides. Ph.D. Thesis, London University Imperial College of Science and Technology, London, UK, 1976. [Google Scholar]
- Barnard, P.A.; Hussey, C.L. Electrochemistry of Tantalum (V) Chloride in the Basic Aluminum Chloride-1-Methyl-3-Ethylimidazolium Chloride Room-Temperature Molten Salt. J. Electrochem. Soc. 1990, 137, 913. [Google Scholar] [CrossRef]
- Koknat, F.W.; Parson, J.A.; Vongvusharintra, A. Metal cluster halide complexes. I. Efficient synthesis of hydrated hexanuclear niobium and tantalum cluster halides M6X14. 8H2O. Inorg. Chem. 1974, 13, 1699–1702. [Google Scholar] [CrossRef]
- Rockenbauer, W. Herstellung von Niob und Tantal durch Schmelzflußelektrolyse. Chem. Ing. Tech. 1969, 41, 159–162. [Google Scholar] [CrossRef]
- Inman, D.; White, S.H. The production of refractory metals by the electrolysis of molten salts; design factors and limitations. J. Appl. Electrochem. 1978, 8, 375–390. [Google Scholar] [CrossRef]
- Fink, C.G.; Ma, C.C. Pure Tungsten Direct from Ore: I. Electrolytic Tungsten from Fused Borax and Fused Phosphate Baths. Trans. Electrochem. Soc. 1943, 84, 33. [Google Scholar] [CrossRef]
- Drossbach, P.; Hashino, T. Die Anodenvörganage bei der Elektrolyse von in Kryolyth gelöster Tonerde II. J. Electrochem. Soc. Jpn. 1965, 33, 229–246. [Google Scholar] [CrossRef]
- Zadra, J.B.; Gomes, J.M. Electrowinning Tungsten and Associated Molybdenum from Scheelite; US Department of the Interior, Bureau of Mines: Washington, DC, USA, 1959; Volume 5554. [Google Scholar]
- Mamantov, G. Molten Salts; Marcel Dekker: New York, NY, USA, 1969. [Google Scholar]
- Senderoff, S.; Mellors, G.W. Electrodeposition of Coherent Deposits of Refractory Metals: VI. Mechanism of Deposition of Molybdenum and Tungsten from Fluoride Melts. J. Electrochem. Soc. 1967, 114, 586. [Google Scholar] [CrossRef]
- Inman, D.; Sethi, R.S.; Spencer, R. The effects of complex ion formation and ionic adsorption on electrode reactions involving metals and metal ions in fused salts. J. Electroanal. Chem. Interfacial Electrochem. 1971, 29, 137–147. [Google Scholar] [CrossRef]
- Inman, D.; Legey, J.C.L.; Spencer, R. The electrochemistry of chromium in molten LiCl+KCl. J. Electroanal. Chem. Interfacial Electrochem. 1975, 61, 289–301. [Google Scholar] [CrossRef]
- Graves, A.D.; Inman, D. Adsorption and the differential capacitance of the electrical double-layer at platinum/halide metal interfaces. Nature 1965, 208, 481–482. [Google Scholar] [CrossRef]
- Zuniga, J.P.; Abdou, M.; Gupta, S.K.; Mao, Y. Molten salt synthesis of complex metal oxide nanoparticles. J. Vis. Exp. JoVE 2018, 140, e58482. [Google Scholar]
- Prieto, G.; Tüysüz, H.; Duyckaerts, N.; Knossalla, J.; Wang, G.-H.; Schüth, F. Hollow Nano- and Microstructures as Catalysts. Chem. Rev. 2016, 116, 14056–14119. [Google Scholar] [CrossRef] [PubMed]
- Hailili, R.; Wang, C.; Lichtfouse, E. Perovskite nanostructures assembled in molten salt based on halogen anions KX (X = F, Cl and Br): Regulated morphology and defect-mediated photocatalytic activity. Appl. Catal. B Environ. 2018, 232, 531–543. [Google Scholar] [CrossRef]
- Kuznetsov, S.A. Electrochemistry of refractory metals in molten salts: Application for the creation of new and functional materials. Pure Appl. Chem. 2009, 81, 1423–1439. [Google Scholar] [CrossRef]
- Nitta, K.; Majima, M.; Inazawa, S.; Kitagawa, K.; Nohira, T.; Hagiwara, R. Electrodeposition of molybdenum from molten salt. SEI Tech. Rev. 2010, 70, 75–78. [Google Scholar]
- Nakajima, H.; Nohira, T.; Hagiwara, R.; Nitta, K.; Inazawa, S.; Okada, K. Electrodeposition of metallic tungsten films in ZnCl2–NaCl–KCl–KF–WO3 melt at 250 °C. Electrochim. Acta 2007, 53, 24–27. [Google Scholar] [CrossRef]
- Malyshev, V.; Gab, A.; Popescu, A.M.; Constantin, V. Electrodeposition of tungsten-molybdenum alloys from tungstate-molybdate melts. Rev. Chem. 2011, 62, 1128–1130. [Google Scholar]
- Izadpanah, S.M.; Aboutalebi, M.R.; Adeli, M. Molten salt electrodeposition of aluminum on mild steel: Effect of process parameters on surface morphology and corrosion properties. Mater. Res. Express 2021, 8, 046518. [Google Scholar] [CrossRef]
- Zhang, M.L.; Chen, Z.; Han, W.; Lv, Y.Z.; Wang, J. Electrochemical formation and phase control of Mg–Li alloys. Chin. Chem. Lett. 2007, 18, 1124–1128. [Google Scholar] [CrossRef]
- Yan, Y.D.; Zhang, M.L.; Xue, Y.; Han, W.; Cao, D.X.; Wei, S.Q. Study on the preparation of Mg–Li–Zn alloys by electrochemical codeposition from LiCl–KCl–MgCl2–ZnCl2 melts. Electrochim. Acta 2009, 54, 3387–3393. [Google Scholar] [CrossRef]
- Ueda, M.; Kigawa, H.; Ohtsuka, T. Co-deposition of Al–Cr–Ni alloys using constant potential and potential pulse techniques in AlCl3–NaCl–KCl molten salt. Electrochim. Acta 2007, 52, 2515–2519. [Google Scholar] [CrossRef]
- Cao, P.; Zhang, M.L.; Han, W.; Yan, Y.D.; Chen, L.J. Electrodeposition of quarternary Mg-Zn-Li-Ca alloys from molten salts. Trans. Nonferrous Met. Soc. China 2013, 3, 861–865. [Google Scholar] [CrossRef]
- Norikawa, Y.; Nohira, T. A New Concept of Molten Salt Systems for the Electrodeposition of Si, Ti, and W. Acc. Chem. Res. 2023, 56, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, H.; Yu, D.; Tian, Q. Molten salt electrolysis of spent nickel-based superalloys with liquid cathode for the selective separation of nickel. Sep. Purif. Technol. 2022, 302, 122168. [Google Scholar] [CrossRef]
- Yang, H.L.; Gao, A.M.; Zhang, Y.Z.; Li, Y.G.; Tang, G.Z.; Jia, K.W.; Wang, F.J. Process Optimization and Structural Characterization of Fe3Si Layer by Pulse Electrodeposition. Adv. Mater. Res. 2010, 129, 1201–1205. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Sun, I.W. Electrodeposition of cobalt and zinc cobalt alloys from a lewis acidic zinc chloride-1-ethyl-3-methylimidazolium chloride molten salt. Electrochim. Acta 2001, 46, 1169–1177. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Zhang, M.; Han, W.; Tian, Y. Study on electrochemical preparation of Al-Li-Y alloys from Y2O3 in LiCl-KCl-AlCl3 molten salts. J. Rare Earths 2011, 29, 378–382. [Google Scholar] [CrossRef]
- Yan, Y.-D.; Li, X.; Zhang, M.-L.; Xue, Y.; Tang, H.; Han, W.; Zhang, Z.-J. Electrochemical Extraction of Ytterbium and Formation of Al–Yb Alloy from Yb2O3 Assisted by AlCl3 in LiCl–KCl Melt. J. Electrochem. Soc. 2012, 159, D649. [Google Scholar] [CrossRef]
- Han, Q.; Liu, K.; Chen, J.; Wei, X. Preparation of the composite LaNi3.7Al1.3/Ni–S–Co alloy film and its HER activity in alkaline medium. Int. J. Hydrogen Energy 2009, 34, 71–76. [Google Scholar] [CrossRef]
- Iida, T.; Nohira, T.; Ito, Y. Electrochemical formation of Sm–Ni alloy films in a molten LiCl–KCl–SmCl3 system. Electrochim. Acta 2001, 46, 2537–2544. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, K.; Zhang, M. Preparation of Mg-Yb alloy film by electrolysis in the molten LiCl-KCl-YbCl3 system at low temperature. J. Rare Earths 2010, 28, 128–133. [Google Scholar] [CrossRef]
- Li, J.; Li, B. Preparation of the TiB2 coatings by electroplating in molten salts. Mater. Lett. 2007, 61, 1274–1278. [Google Scholar] [CrossRef]
- Bing, L.I.; Zhou, F.; Qiu, Z. Preparation of TiB2 coated cathode of Al electrolysis. J. Mater. Sci. Technol. 2001, 17, 171. [Google Scholar]
- Abe, H.; Yoshii, K.; Nishida, K.; Imai, M.; Kitazawa, H. Electroplating of the superconductive boride MgB2 from molten salts. J. Phys. Chem. Solids 2005, 66, 406–409. [Google Scholar] [CrossRef]
- Stern, K.H.; Gadomski, S.T. Electrodeposition of Tantalum Carbide Coatings from Molten Salts. J. Electrochem. Soc. 1983, 130, 300. [Google Scholar] [CrossRef]
- Topor, D.C.; Selman, J.R. Molten Salt Electrodeposition of Refractory Metal Carbide: II. Mechanism and Nucleation Studies. J. Electrochem. Soc. 1993, 140, 352. [Google Scholar] [CrossRef]
- Nitta, K.; Nohira, T.; Hagiwara, R.; Majima, M.; Inazawa, S. Electrodeposition of tungsten from ZnCl2–NaCl–KCl–KF–WO3 melt and investigation on tungsten species in the melt. Electrochim. Acta 2010, 55, 1278–1281. [Google Scholar] [CrossRef]
- Nitta, K.; Majima, M.; Inazawa, S.; Nohira, T.; Hagiwara, R. Electrodeposition of tungsten from Li2WO4-Na2WO4-K2WO4 based Melts. Electrochemistry 2009, 77, 621–623. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, Y.; Sun, N.; Liu, Z.a. Effect of direct current density on microstructure of tungsten coating electroplated from Na2WO4-WO3-NaPO3 system. Appl. Surf. Sci. 2014, 317, 867–874. [Google Scholar] [CrossRef]
- Mehmood, M.; Kawaguchi, N.; Maekawa, H.; Sato, Y.; Yamamura, T.; Kawai, M.; Kikuchi, K.; Furusaka, M. Compact coating of tantalum on tungsten prepared by molten salt electrodeposition. Mater. Trans. 2003, 44, 1659–1662. [Google Scholar] [CrossRef][Green Version]
- Lee, Y.J.; Park, D.J.; Kang, K.S.; Bae, G.G.; Han, M.H.; Lee, J.H. Molten Salt Multi-Anode Reactive Alloy Coating (Marc) of Ta-W Alloy on Sus316l. In Proceedings of the 8th Pacific Rim International Congress on Advanced Materials and Processing; Springer: Cham, Switzerland, 2016; pp. 1975–1981. [Google Scholar] [CrossRef]
- Chernyshev, A.A.; Arkhipov, S.P.; Apisarov, A.P.; Shmygalev, A.S.; Isakov, A.V.; Zaikov, Y.P. Rhenium Electrodeposition and Its Electrochemical Behavior in Molten KF-KBF4-B2O3-KReO4. Materials 2022, 15, 8679. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.a.; Wang, J.; Wang, Z.; Ye, Y.; Bai, S.; Wan, H.; Li, S.; Tang, Y. Morphologies and textures of rhenium coatings electrodeposited in chloride molten salts. Surf. Coat. Technol. 2021, 428, 127887. [Google Scholar] [CrossRef]
- Chernyshev, A.; Apisarov, A.; Shmygalev, A.; Pershin, P.; Kosov, A.; Grishenkova, O.; Isakov, A.; Zaikov, Y. Electrodeposition of Niobium from the CsBr-KBr-NbBr3 Melt. J. Electrochem. Soc. 2021, 168, 072501. [Google Scholar] [CrossRef]
- Cheek, G.T.; Hugh, G.; Trulove, P.C. Electrodeposition of niobium and tantalum from a room-temperature molten salt system. ECS Proc. Vol. 1999, 1999, 527. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).