Overview of the Recent Findings in the Perovskite-Type Structures Used for Solar Cells and Hydrogen Storage
Abstract
1. Introduction
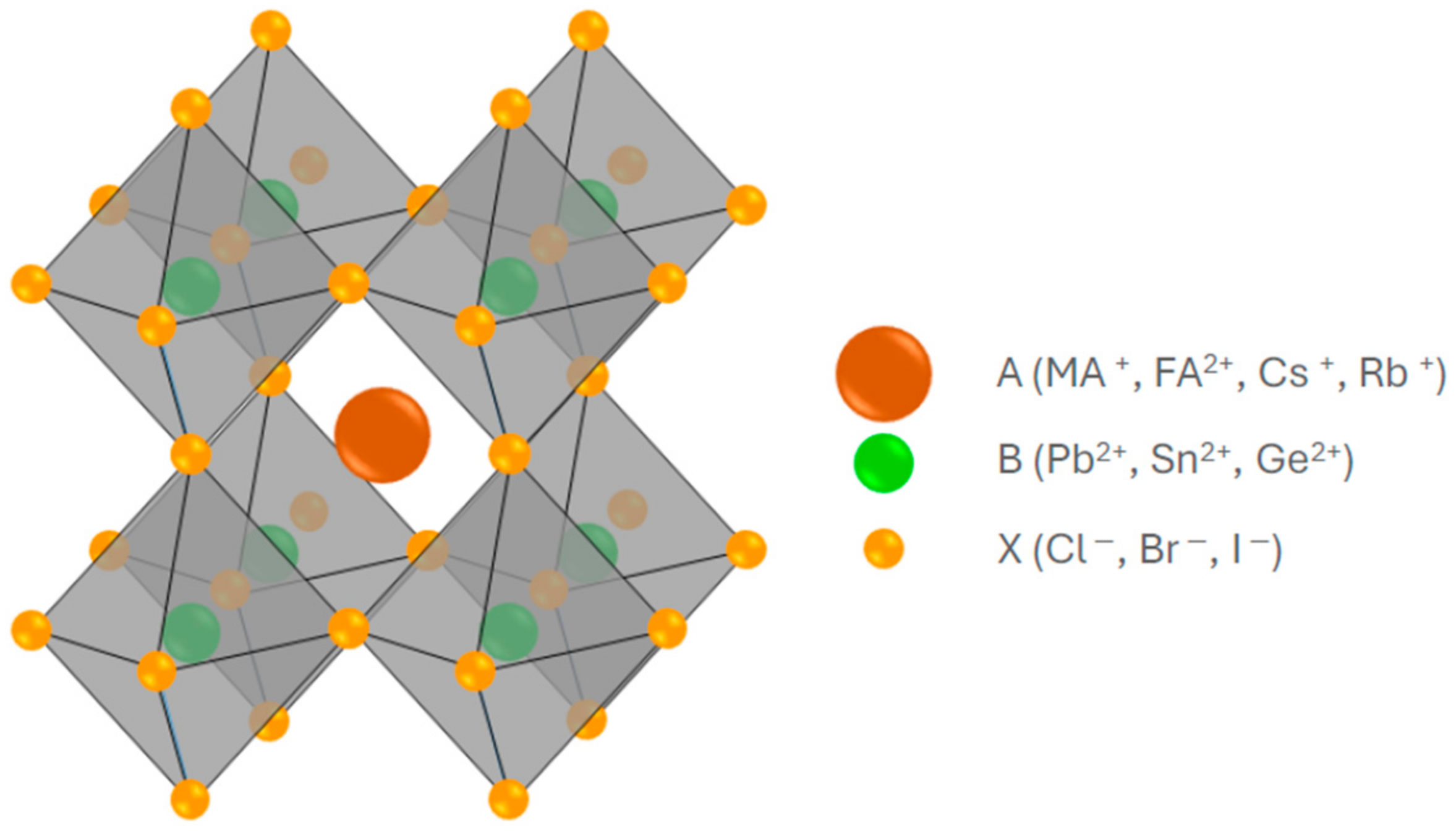
2. Perovskite Materials Used in Photovoltaic
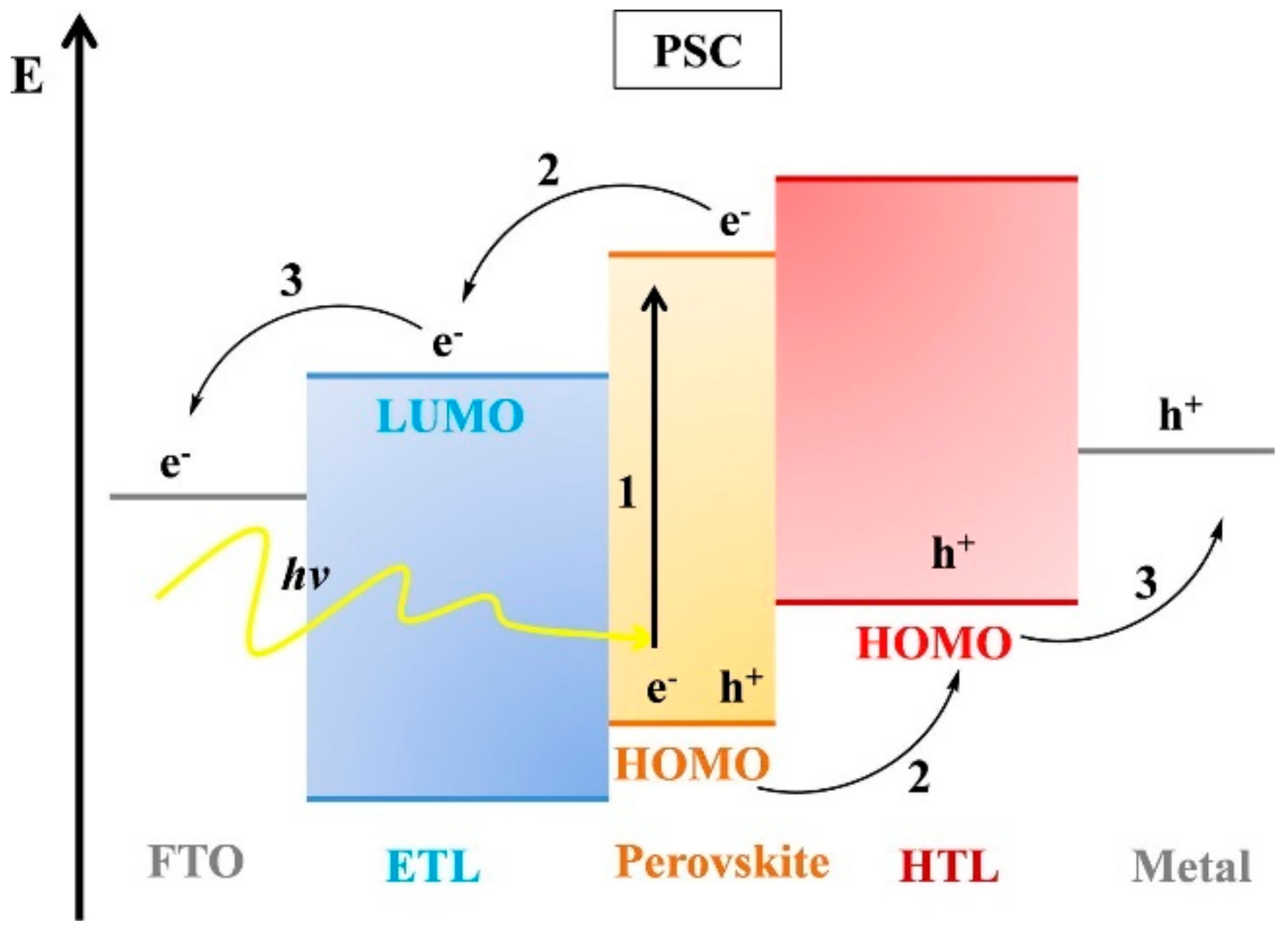
2.1. Perovskite Solar Cells
2.2. Other Solar Cells Systems Utilizing Properties of Perovskite Materials
3. Perovskite-Type Hydrogen Storage—Overview
3.1. Perovskite-Type Hydride
3.2. Perovskite-Type Oxides
4. Perovskite-Type Hydrides Structural, Thermodynamic, and Hydrogen Storage Properties
5. Perovskite-Type Oxides Structural, Thermodynamic, and Hydrogen Storage Properties
6. Future Trends
7. Conclusion Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Ipsakis, D.; Voutetakis, S.; Seferlis, P.; Stergiopoulos, F.; Elmasides, C. Power Management Strategies for a Stand-Alone Power System Using Renewable Energy Sources and Hydrogen Storage. Int. J. Hydrogen Energy 2009, 34, 7081–7095. [Google Scholar] [CrossRef]
- Kane, M. Small Hybrid Solar Power System. Energy 2003, 28, 1427–1443. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamaridarkrim, F.; Hirscher, M. Metal Hydride Materials for Solid Hydrogen Storage: A Review☆. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Endo, N.; Goshome, K.; Tetsuhiko, M.; Segawa, Y.; Shimoda, E.; Nozu, T. Thermal Management and Power Saving Operations for Improved Energy Efficiency within a Renewable Hydrogen Energy System Utilizing Metal Hydride Hydrogen Storage. Int. J. Hydrogen Energy 2021, 46, 262–271. [Google Scholar] [CrossRef]
- Muellerlanger, F.; Tzimas, E.; Kaltschmitt, M.; Peteves, S. Techno-Economic Assessment of Hydrogen Production Processes for the Hydrogen Economy for the Short and Medium Term. Int. J. Hydrogen Energy 2007, 32, 3797–3810. [Google Scholar] [CrossRef]
- Darmawi; Sipahutar, R.; Bernas, S.M.; Imanuddin, M.S. Renewable Energy and Hydropower Utilization Tendency Worldwide. Renew. Sustain. Energy Rev. 2013, 17, 213–215. [Google Scholar] [CrossRef]
- Maradin, D. Advantages and Disadvantages of Renewable Energy Sources Utilization. Int. J. Energy Econ. Policy 2021, 11, 176–183. [Google Scholar] [CrossRef]
- Shetty, C.; Priyam, A. A Review on Tidal Energy Technologies. Mater. Today Proc. 2022, 56, 2774–2779. [Google Scholar] [CrossRef]
- Marques Lameirinhas, R.A.; Torres, J.P.N.; De Melo Cunha, J.P. A Photovoltaic Technology Review: History, Fundamentals and Applications. Energies 2022, 15, 1823. [Google Scholar] [CrossRef]
- Ajayan, J.; Nirmal, D.; Mohankumar, P.; Saravanan, M.; Jagadesh, M.; Arivazhagan, L. A Review of Photovoltaic Performance of Organic/Inorganic Solar Cells for Future Renewable and Sustainable Energy Technologies. Superlattices Microstruct. 2020, 143, 106549. [Google Scholar] [CrossRef]
- Mahmood, Q.; Nazir, G.; Bouzgarrou, S.; Aljameel, A.I.; Rehman, A.; Albalawi, H.; Haq, B.U.; Ghrib, T.; Mera, A. Study of New Lead-Free Double Perovskites Halides Tl2TiX6 (X = Cl, Br, I) for Solar Cells and Renewable Energy Devices. J. Solid State Chem. 2022, 308, 122887. [Google Scholar] [CrossRef]
- Chapin, D.M.; Fuller, C.S.; Pearson, G.L. A New Silicon P-n Junction Photocell for Converting Solar Radiation into Electrical Power. J. Appl. Phys. 1954, 25, 676–677. [Google Scholar] [CrossRef]
- Dannenberg, T.; Vollmer, J.; Passig, M.; Scheiwe, C.; Brunner, D.; Pediaditakis, A.; Jäger, U.; Wang, I.; Xie, W.; Xu, S.; et al. Past, Present, and Future Outlook for Edge Isolation Processes in Highly Efficient Silicon Solar Cell Manufacturing. Sol. RRL 2023, 7, 2200594. [Google Scholar] [CrossRef]
- Fraas, L.M.; O’Neill, M.J. History of Solar Cell Development. In Low-Cost Solar Electric Power; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–12. ISBN 978-3-031-30811-6. [Google Scholar]
- Saga, T. Advances in Crystalline Silicon Solar Cell Technology for Industrial Mass Production. NPG Asia Mater. 2010, 2, 96–102. [Google Scholar] [CrossRef]
- Mercaldo, L.V.; Delli Veneri, P. Silicon Solar Cells: Materials, Technologies, Architectures. In Solar Cells and Light Management; Elsevier: Amsterdam, The Netherlands, 2020; pp. 35–57. ISBN 978-0-08-102762-2. [Google Scholar]
- Andreani, L.C.; Bozzola, A.; Kowalczewski, P.; Liscidini, M.; Redorici, L. Silicon Solar Cells: Toward the Efficiency Limits. Adv. Phys. X 2019, 4, 1548305. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Kawasaki, H.; Yoshida, W.; Irie, T.; Konishi, K.; Nakano, K.; Uto, T.; Adachi, D.; Kanematsu, M.; Uzu, H.; et al. Silicon Heterojunction Solar Cell with Interdigitated Back Contacts for a Photoconversion Efficiency over 26%. Nat. Energy 2017, 2, 17032. [Google Scholar] [CrossRef]
- Kim, S.; Quy, H.V.; Bark, C.W. Photovoltaic Technologies for Flexible Solar Cells: Beyond Silicon. Mater. Today Energy 2021, 19, 100583. [Google Scholar] [CrossRef]
- Kokkonen, M.; Talebi, P.; Zhou, J.; Asgari, S.; Soomro, S.A.; Elsehrawy, F.; Halme, J.; Ahmad, S.; Hagfeldt, A.; Hashmi, S.G. Advanced Research Trends in Dye-Sensitized Solar Cells. J. Mater. Chem. A 2021, 9, 10527–10545. [Google Scholar] [CrossRef]
- Schmidt, J.; Lim, B.; Walter, D.; Bothe, K.; Gatz, S.; Dullweber, T.; Altermatt, P.P. Impurity-Related Limitations of next-Generation Industrial Silicon Solar Cells. In Proceedings of the 2012 IEEE 38th Photovoltaic Specialists Conference (PVSC) PART 2, Austin, TX, USA, 3–8 June 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 1–5. [Google Scholar]
- He, D.; Zeng, M.; Zhang, Z.; Bai, Y.; Xing, G.; Cheng, H.; Lin, Y. Exciton Diffusion and Dissociation in Organic and Quantum-dot Solar Cells. SmartMat 2023, 4, e1176. [Google Scholar] [CrossRef]
- Yi, J.; Zhang, G.; Yu, H.; Yan, H. Advantages, Challenges and Molecular Design of Different Material Types Used in Organic Solar Cells. Nat. Rev. Mater. 2023, 9, 46–62. [Google Scholar] [CrossRef]
- Prajapat, K.; Dhonde, M.; Sahu, K.; Bhojane, P.; Murty, V.; Shirage, P.M. The Evolution of Organic Materials for Efficient Dye-Sensitized Solar Cells. J. Photochem. Photobiol. C Photochem. Rev. 2023, 55, 100586. [Google Scholar] [CrossRef]
- Koné, K.E.; Bouich, A.; Soucase, B.M.; Soro, D. Manufacture of Different Oxides with High Uniformity for Copper Zinc Tin Sulfide (CZTS) Based Solar Cells. J. Mol. Graph. Model. 2023, 121, 108448. [Google Scholar] [CrossRef] [PubMed]
- Aftab, S.; Hussain, S.; Kabir, F.; Aslam, M.; Rajpar, A.H.; Al-Sehemi, A.G. Advances in Flexible Perovskite Solar Cells: A Comprehensive Review. Nano Energy 2024, 120, 109112. [Google Scholar] [CrossRef]
- Manser, J.S.; Christians, J.A.; Kamat, P.V. Intriguing Optoelectronic Properties of Metal Halide Perovskites. Chem. Rev. 2016, 116, 12956–13008. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, M.V.; Protesescu, L.; Bodnarchuk, M.I. Properties and Potential Optoelectronic Applications of Lead Halide Perovskite Nanocrystals. Science 2017, 358, 745–750. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Shrestha, S.; Li, X.; Tsai, H.; Hou, C.-H.; Huang, H.-H.; Ghosh, D.; Shyue, J.-J.; Wang, L.; Tretiak, S.; Ma, X.; et al. Long Carrier Diffusion Length in Two-Dimensional Lead Halide Perovskite Single Crystals. Chem 2022, 8, 1107–1120. [Google Scholar] [CrossRef]
- Miyata, A.; Mitioglu, A.; Plochocka, P.; Portugall, O.; Wang, J.T.-W.; Stranks, S.D.; Snaith, H.J.; Nicholas, R.J. Direct Measurement of the Exciton Binding Energy and Effective Masses for Charge Carriers in Organic–Inorganic Tri-Halide Perovskites. Nat. Phys. 2015, 11, 582–587. [Google Scholar] [CrossRef]
- Hansen, K.R.; McClure, C.E.; Powell, D.; Hsieh, H.; Flannery, L.; Garden, K.; Miller, E.J.; King, D.J.; Sainio, S.; Nordlund, D.; et al. Low Exciton Binding Energies and Localized Exciton–Polaron States in 2D Tin Halide Perovskites. Adv. Opt. Mater. 2022, 10, 2102698. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Huo, X.; Wei, D.; Meng, J.; Dong, J.; Qiao, B.; Zhao, S.; Xu, Z.; Song, D. Bandgap Tuning Strategy by Cations and Halide Ions of Lead Halide Perovskites Learned from Machine Learning. RSC Adv. 2021, 11, 15688–15694. [Google Scholar] [CrossRef]
- Tan, Q.; Li, Z.; Luo, G.; Zhang, X.; Che, B.; Chen, G.; Gao, H.; He, D.; Ma, G.; Wang, J.; et al. Inverted Perovskite Solar Cells Using Dimethylacridine-Based Dopants. Nature 2023, 620, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Bai, Y.; Huang, X.; Li, J.; Wu, Y.; Chen, Y.; Li, K.; Niu, X.; Li, N.; Liu, G.; et al. Anion–π Interactions Suppress Phase Impurities in FAPbI3 Solar Cells. Nature 2023, 623, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Kumar Sinha, N.; Tiwari, S.; Khare, A. A Review on Perovskite Solar Cells: Evolution of Architecture, Fabrication Techniques, Commercialization Issues and Status. Sol. Energy 2020, 198, 665–688. [Google Scholar] [CrossRef]
- Meng, L.; You, J.; Yang, Y. Addressing the Stability Issue of Perovskite Solar Cells for Commercial Applications. Nat. Commun. 2018, 9, 5265. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, R.; Priya, S.; Liu, S. (Frank) Recent Advances in Flexible Perovskite Solar Cells: Fabrication and Applications. Angew. Chem. Int. Ed. 2019, 58, 4466–4483. [Google Scholar] [CrossRef]
- Liang, J.; Wang, C.; Wang, Y.; Xu, Z.; Lu, Z.; Ma, Y.; Zhu, H.; Hu, Y.; Xiao, C.; Yi, X.; et al. All-Inorganic Perovskite Solar Cells. J. Am. Chem. Soc. 2016, 138, 15829–15832. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Guo, W.; Duan, T.; Zhou, Z.; Zhou, Y. All-Inorganic Perovskite Solar Cells Featuring Mixed Group IVA Cations. Nanoscale 2023, 15, 7249–7260. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent Engineering for High-Performance Inorganic–Organic Hybrid Perovskite Solar Cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, S.; Zhu, M.; Li, Z.; Cheng, Z.; Xiang, S.; Zhang, Z. The Role of Grain Boundaries in Organic–Inorganic Hybrid Perovskite Solar Cells and Its Current Enhancement Strategies: A Review. Energy Environ. Mater. 2024, 7, e12696. [Google Scholar] [CrossRef]
- Rao, C.N.R. Perovskites. In Encyclopedia of Physical Science and Technology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 707–714. ISBN 978-0-12-227410-7. [Google Scholar]
- King-Smith, R.D.; Vanderbilt, D. First-Principles Investigation of Ferroelectricity in Perovskite Compounds. Phys. Rev. B 1994, 49, 5828–5844. [Google Scholar] [CrossRef]
- Zhang, H.; Li, N.; Li, K.; Xue, D. Structural Stability and Formability of ABO3-Type Perovskite Compounds. Acta Crystallogr. B 2007, 63, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Bartel, C.J.; Sutton, C.; Goldsmith, B.R.; Ouyang, R.; Musgrave, C.B.; Ghiringhelli, L.M.; Scheffler, M. New Tolerance Factor to Predict the Stability of Perovskite Oxides and Halides. Sci. Adv. 2019, 5, eaav0693. [Google Scholar] [CrossRef] [PubMed]
- Surucu, G.; Gencer, A.; Candan, A.; Gullu, H.H.; Isik, M. CaXH3 (X = Mn, Fe, Co) Perovskite-Type Hydrides for Hydrogen Storage Applications. Int. J. Energy Res. 2020, 44, 2345–2354. [Google Scholar] [CrossRef]
- Schouwink, P.; Ley, M.B.; Tissot, A.; Hagemann, H.; Jensen, T.R.; Smrčok, Ľ.; Černý, R. Structure and Properties of Complex Hydride Perovskite Materials. Nat. Commun. 2014, 5, 5706. [Google Scholar] [CrossRef] [PubMed]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Bannikov, V.V.; Shein, I.R.; Ivanovskii, A.L. Electronic Structure, Chemical Bonding and Elastic Properties of the First Thorium-Containing Nitride Perovskite TaThN3. Phys. Status Solidi RRL Rapid Res. Lett. 2007, 1, 89–91. [Google Scholar] [CrossRef]
- Guo, P.; Ye, Q.; Yang, X.; Zhang, J.; Xu, F.; Shchukin, D.; Wei, B.; Wang, H. Surface & Grain Boundary Co-Passivation by Fluorocarbon Based Bifunctional Molecules for Perovskite Solar Cells with Efficiency over 21%. J. Mater. Chem. A 2019, 7, 2497–2506. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P.; et al. Efficient Perovskite Solar Cells via Improved Carrier Management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Szabó, G.; Park, N.-G.; De Angelis, F.; Kamat, P.V. Are Perovskite Solar Cells Reaching the Efficiency and Voltage Limits? ACS Energy Lett. 2023, 8, 3829–3831. [Google Scholar] [CrossRef]
- Paik, M.J.; Kim, Y.Y.; Kim, J.; Park, J.; Seok, S.I. Ultrafine SnO2 Colloids with Enhanced Interface Quality for High-Efficiency Perovskite Solar Cells. Joule 2024, 8, 2073–2086. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Liu, Z.; Huang, J.; Ma, X.; Liu, Y.; Sun, Q.; Dai, L.; Ahmad, S.; Shen, Y.; et al. Progress and Challenges Toward Effective Flexible Perovskite Solar Cells. Nano-Micro Lett. 2023, 15, 206. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kim, D.H.; Lee, Y.-Y.; Shin, H.-W.; Han, G.S.; Hong, J.S.; Mahmood, K.; Ahn, T.K.; Joo, Y.-C.; Hong, K.S.; et al. Highly Efficient and Bending Durable Perovskite Solar Cells: Toward a Wearable Power Source. Energy Environ. Sci. 2015, 8, 916–921. [Google Scholar] [CrossRef]
- Chung, J.; Shin, S.S.; Hwang, K.; Kim, G.; Kim, K.W.; Lee, D.S.; Kim, W.; Ma, B.S.; Kim, Y.-K.; Kim, T.-S.; et al. Record-Efficiency Flexible Perovskite Solar Cell and Module Enabled by a Porous-Planar Structure as an Electron Transport Layer. Energy Environ. Sci. 2020, 13, 4854–4861. [Google Scholar] [CrossRef]
- Paik, M.J.; Yoo, J.W.; Park, J.; Noh, E.; Kim, H.; Ji, S.-G.; Kim, Y.Y.; Seok, S.I. SnO2—TiO2 Hybrid Electron Transport Layer for Efficient and Flexible Perovskite Solar Cells. ACS Energy Lett. 2022, 7, 1864–1870. [Google Scholar] [CrossRef]
- Gencer, A.; Surucu, G.; Al, S. MgTiO3Hx and CaTiO3Hx Perovskite Compounds for Hydrogen Storage Applications. Int. J. Hydrogen Energy 2019, 44, 11930–11938. [Google Scholar] [CrossRef]
- Ikeda, K.; Kogure, Y.; Nakamori, Y.; Orimo, S. Formation Region and Hydrogen Storage Abilities of Perovskite-Type Hydrides. Prog. Solid State Chem. 2007, 35, 329–337. [Google Scholar] [CrossRef]
- Schmidt, J.; Pettersson, L.; Verdozzi, C.; Botti, S.; Marques, M.A.L. Crystal Graph Attention Networks for the Prediction of Stable Materials. Sci. Adv. 2021, 7, eabi7948. [Google Scholar] [CrossRef]
- Jain, I.P. Hydrogen the Fuel for 21st Century. Int. J. Hydrogen Energy 2009, 34, 7368–7378. [Google Scholar] [CrossRef]
- Veziroğlu, T.N.; Şahi˙n, S. 21st Century’s Energy: Hydrogen Energy System. Energy Convers. Manag. 2008, 49, 1820–1831. [Google Scholar] [CrossRef]
- Ball, M.; Wietschel, M. The Future of Hydrogen—Opportunities and Challenges. Int. J. Hydrogen Energy 2009, 34, 615–627. [Google Scholar] [CrossRef]
- Subramanian, B.; Ismail, S. Production and Use of HHO Gas in IC Engines. Int. J. Hydrogen Energy 2018, 43, 7140–7154. [Google Scholar] [CrossRef]
- Sopena, C.; Diéguez, P.M.; Sáinz, D.; Urroz, J.C.; Guelbenzu, E.; Gandía, L.M. Conversion of a Commercial Spark Ignition Engine to Run on Hydrogen: Performance Comparison Using Hydrogen and Gasoline. Int. J. Hydrogen Energy 2010, 35, 1420–1429. [Google Scholar] [CrossRef]
- Zhang, Y.; Campana, P.E.; Lundblad, A.; Yan, J. Comparative Study of Hydrogen Storage and Battery Storage in Grid Connected Photovoltaic System: Storage Sizing and Rule-Based Operation. Appl. Energy 2017, 201, 397–411. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Wolverton, C.; Siegel, D.J. High Capacity Hydrogenstorage Materials: Attributes for Automotive Applications and Techniques for Materials Discovery. Chem. Soc. Rev. 2010, 39, 656–675. [Google Scholar] [CrossRef]
- Murray, L.J.; Dincă, M.; Long, J.R. Hydrogen Storage in Metal–Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1294. [Google Scholar] [CrossRef]
- Liu, P.; Liang, J.; Xue, R.; Du, Q.; Jiang, M. Ruthenium Decorated Boron-Doped Carbon Nanotube for Hydrogen Storage: A First-Principle Study. Int. J. Hydrogen Energy 2019, 44, 27853–27861. [Google Scholar] [CrossRef]
- Raza, H.H.; Murtaza, G.; Umm-e-Hani; Muhammad, N.; Ramay, S.M. First-Principle Investigation of XSrH3 (X = K and Rb) Perovskite-Type Hydrides for Hydrogen Storage. Int. J. Quantum Chem. 2020, 120, e26419. [Google Scholar] [CrossRef]
- Bouhadda, Y.; Boudouma, Y.; Fennineche, N.; Bentabet, A. Ab Initio Calculations Study of the Electronic, Optical and Thermodynamic Properties of NaMgH3, for Hydrogen Storage. J. Phys. Chem. Solids 2010, 71, 1264–1268. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, Y.; Ding, Z. Research Progress and Application Prospects of Solid-State Hydrogen Storage Technology. Molecules 2024, 29, 1767. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.Y.; Lim, K.L.; Tseng, Y.S.; Chan, S.L.I. A Review on the Characterization of Hydrogen in Hydrogen Storage Materials. Renew. Sustain. Energy Rev. 2017, 79, 1122–1133. [Google Scholar] [CrossRef]
- Xu, N.; Chen, Y.; Chen, S.; Li, S.; Zhang, W. First-Principles Investigation for the Hydrogen Storage Properties of XTiH3 (X = K, Rb, Cs) Perovskite Type Hydrides. Int. J. Hydrogen Energy 2024, 50, 114–122. [Google Scholar] [CrossRef]
- Kojima, Y. Hydrogen Storage Materials for Hydrogen and Energy Carriers. Int. J. Hydrogen Energy 2019, 44, 18179–18192. [Google Scholar] [CrossRef]
- Züttel, A. Hydrogen Storage Methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Kumar, V.; Singh, S.K. High Capacity Hydrogen Storage: Basic Aspects, New Developments and Milestones. Nano Energy 2012, 1, 566–589. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-Storage Materials for Mobile Applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Broom, D.P. Hydrogen Storage Materials: The Characterisation of Their Storage Properties; Green Energy and Technology; Springer London: London, UK, 2011; ISBN 978-0-85729-220-9. [Google Scholar]
- Ikeda, K.; Sato, T.; Orimo, S. Perovskite-Type Hydrides—Synthesis, Structures and Properties. Int. J. Mater. Res. 2008, 99, 471–479. [Google Scholar] [CrossRef]
- Young, K.; Ng, K.; Bendersky, L. A Technical Report of the Robust Affordable Next Generation Energy Storage System-BASF Program. Batteries 2016, 2, 2. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Bernardes, A.M.; Tenório, J.A.S. Spent NiMH Batteries: Characterization and Metal Recovery through Mechanical Processing. J. Power Sources 2006, 160, 1465–1470. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Nakamura, T.; Shibata, E.; Iizuka, A. Adaptation of Minerals Processing Operations for Lithium-Ion (LiBs) and Nickel Metal Hydride (NiMH) Batteries Recycling: Critical Review. Miner. Eng. 2013, 45, 4–17. [Google Scholar] [CrossRef]
- Mohtadi, R.; Orimo, S. The Renaissance of Hydrides as Energy Materials. Nat. Rev. Mater. 2016, 2, 16091. [Google Scholar] [CrossRef]
- Nie, T.; Fang, Z.; Ren, X.; Duan, Y.; Liu, S. Recent Advances in Wide-Bandgap Organic–Inorganic Halide Perovskite Solar Cells and Tandem Application. Nano-Micro Lett. 2023, 15, 70. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.S.; Patil, J.V.; Shao, J.-Y.; Zhong, Y.-W.; Rondiya, S.R.; Dzade, N.Y.; Hong, C.K. Phase-Heterojunction All-Inorganic Perovskite Solar Cells Surpassing 21.5% Efficiency. Nat. Energy 2023, 8, 989–1001. [Google Scholar] [CrossRef]
- Tong, J.; Jiang, Q.; Zhang, F.; Kang, S.B.; Kim, D.H.; Zhu, K. Wide-Bandgap Metal Halide Perovskites for Tandem Solar Cells. ACS Energy Lett. 2021, 6, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Eperon, G.E.; Hörantner, M.T.; Snaith, H.J. Metal Halide Perovskite Tandem and Multiple-Junction Photovoltaics. Nat. Rev. Chem. 2017, 1, 0095. [Google Scholar] [CrossRef]
- Ou, Q.; Bao, X.; Zhang, Y.; Shao, H.; Xing, G.; Li, X.; Shao, L.; Bao, Q. Band Structure Engineering in Metal Halide Perovskite Nanostructures for Optoelectronic Applications. Nano Mater. Sci. 2019, 1, 268–287. [Google Scholar] [CrossRef]
- Liu, X.; Luo, D.; Lu, Z.-H.; Yun, J.S.; Saliba, M.; Seok, S.I.; Zhang, W. Stabilization of Photoactive Phases for Perovskite Photovoltaics. Nat. Rev. Chem. 2023, 7, 462–479. [Google Scholar] [CrossRef]
- Simenas, M.; Gagor, A.; Banys, J.; Maczka, M. Phase Transitions and Dynamics in Mixed Three- and Low-Dimensional Lead Halide Perovskites. Chem. Rev. 2024, 124, 2281–2326. [Google Scholar] [CrossRef]
- Saski, M.; Sobczak, S.; Ratajczyk, P.; Terlecki, M.; Marynowski, W.; Borkenhagen, A.; Justyniak, I.; Katrusiak, A.; Lewiński, J. Unprecedented Richness of Temperature- and Pressure-Induced Polymorphism in 1D Lead Iodide Perovskite. Small 2024, 2403685. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical Management for Colorful, Efficient, and Stable Inorganic–Organic Hybrid Nanostructured Solar Cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-H.; Anaya, M.; Stranks, S.D. Multisource Vacuum Deposition of Methylammonium-Free Perovskite Solar Cells. ACS Energy Lett. 2020, 5, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yousefi Amin, A.A.; Wu, L.; Cao, M.; Zhang, Q.; Ameri, T. Perovskite Nanocrystals: Synthesis, Stability, and Optoelectronic Applications. Small Struct. 2021, 2, 2000124. [Google Scholar] [CrossRef]
- Prochowicz, D.; Saski, M.; Yadav, P.; Grätzel, M.; Lewiński, J. Mechanoperovskites for Photovoltaic Applications: Preparation, Characterization, and Device Fabrication. Acc. Chem. Res. 2019, 52, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Diodati, S.; Nodari, L.; Natile, M.M.; Caneschi, A.; De Julián Fernández, C.; Hoffmann, C.; Kaskel, S.; Lieb, A.; Di Noto, V.; Mascotto, S.; et al. Coprecipitation of Oxalates: An Easy and Reproducible Wet-Chemistry Synthesis Route for Transition-Metal Ferrites. Eur. J. Inorg. Chem. 2014, 2014, 875–887. [Google Scholar] [CrossRef]
- Yu, J.; Ran, R.; Zhong, Y.; Zhou, W.; Ni, M.; Shao, Z. Advances in Porous Perovskites: Synthesis and Electrocatalytic Performance in Fuel Cells and Metal–Air Batteries. Energy Environ. Mater. 2020, 3, 121–145. [Google Scholar] [CrossRef]
- Assirey, E.A.R. Perovskite Synthesis, Properties and Their Related Biochemical and Industrial Application. Saudi Pharm. J. 2019, 27, 817–829. [Google Scholar] [CrossRef]
- Prochowicz, D.; Yadav, P.; Saliba, M.; Kubicki, D.J.; Tavakoli, M.M.; Zakeeruddin, S.M.; Lewiński, J.; Emsley, L.; Grätzel, M. One-Step Mechanochemical Incorporation of an Insoluble Cesium Additive for High Performance Planar Heterojunction Solar Cells. Nano Energy 2018, 49, 523–528. [Google Scholar] [CrossRef]
- Al Amin, N.R.; Lee, C.-C.; Huang, Y.-C.; Shih, C.-J.; Estrada, R.; Biring, S.; Kuo, M.-H.; Li, C.-F.; Huang, Y.-C.; Liu, S.-W. Achieving a Highly Stable Perovskite Photodetector with a Long Lifetime Fabricated via an All-Vacuum Deposition Process. ACS Appl. Mater. Interfaces 2023, 15, 21284–21295. [Google Scholar] [CrossRef]
- Yin, W.-J.; Yang, J.-H.; Kang, J.; Yan, Y.; Wei, S.-H. Halide Perovskite Materials for Solar Cells: A Theoretical Review. J. Mater. Chem. A 2015, 3, 8926–8942. [Google Scholar] [CrossRef]
- Afre, R.A.; Pugliese, D. Perovskite Solar Cells: A Review of the Latest Advances in Materials, Fabrication Techniques, and Stability Enhancement Strategies. Micromachines 2024, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.M.; Butler, K.T.; Brivio, F.; Hendon, C.H.; Van Schilfgaarde, M.; Walsh, A. Atomistic Origins of High-Performance in Hybrid Halide Perovskite Solar Cells. Nano Lett. 2014, 14, 2584–2590. [Google Scholar] [CrossRef] [PubMed]
- Berhe, T.A.; Su, W.-N.; Chen, C.-H.; Pan, C.-J.; Cheng, J.-H.; Chen, H.-M.; Tsai, M.-C.; Chen, L.-Y.; Dubale, A.A.; Hwang, B.-J. Organometal Halide Perovskite Solar Cells: Degradation and Stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Marinova, N.; Valero, S.; Delgado, J.L. Organic and Perovskite Solar Cells: Working Principles, Materials and Interfaces. J. Colloid Interface Sci. 2017, 488, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Desoky, M.M.H.; Bonomo, M.; Buscaino, R.; Fin, A.; Viscardi, G.; Barolo, C.; Quagliotto, P. Dopant-Free All-Organic Small-Molecule HTMs for Perovskite Solar Cells: Concepts and Structure–Property Relationships. Energies 2021, 14, 2279. [Google Scholar] [CrossRef]
- Marchioro, A.; Teuscher, J.; Friedrich, D.; Kunst, M.; Van De Krol, R.; Moehl, T.; Grätzel, M.; Moser, J.-E. Unravelling the Mechanism of Photoinduced Charge Transfer Processes in Lead Iodide Perovskite Solar Cells. Nat. Photonics 2014, 8, 250–255. [Google Scholar] [CrossRef]
- Rong, Y.; Hu, Y.; Mei, A.; Tan, H.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H. Challenges for Commercializing Perovskite Solar Cells. Science 2018, 361, eaat8235. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Yun, H.-S.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled Growth of Perovskite Layers with Volatile Alkylammonium Chlorides. Nature 2023, 616, 724–730. [Google Scholar] [CrossRef]
- Chavan, R.D.; Wolska-Pietkiewicz, M.; Prochowicz, D.; Jędrzejewska, M.; Tavakoli, M.M.; Yadav, P.; Hong, C.K.; Lewiński, J. Organic Ligand-Free ZnO Quantum Dots for Efficient and Stable Perovskite Solar Cells. Adv. Funct. Mater. 2022, 32, 2205909. [Google Scholar] [CrossRef]
- Sani, S.; Usman, A.; Bhatranand, A.; Jiraraksopakun, Y.; Muhammad, K.S.; Yahaya, U. A Study on Defect, Doping, and Performance of ETLs (ZnO, TiO2, and IGZO) for the Lead-Free CsSnCl3 Perovskite Solar Cell by SCAPS-1D Framework. Mater. Today Commun. 2024, 38, 107575. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; et al. Perovskite Solar Cells with Atomically Coherent Interlayers on SnO2 Electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Anaraki, E.H.; Kermanpur, A.; Steier, L.; Domanski, K.; Matsui, T.; Tress, W.; Saliba, M.; Abate, A.; Grätzel, M.; Hagfeldt, A.; et al. Highly Efficient and Stable Planar Perovskite Solar Cells by Solution-Processed Tin Oxide. Energy Environ. Sci. 2016, 9, 3128–3134. [Google Scholar] [CrossRef]
- Dong, Q.; Li, J.; Shi, Y.; Chen, M.; Ono, L.K.; Zhou, K.; Zhang, C.; Qi, Y.; Zhou, Y.; Padture, N.P.; et al. Improved SnO2 Electron Transport Layers Solution-Deposited at Near Room Temperature for Rigid or Flexible Perovskite Solar Cells with High Efficiencies. Adv. Energy Mater. 2019, 9, 1900834. [Google Scholar] [CrossRef]
- Rombach, F.M.; Haque, S.A.; Macdonald, T.J. Lessons Learned from Spiro-OMeTAD and PTAA in Perovskite Solar Cells. Energy Environ. Sci. 2021, 14, 5161–5190. [Google Scholar] [CrossRef]
- Yekani, R.; Wang, H.; Ghamari, P.; Bessette, S.; Sharir-Smith, J.; Gauvin, R.; Demopoulos, G.P. Impact of Photo-Induced Doping of Spiro-OMeTAD as HTL on Perovskite Solar Cell Hysteresis Dynamics. J. Phys. Chem. C 2024, 128, 710–722. [Google Scholar] [CrossRef]
- Bi, H.; Fujiwara, Y.; Kapil, G.; Tavgeniene, D.; Zhang, Z.; Wang, L.; Ding, C.; Sahamir, S.R.; Baranwal, A.K.; Sanehira, Y.; et al. Perovskite Solar Cells Consisting of PTAA Modified with Monomolecular Layer and Application to All-Perovskite Tandem Solar Cells with Efficiency over 25%. Adv. Funct. Mater. 2023, 33, 2300089. [Google Scholar] [CrossRef]
- Li, W.; Martínez-Ferrero, E.; Palomares, E. Self-Assembled Molecules as Selective Contacts for Efficient and Stable Perovskite Solar Cells. Mater. Chem. Front. 2024, 8, 681–699. [Google Scholar] [CrossRef]
- Yi, Z.; Li, X.; Xiong, Y.; Shen, G.; Zhang, W.; Huang, Y.; Jiang, Q.; Ng, X.R.; Luo, Y.; Zheng, J.; et al. Self-assembled Monolayers (SAMs) in Inverted Perovskite Solar Cells and Their Tandem Photovoltaics Application. Interdiscip. Mater. 2024, 3, 203–244. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Wu, C.-G. Bulk Heterojunction Perovskite–PCBM Solar Cells with High Fill Factor. Nat. Photonics 2016, 10, 196–200. [Google Scholar] [CrossRef]
- Gong, C.; Li, H.; Wang, H.; Zhang, C.; Zhuang, Q.; Wang, A.; Xu, Z.; Cai, W.; Li, R.; Li, X.; et al. Silver Coordination-Induced n-Doping of PCBM for Stable and Efficient Inverted Perovskite Solar Cells. Nat. Commun. 2024, 15, 4922. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Leijtens, T.; Siprova, S.; Schlueter, C.; Hörantner, M.T.; Wang, J.T.-W.; Li, C.-Z.; Jen, A.K.-Y.; Lee, T.-L.; Snaith, H.J. C60 as an Efficient N-Type Compact Layer in Perovskite Solar Cells. J. Phys. Chem. Lett. 2015, 6, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Said, A.A.; Aydin, E.; Ugur, E.; Xu, Z.; Deger, C.; Vishal, B.; Vlk, A.; Dally, P.; Yildirim, B.K.; Azmi, R.; et al. Sublimed C60 for Efficient and Repeatable Perovskite-Based Solar Cells. Nat. Commun. 2024, 15, 708. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Biju, V.P.; Qi, Y.; Chen, W.; Liu, Z. Recent Progress in the Development of High-Efficiency Inverted Perovskite Solar Cells. NPG Asia Mater. 2023, 15, 27. [Google Scholar] [CrossRef]
- Tang, H.; Shen, Z.; Shen, Y.; Yan, G.; Wang, Y.; Han, Q.; Han, L. Reinforcing Self-Assembly of Hole Transport Molecules for Stable Inverted Perovskite Solar Cells. Science 2024, 383, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, M.; Carnevali, V.; Zeng, H.; Zeng, M.; Liu, R.; Lempesis, N.; Eickemeyer, F.T.; Luo, L.; Agosta, L.; et al. Buried-Interface Engineering Enables Efficient and 1960-Hour ISOS-L-2I Stable Inverted Perovskite Solar Cells. Adv. Mater. 2024, 36, 2303869. [Google Scholar] [CrossRef]
- Caprioglio, P.; Smith, J.A.; Oliver, R.D.J.; Dasgupta, A.; Choudhary, S.; Farrar, M.D.; Ramadan, A.J.; Lin, Y.-H.; Christoforo, M.G.; Ball, J.M.; et al. Open-Circuit and Short-Circuit Loss Management in Wide-Gap Perovskite p-i-n Solar Cells. Nat. Commun. 2023, 14, 932. [Google Scholar] [CrossRef]
- Imran, T.; Raza, H.; Aziz, L.; Chen, R.; Liu, S.; Jiang, Z.; Gao, Y.; Wang, J.; Younis, M.; Rauf, S.; et al. High Performance Inverted RbCsFAPbI3 Perovskite Solar Cells Based on Interface Engineering and Defects Passivation. Small 2023, 19, 2207950. [Google Scholar] [CrossRef]
- Cao, F.; Bian, L.; Li, L. Perovskite Solar Cells with High-Efficiency Exceeding 25%: A Review. Energy Mater. Devices 2024, 2, 9370018. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H. Detailed Balance Limit of Efficiency of p–n Junction Solar Cells. Renew. Energy 2018, 2, 35–54. [Google Scholar]
- Jošt, M.; Kegelmann, L.; Korte, L.; Albrecht, S. Monolithic Perovskite Tandem Solar Cells: A Review of the Present Status and Advanced Characterization Methods Toward 30% Efficiency. Adv. Energy Mater. 2020, 10, 1904102. [Google Scholar] [CrossRef]
- Bryant, D.; Greenwood, P.; Troughton, J.; Wijdekop, M.; Carnie, M.; Davies, M.; Wojciechowski, K.; Snaith, H.J.; Watson, T.; Worsley, D. A Transparent Conductive Adhesive Laminate Electrode for High-Efficiency Organic-Inorganic Lead Halide Perovskite Solar Cells. Adv. Mater. 2014, 26, 7499–7504. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.; Buonassisi, T.; Egger, D.A.; Hodes, G.; Kronik, L.; Loo, Y.; Lubomirsky, I.; Marder, S.R.; Mastai, Y.; Miller, J.S.; et al. Hybrid Organic–Inorganic Perovskites (HOIPs): Opportunities and Challenges. Adv. Mater. 2015, 27, 5102–5112. [Google Scholar] [CrossRef] [PubMed]
- Tockhorn, P.; Sutter, J.; Cruz, A.; Wagner, P.; Jäger, K.; Yoo, D.; Lang, F.; Grischek, M.; Li, B.; Li, J.; et al. Nano-Optical Designs for High-Efficiency Monolithic Perovskite–Silicon Tandem Solar Cells. Nat. Nanotechnol. 2022, 17, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, H.; Chu, Z.; Xia, R.; Wen, J.; Mo, Y.; Zhu, H.; Luo, H.; Zheng, X.; Huang, Z.; et al. Reducing Perovskite/C60 Interface Losses via Sequential Interface Engineering for Efficient Perovskite/Silicon Tandem Solar Cell. Adv. Mater. 2024, 36, 2308370. [Google Scholar] [CrossRef]
- Zheng, X.; Kong, W.; Wen, J.; Hong, J.; Luo, H.; Xia, R.; Huang, Z.; Luo, X.; Liu, Z.; Li, H.; et al. Solvent Engineering for Scalable Fabrication of Perovskite/Silicon Tandem Solar Cells in Air. Nat. Commun. 2024, 15, 4907. [Google Scholar] [CrossRef]
- Priyanka, E.; Muchahary, D. Performance Improvement of Perovskite/CIGS Tandem Solar Cell Using Barium Stannate Charge Transport Layer and Achieving PCE of 39% Numerically. Sol. Energy 2024, 267, 112218. [Google Scholar] [CrossRef]
- Yu, D.; Pan, M.; Liu, G.; Jiang, X.; Wen, X.; Li, W.; Chen, S.; Zhou, W.; Wang, H.; Lu, Y.; et al. Electron-Withdrawing Organic Ligand for High-Efficiency All-Perovskite Tandem Solar Cells. Nat. Energy 2024, 9, 298–307. [Google Scholar] [CrossRef]
- Sun, H.; Xiao, K.; Gao, H.; Duan, C.; Zhao, S.; Wen, J.; Wang, Y.; Lin, R.; Zheng, X.; Luo, H.; et al. Scalable Solution-Processed Hybrid Electron Transport Layers for Efficient All-Perovskite Tandem Solar Modules. Adv. Mater. 2024, 36, 2308706. [Google Scholar] [CrossRef]
- Wei, R.; Breite, D.; Song, C.; Gräsing, D.; Ploss, T.; Hille, P.; Schwerdtfeger, R.; Matysik, J.; Schulze, A.; Zimmermann, W. Biocatalytic Degradation Efficiency of Postconsumer Polyethylene Terephthalate Packaging Determined by Their Polymer Microstructures. Adv. Sci. 2019, 6, 1900491. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Qian, J.; Liang, Z.; Wu, C.; Wang, K.; Liu, S.; Yang, D. Thermal Radiation Annealing for Overcoming Processing Temperature Limitation of Flexible Perovskite Solar Cells. Adv. Mater. 2024, 2401236. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Zhang, C.; Ye, T.; Wang, K.; Hou, Y.; Zheng, L.; Priya, S.; Liu, S. Highest-Efficiency Flexible Perovskite Solar Module by Interface Engineering for Efficient Charge-Transfer. Adv. Mater. 2023, 35, 2302484. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Choi, S.J.; Sung, H.; Kim, M.; Song, J.W.; Choi, I.W.; Kim, H.-B.; Jo, Y.; Lee, S.; Yoon, S.-Y.; et al. High-Performance and Selective Semi-Transparent Perovskite Solar Cells Using 3D-Structured FTO. Renew. Energy 2024, 222, 119817. [Google Scholar] [CrossRef]
- Jin, Y.; Feng, H.; Fang, Z.; Yang, L.; Liu, K.; Deng, B.; Chen, J.; Chen, X.; Zhong, Y.; Yang, J.; et al. Stabilizing Semi-Transparent Perovskite Solar Cells with a Polymer Composite Hole Transport Layer. Nano Res. 2023, 17, 1500–1507. [Google Scholar] [CrossRef]
- Yoo, J.J.; Lee, J.-W.; Ju, Y.; Kang, B.J.; Kim, Y.; Kim, B.-S.; Kim, Y.Y.; Shin, S.S.; Shin, T.J.; Jeon, N.J. Pyrophosphate Interlayer Improves Performance of Semi-Transparent Perovskite Solar Cells. J. Mater. Chem. A 2024, 12, 12126–12133. [Google Scholar] [CrossRef]
- Tu, Y.; Wu, J.; Xu, G.; Yang, X.; Cai, R.; Gong, Q.; Zhu, R.; Huang, W. Perovskite Solar Cells for Space Applications: Progress and Challenges. Adv. Mater. 2021, 33, 2006545. [Google Scholar] [CrossRef]
- Ho-Baillie, A.W.Y.; Sullivan, H.G.J.; Bannerman, T.A.; Talathi, H.P.; Bing, J.; Tang, S.; Xu, A.; Bhattacharyya, D.; Cairns, I.H.; McKenzie, D.R. Deployment Opportunities for Space Photovoltaics and the Prospects for Perovskite Solar Cells. Adv. Mater. Technol. 2022, 7, 2101059. [Google Scholar] [CrossRef]
- Huan, Z.; Zheng, Y.; Wang, K.; Shen, Z.; Ni, W.; Zu, J.; Shao, Y. Advancements in Radiation Resistance and Reinforcement Strategies of Perovskite Solar Cells in Space Applications. J. Mater. Chem. A 2024, 12, 1910–1922. [Google Scholar] [CrossRef]
- Pérez-del-Rey, D.; Dreessen, C.; Igual-Muñoz, A.M.; Van Den Hengel, L.; Gélvez-Rueda, M.C.; Savenije, T.J.; Grozema, F.C.; Zimmermann, C.; Bolink, H.J. Perovskite Solar Cells: Stable under Space Conditions. Sol. RRL 2020, 4, 2000447. [Google Scholar] [CrossRef]
- Kong, L.; Liu, G. Synchrotron-Based Infrared Microspectroscopy under High Pressure: An Introduction. Matter Radiat. Extrem. 2021, 6, 068202. [Google Scholar] [CrossRef]
- Contreras, L.; Mayacela, M.; Bustillos, A.; Rentería, L.; Book, D. Hydrogen Sorption and Rehydrogenation Properties of NaMgH3. Metals 2022, 12, 205. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, W.; Udovic, T.J.; Rush, J.J.; Yildirim, T. Crystal Chemistry of Perovskite-Type Hydride NaMgH3: Implications for Hydrogen Storage. Chem. Mater. 2008, 20, 2335–2342. [Google Scholar] [CrossRef]
- Reshak, A.H.; Shalaginov, M.Y.; Saeed, Y.; Kityk, I.V.; Auluck, S. First-Principles Calculations of Structural, Elastic, Electronic, and Optical Properties of Perovskite-Type KMgH3 Crystals: Novel Hydrogen Storage Material. J. Phys. Chem. B 2011, 115, 2836–2841. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Noréus, D.; Takeshita, H.; Häussermann, U. Hydrides with the Perovskite Structure: General Bonding and Stability Considerations and the New Representative CaNiH3. J. Solid State Chem. 2005, 178, 3381–3388. [Google Scholar] [CrossRef]
- Naskar, A.; Khanal, R.; Choudhury, S. Role of Chemistry and Crystal Structure on the Electronic Defect States in Cs-Based Halide Perovskites. Materials 2021, 14, 1032. [Google Scholar] [CrossRef] [PubMed]
- Mbonu, I.J.; Louis, H.; Chukwu, U.G.; Agwamba, E.C.; Ghotekar, S.; Adeyinka, A.S. Effects of Metals (X = Be, Mg, Ca) Encapsulation on the Structural, Electronic, Phonon, and Hydrogen Storage Properties of KXCl3 Halide Perovskites: Perspective from Density Functional Theory. Int. J. Hydrogen Energy 2024, 50, 337–351. [Google Scholar] [CrossRef]
- Song, F.; Bai, L.; Moysiadou, A.; Lee, S.; Hu, C.; Liardet, L.; Hu, X. Transition Metal Oxides as Electrocatalysts for the Oxygen Evolution Reaction in Alkaline Solutions: An Application-Inspired Renaissance. J. Am. Chem. Soc. 2018, 140, 7748–7759. [Google Scholar] [CrossRef]
- Deng, G.; Chen, Y.; Tao, M.; Wu, C.; Shen, X.; Yang, H.; Liu, M. Electrochemical Properties and Hydrogen Storage Mechanism of Perovskite-Type Oxide LaFeO3 as a Negative Electrode for Ni/MH Batteries. Electrochim. Acta 2010, 55, 1120–1124. [Google Scholar] [CrossRef]
- Ostadebrahim, M.; Moradlou, O. Electrochemical Hydrogen Storage in LaMO3 (M = Cr, Mn, Fe, Co, Ni) Nano-Perovskites. J. Energy Storage 2023, 72, 108284. [Google Scholar] [CrossRef]
- Mohassel, R.; Soofivand, F.; Dawi, E.A.; Shabani-Nooshabadi, M.; Salavati-Niasari, M. ErMnO3/Er2Mn2O7/ZnO/GO Multi-Component Nanocomposite as a Promising Material for Hydrogen Storage: Facile Synthesis and Comprehensive Investigation of Component Roles. J. Energy Storage 2023, 65, 107285. [Google Scholar] [CrossRef]
- Esaka, T.; Sakaguchi, H.; Kobayashi, S. Hydrogen Storage in Proton-Conductive Perovskite-Type Oxides and Their Application to Nickel–Hydrogen Batteries. Solid State Ion. 2004, 166, 351–357. [Google Scholar] [CrossRef]
- Sakaguchi, H.; Hatakeyama, K.; Kobayashi, S.; Esaka, T. Hydrogenation Characteristics of the Proton Conducting Oxide–Hydrogen Storage Alloy Composite. Mater. Res. Bull. 2002, 37, 1547–1556. [Google Scholar] [CrossRef]
- Henao, J.; Sotelo, O.; Casales-Diaz, M.; Martinez-Gomez, L. Hydrogen Storage in a Rare-Earth Perovskite-Type Oxide La0.6Sr0.4Co0.2Fe0.8O3 for Battery Applications. Rare Met. 2018, 37, 1003–1013. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Rehman, M.A.; Rehman, B.; Sikiru, S.; Qureshi, S.; Ali, E.M.; Awais, M.; Amjad, M.; Iqbal, I.; Rafique, A.; et al. Ab Initio Insight into the Physical Properties of MgXH3 (X = Co, Cu, Ni) Lead-Free Perovskite for Hydrogen Storage Application. Environ. Sci. Pollut. Res. 2023, 30, 113889–113902. [Google Scholar] [CrossRef] [PubMed]
- Mera, A.; Rehman, M.A. First-Principles Investigation for the Hydrogen Storage Properties of AeSiH3 (Ae = Li, K, Na, Mg) Perovskite-Type Hydrides. Int. J. Hydrogen Energy 2024, 50, 1435–1447. [Google Scholar] [CrossRef]
- Siddique, A.; Khalil, A.; Almutairi, B.S.; Tahir, M.B.; Sagir, M.; Ullah, Z.; Hannan, A.; Ali, H.E.; Alrobei, H.; Alzaid, M. Structures and Hydrogen Storage Properties of AeVH3 (Ae = Be, Mg, Ca, Sr) Perovskite Hydrides by DFT Calculations. Int. J. Hydrogen Energy 2023, 48, 24401–24411. [Google Scholar] [CrossRef]
- Hayat, S.; Khalil, R.M.A.; Hussain, M.I.; Rana, A.M.; Hussain, F. First-Principles Investigations of the Structural, Optoelectronic, Magnetic and Thermodynamic Properties of Hydride Perovskites XCuH3 (X = Co, Ni, Zn) for Hydrogen Storage Applications. Optik 2021, 228, 166187. [Google Scholar] [CrossRef]
- Rafique, A.; Usman, M.; Rehman, J.U.; Nazeer, A.; Ullah, H.; Hussain, A. Investigation of Structural, Electronic, Mechanical, Optical and Hydrogen Storage Properties of Cobalt-Based Hydride-Perovskites XCoH3 (X = In, Mn, Sr, Sn, Cd) for Hydrogen Storage Application. J. Phys. Chem. Solids 2023, 181, 111559. [Google Scholar] [CrossRef]
- Song, R.; Chen, Y.; Chen, S.; Xu, N.; Zhang, W. First-Principles to Explore the Hydrogen Storage Properties of XPtH3 (X = Li, Na, K, Rb) Perovskite Type Hydrides. Int. J. Hydrogen Energy 2024, 57, 949–957. [Google Scholar] [CrossRef]
- Xu, N.; Song, R.; Zhang, J.; Chen, Y.; Chen, S.; Li, S.; Jiang, Z.; Zhang, W. First-Principles Study on Hydrogen Storage Properties of the New Hydride Perovskite XAlH3 (X = Na, K). Int. J. Hydrogen Energy 2024, 60, 434–440. [Google Scholar] [CrossRef]
- Xie, D.; Zhang, M.; Liu, Q.; Lin, Y.; Yu, A.; Tang, Y. Organic-Inorganic Conformal Extending High-Purity Metal Nanosheets for Robust Electrochemical Lithium-Ion Storage. Adv. Funct. Mater. 2023, 33, 2306291. [Google Scholar] [CrossRef]
- Shah, S.F.A.; Murtaza, G.; Ismail, K.; Raza, H.H.; Khan, I.J. First Principles Investigation of Transition Metal Hydrides LiXH3 (X = Ti, Mn, and Cu) for Hydrogen Storage. J. Comput. Electron. 2023, 22, 921–929. [Google Scholar] [CrossRef]
- Surucu, G.; Candan, A.; Gencer, A.; Isik, M. First-Principle Investigation for the Hydrogen Storage Properties of NaXH3 (X = Mn, Fe, Co) Perovskite Type Hydrides. Int. J. Hydrogen Energy 2019, 44, 30218–30225. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Bae, H.; Kim, I.-H.; Mathur, L.; Park, J.-Y.; Song, S.-J. High Capacity, Rate-Capability, and Power Delivery at High-Temperature by an Oxygen-Deficient Perovskite Oxide as Proton Insertion Anodes for Energy Storage Devices. J. Electrochem. Soc. 2021, 168, 070540. [Google Scholar] [CrossRef]
- Lahlou Nabil, M.A.; Fenineche, N.; Popa, I.; Sunyol, J.J. Morphological, Structural and Hydrogen Storage Properties of LaCrO3 Perovskite-Type Oxides. Energies 2022, 15, 1463. [Google Scholar] [CrossRef]
- Ullah, M.A.; Riaz, K.N.; Rizwan, M. Computational Evaluation of KMgO3-xHx as an Efficient Hydrogen Storage Material. J. Energy Storage 2023, 70, 108030. [Google Scholar] [CrossRef]
- Parvazian, E.; Watson, T. The Roll-to-Roll Revolution to Tackle the Industrial Leap for Perovskite Solar Cells. Nat. Commun. 2024, 15, 3983. [Google Scholar] [CrossRef]
- Chen, C.; Ran, C.; Yao, Q.; Wang, J.; Guo, C.; Gu, L.; Han, H.; Wang, X.; Chao, L.; Xia, Y.; et al. Screen-Printing Technology for Scale Manufacturing of Perovskite Solar Cells. Adv. Sci. 2023, 10, 2303992. [Google Scholar] [CrossRef]
- Khatoon, S.; Kumar Yadav, S.; Chakravorty, V.; Singh, J.; Bahadur Singh, R.; Hasnain, M.S.; Hasnain, S.M.M. Perovskite Solar Cell’s Efficiency, Stability and Scalability: A Review. Mater. Sci. Energy Technol. 2023, 6, 437–459. [Google Scholar] [CrossRef]
- Noman, M.; Khan, Z.; Jan, S.T. A Comprehensive Review on the Advancements and Challenges in Perovskite Solar Cell Technology. RSC Adv. 2024, 14, 5085–5131. [Google Scholar] [CrossRef]
- Ur Rehman, Z.; Rehman, M.A.; Alomar, S.Y.; Rehman, B.; Awais, M.; Amjad, M.; Sikiru, S.; Ali, E.M.; Hamad, A. Hydrogen Storage Capacity of Lead-Free Perovskite NaMTH3 (MT = Sc, Ti, V): A DFT Study. Int. J. Energy Res. 2024, 2024, 4009198. [Google Scholar] [CrossRef]

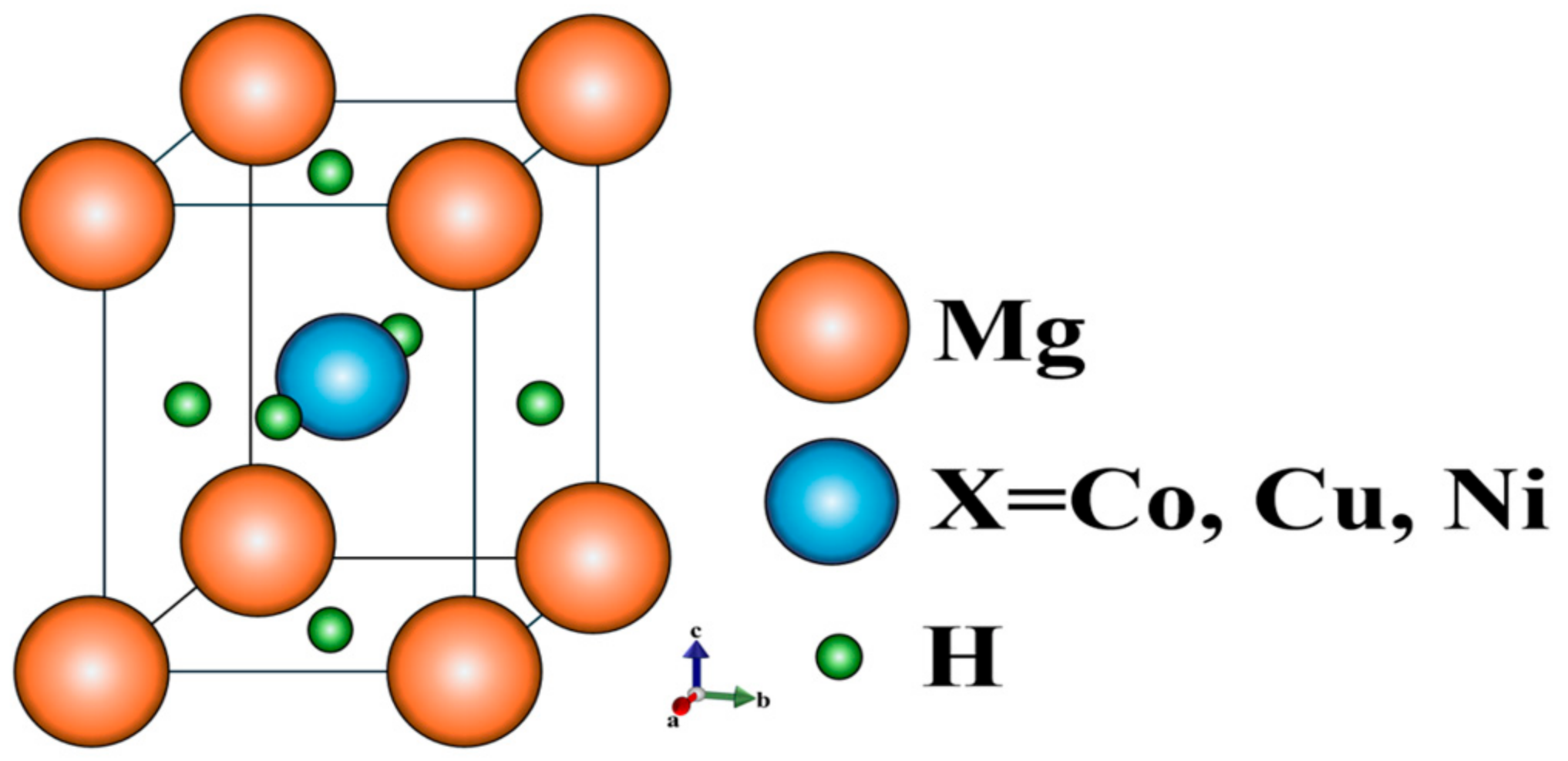

| Compounds | % | · (Å) | · (Å3) | · (g/cm3) | · (eV/Atom) | · (K) |
|---|---|---|---|---|---|---|
| MgCoH3 | 3.64 | 3.32 | 36.44 | 3.93 | −70.93 | 542.69 |
| MgCuH3 | 3.32 | 3.49 | 42.42 | 3.56 | −63.27 | 484.08 |
| MgNiH3 | 3.49 | 3.36 | 37.97 | 3.76 | −68.54 | 524.40 |
| Compounds | % | · (Å) | · (Å3) | · (g/cm3) | · (eV/Atom) |
|---|---|---|---|---|---|
| CaMnH3 | 3.09 | 3.60 | 46.58 | 3.50 | −0.25 |
| CaFeH3 | 3.06 | 3.50 | 42.99 | 3.82 | −0.42 |
| CaCoH3 | 2.97 | 3.48 | 42.16 | 4.03 | −0.44 |
| Compounds | % | · (Å) | · (Å3) | · (g/cm3) | · (eV/Atom) |
|---|---|---|---|---|---|
| LiSiH3 | 7.946 | 4.001 | 64.079 | 1.046 | −17.372 |
| KSiH3 | 4.306 | 3.917 | 60.122 | 1.952 | −15.760 |
| NaSiH3 | 5.588 | 3.986 | 63.363 | 1.429 | −16.134 |
| MgSiH3 | 5.456 | 3.977 | 62.933 | 1.476 | −15.063 |
| Compounds | % | · (Å) | · (Å3) | · (eV/Atom) |
|---|---|---|---|---|
| KSrH3 | 2.33 | 4.77 | 108.50 | −6.60 |
| RbSrH3 | 1.71 | 4.99 | 124.77 | −5.67 |
| Compounds | % | · (Å) | · (Å3) | |
|---|---|---|---|---|
| CoCuH3 | 2.8 | 3.3287 | 36.882 | −1895.3 |
| NiCuH3 | 3.0 | 3.3245 | 36.742 | −5499.0 |
| ZnCuH3 | 2.7 | 3.6129 | 47.160 | −2512.5 |
| Compounds | % | · (Å) | · (Å3) | · (eV/Atom) |
|---|---|---|---|---|
| CdCoH3 | 1.74 | 3.42 | 20.00 | −0.93 |
| InCoH3 | 1.71 | 3.52 | 43.61 | −1.09 |
| MnCoH3 | 2.59 | 3.60 | 46.66 | −0.87 |
| SnCoH3 | 1.68 | 3.59 | 46.27 | −1.31 |
| SrCoH3 | 2.03 | 3.66 | 49.03 | −0.78 |
| Compounds | % | · (Å) | · (Å3) | · (eV/Atom) | · (K) |
|---|---|---|---|---|---|
| LiPtH3 | 1.45 | 3.54 | 44.35 | −0.32 | 237.77 |
| NaPtH3 | 1.35 | 3.63 | 47.96 | −0.31 | 225.39 |
| KPtH3 | 1.26 | 3.80 | 54.83 | −0.22 | 162.43 |
| RbPtH3 | 1.06 | 3.90 | 59.25 | −0.11 | 80.11 |
| Compounds | % | · (Å) | · (Å3) | · (eV/Atom) |
|---|---|---|---|---|
| NaAlH3 | 5.40 | 3.792 | 54.526 | −0.903 |
| KAlH3 | 4.19 | 3.938 | 61.070 | −1.250 |
| Compounds | % | · (Å) | · (GPa) | · (GPa) | · (eV/Atom) |
|---|---|---|---|---|---|
| KTiH3 | 3.36 | 3.999 | 44.938 | 1.767 | −0.285 |
| RbTiH3 | 2.22 | 4.103 | 42.083 | 1.887 | −0.220 |
| CsTuH3 | 1.65 | 4.233 | 37.176 | 1.704 | −0.146 |
| Compounds | % | · (Å) | · (Å3) | · (GPa) | · (eV/Atom) |
|---|---|---|---|---|---|
| KBeH3 | 5.866 | 4.41 | 158.385 | 2.650 | −1.226 |
| KMgH3 | 4.516 | 4.74 | 297.538 | 0.916 | −2.523 |
| KCaH3 | 3.649 | 5.10 | 89.719 | 27.902 | −1.845 |
| Samples | · (nm) | |||
|---|---|---|---|---|
| LaCrO3 | 1.4838 | 0.0044 | 12.060 | 5.8201 |
| LaMnO3 | 8.8778 | 0.0302 | 13.616 | 6.7078 |
| LaFeO3 | 20.997 | 0.1201 | 10.721 | 20.698 |
| LaCoO3 | 8.2064 | 0.0693 | 33.807 | 10.929 |
| LaNiO3 | 4.8519 | 0.0562 | 98.998 | 16.537 |
| Active Material | Substrate | Electrolyte Solution | Reference Electrode | Counter Electrode | Discharge Capacity |
|---|---|---|---|---|---|
| LaCrO3 | - | - | - | - | 6790 mAh/g |
| LaMnO3 | - | - | - | - | 10,500 mAh/g |
| LaFeO3 | Cu electrode | 6M KOH | Ag/AGCl | Pt | 13,500 mAh/g |
| LaCoO3 | - | - | - | - | 8800 mAh/g |
| LaNiO3 | - | - | - | - | 7000 mAh/g |
| Compounds | Calculated Cell Parameters (A) | · (GPa) | · (GPa) |
|---|---|---|---|
| LaCrO3 | 3.85 | 128.07 | 3.2 |
| LaCrO3H6 | 4.43 | 62.26 | 23.48 |
| Compounds | % | a | · (Å) b | c | · (Å3) | · (eV/Atom) | · (K) |
|---|---|---|---|---|---|---|---|
| KMgO3 | - | 4.109 | - | - | 69.37 | −0.023 | - |
| KMg2.7O0.3 | 0.28 | 4.102 | 4.102 | 4.112 | 69.19 | −11.122 | 825 |
| KMg2.4O0.6 | 0.59 | 4.092 | 4.067 | 4.140 | 68.90 | −10.826 | 803 |
| KMg2.1O0.9 | 0.92 | 4.059 | 4.057 | 4.162 | 68.54 | −10.529 | 781 |
| KMg1.8O1.2 | 1.28 | 4.034 | 4.038 | 4.196 | 68.35 | −10.223 | 759 |
| KMg1.5O1.5 | 1.67 | 4.107 | 3.989 | 4.182 | 68.51 | −9.937 | 737 |
| KMg1.2O1.8 | 2.10 | 4.510 | 4.653 | 4.437 | 92.90 | −9.648 | 716 |
| KMg0.9O2.1 | 2.58 | 4.450 | 3.975 | 4.108 | 72.60 | −9.347 | 694 |
| KMg0.6O2.4 | 3.10 | 4.156 | 3.966 | 4.091 | 67.43 | −8.624 | 640 |
| KMg0.3O2.7 | 3.69 | 4.058 | 4.084 | 4.004 | 66.35 | −7.904 | 587 |
| KMgH3 | 4.35 | 4.069 | - | - | 67.36 | −7.606 | 564 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, M.-H.; Neykova, N.; Stachiv, I. Overview of the Recent Findings in the Perovskite-Type Structures Used for Solar Cells and Hydrogen Storage. Energies 2024, 17, 4755. https://doi.org/10.3390/en17184755
Kuo M-H, Neykova N, Stachiv I. Overview of the Recent Findings in the Perovskite-Type Structures Used for Solar Cells and Hydrogen Storage. Energies. 2024; 17(18):4755. https://doi.org/10.3390/en17184755
Chicago/Turabian StyleKuo, Meng-Hsueh, Neda Neykova, and Ivo Stachiv. 2024. "Overview of the Recent Findings in the Perovskite-Type Structures Used for Solar Cells and Hydrogen Storage" Energies 17, no. 18: 4755. https://doi.org/10.3390/en17184755
APA StyleKuo, M.-H., Neykova, N., & Stachiv, I. (2024). Overview of the Recent Findings in the Perovskite-Type Structures Used for Solar Cells and Hydrogen Storage. Energies, 17(18), 4755. https://doi.org/10.3390/en17184755








