An Analysis of Greenhouse Gas Emissions in Electrolysis for Certifying Clean Hydrogen
Abstract
1. Introduction
2. Materials and Methods
- Establishment of Evaluation Criteria: Evaluation criteria for clean hydrogen certification are primarily focused on greenhouse gas emissions generated during the hydrogen production process and are used to assess the environmental efficiency of hydrogen produced using various energy sources.
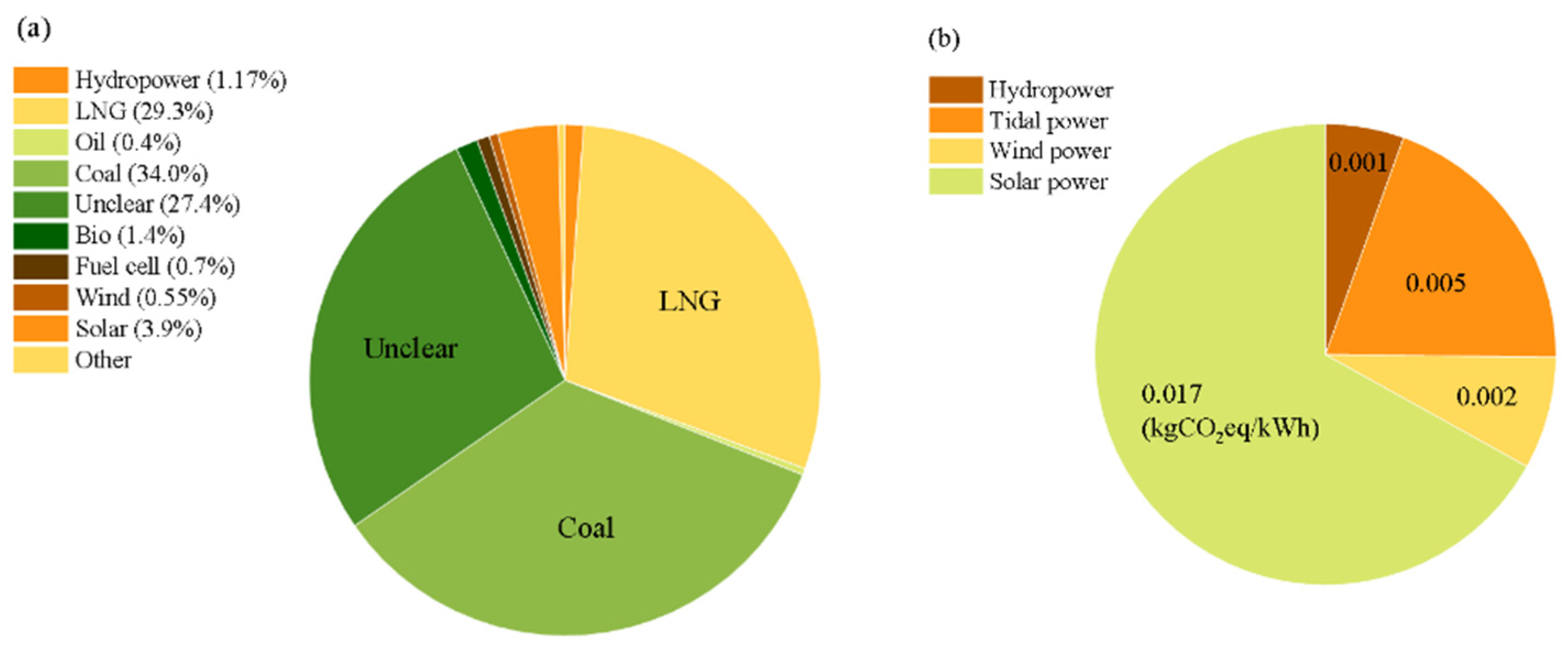
- Classification of Energy Sources: This system distinguishes between hydrogen produced using renewable energy sources (such as solar, wind, and hydroelectric power) and fossil fuels (like coal and natural gas). Hydrogen produced from renewable sources generally exhibits lower greenhouse gas emissions and can achieve higher certification grades.
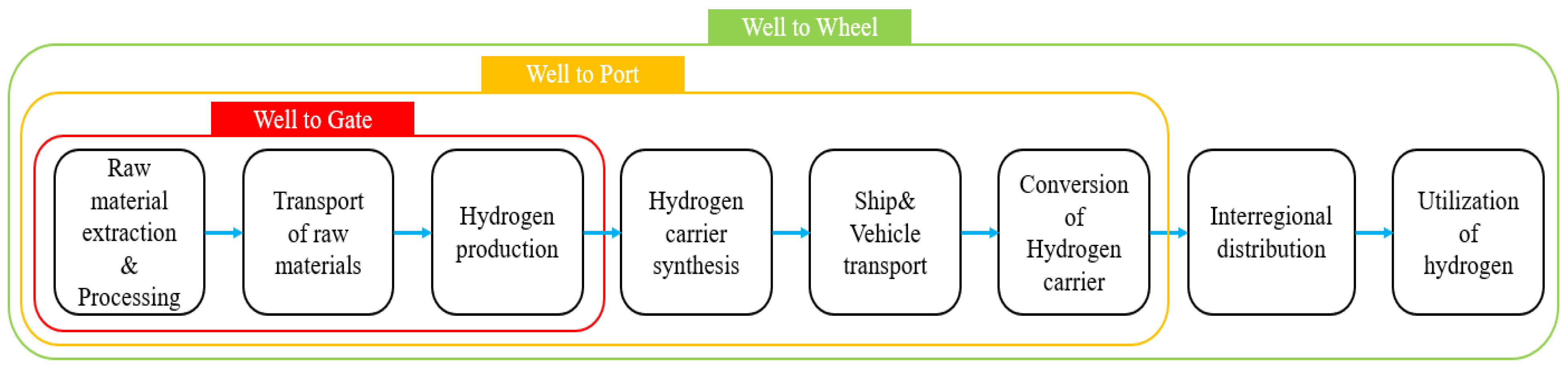
- Evaluation of Production Processes: This aspect involves the evaluation of all greenhouse gas emissions produced during the hydrogen production process, including raw material extraction, electricity consumption, electrolysis processes, compression, and storage.
- Compression and Storage Processes: The environmental impacts of hydrogen compression and storage are also evaluated. High-pressure compression systems can induce additional greenhouse gas emissions, making the development of technologies to minimize these impacts crucial.
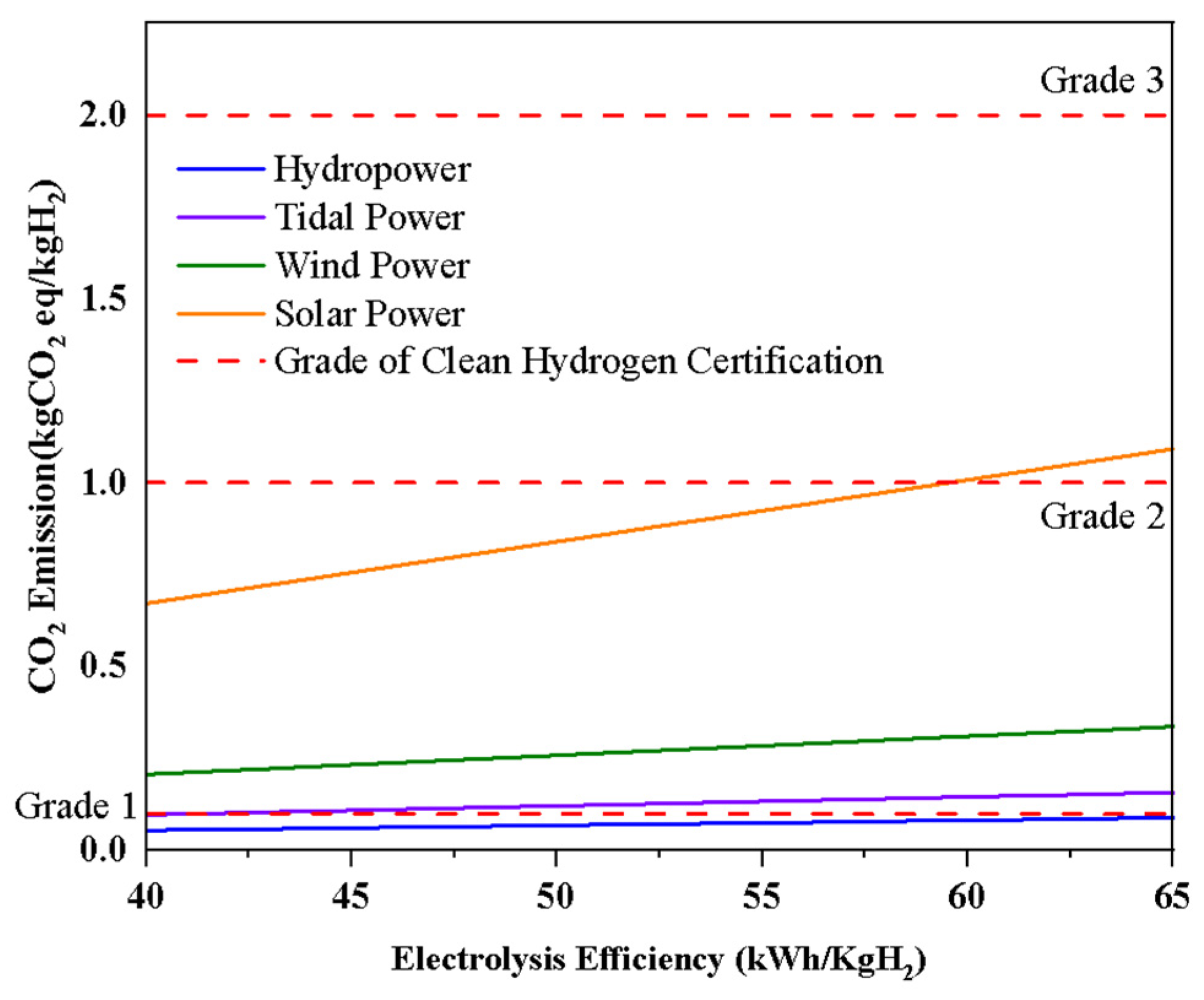
- Certification and Grading: Based on the evaluations above, hydrogen is classified into different grades, typically ranging from Grade 1 to Grade 4. These grades reflect the type and efficiency of energy sources used, with higher grades indicating greater environmental sustainability.
- Global Market Application: South Korea’s Clean Hydrogen Certification System was developed in alignment with internationally recognized standards to maintain competitiveness in the global market and foster growth in the hydrogen economy. As a result, the Clean Hydrogen Certification System can be used to strengthen the sustainability of the hydrogen economy and play a significant role in addressing climate change challenges.
- (1)
- All energy required within the emission calculation scope is supplied by renewable energy generation, excluding fuel used for transportation.
- (2)
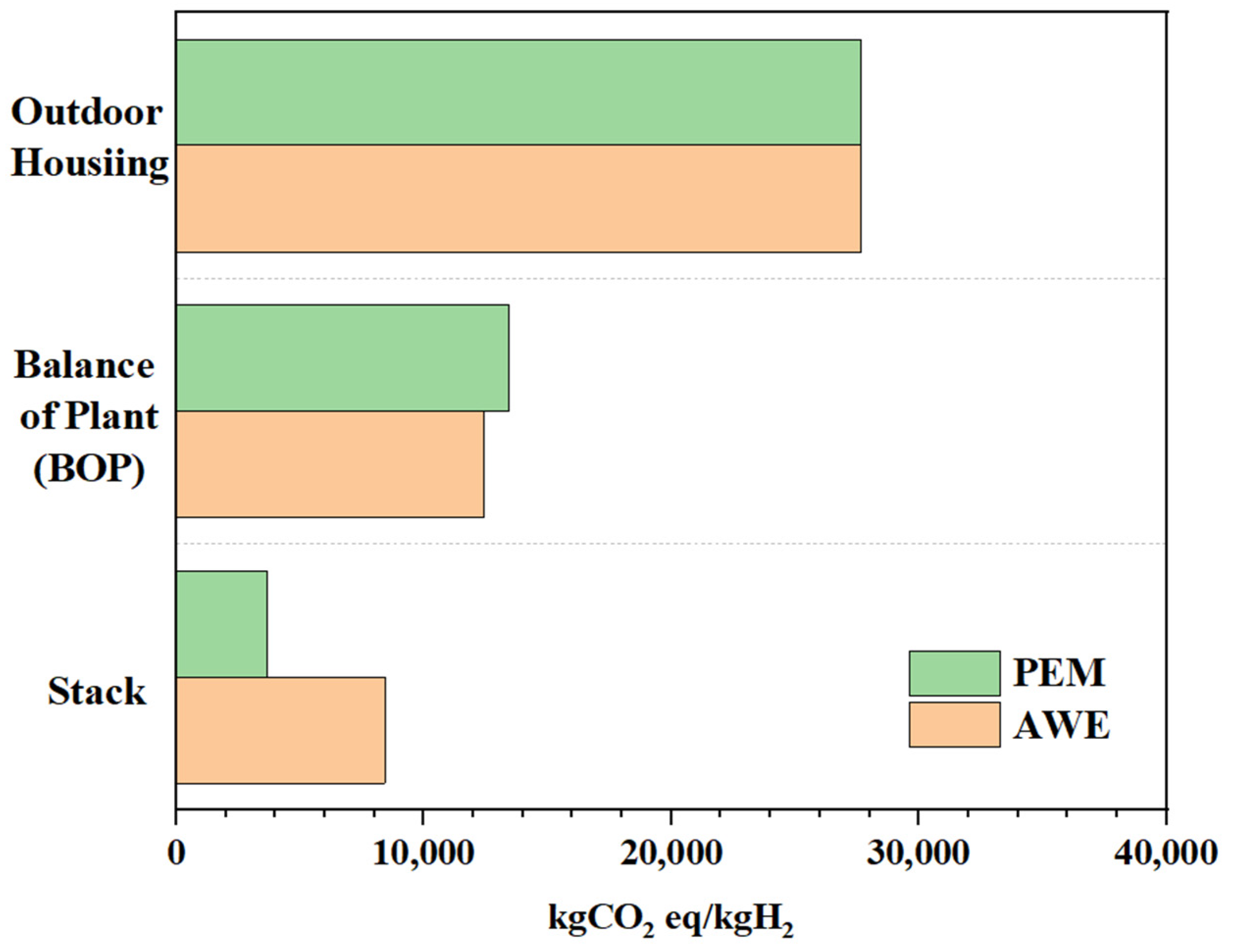
- Within the emission calculation scope, the raw material extraction and processing stages only include the components necessary for electrolysis system production (out-housing, balance of plant, stack).
2.1. Scope of Greenhouse Gas Emission Calculation
2.2. Clean Hydrogen Certification Standard
2.3. CO2 Emission Factor
3. Results and Discussion
3.1. Electricity Grid Rate and CO2 of Renewable Energy in South Korea
3.2. CO2 Emissions According to Electrolysis Feedstock Materials
3.3. CO2 Emissions during Hydrogen Production Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moiceanu, G.; Dinca, M.N. Climate change-greenhouse gas emissions analysis and forecast in Romania. Sustainability 2021, 13, 12186. [Google Scholar] [CrossRef]
- Gao, H.; Wang, X.; Wu, K.; Zheng, Y.; Wang, Q.; Shi, W.; He, M. A review of building carbon emission accounting and prediction models. Buildings 2023, 13, 1617. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, L.; Fernández Carvajal, A.B.; Bujidos-Casado, M. Allocation of greenhouse gas emissions using the fairness principle: A multi-country analysis. Sustainability 2020, 12, 5839. [Google Scholar] [CrossRef]
- Kim, T.H.; Jeong, Y.S. Analysis of energy-related greenhouse gas emission in the Korea’s building sector: Use national energy statistics. Energies 2018, 11, 855. [Google Scholar] [CrossRef]
- Wang, L. Carbon Tax Policy and Technological Innovation for Low-Carbon Emission. In Proceedings of the 2011 International Conference on Management and Service Science, Wuhan, China, 12–14 August 2011; pp. 1–4. [Google Scholar] [CrossRef]
- Tsai, W.-H. Carbon emission reduction Carbon tax, carbon trading, and carbon offset. Energies 2020, 13, 6128. [Google Scholar] [CrossRef]
- Liu, X.; Reddi, K.; Elgowainy, A.; Lohse-Busch, H.; Wang, M.; Rustagi, N. Comparison of well-to-wheels energy use and emissions of a hydrogen fuel cell electric vehicle relative to a conventional gasoline-powered internal combustion engine vehicle. Int. J. Hydrogen Energy 2020, 45, 972–983. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Liu, Z.; Frans, V.F.; Xu, Z.; Qiu, X.; Xu, F.; Li, Y. Assessing the water and carbon footprint of hydropower stations at a national scale. Sci. Total Environ. 2019, 676, 595–612. [Google Scholar] [CrossRef]
- Patel, G.H.; Havukainen, J.; Horttanainen, M.; Soukka, R.; Tuomaala, M. Climate change performance of hydrogen production based on life cycle assessment. Green Chem. 2024, 26, 992–1006. [Google Scholar] [CrossRef]
- Dulău, L.-I. CO2 Emissions of Battery Electric Vehicles and Hydrogen Fuel Cell Vehicles. Clean Technol. 2023, 5, 696–712. [Google Scholar] [CrossRef]
- Kafetzis, A.; Bampaou, M.; Kardaras, G.; Panopoulos, K. Decarbonization of Former Lignite Regions with Renewable Hydrogen: The Western Macedonia Case. Energies 2023, 16, 7029. [Google Scholar] [CrossRef]
- Fearnside, P.M. Greenhouse gas emissions from Brazil’s Amazonian hydroelectric dams. Environ. Res. Lett. 2016, 11, 011002. [Google Scholar] [CrossRef]
- Räsänen, T.A.; Varis, O.; Scherer, L.; Kummu, M. Greenhouse gas emissions of hydropower in the Mekong River Basin. Environ. Res. Lett. 2018, 13, 034030. [Google Scholar] [CrossRef]
- Steinhurst, W.; Knight, P.; Schultz, M. Hydropower greenhouse gas emissions. Conserv. Law Found. 2012, 24, 1–26. Available online: https://www.synapse-energy.com/sites/default/files/SynapseReport.2012-02.CLF+PEW.GHG-from-Hydro.10-056.pdf (accessed on 14 February 2012).
- Ma, Z.; Cai, S.; Ye, W.; Gu, A. Linking emissions trading schemes: Economic valuation of a joint China–Japan–Korea carbon market. Sustainability 2019, 11, 5303. [Google Scholar] [CrossRef]
- Almeida, R.M.; Shi, Q.; Gomes-Selman, J.M.; Wu, X.; Xue, Y.; Angarita, H.; Barros, N.; Forsberg, B.R.; García-Villacorta, R.; Hamilton, S.K.; et al. Reducing greenhouse gas emissions of Amazon hydropower with strategic dam planning. Nat. Commun. 2019, 10, 4281. [Google Scholar] [CrossRef]
- Gan, Y.; Wang, M.; Lu, Z.; Kelly, J. Taking into account greenhouse gas emissions of electric vehicles for transportation de-carbonization. Energy Policy 2021, 155, 112353. [Google Scholar] [CrossRef]
- Bayazıt, Y. The effect of hydroelectric power plants on the carbon emission: An example of Gokcekaya dam, Turkey. Renew. Energy 2021, 170, 181–187. [Google Scholar] [CrossRef]
- Howarth, R.W.; Jacobson, M.Z. How green is blue hydrogen? Energy Sci. Eng. 2021, 9, 1676–1687. [Google Scholar] [CrossRef]
- Longden, T.; Beck, F.J.; Jotzo, F.; Andrews, R.; Prasad, M. ‘Clean’ hydrogen?—Comparing the emissions and costs of fossil fuel versus renewable electricity based hydrogen. Appl. Energy 2022, 306, 118145. [Google Scholar] [CrossRef]
- Dufour, J.; Serrano, D.P.; Galvez, L.; Moreno, J.; Gonzalez, A. Hydrogen production from fossil fuels: Life cycle assessment of technologies with low greenhouse gas emissions. Energy Fuels 2011, 25, 2194–2202. [Google Scholar] [CrossRef]
- Ashwath, J.; Kanishka, S.; Jannan, B.; Janardhan, A.; Shailesh, A.; Rathore, S. Life cycle analysis of hydrogen. AIP Conf. Proc. 2021, 2396, 020008. [Google Scholar] [CrossRef]
- Smitkova, M.; Janíček, F.; Riccardi, J. Life cycle analysis of processes for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 7844–7851. [Google Scholar] [CrossRef]
- Dufour, J.; Serrano, P.; Galvez, L.; Gonzalez, A.; Soria, E.; Fierro, L. Life cycle assessment of alternatives for hydrogen production from renewable and fossil sources. Int. J. Hydrogen Energy 2012, 37, 1173–1183. [Google Scholar] [CrossRef]
- Mann, M.; Spath, P. Life Cycle Assessment of Renewable Hydrogen Production via Wind/Electrolysis: Milestone Completion Report; National Renewable Energy Lab.: Golden, CO, USA, 2004. [Google Scholar] [CrossRef]
- Liu, H.; Liu, S. Life cycle energy consumption and GHG emissions of hydrogen production from underground coal gasification in comparison with surface coal gasification. Int. J. Hydrogen Energy 2021, 46, 9630–9643. [Google Scholar] [CrossRef]
- Kanz, O.; Bruggemann, F.; Ding, K.; Bittkau, K.; Rau, U.; Reinders, A. Life-cycle global warming impact of hydrogen transport through pipelines from Africa to Germany. Sustain. Energy Fuels 2023, 7, 3014–3024. [Google Scholar] [CrossRef]
- Mocoteguy, P.; Brisse, A. A review and comprehensive analysis of degradation mechanisms of solid oxide electrolysis cells. Int. J. Hydrogen Energy 2013, 38, 15887–15902. [Google Scholar] [CrossRef]
- Du, L.; Yang, Y.; Zhou, L.; Liu, M. Greenhouse Gas Reduction Potential and Economics of Green Hydrogen via Water Electrolysis: A Systematic Review of Value-Chain-Wide Decarbonization. Sustainability 2024, 16, 4602. [Google Scholar] [CrossRef]
- Melideo, D.; Ortiz, R.; Weidner, E. Life Cycle Assessment of Hydrogen and Fuel Cell Technologies; JCR Joint Research Centre: Brussels, Belgium, 2020. [Google Scholar] [CrossRef]
- Lee, D.Y.; Elgowainy, A.; Dai, Q. Life cycle greenhouse gas emissions of hydrogen fuel production from chlor-alkali processes in the United States. Appl. Energy 2018, 217, 467–479. [Google Scholar] [CrossRef]
- Rinawati, I.; Keeley, R.; Takeda, S.; Managi, S. Life-cycle. assessment of hydrogen utilization in power generation: A systematic review of technological and methodological choices. Front. Sustain. 2022, 3, 920876. [Google Scholar] [CrossRef]
- Ricks, W.; Xu, Q.; Jenkins, D. Minimizing emissions from grid-based hydrogen production in the United States. Environ. Res. Lett. 2023, 18, 014025. [Google Scholar] [CrossRef]
- Gong, M.; Zhou, W.; Tsai, M.-C.; Zhou, J.; Guan, M.; Lin, M.-C.; Zhang, B.; Hu, Y.; Wang, D.-Y.; Yang, J.; et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 4695. [Google Scholar] [CrossRef]
- Palmer, G.; Roberts, A.; Hoadley, A.; Dargaville, R.; Honnery, D. Life-cycle. greenhouse gas emissions and net energy assessment of large-scale hydrogen production via electrolysis and solar PV. Energy Environ. Sci. 2021, 14, 5113–5131. [Google Scholar] [CrossRef]
- Carvalho, F.; Osipova, L.; Zhou, Y. Life-Cycle Greenhouse Gas Emissions of Hydrogen as a Marine Fuel and Cost of Producing Green Hydrogen in Brazil. 2023. Available online: https://theicct.org/publication/maritime-brazil-hydrogen-costs-mar23/ (accessed on 30 March 2023).
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S.; et al. Non-noble. metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 2019, 10, 5106. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Liu, J.; Zhong, C.; Tu, Y.; Li, P.; Du, L.; Chen, S.; Cui, Z. OH spectator at IrMo intermetallic narrowing activity gap between alkaline and acidic hydrogen evolution reaction. Nat. Commun. 2022, 13, 5497. [Google Scholar] [CrossRef]
- Majasan, O.; Cho, I.; Maier, M.; Shearing, R.; Brett, J. Optimisation of mass transport parameters in a polymer electrolyte membrane electrolyser using factorial design-of-experiment. Front. Energy Res. 2021, 9, 643587. [Google Scholar] [CrossRef]
- Patino, J.; Velasquez, C.; Ramirez, E.; Betancur, R.; Montoya, F.; Chica, E.; Romero-Gómez, P.; Kannan, A.M.; Ramírez, D.; Eusse, P.; et al. Renewable Energy Sources for Green Hydrogen Generation in Colombia and Applicable Case of Studies. Energies 2023, 16, 7809. [Google Scholar] [CrossRef]
- Tenhumberg, N.; Buker, K. Ecological and economic evaluation of hydrogen production by different water electrolysis technologies. Chem. Ing. Tech. 2020, 92, 1586–1595. [Google Scholar] [CrossRef]
- Bareiß, K.; de la Rua, C.; Mockl, M.; Hamacher, T. Life. cycle assessment of hydrogen from proton exchange membrane water electrolysis in future energy systems. Appl. Energy 2019, 237, 862–872. [Google Scholar] [CrossRef]
- Mori, M.; Stropnik, R.; Sekavnik, M.; Lotri, A. Criticality and life-cycle assessment of materials used in fuel-cell and hydrogen technologies. Sustainability 2021, 13, 3565. [Google Scholar] [CrossRef]
- Hardisty, E.; Clark, S.; Hynes, G. Life cycle greenhouse gas emissions from electricity generation: A comparative analysis of Australian energy sources. Energies 2012, 5, 872–897. [Google Scholar] [CrossRef]
- Da Fonseca-Soares, D.; Eliziário, S.A.; Galvinicio, J.D.; Ramos-Ridao, A.F. Life-Cycle Greenhouse Gas (GHG) Emissions Calculation for Urban Rail Transit Systems: The Case of Pernambuco Metro. Appl. Sci. 2023, 13, 8965. [Google Scholar] [CrossRef]
- Duro, J.A.; Giménez-Gómez, J.-M.; Vilella, C. The allocation of CO2 emissions as a claims problem. Energy Econ. 2020, 86, 104652. [Google Scholar] [CrossRef]
- Krishnan, S.; Corona, B.; Kramer, J.; Junginger, M.; Koning, V. Prospective LCA of alkaline and PEM electrolyser systems. Int. J. Hydrogen Energy 2024, 55, 26–41. [Google Scholar] [CrossRef]
- Bakken, H.; Modahl, S.; Engeland, K.; Raadal, L.; Arnøy, S. The life-cycle water footprint of two hydropower projects in Norway. J. Clean. Prod. 2016, 113, 241–250. [Google Scholar] [CrossRef]
- Ang, W.; Su, B. Carbon emission intensity in electricity production: A global analysis. Energy Policy 2016, 94, 56–63. [Google Scholar] [CrossRef]
- Wulf, C.; Kaltschmitt, M. Hydrogen supply chains for mobility environmental and economic assessment. Sustainability 2018, 10, 1699. [Google Scholar] [CrossRef]
- Zhao, G.; Kraglund, R.; Frandsen, L.; Wulff, C.; Jensen, H.; Chen, M.; Graves, R. Life cycle assessment of H2O electrolysis technologies. Int. J. Hydrogen Energy 2020, 45, 23765–23781. [Google Scholar] [CrossRef]
- Qian, S.; Li, L. A Comparison of Well-to-Wheels Energy Use and Emissions of Hydrogen Fuel Cell, Electric, LNG, and Diesel-Powered Logistics Vehicles in China. Energies 2023, 16, 5101. [Google Scholar] [CrossRef]
- Sand, M.; Skeie, R.B.; Sandstad, M.; Krishnan, S.; Myhre, G.; Bryant, H.; Derwent, R.; Hauglustaine, D.; Paulot, F.; Prather, M.; et al. A multi-model assessment of the Global Warming Potential of hydrogen. Commun. Earth Environ. 2023, 4, 203. [Google Scholar] [CrossRef]
- Kawamoto, R.; Mochizuki, H.; Moriguchi, Y.; Nakano, T.; Motohashi, M.; Sakai, Y.; Inaba, A. Estimation of CO2 emissions of internal combustion engine vehicle and battery electric vehicle using LCA. Sustainability 2019, 11, 2690. [Google Scholar] [CrossRef]
- Zhong, X.; Hu, M.; Deetman, S.; Steubing, B.; Lin, X.; Hernandez, A.; Harpprecht, C.; Zhang, C.; Tukker, A.; Behrens, P. Global greenhouse gas emissions from residential and commercial building materials and mitigation strategies to 2060. Nat. Commun. 2021, 12, 6126. [Google Scholar] [CrossRef]
- St-Jacques, M.; Bucking, S.; O’Brien, W. Spatially and temporally sensitive consumption-based emission factors from mixed-use electrical grids for building electrical use. Energy Build. 2020, 224, 110249. [Google Scholar] [CrossRef]
- Hwang, K.; Tang, X. GHG Emissions in Korea’s Renewable Energy Power Generation Sector’s Calculation and Factor Analysis. Soc. Converg. Knowl. Trans. 2022, 10, 111–119. [Google Scholar] [CrossRef]
- Zhong, Z.; Yu, Y.; Zhao, X. Revisiting electric vehicle life cycle greenhouse gas emissions in China: A marginal emission perspective. iScience 2023, 26, 106565. [Google Scholar] [CrossRef]
- Marrasso, E.; Roselli, C.; Sasso, M. Electric efficiency indicators and carbon dioxide emission factors for power generation by fossil and renewable energy sources on hourly basis. Energy Convers. Manag. 2019, 196, 1369–1384. [Google Scholar] [CrossRef]
- Parkinson, B.; Balcombe, P.; Speirs, F.; Hawkes, D.; Hellgardt, K. Levelized cost of CO2 mitigation from hydrogen production routes. Energy Environ. Sci. 2019, 12, 19–40. [Google Scholar] [CrossRef]
- Zhao, G.; Pedersen, S. Life cycle assessment of hydrogen production and consumption in an isolated territory. Procedia CIRP 2018, 69, 529–533. [Google Scholar] [CrossRef]
- Kolahchian Tabrizi, M.; Famiglietti, J.; Bonalumi, D.; Campanari, S. The Carbon Footprint of Hydrogen Produced with State-of-the-Art Photovoltaic Electricity Using Life-Cycle Assessment Methodology. Energies 2023, 16, 5190. [Google Scholar] [CrossRef]
- Hai, G.; Xue, X.; Wu, Z.; Zhang, C.; Liu, X.; Huang, X. High-throughput calculation-based rational design of Fe-doped MoS2 nanosheets for electrocatalytic pH-universal overall water splitting. J. Energy Chem. 2024, 91, 194–202. [Google Scholar] [CrossRef]
- Hai, G.; Gao, H.; Huang, X.; Tan, L.; Xue, X.; Feng, S.; Wang, G. An efficient factor for fast screening of high-performance two-dimensional metal–organic frameworks towards catalyzing the oxygen evolution reaction. Chem. Sci. 2022, 13, 4397–4405. [Google Scholar] [CrossRef]
- Mir, A.; Upadhyay, S.; Pandey, P. A review on recent advances and progress in Mo2C@C: A suitable and stable electrocatalyst for HER. Int. J. Hydrogen Energy 2023, 48, 13044–13067. [Google Scholar] [CrossRef]
- de Kleijne, K.; de Coninck, H.; van Zelm, R.; Huijbregts, M.A.J.; Hanssen, S.V. The many greenhouse gas footprints of green hydrogen. Sustain. Energy Fuels 2022, 6, 4383–4387. [Google Scholar] [CrossRef]
- Herath, I.; Deurer, M.; Horne, D.; Singh, R.; Clothier, B. The water footprint of hydroelectricity: A methodological comparison from a case study in New Zealand. J. Clean. Prod. 2011, 19, 1582–1589. [Google Scholar] [CrossRef]
- Granovskii, M.; Dincer, I.; Rosen, A. Greenhouse gas emissions reduction by use of wind and solar energies for hydrogen and electricity production: Economic factors. Int. J. Hydrogen Energy 2007, 32, 927–931. [Google Scholar] [CrossRef]
- Dufour, J.; Serrano, P.; Galvez, L.; Moreno, J.; Garcia, C. Life cycle assessment of processes for hydrogen production. Environmental feasibility and reduction of greenhouse gases emissions. Int. J. Hydrogen Energy 2009, 34, 1370–1376. [Google Scholar] [CrossRef]
- Guo, Y.; Shi, E.; Yan, R.; Wei, W. System based greenhouse emission analysis of off-site prefabrication: A comparative study of residential projects. Sci. Rep. 2023, 13, 10689. [Google Scholar] [CrossRef]
- Nnabuife, G.; Darko, K.; Obiako, C.; Kuang, B.; Sun, X.; Jenkins, K. A comparative analysis of different hydrogen production methods and their environmental impact. Clean Technol. 2023, 5, 1344–1380. [Google Scholar] [CrossRef]
- Kone, C.; Buke, T. Factor analysis of projected carbon dioxide emissions according to the IPCC based sustainable emission scenario in Turkey. Renew. Energy 2019, 133, 914–918. [Google Scholar] [CrossRef]
- Liang, D.; Tian, Z.; Ren, F.; Pan, J. Installed hydropower capacity and carbon emission reduction efficiency based on the EBM method in China. Front. Energy Res. 2020, 8, 82. [Google Scholar] [CrossRef]
- Yoo, E.; Kim, M.; Song, H. Well-to-wheel analysis of hydrogen fuel-cell electric vehicle in Korea. Int. J. Hydrogen Energy 2018, 43, 19267–19278. [Google Scholar] [CrossRef]
- Kim, D.; Choi, Y.; Oh, J.; Park, C. Analysis of Carbon Emission Effects and Hydrogen Prices for Overseas Green Hydrogen Imports by Development of Green Ship. J. Hydrog. New Energy 2023, 35, 1–13. [Google Scholar] [CrossRef]
- Jeong, C.; Lee, H.; Roh, H.; Park, J.B. Scenario analysis of the GHG emissions in the electricity sector through 2030 in South Korea considering updated NDC. Energies 2022, 15, 3310. [Google Scholar] [CrossRef]
- Nong, D.; Simshauser, P.; Nguyen, B. Greenhouse gas emissions vs CO2 emissions: Comparative analysis of a global carbon tax. Appl. Energy 2021, 298, 117223. [Google Scholar] [CrossRef]
- Chu, W.; Vicidomini, M.; Calise, F.; Duić, N.; Østergaard, P.A.; Wang, Q.; da Graça Carvalho, M. Review of Hot Topics in the Sustainable Development of Energy, Water, and Environment Systems Conference in 2022. Energies 2023, 16, 7897. [Google Scholar] [CrossRef]
- Wang, C.; Wang, W.; Huang, R. Supply chain enterprise operations and government carbon tax decisions considering carbon emissions. J. Clean. Prod. 2017, 152, 271–280. [Google Scholar] [CrossRef]
- Salekpay, F. The Allocation of Greenhouse Gas Emission in European Union through Applying the Claims Problems Approach. Games 2023, 14, 9. [Google Scholar] [CrossRef]
- Kim, J.; Tromp, N. Analysis of carbon emissions embodied in South Korea’s international trade: Production-based and consumption-based perspectives. J. Clean. Prod. 2021, 320, 128839. [Google Scholar] [CrossRef]
- Ha, S.; Tae, S.; Kim, R. A study on the limitations of South Korea’s national roadmap for greenhouse gas reduction by 2030 and suggestions for improvement. Sustainability 2019, 11, 3969. [Google Scholar] [CrossRef]
- Kim, S. Decomposition analysis of greenhouse gas emissions in Korea’s transportation sector. Sustainability 2019, 11, 1986. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K. Decomposition analysis of the greenhouse gas emissions in Korea’s electricity generation sector. Carbon Manag. 2016, 7, 249–260. [Google Scholar] [CrossRef]
- Choi, W.; Yoo, E.; Seol, E.; Kim, M.; Song, H. Greenhouse gas emissions of conventional and alternative vehicles: Predictions based on energy policy analysis in South Korea. Appl. Energy 2020, 265, 114754. [Google Scholar] [CrossRef]
- Lotrič, A.; Sekavčnik, M.; Kuštrin, I.; Mori, M. Life-cycle assessment of hydrogen technologies with the focus on EU critical raw materials and end-of-life strategies. Int. J. Hydrogen Energy 2021, 46, 10143–10160. [Google Scholar] [CrossRef]

| Country | System Boundary | Emission Benchmark (kgCO2eq/kgH2) | Purity, Pressure | Support Budget |
|---|---|---|---|---|
| USA | Well-to-gate | 4 | 99%, 3 MPa | USD 13 Billion |
| EU | Well-to-Wheel | 3.4 | 99%, 3 MPa | USD 560 Million |
| UK | Well-to-Gate | 2.4 | 99%, 3 MPa | USD 10 Million |
| Japan | Well-to-Gate | 3.4 | USD 44 Billion |
| Grade | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| CO2 Emission | 0~0.1 | 0.1~1 | 1~2 | 2~4 |
| Component | Materials | AWE | PEM |
|---|---|---|---|
| Stack | Carbon steel | 822 | 6 |
| Stainless steel | 1656 | 993 | |
| Aluminum | 244 | 1 | |
| Transition metals | 144 | 74 | |
| Polypropylene | 10 | 6 | |
| Thermoplastics | 240 | 10 | |
| Polyvinylchloride | 26 | - | |
| etc. | 4 | 71 | |
| BOP (Balance of Plant) | Carbon steel | 1452 | 1648 |
| Carbon steel sheet | 497 | 626 | |
| Stainless steel | 753 | 796 | |
| Aluminum | 509 | 526 | |
| Transition metals | 772 | 894 | |
| Polypropylene | 39 | 14 | |
| Polyester | 49 | 1 | |
| Thermoplastics | 157 | 164 | |
| Polyvinylchloride | 20 | 13 | |
| Ceramic | 63 | 63 | |
| Silica | 108 | 65 | |
| Acrylonitrile butadiene styrene | 92 | - | |
| Thermoplastics | 157 | - | |
| etc. | 34 | 52 | |
| Outdoor Housing | Carbon steel | 11,040 | 11,040 |
| Carbon steel sheet | 19 | 19 | |
| Stainless steel | 195 | 195 | |
| Transition metals | 241 | 241 | |
| Thermoplastics | 34 | 34 | |
| Fluorescent lamps | 85 | 85 | |
| Exterior paint | 330 | 330 | |
| etc. | 39 | 9 |
| Region | Distance (km) | GHG Emissions (kgCO2eq) | |
|---|---|---|---|
| AWE | PEM | ||
| Seoul | 161 | 638 | 579 |
| Wonju | 167 | 661 | 600 |
| Cheongju | 46 | 182 | 165 |
| Daejeon | 30 | 119 | 108 |
| Jeonju | 84 | 333 | 302 |
| Gwangju | 168 | 665 | 604 |
| Daegu | 153 | 606 | 550 |
| Busan | 260 | 1030 | 935 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Min, I.; Lee, J.; Yang, H. An Analysis of Greenhouse Gas Emissions in Electrolysis for Certifying Clean Hydrogen. Energies 2024, 17, 3698. https://doi.org/10.3390/en17153698
Kim Y, Min I, Lee J, Yang H. An Analysis of Greenhouse Gas Emissions in Electrolysis for Certifying Clean Hydrogen. Energies. 2024; 17(15):3698. https://doi.org/10.3390/en17153698
Chicago/Turabian StyleKim, Yunji, Inhong Min, Jieun Lee, and Heena Yang. 2024. "An Analysis of Greenhouse Gas Emissions in Electrolysis for Certifying Clean Hydrogen" Energies 17, no. 15: 3698. https://doi.org/10.3390/en17153698
APA StyleKim, Y., Min, I., Lee, J., & Yang, H. (2024). An Analysis of Greenhouse Gas Emissions in Electrolysis for Certifying Clean Hydrogen. Energies, 17(15), 3698. https://doi.org/10.3390/en17153698







