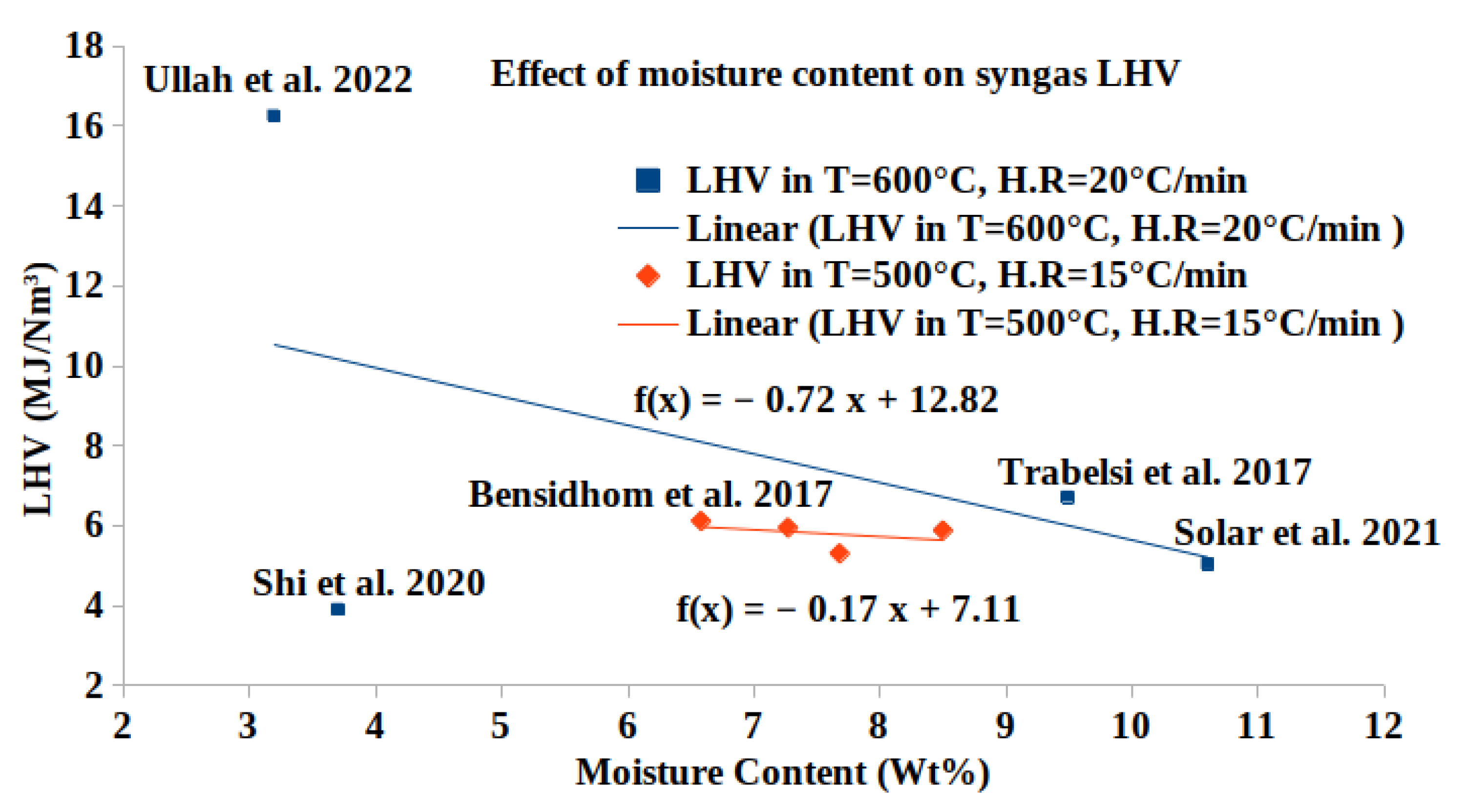
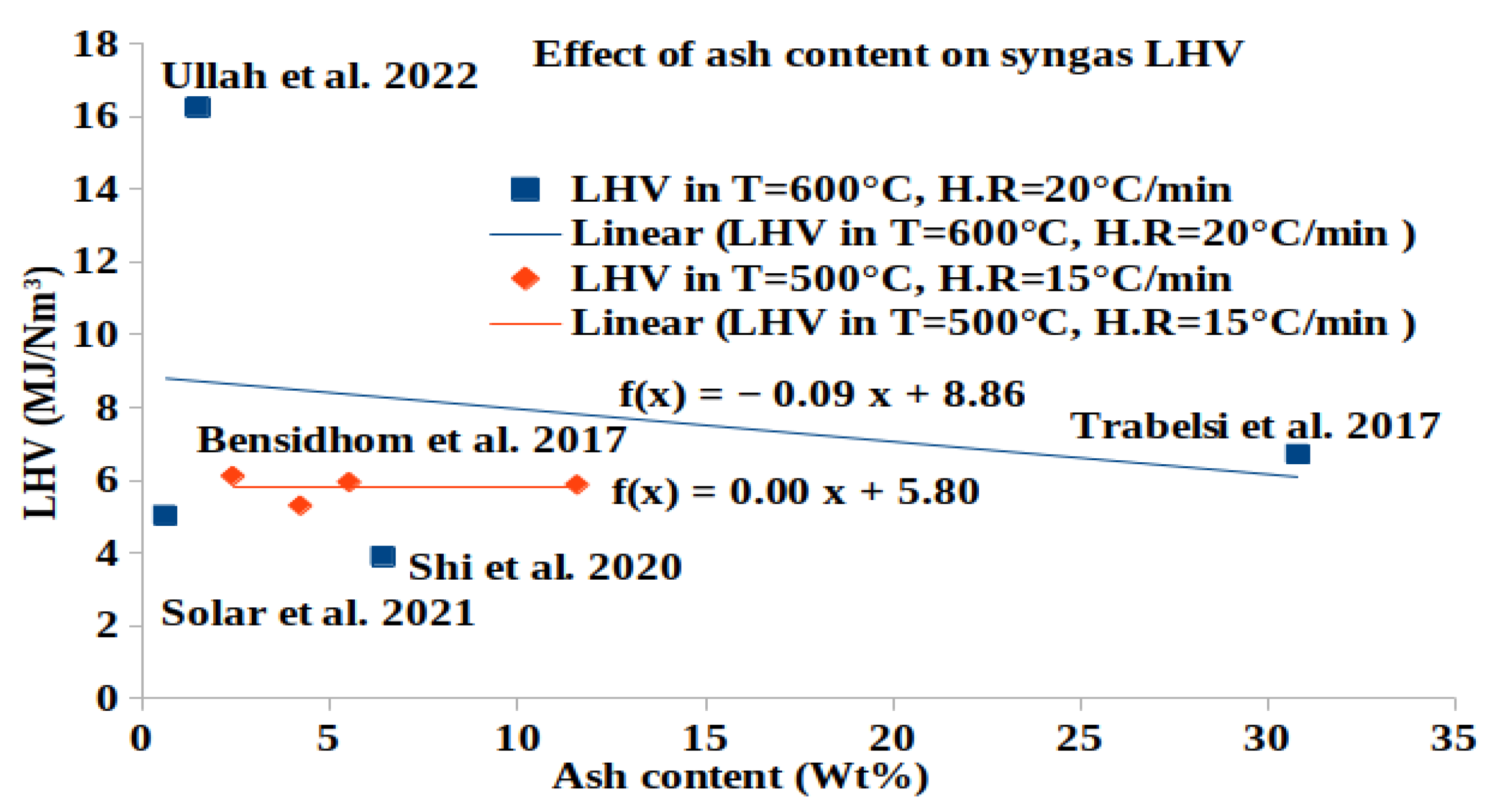
A Comprehensive Review of Syngas Production, Fuel Properties, and Operational Parameters for Biomass Conversion
Abstract
1. Introduction
2. Pyrolytic Generation of Syngas
3. Gasification for Syngas Production
3.1. The Gasifying Agent
3.2. Equivalence Ratio
| Biomass | GT | T (°C) | GA | Catalyst | ER | LHV | H2/CO | CO/CO2 | TY | GY |
|---|---|---|---|---|---|---|---|---|---|---|
| Pine sawdust [63] | fluidized-bed | 800 | air–steam mixture | dolomite and nickel | 0.3 | - | 1.54 | 0.9 | 13.85 | 1.56 |
| Pine sawdust [63] | fluidized-bed | 800 | air–steam mixture | dolomite and nickel | 0.25 | - | 1.46 | 0.99 | 19.05 | 1.54 |
| Bamboo [24] | updraft fixed-bed | 800 | steam | no | 0.2 | 6.2 | 0.71 | 0.43 | - | - |
| Wood residue [70] | fluidized-bed | 823 | mixture of oxygen and steam | Ni/Al2O3 (20 wt%) | 0.17 | - | 1.22 | 0.48 | - | 1.31 |
| Palletized Napier grass [64] | fluidized-bed gasifier | 800 | air | no | 0.2 | - | 3.44 | 0.5 | - | 1.93 |
| White pine [71] | fluidized-bed | 750 | air–steam | no | 0.3 | 4.65 | 3.9 | 0.18 | - | 2.18 |
| Citrus peels [71] | fluidized-bed | 750 | air–steam | no | 0.3 | 3.88 | 2.4 | 0.5 | - | 2.38 |
| Posidonia Oceanica [71] | fluidized-bed | 750 | air–steam | no | 0.3 | 2.67 | 6.0 | 0.2 | - | 2.7 |
| Poultry litter [65] | bubbling fluidized-bed | 750 | air | no | 0.17 | 4.22 | 0.9 | 1.1 | 4.87 | 1.22 |
| Poultry litter [65] | 0.21 | 4.2 | 0.89 | 0.98 | 4.25 | 1.247 | ||||
| Beech wood [65] | bubbling fluidized-bed | 750 | air | no | 0.18 | 4.96 | 0.5 | 1.14 | 7.52 | 0.86 |
| Beech wood [65] | bubbling fluidized-bed | 750 | air | no | 0.225 | 4.86 | 0.55 | 1.0 | 7.44 | 0.948 |
| Beech wood [65] | bubbling fluidized-bed | 750 | air | no | 0.27 | 4.82 | 0.46 | 0.95 | 6.72 | 0.976 |
| Rice husks [66] | autothermal cyclone gasifier | 853 | air | no | 0.23 | 4.23 | 0.12 | 1.45 | 6.85 | - |
| Rice husks [66] | autothermal cyclone gasifier | 888 | air | no | 0.29 | 3.99 | 0.1438 | 1.28 | 5.07 | - |
| Rice husks [66] | autothermal cyclone | 908 | air | no | 0.32 | 3.63 | 0.1435 | 1.14 | 4.96 | - |
| Birchwood [67] | fixed-bed downdraft | - | air | no | 0.2 | 3.72 | 0.47 | 1.63 | - | 1.76 |
| Birchwood [67] | fixed-bed downdraft | - | air | no | 0.35 | 3.82 | 0.31 | 1.38 | - | 2.6 |
| Birchwood [67] | fixed-bed downdraft | - | air | no | 0.5 | 3.61 | 0.27 | 1.27 | - | 2.56 |
| Oil palm fronds [68] | downdraft gasifier | air | no | 0.37 | 4.83 | 0.57 | 1.81 | - | - | |
| Olive kernel [69] | downdraft fixed-bed | - | air | no | 0.14 | 10.38 | 1.53 | 0.51 | - | - |
| Olive kernel [69] | downdraft fixed-bed | - | air | no | 0.21 | 10.5 | 1.69 | 0.48 | - | - |
| Olive kernel [69] | downdraft fixed-bed | - | air | no | 0.41 | 8.75 | 1.53 | 0.42 | - | - |
| Olive tree cuttings [69] | downdraft fixed-bed | - | air | no | 0.14 | 11.32 | 1.38 | 0.45 | - | - |
| Olive tree cuttings [69] | downdraft fixed-bed | - | air | no | 0.21 | 10.38 | 1.71 | 0.41 | - | - |
| Olive tree cuttings [69] | downdraft fixed-bed | - | air | no | 0.41 | 8.06 | 1.53 | 0.25 | - | - |
3.3. The Effect of the Form of the Biomass
3.3.1. The Gasification of the Pellets
3.3.2. The Gasification of the Briquettes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency. 2024. Available online: https://www.iea.org/countries/tunisia (accessed on 20 March 2023).
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar]
- Johnsson, F.; Kjärstad, J.; Rootzén, J. The threat to climate change mitigation posed by the abundance of fossil fuels. Clim. Policy 2019, 19, 258–274. [Google Scholar] [CrossRef]
- IEA. Global Energy-Related Greenhouse Gas Emissions, 2000–2022. 2023. Available online: https://www.iea.org/data-and-statistics/charts/global-energy-related-greenhouse-gas-emissions-2000-2022 (accessed on 2 March 2023).
- Ram, M.; Child, M.; Aghahosseini, A.; Bogdanov, D.; Lohrmann, A.; Breyer, C. A comparative analysis of electricity generation costs from renewable, fossil fuel and nuclear sources in G20 countries for the period 2015–2030. J. Clean. Prod. 2018, 199, 687–704. [Google Scholar] [CrossRef]
- Babu, B.V. Biomass pyrolysis: A state-of-the-art review. Biofuels Bioprod. Biorefin. 2008, 2, 393–414. [Google Scholar] [CrossRef]
- IEA. Available online: https://www.iea.org/world (accessed on 20 March 2023).
- Liu, W.; Liu, C.; Gogoi, P.; Deng, Y. Overview of Biomass Conversion to Electricity and Hydrogen and Recent Developments in Low-Temperature Electrochemical Approaches. Engineering 2020, 6, 1351–1363. [Google Scholar] [CrossRef]
- Trabelsi, A.B.H.; Ghrib, A.; Zaafouri, K.; Friaa, A.; Ouerghi, A.; Naoui, S.; Belayouni, H. Hydrogen-Rich Syngas Production from Gasification and Pyrolysis of Solar Dried Sewage Sludge: Experimental and Modeling Investigations. BioMed Res. Int. 2017, 2017, 7831470. [Google Scholar] [CrossRef]
- Trabelsi, A.B.H.; Zaafouri, K.; Baghdadi, W.; Naoui, S.; Ouerghi, A. Second generation biofuels production from waste cooking oil via pyrolysis process. Renew. Energy 2018, 126, 888–896. [Google Scholar] [CrossRef]
- Hassen-Trabelsi, A.B.; Kraiem, T.; Naoui, S.; Belayouni, H. Pyrolysis of waste animal fats in a fixed-bed reactor: Production and characterization of bio-oil and bio-char. J. Waste Manag. 2014, 34, 210–218. [Google Scholar] [CrossRef]
- Aissaoui, M.H.; Trabelsi, A.B.H.; Bensidhom, G.; Ceylan, S.; Leahy, J.J.; Kwapinski, W. Sustainable Valorization of Olive Pomace Waste to Renewable Biofuels, Biomaterials and Biochemicals via Pyrolysis Process: Experimental and Numerical Investigation. 2021. Available online: https://www.researchgate.net/publication/354942727_Sustainable_Valorization_of_Olive_Pomace_Waste_to_Renewable_Biofuels_Biomaterials_and_Biochemicals_Via_Pyrolysis_Process_Experimental_and_Numerical_Investigation (accessed on 20 March 2023).
- Grioui, N.; Halouani, K.; Agblevor, F.A. Bio-oil from pyrolysis of Tunisian almond shell: Comparative study and investigation of aging effect during long storage. Energy Sustain. Dev. 2014, 21, 100–112. [Google Scholar] [CrossRef]
- Bensidhom, G.; Hassen-Trabelsi, A.B.; Alper, K.; Sghairoun, M.; Zaafouri, K.; Trabelsi, I. Pyrolysis of Date palm waste in a fixed-bed reactor: Characterization of pyrolytic products. Bioresour. Technol. 2017, 247, 363–369. [Google Scholar] [CrossRef]
- Yang, Y.; Qian, X.; Alamu, S.O.; Brown, K.; Lee, S.W.; Kang, D.-H. Qualities and Quantities of Poultry Litter Biochar Characterization and Investigation. Energies 2024, 17, 2885. [Google Scholar] [CrossRef]
- Vendra Singh, S.; Chaturvedi, S.; Dhyani, V.C.; Kasivelu, G. Pyrolysis temperature influences the characteristics of rice straw and husk biochar and sorption/desorption behaviour of their biourea composite. Bioresour. Technol. 2020, 314, 123674. [Google Scholar] [CrossRef] [PubMed]
- Khiari, B.; Jeguirim, M. Pyrolysis of Grape Marc from Tunisian Wine Industry: Feedstock Characterization, Thermal Degradation and Kinetic Analysis. Energies 2018, 11, 730. [Google Scholar] [CrossRef]
- Ruiz, J.A.; Juárez, M.C.; Morales, M.P.; Muñoz, P.; Mendívil, M.A. Biomass gasification for electricity generation: Review of current technology barriers. Renew. Sustain. Energy Rev. 2013, 18, 174–183. [Google Scholar] [CrossRef]
- Ongen, A.; Ozcan, H.K.; Ozbas, E.E. Gasification of biomass and treatment sludge in a fixed bed gasifier. Int. J. Hydrogen Energy 2016, 41, 8146–8153. [Google Scholar] [CrossRef]
- Plis, P.; Wilk, R.K. Theoretical and experimental investigation of biomass gasification process in a fixed bed gasifier. Energy 2011, 36, 3838–3845. [Google Scholar] [CrossRef]
- Aydin, E.S.; Yucel, O.; Sadikoglu, H. Experimental study on hydrogen-rich syngas production via gasification of pine cone particles and wood pellets in a fixed bed downdraft gasifier. Int. J. Hydrogen Energy 2019, 44, 17389–17396. [Google Scholar] [CrossRef]
- Khlifi, S.; Lajili, M.; Perré, P.; Pozzobon, V. A Numerical Study of Turbulent Combustion of a Lignocellulosic Gas Mixture in an Updraft Fixed Bed Reactor. Sustainability 2022, 14, 16587. [Google Scholar] [CrossRef]
- Luo, S.; Zhou, Y.; Yi, C. Syngas production by catalytic steam gasification of municipal solid waste in fixed-bed reactor. Energy 2012, 44, 391–395. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, C.; Ying, Z.; Wang, B. Experimental study on gasification performance of bamboo and PE from municipal solid waste in a bench-scale fixed bed reactor. Energy Convers. Manag. 2016, 117, 393–399. [Google Scholar] [CrossRef]
- Kruesi, M.; Jovanovic, Z.R.; dos Santos, E.C.; Yoon, H.C.; Steinfeld, A. Solar-driven steam-based gasification of sugarcane bagasse in a combined drop-tube and fixed-bed reactor-Thermodynamic, kinetic, and experimental analyses. Biomass Bioenergy 2013, 52, 173–183. [Google Scholar] [CrossRef]
- Whitty, K.J.; Zhang, H.R.; Eddings, E.G. Emissions from syngas combustion. Combust. Sci. Technol. 2008, 180, 1117–1136. [Google Scholar] [CrossRef]
- Zribi, M.; Lajili, M.; Escudero-Sanz, F.J. Hydrogen enriched syngas production via gasification of biofuels pellets/powders blended from olive mill solid wastes and pine sawdust under different water steam/nitrogen atmospheres. Int. J. Hydrogen Energy 2018, 44, 11280–11288. [Google Scholar] [CrossRef]
- Demirbas, A.; Arin, G. An Overview of Biomass Pyrolysis. Energy Sources 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Andican, A.; Faizal, M.; Said, M.; Aprianti, N. Synthetic Gas Production from Fine Coal Gasification Using Low-Cost Catalyst. J. Ecol. Eng. 2022, 23, 64–72. [Google Scholar] [CrossRef]
- David, E. Evaluation of Hydrogen Yield Evolution in Gaseous Fraction and Biochar Structure Resulting from Walnut Shells Pyrolysis. Energies 2020, 13, 6359. [Google Scholar] [CrossRef]
- Xie, Y.; Zeng, K.; Flamant, G.; Yang, H.; Liu, N.; He, X.; Yang, X.; Nzihou, A.; Chen, H. Solar pyrolysis of cotton stalk in molten salt for bio-fuel production: Review. Energy 2019, 179, 1124–1132. [Google Scholar] [CrossRef]
- Sedmihradská, A.; Pohořelý, M.; Jevič, P.; Skoblia, S.; Beňo, Z.; Farták, J.; Čech, B.; Hartman, M. Pyrolysis of wheat and barley straw. Res. Agric. Eng. 2020, 66, 8–17. [Google Scholar] [CrossRef]
- Mansouri, S.; Khiari, R.; Bendouissa, N.; Saadallah, S.; Mhenni, F.; Mauret, E. Chemical composition and pulp characterization of Tunisian vine stems. Ind. Crop. Prod. 2012, 36, 22–27. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Evangelopoulos, P.; Sieradzka, M.; Zajemska, M.; Magdziarz, A. Pyrolysis of agricultural waste biomass towards production of gas fuel and high-quality char: Experimental and numerical investigations. Fuel 2021, 296, 120611. [Google Scholar] [CrossRef]
- Qian, X.; Xue, J.; Yang, Y.; Lee, S.W. Thermal Properties and Combustion-Related Problems Prediction of Agricultural Crop Residues. Energies 2021, 14, 4619. [Google Scholar] [CrossRef]
- Kraiem, N.; Jeguirim, M.; Limousy, L.; Lajili, M.; Dorge, S.; Michelin, L.; Said, R. Impregnation of olive mill wastewater on dry biomasses: Impact on chemical properties and combustion performances. Energy 2014, 78, 479–489. [Google Scholar] [CrossRef]
- Lajili, M.; Guizani, C.; Sanz, F.J.E.; Jeguirim, M. Fast pyrolysis and steam gasification of pellets prepared from olive oil mill residues. Energy 2018, 150, 61–68. [Google Scholar] [CrossRef]
- Kraiem, N.; Lajili, M.; Limousy, L.; Said, R.; Jeguirim, M. Energy recovery from Tunisian agri-food wastes: Evaluation of combustion performance and emissions characteristics of green pellets prepared from tomato residues and grape marc. Energy 2016, 107, 409–418. [Google Scholar] [CrossRef]
- Limousy, L.; Jeguirim, M.; Dutournié, P.; Kraiem, N.; Lajili, M.; Said, R. Gaseous Products and particulate matter emissions of biomass residential boiler fired with spent coffee grounds pellets. Fuel 2016, 107, 323–329. [Google Scholar] [CrossRef]
- Ullah, F.; Zhang, L.; Ji, G.; Irfan, M.; Ma, D.; Li, A. Experimental analysis on products distribution and characterization of medical waste pyrolysis with a focus on liquid yield quantity and quality. Sci. Total Environ. 2022, 829, 154692. [Google Scholar] [CrossRef]
- Salem, A.M.; Dhami, H.S.; Paul, M.C. Syngas Production and Combined Heat and Power from Scottish Agricultural Waste Gasification—A Computational Study. Sustainability 2022, 14, 3745. [Google Scholar] [CrossRef]
- Shi, K.; Yan, J.; Menéndez, J.A.; Luo, X.; Yang, G.; Chen, Y.; Lester, E.; Wu, T. Production of H2-Rich Syngas from Lignocellulosic Biomass Using Microwave-Assisted Pyrolysis Coupled with Activated Carbon Enabled Reforming. Front. Chem. 2020, 8, 3. [Google Scholar] [CrossRef]
- Solar, J.; Caballero, B.M.; López-Urionabarrenechea, A.; Acha, E.; Arias, P.L. Pyrolysis of Forestry Waste in a Screw Reactor with Four Sequential Heating Zones: Influence of Isothermal and Nonisothermal Profiles. Ind. Eng. Chem. Res. 2021, 60, 18627–18639. [Google Scholar] [CrossRef]
- Shehzad, A.; Bashir, M.J.K.; Horttanainen, M.; Manttari, M.; Havukainen, J.; Abbas, G. Modeling and comparative assessment of bubbling fluidized bed gasification system for syngas production—A gateway for a cleaner future in Pakistan. Environ. Technol. 2017, 39, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Atnaw, S.M.; Sulaiman, S.A.; Yusup, S. Influence of Fuel Moisture Content and Reactor Temperature on the Calorific Value of Syngas Resulted from Gasification of Oil Palm Fronds. Sci. World J. 2014, 2014, 121908. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Chi, Y.; Tang, Y.; Ni, M.; Nzihou, A.; Weiss-Hortala, E.; Huang, Q. Effect of Operating Parameters and Moisture Content on Municipal Solid Waste Pyrolysis and Gasification. Energy Fuels. 2016, 30, 3994–4001. [Google Scholar] [CrossRef]
- Chen, J.; Gao, S.; Xu, F.; Xu, W.; Yang, Y.; Kong, D.; Wang, Y.; Yao, H.; Chen, H.; Zhu, Y.; et al. Influence of moisture and feedstock form on the pyrolysis behaviors, pyrolytic gas production, and residues micro-structure evolutions of an industrial sludge from a steel production enterprise. Energy 2022, 248, 123603. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, S.; Huang, S.; Tang, J.; Du, T.; Cheng, X.; Huang, R.; Chen, J.-Y. Effects of water content on evaporation and combustion characteristics of water emulsified diesel spray. Appl. Energy 2018, 226, 397–407. [Google Scholar] [CrossRef]
- Fraia, D.S.; Uddin, M.R. Energy Recovery from Waste Paper and Deinking Sludge to Support the Demand of the Paper Industry: A Numerical Analysis. Sustainability 2022, 14, 4669. [Google Scholar] [CrossRef]
- Puri, L.; Hu, Y.; Naterer, G. Critical review of the role of ash content and composition in biomass pyrolysis. Front. Fuels 2024, 2, 1378361. [Google Scholar] [CrossRef]
- Rafiq, M.K.; Bachmann, R.T.; Rafiq, M.T.; Shang, Z.; Joseph, S.; Long, R.L. Influence of Pyrolysis Temperature on Physico-Chemical Properties of Corn Stover (Zea mays L.) Biochar and Feasibility for Carbon Capture and Energy Balance. PLoS ONE 2016, 11, e0156894. [Google Scholar] [CrossRef] [PubMed]
- Kardaś, D.; Kluska, J.; Kazimierski, P. The course and effects of syngas production from beechwood and RDF in updraft reactor in the light of experimental tests and numerical calculations. Therm. Sci. Eng. Prog. 2018, 8, 136–144. [Google Scholar] [CrossRef]
- Samiran, N.A.; Jaafar, M.N.M.; Ng, J.-H.; Lam, S.S.; Chong, C.T. Progress in biomass gasification technique—With focus on Malaysian palm biomass for syngas production. Renew. Sustain. Energy Rev. 2016, 62, 1047–1062. [Google Scholar] [CrossRef]
- Santos, S.M.; Assis, A.C.; Gomes, L.; Nobre, C.; Brito, P. Waste Gasification Technologies: A Brief Overview. Waste 2023, 1, 140–165. [Google Scholar] [CrossRef]
- Havilah, P.R.; Sharma, A.K.; Govindasamy, G.; Matsakas, L.; Patel, A. Biomass Gasification in Downdraft Gasifiers: A Technical Review on Production, Up-Gradation and Application of Synthesis Gas. Energies 2022, 15, 3938. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Zawawi, N.A.; Kasim, F.H.; Inayat, A.; Khasri, A. Assessing the gasification performance of biomass: A review on biomass gasification process conditions, optimization, and economic evaluation. Renew. Sustain. Energy Rev. 2016, 53, 1333–1347. [Google Scholar] [CrossRef]
- Hu, X.; Bai, F.; Yu, C.; Yan, F. Experimental Study of the Laminar Flame Speeds of the CH4/H2/CO/CO2/N2 Mixture and Kinetic Simulation in Oxygen-Enriched Air Condition. ACS Omega 2020, 5, 33372–33379. [Google Scholar] [CrossRef]
- Fiore, M.; Magi, V.; Viggiano, A. Internal combustion engines powered bysyngas: A review. Appl. Energy 2020, 276, 115415. [Google Scholar] [CrossRef]
- Gallucci, F.; Liberatore, R.; Sapegno, L.; Volponi, E.; Venturini, P.; Rispoli, F.; Paris, E.; Carnevale, M.; Colantoni, A. Influence of Oxidant Agent on Syngas Composition: Gasification of Hazelnut Shells through an Updraft Reactor. Energies 2020, 13, 102. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.; Miranda, M.; Neves, D.; Varela, F.; Santos, J. Effect of gasification agent on co-gasification of rice production wastes mixtures. Fuel 2016, 180, 407–416. [Google Scholar] [CrossRef]
- Dai, J.; Saayman, J.; Grace, J.R.; Ellis, N. Gasification of Woody Biomass. Annu. Rev. Chem. Biomol. Eng. 2015, 6, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Yuan, Z.; Wu, C.; Ma, L.; Chen, Y.; Tsubaki, N. Bio-syngas production from biomass catalytic gasification. Energy Convers. Manag. 2007, 48, 1132–1139. [Google Scholar] [CrossRef]
- Khezri, R.; Azlinaa, W.; Tan, H.B. An Experimental Investigation of Syngas Composition from Small-Scale Biomass Gasification. Int. J. Biomass Renew. 2016, 5, 6–13. [Google Scholar] [CrossRef]
- Katsaros, G.; Pandey, D.S.; Horvat, A.; Almansa, G.A.; Fryda, L.E.; Leahy, J.J.; Tassou, S.A. Experimental investigation of poultry litter gasification and co-gasification with beech wood in a bubbling fluidised bed reactor—Effect of equivalence ratio on process performance and tar evolution. Fuel 2020, 262, 116660. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, D.; Zhang, Z.; Sun, S.; Che, H.; Luan, J. Experimental Study on Autothermal Cyclone Air Gasification of Biomass. J. Energy Resour. Technol. 2017, 140, 042001. [Google Scholar] [CrossRef]
- Jayathilake, R.; Rudra, S. Numerical and Experimental Investigation of Equivalence Ratio (ER) and Feedstock Particle Size on Birchwood Gasification. Energies 2017, 10, 1232. [Google Scholar] [CrossRef]
- Atnaw, S.M.; Sulaiman, S.A.; Yusup, S. Syngas production from downdraft gasification of oil palm fronds. Energy 2013, 61, 491–501. [Google Scholar] [CrossRef]
- Skoulou, V.; Zabaniotou, A.; Stavropoulos, G.; Sakelaropoulos, G. Syngas production from olive tree cuttings and olive kernels in a downdraft fixed-bed gasifier. Int. J. Hydrogen Energy 2018, 33, 1185–1194. [Google Scholar] [CrossRef]
- Peng, W.X.; Wang, L.S.; Mirzaee, M.; Ahmadi, H.; Esfahani, M.J.; Fremaux, S. Hydrogen and syngas production by catalytic biomass gasification. Energy Convers. Manag. 2017, 135, 270–273. [Google Scholar] [CrossRef]
- Maisano, S.; Urbani, F.; Cipitı, F.; Freni, F.; Chiodo, V. Syngas production by BFB gasification: Experimental comparison of different biomasses. Int. J. Hydrogen Energy 2018, 44, 4414–4422. [Google Scholar] [CrossRef]
- Meng, F.; Meng, J.; Zhang, D. Influence of higher equivalence ratio on the biomass oxygen gasification in a pilot scale fixed bed gasifier. J. Renew. Sustain. Energy 2018, 10, 053101. [Google Scholar] [CrossRef]
- Yoon, S.J.; Son, Y.-I.; Kim, Y.-K.; Lee, J.-G. Gasification and power generation characteristics of rice husk and rice husk pellet using a downdraft fixed-bed gasifier. Renew. Energy 2012, 42, 163–167. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, L.; Peng, X.; Mao, M.; Chi, Y.; Wang, F. Effect of Sludge Pellets Addition on Combustion Characteristics and Ash Behaviour of Municipal Solid Waste. Waste Biomass Valorization 2020, 11, 5351–5361. [Google Scholar] [CrossRef]
- Khlifi, S.; Lajili, M.; Belghith, S.; Mezlini, S.; Tabet, F.; Jeguirim, M. Briquettes production from olive mill waste under optimal temperature and pressure conditions: Physico-chemical and mechanical characterizations. Energies 2020, 13, 1214. [Google Scholar] [CrossRef]
- Khardiwar, M.S.; Dubey, A.K.; Mahalle, D.M.; Kumar, S. Study on Physical and Chemical Properties of crop Residues briquettes for gasification. Int. J. Renew. Energy Technol. Res. 2013, 2, 237–248. [Google Scholar]
- Timsina, R.; Thapa, R.K.; Moldestad, B.M.E.; Jaiswal, R.; Bhattarai, A.; Jecmenica, M.; Eikeland, M.S. Experimental evaluation of wood and grass pellets in a bubbling fluidized bed gasifier. Energy Rep. 2023, 9, 4049–4058. [Google Scholar] [CrossRef]
- Mikeska, M.; Najser, J.; Peer, V.; Frantık, J.; Kielar, J. Quality assessment of gas produced from different types of biomass pellets in gasification process. Energy Explor. Exploit. 2020, 38, 406–416. [Google Scholar] [CrossRef]
- Kurkela, E.; Kurkela, M.; Hiltunen, I. Production of Synthesis Gas from Biomass Residues by Staged Fixed-bed Gasification—Results from Pilot Test Campaigns. Chem. Eng. Trans. 2021, 86, 7–12. [Google Scholar] [CrossRef]
- Yasuda, H.; Murakami, T. Visualization of solid distribution with heterogeneity inside fixed bed gasifier. J. Mater. Cycles Waste Manag. 2020, 22, 1561–1568. [Google Scholar] [CrossRef]
- Sharma, T.; Maya, D.M.Y.; Nascimento, F.R.M.; Shi, Y.; Ratner, A.; Lora, E.E.S.; Neto, L.J.M.; Palacios, J.C.E.; Andrade, R.V. An Experimental and Theoretical Study of the Gasification of Miscanthus Briquettes in a Double-Stage Downdraft Gasifier: Syngas, Tar, and Biochar Characterization. Energies 2018, 11, 3225. [Google Scholar] [CrossRef]
- Arjharn, W.; Hinsui, T.; Liplap, P.; Raghavan, G.S.V. Evaluation of an Energy Production System from Sewage Sludge Using a Pilot-Scale Downdraft Gasifier. Energy Fuels 2013, 27, 229–236. [Google Scholar] [CrossRef]
- Singla, M.; Singh, M.; Dogra, R. Experimental investigation of imbert downdraft gasifier using rice straw briquettes. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 1–11. [Google Scholar] [CrossRef]
- Dogru, M. Experimental Results of Olive Pits Gasification in a Fixed Bed downdraft Gasifier System. Int. J. Green Energy 2013, 10, 348–361. [Google Scholar] [CrossRef]
- Raj, R.; Singh, D.K.; Tirkey, J.V. Gasifier-engine performance analysis using Co-gasification of mahua wood waste and sawdust briquette blend: An experimental and optimization approach. Biomass Convers. Biorefin. 2023, 1–24. [Google Scholar] [CrossRef]
- Rasmussena, N.B.K.; Aryal, N. Syngas production using straw pellet gasification in fluidized bed allothermal reactor under different temperature conditions. Fuel 2019, 263, 116706. [Google Scholar] [CrossRef]
- Wiyono, A.; Saw, L.H.; Anggrainy, R.; Husen, A.S.; Purnawan; Rohendi, D.; Gandidi, I.M.; Adanta, D.; Pambudi, N.A. Enhancement of syngas production via co-gasification and renewable densified fuels (RDF) in an open-top downdraft gasifier: Case study of Indonesian waste. Case Stud. Therm. Eng. 2021, 27, 101205. [Google Scholar] [CrossRef]
- Schweitzer, D.; Gredinger, A.; Schmid, M.; Waizmann, G.; Beirow, M.; Sporl, R.; Scheffknecht, G. Steam gasification of wood pellets, sewage sludge and manure: Gasification performance and concentration of impurities. Biomass Bioenergy 2017, 111, 308–319. [Google Scholar] [CrossRef]
- Nobre, C.; Longo, A.; Vilarinho, C.; Gonçalves, M. Gasification of pellets produced from blends of biomass wastes and refuse derived fuel chars. Renew. Energy 2020, 154, 1294–1303. [Google Scholar] [CrossRef]
- Sulaiman, S.A.; Razali, N.H.M.; Konda, R.E.; Atnaw, S.M.; Moni, M.N.Z. On the Diversification of Feedstock in Gasification of oil Palm Fronds. J. Mech. Eng. Sci. 2014, 6, 907–915. [Google Scholar] [CrossRef]
| Sources | Ultimate Analysis | Proximate Analysis (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | Moisture Content | Ash Content | Volatile Matter | Fixed Carbon | |
| Sewage sludge [9] | 48.21 | 8.17 | 10.15 | 1.71 | 0.96 | 9.49 | 30.80 | 58.81 | 0.90 |
| Olive pomace waste [12] | 47.34 | 6.6 | 41.31 | 2.73 | - | 3.44 | 2.02 | 73.22 | 21.32 |
| Date palm leaflets [14] | 43.14 | 7.49 | 37.59 | 0.2 | - | 8.50 | 11.58 | 72.28 | 7.64 |
| Date palm rachis [14] | 39.95 | 7.19 | 47.2 | 0.16 | - | 7.27 | 5.50 | 78.11 | 9.12 |
| Empty fruit bunch [14] | 43.49 | 7.51 | 44.61 | 0.19 | - | 7.68 | 4.20 | 81.20 | 6.92 |
| Date palm glaich [14] | 43.65 | 7.59 | 46.2 | 0.16 | - | 6.58 | 2.40 | 83.84 | 7.18 |
| Walnut shells [31] | 49.26 | 5.38 | 43.9 | 0.28 | - | 7.85 | 1.18 | 80.21 | 10.76 |
| Bamboo [43] | 49.9 | 6.5 | 30.6 | 6.0 | 0.6 | 3.7 | 6.4 | 74.0 | 15.9 |
| Pinus radiata [44] | 47.8 | 7.6 | 44 | 0 | 0 | 10.6 | 0.6 | 70.7 | 18.1 |
| Cotton stalk [32] | 46.56 | 6.04 | 42.01 | 0.79 | - | 1.86 | 4.60 | 77.5 | 16.04 |
| Wheat straw [33] | 42.36 | 5.27 | 45.15 | 1.12 | >0.1 | 8.40 | 6.00 | 62.40 | 23.30 |
| Barley straw [33] | 42.44 | 5.25 | 44.73 | 1.18 | >0.1 | 8.60 | 6.30 | 62.10 | 23.10 |
| Medical waste [41] | 59.0 | 8.0 | 24.53 | 2.0 | 5.0 | 3.19 | 1.47 | 92.72 | 2.62 |
| Waste cooking oil [10] | 75.61 | 13.27 | 9.79 | 0.12 | 0.09 | - | 1.12 | - | - |
| Lamb fatty wastes [11] | 74.63 | 12.11 | 12.50 | 0.15 | 0.27 | - | 0.34 | - | - |
| Almond shells [13] | 45.64 | 6.19 | 45.43 | <0.5 | <0.05 | 7 | 2.71 | - | - |
| Source | Conditions of Syngas Production | Syngas | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (°C/min) | Temperature (°C) | Residence Time (min) | GY | Composition (Vol %) | LHV (MJ/Nm3) | |||||
| (wt.%) | CH4 | CO2 | H2 | CO | C2H6 | |||||
| Sewage sludge [9] | 20 | 600 | 30 | 21.77 | 17.16 | 2.31 | 74.69 | 3.69 | 2.17 | 6.716 |
| Olive pomace waste [12] | 15 | 600 | 40 | 37 | 10.54 | 0.34 | 52.08 | 35.65 | 1.39 | 6.213 |
| Date palm leaflets [14] | 15 | 500 | 54 | 46 | 7.14 | 0.26 | 30.36 | 61.65 | 0.6 | 5.88 |
| Date palm rachis [14] | 15 | 500 | 54 | 39 | 6.37 | 0.48 | 12.68 | 79.81 | 0.67 | 5.95 |
| Empty fruit bunch [14] | 15 | 500 | 54 | 40 | 5.09 | 0.11 | 70.89 | 23.63 | 0.28 | 5.305 |
| Date palm glaich [14] | 15 | 500 | 54 | 43 | 9.92 | 0.33 | 39.53 | 49.45 | 0.77 | 6.116 |
| Walnut shells [31] | 5 | 600 | 120 | 15 | 13 | 29.84 | 16.66 | 40.48 | - | 4.861 |
| Walnut shells [31] | 5 | 500 | 100 | 8.9 | 8.56 | 37.8 | 15.77 | 39.77 | - | 4.113 |
| Bamboo [43] | 20 | 600 | 30 | - | 1.01 | 23.8 | 30.11 | 45.06 | - | 3.905 |
| Bamboo [43] | 20 | 700 | 35 | - | 4.64 | 16.96 | 43.73 | 34.64 | - | 4.51 |
| Pinus radiata [44] | 20 | 600 | 30 | 35.6 | 16.8 | 37.5 | 20.4 | 24.2 | 1.1 | 5.037 |
| Pinus radiata [44] | 17 | 500 | 30 | 26.4 | 13.8 | 48.5 | 20.1 | 14.0 | 3.6 | 4.7 |
| Cotton stalk [32] | 10 | 550 | 30 | 28.2 | 11.74 | 45.89 | 6.98 | 30.68 | 4.68 | 4.98 |
| wheat straw [33] | 7 | 600 | 90 | 20.16 | 8.75 | 51.55 | 9.54 | 28.12 | 2.03 | 3.79 |
| wheat straw [33] | 6 | 500 | 90 | 18.91 | 7.75 | 56.69 | 4.04 | 29.53 | 1.98 | 3.45 |
| barley straw [33] | 7 | 600 | 90 | 19.1 | 10.33 | 49.1 | 10.55 | 27.84 | 2.18 | 4.10 |
| barley straw [33] | 6 | 500 | 90 | 20.26 | 8.86 | 55.06 | 3.91 | 29.76 | 2.41 | 3.74 |
| Medical waste [41] | 20 | 600 | 30 | 37.3 | 27.77 | 7.36 | 11.27 | 13.6 | 40.0 | 16.25 |
| Waste cooking oil [10] | 15 | 650 | - | 15.9 | 22.85 | 17.59 | 52.85 | 6.21 | 0.5 | 6.301 |
| Lamb fatty wastes [11] | 15 | 500 | 50 | 62 | - | - | - | - | - | - |
| Almond shells [13] | 10 | 537 | 50 | 47 | - | - | - | - | - | - |
| Densified Biomass | Diameter (mm) | Length (mm) | References |
|---|---|---|---|
| Wood pellets | 6 | 30–40 | [77] |
| Grass pellets | 7 | 30–40 | [77] |
| Wood pellets | 7 | 20 | [21] |
| Pellets from spruce wood, hay, and wheat straw | 8 | - | [78] |
| Wood, bark, and sunflower husk pellets | 8–10 | - | [79] |
| Wood pellets | 6 | 10–25 | [80] |
| Miscanthus briquettes | 20 | 30 | [81] |
| Soybean briquettes | 60 | 60–85 | [76] |
| Pigeon pea briquettes | 30 | 65–90 | [76] |
| Soybean–pigeon pea mixture briquettes | 60 | 65–80 | [76] |
| Sewage sludge briquettes | 45 | 40–50 | [82] |
| Rice straw briquettes (binder: cotton stalk) | - | 45–50 | [83] |
| Olive pits briquettes | 50 | 70 | [84] |
| Sawdust briquette | 95 | 30–35 | [85] |
| Biomass | GT | T (°C) | GA | Catalyst | ER | LHV | H2/CO | CO/CO2 | CCE (%) | CGE (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Wood–coconut fiber pellets [87] | downdraft gasifier | 900 | air | no | 0.32 | 3.58 | 7.1 | 0.16 | - | - |
| Rice husk pellets [87] | downdraft gasifier | 700 | air | no | 0.32 | 2.8 | 3.7 | 0.32 | - | - |
| Wood pellets [21] | downdraft | 877 | air | no | 0.278 | - | 0.84 | 3.76 | - | 87.6 |
| Spruce wood pellets [78] | fix-bed gasifier | 800 | air | no | n.d | 5.78 | 0.41 | 2.65 | - | - |
| Hay pellets [78] | fix-bed gasifier | 800 | air | no | n.d | 4.48 | 0.44 | 1.37 | - | - |
| Wheat straw pellets [78] | fix-bed gasifier | 800 | air | no | n.d | 3.93 | 0.44 | 1.32 | - | - |
| Wood pellets [88] | dual fluidized-bed | 800 | steam | no | n.d | - | 1.9 | 0.807 | - | - |
| Pine waste pellets (vol. %, dry) [89] | bubbling fluidized-bed | 800 | air | no | 0.25 | 5.4 | 0.37 | 1.25 | 96.1 | 72.9 |
| Straw pellets [86] | allothermal gasifier | 824 | steam | (Mg2+, Fe2+)2SiO4 | n.d | 7.9 | 0.79 | 0.5 | - | - |
| Wood pellets [86] | allothermal gasifier | 790 | steam | (Mg2+, Fe2+)2SiO4 | n.d | 8.0 | 0.83 | 0.6 | - | - |
| Biomass | GT | T (°C) | GA | Catalyst | ER | LHV | H2/CO | CO/CO2 | CCE (%) | CGE (%) | TY (g/Nm3) | GY (Nm3/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sewage sludge briquettes [82] | downdraft gasifier | 780 | air | no | 0.24 | 4.87 | 1.2 | 1.1 | - | - | 0.07678 | 1.47 |
| Olive pits briquettes [84] | downdraft fixed-bed | >1000 | air | no | - | 5.04 | 0.78 | 1.27 | >97 | 68.21 | 0.796 | - |
| Sawdust briquettes [85] | downdraft gasifier | 800–900 | air | no | - | 3.21 | 0.72 | 1.57 | - | - | 4 | - |
| Corn stalk briquettes [72] | fixed-bed gasifier | 923 | oxygen | no | 0.32 | 11.2 | 0.7 | 4.07 | 73.93 | - | - | - |
| Fronds briquettes (oil palm) [90] | updraft gasifier | 734 | air | no | - | - | 0.25 | 1 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khlifi, S.; Pozzobon, V.; Lajili, M. A Comprehensive Review of Syngas Production, Fuel Properties, and Operational Parameters for Biomass Conversion. Energies 2024, 17, 3646. https://doi.org/10.3390/en17153646
Khlifi S, Pozzobon V, Lajili M. A Comprehensive Review of Syngas Production, Fuel Properties, and Operational Parameters for Biomass Conversion. Energies. 2024; 17(15):3646. https://doi.org/10.3390/en17153646
Chicago/Turabian StyleKhlifi, Saaida, Victor Pozzobon, and Marzouk Lajili. 2024. "A Comprehensive Review of Syngas Production, Fuel Properties, and Operational Parameters for Biomass Conversion" Energies 17, no. 15: 3646. https://doi.org/10.3390/en17153646
APA StyleKhlifi, S., Pozzobon, V., & Lajili, M. (2024). A Comprehensive Review of Syngas Production, Fuel Properties, and Operational Parameters for Biomass Conversion. Energies, 17(15), 3646. https://doi.org/10.3390/en17153646