CO2 Corrosion of Downhole Sand Control Screen: Experiments, Model, and Application
Abstract
1. Introduction
2. Experimental
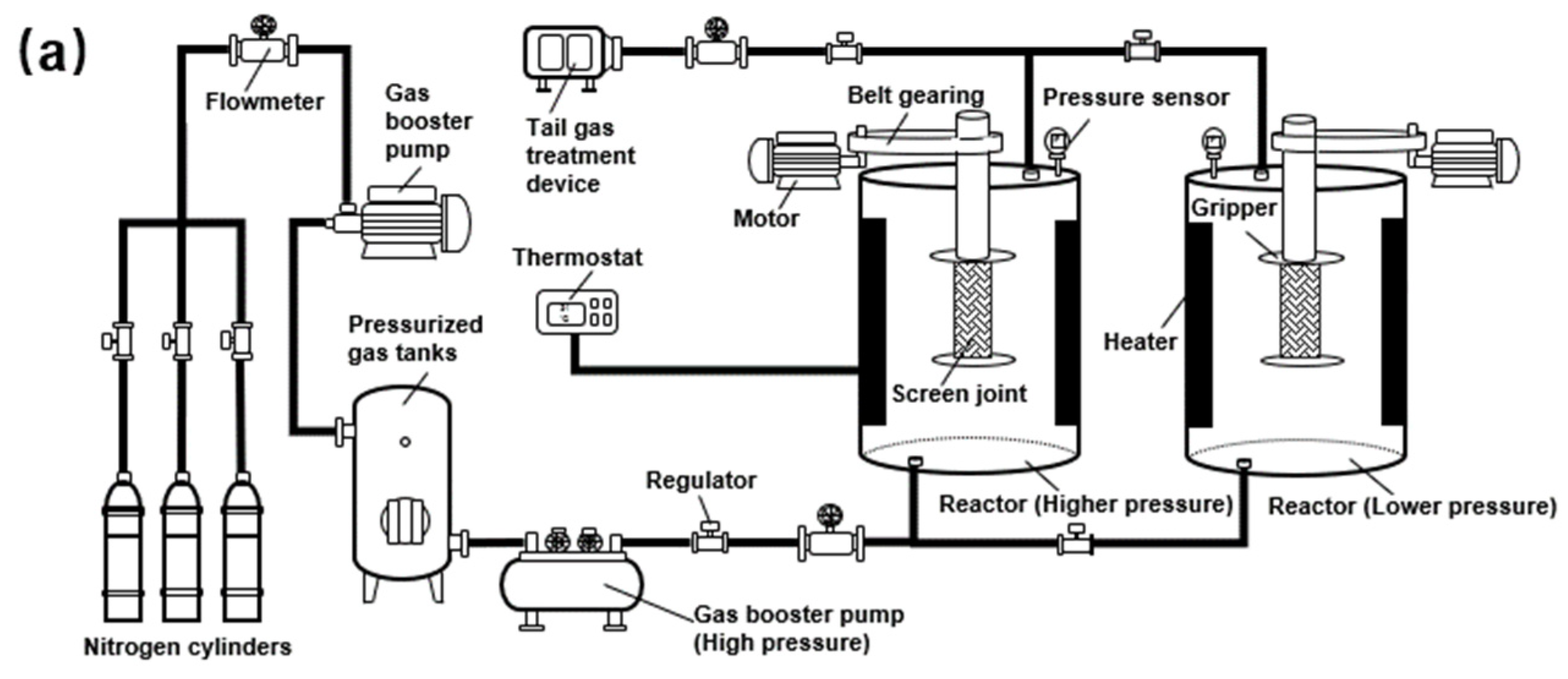
2.1. Apparatus
2.2. Materials and Preparation
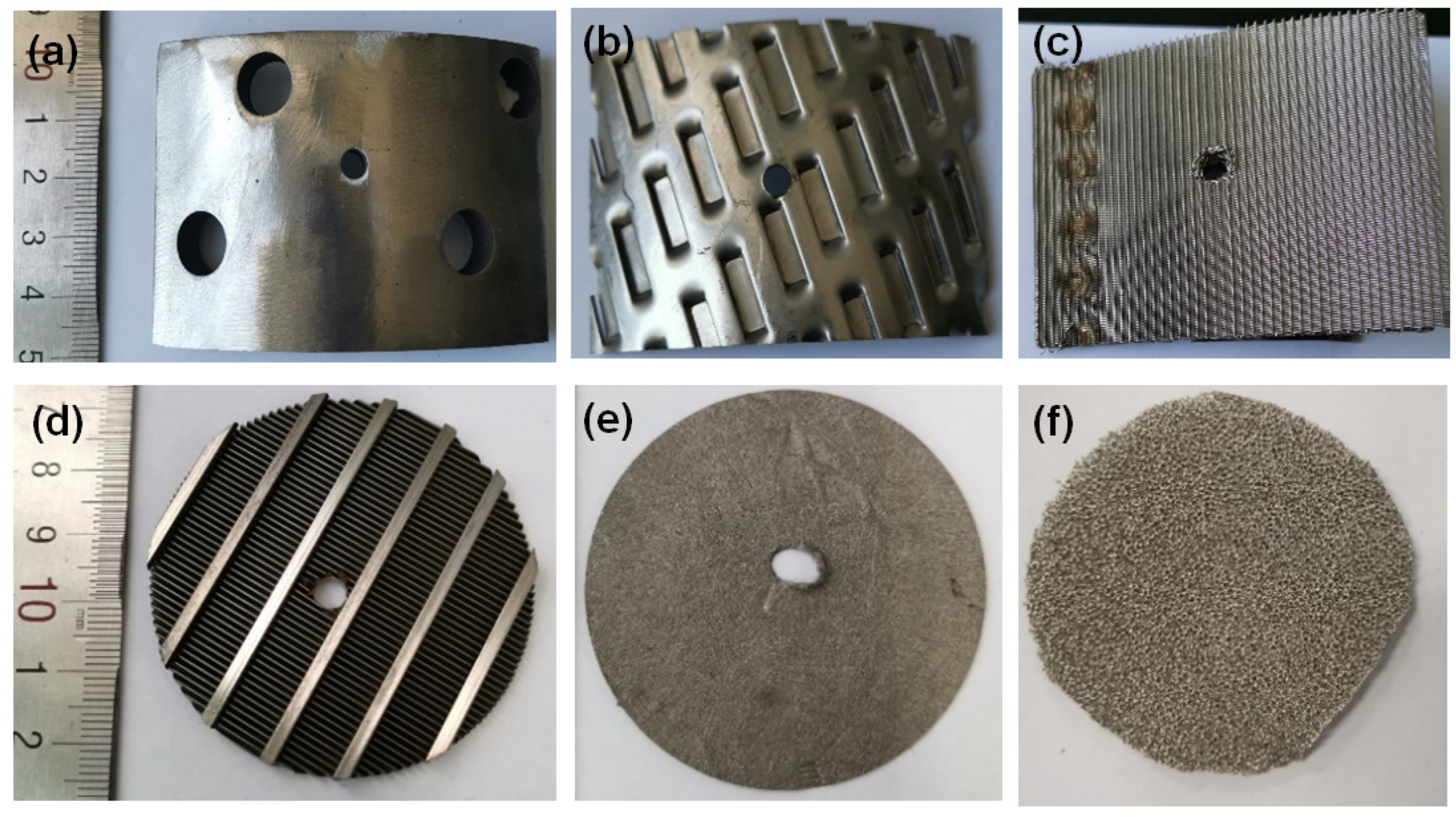
2.2.1. Corrosion Samples
2.2.2. Fluids
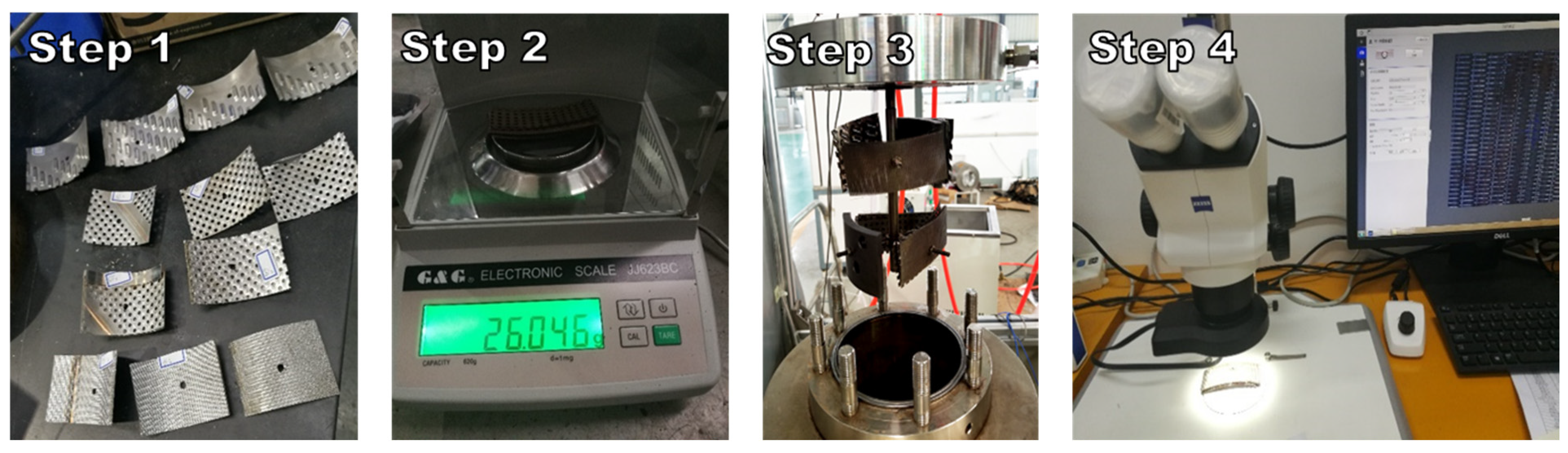
2.3. Procedure
2.4. Experimental Evaluation Methods
- (1)
- Corrosion weight loss rate is the proportion of mass lost by corrosion per unit time:
- (2)
- The mass corrosion rate is the mass lost by corrosion per unit area per unit time:
- (3)
- The depth corrosion rate is the corrosion depth or thickness of the media surface per unit time:
3. Results and Discussion
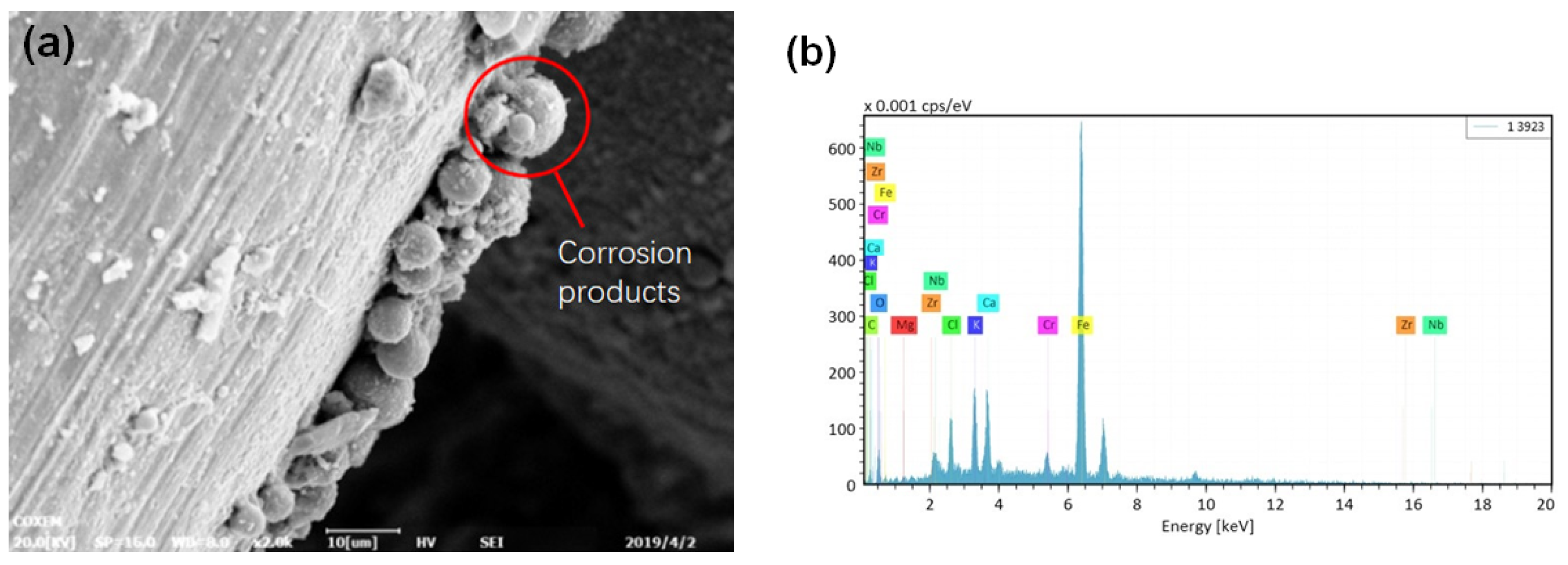
3.1. Microscopic Morphology and Product Analysis
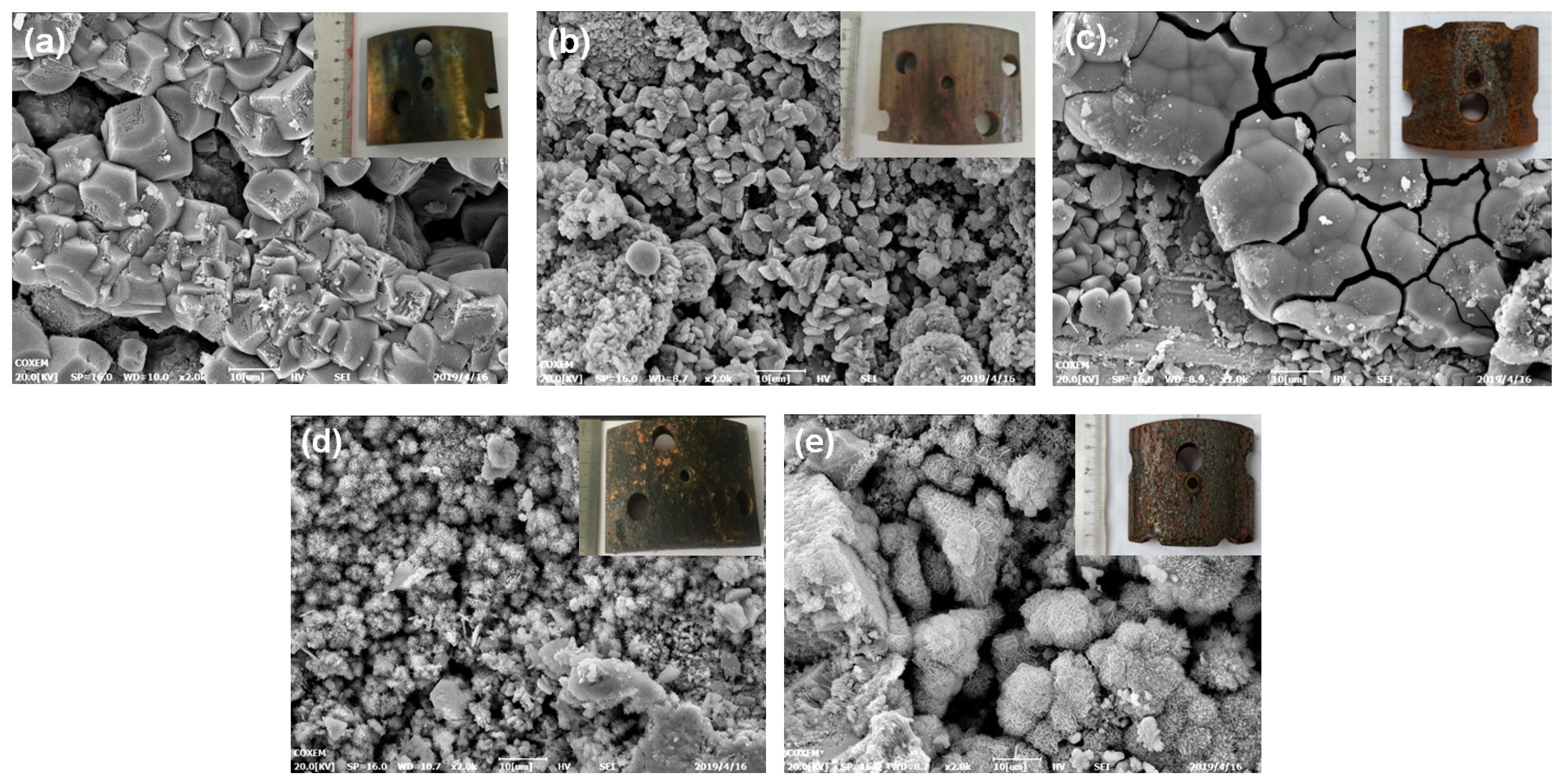
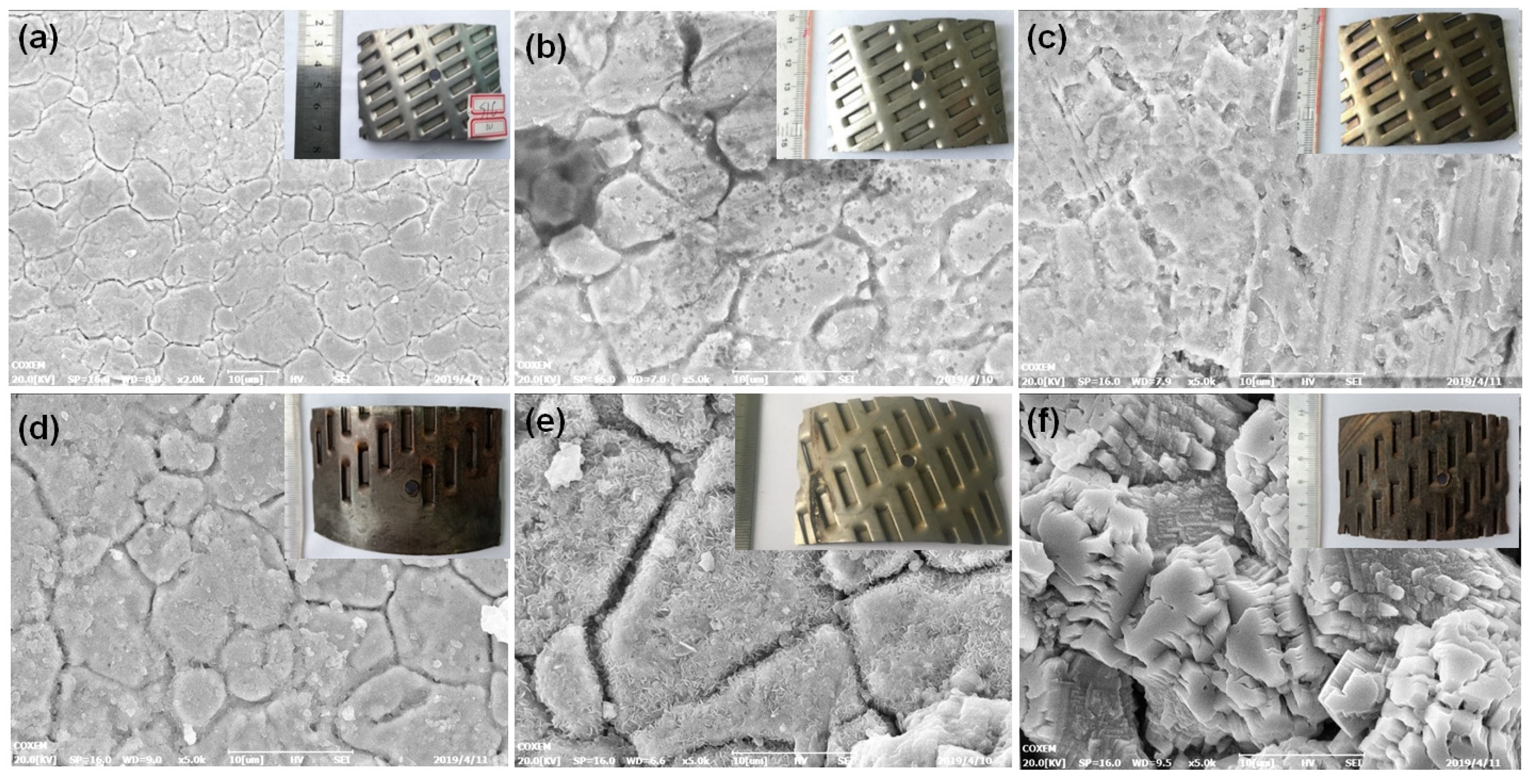
3.1.1. Corrosion Morphology of Basic Pipe
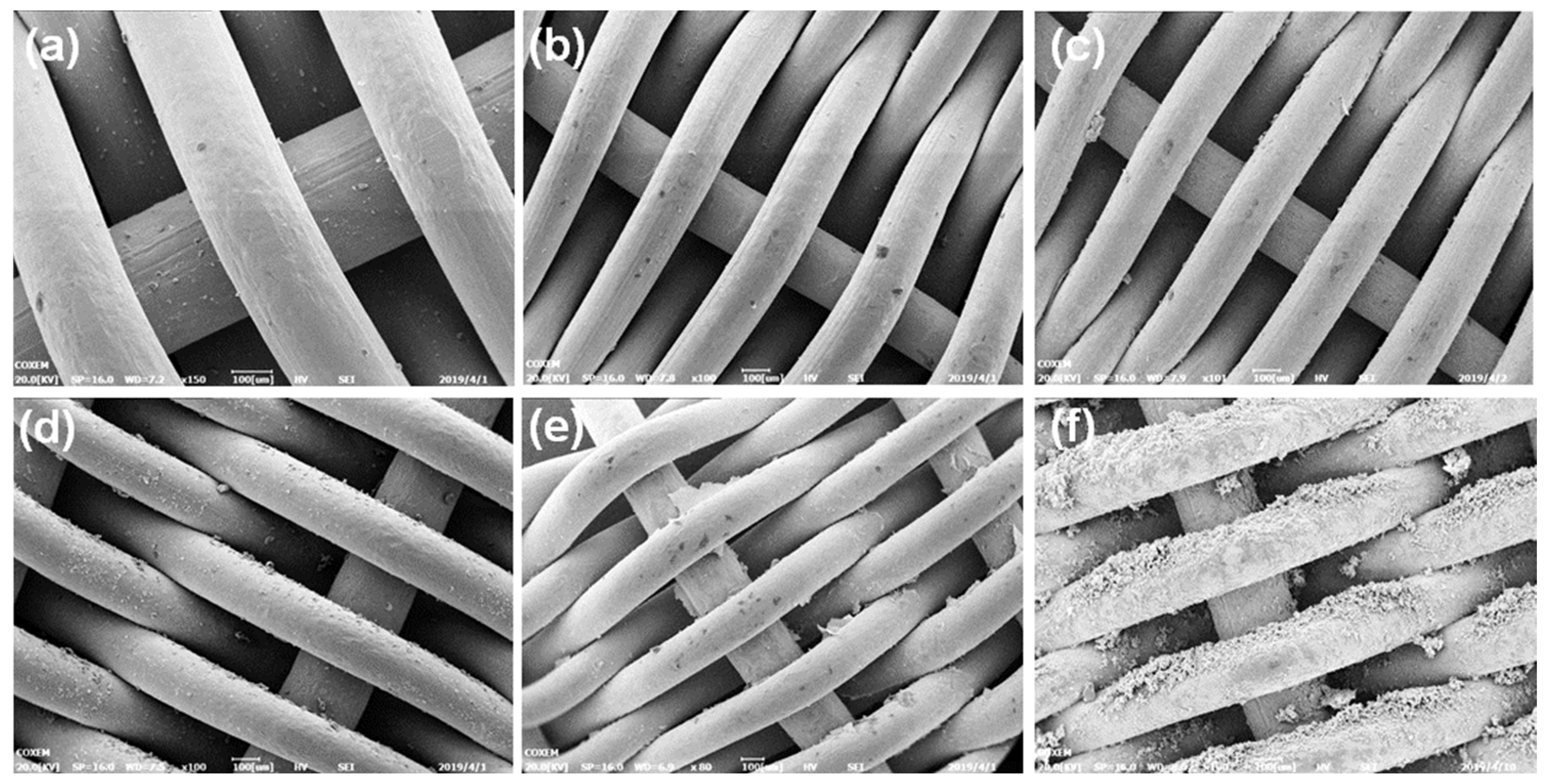
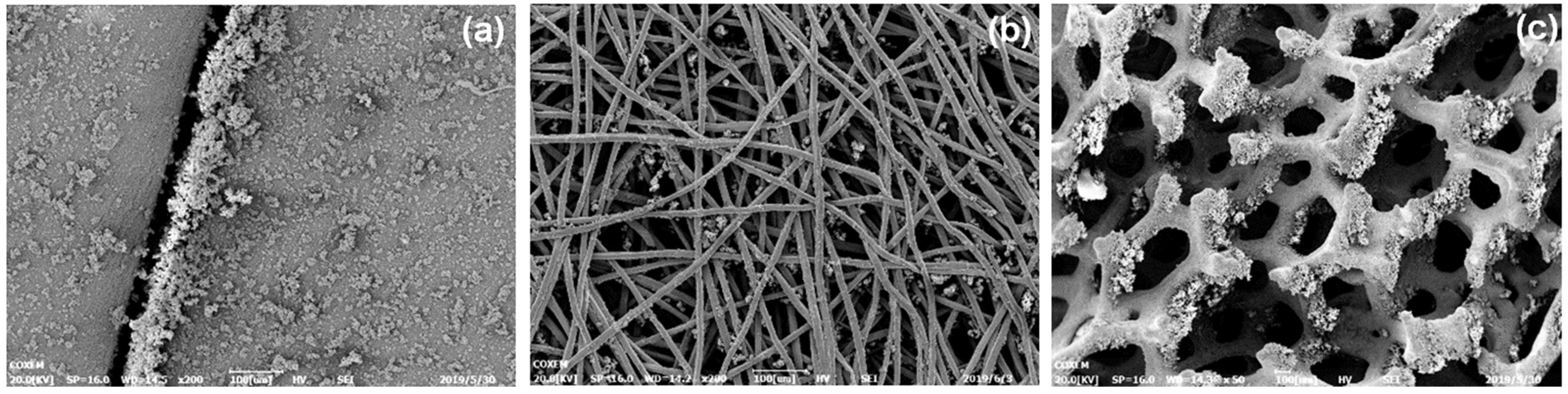
3.1.2. Corrosion Morphology of Sand Retaining Media
3.1.3. Corrosion Morphology of Protective Shroud
3.1.4. Corrosion Morphology of the Screen as a Whole
3.2. Corrosion Rate Analysis
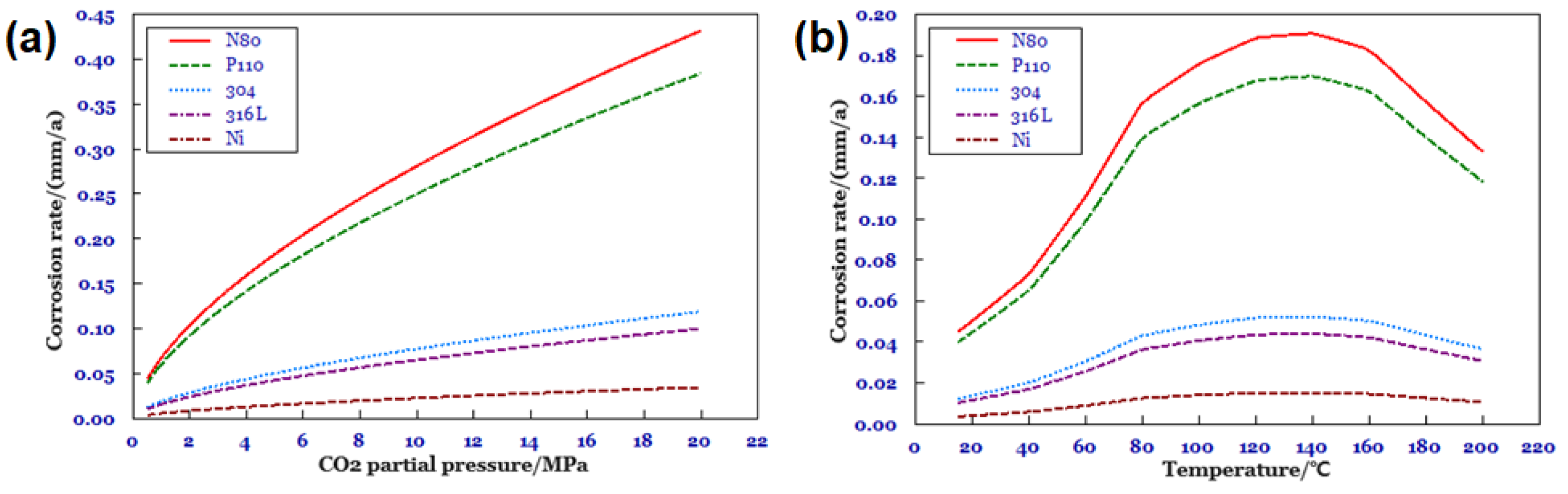
3.2.1. Effect of Corrosion Rate under Different CO2 Partial Pressures
3.2.2. Effect of Corrosion Rate at Different Temperatures
3.2.3. Effect of Corrosion Rate under Different Water–Gas Ratios
4. Prediction Method of Screen Corrosion Rate
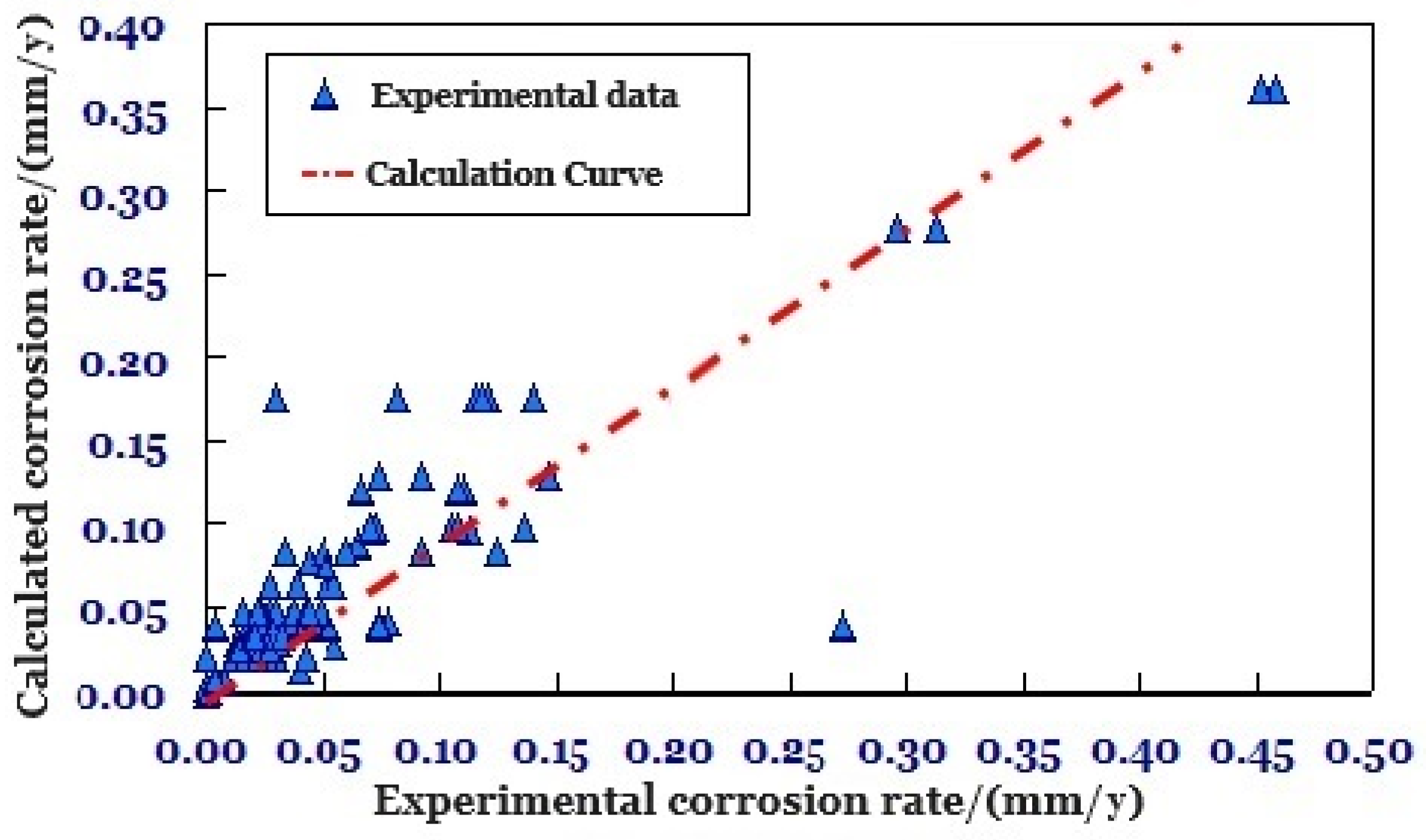
4.1. Modification of the Corrosion Rate Model
4.2. Evaluation Method of Corrosion Life of Bottom-Hole Screen Pipe
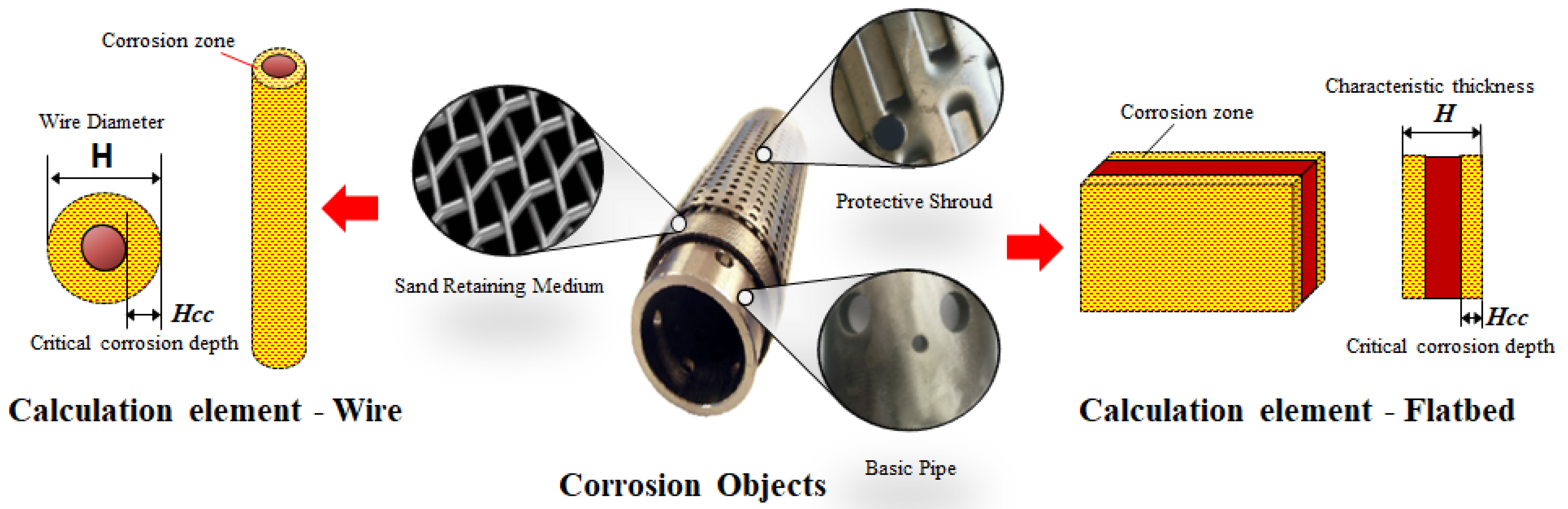
4.2.1. Characteristic Calculation Elements of Screen
4.2.2. Corrosion Evaluation Method of Screen
5. Wellbore Screen Optimization Applications
5.1. Typical Gas Reservoir Conditions and Screen Data Used
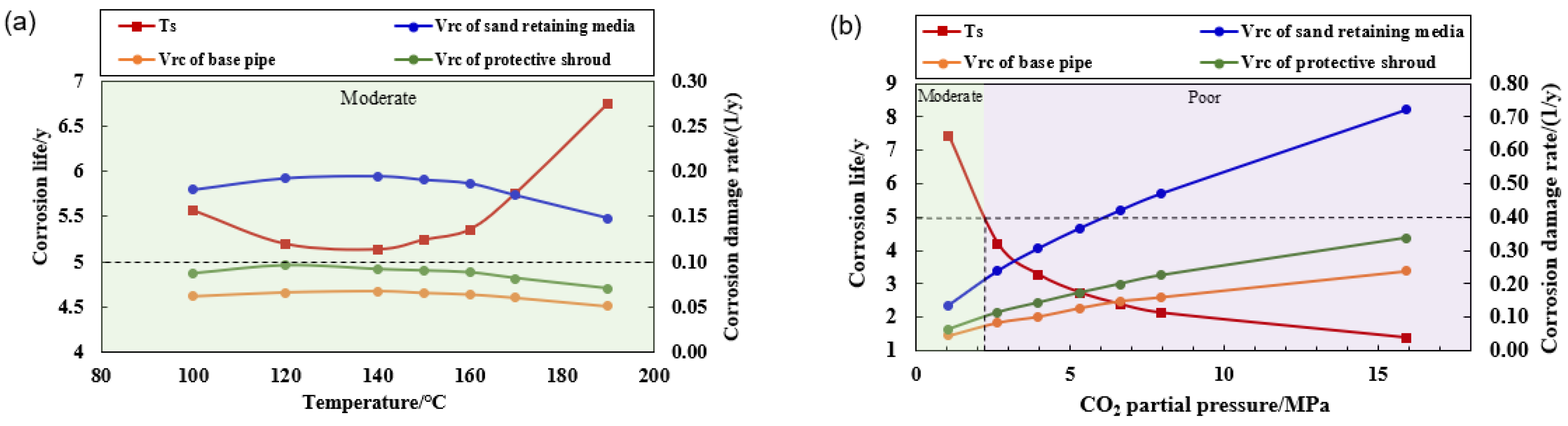
5.2. Evaluation Results of Corrosion Resistance for Screens in HTHP Environments
6. Conclusions
- (1)
- Through systematic experiments to reveal the corrosion law of screen components under HTHP environment. N80 base pipe screen corrosion rate is the highest under 140–150 °C; this condition is exactly the gas field in the South China Sea with bottoming temperature conditions, and the risk of corrosion is high. The corrosion rate of 316L screen media is the slowest, but due to the media feature size being small, corrosion damage is the fastest, and this is a high corrosion risk component.
- (2)
- A corrosion evaluation method for screen tubing in HTHP gas reservoirs is constructed with corrosion experimental evaluation, wellbore corrosion damage evaluation, and corrosion resistance evaluation as the core. The new evaluation method considers the corrosion rate and structural parameters of screen tubing with complex structure, and the overall compliance rate with the experiment is higher than 90%, which provides key support for the evaluation of screen tubing corrosion. It is recommended to utilize the method in similar HTHP environments, regularly update it with new data to enhance accuracy, and implement comprehensive monitoring systems for early detection and intervention.
- (3)
- The corrosion rate of the screen tube was predicted using actual field data. For a typical Well X, due to the high CO2 content (4%), the risk of screen tube corrosion is relatively high, and the screen retaining media is a corrosion failure part, with a minimum corrosion life of about 5 years, and the life of the outer protective shroud is about 11–12 years. It is recommended to consider improving the corrosion resistance of the screen in terms of optimizing the structure of the outer protective shroud and the structural parameters of the screen.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| HTHP | High temperature high-pressure |
| EDS | Energy dispersive spectrometer |
| SEM | Scanning electron microscopy |
| CO2 | Carbon dioxide |
| Fe3C | Triiron carbide |
| FeCO3 | Iron carbonate |
| H2CO3 | Carbonic acid |
| pH | Potential of hydrogen |
| 13Cr | AISI 13Cr stainless steel |
| 316L | AISI 316L stainless steel |
| 304 | AISI 304 stainless steel |
| N80 | API N80 casing steel |
| P110 | API P110 casing steel |
| Ni | Nickel |
| K+ | Potassium ion |
| Na+ | Sodium ion |
| Mg2+ | Magnesium ion |
| Cl− | Chloride ion |
| SO42− | Sulfate ion |
| HCO3− | Bicarbonate ion |
References
- Cui, Z.D.; Wu, S.L.; Zhu, S.L.; Yang, X.J. Study on corrosion properties of pipelines in simulated produced water saturated with supercritical CO2. Appl. Surf. Sci. 2006, 252, 2368–2374. [Google Scholar] [CrossRef]
- Elgaddafi, R.; Naidu, A.; Ahmed, R.; Shah, S.; Hassani, S.; Osisanya, S.O.; Saasen, A. Modeling and experimental study of CO2 corrosion on carbon steel at elevated pressure and temperature. J. Nat. Gas Sci. Eng. 2015, 27, 1620–1629. [Google Scholar] [CrossRef]
- Yevtushenko, O.; Bettge, D.; Bohraus, S.; Bäßler, R.; Pfennig, A.; Kranzmann, A. Corrosion behavior of steels for CO2 injection. Process Saf. Environ. Prot. 2014, 92, 108–118. [Google Scholar] [CrossRef]
- Nešić, S. Key issues related to modelling of internal corrosion of oil and gas pipelines—A review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Nyborg, R. Controlling internal corrosion in oil and gas pipeline. Bus. Brief. Explor. Prod. Oil Gas Rev. 2005, 2, 70–74. [Google Scholar]
- Aroyehun, M.E.; Oko, F.N.; Onyeanusi, O.; Oguntade, T.; Kabara, A.; Dimkpa, B. Comparative Study of Sand Control Methods in Selected Niger-Delta Sand Stone Reservoirs. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 6–8 August 2018. [Google Scholar]
- Garolera, D.; Carol, I.; Papanastasiou, P. Application of zero-thickness interface elements to sanding prediction analysis. J. Pet. Sci. Eng. 2020, 190, 107052. [Google Scholar] [CrossRef]
- Hyodo, M.; Nakata, Y.; Yoshimoto, N. Challenge for methane hydrate production by geotechnical engineering. Jpn Geotech. Soc. Spec. Publ. 2016, 2, 62–75. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Roostaei, M.; Fattahpour, V.; Sutton, C.; Fermaniuk, B.; Zhu, D.; Jung, H.; Li, J.; Sun, C.; Gong, L.; et al. Standalone Sand Control Failure: Review of Slotted Liner, Wire Wrap Screen, and Premium Mesh Screen Failure Mechanism. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 4–26 September 2018. [Google Scholar]
- Peng, Y.; Fu, G.; Sun, X.; Sun, B.; Wang, J.; Zhang, W.; Du, Q. Optimization design of screen pipes hole arrangement parameter based on collapse strength. Thin-Walled Struct. 2022, 171, 108647. [Google Scholar] [CrossRef]
- Peng, Y.; Fu, G.; Sun, B.; Chen, J.; Zhang, W.; Ren, M.; Zhang, H. Data-driven collapse strength modelling for the screen pipes with internal corrosion defect based on finite element analysis and tree-based machine learning. Ocean Eng. 2023, 279, 114400. [Google Scholar] [CrossRef]
- Ren, C.; Liu, D.; Bai, Z.; Li, T. Corrosion behavior of oil tube steel in simulant solution with hydrogen sulfide and carbon dioxide. Mater. Chem. Phys. 2005, 93, 305–309. [Google Scholar] [CrossRef]
- Kinsella, B.; Tan, Y.J.; Bailey, S. Electrochemical Impedance Spectroscopy and Surface Characterization Techniques to Study Carbon Dioxide Corrosion Product Scales. Corrosion 1998, 54, 835–842. [Google Scholar] [CrossRef]
- Lu, M.X.; Bai, Z.Q.; Zhao, X. Actuality and typical cases for corrosion in the process of extraction, gathering, storgage and transmission for oil and gas. Corros. Prot. 2002, 23, 105–113. [Google Scholar]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef]
- López, D.A.; Schreiner, W.H.; de Sánchez, S.R.; Simison, S.N. The influence of inhibitors molecular structure and steel microstructure on corrosion layers in CO2 corrosion: An XPS and SEM characterization. Appl. Surf. Sci. 2004, 236, 77–97. [Google Scholar] [CrossRef]
- Xiao, R.; Zhao, C.; Zou, Z.; Chen, Z.; Tian, L.; Xu, H.; Tang, H.; Liu, Q.; Lin, Z.; Yang, X. In situ fabrication of 1D CdS nanorod/2D Ti3C2 MXene nanosheet Schottky heterojunction toward enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 268, 118382. [Google Scholar] [CrossRef]
- Ma, W.; Wang, H.; Guo, H.; Cai, L.; Li, X.; Hua, Y. A synergistic experimental and computational study on CO2 corrosion of X65 carbon steel under dispersed droplets within oil/water mixtures. Corros. Commun. 2023, 12, 64–75. [Google Scholar] [CrossRef]
- Hong, T.; Sun, Y.H.; Jepson, W.P. Study on corrosion inhibitor in large pipelines under multiphase flow using EIS. Corros. Sci. 2002, 44, 101–112. [Google Scholar] [CrossRef]
- Moreira, R.M.; Franco, C.V.; Joia, C.J.B.M.; Giordana, S.; Mattos, O.R. The effects of temperature and hydrodynamics on the CO2 corrosion of 13Cr and 13Cr5Ni2Mo stainless steels in the presence of free acetic acid. Corros. Sci. 2004, 46, 2987–3003. [Google Scholar] [CrossRef]
- López, D.A.; Simison, S.N.; de Sánchez, S.R. The influence of steel microstructure on CO2 corrosion. EIS studies on the inhibition efficiency of benzimidazole. Electrochim. Acta 2003, 48, 845–854. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Sun, L.; Fan, H. Effects of pH and Cl− concentration on corrosion behavior of the galvanized steel in simulated rust layer solution. Corros. Sci. 2012, 65, 520–527. [Google Scholar] [CrossRef]
- Moreno, M.; Morris, W.; Alvarez, M.G.; Duffó, G.S. Corrosion of reinforcing steel in simulated concrete pore solutions: Effect of carbonation and chloride content. Corros. Sci. 2004, 46, 2681–2699. [Google Scholar] [CrossRef]
- Otmacic Curkovic, H.; Stupnisek-Lisac, E.; Takenouti, H. The influence of pH value on the efficiency of imidazole based corrosion inhibitors of copper. Corros. Sci. 2010, 52, 398–405. [Google Scholar] [CrossRef]
- Nordsveen, M.; Nešić, S.; Nyborg, R.; Stangeland, A. A Mechanistic Model for Carbon Dioxide Corrosion of Mild Steel in the Presence of Protective Iron Carbonate Films—Part 1: Theory and Verification. Corrosion 2003, 59, 443–456. Available online: https://onepetro.org/corrosion/article/116412/A-Mechanistic-Model-for-Carbon-Dioxide-Corrosion (accessed on 30 June 2003). [CrossRef]
- Snesic, S.; George, K.; Wang, S. High Pressure CO2 Corrosion Electrochemistry and the Effect of Acetic Acid. In Proceedings of the Corrosion 2004, New Orleans, LA, USA, 28 March–1 April 2004. [Google Scholar]
- Choi, Y.-S.; Farelas, F.; Nešic, S.; Magalhães, A.A.O.; Andrade, C.d.A. Corrosion Behavior of Deep Water Oil Production Tubing Material under Supercritical CO2 Environment: Part I. Effect of Pressure and Temperature. In Proceedings of the Corrosion 2013, Orlando, FL, USA, 17–21 March 2013. [Google Scholar]
- Sun, W.; Nešić, S. A Mechanistic Model of Uniform Hydrogen Sulfide/Carbon Dioxide Corrosion of Mild Steel. Corrosion 2009, 65, 291–307. [Google Scholar] [CrossRef]
- Seiersten, M. Materials Selection for Separation, Transportation and Disposal of CO2. In Proceedings of the Corrosion 2001, Houston, TX, USA, 11–16 March 2001. [Google Scholar]
- Nešić, S.; Lee, K.; Ruzic, V. A mechanistic model of iron carbonate film growth and the effect on CO2 corrosion of mild steel. In Proceedings of the Corrosion 2002, Denver, CO, USA, 7–11 April 2002. [Google Scholar]
- Forero, B.; Núñez, M.M.G.; Bott, I. Analysis of the Corrosion Scales Formed on API 5L X70 and X80 Steel Pipe in the Presence of CO2. Mater. Res. 2014, 17, 461–471. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Radaelli, M. Corrosion rate of high CO2 pressure pipeline steel for carbon capture transport and storage. La Metall. Ital. 2014, 106, 21–27. [Google Scholar]
- Hassani, S.; Vu, T.N.; Rosli, N.R.; Esmaeely, S.N.; Choi, Y.-S.; Young, D.; Nesic, S. Wellbore integrity and corrosion of low alloy and stainless steels in high pressure CO2 geologic storage environments: An experimental study. Int. J. Greenh. Gas Control 2014, 23, 30–43. [Google Scholar] [CrossRef]
- Nazari, M.H.; Allahkaram, S.R.; Kermani, M.B. The effects of temperature and pH on the characteristics of corrosion product in CO2 corrosion of grade X70 steel. Mater. Des. 2010, 31, 3559–3563. [Google Scholar] [CrossRef]
- Yin, Z.F.; Feng, Y.R.; Zhao, W.Z.; Bai, Z.Q.; Lin, G.F. Effect of temperature on CO2 corrosion of carbon steel. Surf. Interface Anal. 2009, 41, 517–523. [Google Scholar] [CrossRef]
- Farelas, F.; Choi, Y.S.; Nešić, S.; Magalhães, A.A.O.; de Azevedo Andrade, C. Corrosion Behavior of Deep Water Oil Production Tubing Material Under Supercritical CO2 Environment: Part 2—Effect of Crude Oil and Flow. Corrosion 2013, 70, 137–145. [Google Scholar] [CrossRef]
- Li, D.; Ma, W.; Han, D.; Zhang, L.; Lu, M.; Wang, L. Effects of temperature on CO2 corrosion of tubing and casing steel. In Proceedings of the NACE—International Corrosion Conference Series, Orlando, FL, USA, 17–21 March 2013. [Google Scholar]
- Yevtushenko, O.; Bäßler, R.; Pfennig, A. Corrosion behaviour of Cr13 steel in CO2 saturated brine with high chloride concentration. Mater. Corros. 2012, 63, 517–521. [Google Scholar] [CrossRef]
- Pfennig, A.; Kranzmann, A. The Role of Pit Corrosion in Engineering the Carbon Storage Site at Ketzin, Germany; WIT Press: Billerica, MA, USA, 2010; pp. 109–119. [Google Scholar]
- Carvalho, D.S.; Joia, C.J.B.; Mattos, O.R. Corrosion rate of iron and iron–chromium alloys in CO2 medium. Corros. Sci. 2005, 47, 2974–2986. [Google Scholar] [CrossRef]
- Linter, B.R.; Burstein, G.T. Reactions of pipeline steels in carbon dioxide solutions. Corros. Sci. 1999, 41, 117–139. [Google Scholar] [CrossRef]
- Gopal, M.; Rajappa, S.; Zhang, R. Modeling the Diffusion Effects Through the Iron Carbonate Layer in the Carbon Dioxide Corrosion of Carbon Steel. In Proceedings of the Corrosion 98, San Diego, CA, USA, 22–27 March 1998. [Google Scholar]
- Pfennig, A.; Bäßler, R. Effect of CO2 on the stability of steels with 1% and 13% Cr in saline water. Corros. Sci. 2009, 51, 931–940. [Google Scholar] [CrossRef]
- Yan, B.; Dong, C.; Huang, L.; Wei, A.; Yan, Q.; Fang, D.; Zhong, Y.; Wang, L. Experimental Evaluation of Dynamic Mechanical Sand Screen Corrosion for the High Temperature Pressure Gas Reservoir in South China Sea. Spec. Oil Gas Reserv. 2020, 27, 148–156. Available online: https://kns.cnki.net/kcms/detail/21.1357.TE.20200302.0842.002.html (accessed on 2 March 2020).
- Peng, Y.; Fu, G.; Sun, B.; Sun, X.; Chen, J.; Estefen, S.F. Bending Deformation and Ultimate Moment Calculation of Screen Pipes in Offshore Sand Control Completion. J. Mar. Sci. Eng. 2023, 11, 754. [Google Scholar] [CrossRef]
- Dong, C.; Gao, K.; Dong, S.; Shang, X.; Wu, Y.; Zhong, Y. A new integrated method for comprehensive performance of mechanical sand control screens testing and evaluation. J. Pet. Sci. Eng. 2017, 158, 775–783. [Google Scholar] [CrossRef]
- Mondal, S.; Wu, C.-H.; Sharma, M.M.; Chanpura, R.A.; Parlar, M.; Ayoub, J.A. Characterizing, Designing, and Selecting Metal Mesh Screens for Standalone-Screen Applications. SPE Drill. Complet. 2016, 31, 085–094. [Google Scholar] [CrossRef]
- Loizzo, M.; Bressers, P.; Benedictus, T.; Le Guen, Y.; Poupard, O. Assessing CO2 interaction with cement and steel over a two-year injection period: Current state and future risks for the MovECBM project in Poland. Energy Procedia 2009, 1, 3579–3586. [Google Scholar] [CrossRef][Green Version]
- Nor, A.M.; Suhor, M.F.; Mohamed, M.F.; Singer, M.; Nesic, S. Corrosion of Carbon Steel In High CO2 Environment: Flow Effect. In Proceedings of the Corrosion 2011, Houston, TX, USA, 13–17 March 2011. [Google Scholar]
- Johnson, M.L. Ferrous Carbonate Precipitation Kinetics: A Temperature Ramped Approach; NACE International: Houston, TX, USA, 1991. [Google Scholar]
- Valdes, A.; Case, R.; Ramirez, M.; Ruiz, A. The Effect of Small Amounts of H2S on CO2 Corrosion of a Carbon Steel. In Proceedings of the Corrosion 98, San Diego, CA, USA, 22–27 March 1998. [Google Scholar]
- NORSOK Standard M-506; CO2 Corrosion Rate Calculation Model. Norwegian Technology Center: Oslo, Norway, 1998; Rev. 1.



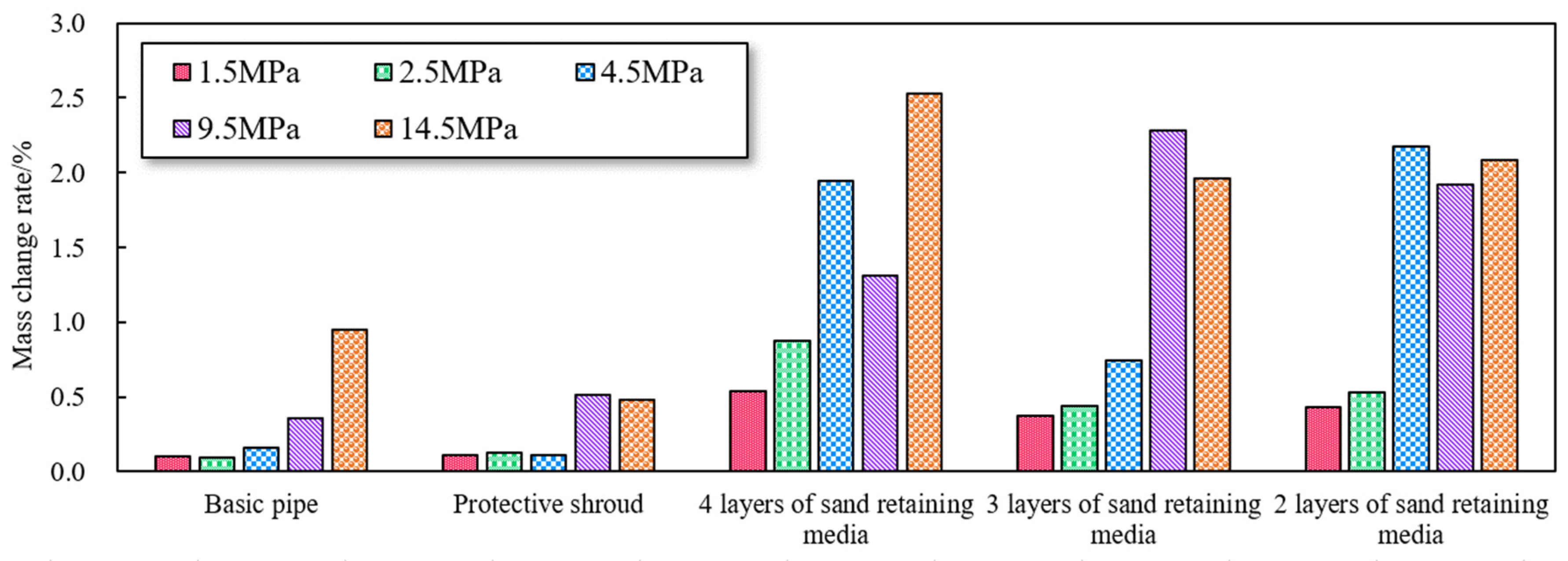
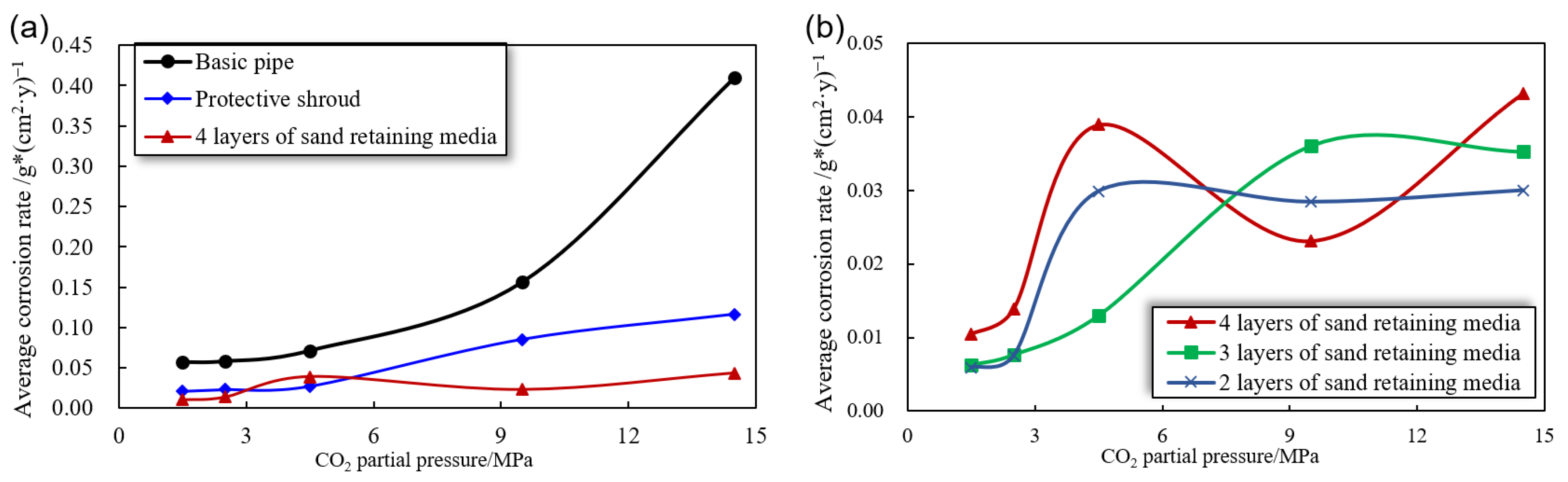
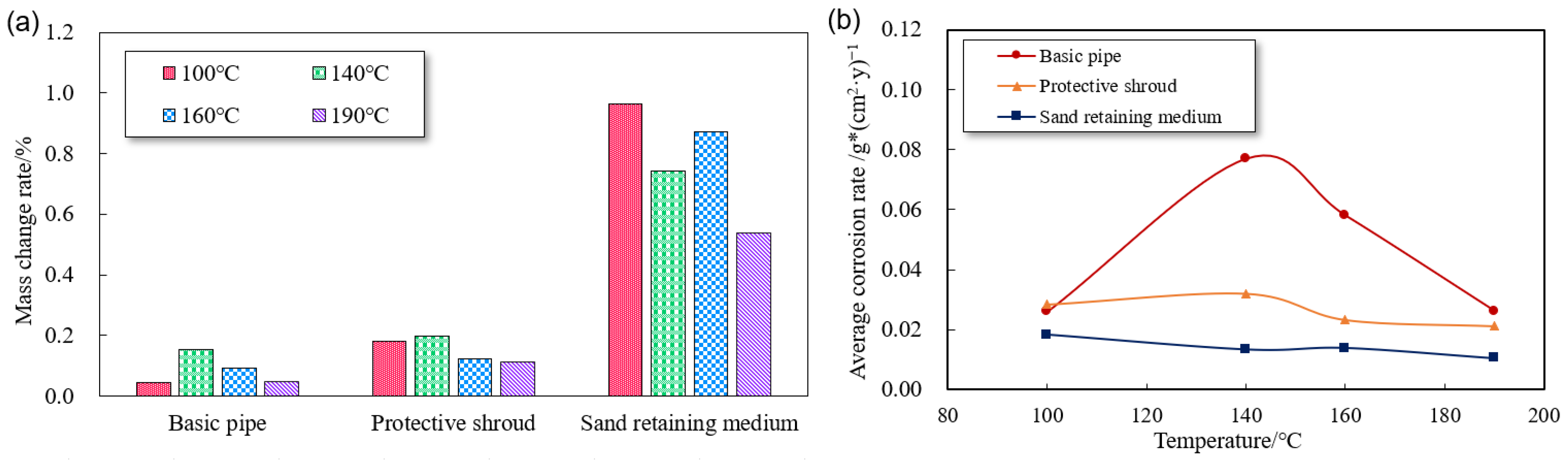
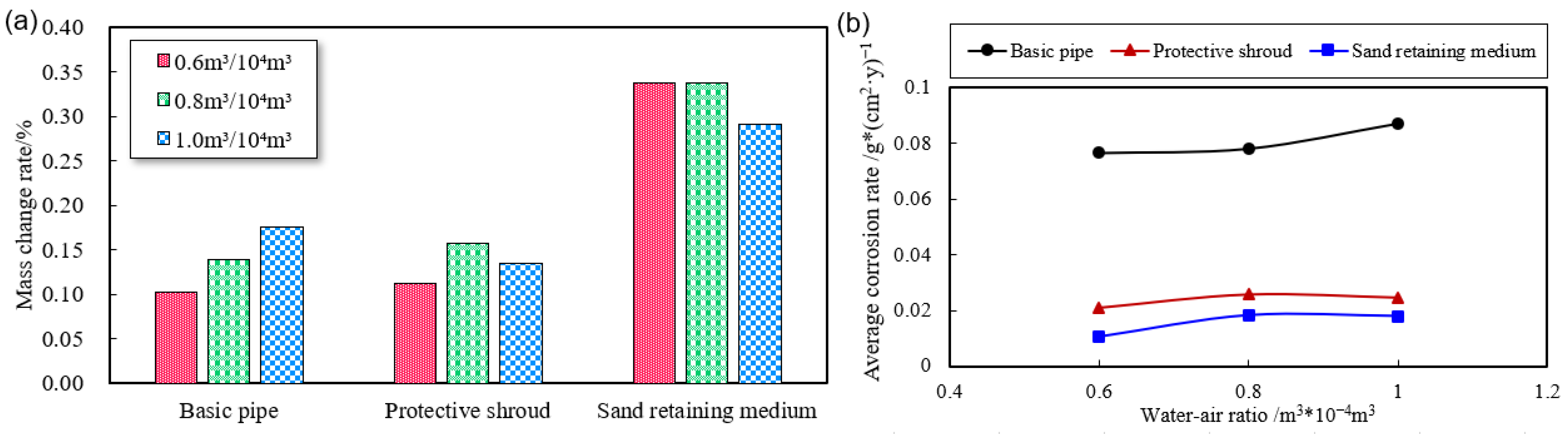
| Ions | K+ and Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− |
| Content (mg/L) | 5652 | 40 | 17 | 6402 | 1300 | 2539 |
| Temperature °C | pH | ||
|---|---|---|---|
| 20 | 4.762 | pH < 4.6 | f(pH) = 2.0676 − (0.2309 pH) |
| 4.6 ≤ pH | f(pH) = 5.1885 − (1.2353 pH) + (0.0708 pH2) | ||
| 40 | 8.927 | pH < 4.6 | f(pH) = 2.0676 − (0.2309 pH) |
| 4.6 ≤ pH | f(pH) = 5.1885 − (1.2353 pH) + (0.0708 pH2) | ||
| 60 | 10.695 | pH < 4.6 | f(pH) = 1.836 − (0.1818 pH) |
| 4.6 ≤ pH | f(pH) = 15.444 − (6.1291 pH) + (0.8204 pH2) − (0.0371 pH3) | ||
| 80 | 9.949 | pH < 4.6 | f(pH) = 2.6727 − (0.3636 pH) |
| 4.6 ≤ pH | f(pH) = 331.68 e(−1.2618 pH) | ||
| 90 | 6.250 | pH < 4.6 | f(pH) = 3.1355 − (0.4673 pH) |
| 4.6 ≤ pH < 5.6 | f(pH) = 21,254 × e(−2.1811 pH) | ||
| 5.6 ≤ pH | f(pH) = 0.4014 − (0.0538 pH) | ||
| 120 | 7.770 | pH < 4.3 | f(pH) = 1.5375 − (0.125 pH) |
| 4.3 ≤ pH < 5 | f(pH) = 5.9757 − (1.157 pH) | ||
| 5 ≤ pH | f(pH) = 0.546125 − (0.071225 pH) | ||
| 150 | 5.203 | pH < 3.8 | f(pH) = 1 |
| 3.8 ≤ pH < 5 | f(pH) = 17.634 − (7.0945 pH) + (0.715 pH2) | ||
| 5 ≤ pH | f(pH) = 0.037 |
| Screen pipe Assemblies | Material | β1 (Material Impact Factor) | β2 (Structural Impact Factor) |
|---|---|---|---|
| Base pipe | N80 | 1.0 | 1.0 |
| Protective shroud | 304 | 0.28 | 0.43 |
| Sand retaining medium | Ni | 0.11 | 0.32 |
| P110 | 0.89 | 0.42 | |
| 316L | 0.23 | 0.88 |
| Qualitative Indicators for Evaluation | Excellent | Good | Moderate | Poor |
|---|---|---|---|---|
| Corrosion damage rate VRc (1/y) | <0.067 | 0.067–0.1 | 0.1–0.2 | >0.2 |
| Corrosion damage life Ts (y) | >15 | 10–15 | 5–10 | <5 |
| Item | Temperature | Reservoir Pressure | CO2 Content | CO2 Partial Pressure | Water–Air Ratio | Cl− Content |
|---|---|---|---|---|---|---|
| Data | 142–188 °C | 53 MPa | 3.4–4.0% | 2–12 MPa | 0.6 m3/104 m3 | 6400 mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Dong, C.; Li, X.; Bai, H.; Yin, B.; Li, H.; Shen, K. CO2 Corrosion of Downhole Sand Control Screen: Experiments, Model, and Application. Energies 2024, 17, 3316. https://doi.org/10.3390/en17133316
Zhou B, Dong C, Li X, Bai H, Yin B, Li H, Shen K. CO2 Corrosion of Downhole Sand Control Screen: Experiments, Model, and Application. Energies. 2024; 17(13):3316. https://doi.org/10.3390/en17133316
Chicago/Turabian StyleZhou, Bo, Changyin Dong, Xiaobo Li, Haobin Bai, Bin Yin, Huaiwen Li, and Kaixiang Shen. 2024. "CO2 Corrosion of Downhole Sand Control Screen: Experiments, Model, and Application" Energies 17, no. 13: 3316. https://doi.org/10.3390/en17133316
APA StyleZhou, B., Dong, C., Li, X., Bai, H., Yin, B., Li, H., & Shen, K. (2024). CO2 Corrosion of Downhole Sand Control Screen: Experiments, Model, and Application. Energies, 17(13), 3316. https://doi.org/10.3390/en17133316






