1. Introduction
The negative impact of industrial petroleum processes on the environment, especially in terms of global warming, and aquatic and terrestrial pollution has increased the search for alternative nonpetroleum processes to keep existing petrochemical and allied businesses running. Ethylene glycol (EG) production is one process that is currently being affected and will be severely affected in the near future as the global reliance on fossil fuels declines. EG is an important petrochemical product that is widely used for the manufacture of antifreeze and coolant in automobiles, de-icing fluid for windshields of aircraft, desiccant for natural gas production, and a precursor for the manufacture of polyester fibers and resins: predominantly poly(ethylene terephthalate) (PET) [
1]. EG has an annual production of over 25 million metric tons, and its demand has been estimated at a rate of 5% per year [
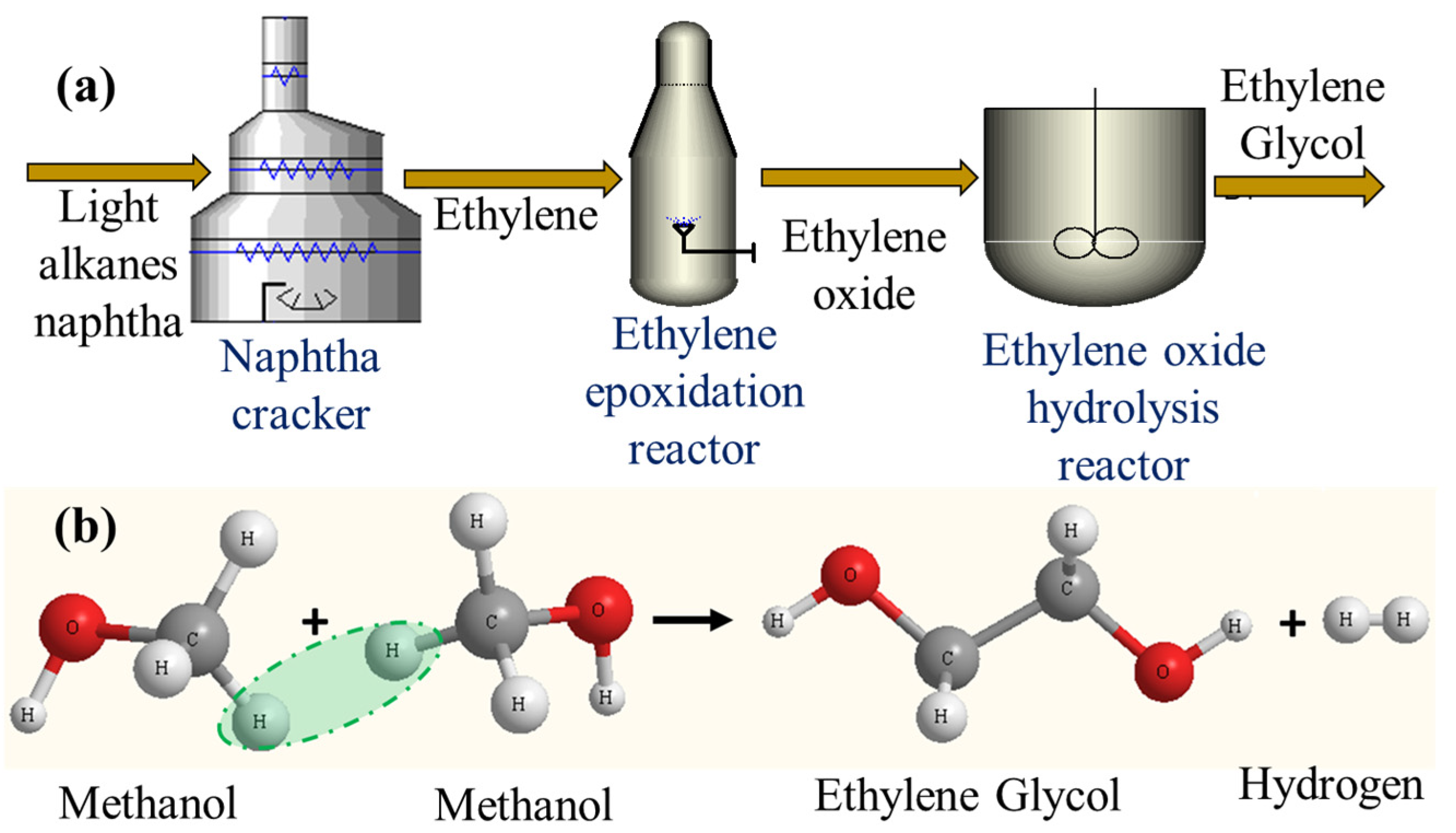
2]. At present, EG is primarily produced at the industrial scale in a multistep process via the cracking of petroleum-derived naphtha to produce ethylene, followed by ethylene epoxidation to ethylene oxide (EO), and the subsequent hydrolysis of EO to EG (
Figure 1a). As the future reliance on crude oil resources shrinks, the synthesis of EG using other alternative approaches such as the conversion of syngas to EG through dimethyl oxalate (DMO) attracts more and more interest [
1]. As expected, these two multistep processes suffer from both high energy demand and a long production chain, thus reducing efficiency and increasing production costs. A robust and economically attractive route to produce EG must reduce the number of processing steps, utilities, and production costs and improve safety, i.e., process intensification (PI). In the course of the author’s search for appropriate feedstock and thermodynamic process simulation studies, process-intensified, one-step EG production using cheap and highly abundant methanol (MeOH) as the feedstock, i.e., the direct conversion of MeOH to EG (MeOH-2-EG), was developed. This process can be achieved in a single step, unlike the multistep status quo; in addition, hydrogen (H
2), which is clean energy, is obtained as the by-product (
Figure 1b). Furthermore, this proposed route will reduce the pressure on the ethylene market, which is presently being gradually overstretched by the recent increase in polyethylene (made from ethylene monomers) demands from the automobile and aircraft industries for replacing several replaceable noncritical metallic components/parts with polymers. Recently, automobile and aircraft manufacturers have opted to replace several replaceable automobile and airplane metallic parts with polymers to reduce their static weight and eventually reduce fuel consumption in order to reduce the carbon dioxide (CO
2) emissions released from exhausts into the atmosphere.
In recent times, due to the global clamor for a green and sustainable environment, there are growing interests in the utilization of nonpetroleum carbon resources, in particular, shale gas (methane, CH
4) and CO
2 for the sustainable production of chemicals, therefore, increasing the importance of MeOH synthesis within the C1 chemistry domain [
3,
4]. According to the Market.us [
5], the MeOH market is witnessing substantial growth, with a projected value of USD 66.2 billion at a CAGR of 5.83%. Presently, this increment in MeOH production has led to a significant reduction in prices due to a surplus in supply beyond the demand in the international market. For example, available data showed that between March 2022 and July 2023, in Europe and the US, FOB Rdam and FOB USG methanol prices have dropped from an average of USD 460/mt and EUR 455 to about USD 230/mt and EUR 190, respectively [
6]. This trend in methanol prices is also seen in China, Southeast Asia, and the Middle East. Thus, there is a strong incentive to develop new methods or routes for the effective valorization of MeOH via C–C coupling with high selectivity within the framework of process intensification and circular economy [
7]. The typical conversion of MeOH usually involves the activation of its C–H, C–O, and/or O–H bonds. The system that can selectively activate the relatively stable and poorly reactive C–H bond of MeOH while preserving the C–O and O–H bonds to guarantee a C–C coupling is challenging. Unfortunately, this is the sole route for achieving MeOH-2-EG (
Figure 1b). Furthermore, the preliminary thermodynamic simulation results established that the reaction is not feasible at any temperature according to the minimization of Gibb’s free energy based on the chemical reaction equilibria theory (
Figure 2) for different reaction phases (liquid or gas) for MeOH and EG, viz.:
Case 1: CH3OH(l) → C2H6O2(l) + H2(g)
Case 2: CH3OH(g) → C2H6O2(l) + H2(g)
Case 3: CH3OH(g) → C2H6O2(g) + H2(g)
The only case with seeming propensity is
Case 1 with
ΔG < 0 between 250 and 450 °C. However, maintaining MeOH at the liquid phase within 250 and 450 °C is not thermodynamically feasible since the critical temperature (
Tc) of MeOH is ~240 °C (
Case ⸎,
Figure 2). The non-feasibility of
Cases 2–3, despite the seamless conversion of MeOH to other C1 products like formaldehyde (CH
2O) over supported silver catalysts, can be due to the atomic bond strengths since the C–H bond’s energy is much higher than that of the C–O bond, although it is lower than that of O–H bonds in a MeOH molecule (
Table 1). Another possibility is the relatively high energy demand of MeOH selective dehydrogenation (
ΔH = +84 kJ mol
−1) compared to the dehydrogenation of higher alcohols, such as ethanol (
ΔH = +68 kJ mol
−1) [
8,
9,
10]. Thus, it appears that the use of MeOH as a C1-feedstock for catalytic C–C coupling is restricted to dehydrative oligomerization processes like the MeOH-to-gasoline (MTG), MeOH-to-olefins (MTO), and the Monsanto and Cativa processes of methanol carbonylation, which are the second-largest volume applications of homogenous catalysis [
9]. This implied that the selective activation of the stable
sp3 α-C–H bond in MeOH without breaking the C–O bonds at elevated reaction temperatures is challenging in synthetic chemistry; hence, it is of high academic significance [
4].
So far, among the few findings in the literature that have attempted direct MeOH-2-EG, there are the works of Xie et al. [
4], wherein a visible light-driven dehydrogenative coupling of MeOH-2-EG was reported. They showed that over a molybdenum disulfide nanofoam-modified cadmium sulfide nanorod catalyst, EG was formed at a high efficiency, with 90% selectivity and hydrogen as the side product. They showed that the photoexcited holes on a cadmium sulfide nanorod led to preferential C–H bond activations instead of the O–H bond in methanol via a concerted proton–electron transfer mechanism, thus forming hydroxymethyl radicals (*CH
2OH) that readily desorb from catalyst surfaces for subsequent coupling. Considering process economics, facile process design, and more importantly, EG yields, the intervention of the visible light-driven dehydrogenative coupling of MeOH-2-EG will be problematic due to low throughput.
To surmount the challenge of selective C–H bond scission while preserving O–H and C–O bonds, the idea of dielectric barrier discharge (DBD) plasma becomes attractive due to its capacity for selective high-energy scission based on the Siemens reports of its first experimental investigation on simple barrier discharges in 1857. DBD plasma can initiate a series of ionization and chemical processes that are far from thermodynamic constraints under ambient operating conditions [
6]. Previously, DBD was employed for methanol conversion with the original intention of obtaining hydrogen-rich syngas; incidentally, a trace amount of EG was found in the product effluent. This serendipity prompted another deliberate effort by Zhang et al. [
11] in a systematic study exploring the direct synthesis of EG from the plasma of methanol vapor; interestingly, encouraging results were obtained using hydrogen as a carrier gas in a non-catalytic double dielectric barrier discharge (DDBD) reactor. Their results demonstrated that as the input power was increased from 8.5 W to 28.6 W, the conversion of MeOH increased from 6.1% to 30.2%, while EG selectivity decreased from 69.8% to 38.0%. The elevation of input power mainly increased undesired CO and CH
4 by-products. Poor EG selectivity can be explained in terms of the atom bond strengths mentioned above and the bond length in
Table 1 wherein the C–H bonds have intermediate bond lengths. Since the literature has indicated that chemical reactions in DBD are governed by the electron’s temperature instead of the thermal processes or gas temperature, several drawbacks such as the low conversion of reactants, poor selectivity of desired products, and low energy efficiency are thus inevitable under DBD plasma alone. Thus, to tailor the product distribution, i.e., to improve the desired selectivity, the combination of DBD plasma and catalysis becomes attractive.
Recently, the author explored similar plasma-assisted catalysis (PAC) to convert CH
4 into C
2H
4 and H
2 in a single step, which hitherto is not thermodynamically feasible due to the symmetricity of the four high-energy C–H bonds of CH
4 [
12]. Serendipity offers a future option for CH
4 conversion (
XCH4) to light olefins to replace the state-of-the-art steam reforming of CH
4 relative to syngas at elevated temperatures (700–1100 °C) and the subsequent conversion of the syngas to olefins (at 200–280 °C), with a large attendant volume of wastewater. The findings showed that
XCH4 increased from 36.7% under plasma conditions without any catalysts to an average of above 55% over a series of Ni catalysts that were functionalized with oxalate ligands to downsize the Ni particle size and was supported on either HCl- or NaOH-treated kaolin supports, and it was calcined at either 700 or 800 °C. Furthermore, Ni catalysts calcined at 800 °C, and those supported on NaOH-treated kaolin showed a higher
XCH4, ethylene–ethane ratio (C
2=/C
2–) and hydrogen yield (
YH2), which are all attributed to the catalyst’s strong metal–support interaction. When the Ni catalysts were replaced with a ceria alloyed platinum catalyst,
XCH4 increased to 73.5% with a high
YH2 of 33.8% and a C
2=/C
2– ratio of ca. 12.3 compared to an average
YH2 of ~20% and C
2=/C
2– ratio ca. 0.032 observed over the Ni-based catalysts. Conclusively, while only C
2H
6 and minuscule H
2 without any C
2H
4 formation were observed under a non-catalytic plasma system, substantially, larger amounts of C
2H
4 and H
2 were obtained under the PAC using alloyed Pt catalysts than compared to Ni catalysts from CH
4, which otherwise was not thermodynamically feasible.