Abstract
In recent decades, growing energy demand, coupled with concerns about climate change, has led to the exploration of sustainable energy sources. Among these, biomass gasification stands out as a promising method for generating heat and power. This research delves into the potential impact of biomass gasification within the global energy landscape, focusing particularly on its application in cogeneration plants. Utilizing Aspen Plus software V10, this study undertook the modeling and optimization of a biomass cogeneration plant. Through simulation, it was found that a biomass flow rate of 5 kg/s yielded 6.172 MW of power output. Additionally, the study revealed several key factors that influence power generation: increasing biomass and airflow rates, increasing gasification temperature, and reducing water flow rate. By doubling the biomass flow rate to 10 kg/s and increasing the temperature to 800 °C, power generation increases by 41.75%. Moreover, the study demonstrates that Portuguese municipal waste is an efficient source of energy production, with higher cold gas and overall efficiencies compared to forest and vine-pruning residues.
1. Introduction
For many years, industries have relied mainly on fossil fuels as their main energy source [1]. However, this dependence has triggered significant climate shifts and the depletion of fossil fuel reserves, posing pressing challenges for society today. Notably, the combustion of fossil fuels stands out as one of the foremost contributors to environmental issues [2].
In a concerted effort to address these challenges, the European Council has set forth key targets to be met by 2030. These targets include reducing greenhouse gas emissions by at least 55% from 1990 levels, achieving a renewable energy share of between 32% and up to 42.5%, improving energy efficiency by 32.5% to 36%, and attaining a primary energy consumption efficiency of 39% [3].
Renewable energy sources (RES) present a compelling alternative to fossil fuels due to their minimal environmental impact [4]. Between 2010 and 2021, renewable energy witnessed unparalleled growth, boasting an average annual increase of 0.8 percentage points. This increase in the deployment of renewable energy is a crucial factor in mitigating the increase in global temperature, with the aim of keeping it below 1.5 °C. However, to realize the goal of achieving net zero emissions by 2050, the pace of renewable electricity adoption must accelerate substantially [5].
In recent years, biomass has emerged as a significant contender among RES for heat and power production due to its versatility. Biomass can be utilized in solid, liquid, and gaseous forms [2]. Moreover, it is often regarded as a carbon-neutral feedstock because it originates from plants. Through photosynthesis, plants convert carbon dioxide from the air into oxygen [6].
Biomass fuels can be categorized into six primary classes: wood and woody materials (including both hard and soft woods); herbaceous fuels, such as straw and grasses; waste materials, including sewage sludge and refuse-derived fuel (RDF); industrial derivatives; aquatic biomass, such as kelp and energy crops specifically cultivated for energy production purposes [7,8].
Biofuels can also be categorized into four categories: conventional or first-generation biofuels, derived from food crops like sugar, starch, and vegetable oils, as well as land-using feedstock; second-generation biofuels, sourced from non-food crops such as wheat straw, municipal waste, and algae; third-generation biofuels, obtained from microalgae and microbial sources like bio-oils and biogas; and lastly, fourth-generation biofuels, which involve advanced technologies like carbon dioxide capture for production [9].
One of the primary biomass conversion techniques is thermochemical conversion, which includes pyrolysis, gasification, and combustion [10]. Pyrolysis offers several advantages, including lower levels of secondary pollution, cost-effectiveness, high efficiency, and a higher net calorific value. Nevertheless, it also has limitations such as favoring small-sized particles and requiring a long residence time [9]. Combustion, while showing potential for future applications and being relatively cost-effective compared to other techniques, has drawbacks such as emissions of pollutant gases, ash deposition, slagging of inorganic components, the need for a drying pre-treatment, and limited product versatility [9]. On the other hand, gasification stands out as one of the most advanced processes for biomass conversion into fuel. The resulting gaseous product, known as syngas, mainly comprises carbon monoxide (CO), hydrogen (H2), carbon dioxide (CO2), and methane (CH4). Common gasifying agents include air or oxygen, steam, or CO2, with temperatures typically exceeding 700 °C [11,12,13]. Syngas produced by gasification can be utilized for heat and power production. Gasification offers several advantages over other techniques, including the production of versatile and synthetic gas, the recovery of useful resources, uniform temperature distribution, and efficient heat transfer. However, it also has limitations such as tar release, high capital and equipment maintenance costs, the requirement for high heat energy, and the need for feed rate optimization [9].
A wide array of biomass gasification reactors has been developed, typically falling into three generic types: fixed bed, fluidized bed (FB), and entrained flow gasifiers [14].
Fixed bed gasifiers are commonly employed in small to medium power plants and can be further categorized into three reactor types: updraft, downdraft, and cross-draft. For instance, the Harboøre gasification plant in Denmark utilizes an updraft gasifier, boasting a power production of approximately 500 MWh per month and a total grid supply exceeding 32,000 MWh. The gasifiers have operated for a total of 110,000 h, while the engine has run for 70,000 h. Meanwhile, a modified version of the downdraft gasifier is utilized in the Viking gasification plant in Denmark, with two units installed—one in Hadsund generating 200 kWe and the other in Hillerod generating 500 kWe. However, cross-draft reactors exhibit overall low efficiency and are unable to produce producer gas of acceptable quality, resulting in their exclusion from further development [14].
FB gasification reactors are typically employed in medium-to-large-scale applications, with variations including bubbling fluidized bed (BFB), circulating fluidized bed (CFB), and dual fluidized bed (DFB) reactors. For example, the Winkler gasifier developed in Germany is an application of BFB gasification reactors. On the other hand, CFB gasifier reactors, such as the Varnamo gasifier built in Sweden, are suitable for gas turbine applications. However, economic reasons led to the mothballing of the Varnamo plant after 8000 h of gasifier operation and 3600 h of turbine operation in 2000 [14]. DFB reactors, which often integrate BFB and CFB designs [15], are employed in plants like the Gussing gasification plant in Austria. While the Gussing technical concept was once considered the most successful allothermal gasification system globally, economic factors led to the plant’s shutdown after nearly 100,000 h of operation [14]. Another example of the utilization of the FB gasification reactors is the Renugas plant in Maui, Hawaii. However, this plant encountered major problems such as the low bulk density of the bagasse used, which led to difficulties in the feeding operation. Additionally, interruptions caused by residual sugars in the bagasse dust affected the operation. Consequently, the Renugas plant in Maui had to be discontinued due to these problems and lack of funds [16]. A BFB reactor based on the Renugas technology was built in Skive, Denmark. The plant’s design started in 2004 and it commenced operating in 2008. Although this plant encountered several issues, the project managed to overcome these difficulties and is still in operation [16].
Entrained flow gasifiers are highly efficient and well-suited for large-scale applications. Examples include the Koppers–Toptzek, Texaco, and Shell gasifiers [14].
Project Arable Biomass Renewable Energy (ARBRE) in the UK, which aimed to demonstrate electricity generation from energy crops using a CFB gasification reactor, never reached commercial operation. This failure was attributed to technical problems with the gasification technology, poor management, and financial issues [17].
In Europe today, a significant number of gasification plants are operational for energy generation, including the Swindon Advanced Biofuels Plant in the UK, the Pelletvergaseer AEW Rheinfelden in Switzerland, and the BioTfuel pilot in France. However, some plants are no longer operational, such as the CHP PratteIn, which ceased operations in 2014 due to technical issues, and the Wood co-gasification in IGCC, which closed in 2013 due to low energy prices and high operational costs [18]. Worldwide, the Combustion Gasification and Propulsion Laboratory (CGOL) located at the Indian Institute of Science (IISc) is involved in researching and developing gasification techniques. Currently, their focus is on the conversion of biomass to produce hydrogen for industrial applications [19]. Another example is Carbogas, located in Brazil. They specialize in the production of coal, biomass, and waste gasification plants to generate fuel gases for use in ceramic, steel, and other furnaces. Currently, they are working towards a sustainable future by leveraging renewable resources and environmental conservation [20].
Numerical models are commonly employed in process simulations due to their ability to provide accurate predictions of system behavior under various conditions. While empirical experiences are valuable for understanding complex processes, they may struggle to account for all variables involved. In contrast, numerical models can comprehensively consider factors such as temperature, pressure, and the species involved, offering detailed insights into system behavior. The information obtained from numerical models can facilitate further studies, enabling optimization of system performance, reduction in energy consumption, and enhancement of the quality of the final product. Moreover, numerical models offer cost-saving benefits by allowing visualization and verification of the effects of new equipment on the process before purchase, mitigating the risk associated with investing in expensive equipment.
In addition to power and heat, syngas can be used to produce hydrogen, for fertilizer production, CO2 capture, and sustainable aviation fuels, just to name a few. However, in this study, the focus is on the production of energy and heat.
In the literature, Aspen Plus software has been extensively utilized to investigate gasification and cogeneration models. For instance, Okati et al. [21] conducted a study exploring the influence of temperature, equivalence ratio, steam-to-waste ratio, and different ratios of polypropylene in the feedstock on the quality of syngas and hydrogen produced. Their findings revealed that higher quantities of polyethylene in the waste, along with low air-to-waste ratios and equivalence ratios, resulted in a higher molar fraction of hydrogen. Additionally, they noted that the impact of steam injection and temperature variations on hydrogen production was minimal.
Similarly, Jana and De [22] employed Aspen Plus to analyze the performance of cogeneration plants with and without CO2 capture. Their results suggested that post-combustion CO2 capture is more effective than pre-combustion CO2 capture for plants with negative CO2 emissions. They emphasized the need to optimize the degree of capture, as it significantly influences the overall thermodynamic and economic performance of the plant.
In another study, Tavares et al. [23] presented a numerical methodology utilizing Aspen Plus to simulate the gasification of Portuguese forest residues. Their investigation concluded that higher temperatures favor the production of gas rich in hydrogen and with a higher heating value. Additionally, they found that using steam instead of air as a gasifying agent increases the hydrogen content and calorific value of the produced gas.
Pitrez et al. [24] introduced a computational model replicating high-temperature treatment for COVID-19 waste through plasma gasification using Aspen Plus. They augmented the model with an additional Gibbs reactor to enhance the calorific value of the syngas produced. Their findings indicated that the inclusion of the extra Gibbs reactor raised the calorific value of the syngas from 4.97 to 5.19 MJ/m3. This increase was attributed to elevated molar fractions of CO and CH4, coupled with a decrease in the molar fraction of H2. Furthermore, the study revealed variations in waste composition among different regions. Waste from Korea exhibited higher hydrogen molar fractions, while waste from Turkey yielded higher carbon monoxide molar fractions, and waste from Lithuania showed the highest methane molar fractions. The lowest syngas calorific values were observed for Turkish waste, whereas the highest values were recorded for Korean waste.
Calì et al. [25] conducted an experiment aimed at optimizing and developing a fixed-bed updraft gasification process for biomass-based power generation. Their study particularly emphasized the optimization of an integrated double-stage wastewater management system, aiming to minimize both liquid residues and water makeup. Through their investigation, they identified the optimal process parameters for operating the syngas cleaning system, resulting in a remarkable 60% reduction in wastewater disposal.
The utilization of gasification of biomass to produce heat and energy can be a solution for the environmental problems associated with greenhouse gas and for energy production, since it offers a triple advantage: mitigating the risk of uncontrolled forest fires by cleaning rural areas, harnessing energy from waste recovery, and diminishing reliance on fossil fuels. Additionally, it contributes to curtailing greenhouse gas emissions, a matter of significant concern in recent decades.
This study builds upon the model developed by Rakaz and Abdulrazik [26] to assess the influence of the gasification process on a cogeneration plant utilizing biomass to achieve a targeted power output of 1.3 MW and optimize its performance. Their findings indicate that it is feasible to reach the desired power of 1.3 MW. Moreover, the power generated increases with higher biomass and air flow rates, as well as with increased boiler temperature. Conversely, increasing the water flow rate leads to a decrease in power generated. The study also underscores the potential of biomass-based cogeneration plants to replace conventional fossil fuels.
2. Materials and Methods
The research aimed to select a suitable Process Flow Diagram (PFD) for biomass cogeneration based on the literature. Figure 1 depicts the PFD equivalent to that provided by Rakaz and Abdulrazik [26], which utilizes biomass as fuel.
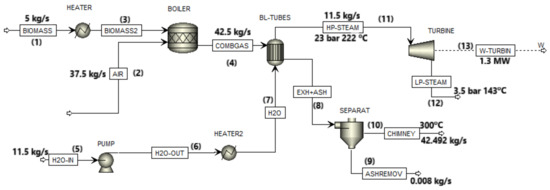
Figure 1.
Process flow diagram of cogeneration plant.
In this configuration, biomass is heated (1) before fueling a water tube boiler (3) to generate both heat and power via steam. Forced draft fans (2) supply air to the boiler and the combustion gases are directed to the heat exchanger (4). Simultaneously, water is pumped (5) and heated (6) before being sent into the heat exchanger (7). The combustion chamber’s heat raises the water temperature, producing steam at 23 bar and 222 °C. This superheated steam drives a turbine generator (11). The turbine generator exhausts steam at 143 °C and 3.5 bar, which is directed to a steam manifold for various processes (12), such as to sterilization process, to digester, to clarification tank, and to CPO (crude palm oil) storage tank. The ashes and the exhaust gas leave the combustion chamber (8) and the ashes are removed (9), while exhaust gas is released through a chimney (10). The generated power must meet energy requirements across different areas (13). Specifically, 180 kW is allocated for the boiler, 70 kW for loading ramps, 100 kW for threshing machinery, 160 kW for a screw press, 110 kW for decarpeting, 160 kW for a kernel plant, and 210 kW for clarification and oil purification. Additionally, 150 kW is needed for each process of effluent processing and EFB (empty fruit bunch) composting press. Finally, 52 kW is designated for domestic use, totaling approximately 1.3 MW to satisfy the plant’s needs.
Simulation Model
The cogeneration system model was implemented using Aspen Plus, a software developed by Aspentech [27]. Aspen Plus is widely utilized for designing and simulating various industrial processes. It offers a range of blocks including heat exchangers, mixers/splitters, pumps, compressors, turbines, and more, enabling comprehensive simulations of complex systems.
There are several approaches to conducting simulations using Aspen Plus software, but it is essential to understand the properties of the processes being simulated beforehand. Additionally, familiarity with the behavior of various process components and providing initial process conditions is crucial.
The simulation process in Aspen Plus can generally be divided into three phases:
- Definition of the chemical components involved in the simulation;
- Setting of the PFD by setting the blocks and defining the connections between them;
- Setting the main parameters of all flows (temperature, pressure, etc.) and operating conditions of each block.
In this work, the MIXCINC (mixed criticality incremental stream computation) stream class was used. This choice was made because the process involves a combination of nonconventional components (such as biomass and ash), solids (such as carbon), and conventional components including hydrogen (H2), oxygen (O2), nitrogen (N2), water (H2O), sulfur (S2), methane (CH4), carbon monoxide (CO), and carbon dioxide (CO2).
For the nonconventional components, the algorithms HCOALGEN and DCOALIGT were utilized for determining the enthalpy and density, respectively. The HCOALGEN model necessitates three component attributes for these nonconventional components: the proximate analysis results (PROXANAL), the ultimate analysis results (ULTANAL), and the sulfur analysis results (SULFANAL) of the material utilized, as denoted in Aspen Plus. The proximate analysis provides data on the moisture, ash, volatile matter, and fixed carbon content. Meanwhile, the ultimate analysis offers the weight composition of the material concerning ash, carbon, hydrogen, nitrogen, chlorine, sulfur, and oxygen, whereas the sulfur analysis furnishes the sulfur content categorized into pyritic, sulfate, and organic sulfur.
On the other hand, the DCOALIGT model solely requires two of these components: ULTANAL and SULFANAL.
The components involved in this work were defined by using the Peng–Robinson equation of state as property method [28]:
where P is the pressure, R the gas constant, T the absolute temperature, is the molar volume, and are the energy parameter and the size parameter, respectively, and is a dimensionless function of reduced temperature () and acentric conversion factor ():
Figure 2 shows the model of the biomass cogeneration plant developed in Aspen Plus. In the original study by Rakaz and Abdulrazik [26], a boiler was employed to simulate the combustion of biomass. However, in the present work, a gasification process is utilized instead.
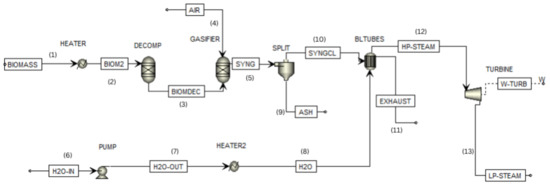
Figure 2.
Flowsheet of the biomass cogeneration plant.
In the configuration shown in Figure 2, the process begins with the introduction of biomass into a heater (1). Once heated, the biomass is directed to a decomposer (2) and undergoes decomposition to break it down into its constituent elements. Subsequently, the decomposed biomass is directed to the gasifier (3), where the gasification process will take place. Air is supplied to the gasifier as a gasifying agent (4). The product resulting from gasification, the synthesis gas, is then sent to a separator (5) to remove the ash content (9), and the resulting clean synthesis gas is directed to the heat exchanger (10). Simultaneously, the water is pressurized in a pump (6) before being directed to a heater (7). From there, the water flows to the heat exchanger (8). Exhaust gases (11) are released inside the heat exchanger, while high-pressure steam is channeled to a turbine (12) for power generation. Furthermore, the low-pressure steam will be directed to a steam collector (13) for various processes.
In Figure 2, the process is divided into two parts before reaching the heat exchanger (BLTUBES). One part involves the decomposition and gasification of biomass, sourced from a splitter (SPLIT), while the other part, where water is pumped and heated, originates from a heater (HEATER2). The combination of the gasifier and heat exchanger represents a water tube boiler in Aspen Plus, as there is no direct water tube boiler system available in the software [24].
In the first route, 5 kg/s of biomass enters the heater (HEATER) at 25 °C and 1 bar and exits the heater at 800 °C and 1 bar. Subsequently, the biomass is directed to a RYELD reactor (DECOMP), where it undergoes decomposition into its constituent elements. These elements include carbon (C), hydrogen (H2), oxygen (O2), nitrogen (N2), sulfur (S2), and ash. The yield distribution is specified according to the ultimate analysis of the biomass. The composition of the biomass used in this study can be found in Table 1.

Table 1.
Portuguese municipal solid waste proximate and ultimate analysis [29].
The gasification phase is conducted in an RGibbs reactor (GASIFIER) to simulate the process in a BFB gasification reactor. This reactor utilizes Gibbs free energy minimization as a model for chemical equilibrium to replicate the gasification process accurately. The total Gibbs free energy is more suitable for complex problems since no chemical reaction needs to be known to find the solution. The Gibbs free energy minimization helps to predict the equilibrium conditions of the system, and can be defined as [30]:
where is the total Gibbs free energy, is the number of moles of species , and is the chemical potential of species .
In a second inlet stream, 37.5 kg/s of air containing 21% oxygen and 79% nitrogen at 25 °C and 1 bar is also introduced into the gasifier.
The reactions considered for the gasification are [29,31,32]:
- Boudouard
C + CO2 → 2CO ΔH = 172 kJ/mol
- Water–gas
C + H2O → CO + H2 ΔH = 131 kJ/mol
- Methanation
C + 2H2 → CH4 ΔH = −75 kJ/mol
- Shift
CO2 + H2 ↔ CO + H2O ΔH = −41 kJ/mol
- Steam reforming
CH4 + H2O ↔ CO + 3H2 ΔH = 206 kJ/mol
The gasification process takes place at 800 °C and 1 bar. The resulting raw syngas (SYNG) is then directed to an SSPLIT separator (SPLIT), where the solid components are separated from the gas. The clean syngas (SYNGCL) is subsequently directed to the heat exchanger (BLTUBES), which symbolizes the water tubes in the water tube boiler. The gas produced is connected to the hot inlet of the heat exchanger.
Simultaneously, in the second route, 11.5 kg/s of water at 25 °C and 2 bar is pumped (PUMP) to a pressure of 45 bar. This pressurized water is then heated to 98 °C in a heater (HEATER2) before entering the heat exchanger as its cold inlet.
A shortcut, counter-current heat exchanger was employed with a minimum temperature approach of 1 °C, while the hot stream outlet temperature was set at 100 °C. For pressure drop calculations, an outlet pressure of 1 bar was considered. The exhaust gas from the hot outlet stream of the heat exchanger is released. Additionally, high-pressure steam from the cold outlet stream of the heat exchanger, at 478 °C and 45 bar, is directed to a turbine (TURBINE).
The turbine was assumed to be isentropic, operating at 4 bar with an isentropic efficiency of 88% and a mechanical efficiency of 99%, consistent with the values used by Cirillo et al. [2]. The exhaust gas released will be directed to a steam manifold for plant heating purposes, while the electricity generated will be transferred to the main switch box for power distribution, as depicted in Figure 1.
3. Results
Figure 3 illustrates the flowsheet of the biomass cogeneration plant after the simulation, displaying the pressure and temperature at each step. The power generated is recorded as 6.172 MW for a mass flow rate of 5 kg/s of biomass. In comparison, Rakaz and Abdulrazik [26] achieved a power output of 2.170 MW for the same biomass flow rate and a temperature of 800 °C in the boiler. This indicates an increase of 4.002 MW in the power generated for the same mass flow rate of biomass and temperature in the gas production process.
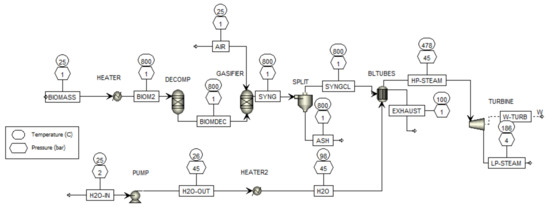
Figure 3.
Biomass cogeneration plant with temperature and pressure.
In a separate study, Mahidin et al. [33] conducted a simulation and optimization of the same plant (Figure 1). They presented five scenarios and concluded that the appropriate flow rate for biomass was 5 kg/s and for air was 58.5 kg/s. The highest power generated in their study was 4.46 MW, which is 2.29 MW higher than the values obtained by Rakaz and Abdulrazik [26], but 1.712 MW lower than the value obtained in the present study.
As can be seen in Figure 1, the plant needs 1.3 MW of energy. That means that the energy produced in the turbine is more than enough to fulfill the necessities of the plant.
To optimize the system, various changes were considered, including adjustments to factors such as the water flow rate, biomass flow rate, air flow rate, and variations in the gasification temperature.
Initially, the variation in the biomass flow rate was examined, spanning values from 1 kg/s to 10 kg/s. The results indicate a direct relationship between the biomass flow rate and the energy produced by the system, as shown in Table 2. For instance, at a biomass flow rate of 1 kg/s, the power generated equals 4.573 MW. Conversely, employing a biomass flow rate of 10 kg/s yields a power output of 8.749 MW, representing an increase of approximately 91.32% compared to the 5 kg/s biomass flow rate.

Table 2.
Power generated for different biomass flow rates.
Furthermore, the comparison with the power output at a biomass flow rate of 5 kg/s reveals an increase of approximately 41.75% with the utilization of 10 kg/s biomass flow rate. This demonstrates that a higher biomass flow rate results in a greater fuel supply, leading to increased gas production and consequently, higher power output. Consequently, the optimal biomass flow rate was determined to be 10 kg/s.
Considering a biomass flow rate of 10 kg/s, the impact of varying water flow rates on the power generation of the system was investigated. Water flow rates ranging from 11.5 kg/s to 13.5 kg/s were studied, consistent with the range used by Rakaz and Abdulrazik [26]. The results are presented in Table 3.

Table 3.
Power generated for different water flow rates.
It is evident from the table that the power generated decreases with an increase in the water flow rate. For instance, at a water flow rate of 11.5 kg/s, the power generated is 8.749 MW, whereas, at 13.5 kg/s, the power generated decreases to 7.578 MW, representing a reduction of approximately 13.38%.
This decline in power generation with higher water flow rates can be attributed to excessive cooling of the gasification reactor. Excessive water flow rates may lead to a reduction in gasification efficiency, thereby indirectly diminishing power generation.
Based on these results, the optimal water flow rate is 11.5 kg/s. Next, considering a biomass flow rate and water flow rate of 10 kg/s and 11.5 kg/s, respectively, the influence of the airflow rate on the power generated was studied. For this, a range of values between 35.5 kg/s and 37.5 kg/s for the air flow rate was considered, Table 4.

Table 4.
Power generated for different air flow rates.
From the results, it is evident that the power generated decreases as the airflow rate decreases. For instance, at an airflow rate of 37.5 kg/s, the power generated is 8.749 MW, whereas a decrease in the airflow rate to 35.5 kg/s results in a power output of 8.223 MW, representing a decrease of approximately 6.01%. Therefore, it can be concluded that the optimal air flow rate is 37.5 kg/s. This phenomenon occurs because increasing the air flow rate enhances the gasification efficiency, allowing for more efficient conversion of biomass into power.
Finally, the effect of the temperature of the gasification process on the power generated was studied. The range of temperatures considered was from 700 °C to 800 °C. The results can be consulted in Table 5.

Table 5.
Power generated for different gasification temperatures.
From the results obtained, we can see that the power generated by the system decreases with the decrease in the temperature. For a temperature of 800 °C, the power generated is 8.749 MW, but for a temperature of 700 °C, the power generated is 6.404 MW, which represents a decrease of approximately 26.80%. Higher temperatures, usually promote faster reactions and conversion of biomass components. As a result, the power generation increases with the increase in temperature.
Based on these results, the optimal biomass flow rate is 10 kg/s. The water flow rate, air flow rate, and gasification temperature can be kept constant at 11.5 kg/s, 37.5 kg/s, and 800 °C, respectively.
The results obtained were compared with those presented in the literature by Rakaz and Abdulrazik [26].
Figure 4 illustrates the power generated for different biomass flow rates and water flow rates. It is evident that the power generated increases with an increase in biomass flow rate and a decrease in water flow rate. The maximum power generated in the current study is 8.749 MW for a biomass flow rate of 10 kg/s and a water flow rate of 13.5 kg/s. In contrast, Rakaz and Abdulrazik [26] achieved a maximum power output of 1.764 MW for a biomass flow rate of 5 kg/s and a water flow rate of 11.5 kg/s.
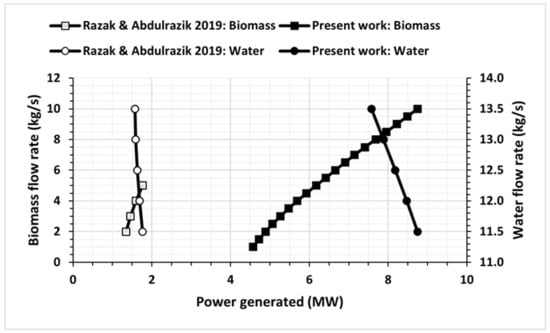
Figure 4.
Power generated at different biomass and water flow rates compared with the results obtained by Rakaz and Abdulrazik [26].
Figure 5 displays the power generated for different temperatures and airflow rates. The power generated increases with an increase in air flow rate and temperature. Notably, Rakaz and Abdulrazik [26] achieved a maximum power output of 2.170 MW by increasing the boiling temperature from 700 °C to 800 °C.
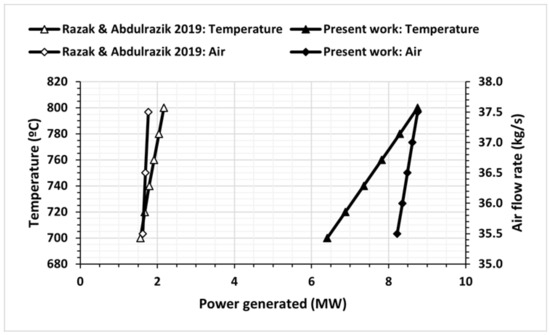
Figure 5.
Power generated at different temperatures and air flow rates compared with the results obtained by Rakaz and Abdulrazik [26].
These comparisons demonstrate that the results obtained in the present study align with those of Rakaz and Abdulrazik [26]. Both studies confirm that the power generated increases with an increase in biomass flow rate, temperature, and airflow rate, and a decrease in water flow rate. The findings of this study indicate that employing the gasification process yields higher energy generation compared to the combustion process. However, after replacing the combustion chamber with the gasification system, it was found that gasification allows better performance in obtaining power and, in addition, allows the formation of syngas.
Since the plant only needs 1.3 MW to operate, the additional power produced can be sold to the national power grid. Furthermore, the excess of power produced can possibly help with the fossil fuel dependency problem and minimize environmental degradation.
It was also observed that for a biomass flow superior to 10 kg/s, the simulation was no longer doable due to air becoming a limiting reactant.
The energy produced also relies on the thermodynamic properties of biomass, including proximate and ultimate analyses, moisture content, low heating values, etc., alongside various other parameters. To investigate the impact of these thermodynamic properties, a comparison was made between the results obtained for Portuguese municipal solid waste, as shown in Table 1, and those for forest residues and vine-pruning residues, as presented in Table 6. The operating conditions for flow rates and temperature are as previously stated.

Table 6.
Proximate and ultimate analysis of forest residues and vine-pruning residues [34].
To calculate the efficiency of the system, the cold gas efficiency (CGE) was calculated using the equation below [35]:
The LHV of the syngas can be determined by using the following expression [35]:
where represents the mole fraction of the syngas compounds.
The total efficiency was determined by using the following equation, taking into account the power generated () and the lower heating value of the biomass [35]:
The results, as shown in Table 7, reveal that the highest power generation occurs with Portuguese. Specifically, power generation for forest residues and vine-pruning residues when compared to municipal solid waste is 5.49% and 5.71% lower, respectively.

Table 7.
CGE, total efficiency, and power generated for the three biomasses.
Regarding cold gas efficiency (CGE), Portuguese municipal waste demonstrates an efficiency of 50.67%, while forest residues and vine-pruning residues exhibit efficiencies of 15.67% and 5.83%, respectively. This substantial decrease in CGE highlights Portuguese municipal waste as a notably more efficient source of energy generation. Several factors, including waste composition (Table 1 and Table 6), may contribute to this efficiency difference.
For total efficiency, Portuguese municipal waste, forest residues, and vine-pruning residues showcase efficiencies of 6.08%, 3.91%, and 5.46%, respectively. This suggests that the energy generation process using Portuguese municipal waste is relatively efficient overall. Additionally, despite vine-pruning residues having a low CGE, their overall energy generation process remains efficient compared to forest residues.
4. Conclusions
In this study, the optimization of a biomass cogeneration plant was investigated by replacing the combustion chamber with a gasification system. It was found that the use of a gasification process yielded an energy production of 6.172 MW with a biomass flow of 5 kg/s, which exceeds the plant’s energy needs by 4.7 times (1.3 MW). This energy production could be increased by 41.75% with a biomass flow of 10 kg/s. The optimal flow rates determined were 10 kg/s for biomass, 11.5 kg/s for water, and 37.5 kg/s for air, with a gasification temperature of 800 °C.
Replacing the combustion chamber with a gasification system was found to improve power generation performance in addition to producing syngas.
Moreover, the power generated was observed to be influenced by biomass, water, and airflow rates, as well as gasification temperature. Power generation increased with higher biomass rates, airflow, and gasification temperature, while it decreased with increasing water flow rate. Additionally, thermodynamic properties such as proximate and ultimate analysis, moisture content, low heating value, etc., alongside efficiency, play crucial roles in analyzing power generation. It was evident that Portuguese municipal waste presented higher values of power generated, cold gas efficiency (CGE), and total efficiency when compared with forest residues and vine-pruning residues. This disparity could be attributed to several factors, including the composition of the waste.
By generating both energy and heat, these systems can make more efficient use of the fuel, which could lead to significant cost savings over time. The electricity generated can reduce the amount of energy that needs to be purchased from the grid and the heat produced can also be used, reducing the need for additional fuel to meet thermal energy demands. However, the economic analysis is always important. The analysis of the payback period, return on investment, and net present value are essential to determine whether the benefits of increased power production and overall efficiency outweigh the initial and ongoing costs.
The study also suggests that biomass cogeneration can be an alternative to solid fuels for energy production. Such a change could significantly contribute to a greener and cleaner environment, in addition to presenting some benefits, such as mitigating the risk of uncontrolled forest fires through the cleaning of rural areas and the use of forest residues for energy production.
Author Contributions
Conceptualization, F.N., A.A.S. and A.R.; methodology, F.N. and A.A.S.; software, F.N. and A.A.S.; validation, A.A.S. and A.R.; investigation, F.N.; writing—original draft preparation, F.N.; writing—review and editing, F.N., A.A.S. and A.R.; supervision, A.A.S. and A.R.; funding acquisition, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by National Funds by FCT—Portuguese Foundation for Science and Technology, under project PCIF/GVB/0169/2019.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Wu, Q.; Qiang, T.; Zeng, G.; Zhang, H.; Huang, Y.; Wang, Y. Sustainable and renewable energy from biomass wastes in palm oil industry: A case study in Malaysia. Int. J. Hydrogen Energy 2017, 42, 23871–23877. [Google Scholar] [CrossRef]
- Cirillo, D.; Palma, M.; Villetta, M.; Macaluso, A.; Mauro, A.; Vanoli, L. A novel biomass gasification micro-cogeneration plant: Experimental and numerical analysis. Energy Convers. Manag. 2021, 243, 114349. [Google Scholar] [CrossRef]
- 2023 Climante & Energy Framework. Available online: https://climate.ec.europa.eu/eu-action/climate-strategies-targets/2030-climate-energy-framework_en (accessed on 6 November 2023).
- Passey, R.; Spooner, T.; MacGill, I.; Watt, M.; Syngellakis, K. The potential impact of grid-connected distributed generation and how to address them: A review of technical and non-technical factors. Energy Policy 2011, 39, 6280–6290. [Google Scholar] [CrossRef]
- Monteiro, E.; Ferreira, S. Some perspectives for the gasification process in the energy transition world scenario. Energies 2023, 16, 5543. [Google Scholar] [CrossRef]
- Ramos, A.; Rouboa, A. Syngas production strategies from biomass gasification: Numerical studies for operational conditions and quality indexes. Renew. Energy 2020, 155, 1211–1221. [Google Scholar] [CrossRef]
- Jenkins, B.; Baxter, L.; Miler, T., Jr.; Miles, T. Combustion proprieties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Khan, A.; de Jong, W.; Jansens, P.; Spliethoff, H. Biomass combustion in fluidized bed boilers: Potential problems and remedies. Fuel Process. Technol. 2009, 90, 21–50. [Google Scholar] [CrossRef]
- Ramos, A.; Monteiro, E.; Rouboa, A. Biomass pre-treatment techniques for the production of biofuels using thermal conversion methods—A review. Energy Convers. Manag. 2022, 270, 116271. [Google Scholar] [CrossRef]
- Velvizhi, G.; Jacqueline, P.; Shetti, N.; Latha, K.; Mohanakrishna, G.; Aminabhavi, T. Emerging trends and advances in valorization of lignocellulosic biomass to biofuels. J. Environ. Manag. 2023, 345, 118527. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of the lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Kuo, P.; Wu, W. Design, optimization and energetic efficiency of producing hydrogen-rich gas from biomass steam gasification. Energies 2015, 8, 94–110. [Google Scholar] [CrossRef]
- Razmi, A.; Afshar, H.; Pourahmadiyan, A.; Torabi, M. Investigation of a combined heat and power (CHP) system based on biomass and compressed air energy storage (CAES). Sustain. Energy Technol. Assess. 2021, 46, 101253. [Google Scholar] [CrossRef]
- Oliveira, M.; Ramos, A.; Ismail, T.; Monteiro, E.; Rouboa, A. A review on plasma gasification of solid residues: Recent advances and developments. Energies 2022, 15, 1475. [Google Scholar] [CrossRef]
- Karl, J.; Proll, T. Steam gasification of biomass in dual fluidized bed gasifiers: A review. Renew. Sustain. Energy Rev. 2018, 98, 64–78. [Google Scholar] [CrossRef]
- Motta, I.; Miranda, N.; Filho, R.; Maciel, M. Biomass gasification in fluidized beds: A review of biomass moisture content and operating pressure effects. Renew. Sust. Energy Rev. 2018, 94, 998–1023. [Google Scholar] [CrossRef]
- Piterou, A.; Shackley, S.; Upham, P. Project ARBRE: Lessons for bio-energy developers and policy-makers. Energy Policy 2008, 36, 2044–2050. [Google Scholar] [CrossRef]
- Task 33: Gasification of Biomass and Waste|Database. Available online: https://task33.ieabioenergy.com/ (accessed on 20 April 2024).
- Combustion Gasification & Propulsion Laboratory (CGPL). Available online: https://cgpl.iisc.ac.in/index.html# (accessed on 24 April 2024).
- Carbogas. Available online: http://carbogas.com.br/default.asp (accessed on 24 April 2024).
- Okati, A.; Khani, M.; Shokri, B.; Monteiro, E.; Rouboa, A. On the operating parameters for hydrogen-rich syngas production in a plasma co-gasification process of municipal solid wastes and polypropylene using a constrained model in Aspen plus. J. Energy Inst. 2023, 107, 101173. [Google Scholar] [CrossRef]
- Jana, K.; De, S. Biomass integrated gasification combined cogeneration with or without CO2 capture—A comparative thermodynamic study. Renew. Energy 2014, 72, 243–252. [Google Scholar] [CrossRef]
- Tavares, R.; Monteiro, E.; Tabet, F.; Rouboa, A. Numerical investigation of optimum operating conditions for syngas and hydrogen production from biomass gasification using Aspen Plus. Renew. Energy 2020, 146, 1309–1314. [Google Scholar] [CrossRef]
- Pitrez, P.; Monteiro, E.; Rouboa, A. Numerical analysis of plasma gasification of hazardous waste using Aspen Plus. Energy Rep. 2023, 9, 418–426. [Google Scholar] [CrossRef]
- Calì, G.; Deiana, P.; Bassano, C.; Meloni, S.; Maggio, E.; Mascia, M.; Pettinau, A. Syngas Production, Clean-Up and Wastewater Management in a Demo-Scale Fixed-Bed Updraft Biomass Gasification Unit. Energies 2020, 13, 2594. [Google Scholar] [CrossRef]
- Razak, N.; Abdulrazik, A. Modelling and optimization of biomass-based cogeneration plant. In Proceedings of the 2019 9th International Conference on Future Environment and Energy, Osaka, Japan, 9–11 January 2019. [Google Scholar]
- Aspen Plus|Leading Process Simulation Software|AspenTech. Available online: https://www.aspentech.com/en/products/engineering/aspen-plus (accessed on 5 November 2023).
- Noh, J.; Fulgueras, A.; Sebastian, L.; Lee, H.; Kim, D.; Cho, J. Estimation of the thermodynamic properties of hydrogen isotopes and modeling of hydrogen isotope systems using Aspen Plus simulator. J. Ind. Eng. Chem. 2017, 46, 1–8. [Google Scholar] [CrossRef]
- Cardoso, J.; Silva, V.; Eusébio, D.; Brito, P. Hydrodynamic Modelling of Municipal Solid Waste Residues in a Pilor Scale Fluidized Bed Reactor. Energies 2017, 10, 1773. [Google Scholar] [CrossRef]
- Jarungthammachote, S.; Dutta, A. Equilibrium modeling of gasification: Gibbs free energy minimization approach and its application to spouted bed and spout-fluid bed gasifiers. Energy Convers. Manag. 2008, 49, 1345–1356. [Google Scholar] [CrossRef]
- Kuo, P.; Wu, W. Thermodynamic analysis of a combined heat and power system with CO2 utilization based on co-gasification of biomass and coal. Chem. Eng. Sci. 2016, 142, 201–214. [Google Scholar] [CrossRef]
- González-Vásquez, M.; Rubiera, F.; Pevida, C.; Pio, D.; Tarelho, L. Thermodynamic Analysis of Biomass Gasification Using Aspen Plus: Comparison of Stoichiometric and Non-Stoichiometric Models. Energies 2021, 14, 189. [Google Scholar] [CrossRef]
- Mahidin, M.; Erdiwansyah, E.; Husin, H.; Hisbullah, H.; Hayati, A.; Zhafran, M.; Sidiq, M.; Rinaldi, A.; Fitra, B.; Tarima, R.; et al. Utilization of oil palm biomass as a renewable and sustainable energy source in Aceh provice. J. Adv. Res. Fluid Mech. Therm. Sci. 2020, 67, 97–108. [Google Scholar]
- Silva, V.; Monteiro, E.; Couto, N.; Brito, P.; Rouboa, A. Analysis of syngas quality from Portuguese biomasses: An experimental and numerical study. Energy Fuels 2014, 28, 5766–5777. [Google Scholar] [CrossRef]
- HajiHashemi, M.; Mazhkoo, S.; Dadfar, H.; Livani, E.; Varnosefaderani, A.; Pourali, O.; Nobar, S.; Dutta, A. Combined heat and power production in a pilot-scale biomass gasification system: Experimental study and kinetic simulation using Aspen Plus. Energy 2023, 276, 127506. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).