Biogas Upgrading Technology: Conventional Processes and Emerging Solutions Analysis
Abstract
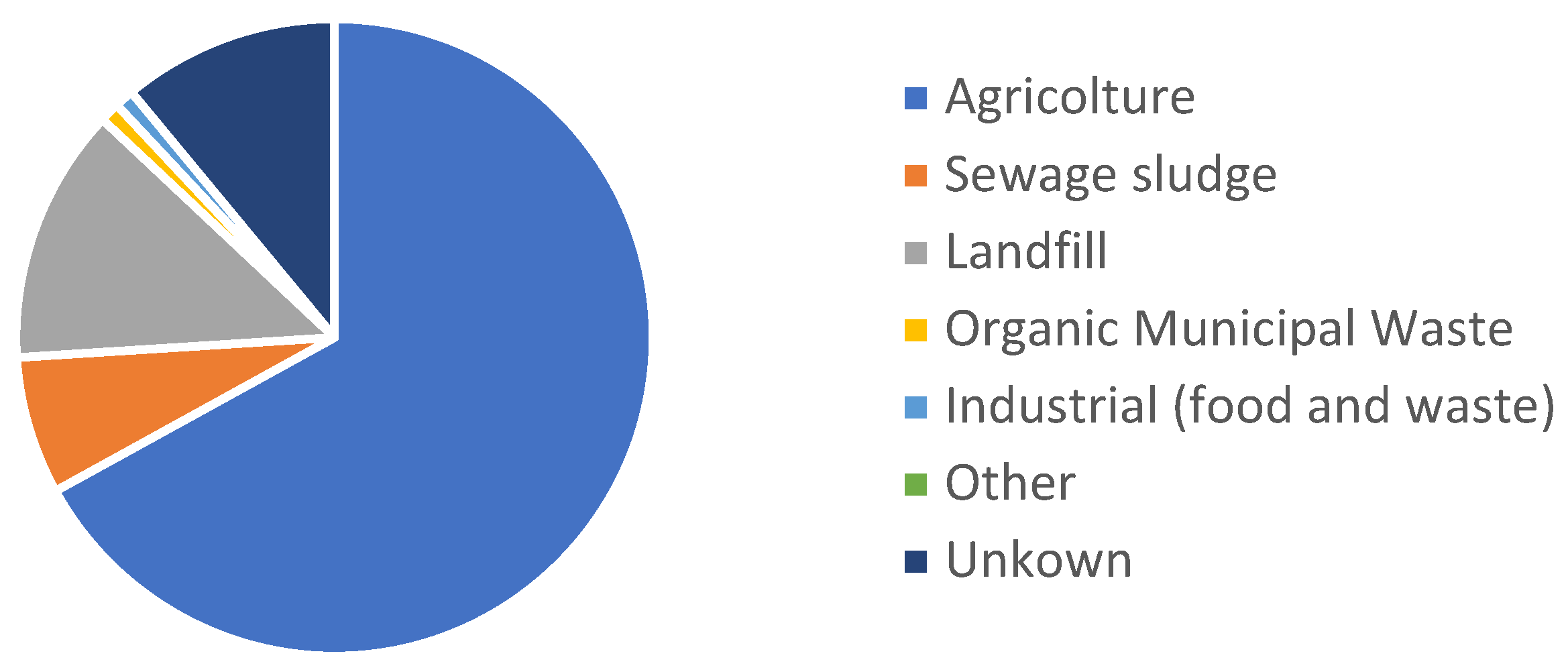
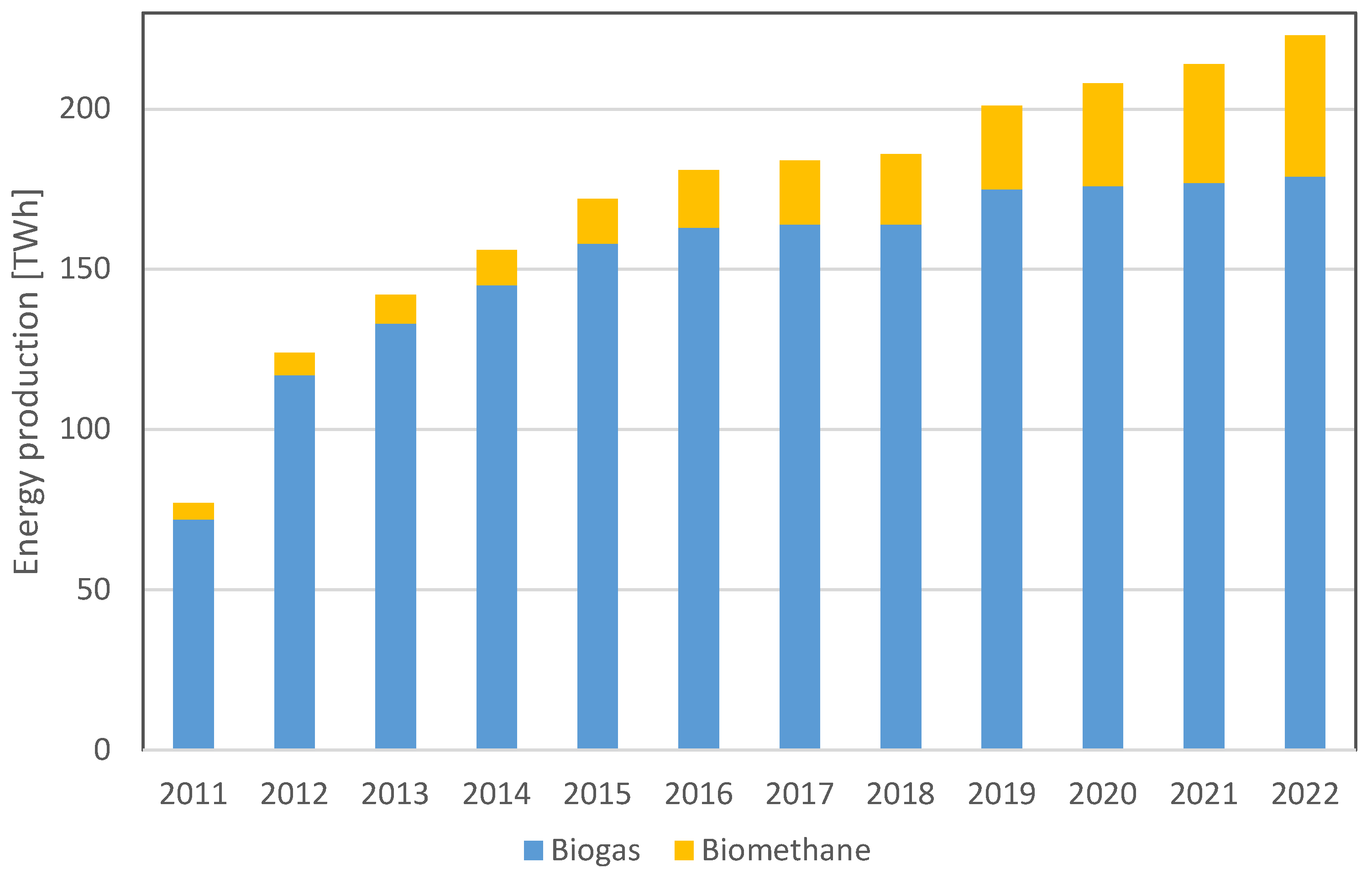
1. Introduction
2. Biogas Pretreatment before the Upgrading System: Cleaning Technologies
2.1. H2S Removal System
2.1.1. Biofiltration
2.1.2. Bioscrubber
2.2. NH3 Removal System
- Bioreactor: Ammonia removal takes place in a bioreactor, whereas gas–liquid mass transfer occurs in a scrubber. Providing adequate time for gas-phase NH3 to interact with the scrubbing liquid enables NH3 to dissolve as NH4+ in the aqueous solution [29].
- Biological ammonium oxidation: This is an anaerobic and exothermic process, so the temperature control is a crucial aspect of the technology. This approach is commonly used to treat wastewater with a high percentage of ammonia. It is frequently used for removing gaseous NH3 as well [30].
- Bioconversion: This can be divided into two phases. Initially, bacteria convert NH3 into nitrite (NO2), as can be seen in Equation (1); then, NO2 is converted into nitrate (NO3), as illustrated in Equation (2).
- Ammonia acts as the electron donor in these bioconversion process, while CO2 serves as the carbon source and O2 functions as the electron acceptor. The pH has a crucial role in microbial development and the effective conversion of NH3 through mass transfer from the gaseous to liquid phase [30].
- Biofiltration: Examples include BFs and BTFs. Biofiltration is similar to the process described in the H2S removal section. Biofilters are mainly utilized to address exhaust air with elevated levels of NH3 emissions from agricultural and livestock operations. [31].
2.3. Siloxanes and VOCs Removal
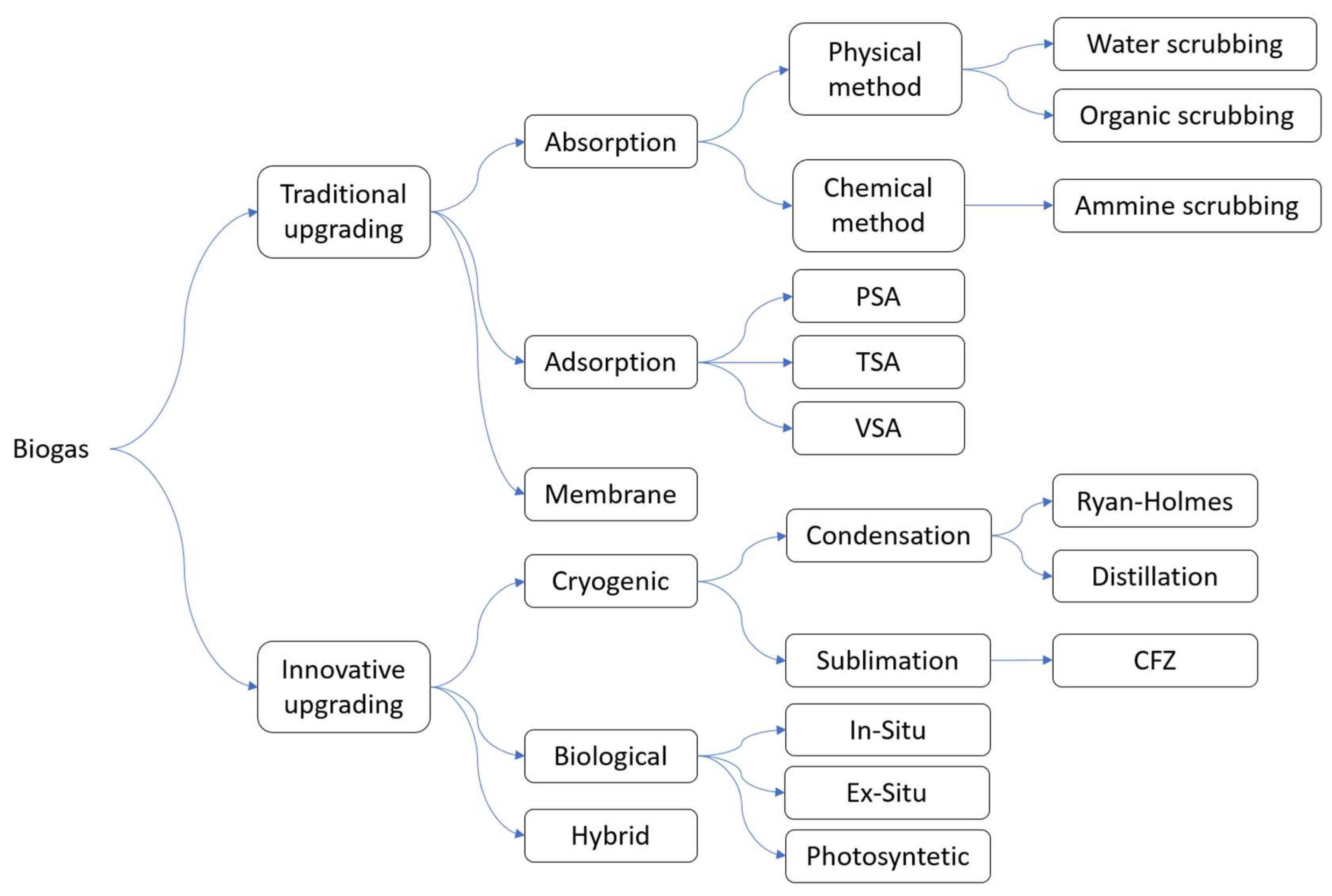
3. Upgrading Technologies
3.1. Absorption
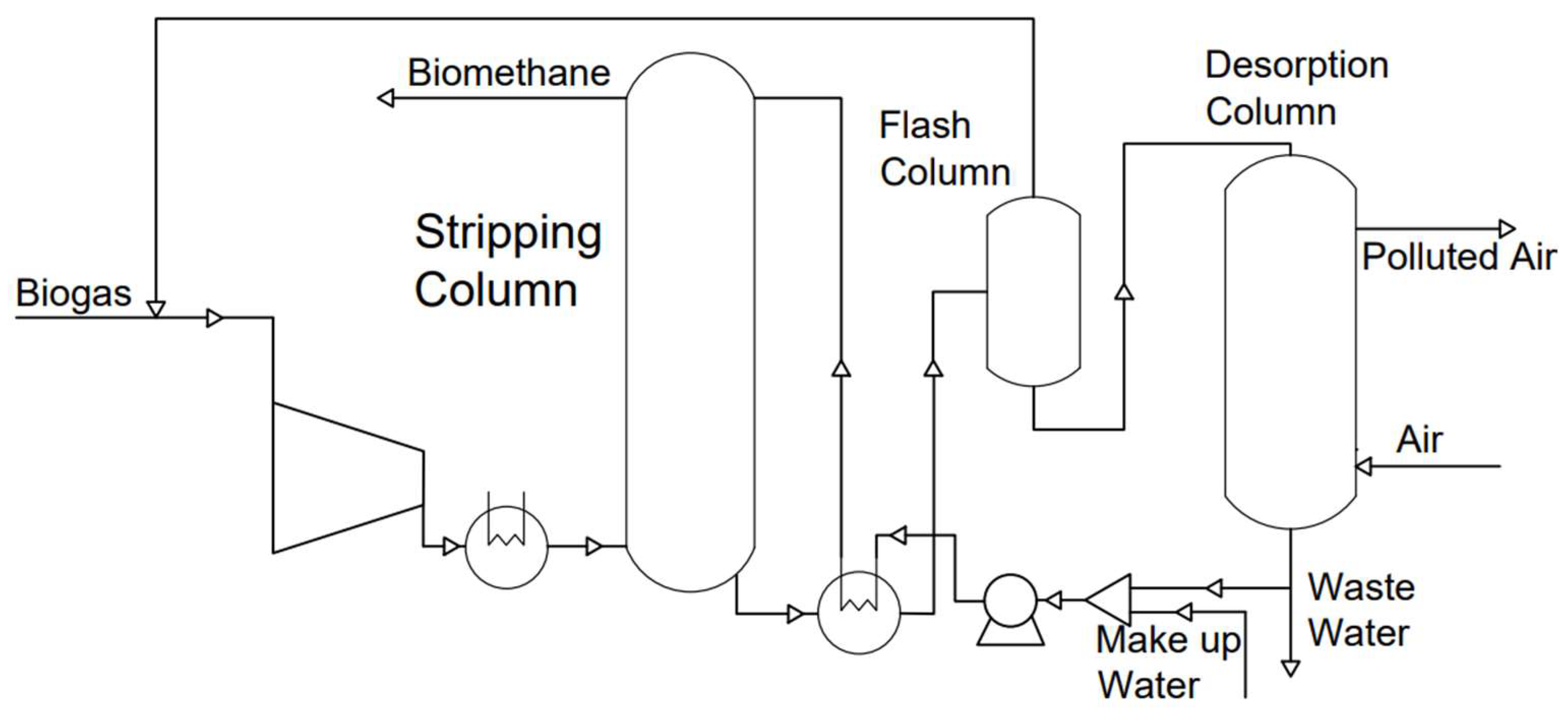
3.1.1. Water Scrubbing
3.1.2. Organic Physical Scrubbing
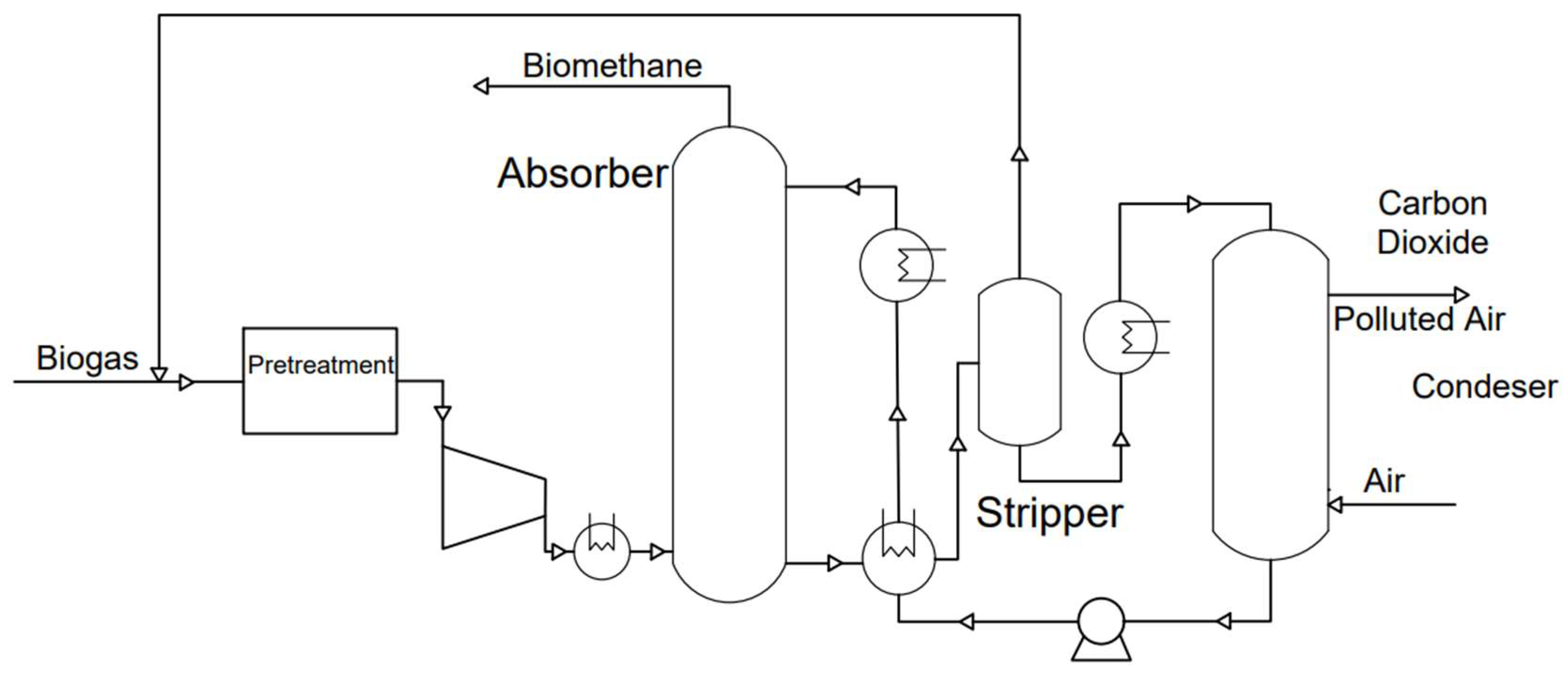
3.1.3. Chemical Absorption
3.2. Adsorption
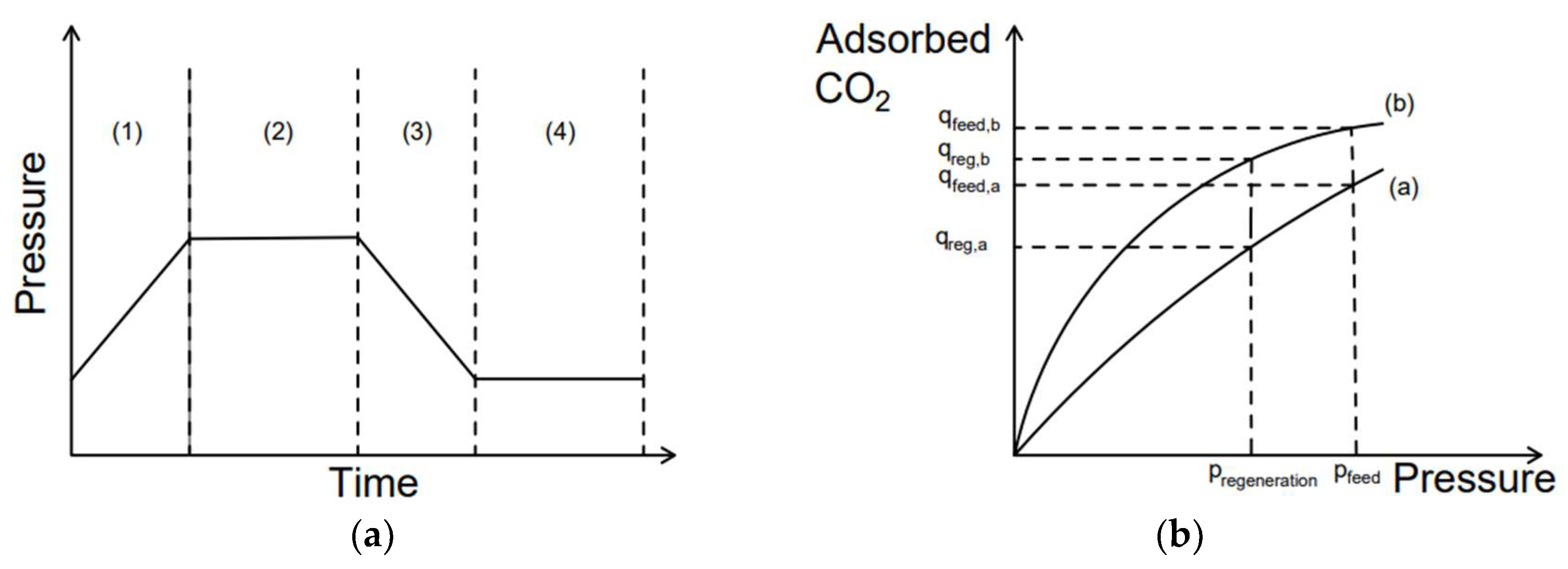
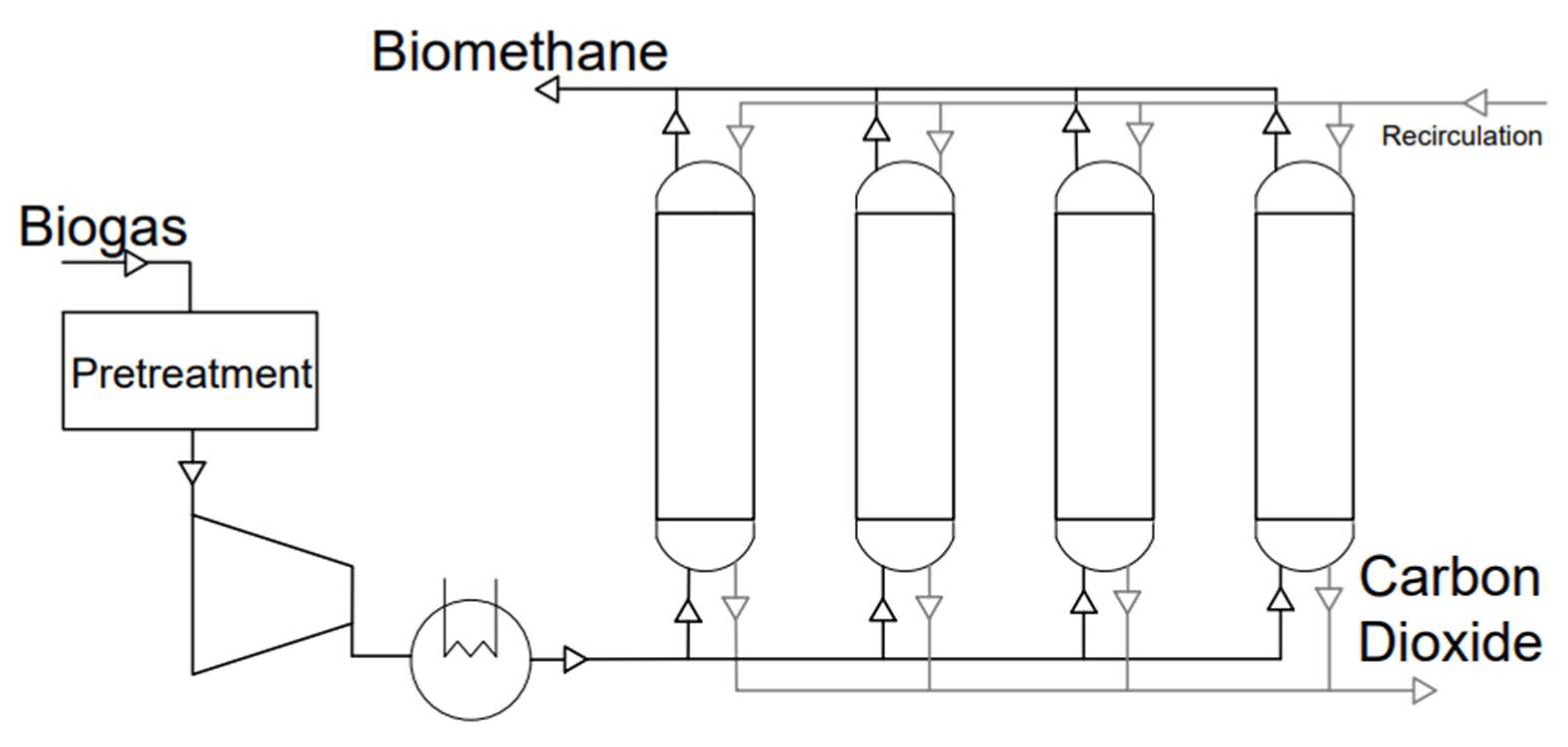
Pressure Swing Adsorption
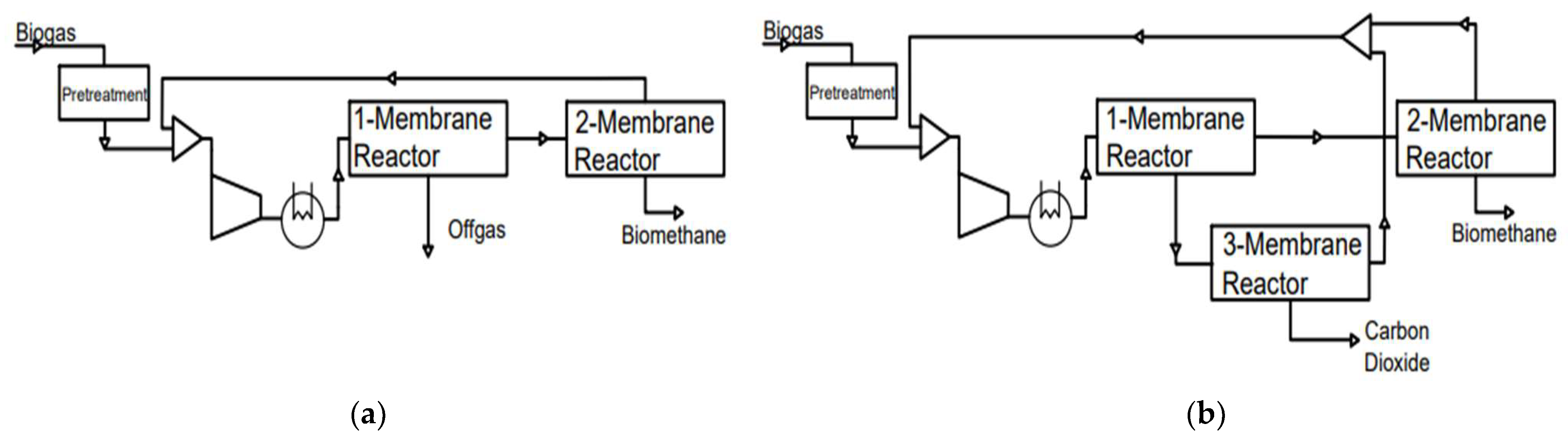
3.3. Membrane Separation
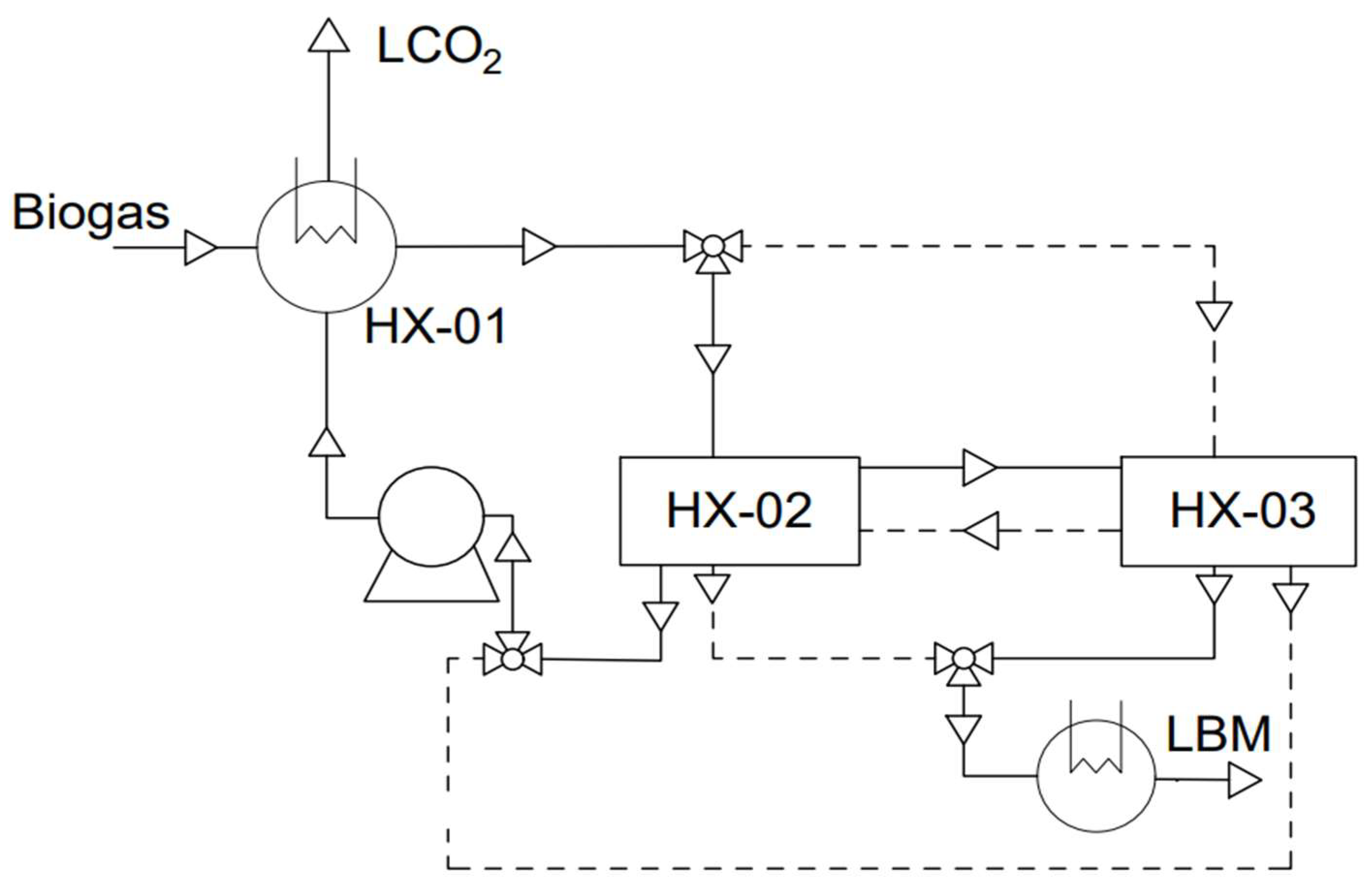
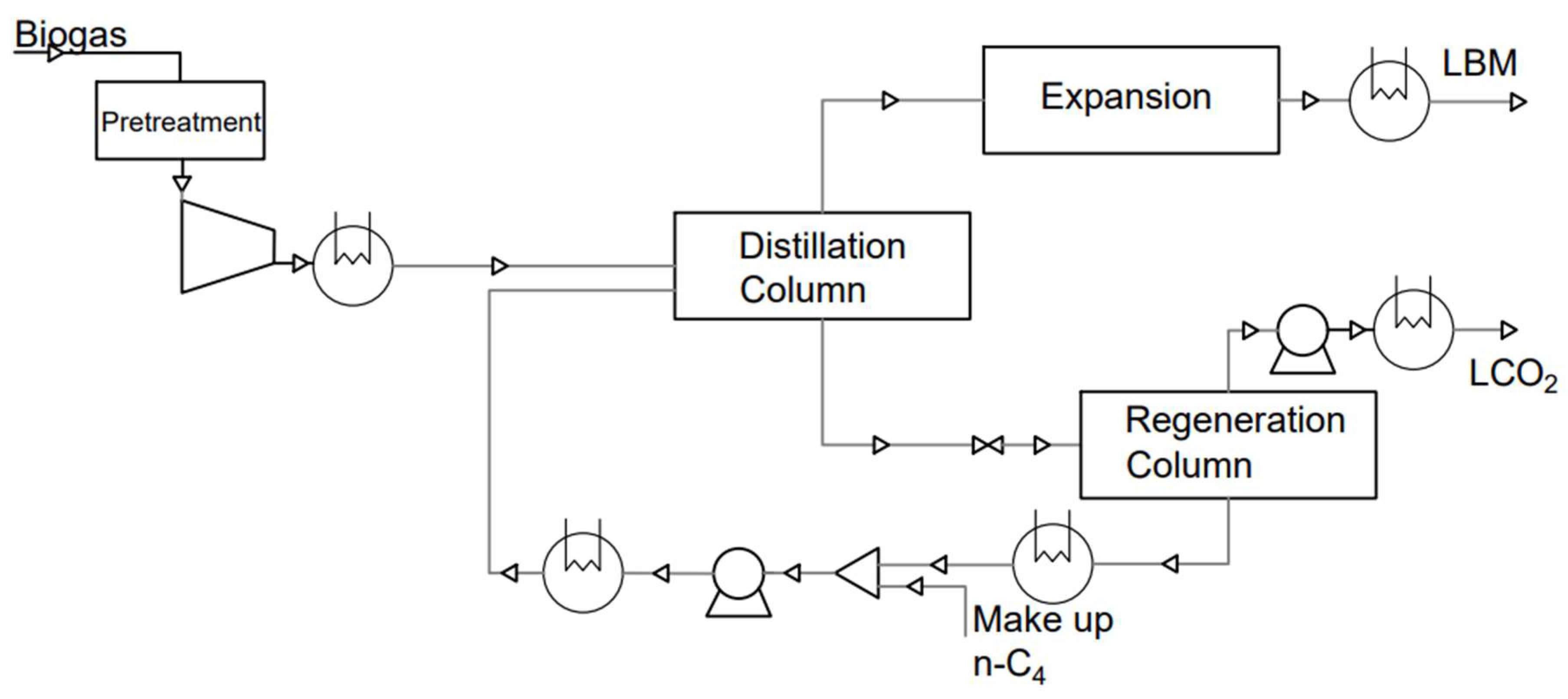
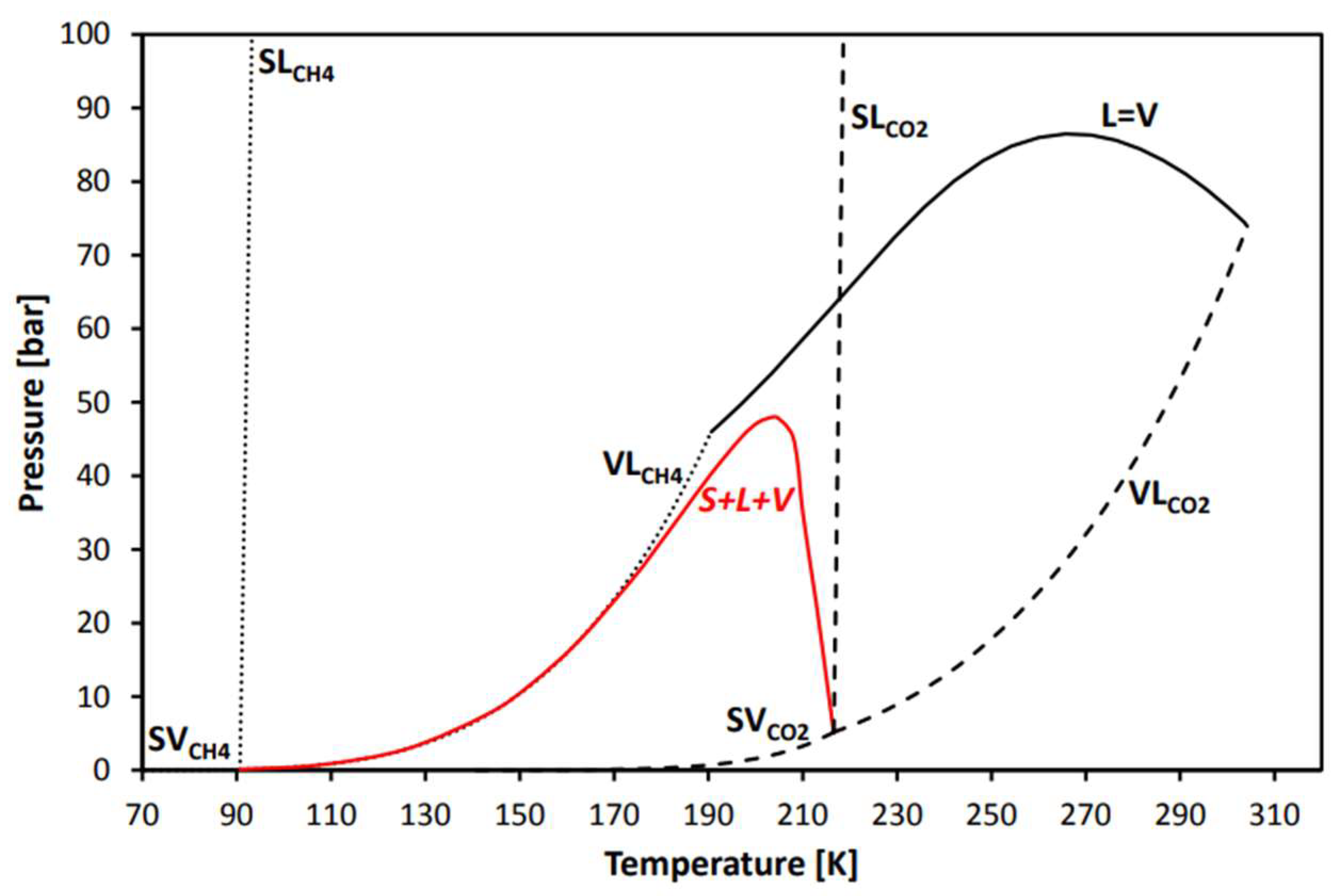
3.4. Cryogenic Separation
3.4.1. Controlled Freeze Zone (CFZ)
3.4.2. The Ryan–Holmes Process
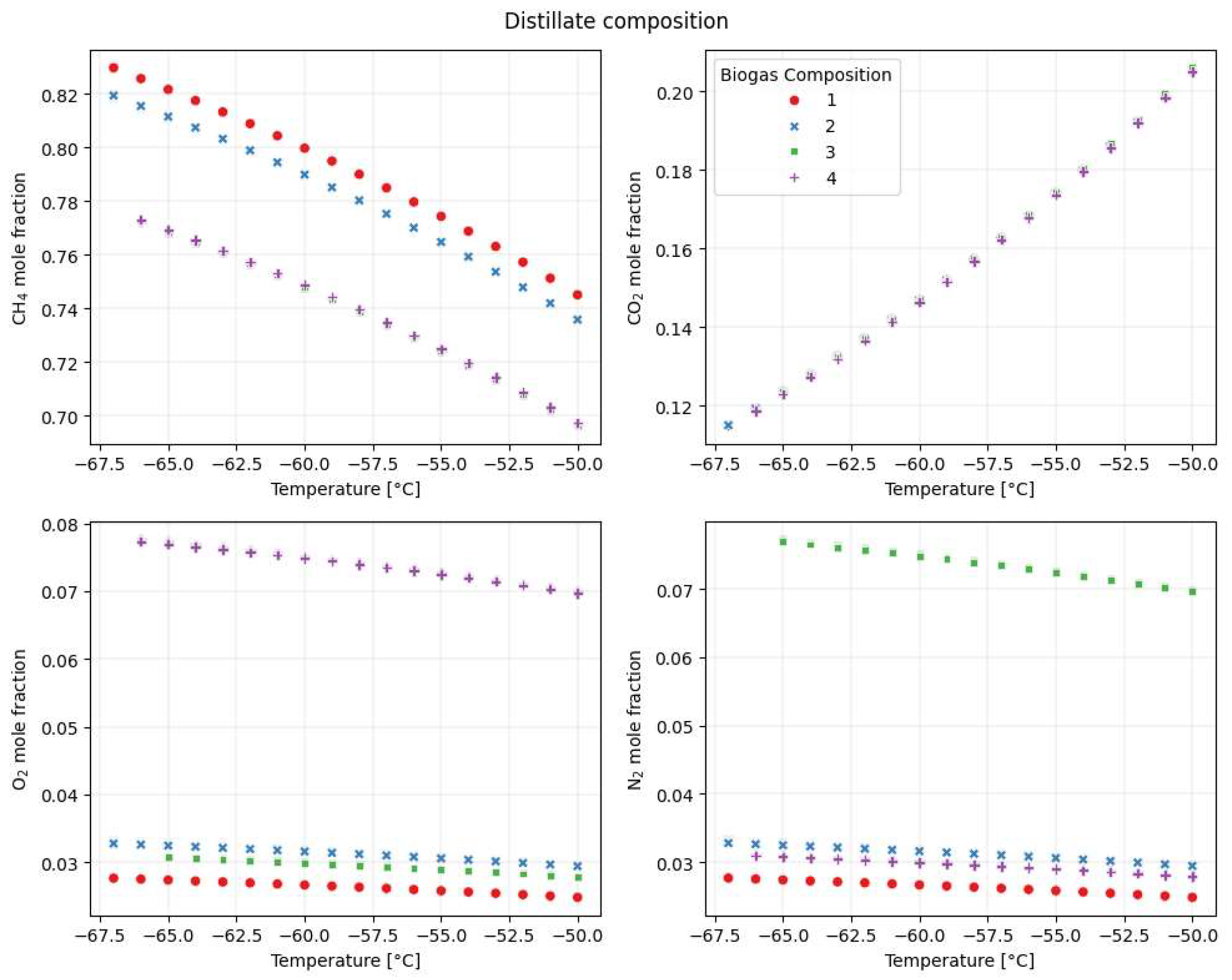
3.4.3. Cryogenic Distillation
3.5. Upgrading Technology Recap
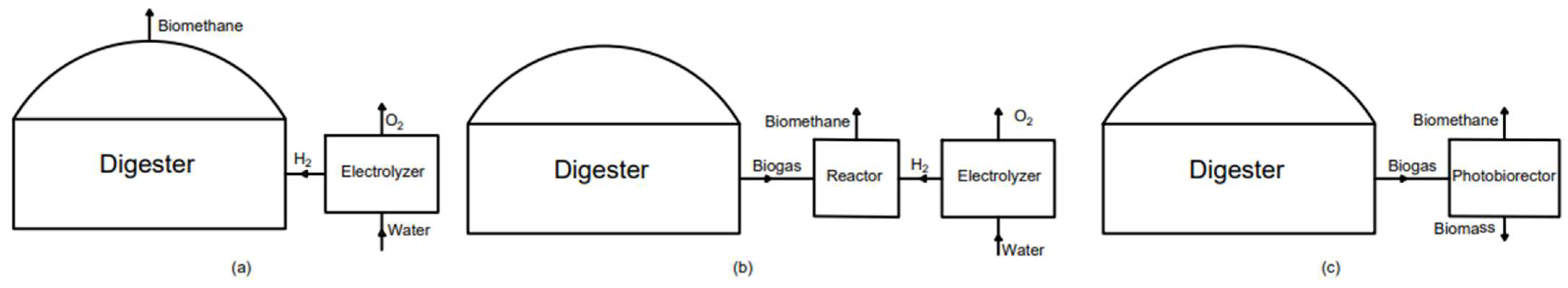
3.6. Biological Upgrading
3.6.1. In Situ Methanation
3.6.2. Ex Situ Methanation
3.6.3. Photosynthetic Upgrading Process
3.7. Hybrid Upgrading Technologies
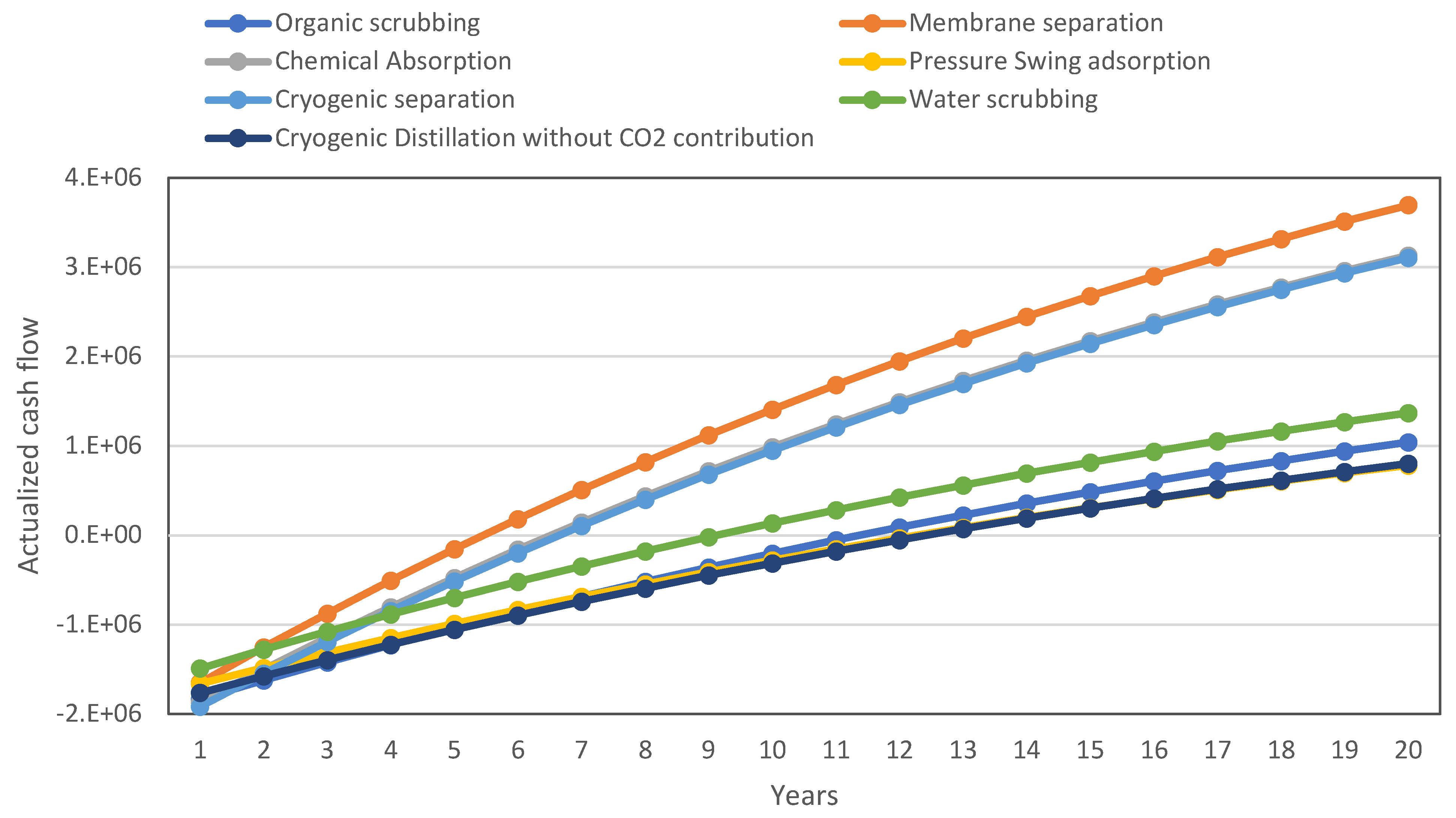
4. A Basic Economic Analysis for the Technology Comparison
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| AD | anaerobic digestion |
| BF | biofilters |
| BS | bioscrubber |
| BTS | biotrickling filters |
| CA | chemical absorption |
| CA | Cellulose Acetate |
| CD | cryogenic distillation |
| CFZ | controlled freezing zone |
| CHP | cogenerative heat and power |
| DEA | Diethanolamine |
| GHG | greenhouse gas emission |
| GWP | Global Warming Potential |
| HETP | Height Equivalent to a Theoretical Plate |
| LBM | liquefied biomethane |
| MEA | Monoethanolamine |
| MOF | Metal-organic frameworks |
| MS | membrane separation |
| NFM | N-Formylmorpholine |
| NH3 | ammonia |
| NMP | N-Methylpyrrolidone |
| OFMSW | organic fraction of municipal solid waste |
| OS | organic scrubbing |
| PC | Polycarbonate |
| PI | Polymide |
| PSA | pressure swing adsorption |
| RTO | regenerative thermal oxidizer |
| TSA | temperature swing adsorption |
| VOCs | volatile organic compounds |
| VSA | vacuum swing adsorption |
| WS | water scrubbing |
References
- Renewable Capacity Statistics 2022. Available online: https://www.irena.org/publications/2022/Apr/Renewable-Capacity-Statistics-2022 (accessed on 10 April 2024).
- European Climate Law—European Commission. Available online: https://climate.ec.europa.eu/eu-action/european-climate-law_en (accessed on 28 March 2024).
- Achinas, S.; Willem Euverink, G.J. Rambling Facets of Manure-Based Biogas Production in Europe: A Briefing. Renew. Sustain. Energy Rev. 2020, 119, 109566. [Google Scholar] [CrossRef]
- Johansson, V.; Lehtveer, M.; Göransson, L. Biomass in the Electricity System: A Complement to Variable Renewables or a Source of Negative Emissions? Energy 2019, 168, 532–541. [Google Scholar] [CrossRef]
- Tshikovhi, A.; Motaung, T.E. Technologies and Innovations for Biomass Energy Production. Sustainability 2023, 15, 12121. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel Ethanol Production from Lignocellulosic Biomass: An Overview on Feedstocks and Technological Approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Pham, T.P.T.; Kaushik, R.; Parshetti, G.K.; Mahmood, R.; Balasubramanian, R. Food Waste-to-Energy Conversion Technologies: Current Status and Future Directions. Waste Manag. 2015, 38, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Advanced Thermochemical Conversion Technologies Used for Energy Generation: Advancement and Prospects—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0016236122009644?via%3Dihub (accessed on 10 April 2024).
- Microbial Conversion of Waste Biomass into Bioethanol: Current Challenges and Future Prospects|Biomass Conversion and Biorefinery. Available online: https://link.springer.com/article/10.1007/s13399-021-01824-z (accessed on 10 April 2024).
- Verbeeck, K.; Buelens, L.C.; Galvita, V.V.; Marin, G.B.; Geem, K.M.V.; Rabaey, K. Upgrading the Value of Anaerobic Digestion via Chemical Production from Grid Injected Biomethane. Energy Environ. Sci. 2018, 11, 1788–1802. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Berni, M.; Dragone, G.; Mussatto, S.I.; Forster-Carneiro, T. Anaerobic Digestion Process: Technological Aspects and Recent Developments. Int. J. Environ. Sci. Technol. 2018, 15, 2033–2046. [Google Scholar] [CrossRef]
- EBA Statistical Report 2023|European Biogas Association. Available online: https://www.europeanbiogas.eu/eba-statistical-report-2023/ (accessed on 6 March 2024).
- Florio, C.; Fiorentino, G.; Corcelli, F.; Ulgiati, S.; Dumontet, S.; Güsewell, J.; Eltrop, L. A Life Cycle Assessment of Biomethane Production from Waste Feedstock Through Different Upgrading Technologies. Energies 2019, 12, 718. [Google Scholar] [CrossRef]
- Marconi, P.; Rosa, L. Role of Biomethane to Offset Natural Gas. Renew. Sustain. Energy Rev. 2023, 187, 113697. [Google Scholar] [CrossRef]
- Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources (Recast) (Text with EEA Relevance). 2018, Volume 328. Available online: https://eur-lex.europa.eu/eli/dir/2018/2001/oj (accessed on 3 March 2024).
- Decreto Direttoriale n. 23 Del 13 Gennaio 2023 Di Approvazione Delle Regole Applicative Del Decreto Ministeriale n. 340 Del 15 Settembre 2022 Recante Disposizioni per l’incentivazione Del Biometano Immesso Nella Rete Del Gas Naturale | Ministero Dell’Ambiente e Della Sicurezza Energetica. Available online: https://www.mase.gov.it/bandi/decreto-direttoriale-n-23-del-13-gennaio-2023-di-approvazione-delle-regole-applicative-del (accessed on 10 April 2024).
- Das, J.; Ravishankar, H.; Lens, P.N.L. Biological Biogas Purification: Recent Developments, Challenges and Future Prospects. J. Environ. Manag. 2022, 304, 114198. [Google Scholar] [CrossRef]
- Syed, M.; Soreanu, G.; Falletta, P.; Béland, M. Removal of Hydrogen Sulfide from Gas Streams Using Biological Processes—A Review. Can. Biosyst. Eng./Le Genie Des Biosyst. Au Can. 2006, 48, 2.1–2.13. [Google Scholar]
- Sun, Z.; Pang, B.; Xi, J.; Hu, H.-Y. Screening and Characterization of Mixotrophic Sulfide Oxidizing Bacteria for Odorous Surface Water Bioremediation. Bioresour. Technol. 2019, 290, 121721. [Google Scholar] [CrossRef] [PubMed]
- Di Capua, F.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Electron Donors for Autotrophic Denitrification. Chem. Eng. J. 2019, 362, 922–937. [Google Scholar] [CrossRef]
- Das, J.; Rene, E.R.; Dupont, C.; Dufourny, A.; Blin, J.; van Hullebusch, E.D. Performance of a Compost and Biochar Packed Biofilter for Gas-Phase Hydrogen Sulfide Removal. Bioresour. Technol. 2019, 273, 581–591. [Google Scholar] [CrossRef]
- Kennes, C.; Veiga, M.C. Inert Filter Media for the Biofiltration of Waste Gases—Characteristics and Biomass Control. Rev. Environ. Sci. Biotechnol. 2002, 1, 201–214. [Google Scholar] [CrossRef]
- DMT Clear Gas Solutions: Biogas Upgrading to Renewable Natural Gas. Available online: https://www.dmt-cgs.com/ (accessed on 29 March 2024).
- Biogasclean | Biogas Cleaning, H2S Removal & Desulphurization. Available online: https://biogasclean.com/ (accessed on 29 March 2024).
- Le Borgne, S.; Baquerizo, G. Microbial Ecology of Biofiltration Units Used for the Desulfurization of Biogas. ChemEngineering 2019, 3, 72. [Google Scholar] [CrossRef]
- Ren, B.; Zhao, Y.; Lyczko, N.; Nzihou, A. Current Status and Outlook of Odor Removal Technologies in Wastewater Treatment Plant. Waste Biomass Valor 2019, 10, 1443–1458. [Google Scholar] [CrossRef]
- Technologies. Available online: https://www.veoliawatertechnologies.com/en/technologies-1 (accessed on 29 March 2024).
- Home—PAQUES. Available online: https://en.paques.nl/ (accessed on 29 March 2024).
- Wang, Y.; Chang, M.; Pan, Y.; Zhang, K.; Lyu, L.; Wang, M.; Zhu, T. Performance Analysis and Optimization of Ammonium Removal in a New Biological Folded Non-Aerated Filter Reactor. Sci. Total Environ. 2019, 688, 505–512. [Google Scholar] [CrossRef]
- Pal, P. Chapter 3—Biological Treatment Technology. In Industrial Water Treatment Process Technology; Pal, P., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 65–144. ISBN 978-0-12-810391-3. [Google Scholar]
- Van der Heyden, C.; Volcke, E.I.P.; Brusselman, E.; Demeyer, P. Comparative 1-Year Performance Study of Two Full-Scale Biotrickling Filters for Ammonia Removal Including Nitrous Oxide Emission Monitoring. Biosyst. Eng. 2019, 188, 178–189. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Technical Overview of Volatile Organic Compounds. Available online: https://www.epa.gov/indoor-air-quality-iaq/technical-overview-volatile-organic-compounds (accessed on 2 April 2024).
- Accettola, F.; Guebitz, G.M.; Schoeftner, R. Siloxane Removal from Biogas by Biofiltration: Biodegradation Studies. Clean Technol. Environ. Policy 2008, 10, 211–218. [Google Scholar] [CrossRef]
- Boada, E.; Santos-Clotas, E.; Bertran, S.; Cabrera-Codony, A.; Martín, M.J.; Bañeras, L.; Gich, F. Potential Use of Methylibium Sp. as a Biodegradation Tool in Organosilicon and Volatile Compounds Removal for Biogas Upgrading. Chemosphere 2020, 240, 124908. [Google Scholar] [CrossRef] [PubMed]
- Calbry-Muzyka, A.; Madi, H.; Rüsch-Pfund, F.; Gandiglio, M.; Biollaz, S. Biogas Composition from Agricultural Sources and Organic Fraction of Municipal Solid Waste. Renew. Energy 2022, 181, 1000–1007. [Google Scholar] [CrossRef]
- Paolini, V.; Petracchini, F.; Carnevale, M.; Gallucci, F.; Perilli, M.; Esposito, G.; Segreto, M.; Occulti, L.G.; Scaglione, D.; Ianniello, A.; et al. Characterisation and Cleaning of Biogas from Sewage Sludge for Biomethane Production. J. Environ. Manag. 2018, 217, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Santos-Clotas, E.; Cabrera-Codony, A.; Boada, E.; Gich, F.; Muñoz, R.; Martín, M.J. Efficient Removal of Siloxanes and Volatile Organic Compounds from Sewage Biogas by an Anoxic Biotrickling Filter Supplemented with Activated Carbon. Bioresour. Technol. 2019, 294, 122136. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, Z.; Hao, Z. Recent Advances in Technologies for the Removal of Volatile Methylsiloxanes: A Case in Biogas Purification Process. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2257–2313. [Google Scholar] [CrossRef]
- Yang, L.; Corsolini, S.I. Online Removal of Volatile Siloxanes in Solid-State Anaerobic Digester Biogas Using a Biofilter and an Activated Carbon Filter. J. Environ. Chem. Eng. 2019, 7, 103284. [Google Scholar] [CrossRef]
- Desotec. Available online: https://www.desotec.com/it/ (accessed on 19 April 2024).
- Global Activated Carbon Producer NORIT—Purity for Life. Available online: https://norit.com (accessed on 19 April 2024).
- Ardolino, F.; Cardamone, G.F.; Parrillo, F.; Arena, U. Biogas-to-Biomethane Upgrading: A Comparative Review and Assessment in a Life Cycle Perspective. Renew. Sustain. Energy Rev. 2021, 139, 110588. [Google Scholar] [CrossRef]
- Incer-Valverde, J.; Korayem, A.; Tsatsaronis, G.; Morosuk, T. “Colors” of Hydrogen: Definitions and Carbon Intensity. Energy Convers. Manag. 2023, 291, 117294. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Understanding Global Warming Potentials. Available online: https://www.epa.gov/ghgemissions/understanding-global-warming-potentials (accessed on 11 April 2024).
- Huonder, A.; Olsen, D. Methane Emission Reduction Technologies for Natural Gas Engines: A Review. Energies 2023, 16, 7054. [Google Scholar] [CrossRef]
- Angelidaki, I.; Xie, L.; Luo, G.; Zhang, Y.; Oechsner, H.; Lemmer, A.; Munoz, R.; Kougias, P.G. Chapter 33—Biogas Upgrading: Current and Emerging Technologies. In Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels, 2nd ed.; Pandey, A., Larroche, C., Dussap, C.-G., Gnansounou, E., Khanal, S.K., Ricke, S., Eds.; Biomass, Biofuels, Biochemicals; Academic Press: Cambridge, MA, USA, 2019; pp. 817–843. ISBN 978-0-12-816856-1. [Google Scholar]
- IUPAC-NIST Solubilities Database. Available online: https://srdata.nist.gov/solubility/sol_detail.aspx?sysID=62_1 (accessed on 11 April 2024).
- Bauer, F.; Hulteberg, C.; Persson, T.; Tamm, D. Biogas Upgrading—Review of Commercial Technologies; Svenskt Gastekniskt Center AB: Malmö, Sweden, 2013. [Google Scholar]
- Struk, M.; Kushkevych, I.; Vítězová, M. Biogas Upgrading Methods: Recent Advancements and Emerging Technologies. Rev. Environ. Sci. Biotechnol. 2020, 19, 651–671. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Tarannum, K.; Chowdhury, A.T.; Rafa, N.; Nuzhat, S.; Kumar, P.S.; Vo, D.-V.N.; Lichtfouse, E.; Mahlia, T.M.I. Biogas Upgrading, Economy and Utilization: A Review. Environ. Chem. Lett. 2021, 19, 4137–4164. [Google Scholar] [CrossRef]
- Upadhyay, A.; Kovalev, A.A.; Zhuravleva, E.A.; Kovalev, D.A.; Litti, Y.V.; Masakapalli, S.K.; Pareek, N.; Vivekanand, V. Recent Development in Physical, Chemical, Biological and Hybrid Biogas Upgradation Techniques. Sustainbility 2023, 15, 476. [Google Scholar] [CrossRef]
- Firdaus, M.; Kolmetz, K.; Dwijayanti, A.; Hoon, C.Y. Distillation Column Selection, Sizing and Troubleshooting, Kolmetz Handbook of Process Equipment Design. 2013. Available online: https://www.researchgate.net/publication/336591526_Distillation_Column_Selection_Sizing_and_Troubleshooting_Kolmetz_Handbook_of_Process_Equipment_Design (accessed on 5 March 2024).
- Ryckebosch, E.; Drouillon, M.; Vervaeren, H. Techniques for Transformation of Biogas to Biomethane. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Aghel, B.; Behaein, S.; Wongwises, S.; Shadloo, M.S. A Review of Recent Progress in Biogas Upgrading: With Emphasis on Carbon Capture. Biomass Bioenergy 2022, 160, 106422. [Google Scholar] [CrossRef]
- Khan, M.U.; Lee, J.T.E.; Bashir, M.A.; Dissanayake, P.D.; Ok, Y.S.; Tong, Y.W.; Shariati, M.A.; Wu, S.; Ahring, B.K. Current Status of Biogas Upgrading for Direct Biomethane Use: A Review. Renew. Sustain. Energy Rev. 2021, 149, 111343. [Google Scholar] [CrossRef]
- Carranza-Abaid, A.; Wanderley, R.R.; Knuutila, H.K.; Jakobsen, J.P. Analysis and Selection of Optimal Solvent-Based Technologies for Biogas Upgrading. Fuel 2021, 303, 121327. [Google Scholar] [CrossRef]
- Sun, Q.; Li, H.; Yan, J.; Liu, L.; Yu, Z.; Yu, X. Selection of Appropriate Biogas Upgrading Technology—A Review of Biogas Cleaning, Upgrading and Utilisation. Renew. Sustain. Energy Rev. 2015, 51, 521–532. [Google Scholar] [CrossRef]
- Korson, L.; Drost-Hansen, W.; Millero, F.J. Viscosity of Water at Various Temperatures. J. Phys. Chem. 1969, 73, 34–39. [Google Scholar] [CrossRef]
- Xiang, H.W.; Laesecke, A.; Huber, M.L. A New Reference Correlation for the Viscosity of Methanol. J. Phys. Chem. Ref. Data 2006, 35, 1597–1620. [Google Scholar] [CrossRef]
- Langan, J.R.; Salmon, G.A. Physical Properties of N-Methylpyrrolidinone as Functions of Temperature. J. Chem. Eng. Data 1987, 32, 420–422. [Google Scholar] [CrossRef]
- Cavaignac, R.S.; Ferreira, N.L.; Guardani, R. Techno-Economic and Environmental Process Evaluation of Biogas Upgrading via Amine Scrubbing. Renew. Energy 2021, 171, 868–880. [Google Scholar] [CrossRef]
- Bishnoi, S.; Rochelle, G.T. Absorption of Carbon Dioxide into Aqueous Piperazine: Reaction Kinetics, Mass Transfer and Solubility. Chem. Eng. Sci. 2000, 55, 5531–5543. [Google Scholar] [CrossRef]
- Rufford, T.E.; Smart, S.; Watson, G.C.Y.; Graham, B.F.; Boxall, J.; Diniz da Costa, J.C.; May, E.F. The Removal of CO2 and N2 from Natural Gas: A Review of Conventional and Emerging Process Technologies. J. Pet. Sci. Eng. 2012, 94–95, 123–154. [Google Scholar] [CrossRef]
- Choi, W.-J.; Seo, J.-B.; Jang, S.-Y.; Jung, J.-H.; Oh, K.-J. Removal Characteristics of CO2 Using Aqueous MEA/AMP Solutions in the Absorption and Regeneration Process. J. Environ. Sci. 2009, 21, 907–913. [Google Scholar] [CrossRef]
- Method and Apparatus for Fractionating Gaseous Mixtures by Adsorption—EXXON RESEARCH ENGINEERING CO. Available online: https://www.freepatentsonline.com/2944627.html (accessed on 12 April 2024).
- Saha, D.; Bao, Z.; Jia, F.; Deng, S. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and Zeolite 5A. Environ. Sci. Technol. 2010, 44, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Ritter, J.A.; Yang, R.T. Equilibrium Adsorption of Multicomponent Gas Mixtures at Elevated Pressures. Ind. Eng. Chem. Res. 1987, 26, 1679–1686. [Google Scholar] [CrossRef]
- Bao, Z.; Alnemrat, S.; Yu, L.; Vasiliev, I.; Ren, Q.; Lu, X.; Deng, S. Kinetic Separation of Carbon Dioxide and Methane on a Copper Metal–Organic Framework. J. Colloid Interface Sci. 2011, 357, 504–509. [Google Scholar] [CrossRef]
- Membrane Technology and Applications—Richard W. Baker—Google Libri. Available online: https://books.google.it/books?hl=it&lr=&id=EyXgEAAAQBAJ&oi=fnd&pg=PA525&ots=i4UnaEFoQT&sig=lQxVRVPJBFOQvutNTRL8wAVOtXA&redir_esc=y#v=onepage&q&f=false (accessed on 12 April 2024).
- Gabrielli, P.; Gazzani, M.; Mazzotti, M. On the Optimal Design of Membrane-Based Gas Separation Processes. J. Membr. Sci. 2017, 526, 118–130. [Google Scholar] [CrossRef]
- UBE CO2 Separator for Biogas Upgrading. Available online: https://ube.es/products/biogas-upgrading-biomethane-co2-separator/ (accessed on 12 April 2024).
- Basu, S.; Khan, A.L.; Cano-Odena, A.; Liu, C.; Vankelecom, I.F.J. Membrane-Based Technologies for Biogas Separations. Chem. Soc. Rev. 2010, 39, 750–768. [Google Scholar] [CrossRef]
- Pellegrini, L.A.; De Guido, G.; Langé, S. Biogas to Liquefied Biomethane via Cryogenic Upgrading Technologies. Renew. Energy 2018, 124, 75–83. [Google Scholar] [CrossRef]
- Northrop, P.S.; Valencia, J.A. The CFZ™ Process: A Cryogenic Method for Handling High-CO2 and H2S Gas Reserves and Facilitating Geosequestration of CO2 and Acid Gases. Energy Procedia 2009, 1, 171–177. [Google Scholar] [CrossRef]
- Parker, P.M.E.; Northrop, S.; Valencia, J.A.; Foglesong, R.E.; Duncan, W.T. CO2 Management at ExxonMobil’s LaBarge Field, Wyoming, USA. Energy Procedia 2011, 4, 5455–5470. [Google Scholar] [CrossRef]
- Lastari, F. Ryan-Holmes and Modified Ryan-Holmes Processes for LNG Production. Ph.D. Thesis, Curtin University, Perth, Australia, 2009. [Google Scholar]
- Baccanelli, M.; Langé, S.; Rocco, M.; Pellegrini, L.; Colombo, E. Low Temperature Techniques for Natural Gas Purification and LNG Production: An Energy and Exergy Analysis. Appl. Energy 2016, 180, 546–559. [Google Scholar] [CrossRef]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. Low-Temperature Distillation Process for CO2/CH4 Separation: A Study for Avoiding CO2 Freeze-Out. J. Heat Transf. 2018, 140, 042001. [Google Scholar] [CrossRef]
- Yousef, A.M.; Eldrainy, Y.A.; El-Maghlany, W.M.; Attia, A. Biogas Upgrading Process via Low-Temperature CO2 Liquefaction and Separation. J. Nat. Gas Sci. Eng. 2017, 45, 812–824. [Google Scholar] [CrossRef]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. New Approach for Biogas Purification Using Cryogenic Separation and Distillation Process for CO2 Capture. Energy 2018, 156, 328–351. [Google Scholar] [CrossRef]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. Upgrading Biogas to Biomethane and Liquid CO2: A Novel Cryogenic Process. Fuel 2019, 251, 611–628. [Google Scholar] [CrossRef]
- Live Carbon Prices Today, Carbon Price Charts • Carbon Credits. Carbon Credits. 2024. Available online: https://carboncredits.com/carbon-prices-today/ (accessed on 6 March 2024).
- Aspen HYSYS | Process Simulation Software | AspenTech. Available online: https://www.aspentech.com/en/products/engineering/aspen-hysys (accessed on 23 February 2024).
- Welcome to Python.Org. Available online: https://www.python.org/ (accessed on 23 February 2024).
- Smith, R.; Inomata, H.; Peters, C. Chapter 6—Equations of State and Formulations for Mixtures. In Supercritical Fluid Science and Technology; Smith, R., Inomata, H., Peters, C., Eds.; Introduction to Supercritical Fluids; Elsevier: Amsterdam, The Netherlands, 2013; Volume 4, pp. 333–480. [Google Scholar]
- Xie, L.; Xu, J.; Zhang, Y.; He, Y. Biogas Upgrading. In Advances in Bioenergy; Elsevier: Amsterdam, The Netherlands, 2020; Volume 5, pp. 309–344. ISBN 978-0-12-820744-4. [Google Scholar]
- Liu, C.; Xiao, J.; Li, H.; Chen, Q.; Sun, D.; Cheng, X.; Li, P.; Dang, Y.; Smith, J.A.; Holmes, D.E. High Efficiency In-Situ Biogas Upgrading in a Bioelectrochemical System with Low Energy Input. Water Res. 2021, 197, 117055. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, M.; Aghbashlo, M.; Valijanian, E.; Kazemi Shariat Panahi, H.; Nizami, A.-S.; Ghanavati, H.; Sulaiman, A.; Mirmohamadsadeghi, S.; Karimi, K. A Comprehensive Review on Recent Biological Innovations to Improve Biogas Production, Part 2: Mainstream and Downstream Strategies. Renew. Energy 2020, 146, 1392–1407. [Google Scholar] [CrossRef]
- Fu, S.; Angelidaki, I.; Zhang, Y. In Situ Biogas Upgrading by CO2-to-CH4 Bioconversion. Trends Biotechnol. 2021, 39, 336–347. [Google Scholar] [CrossRef]
- McPhy: Clean Hydrogen Production and Distribution Equipment. Available online: https://mcphy.com/en/ (accessed on 17 April 2024).
- Chi siamo—H2 Energy Srl 2023. Available online: https://www.h2e.it/2023/ (accessed on 6 March 2024).
- Lai, C.-Y.; Zhou, L.; Yuan, Z.; Guo, J. Hydrogen-Driven Microbial Biogas Upgrading: Advances, Challenges and Solutions. Water Res. 2021, 197, 117120. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; O’Shea, R.; Lin, R.; Murphy, J.D. A Comparative Evaluation of Design Factors on Bubble Column Operation in Photosynthetic Biogas Upgrading. Biofuel Res. J. 2021, 8, 1351–1373. [Google Scholar] [CrossRef]
- Rodero, M.d.R.; Carvajal, A.; Castro, V.; Navia, D.; de Prada, C.; Lebrero, R.; Muñoz, R. Development of a Control Strategy to Cope with Biogas Flowrate Variations during Photosynthetic Biogas Upgrading. Biomass Bioenergy 2019, 131, 105414. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Park, J.; Heubeck, S.; Ralph, P.J.; Craggs, R.J. Size Matters—Microalgae Production and Nutrient Removal in Wastewater Treatment High Rate Algal Ponds of Three Different Sizes. Algal Res. 2020, 45, 101734. [Google Scholar] [CrossRef]
- Meier, L.; Barros, P.; Torres, A.; Vilchez, C.; Jeison, D. Photosynthetic Biogas Upgrading Using Microalgae: Effect of Light/Dark Photoperiod. Renew. Energy 2017, 106, 17–23. [Google Scholar] [CrossRef]
- Kapoor, R.; Ghosh, P.; Kumar, M.; Vijay, V.K. Evaluation of Biogas Upgrading Technologies and Future Perspectives: A Review. Environ. Sci. Pollut. Res. 2019, 26, 11631–11661. [Google Scholar] [CrossRef] [PubMed]
- Bassani, I.; Kougias, P.G.; Angelidaki, I. In-Situ Biogas Upgrading in Thermophilic Granular UASB Reactor: Key Factors Affecting the Hydrogen Mass Transfer Rate. Bioresour. Technol. 2016, 221, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Bassani, I.; Kougias, P.G.; Treu, L.; Porté, H.; Campanaro, S.; Angelidaki, I. Optimization of Hydrogen Dispersion in Thermophilic Up-Flow Reactors for Ex Situ Biogas Upgrading. Bioresour. Technol. 2017, 234, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Rachbauer, L.; Voitl, G.; Bochmann, G.; Fuchs, W. Biological Biogas Upgrading Capacity of a Hydrogenotrophic Community in a Trickle-Bed Reactor. Appl. Energy 2016, 180, 483–490. [Google Scholar] [CrossRef]
- Song, C.; Fan, Z.; Li, R.; Liu, Q.; Kitamura, Y. Efficient Biogas Upgrading by a Novel Membrane-Cryogenic Hybrid Process: Experiment and Simulation Study. J. Membr. Sci. 2018, 565, 194–202. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Ji, N.; Deng, S.; Zhao, J.; Li, Y.; Song, Y.; Li, H. Alternative Pathways for Efficient CO2 Capture by Hybrid Processes—A Review. Renew. Sustain. Energy Rev. 2018, 82, 215–231. [Google Scholar] [CrossRef]
- Francisco López, A.; Lago Rodríguez, T.; Faraji Abdolmaleki, S.; Galera Martínez, M.; Bello Bugallo, P.M. From Biogas to Biomethane: An In-Depth Review of Upgrading Technologies That Enhance Sustainability and Reduce Greenhouse Gas Emissions. Appl. Sci. 2024, 14, 2342. [Google Scholar] [CrossRef]
- Sulewski, P.; Ignaciuk, W.; Szymańska, M.; Wąs, A. Development of the Biomethane Market in Europe. Energies 2023, 16, 2001. [Google Scholar] [CrossRef]
- EU Carbon Permits-Price-Chart-Historical Data-News. Available online: https://tradingeconomics.com/commodity/carbon (accessed on 15 April 2024).
- Ongis, M.; Di Marcoberardino, G.; Manzolini, G.; Gallucci, F.; Binotti, M. Membrane Reactors for Green Hydrogen Production from Biogas and Biomethane: A Techno-Economic Assessment. Int. J. Hydrogen Energy 2023, 48, 19580–19595. [Google Scholar] [CrossRef]
- Jarre, M.; Noussan, M.; Poggio, A. Operational Analysis of Natural Gas Combined Cycle CHP Plants: Energy Performance and Pollutant Emissions. Appl. Therm. Eng. 2016, 100, 304–314. [Google Scholar] [CrossRef]




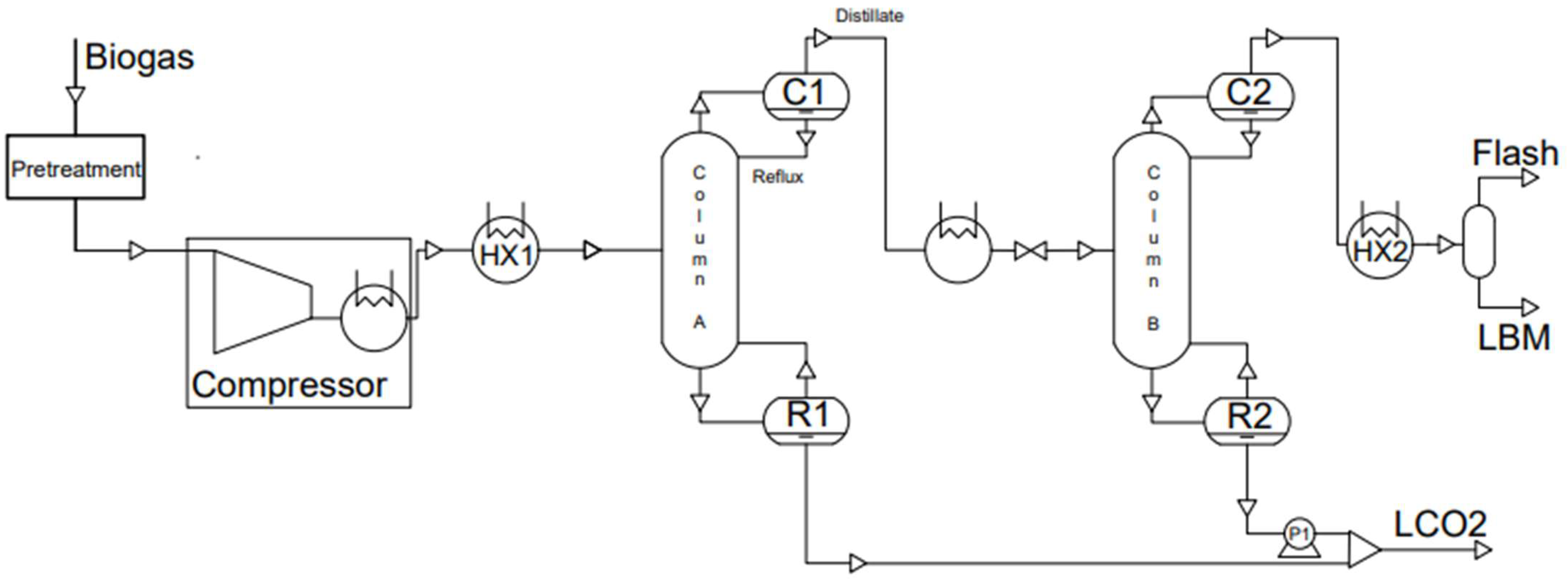
| Landfilled | AD from Agricultural | AD from Waste | Natural Gas | |
|---|---|---|---|---|
| CH4 (v%) | 40–70 | 49–69 | 44–67 | 85–92 |
| CO2 (v%) | 25–40 | 29–44 | 30–44 | 0.2–1.5 |
| N2 (v%) | 0–17 | 0.6–13 | 0.1–6 | 0.3 |
| O2 (v%) | 0–3 | 0.2–3 | 0.1–3 |
| Bacteria | Final Product | Reference |
|---|---|---|
| Cholorobium limicola | CH2O, H2O, SO42− | [18] |
| Thiobacillus thioparus | H+, SO42−, S0 | [19] |
| Thiobacillus denitrificans | NH4+, H2O, S0 | [20] |
| Technology | Solvent | Viscosity at Absorption Condition [mbarx s] × 10−8 | Viscosity at Desorption Condition [mbarx s] × 10−8 | |
|---|---|---|---|---|
| Water scrubbing | Water | 1.31 (at 10 °C) | 0.5 (at 47 °C) | [58] |
| Organic physical scrubbing | Methanol | 3.25 (at 60 °C) | 0.8 (at 0 °C) | [59] |
| NMP | 1.5 (at 15 °C) | 1.1 (at 54 °C) | [60] | |
| NFM | 17.1 (at −20 °C) | 8.3 (at 25 °C) | [56] |
| Amine Type | Absorption Capacity [molCO2/molAmine] | Advantages | Disadvantages | |
|---|---|---|---|---|
| Monoethanolamine (MEA) | 0.45–0.52 |
|
| [63] |
| Diethanolamine (DEA) | 0.21–0.81 |
|
| [63] |
| N-methyldiethanolamine | 0.20–0.81 |
|
| [63] |
| 2-Amino-2-methyl-1-propanol | 0.84 |
|
| [64] |
| Adsorbent Material | Type | CO2/CH4 Equilibrium Selectivity | CO2/CH4 Kinetic Selectivity | |
|---|---|---|---|---|
| Zeolite | Equilibrium | 5.19 | 3.6 | [66] |
| Activated carbon | Equilibrium | 3.29 | [67] | |
| Metal-organic frameworks (Cu-MOF) | Kinetic | 1.86 | 9.7 | [68] |
| Material | CO2/CH4 Selectivity | CO2 Permeability [10−10 cm3(STP) · cm/cm2 · s · cmHg] | |
|---|---|---|---|
| Cellulose Acetate (CA) | 30 | 10 | [69,72] |
| Polycarbonate (PC) | 32.5 | [72] | |
| Polymide (PI) | 42.8 | 13 | [69,72] |
| Min Value | Max Value | |
|---|---|---|
| Condenser temperature [°C] | −80 | −50 |
| Reflux ratio | 1.4 | 3 |
| Column pressure [bar] | 5050 | 7050 |
| Number of column theoretical plate | 11 | 25 |
| CH4 [%] | CO2 [%] | N2 [%] | O2 [%] | |
|---|---|---|---|---|
| Composition 1 | 60 | 36 | 2 | 2 |
| Composition 2 | 50 | 46 | 2 | 2 |
| Composition 3 | 50 | 43 | 5 | 2 |
| Composition 4 | 50 | 43 | 2 | 5 |
| Condenser Temperature | Pressure | Number of Stage | Reflux Ratio | CH4 in Biogas | |
|---|---|---|---|---|---|
| CH4 in distillate | −0.98 | <10−5 | <10−5 | <10−5 | 0.18 |
| Distillate molar flow | 0.39 | <10−5 | <10−5 | <10−5 | 0.92 |
| WS | OS | CA | PSA | MS | CD | |
|---|---|---|---|---|---|---|
| CH4 purity | 95–98 | 97–99 | 96–99.5 | 95–99 | 95–99 | 97–99.9 |
| Chemical dangers | No | Yes | Yes | No | No | No |
| Water pretretment | No | Yes | No | Yes | No | Yes |
| Cleaning pretratment | No | Yes | Yes | Yes | Yes | Yes |
| Offgas treatment | Yes | Yes | Yes | Yes | Yes | No |
| Operational pressure | 4–10 | 4–8 | 1–2 | 4–10 | 6–12 | 50–80 |
| Output pressure | 4–10 | 2–7 | 1–2 | 2–4 | 6–8 | Depend on the final product |
| Thermal energy requirment | No | Yes | Yes | Yes/No | No | Yes |
| water use | Yes | No | No | No | No | No |
| Producers | Malmberg Greenlane | HAASE Umwelttechnik GmbH | Hera Cleantech | Mahler Energietechnik | Air Liquide AB Holding S.p.A Prodeval | GtS Future Energy |
| Type | Temperature [°C] | Retentation Time | CH4 Purity [%] | |
|---|---|---|---|---|
| In situ | 38 | 20 days | 96 | [97] |
| In situ | 55 | 5–20 days | 82 | [98] |
| Ex situ | 55 | 4–15 h | 89.5–96.3 | [99] |
| Ex situ | 37 | 3.5 h | 96 | [100] |
| Biogas production [Nm3/h] | 1000 | |
| Biogas composition [%mol CH4/CO2] | 60/40 | |
| Equivalent hour [h/y] | 8000 | |
| OPEX/CAPEX | 5% | |
| Incentivized electricity price [€/MWh] | 124 | [17] |
| Carbon dioxide price [€/ton] | 30 | [105] |
| Biogas production cost [€/Nm3] | 0.2712 | [106] |
| Conversion efficiency | 50% | [107] |
| Biomethane PCI [kWh/Nm3] | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galloni, M.; Di Marcoberardino, G. Biogas Upgrading Technology: Conventional Processes and Emerging Solutions Analysis. Energies 2024, 17, 2907. https://doi.org/10.3390/en17122907
Galloni M, Di Marcoberardino G. Biogas Upgrading Technology: Conventional Processes and Emerging Solutions Analysis. Energies. 2024; 17(12):2907. https://doi.org/10.3390/en17122907
Chicago/Turabian StyleGalloni, Matteo, and Gioele Di Marcoberardino. 2024. "Biogas Upgrading Technology: Conventional Processes and Emerging Solutions Analysis" Energies 17, no. 12: 2907. https://doi.org/10.3390/en17122907
APA StyleGalloni, M., & Di Marcoberardino, G. (2024). Biogas Upgrading Technology: Conventional Processes and Emerging Solutions Analysis. Energies, 17(12), 2907. https://doi.org/10.3390/en17122907





