Non-Normative Oxidation Stability Indication of FAME Produced from Rapeseed and Used Cooking Oil
Abstract
1. Introduction
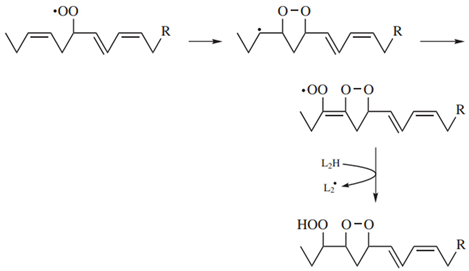


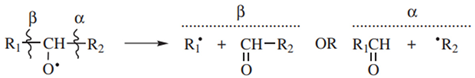


2. Materials and Methods
3. Results and Discussion
3.1. Properties of the Tested FAMEs
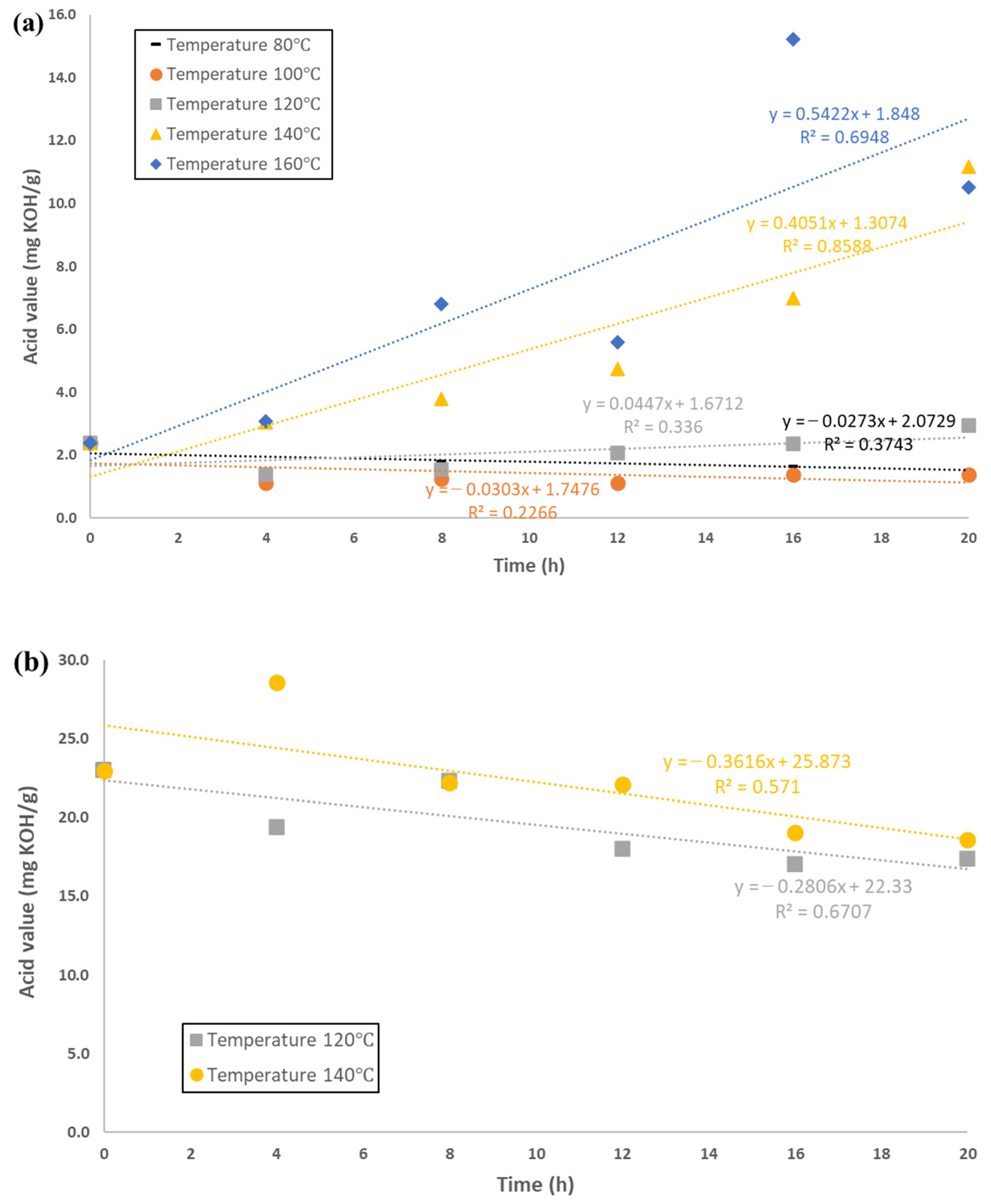
3.2. Change in the Values of Acids, Peroxides and Anisidine in the Samples Tested
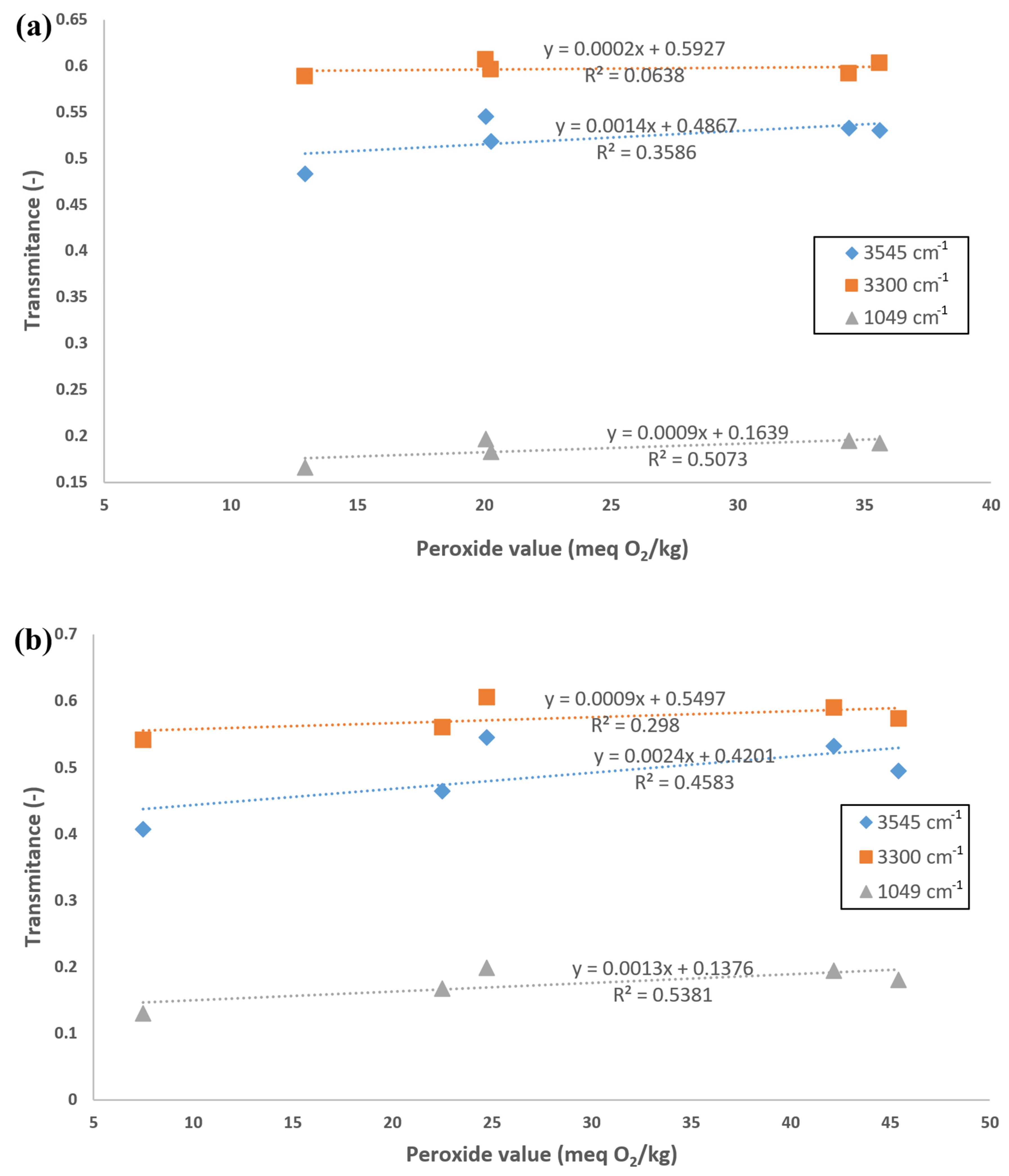
3.3. Application of IR Analysis in the Study of FAME Aging under the Influence of Temperature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ershov, M.A.; Savelenko, V.D.; Makhova, U.A.; Makhmudova, A.E.; Zuikov, A.V.; Kapustin, V.M.; Abdellatief, T.M.M.; Burov, N.O.; Geng, T.; Abdelkareem, M.A.; et al. Current Challenge and Innovative Progress for Producing HVO and FAME Biodiesel Fuels and Their Applications. Waste Biomass Valorization 2023, 14, 505–521. [Google Scholar] [CrossRef]
- van Grinsven, A.; van den Toorn, E.; van der Veen, R.; Kampman, B. Used Cooking Oil (UCO) as Biofuel Feedstock in the EU; CE Delft: Delft, the Netherlands, 2020. [Google Scholar]
- Pińkowska, H. Technical Aspects of Biodiesla Production—New Research Directions; Wrocław University of Economics: Wrocław, Poland, 2008. (In Polish) [Google Scholar]
- Juárez, M.D.; Osawa, C.C.; Acuña, M.E.; Sammán, N.; Gonçalves, L.A.G. Degradation in soybean oil, sunflower oil and partially hydrogenated fats after food frying, monitored by conventional and unconventional methods. Food Control 2011, 22, 1920–1927. [Google Scholar] [CrossRef]
- Dabi, M.; Saha, U.K. Application potential of vegetable oils as alternative to diesel fuels in compression ignition engines: A review. J. Energy Inst. 2019, 92, 1710–1726. [Google Scholar] [CrossRef]
- Jakóbiec, J.; Ambrozik, A. Selected physicochemical and functional properties of methyl esters of fatty acids of rapeseed oil as engine fuel. Inżynieria Rol. 2008, 9, 107–115. (In Polish) [Google Scholar]
- Kumar Saluja, R.; Kumar, V.; Sham, R. Stability of biodiesel—A review. Renew. Sustain. Energy Rev. 2016, 62, 866–881. [Google Scholar] [CrossRef]
- Zuleta, E.C.; Baena, L.; Riosa, L.A.; Calderón, J.A. The Oxidative Stability of Biodiesel and its Impact on the Deterioration of Metallic and Polymeric Materials: A Review. J. Braz. Chem. Soc. 2012, 23, 2159–2175. [Google Scholar] [CrossRef]
- Tomić, M.; Đurišić-Mladenović, N.; Mićić, R.; Simikić, M.; Savin, L. Effects of accelerated oxidation on the selected fuel properties and composition of biodiesel. Fuel 2019, 235, 269–276. [Google Scholar] [CrossRef]
- Agarwal, S.; Singhal, S.; Singh, M.; Arora, S.; Tanwer, M. Role of Antioxidants in Enhancing Oxidation Stability of Biodiesels. ACS Sustain. Chem. Eng. 2018, 6, 11036–11049. [Google Scholar] [CrossRef]
- Puzanowska-Tarasiewicz, H.; Starczewskam, B.; Kuźmickam, L. Reactive oxygen forms. Bromatol. I Chem. Toksykol. 2008, 41, 1007–1015. (In Polish) [Google Scholar]
- Min, D.B.; Boff, J.M. Chemistry and Reaction of Singlet Oxygen in Foods. Compr. Rev. Food Sci. Food Saf. 2006, 1, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Schaich, K.M. Lipid Oxidation: Theoretical Aspects. In Bailey’s Industrial Fat and Oil Products; Shahidi, F., Ed.; John Wiley: New York, NY, USA, 2005; Volume 1, pp. 269–355. [Google Scholar]
- Eldin, A.K. Lipid Oxidation Pathways; AOCS Press: Champaign, IL, USA, 2003. [Google Scholar]
- Alves-Fortunato, M.; Ayoub, E.; Bacha, K.; Mouret, A.; Dalmazzone, C. Fatty Acids Methyl Esters (FAME) autoxidation: New insights on insoluble deposit formation process in biofuels. Fuel 2020, 268, 117074. [Google Scholar] [CrossRef]
- Paligova, J.; Jorikova, L.; Cvengros, J. Study of FAME stability. Energy Fuels 2008, 22, 1991–1996. [Google Scholar] [CrossRef]
- Sampaio, K.A.; Ayala, J.V.; Silva, S.M.; Ceriani, R.; Verhe, R.; Meirelles, A.J.A. Thermal Degradation Kinetics of Carotenoids in Palm Oil. J. Am. Oil Chem. Soc. 2013, 90, 191–198. [Google Scholar] [CrossRef]
- Chung, H.Y. Oxidative degradation kinetics of tocopherols during heating. J. Food Sci. Nutr. 2007, 12, 115–118. [Google Scholar] [CrossRef]
- Sabliov, C.M.; Fronczek, C.; Astete, C.E.; Khachaturyan, M.; Khachatryan, L.; Leonardi, C. Effects of Temperature and UV Light on Degradation of a-Tocopherol in Free and Dissolved Form. J. Am. Oil Chem. Soc. 2009, 86, 895–902. [Google Scholar] [CrossRef]
- Morales, A.; Marmesat, S.; Dobarganes, M.C.; Marquez-Ruiz, M.; Velasco, J. Formation of Hydroperoxy-, Keto- and Hydroxy-Dienes in FAME from Oils: Influence of Temperature and Addition of a-Tocopherol. J. Am. Oil Chem. Soc. 2012, 89, 675–684. [Google Scholar] [CrossRef]
- Grabowski, P.; Szwarczyńska, A.; Nowakowska, A. Effect of FAME type on the rate of aging in the presence of oxygen. A preliminary study. Przemysł Chem. 2024, 103, 769–775. [Google Scholar]
- EN ISO 3675:1998; Crude Petroleum and Liquid Petroleum Products—Laboratory Determination of Density—Hydrometer Method. ISO: Geneva, Switzerland, 1998.
- EN ISO 3104:2020; Petroleum Products—Transparent and Opaque Liquids—Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity. ISO: Geneva, Switzerland, 2020.
- EN 14103:2020; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Ester and Linolenic acid Methyl Ester Contents. ISO: Geneva, Switzerland, 2020.
- EN ISO 3960:2017; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination. ISO: Geneva, Switzerland, 2017.
- EN ISO 6885:2016; Animal and Vegetable Fats and Oils—Determination of Anisidine Value. ISO: Geneva, Switzerland, 2016.
- EN 14104:2003; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Acid Value. ISO: Geneva, Switzerland, 2003.
- EN 14214:2012+A2:2019; Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods. ISO: Geneva, Switzerland, 2019.
- Jokiniemi, T.; Ahokas, J. A review of production and use of first generation biodiesel in agriculture. Agron. Res. 2013, 11, 239–248. [Google Scholar]
- Mathur, S.; Waswani, H.; Singh, D.; Ranjan, R. Alternative Fuels for Agriculture Sustainability: Carbon Footprint and Economic Feasibility. AgriEngineering 2022, 4, 993–1015. [Google Scholar] [CrossRef]



| Parameters | Norm Values28) | FAMEs from Unrefined Oil | FAMEs from Used Cooking Oil |
|---|---|---|---|
| Density in 15 °C, kg/m3 | 860–900 | 883 ± 3 | 886 ± 4 |
| Kinematic viscosity in 40 °C, mm2/s | 3.50–5.00 | 4.31 ± 0.11 | 5.32 ± 0.18 |
| Total concentration of methyl esters, % | min. 96.5 | 95.7 ± 0.7 | 94.5 ± 1.2 |
| Peroxide value, meq O2/kg | Non-normative parameter | 9.85 ± 0.31 | 7.40 ± 0.24 |
| Anisidine value, AnV | Non-normative parameter | 3.183 ± 0.307 | 15.790 ± 0.531 |
| Acid value, mg KOH/g | max. 0. | 2.38 ± 0.21 | 22.98 ± 0.46 |
| Color | Non-normative parameter | Orange | yellow |
| Temperature, °C | Zero-Order Kinetic Equation | R2 | First-Order Kinetic Equation | R2 |
|---|---|---|---|---|
| FAMEs from unrefined rapeseed oil | ||||
| 80 | c = 0.8489t + 8.2459 | 0.9449 | log(c) = 0.0255t + 0.9279 | 0.9276 |
| 100 | c = 1.6972t + 11.216 | 0.9737 | log(c) = 0.0241t + 1.2891 | 0.9117 |
| 120 | c = 1.6656t + 13.824 | 0.9555 | log(c) = 0.018t + 1.3835 | 0.9639 |
| 140 | c = 0.5392t + 12.741 | 0.8099 | log(c) = 0.0071t + 1.2378 | 0.906 |
| 160 | c = −0.1039t + 11.315 | 0.0898 | log(c) = −0.0095t + 1.0777 | 0.2514 |
| FAMEs from rapeseed oil used for cooking | ||||
| 120 | c = 0.3293t + 8.5391 | 0.5331 | log(c) = 0.0096t + 0.9793 | 0.3106 |
| 140 | c = −0.0238t + 7.4333 | 0.1271 | log(c) = −0.0016t + 0.8729 | 0.0960 |
| Temperature, °C | Zero-Order Kinetic Equation | R2 | First-Order Kinetic Equation | R2 |
|---|---|---|---|---|
| FAMEs from unrefined rapeseed oil | ||||
| 80 | c = 0.5167t + 2.1449 | 0.9114 | log(c) = 0.0353t + 0.4401 | 0.9328 |
| 100 | c = 0.462t + 1.6607 | 0.9000 | log(c) = 0.0471t + 0.1982 | 0.8975 |
| 120 | c = 0.6974t + 1.2477 | 0.9418 | log(c) = 0.0511t + 0.2652 | 0.8975 |
| 140 | c = 5.0258t + 19.949 | 0.8754 | log(c) = 0.023t + 1.6249 | 0.7749 |
| 160 | c = 17.403t + 53.317 | 0.8991 | log(c) = 0.0252t + 2.1071 | 0.7971 |
| FAMEs from rapeseed oil used for cooking | ||||
| 120 | c = 6.7035t + 19.887 | 0.9676 | log(c) = 0.0455t + 1.3857 | 0.8630 |
| 140 | c = 4.4838t + 23.747 | 0.9523 | log(c) = 0.0379t + 1.3851 | 0.8214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowski, P.; Szwarczyńska, A. Non-Normative Oxidation Stability Indication of FAME Produced from Rapeseed and Used Cooking Oil. Energies 2024, 17, 4210. https://doi.org/10.3390/en17174210
Grabowski P, Szwarczyńska A. Non-Normative Oxidation Stability Indication of FAME Produced from Rapeseed and Used Cooking Oil. Energies. 2024; 17(17):4210. https://doi.org/10.3390/en17174210
Chicago/Turabian StyleGrabowski, Pawel, and Angelika Szwarczyńska. 2024. "Non-Normative Oxidation Stability Indication of FAME Produced from Rapeseed and Used Cooking Oil" Energies 17, no. 17: 4210. https://doi.org/10.3390/en17174210
APA StyleGrabowski, P., & Szwarczyńska, A. (2024). Non-Normative Oxidation Stability Indication of FAME Produced from Rapeseed and Used Cooking Oil. Energies, 17(17), 4210. https://doi.org/10.3390/en17174210








