Automotive e-Fuels via Hydrocracking of FT-Wax: e-Gasoline and e-Diesel Production
Highlights
- Hydrocracking activity of Ni-W was better over zeolite-supported Ni-W considering e-gasoline selectivity and hydrogen consumption
- Optimal hydrocracking operation achieved at 8.3 MPa pressure, 603 K temperature, and 2500 scfb H2/oil
- E-gasoline and e-diesel were produced via solar hydrogen fed hydrocracking of Fischer–Tropsch wax
- E-gasoline and e-diesel abide by key EN228 and EN590 specs respectively
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. Testing Infrastructure
2.3. Experimental Procedure
2.4. Analysis
2.5. Storage Stability Study
3. Results
3.1. Evaluation of Ni-W Catalyst
3.2. Evaluation of NiW Zeolite-Supported Catalyst
3.3. Evaluation of NiW Al2O3—SiO2 Supported Catalyst
3.4. Product Evaluation
3.5. Storage Stability Study
4. Discussion
5. Conclusions
- −
- The optimum conditions for the NiW catalyst include a 8.3 MPa pressure, 603 K temperature, 1 hr−1 LHSV, and 2500 scfb H2/oil ratio.
- −
- The optimum conditions for the NiW zeolite-supported catalyst include a 8.3 MPa pressure, 588 K temperature, 1 hr−1 LHSV, and 3000 scfb H2/oil ratio.
- −
- Crushing the NiW Al2O3—SiO2-supported catalyst into smaller-diameter particles to fit into the reactor destroyed its activity, leading to no hydrocracking reactions.
- −
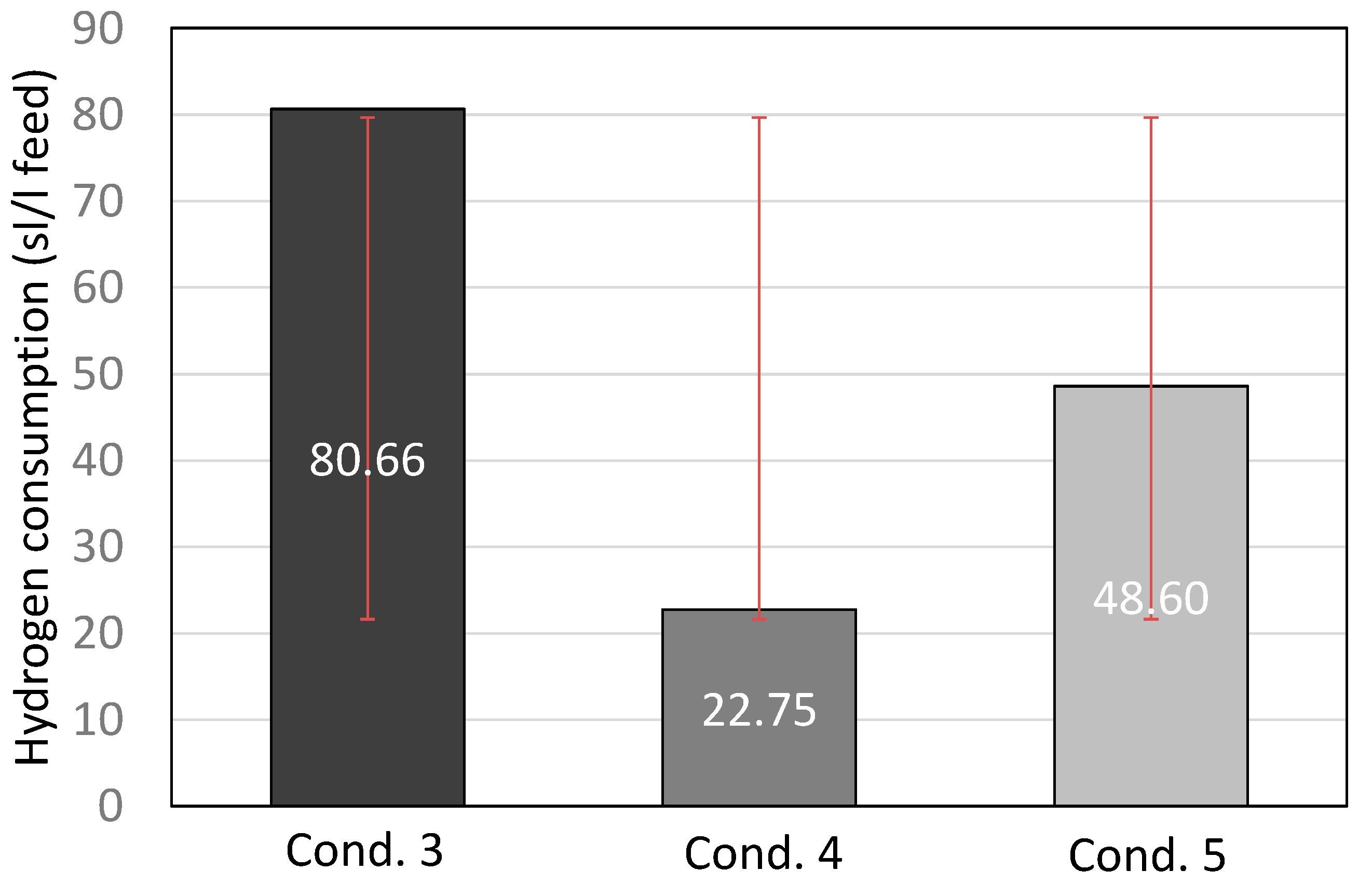
- The optimum Ni-W catalyst product rendered a higher e-gasoline content and lower residue content compared to that of the Ni-W zeolite-supported catalyst, while the hydrogen consumption was about 40% lower compared to that achieved by the NiW zeolite-supported catalyst.
- −
- High-quality e-gasoline, e-diesel, and heavy e-fuel were produced via the hydrocracking of wax from Fischer–Tropsch synthesis, also exhibiting significant stability over a maximum storage period of 4 months in the examined conditions.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations—Symbols
| CERTH | Centre for Research & Technology Hellas |
| CLG | Chemical Looping Gasification |
| CPERI | Chemical Process & Energy Resources Institute |
| DHD | Dehydrogenation |
| DMDS | Di-methyl-di-sulfide |
| DOS | Days On Stream |
| FT | Fischer–Tropsch |
| GHG | Green House Gas emissions |
| HAGO | Heavy Atmospheric Gas Oil |
| HD | Hydrogenation |
| HHV | High Heating Value |
| I.D. | Inlet diameter (referred to hydrotreating reactor) |
| KIST | Korea Institute of Science and Technology |
| LAGO | Light Atmospheric Gas Oil |
| LEFH | Laboratory of Environmental Fuels and Hydrocarbons |
| LHSV | Liquid Hourly Space Velocity |
| LPG | Liquified Petroleum Gas |
| NA | Not Available |
| RON | Research Octane Number |
| RVP | Reid vapor pressure |
| TAN | Total Acid Number |
| TRL | Technology Readiness Level |
| VGO | Vacuum Gas Oil |
| WC | Water Content |
| WHSV | Weight Hourly Space Velocity |
References
- Concawe, Environmental Science for European Refining, Aramco. In E-Fuels: A Technoeconomic Assessment of European Domestic Production and Imports Towards 2050; Report No. 17/22; Concawe: Brussels, Belgium, 2022.
- Chemical Looping Gasification for Sustainable Production of Biofuels—CLARA. Available online: www.clara-h2020.eu (accessed on 18 November 2023).
- Suo, Y.; Yao, Y.; Zhang, Y.; Xing, S.; Yuan, Z.Y. Recent advances in cobalt-based Fischer-Tropsch synthesis catalysts. J. Ind. Eng. Chem. 2022, 115, 92–119. [Google Scholar] [CrossRef]
- Sage, V.; Sun, Y.; Hazewinkel, P.; Bhatelia, T.; Braconnier, L.; Tang, L.; Chiang, K.; Batten, M.; Burke, N. Modified product selectivity in Fischer-Tropsch synthesis by catalyst pre-treatment. Fuel Process. Technol. 2017, 167, 183–192. [Google Scholar] [CrossRef]
- Leckel, D.; Liwanga-Ehumbu, M. Diesel-selective hydrocracking of an iron-based-Fischer-Tropsch wax fraction (C15-C45) using a MoO3-modified noble metal catalyst. Energy Fuels 2006, 20, 2330–2336. [Google Scholar] [CrossRef]
- Tomasek, S.; Lonyi, F.; Valyon, J.; Wollmann, A.; Hancsók, J. Hydrocracking of Fischer–Tropsch Paraffin Mixtures over Strong Acid Bifunctional Catalysts to Engine Fuels. ACS Omega 2020, 5, 26413–26420. [Google Scholar] [CrossRef]
- Bouchy, C.; Hastoy, G.; Guillon, E.; Martens, J.A. Fischer-Tropsch Waxes Upgrading via Hydrocracking and Selective Hydroisomerization. Oil Gas Sci. Technol.-Rev. IFP 2009, 64, 91–112. [Google Scholar] [CrossRef]
- Pleyer, O.; Petr, S.; Dan, V.; Jiří, H.; Radek, Č. Hydrocracking of fischer-tropsch wax. PALIVA 2020, 12, 26–33. [Google Scholar] [CrossRef]
- Hodala, J.L.; Jung, J.S.; Yang, E.H.; Hong, G.H.; Noh, Y.S.; Moon, D.J. Hydrocracking of FT-wax to fuels over non-noble metal catalysts. Fuel 2016, 185, 339–347. [Google Scholar] [CrossRef]
- Li, T.; Tao, Z.; Hu, C.; Zhao, C.; Yi, F.; Zhao, G.; Zhang, L.; Yang, Y. Brønsted acidity of amorphous silica-aluminas for hydrocracking of Fischer-Tropsch wax into diesel fractions. Appl. Catal. A Gen. 2022, 630, 118439. [Google Scholar] [CrossRef]
- Fratczak, J.; Carmona, H.P.; Tisler, Z.; Herrador, J.M.H.; Gholami, Z. Hydrocracking of heavy fischer-tropsch wax distillation residues and its blends with vacuum gas oil using phonolite-based catalysts. Molecules 2021, 26, 7172. [Google Scholar] [CrossRef]
- Gregor, J.H. Fischer-Tropsch products as liquid fuels or chemicals. Catal. Lett. 1990, 7, 317–331. [Google Scholar] [CrossRef]
- Gamba, S.; Pellegrini, L.A.; Calemma, V.; Gambaro, C. Liquid fuels from Fischer–Tropsch wax hydrocracking: Isomer distribution. Catal. Today 2010, 156, 58–64. [Google Scholar] [CrossRef]
- Calemma, V.; Gambaro, C.; Parker, W.O., Jr.; Carbone, R.; Giardino, R.; Scorletti, P. Middle distillates from hydrocracking of FT waxes: Composition, characteristics and emission properties. Catal. Today 2010, 149, 40–46. [Google Scholar] [CrossRef]
- Yan, P.-H.; Tao, Z.-C.; Hao, K.; Wang, Y.-D.; Yang, Y.; Li, Y.-W. Effect of impregnation methods on nickel-tungsten catalysts and its performance on hydrocracking Fischer-Tropsch wax. J. Fuel Chem. Technol. 2013, 41, 691–697. [Google Scholar] [CrossRef]
- O’Connell, A.; Georgiadou, M.; Schleker, T. Advanced Alternative Fuels. Technolgy Development Report; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar] [CrossRef]
- Standards EN 590; Automotive Fuels—Diesel—Requirements and Test Methods. European Committee: Brussels, Belgium, 2009.
- Standard ISO/IEC 19540-1; Information Technology—Object Management Group Unified Architecture Framework (OMG UAF)—Part 1: Domain Metamodel (DMM). ISO and IEC Joint Technical Committee: Washington, DC, USA, 2022.
- Dimitriadis, A.; Seljak, T.; Vihar, R.; Baškovič, U.Z.; Dimaratos, A.; Bezergianni, S.; Samaras, Z.; Katrašnik, T. Improving PM-NOx trade-off with paraffinic fuels: A study towards diesel engine optimization with HVO. Fuel 2020, 265, 116921. [Google Scholar] [CrossRef]
- Kirsch, H.; Sommer, U.; Pfeifer, P.; Dittmeyer, R. Power-to-fuel conversion based on reverse water-gas-shift, Fischer-Tropsch Synthesis and hydrocracking: Mathematical modeling and simulation in Matlab/Simulink. Chem. Eng. Sci. 2020, 227, 115930. [Google Scholar] [CrossRef]
- Kirsch, H.; Lochmahr, N.; Staudt, C.; Pfeifer, P.; Dittmeyer, R. Production of CO2-neutral liquid fuels by integrating Fischer-Tropsch synthesis and hydrocracking in a single micro-structured reactor: Performance evaluation of different configurations by factorial design experiments. Chem. Eng. J. 2020, 393, 124553. [Google Scholar] [CrossRef]
- Vedachalam, S.; Boahene, P.; Dalai, A.K. Production of jet fuel by hydrorefining of Fischer-Tropsch wax over Pt/Al-TUD-1 bifunctional catalyst. Fuel 2021, 300, 121008. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Chrysikou, L.P.; Meletidis, G.; Terzis, G.; Auersvald, M.; Kubicka, D.; Bezergianni, S. Bio-based refinery intermediate production via hydrodeoxygenation of fast pyrolysis bio-oil. Renew. Energy 2021, 168, 593–605. [Google Scholar] [CrossRef]
- Ziogou, C.; Ipsakis, D.; Seferlis, P.; Bezergianni, S.; Papadopoulou, S.; Voutetakis, S. Optimal production of renewable hydrogen based on an efficient energy management strategy. Energy 2013, 55, 58–67. [Google Scholar] [CrossRef]
- Halmenschlager, G.M.; Brar, M.; Apan, I.T.; Klerk, A. Hydrocracking vacuum gas oil with wax. Catal. Today 2020, 353, 187–196. [Google Scholar] [CrossRef]
- D7169-23; Standard Test Method for Boiling Point Distribution of Samples with Residues Such as Crude Oils and Atmospheric and Vacuum Residues by High Temperature Gas Chromatography. ASTM International: West Conshohocken, PA, USA, 2023.
- D4294-21; Standard Test Method for Sulfur in Petroleum and Petroleum Products by Energy Dispersive X-ray Fluorescence Spectrometry. ASTM International: West Conshohocken, PA, USA, 2021.
- D6304-20; Standard Test Method for Determination of Water in Petroleum Products, Lubricating Oils, and Additives by Coulometric Karl Fischer Titration. ASTM International: West Conshohocken, PA, USA, 2020.
- E203-16; Standard Test Method for Water Using Volumetric Karl Fischer Titration. ASTM International: West Conshohocken, PA, USA, 2016.
- D445-21; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2021.
- D976-06; Standard Test Method for Calculated Cetane Index of Distillate Fuels. ASTM International: West Conshohocken, PA, USA, 2006.
- Standard EN 15751:2014; Automotive Fuels—Fatty Acid Methyl Ester (FAME) Fuel and Blends with Diesel Fuel—Determination of Oxidation Stability by Accelerated Oxidation Method. European Committee: Brussels, Belgium, 2014.
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Chrysikou, L.P.; Litinas, A.; Bezergianni, S. Assessment of biodiesel stability under long-term storage and dynamic accelerated oxidation: A comparison approach. Clean Technol. Environ. Policy 2022, 24, 2583–2593. [Google Scholar] [CrossRef]
- Sie, S.T. Acid-catalyzed cracking of paraffinic hydrocarbons. 1. Discussion of existing mechanism and proposal of a new mechanism. Ind. Eng. Chem. Res. 1992, 31, 1881–1889. [Google Scholar] [CrossRef]
- Sie, S.T. Acid-catalyzed cracking of paraffinic hydrocarbons. 2. Evidence for the protonated cyclopropane mechanism from catalytic cracking experiments. Ind. Eng. Chem. Res. 1993, 32, 397–402. [Google Scholar] [CrossRef]
- Sie, S.T. Acid-catalyzed cracking of paraffinic hydrocarbons. 3. Evidence for the protonated cyclopropane mechanism from hydrocracking/hydroisomerization experiments. Ind. Eng. Chem. Res. 1993, 32, 403–408. [Google Scholar] [CrossRef]
- Khuong, L.S.; Zulkifli, N.W.M.; Masjuki, H.H.; Niza Mohamad, E.; Arslan, A.; Mosarof, M.H.; Azham, A. A review on the effect of bioethanol dilution on the properties and performance of automotive lubricants in gasoline engines. RSC Adv. 2016, 6, 66847. [Google Scholar] [CrossRef]
- Gómez, N.; Banks, S.W.; Nowakowski, D.J.; Rosas, J.G.; Cara, J.; Sánchez, M.E.; Bridgwater, A.V. Effect of temperature on product performance of a high ash biomass during fast pyrolysis and its bio-oil storage evaluation. Fuel Process. Technol. 2018, 172, 97–105. [Google Scholar] [CrossRef]
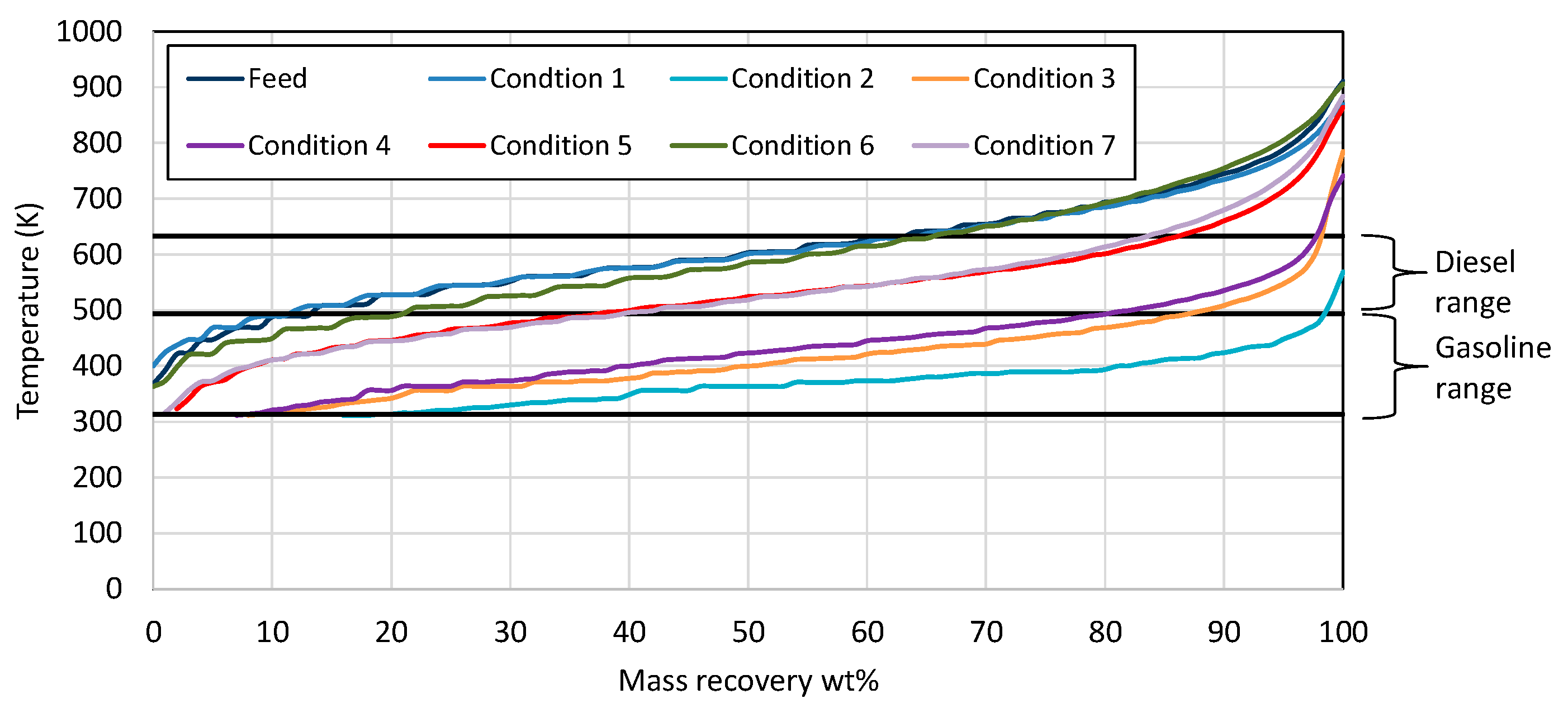
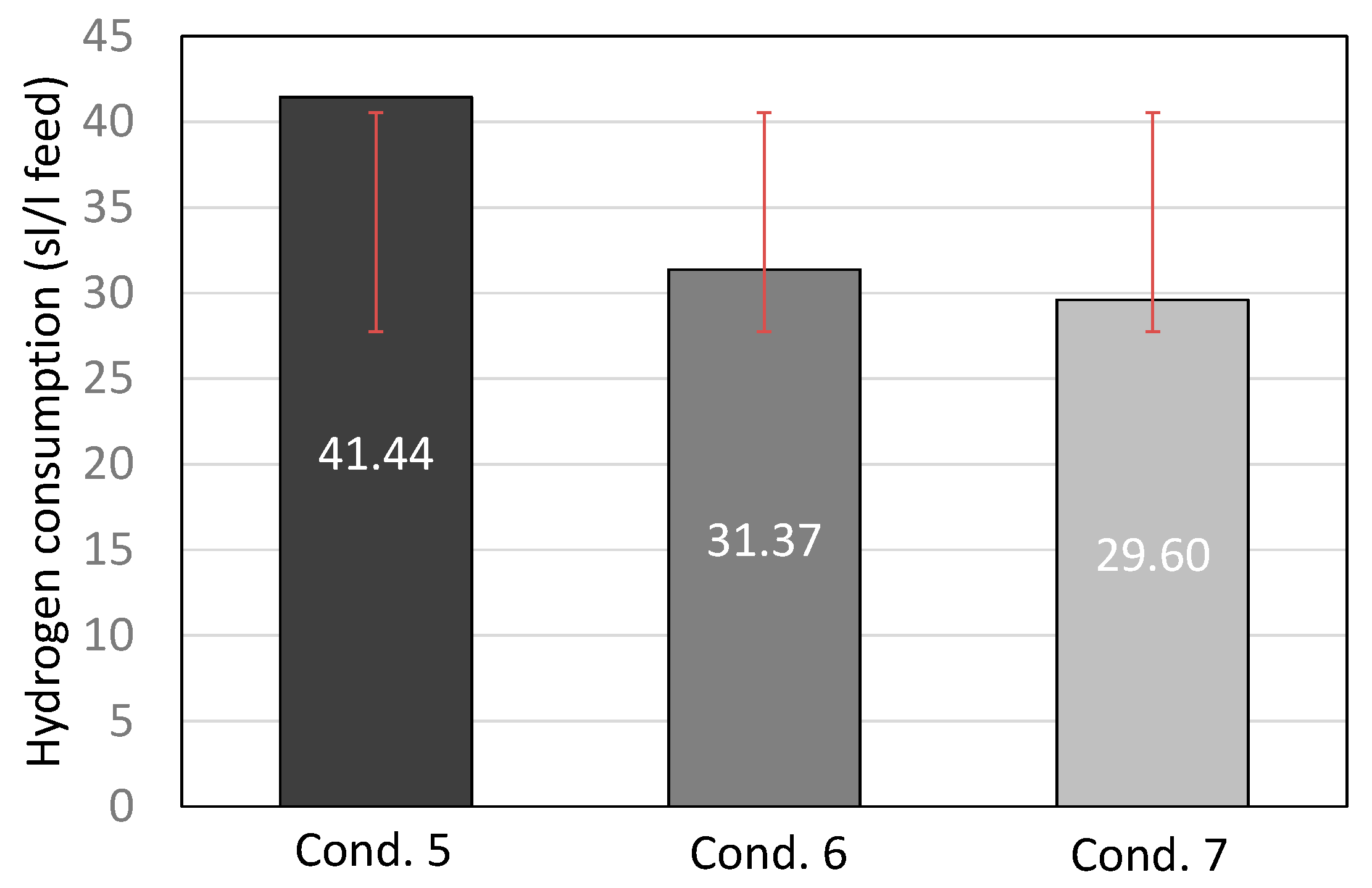
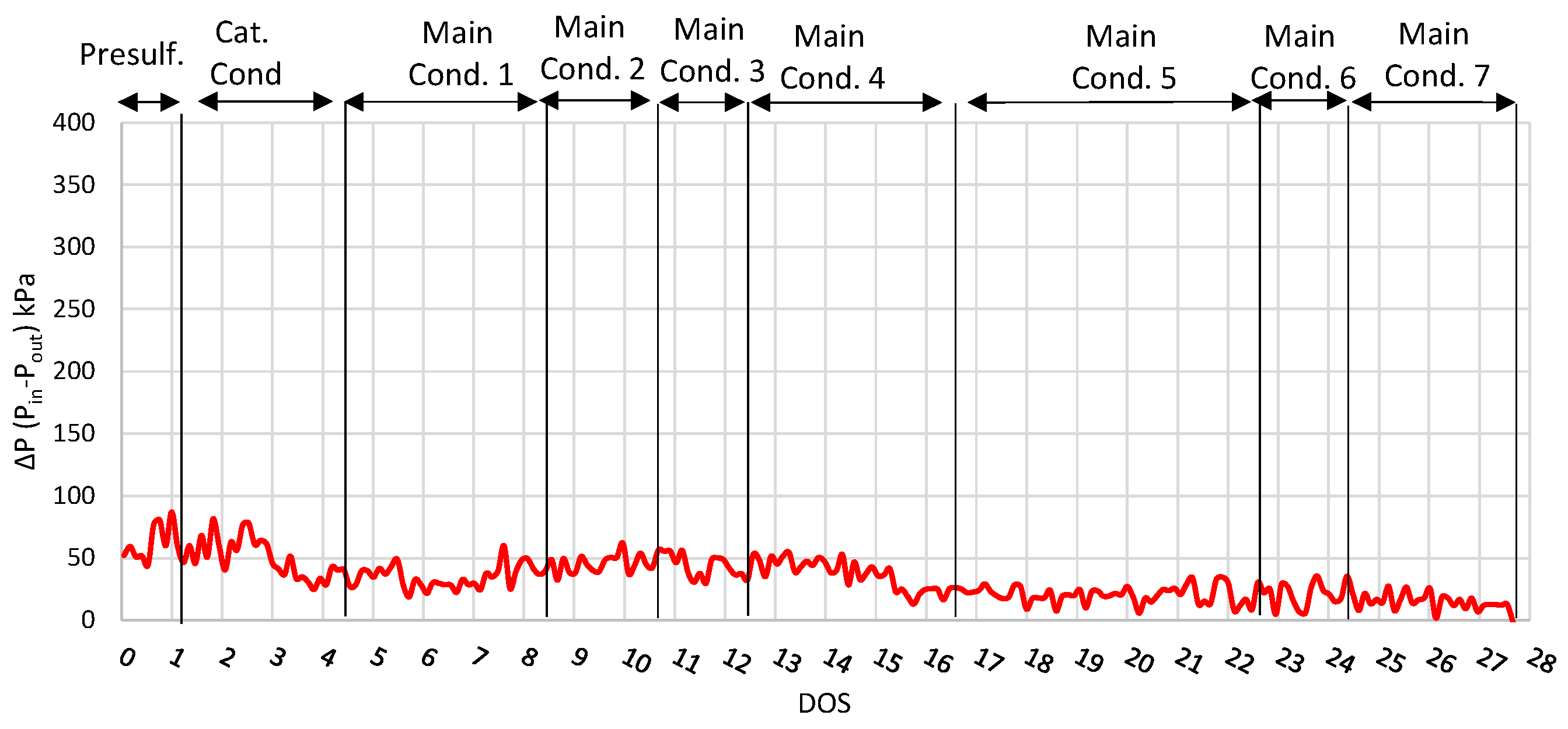
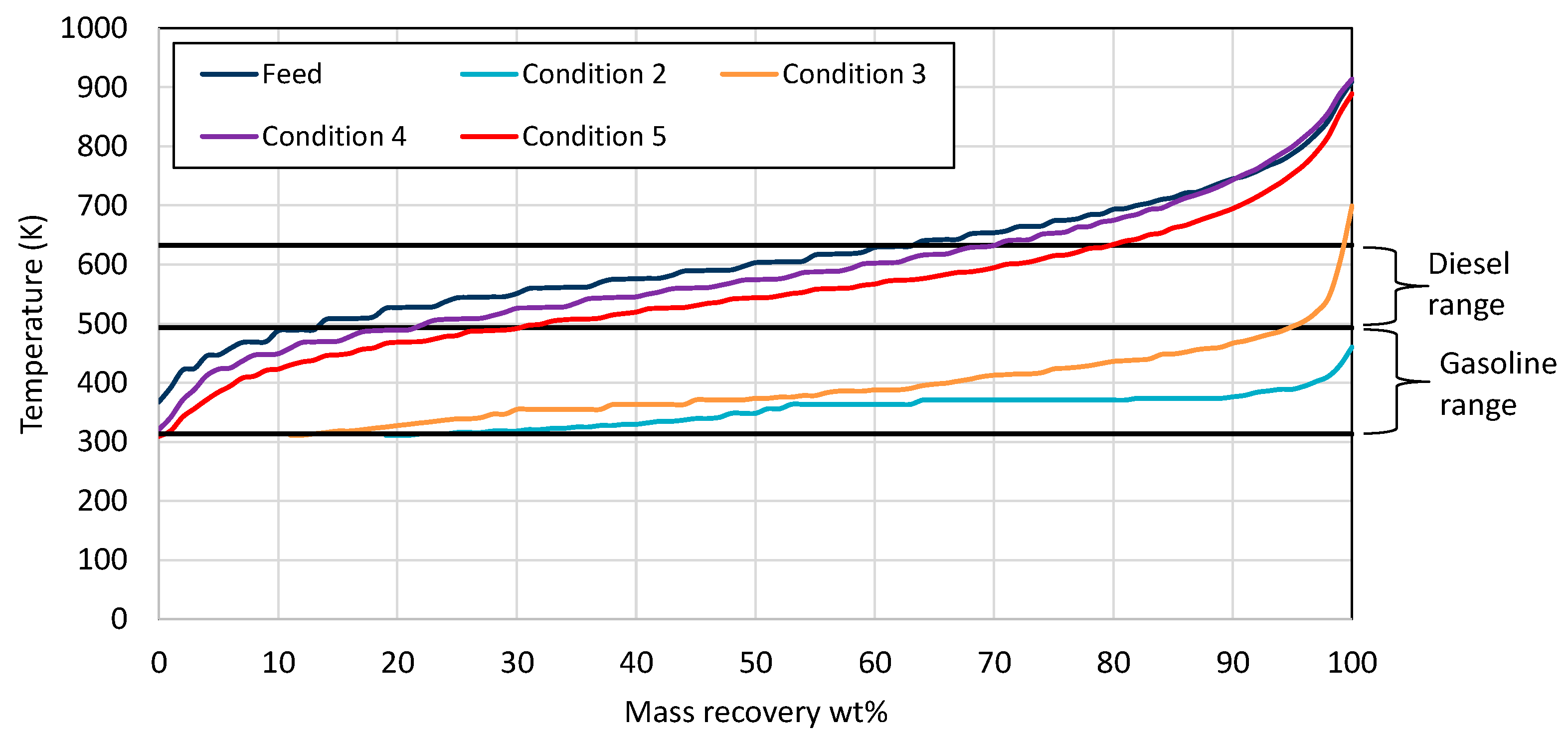
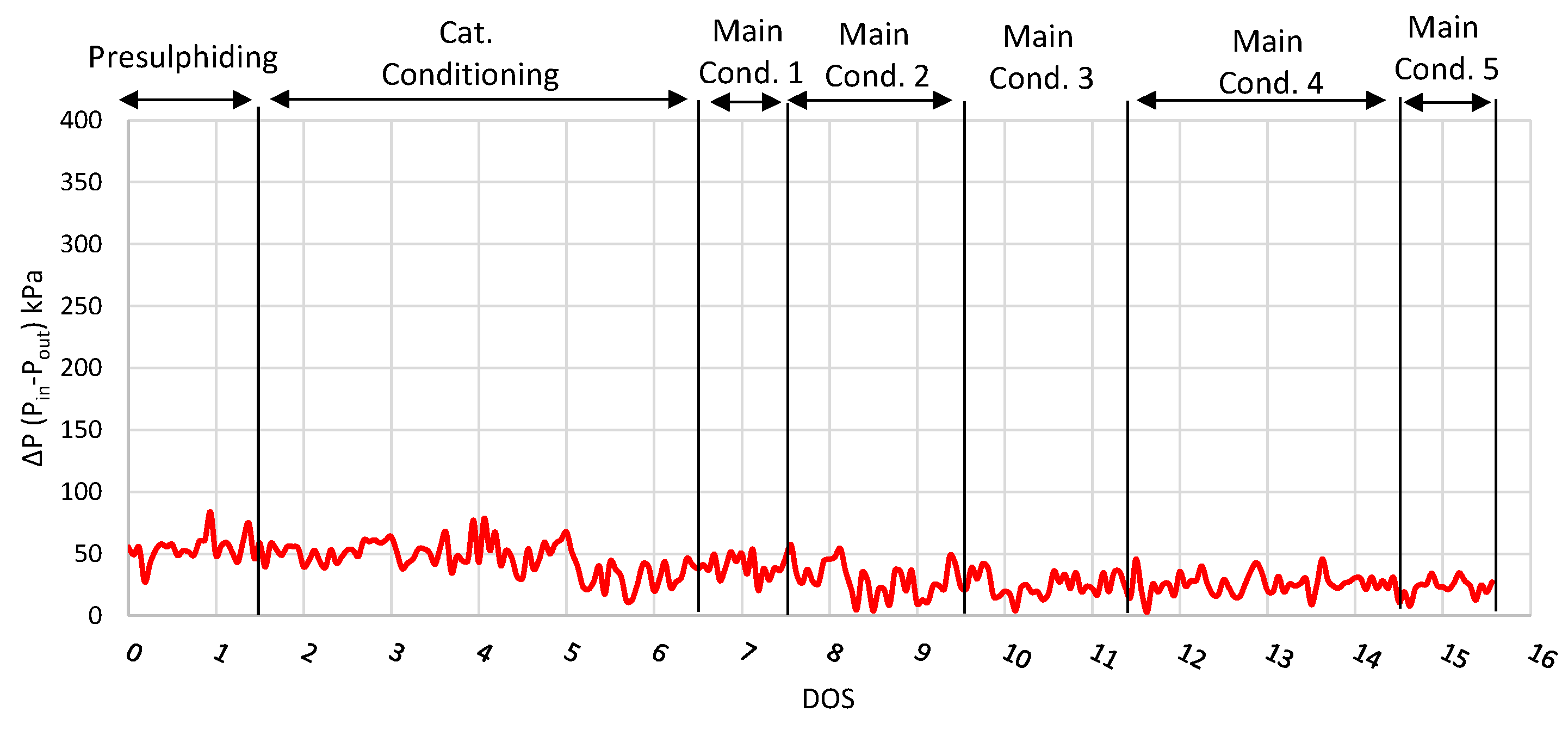
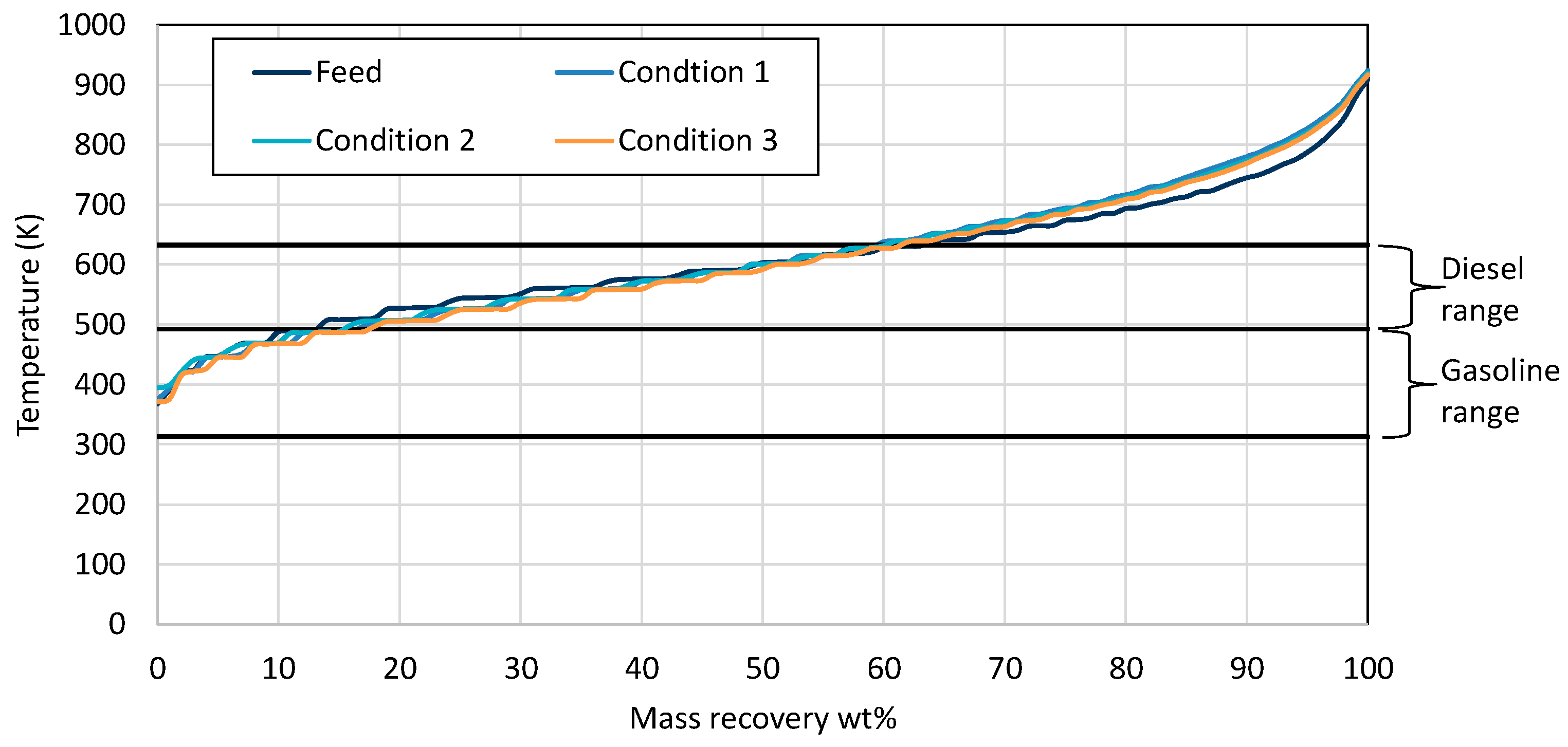
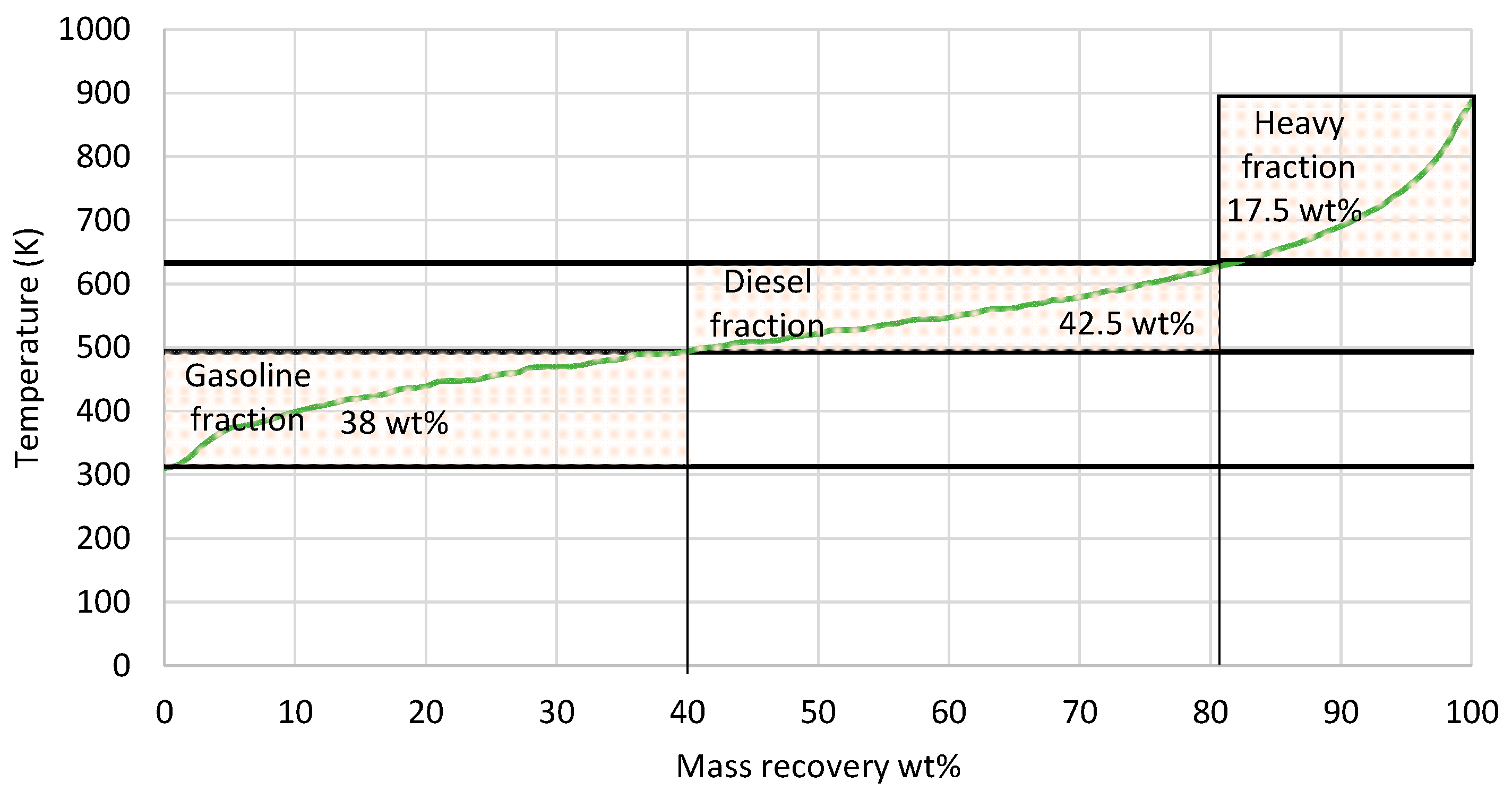
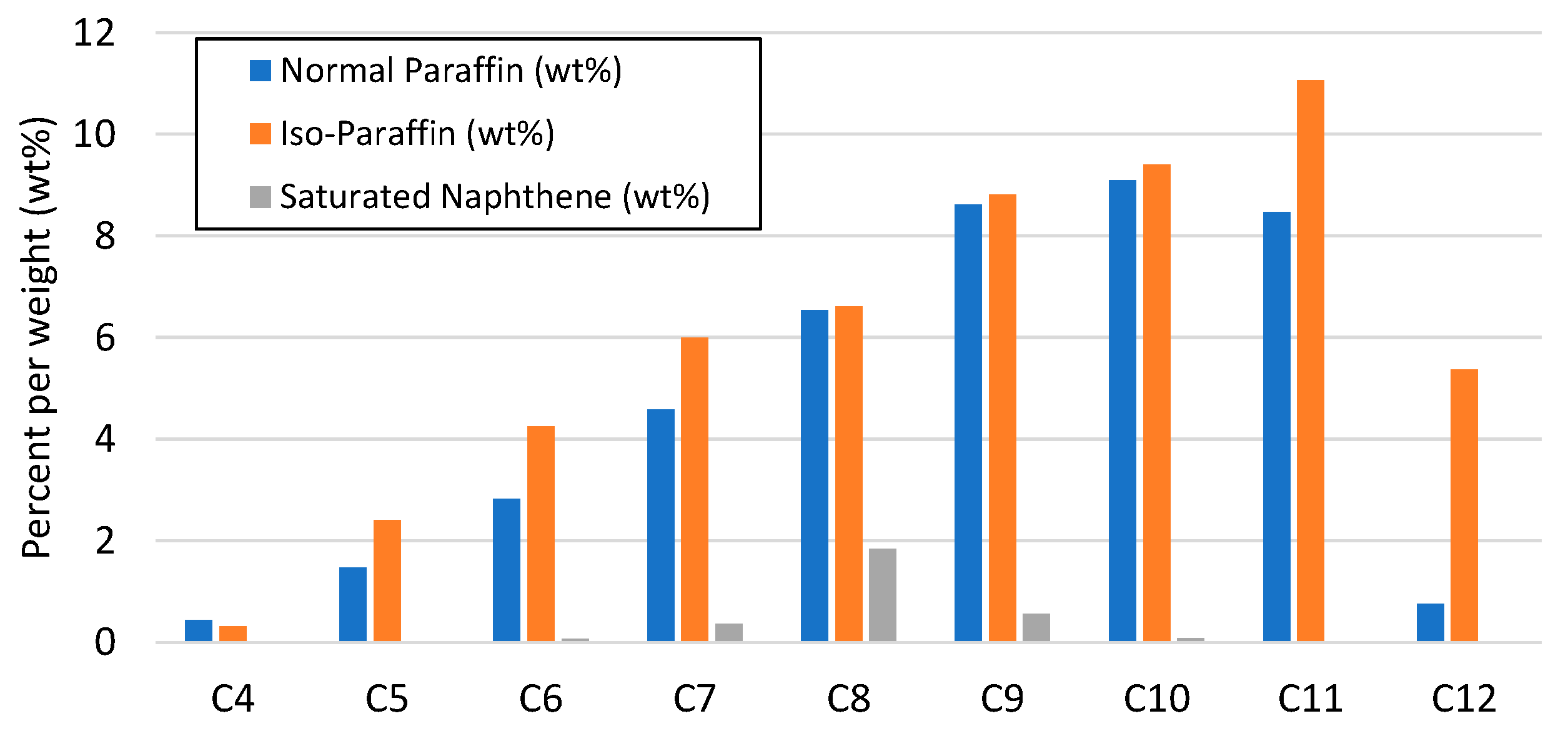
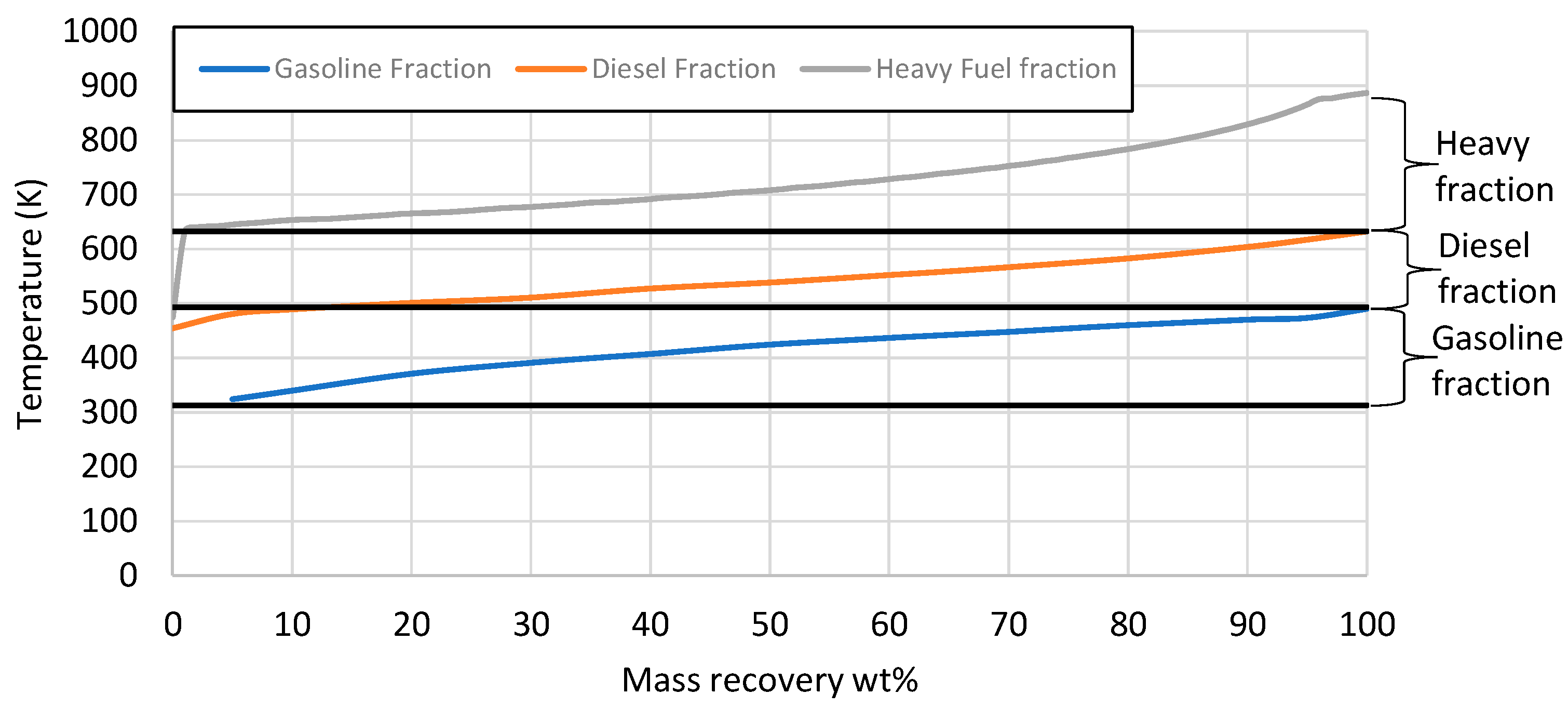
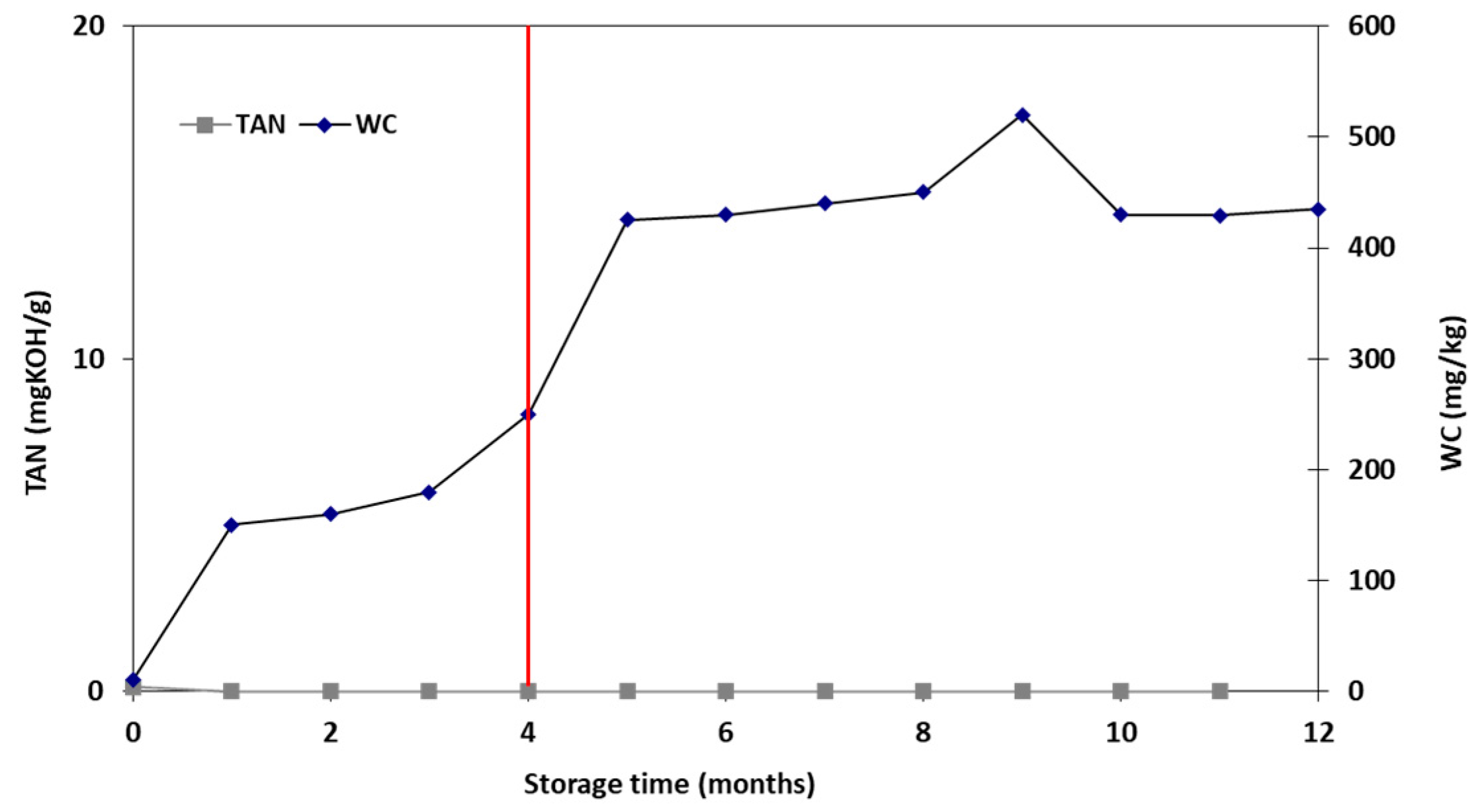
| Properties | Units | FT-Wax | Analysis Method |
|---|---|---|---|
| Density at 288 K | g/mL | 0.7897 | ASTM D-4052 |
| Sulfur dry basis | wppm | 2.5 | ASTM D-5453 |
| Viscosity at 313 K | cSts | 2.339 | ASTM D445 |
| TAN | mgKOH/g | 0.2 | ASTM D-664 |
| Hydrogen dry basis | wt% | 14.56 | ASTM D-5291 |
| Carbon dry basis | wt% | 84.4 | ASTM D-5291 |
| Oxygen dry basis | wt% | 1.04 | Calculated by difference |
| Nitrogen dry basis | wt% | 0.5 | ASTM D-4629 |
| Gasoline | wt% | 13 | ASTM D-7169 |
| Diesel | wt% | 50 | ASTM D-7169 |
| Residue | wt% | 37 | ASTM D-7169 |
| Unit | Catalyst Conditioning | Cond. 1 | Cond. 2 | Cond. 3 | Cond. 4 | Cond. 5 | Cond. 6 | Cond. 7 | |
|---|---|---|---|---|---|---|---|---|---|
| DOS | - | 1–3 | 4–7 | 8–10 | 11 | 12–15 | 16–21 | 22–23 | 24–25 |
| Pres. | MPa | 14.8 | 10.3 | 13.8 | 13.8 | 12.4 | 8.3 | 8.3 | 8.3 |
| Temp. | K | 613 | 623 | 653 | 633 | 623 | 603 | 573 | 603 |
| H2/Oil | scfb | 3640 | 3000 | 3000 | 3000 | 3000 | 3000 | 3000 | 2500 |
| LHSV | hr−1 | 2.18 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Feed | - | HAGO | wax | wax | wax | wax | wax | wax | wax |
| Units | Cond. 1 | Cond. 2 | Cond. 3 | Cond. 4 | Cond. 5 | Cond. 6 | Cond. 7 | ||
|---|---|---|---|---|---|---|---|---|---|
| Hydrogen | H2 | v/v % | 99.89 | 92.85 | - | 97.52 | 98.71 | 99.86 | 98.54 |
| Methane | CH4 | v/v % | 0.02 | 0.68 | - | 0.16 | 0.04 | 0.01 | 0.04 |
| Propane | C3H8 | v/v % | 0.01 | 2.05 | - | 0.01 | 0.21 | 0.00 | 0.20 |
| Isobutane | i-C4H10 | v/v % | 0.00 | 1.88 | - | 0.66 | 0.36 | 0.00 | 0.29 |
| N-Butane | n-C4H10 | v/v % | 0.01 | 1.05 | - | 0.33 | 0.18 | 0.01 | 0.18 |
| Isopentane | i-C5H12 | v/v % | 0.00 | 0.76 | - | 0.34 | 0.21 | 0.00 | 0.24 |
| N-Pentane | n-C5H12 | v/v % | 0.00 | 0.32 | - | 0.13 | 0.08 | 0.02 | 0.12 |
| C6+ | C6+ | v/v % | 0.00 | 0.32 | - | 0.23 | 0.14 | 0.06 | 0.31 |
| Nitrogen | N2 | v/v % | 0.06 | 0.07 | - | 0.48 | 0.05 | 0.05 | 0.07 |
| Units | Catalyst Conditioning | Cond. 1 | Cond. 2 | Cond. 3 | Cond. 4 | Cond. 5 | ||
|---|---|---|---|---|---|---|---|---|
| DOS | - | 1–3 | 4–5 | 6 | 7–8 | 9–10 | 11–13 | 14–15 |
| Pres. | MPa | 14.8 | 14.8 | 8.3 | 8.3 | 8.3 | 8.3 | 8.3 |
| Temp. | K | 613 | 653 | 603 | 653 | 603 | 573 | 588 |
| H2/oil | scfb | 3640 | 3640 | 2500 | 3000 | 2500 | 3000 | 3000 |
| LHSV | hr−1 | 2.18 | 2.18 | 1 | 1 | 1 | 1 | 1 |
| Feed | - | HAGO | HAGO | wax | wax | wax | wax | wax |
| Units | Cond. 1 | Cond. 2 | Cond. 3 | Cond. 4 | Cond. 5 | ||
|---|---|---|---|---|---|---|---|
| Hydrogen | H2 | v/v % | - | - | 93.49 | 99.33 | 98.84 |
| Methane | CH4 | v/v % | - | - | 0.07 | 0.01 | 0.01 |
| Propane | C3H8 | v/v % | - | - | 1.73 | 0.08 | 0.19 |
| Isobutane | i-C4H10 | v/v % | - | - | 2.36 | 0.15 | 0.32 |
| N-Butane | n-C4H10 | v/v % | - | - | 1.05 | 0.07 | 0.15 |
| Isopentane | i-C5H12 | v/v % | - | - | 0.00 | 0.13 | 0.21 |
| N-Pentane | n-C5H12 | v/v % | - | - | 0.47 | 0.04 | 0.07 |
| C6+ | C6+ | v/v % | - | - | 0.72 | 0.15 | 0.14 |
| Nitrogen | N2 | v/v % | - | - | 0.10 | 0.05 | 0.05 |
| Units | Cat. Conditioning | Cat. Conditioning | Cond. 1 | Cond. 2 | Cond. 3 | |
|---|---|---|---|---|---|---|
| DOS | - | 1–3 | 4–5 | 6 | 7–8 | 9–10 |
| Pres. | MPa | 14.8 | 13.8 | 8.3 | 13.8 | 10.3 |
| Temp. | K | 613 | 653 | 603 | 623 | 623 |
| H2/oil | scfb | 3640 | 3000 | 2500 | 2500 | 3000 |
| LHSV | hr−1 | 2.18 | 1 | 1 | 1 | 1 |
| Feed | - | HAGO | wax | wax | wax | wax |
| Units | Cond. 1 | Cond. 2 | Cond. 3 | |
|---|---|---|---|---|
| Hydrogen | v/v % | 99.84 | 99.72 | 99.72 |
| Methane | v/v % | 0.01 | 0.04 | 0.04 |
| Ethane | v/v % | 0.00 | 0.02 | 0.02 |
| N-Butane | v/v % | 0.01 | 0.03 | 0.03 |
| N-Pentane | v/v % | 0.02 | 0.03 | 0.03 |
| C6+ | v/v % | 0.03 | 0.06 | 0.06 |
| Carbon dioxide | v/v % | 0.01 | 0.04 | 0.03 |
| Nitrogen | v/v % | 0.06 | 0.06 | 0.05 |
| Properties | Units | Gasoline EN 228 | e-Gasoline Fraction | Diesel EN 590 | e-Diesel Fraction | Analysis/Method |
|---|---|---|---|---|---|---|
| Kinematic viscosity at 313 K | mm2s−1 | - | - | 2.0–4.5 | 2.427 | ASTM D445 |
| Density at 288 K | Kg m−3 | 720–775 | 716 | 820–835 | 774 | ASTM D4052 |
| RVP at 311 K (summer grade) | kPa | 45–60 | 26.29 | - | - | ASTM D323 |
| Cetane number, min | - | - | - | Min. 51 | 77.38 * | ASTM D613 |
| RON | - | Min. 95 | NA ** | - | - | ASTM D2699 |
| Distillation temperature T10 | K | Max. 343 | 340 | - | 490 | ASTM D86 |
| T50 | K | 339–383 | 424 | - | 538 | ASTM D86 |
| T90 | K | Max. 463 | 470 | 555–611 | 604 | ASTM D86 |
| Oxidation stability at 383 K | For gasoline: minutes For diesel: g m−3 | Min. 144 | NA ** | Max. 25 | NA ** | Gasoline: ASTM D525 Diesel: EN ISO 12205 |
| Oxygen content | wt% | Max. 2.7 | 0.0 | - | 0.0 | ASTM D4815 |
| Benzene | v/v % | Max. 1.0 | NA ** | - | - | ASTM D4420 |
| Sulfur content | wt% | Max. 0.001 | 0.002 | Max. 0.001 | 0.001 | ASTM D381 |
| Flash point | K | - | - | Min. 329 | 368 | ASTM D93 |
| Lubricity at 333 K | μm | - | - | Max. 460 | - | ASTM D6079 |
| Ash | wt% | - | - | Max. 0.01 | 0 | ASTM D482 |
| Water and sediment | v/v % | - | - | Max. 0.05 | 0.0029 | ASTM D2709 |
| Calorific value | MJ/kg | 34.84 | NA ** | 43.8 | 47.35 | Calculated |
| Stoichiometric air fuel ratio | - | 1/14.7 | NA ** | 1/14.67 | NA ** | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriadis, A.; Chrysikou, L.P.; Bezergianni, S. Automotive e-Fuels via Hydrocracking of FT-Wax: e-Gasoline and e-Diesel Production. Energies 2024, 17, 2756. https://doi.org/10.3390/en17112756
Dimitriadis A, Chrysikou LP, Bezergianni S. Automotive e-Fuels via Hydrocracking of FT-Wax: e-Gasoline and e-Diesel Production. Energies. 2024; 17(11):2756. https://doi.org/10.3390/en17112756
Chicago/Turabian StyleDimitriadis, Athanasios, Loukia P. Chrysikou, and Stella Bezergianni. 2024. "Automotive e-Fuels via Hydrocracking of FT-Wax: e-Gasoline and e-Diesel Production" Energies 17, no. 11: 2756. https://doi.org/10.3390/en17112756
APA StyleDimitriadis, A., Chrysikou, L. P., & Bezergianni, S. (2024). Automotive e-Fuels via Hydrocracking of FT-Wax: e-Gasoline and e-Diesel Production. Energies, 17(11), 2756. https://doi.org/10.3390/en17112756







