A Strategy for Enhanced Carbon Storage: A Hybrid CO2 and Aqueous Formate Solution Injection to Control Buoyancy and Reduce Risk
Abstract
1. Introduction
- No geomechanical or caprock modeling;
- A pure CO2 stream is injected at a typical commercial rate;
- No consideration of surface facility modeling. Focus on the subsurface flow and the impact of formate on CO2 fate and transport in a saline aquifer.
2. Modeling Geochemical Reactions
- The diffusion coefficient (D) for super-critical CO2 in brine equals 3.65 × 10−5 cm2/s, according to Ahmadi et al. [32]. This coefficient is applied to compute the effective CO2 diffusion (Deff) considering a porous medium with a tortuosity τ.
- The solubility of CO2 in brine can be estimated using the method proposed by Li and Nghiem [33], which is based on Henry’s law. This model calculates Henry’s constant based on Equation (2), which is a function of pressure and temperature. However, the influence of salt on the solubility of CO2 in the aqueous phase is taken into account through the use of a salting-out coefficient [34].where: Henry’s constant at current pressure (p) and temperature (T);: Henry’s constant at reference pressure (p*) and temperature (T);: partial molar volume at infinite dilution;R: universal gas constant;i: species dissolved in water (CO2 in this work).
- Reactions between a formate ion and other species in brine using kinetic parameters from the MINTEQ database [37]:
- Reactions with primary minerals using kinetic parameters from PHREEQC for the Transition-State-Theory (TST)-derived rate laws:
- -
- In the synthetic aquifer model:Calcite [CaCO3] + H+ = Ca2+ + HCO3−
- -
- In the real aquifer model:
- Permeability alteration due to mineral precipitation or dissolution was computed by applying the Kozeny–Carman equation with an exponent value of 3, as Zeidouni et al. [38] recommended as follows:andwhere the resistance factor rf is modeled by the Kozeny–Carman equation or the power law relationship; kn and kk refer to permeability at previous (n) and current (k) timesteps, respectively. The porosity, φ, in (14) is calculated as follows:where:φ* is the reference porosity without mineral precipitation/dissolution;Nj is the total moles of mineral j per bulk volume at the current time;is the total moles of mineral j per bulk volume at the initial time;ρm,j is the mineral molar density;cf is the rock compressibility;p* is the reference pressure.
- The aqueous formate solution used in this study considered the formulation of Wang et al. [21], with a formate concentration of 30 wt% in brine, with a total salinity of 468,333 ppm (Na+: 159,236 ppm; Cl−: 9097 ppm; HCOO−: 300,000 ppm) and pH adjusted to 7. The resulting density of this formate solution is around 2300 kg/m3 with a viscosity of about 3 cP at the real average reservoir pressure and temperature conditions, as will be detailed in the following sections.
3. Case Study 1: Synthetic Model
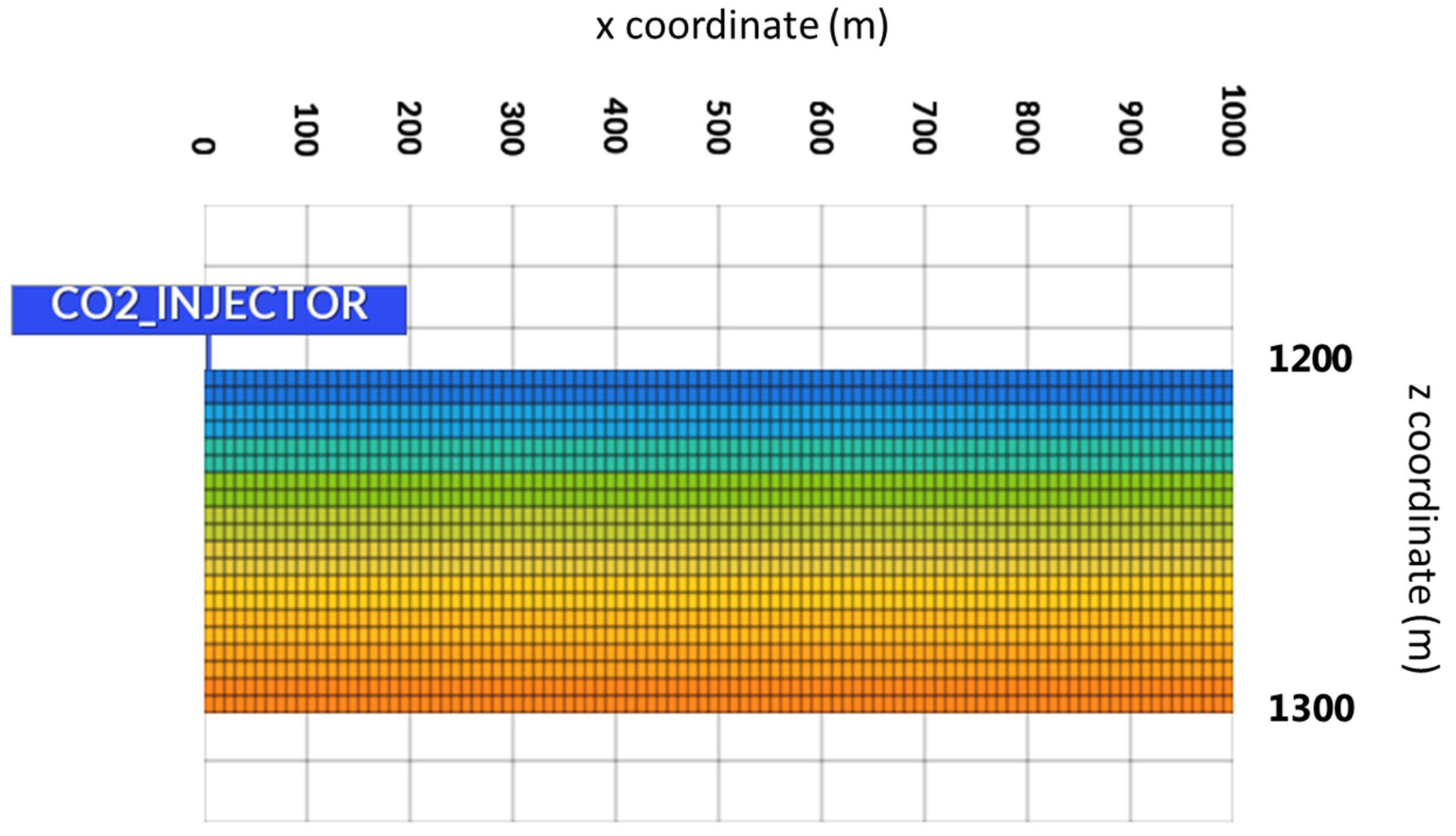
3.1. Geological Model
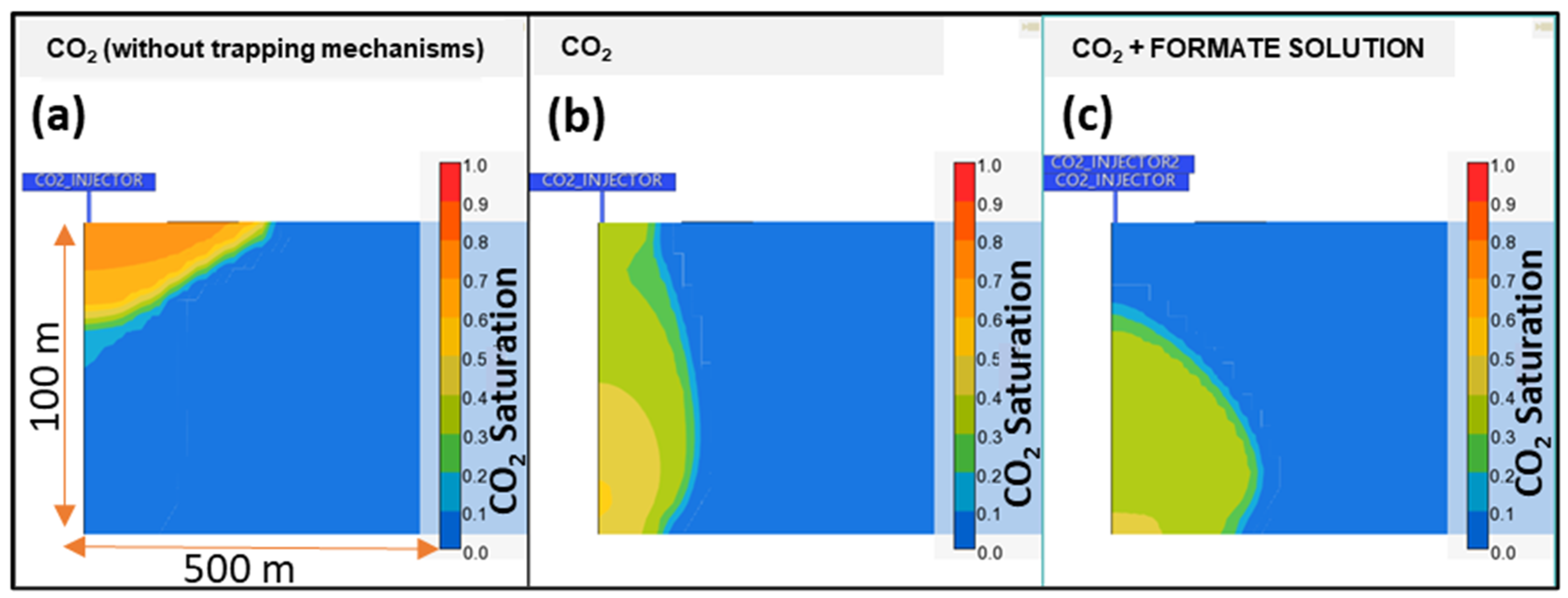
3.2. Results
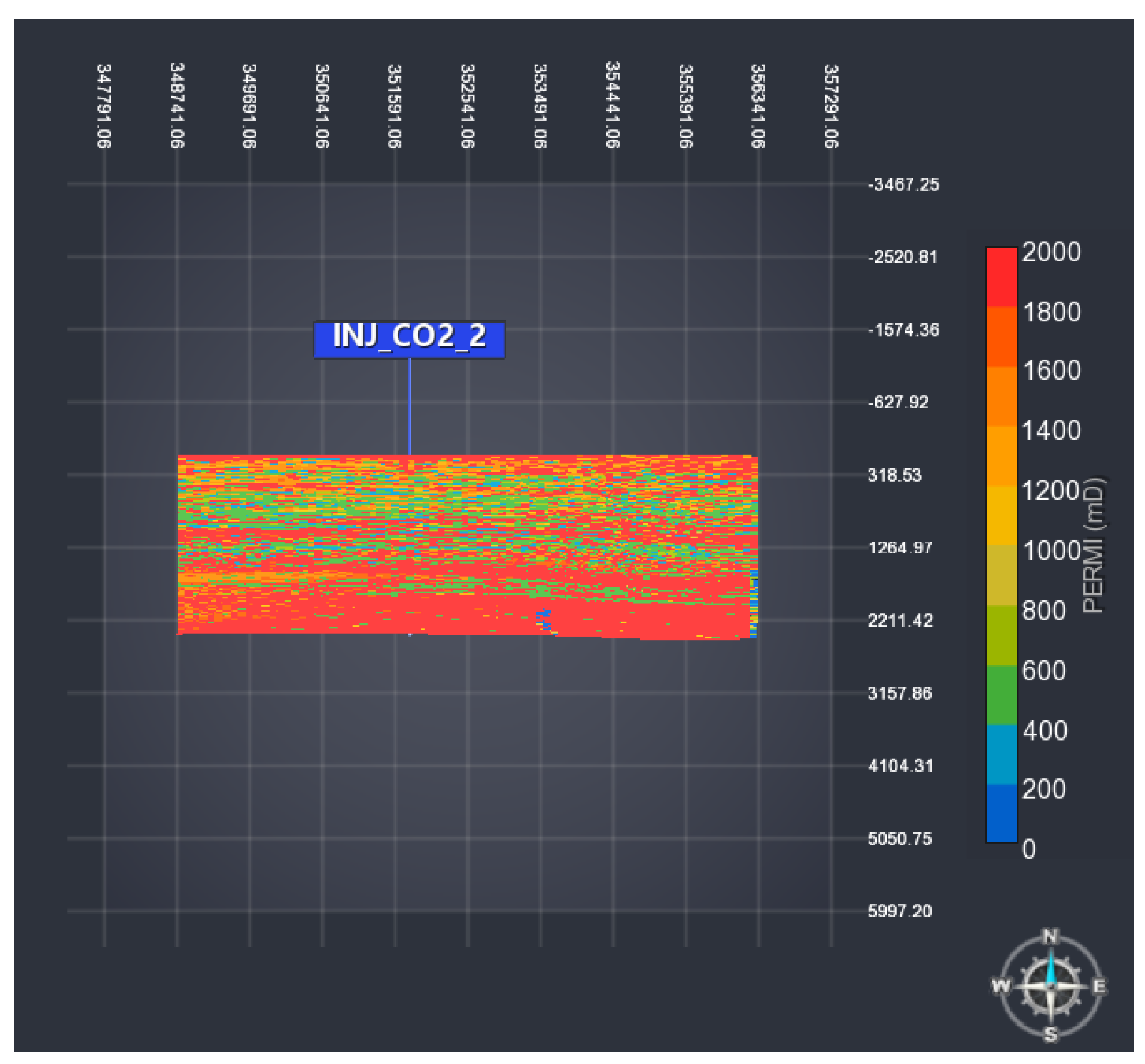
4. Case Study 2: Real Aquifer
4.1. Geological Model
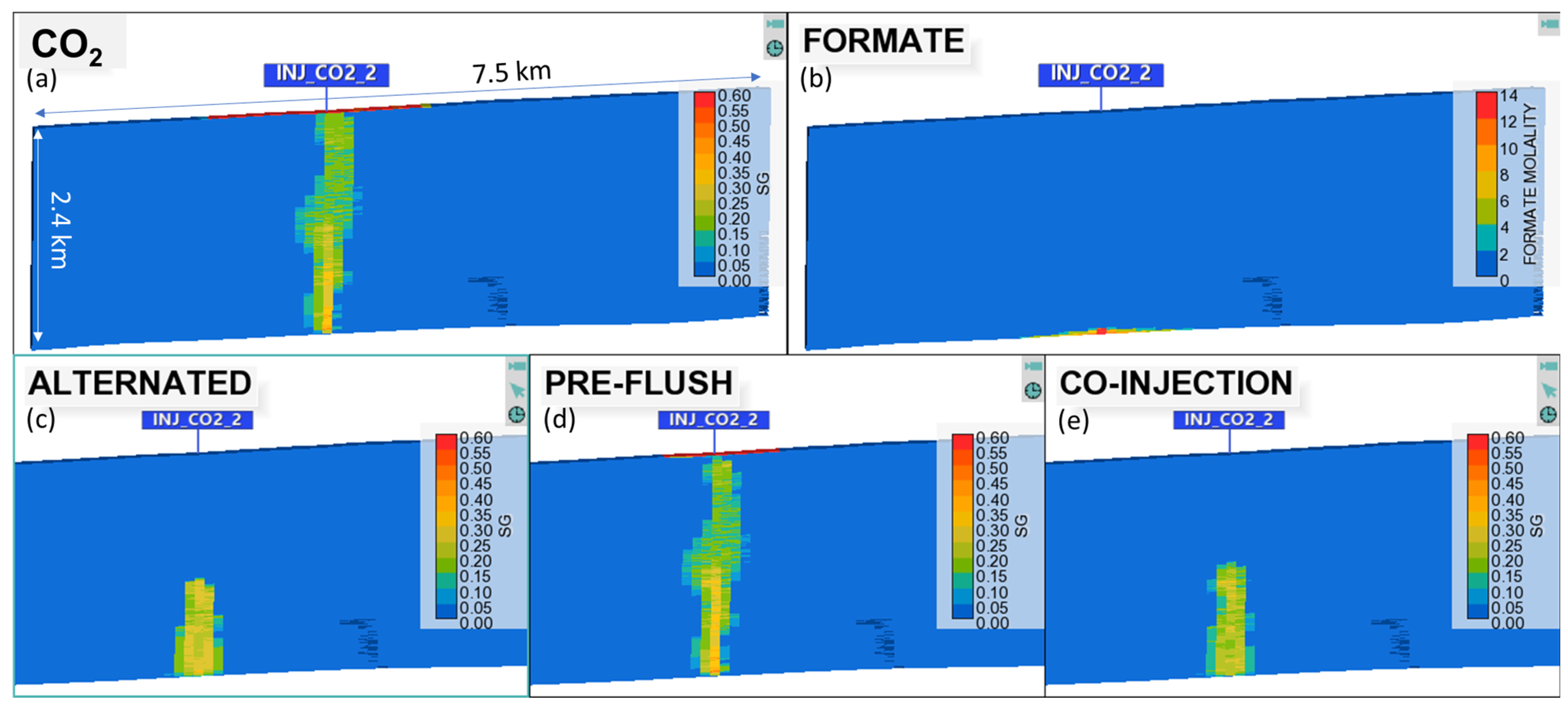
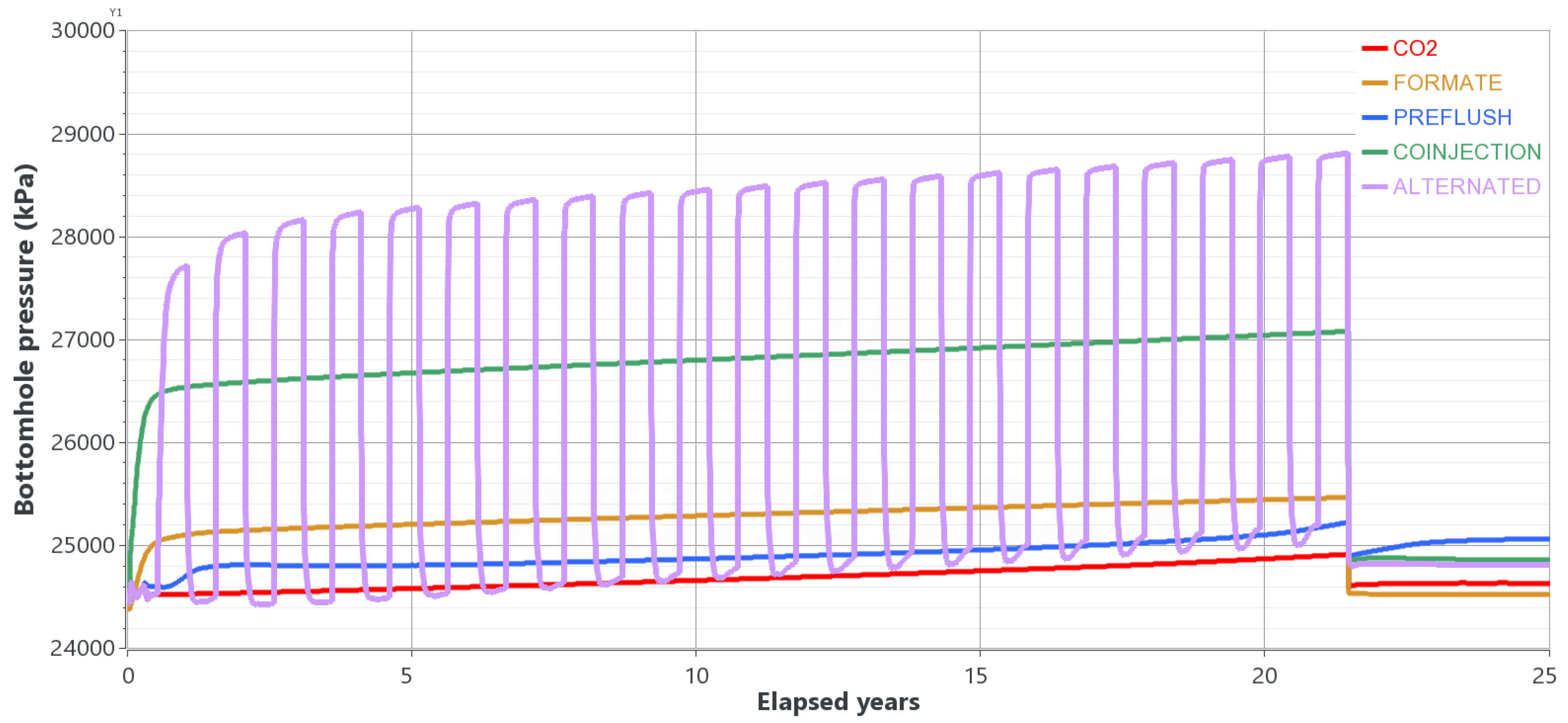
4.2. Results
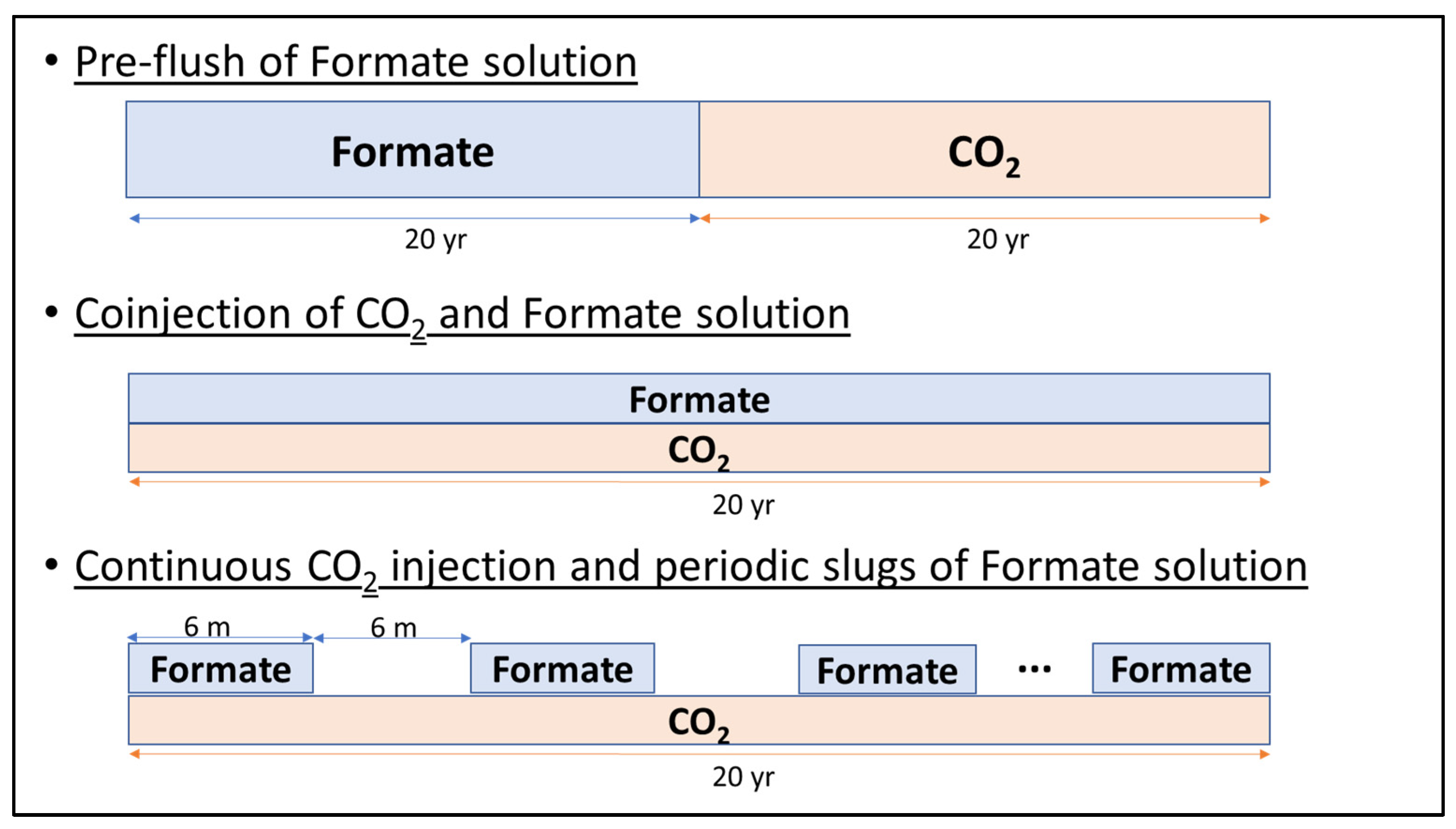
- Start injecting the same volume of CO2 and formate solution. In this case, 1.5 million metric tons/yr of CO2 was assumed based on the field project design;
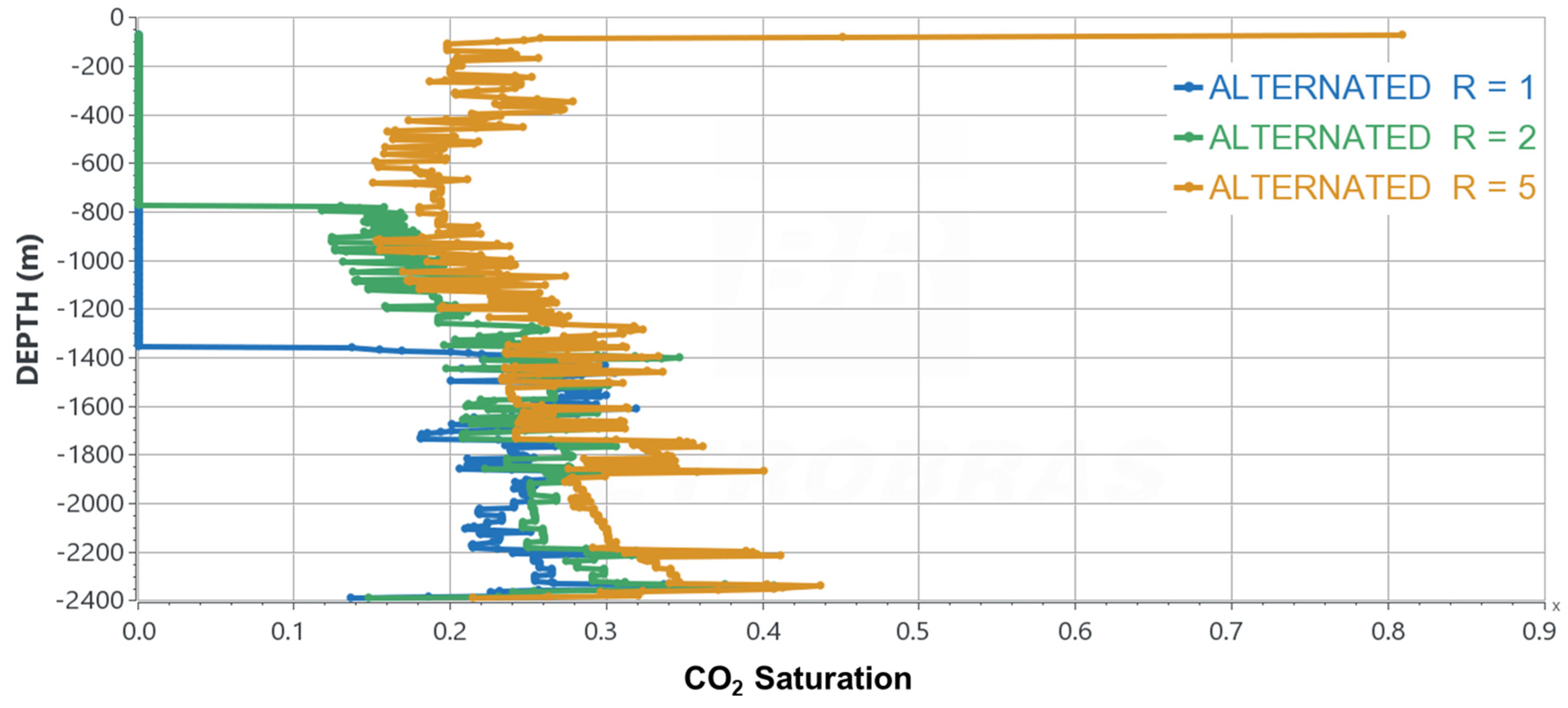
- Evaluate different CO2/formate volumetric ratios (R);
- With the best R-value, test different hybrid strategies: (i) pre-flush of formate preceding the CO2 injection, called “pre-flush”; (ii) simultaneous and continuous injections of both fluids, called “co-injection”; (iii) continuous CO2 injection and alternating and simultaneous formate injection with 6-month slugs, called “alternated.” Figure 9 illustrates the different injection strategies.
4.3. Sensitivity to the Formate Volume
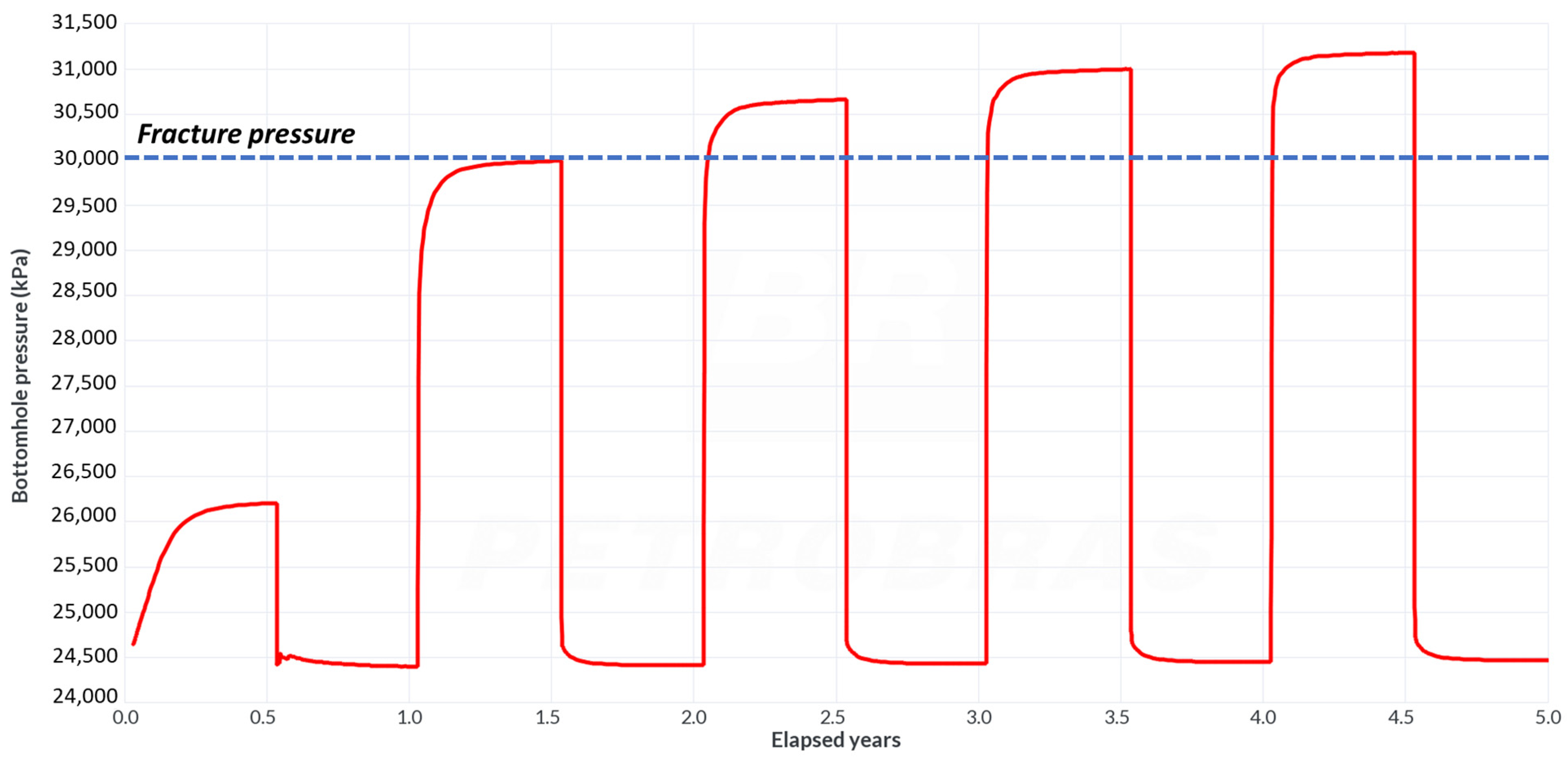
4.4. Sensitivity to Rock Permeability
5. Conclusions
- The co-injection of an aqueous formate solution could make the gravity-dominant regime less intense during CO2 injection for carbon storage purposes, especially when the CO2 and formate solution volumes are approximately the same;
- Three injection strategies were evaluated against the single fluid injection (only CO2 or formate). The best strategies combine the simultaneous injection of the two fluids, either continuous or alternated slugs of formate solution. Both cases prevent a CO2 plume from rising to the surface and keep the plume stationary over hundreds of years of redistribution;
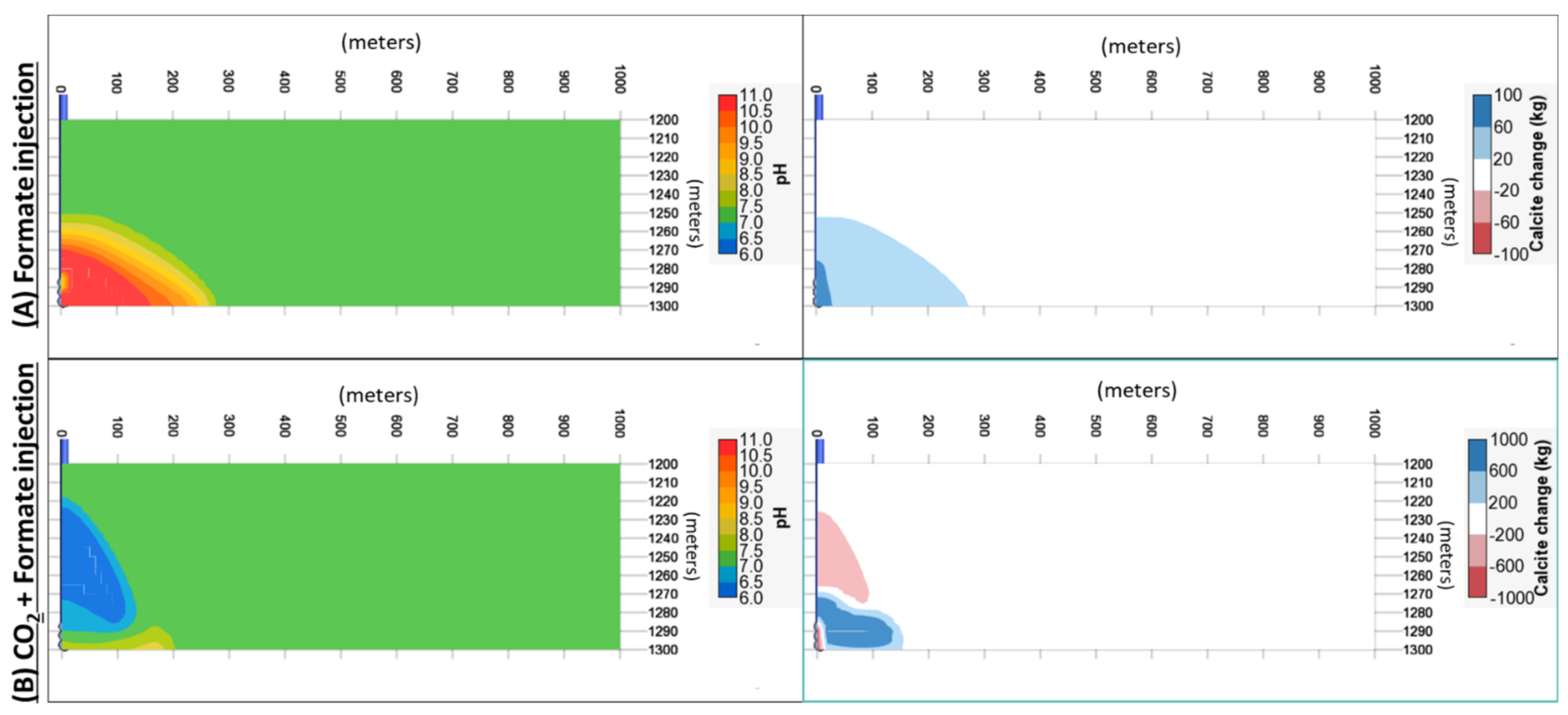
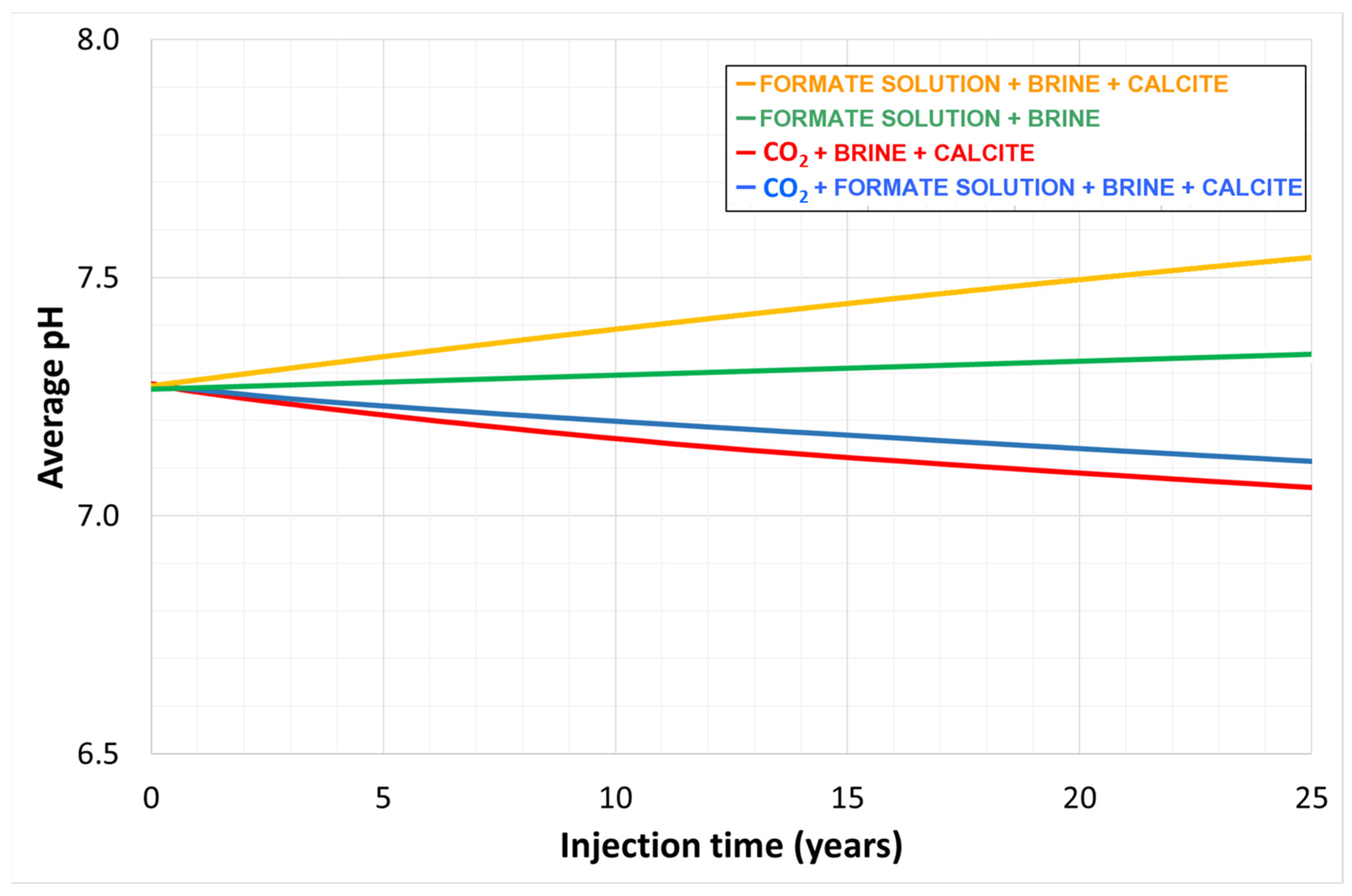
- A secondary benefit of the formate solution is the pH buffering, which results in a suppressed change in pH when CO2 is injected. This mechanism induced calcite precipitation from brines in the studied case. Thus, it could be an additional and permanent CO2 trapping mechanism in this buffer zone in carbonate rocks.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| C | Land’s constant, dimensionless |
| cf | rock compressibility, kPa−1 |
| D | diffusion coefficient, cm²/s |
| Deff | effective diffusion coefficient, cm²/s |
| k | current absolute permeability, mD [9.869 × 10−4 μm2] |
| Henry’s constant at current pressure (p) and temperature (T), dimensionless | |
| Henry’s constant at reference pressure (p*) and temperature (T), dimensionless | |
| Nj | the total moles of mineral j, gmol/m³ |
| p | pressure, kPa |
| R | universal gas constant, 8.314 kPa·L/mol·K |
| rf | resistance factor, dimensionless |
| Sgt | trapped gas saturation, dimensionless |
| Sg max | maximum gas saturation, dimensionless |
| partial molar volume at infinite dilution, L/mol | |
| Greek Symbols | |
| current porosity | |
| ρm | mineral molar density, gmol/m3 |
| density, kg/m³ | |
| τ | tortuosity, dimensionless |
References
- Birol, D.F. World Energy Outlook 2022; IEA Publications: Paris, France, 2022. [Google Scholar]
- Delshad, M.; Kong, X.; Tavakoli, R.; Hosseini, S.A.; Wheeler, M.F. Modeling and Simulation of Carbon Sequestration at Cranfield Incorporating New Physical Models. Int. J. Greenh. Gas Control. 2013, 18, 463–473. [Google Scholar] [CrossRef]
- Han, W.S.; McPherson, B.J.; Lichtner, P.C.; Wang, F.P. Evaluation of Trapping Mechanisms in Geologic CO2 Sequestration: Case Study of SACROC Northern Platform, a 35-Year CO2 Injection Site. Am. J. Sci. 2010, 310, 282–324. [Google Scholar] [CrossRef]
- Nghiem, L.; Shrivastava, V.; Kohse, B.; Hassam, M.; Yang, C. Simulation of Trapping Processes for CO2 Storage in Saline Aquifers. In Proceedings of the Canadian International Petroleum Conference, Calgary, AB, Canada, 16–18 June 2009; Petroleum Society of Canada: Calgary, AB, Canada, 2009. [Google Scholar]
- Rackley, S.A. Carbon Capture and Storage, 2nd ed.; Butterworth-Heinemann: Cambridge, MA, USA, 2017; ISBN 978-0-12-812041-5. [Google Scholar]
- Duan, Z.; Sun, R. An Improved Model Calculating CO2 Solubility in Pure Water and Aqueous NaCl Solutions from 273 to 533 K and from 0 to 2000 Bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Portier, S.; Rochelle, C. Modelling CO2 Solubility in Pure Water and NaCl-Type Waters from 0 to 300 °C and from 1 to 300 Bar. Chem. Geol. 2005, 217, 187–199. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Zitha, P.L.J.; Bruining, J. Enhanced Mass Transfer of CO2 into Water: Experiment and Modeling. Ind. Eng. Chem. Res. 2009, 48, 6423–6431. [Google Scholar] [CrossRef]
- Neufeld, J.A.; Hesse, M.A.; Riaz, A.; Hallworth, M.A.; Tchelepi, H.A.; Huppert, H.E. Convective Dissolution of Carbon Dioxide in Saline Aquifers. Geophys. Res. Lett. 2010, 37, 22. [Google Scholar] [CrossRef]
- Taheri, A.; Torsæter, O.; Lindeberg, E.; Hadia, N.J.; Wessel-Berg, D. Effect of Convective Mixing Process on Storage of CO2 in Saline Aquifers with Layered Permeability. Adv. Chem. Res. 2021, 3, 1–21. [Google Scholar] [CrossRef]
- Amarasinghe, W.; Farzaneh, S.; Fjelde, I.; Sohrabi, M.; Guo, Y. A Visual Investigation of CO2 Convective Mixing in Water and Oil at the Pore Scale Using a Micromodel Apparatus at Reservoir Conditions. Gases 2021, 1, 53–67. [Google Scholar] [CrossRef]
- Elenius, M.T.; Voskov, D.V.; Tchelepi, H.A. Interactions between Gravity Currents and Convective Dissolution. Adv. Water Resour. 2015, 83, 77–88. [Google Scholar] [CrossRef]
- Bachu, S. CO2 Storage in Geological Media: Role, Means, Status and Barriers to Deployment. Prog. Energy Combust. Sci. 2008, 34, 254–273. [Google Scholar] [CrossRef]
- Xu, T.; Yue, G.; Wang, F.; Liu, N. Using Natural CO2 Reservoir to Constrain Geochemical Models for CO2 Geological Sequestration. Appl. Geochem. 2014, 43, 22–34. [Google Scholar] [CrossRef]
- Hovorka, S. Optimization of Geological Environments for Carbon Dioxide Disposal in Saline Aquifers in the United States (Part One); University of Texas: Austin, TX, USA, 2008; p. 990445. [Google Scholar]
- Bump, A.P.; Bakhshian, S.; Ni, H.; Hovorka, S.D.; Olariu, M.I.; Dunlap, D.; Hosseini, S.A.; Meckel, T.A. Composite Confining Systems: Rethinking Geologic Seals for Permanent CO2 Sequestration. Int. J. Greenh. Gas Control. 2023, 126, 103908. [Google Scholar] [CrossRef]
- Wasik, D.O.; Polat, H.M.; Ramdin, M.; Moultos, O.A.; Calero, S.; Vlugt, T.J.H. Solubility of CO2 in Aqueous Formic Acid Solutions and the Effect of NaCl Addition: A Molecular Simulation Study. J. Phys. Chem. C 2022, 126, 19424–19434. [Google Scholar] [CrossRef] [PubMed]
- Oyenowo, O.P.; Wang, H.; Okuno, R.; Mirzaei-Paiaman, A.; Sheng, K. Geochemical Impact on Rock Wettability in Injection of High-Concentration Formate Solution for Enhanced Geologic Carbon Storage and Oil Recovery. In Proceedings of the SPE International Conference on Oilfield Chemistry, The Woodlands, TX, USA, 28–29 June 2023; p. D021S010R005. [Google Scholar]
- Oyenowo, O.P.; Sheng, K.; Abeykoon, G.A.; Argüelles-Vivas, F.J.; Okuno, R. A Case Study of Using Aqueous Formate Solution for Carbon Sequestration and Geological Storage. GeoGulf Trans. 2021, 71, 203–215. [Google Scholar]
- Baghishov, I.; Abeykoon, G.A.; Wang, M.; Oyenowo, O.P.; Argüelles-Vivas, F.J.; Okuno, R. A Mechanistic Comparison of Formate, Acetate, and Glycine as Wettability Modifiers for Carbonate and Shale Formations. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129849. [Google Scholar] [CrossRef]
- Wang, H.; Precious Oyenowo, O.; Okuno, R. Aqueous Formate Solution for Enhanced Water Imbibition in Oil Recovery and Carbon Storage in Carbonate Reservoirs. Fuel 2023, 345, 128198. [Google Scholar] [CrossRef]
- Oyenowo, O.P.; Wang, H.; Mirzaei-Paiaman, A.; Carrasco-Jaim, O.A.; Sheng, K.; Okuno, R. Geochemical Impact of High-Concentration Formate Solution Injection on Rock Wettability for Enhanced Oil Recovery and Geologic Carbon Storage. Energy Fuels 2024, 38, 6138–6155. [Google Scholar] [CrossRef]
- ChemAnalyst. Formic Acid Market Analysis; ChemAnalyst: Noida, India, 2023. [Google Scholar]
- Xu, D.; Li, K.; Jia, B.; Sun, W.; Zhang, W.; Liu, X.; Ma, T. Electrocatalytic CO2 Reduction towards Industrial Applications. Carbon Energy 2023, 5, e230. [Google Scholar] [CrossRef]
- Fernández-Caso, K.; Díaz-Sainz, G.; Alvarez-Guerra, M.; Irabien, A. Electroreduction of CO2: Advances in the Continuous Production of Formic Acid and Formate. ACS Energy Lett. 2023, 8, 1992–2024. [Google Scholar] [CrossRef]
- Gao, T.; Xia, B.; Yang, K.; Li, D.; Shao, T.; Chen, S.; Li, Q.; Duan, J. Techno-Economic Analysis and Carbon Footprint Accounting for Industrial CO2 Electrolysis Systems. Energy Fuels 2023, 37, 17997–18008. [Google Scholar] [CrossRef]
- Ramdin, M.; Morrison, A.R.T.; De Groen, M.; Van Haperen, R.; De Kler, R.; Irtem, E.; Laitinen, A.T.; Van Den Broeke, L.J.P.; Breugelmans, T.; Trusler, J.P.M.; et al. High-Pressure Electrochemical Reduction of CO2 to Formic Acid/Formate: Effect of pH on the Downstream Separation Process and Economics. Ind. Eng. Chem. Res. 2019, 58, 22718–22740. [Google Scholar] [CrossRef]
- Yang, H.; Kaczur, J.J.; Sajjad, S.D.; Masel, R.I. Performance and Long-Term Stability of CO2 Conversion to Formic Acid Using a Three-Compartment Electrolyzer Design. J. CO2 Util. 2020, 42, 101349. [Google Scholar] [CrossRef]
- Oyenowo, O.P.; Sheng, K.; Okuno, R. Simulation Case Studies of Aqueous Formate Solution for Geological Carbon Storage. Fuel 2023, 334, 126643. [Google Scholar] [CrossRef]
- Dermanaki Farahani, Z.; Khorsand Movaghar, M.R. Improving Oil Recovery Using Miscible Selective Simultaneous Water Alternating Gas (MSSWAG) Injection in One of the Iranian Reservoirs. Arab. J. Sci. Eng. 2018, 43, 2521–2535. [Google Scholar] [CrossRef]
- CMG. GEM Compositional & Unconventional Simulator, version 2022.10; Windows, CMG: Calgary, AB, Canada, 2022. [Google Scholar]
- Ahmadi, H.; Erfani, H.; Jamialahmadi, M.; Soulgani, B.S.; Dinarvand, N.; Sharafi, M.S. Corrigendum to “Experimental Study and Modelling on Diffusion Coefficient of CO2 in Water” Fluid Phase Equilibria 523 (2020) 112,584. Fluid Phase Equilibria 2021, 529, 112869. [Google Scholar] [CrossRef]
- Li, Y.-K.; Nghiem, L.X. Phase Equilibria of Oil, Gas and Water/Brine Mixtures from a Cubic Equation of State and Henry’s Law. Can. J. Chem. Eng. 1986, 64, 486–496. [Google Scholar] [CrossRef]
- Bakker, R.J. Package FLUIDS 1. Computer Programs for Analysis of Fluid Inclusion Data and for Modelling Bulk Fluid Properties. Chem. Geol. 2003, 194, 3–23. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Thorstenson, D.C.; Plummer, L.N. PHREEQE: A Computer Program for Geochemical Calculations; U.S. Geological Survey: Denver, CO, USA, 1980.
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3: A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey Techniques and Methods, Book 6; U.S. Geological Survey: Denver, CO, USA, 2013; p. 497.
- Allison, J.D.; Brown, D.S.; Novo-Gradac, K.J. MINTEQA2/PRODEFA2: A Geochemical Assessment Model for Environmental Systems: Version 3.0 User’s Manual; Environmental Research Laboratory, Office of Research and Development, US Environmental Protection Agency: Washington, DC, USA, 1991. [Google Scholar]
- Zeidouni, M.; Pooladi-Darvish, M.; Keith, D. Analytical Solution to Evaluate Salt Precipitation during CO2 Injection in Saline Aquifers. Int. J. Greenh. Gas Control. 2009, 3, 600–611. [Google Scholar] [CrossRef]
- Machado, M.V.B.; Delshad, M.; Sepehrnoori, K. A Practical and Innovative Workflow to Support the Numerical Simulation of CO2 Storage in Large Field-Scale Models. SPE Reserv. Eval. Eng. 2023, 26, 1541–1552. [Google Scholar] [CrossRef]
- Machado, M.V.B.; Delshad, M.; Sepehrnoori, K. Modeling Self-Sealing Mechanisms in Fractured Carbonates Induced by CO2 Injection in Saline Aquifers. ACS Omega 2023, 8, 48925–48937. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.B.; Khanal, A.; Delshad, M. Unveiling the Essential Parameters Driving Mineral Reactions during CO2 Storage in Carbonate Aquifers through Proxy Models. Appl. Sci. 2024, 14, 1465. [Google Scholar] [CrossRef]
- Carlson, F.M. Simulation of Relative Permeability Hysteresis to the Nonwetting Phase; SPE: San Antonio, TX, USA, 1981. [Google Scholar]
- Land, C.S. Calculation of Imbibition Relative Permeability for Two- and Three-Phase Flow from Rock Properties. Soc. Pet. Eng. J. 1968, 8, 149–156. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, T.; Pruess, K. The Effects of Gas-Fluid-Rock Interactions on CO2 Injection and Storage: Insights from Reactive Transport Modeling. Energy Procedia 2009, 1, 1783–1790. [Google Scholar] [CrossRef]
- Brêda, T.C.; Mello, C.L.; Silva Junior, G.C.d.; Alves, M.d.G.; Neiva, E.B. Caracterização Petrográfica Da Formação Emborê Com Base Em Dados Dos Poços 2-CST-1-RJ e 9-BRF-1D-RJ (Região Emersa Da Bacia de Campos). Geol. USP. Sér. Cient. 2018, 18, 45–58. [Google Scholar] [CrossRef]
- PETROBRAS (PETROBRAS, Rio de Janeiro, Brazil). Personal Communication. 2023.
- Agarwal, A.S.; Zhai, Y.; Hill, D.; Sridhar, N. The Electrochemical Reduction of Carbon Dioxide to Formate/Formic Acid: Engineering and Economic Feasibility. ChemSusChem 2011, 4, 1301–1310. [Google Scholar] [CrossRef]
- Hosa, A.; Esentia, M.; Stewart, J.; Haszeldine, S. Injection of CO2 into Saline Formations: Benchmarking Worldwide Projects. Chem. Eng. Res. Des. 2011, 89, 1855–1864. [Google Scholar] [CrossRef]
- Metz, B.; Davidson, O.; de Coninck, H.; Loos, M.; Meyer, L. (Eds.) IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005; ISBN 978-0-521-86643-9.





| Total model pore volume | 180,000 m3 |
| Average horizontal permeability | 100 mD |
| Ratio of vertical/horizontal permeabilities | 0.10 |
| Average porosity | 0.18 |
| Initial pressure @ datum | 8.963 MPa |
| Temperature Initial pH | 41 °C 7.3 |
| CO2 injection rate Aqueous formate solution injection rate | 1.0 metric tons/d 0.65 m3/d |
| Relative permeability curves Capillary pressure curves | Figure 2 Figure 2 |
| Ions | Concentration (ppm) |
|---|---|
| H+ | 1.4872 × 10−5 |
| Ca2+ | 11,307 |
| Na+ | 17,763 |
| Cl− HCO3− | 39,604 425 |
| Ions | Concentration (ppm) |
|---|---|
| H+ | 1.4767 × 10−4 |
| Ca2+ | 998 |
| Mg2+ | 627 |
| Na+ | 27,094 |
| Cl− HCO3− | 42,685 1337 |
| Case | Cumulative CO2 Injected | |
|---|---|---|
| Original Permeability (Mean: 1500 mD) | Reduced Permeability (Mean: 150 mD) | |
| Alternated | 30 million metric tons | 3.10 million metric tons |
| Co-injection | 30 million metric tons | 1.85 million metric tons |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa Machado, M.V.; Delshad, M.; Carrasco Jaim, O.A.; Okuno, R.; Sepehrnoori, K. A Strategy for Enhanced Carbon Storage: A Hybrid CO2 and Aqueous Formate Solution Injection to Control Buoyancy and Reduce Risk. Energies 2024, 17, 2680. https://doi.org/10.3390/en17112680
Barbosa Machado MV, Delshad M, Carrasco Jaim OA, Okuno R, Sepehrnoori K. A Strategy for Enhanced Carbon Storage: A Hybrid CO2 and Aqueous Formate Solution Injection to Control Buoyancy and Reduce Risk. Energies. 2024; 17(11):2680. https://doi.org/10.3390/en17112680
Chicago/Turabian StyleBarbosa Machado, Marcos Vitor, Mojdeh Delshad, Omar Ali Carrasco Jaim, Ryosuke Okuno, and Kamy Sepehrnoori. 2024. "A Strategy for Enhanced Carbon Storage: A Hybrid CO2 and Aqueous Formate Solution Injection to Control Buoyancy and Reduce Risk" Energies 17, no. 11: 2680. https://doi.org/10.3390/en17112680
APA StyleBarbosa Machado, M. V., Delshad, M., Carrasco Jaim, O. A., Okuno, R., & Sepehrnoori, K. (2024). A Strategy for Enhanced Carbon Storage: A Hybrid CO2 and Aqueous Formate Solution Injection to Control Buoyancy and Reduce Risk. Energies, 17(11), 2680. https://doi.org/10.3390/en17112680










