Assessment of the Biogenic Souring in Oil Reservoirs under Secondary and Tertiary Oil Recovery
Abstract
1. Introduction
- Provide a survey of recent studies on the understanding of biogenic reservoir souring, the controlling parameters, and its mitigation;
- Emphasize the importance of mineral scavenging for the retention of H2S in the reservoir based on a theoretical approach as well as laboratory measurements;
- Underline laboratory and numerical modeling efforts, approaches, and success rates, exemplified via two application cases on a waterflooding and a microbial enhanced oil recovery (MEOR) technology project;
- Use of the Monte Carlo method as a pragmatic reservoir modeling approach with the associated uncertainties;
- Present and discuss a workflow that can be used in similar studies to assess the risks of biogenic souring and potential mitigation measures.
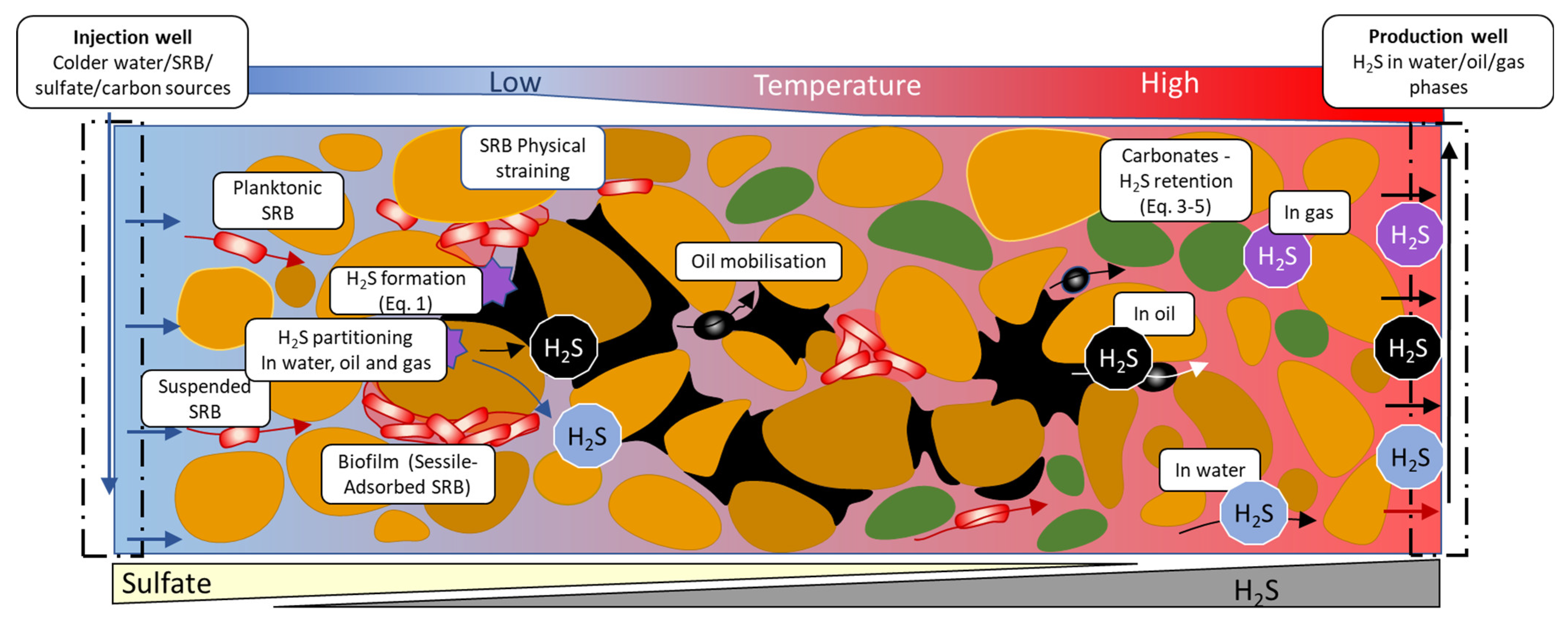
2. Biogenic Souring: Mechanisms, and Impacts on Reservoir–Oil Production
3. Controlling Parameters of the Biogenic Souring in Oil Reservoirs
3.1. Microbiology: SRM in the Reservoir and/or Injected Water
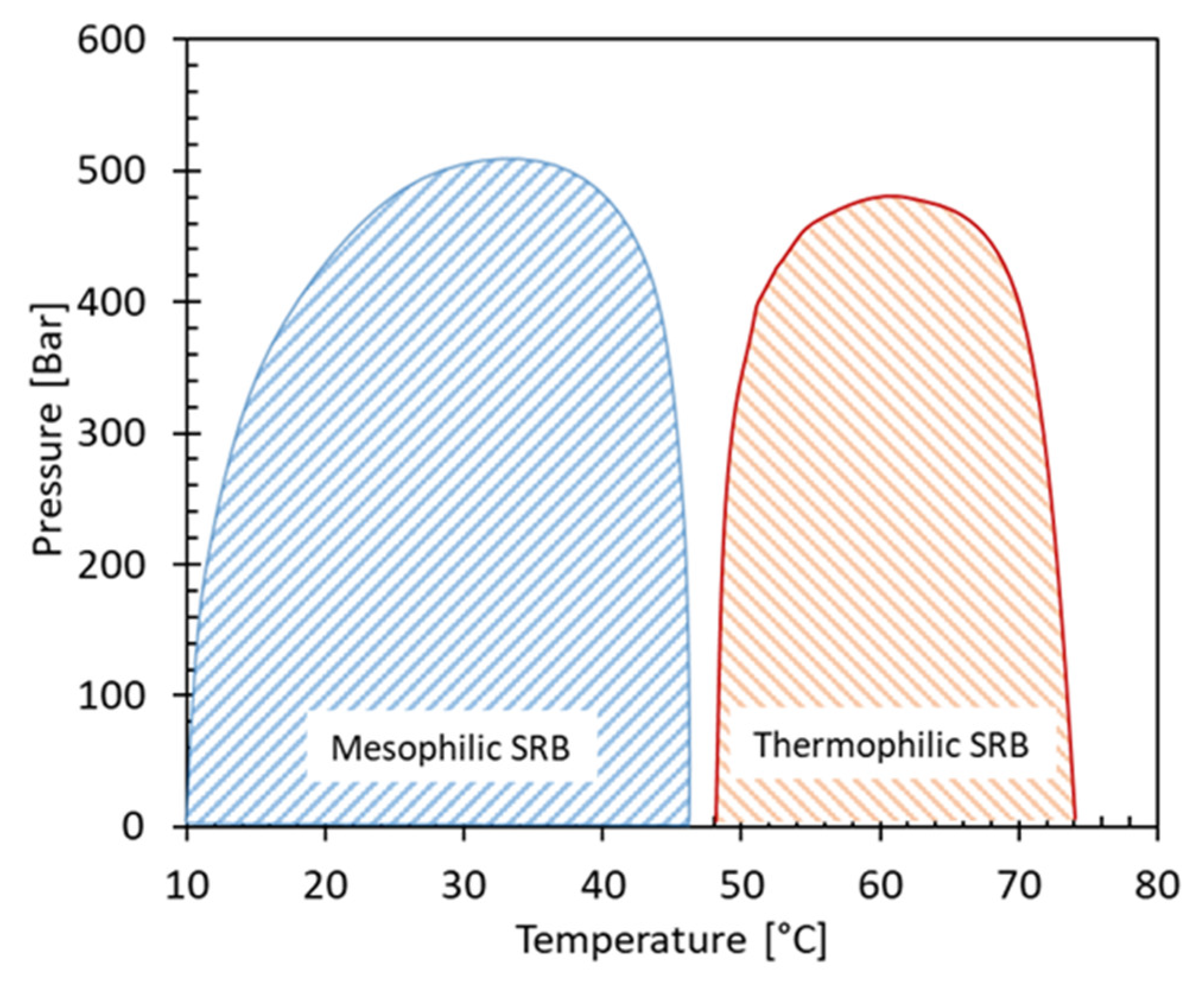
3.2. Pressure and Temperature
3.3. Chemical–Physicochemical Conditions
3.3.1. Source of Carbon, Energy, and Nutrients
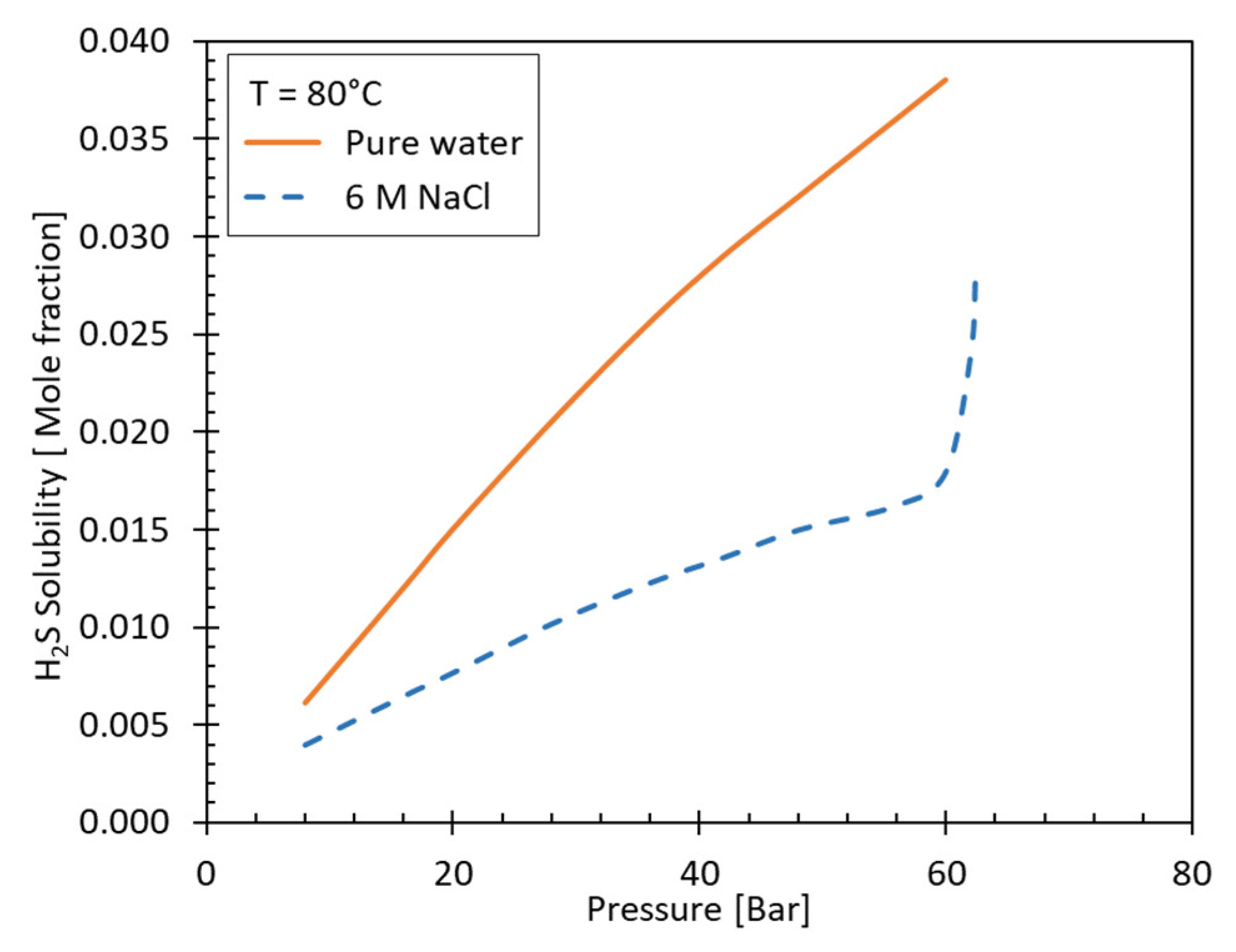
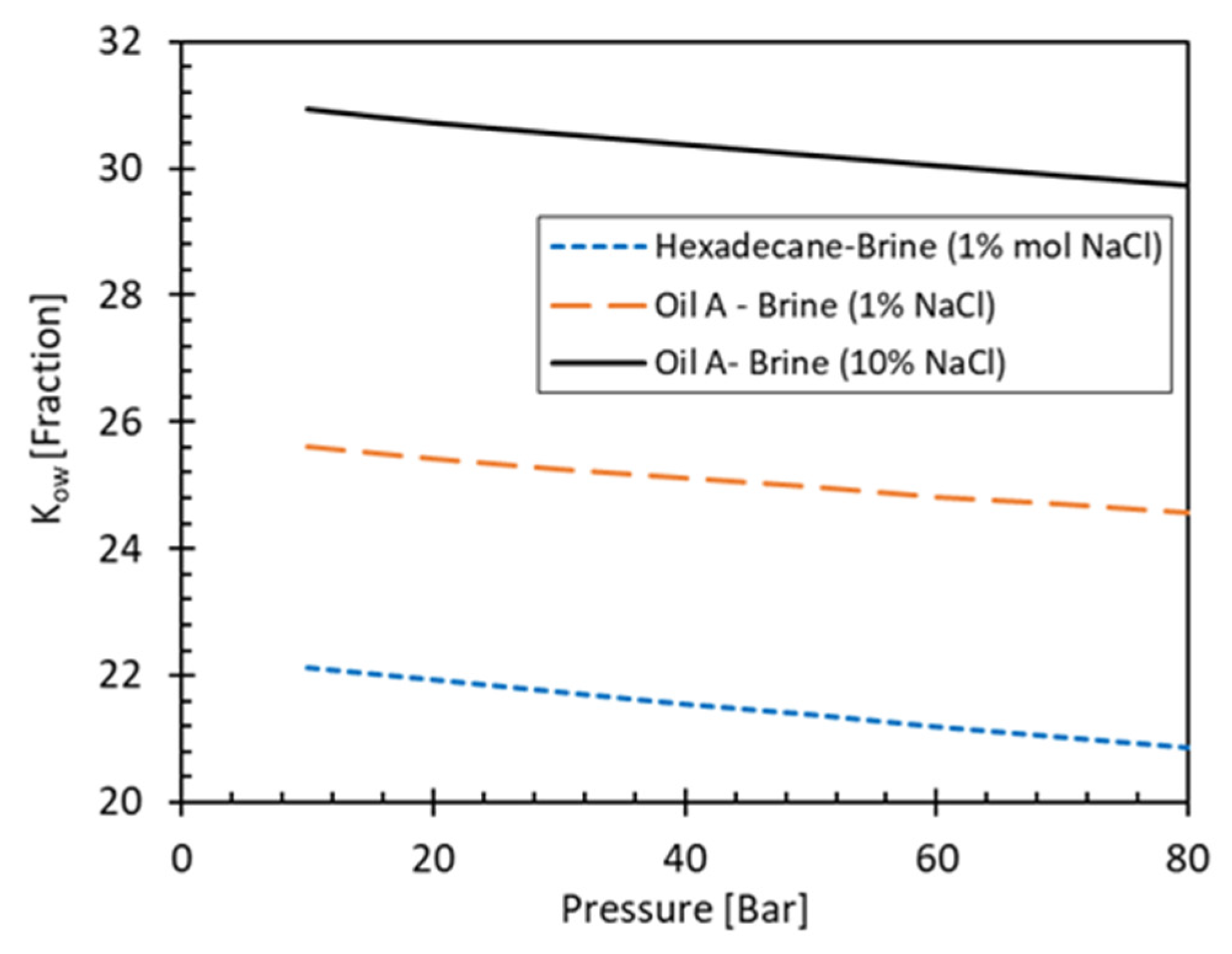
3.3.2. Salinity
3.3.3. Partitioning
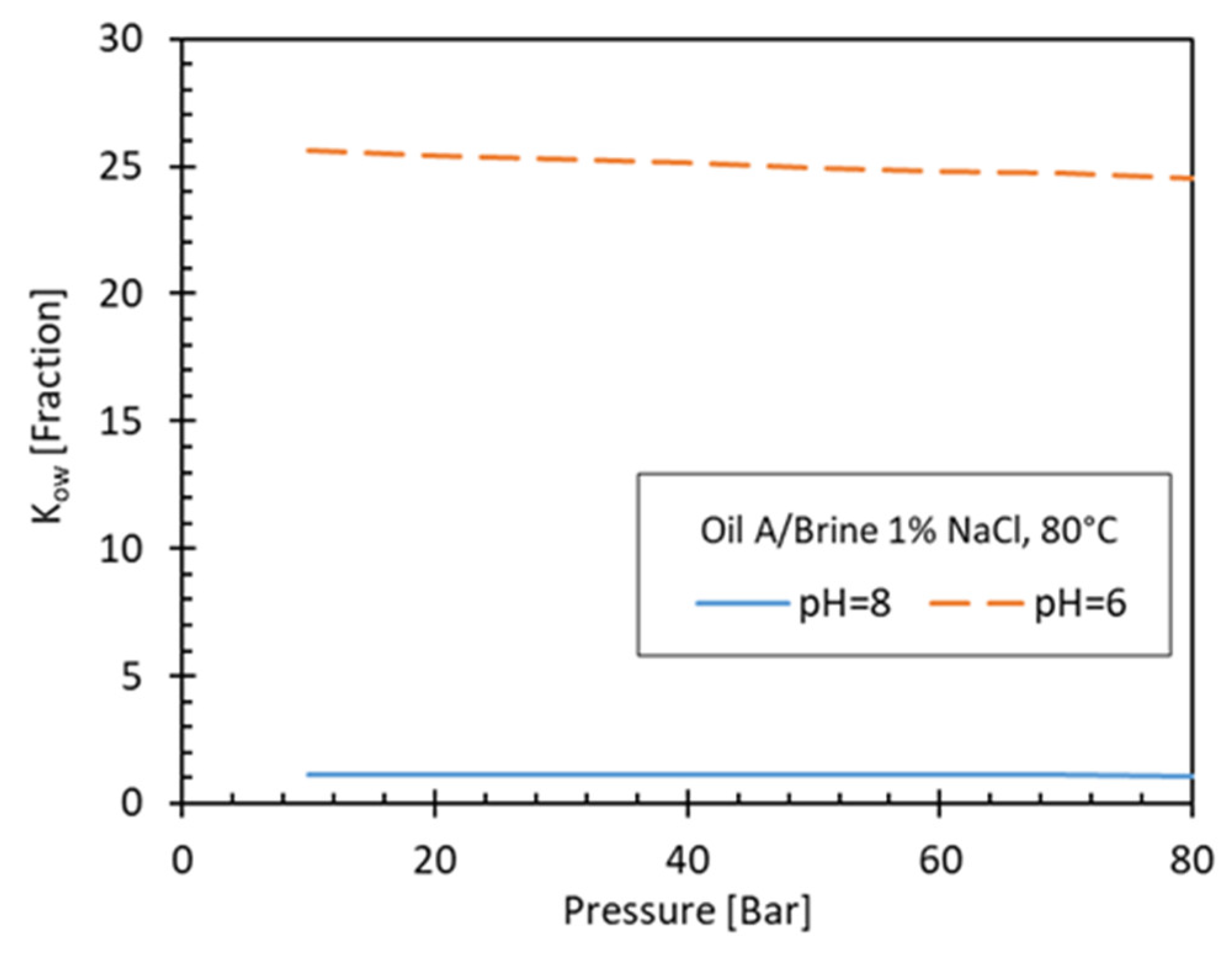
3.3.4. pH
3.3.5. Rock Mineralogy/Formation Water Composition
3.4. Petrophysical Conditions
3.4.1. Permeability
3.4.2. Heterogeneity
4. Laboratory and Numerical Modeling Efforts—Types and Applications
4.1. Conceptual Reservoir Models of Biogenic Souring
4.1.1. Mixing Type Souring Pattern
4.1.2. Biofilm Formation
4.1.3. Thermal Viability Shell (TVS)
4.2. Model Implementations
5. Case Analyses
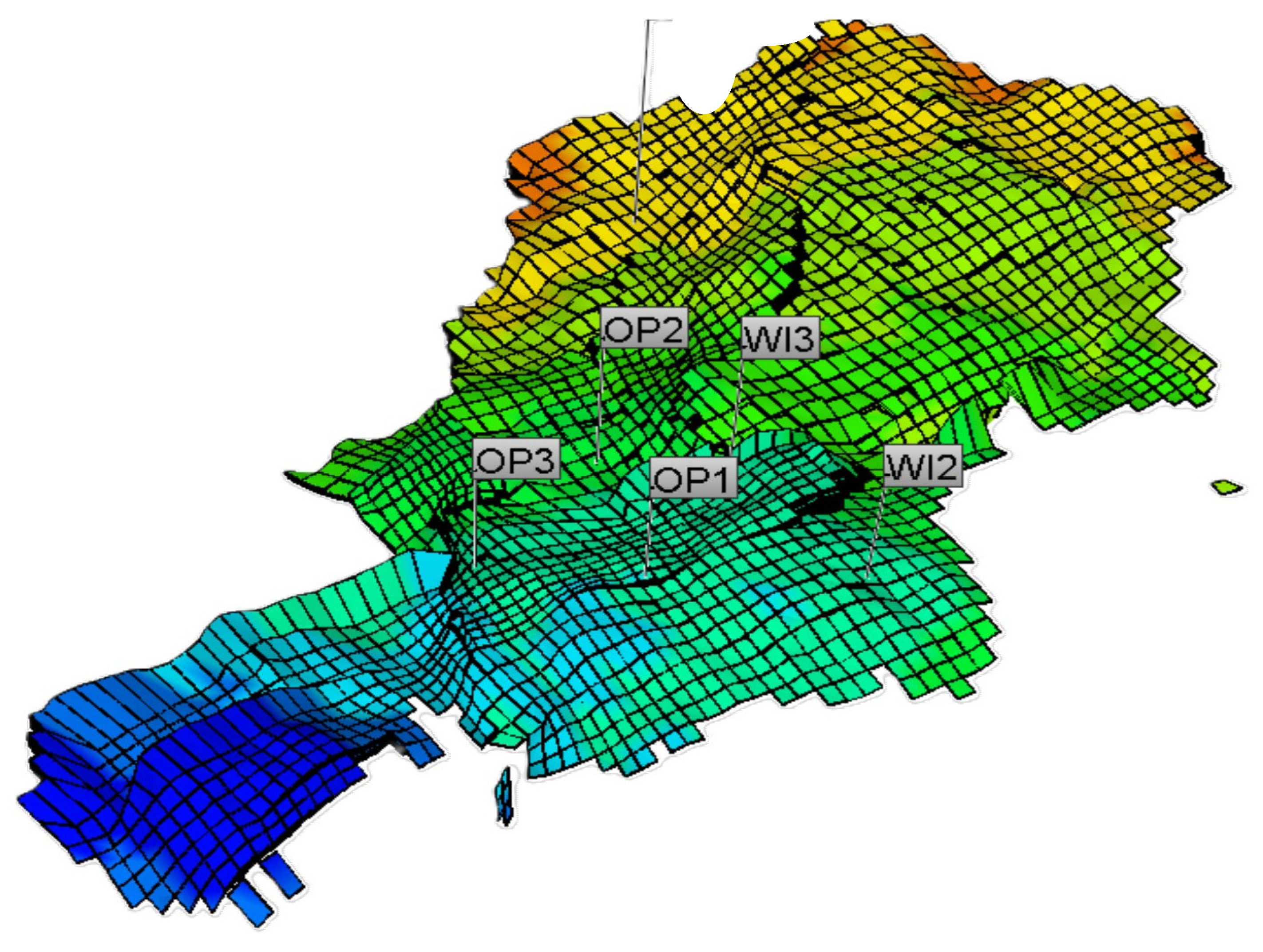
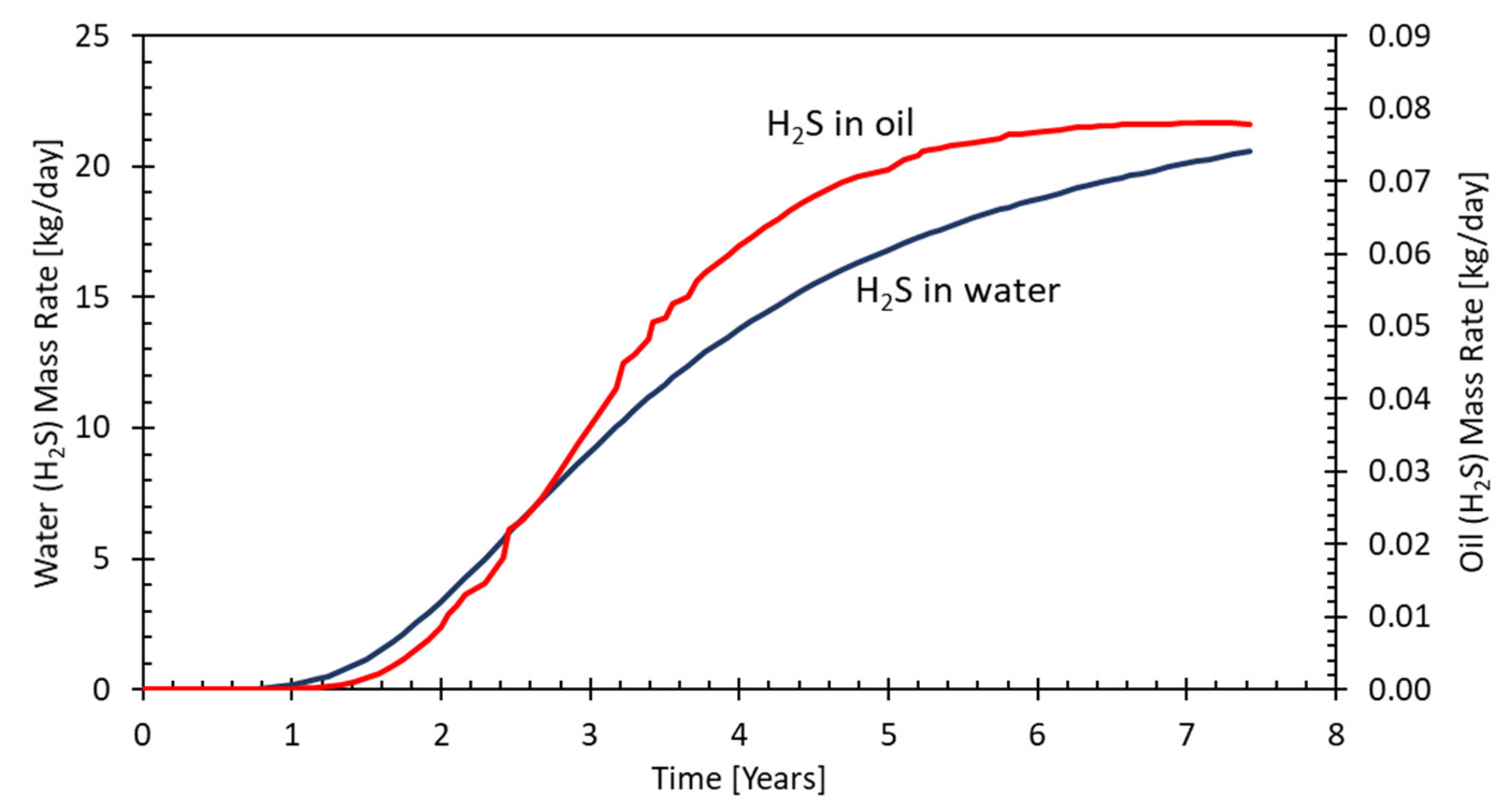
5.1. Case 1: A Field in North Sea under Seawater Flood
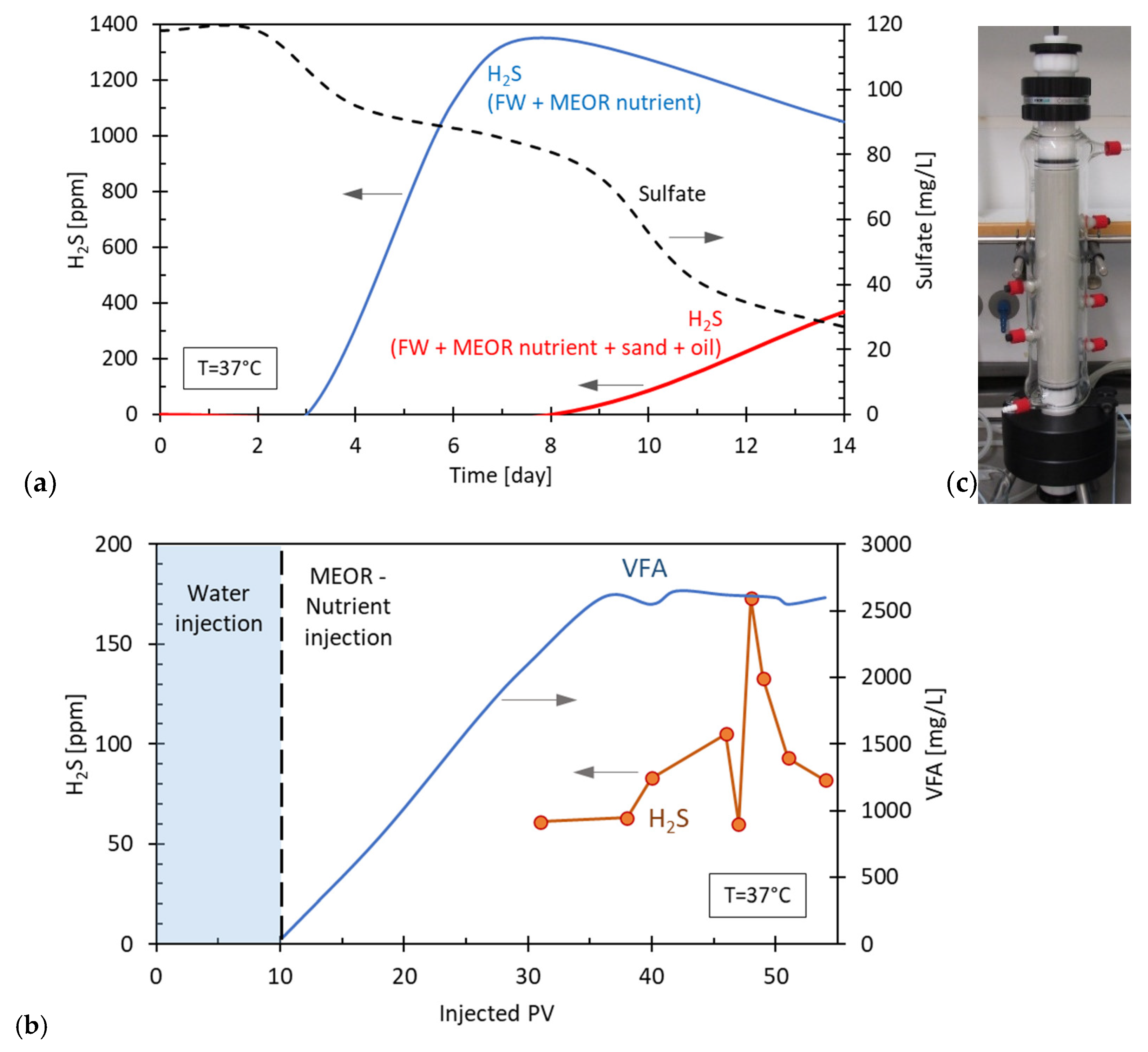
5.2. Case 2: An Onshore Field on a MEOR Application
- -
- Sulfate content of the injection/formation water: The sulfate content of both the injection and formation water can be determined from the water analyses of the injection and production waters. However, if sulfate is introduced via the injection water, and if the formation water does not contain sulfate in the first place, it might be challenging to estimate the timely and spatial distribution of sulfate in the reservoir;
- -
- Amount of substrate available for SRB in the reservoir is a critical parameter and independent of whether SRB are found in injection water and/or found in the reservoir. In the case, of a typical MEOR application, VFAs are generated as the product of in situ fermentation reactions and these are available in excess for the complete reduction of sulfate. In this case, they cannot be used in an uncertainty analysis;
- -
- Partitioning coefficients: The partitioning of the H2S between the phases in the reservoir as well as in the wellbore can be determined well if the compositions and thermodynamic conditions are known. Yet, a careful examination of the existing literature implies that there is still a margin of uncertainties, especially for the oil/water partitioning coefficients.
6. Mitigation of Biogenic Souring
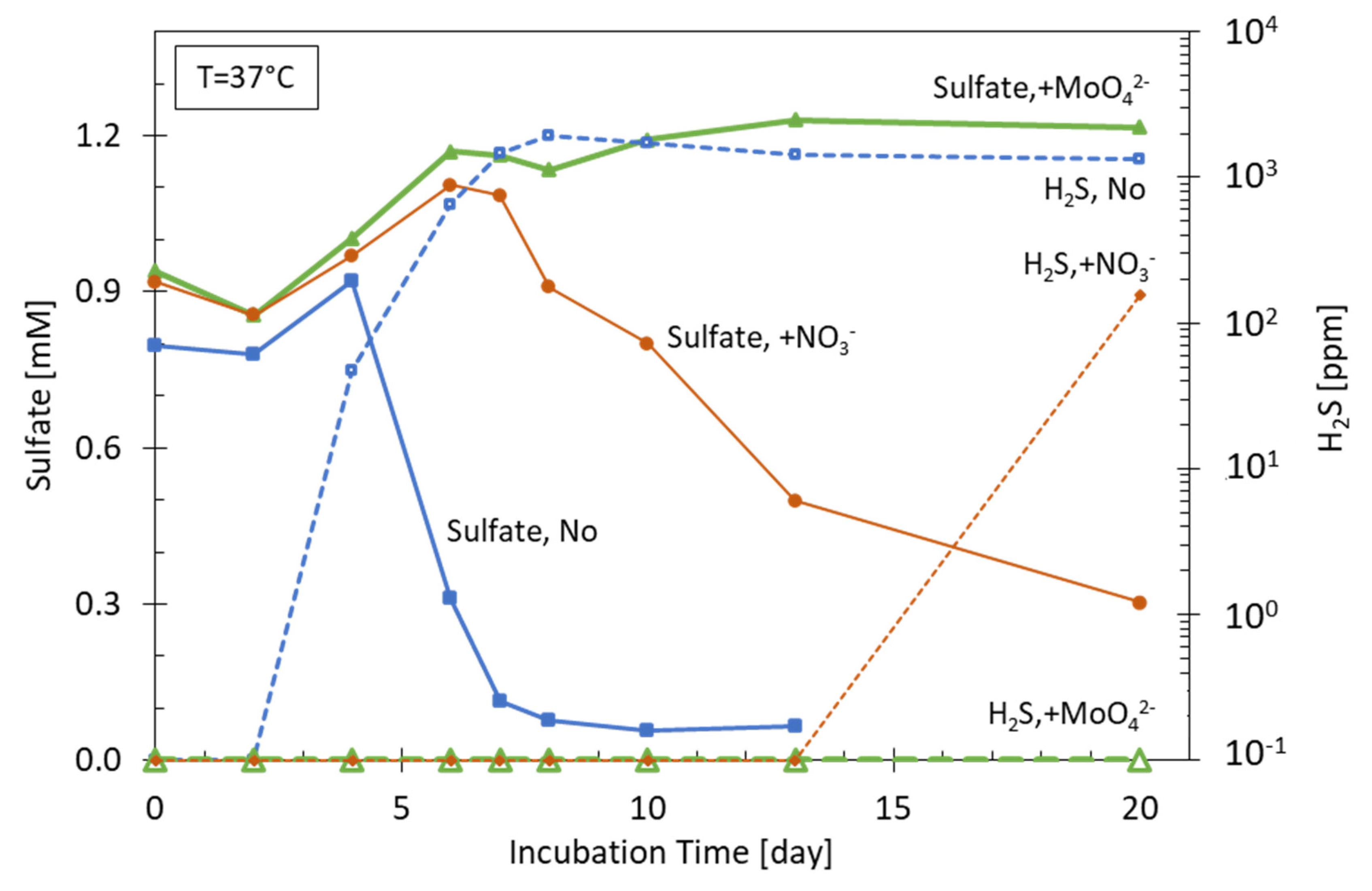
6.1. Nitrate/Nitrite
- -
- The fundamental inhibition mechanisms are well understood, but the complex biological, biochemical, and abiotic interactions in a reservoir cannot be predicted fully. In recent studies [56], the inhibitory effect previously claimed to be due to nitrate injection on the Gullfaks field could be assigned to mixing and biofilm models instead and was potentially not related to the nitrate application;
- -
- Continuous and well-defined dosages of high nitrate concentrations are needed for direct inhibition (>10 mM). A sub-optimal or insufficient nitrate treatment strategy may result in higher H2S production from production wells despite a lower total amount of generated H2S inside the reservoir; but unconsumed nitrate in the case of excessive nitrate injection could increase the need for post-production water treatment [106];
- -
- -
- Also, depending on the composition of the reservoir brine (e.g., presence of dissolved calcite and/or iron) various types of mineral precipitation can be observed. Nitrite injected or formed as a fist intermediate during nitrate injection is very reactive towards Fe(II) and can lead to Fe(II) oxidation and the subsequent formation of low-soluble Fe(III) compounds. Concurrent to iron reduction, calcite minerals can be precipitated; the precipitation of calcite during bacterial activity is known as microbially induced calcite precipitation (MICP). Precipitation of minerals in porous media decreases the porosity and permeability, thus decreasing the injection-production capacity of the reservoir. The mechanism of calcite precipitation during anaerobic denitrification is not completely understood, but it is supposed to be a combination of several effects including a local pH increase, the production of CO2 (see Equations (2)–(4)), the degradation of Ca2+-complexing organic acids, and enhanced nucleation via exopolymeric substances (EPS) [108];
- -
6.2. Molybdate
6.3. Perchlorate
6.4. Other Inhibitors
6.5. Limits and Perspectives
7. Workflow for the Assessment of Biogenic Souring
7.1. Step 1: Preliminary Assessment
7.2. Step 2: Modeling and Quantification
7.3. Step 3: Monitoring
8. Conclusions
- -
- Technological developments in microbiology and petroleum industry simulation tools and techniques provide a sufficiently good analytical background for the prediction of H2S formation and its spatial and temporal distribution in the reservoir. The predictive capability depends heavily on the reliability and representativeness of the model created. The process of calibrating the simulation models requires high-quality, reliable data from theoretical work, chemical–physical modeling, and field measurements;
- -
- H2S retention in the reservoir can be significant if reservoir rock contains iron-bearing minerals like siderite; as shown in a MEOR case analysis, even minor siderite content can inhibit the H2S generation totally;
- -
- Considering the uncertainties in the associated inputs as well as in the geological unknowns, the use of the Monte Carlo method is suggested for numerical evaluation, where the uncertainties of each associated parameter can be considered to define the H2S production in terms of a probability distribution. State-of-the-art commercial reservoir simulators provide user friendly interactive codes for such analysis;
- -
- Nitrate and nitrite are the best-known and best-studied chemicals for biogenic souring inhibition, although their reported effectiveness in field applications is controversial. Molybdate and perchlorate are the emerging candidates for SRB inhibition, but further investigation is needed for environmentally friendly, safe, and economical applications;
- -
- A workflow for assessing and managing biogenic souring in oil reservoirs during secondary and tertiary oil recovery is presented. The workflow provides a continuous cyclic process to improve the quality of the assessment and decisions based on new laboratory and field data in each cycle;
- -
- Further research is required for environmentally friendly, safe, and economical applications. This includes advanced testing of existing inhibitors and the development of new chemicals that can effectively combat reservoir souring and its severe impact on production. Easy-to-use analytical methods to detect the chemicals produced and using them to calibrate numerical simulation codes are essential. Reports on field applications to monitor H2S production in operations would certainly support the research work.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| avr | Average |
| BAC | Benzalkonium chloride |
| BTEX | Benzene, toluene, ethylbenzene, and xylene (light aromatics) |
| DOC | Dissolved organic carbon |
| DP | Dykstra–Parsons coefficient |
| EIA | Environmental impact assessment |
| Eq. | Equation |
| FW | Formation water |
| HSE | Health, safety, and environment |
| k | Permeability, mD |
| Kow,og,wg | Partitioning coefficient (of H2S), oil–water, oil–gas, water–gas, and fraction |
| KS | Monod equation; half rate constant at which the rate is rmax/2, g/L |
| m | Milli |
| M | Molar |
| MEOR | Microbial enhanced oil recovery |
| MIC | Microbially induced corrosion |
| MICP | Microbial-induced calcite precipitation |
| MPN | Most probable number |
| NRB | Nitrate-reducing bacteria |
| P | Pressure, bar |
| P10 | Monte Carlo simulation, low-probability case |
| P50 | Monte Carlo simulation, mean-probability case |
| P90 | Monte Carlo simulation, high-probability case |
| ppmv | Parts per million in gas (vapor) phase |
| ppmw | Parts per million in water phase |
| PV | Pore volume, m3 |
| PWRI | Produced water re-injection |
| qFISH | Quantitative Fluorescent In Situ Hybridization |
| qPCR | Quantitative Polymerase Chain Reaction |
| rg | Monod equation; specific growth rate, hour−1 |
| RINC | Required inhibitory nitrate concentration; mM |
| rmax | Monod equation; maximum growth rate, hour−1 |
| RT-qPCR | Reverse-transcription qPCR |
| S | Monod equation; (limiting) substrate concentration, g/L |
| SCI | Sulfur cycle intermediates |
| SRA | Sulfate-reducing archaea |
| SRB | Sulfate-reducing bacteria |
| SRM | Sulfate-reducing microorganism |
| T | Temperature, °C |
| TDS | Total dissolved solids, g/L |
| TVS | Thermal viability shell |
| VFA | Volatile fatty acids |
| wt. | Weight |
References
- Gaspar, J.; Davis, D.; Camacho, C.; Alvarez, P.J.J. Biogenic versus Thermogenic H2S Source Determination in Bakken Wells: Considerations for Biocide Application. Environ. Sci. Technol. Lett. 2016, 3, 127–132. [Google Scholar] [CrossRef]
- WHO. WHO Hydrogen Sulfide; European Series; WHO Regional Publications: Copenhagen, Denmark, 2000; pp. 1–7. [Google Scholar]
- Anchliya, A. New Nitrate-Based Treatments—A novel approach to control hydrogen sulfide in reservoir and to increase oil recovery. In Proceedings of the SPE Europec Featured at EAGE Conference and Exhibition, Vienna, Austria, 12–15 June 2006. [Google Scholar] [CrossRef]
- Carlson, H.K.; Stoeva, M.K.; Justice, N.B.; Sczesnak, A.; Mullan, M.R.; Mosqueda, L.A.; Kuehl, J.V.; Deutschbauer, A.M.; Arkin, A.P.; Coates, J.D. Monofluorophosphate is a selective inhibitor of respiratory Sulfate-Reducing microorganisms. Environ. Sci. Technol. 2015, 49, 3727–3736. [Google Scholar] [CrossRef] [PubMed]
- Bastin, E.S.; Greer, F.E.; Merritt, C.A.; Moulton, G.F. The presence of sulphate reducing bacteria in oil field waters. Science 1926, 63, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Bass, C. ZoBell’s contribution to petroleum microbiology. In Proceedings of the 8th International Symposium on Microbial Ecology, Halifax, NS, Canada, 9–14 August 1998. [Google Scholar]
- Almeida, P.F.; Almeida, R.C.; Carvalho, E.B.; Ramos-de-Souza, E.; Carvalho, A.S.; Silva, C.H.T.P.; Taft, C.A. Overview of sulfate-reducing bacteria and strategies to control biosulfide generation in oil waters. In Modern Biotechnology in Medicinal Chemistry and Industry; Research Signpost: Thiruvananthapuram, India, 2006; ISBN 81-308-0132-9. [Google Scholar]
- Hubert, C. Microbial Ecology of Oil Reservoir Souring and its Control by Nitrate Injection. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 2753–2766. [Google Scholar] [CrossRef]
- Xue, Y.; Voordouw, G.; Gieg, L.M. Laboratory protocols for investigating microbial souring and potential treatments in crude oil reservoirs. In Hydrocarbon and Lipid Microbiology Protocols: Activities and Phenotypes; Springer: Berlin/Heidelberg, Germany, 2015; pp. 183–210. [Google Scholar] [CrossRef]
- Skovhus, T.L.; Whitby, C. Oilfield Microbiology; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781138057753. [Google Scholar]
- Hagar, H.S.; Foroozesh, J.; Kumar, S.; Zivar, D.; Banan, N.; Dzulkarnain, I. Microbial H2S generation in hydrocarbon reservoirs: Analysis of mechanisms and recent remediation technologies. J. Nat. Gas Sci. Eng. 2022, 106, 104729. [Google Scholar] [CrossRef]
- Burger, E.D.; Jenneman, G.E.; Bache, Ø.; Jensen, T.B.; Soerensen, S. A mechanistic model to evaluate reservoir souring in the ekofisk field. In Proceedings of the SPE International Conference on Oilfield Chemistry, The Woodlands, TX, USA, 2–4 February 2005. [Google Scholar] [CrossRef]
- Jurelevicius, D.; von der Weid, I.; Korenblum, E.; Valoni, E.; Penna, M.; Seldin, L. Effect of nitrate injection on the bacterial community in a water-oil tank system analyzed by PCR-DGGE. J. Ind. Microbiol. Biotechnol. 2008, 35, 251–255. [Google Scholar] [CrossRef]
- Arensdorf, J.J.; Miner, K.; Ertmoed, R.; Clay, B.; Stadnicki, P.; Voordouw, G. Mitigation of reservoir souring by nitrate in a Produced Water Re-Injection System in Alberta. In Proceedings of the SPE International Conference on Oilfield Chemistry, The Woodlands, TX, USA, 20–24 April 2009. [Google Scholar] [CrossRef]
- Skjevrak, I.; Standnes, D.; Thomsen, U.; Xu, J.; Håland, K.; Kjølhamar, A.; Munkerud, P. Field observations of reservoir souring development and implications for the Extended Growth Zone (EGZ) souring model. J. Pet. Sci. Eng. 2021, 204, 108721. [Google Scholar] [CrossRef]
- Abd Rahman, H.; Sedaralit, M.F.; Zainal, S.; de Rezende, J.R. Modelling reservoir souring mitigation strategy based on dynamic microorganisms interactions. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 1–3 November 2022. [Google Scholar] [CrossRef]
- Hitzman, D.O.; Sperl, G.T. A new microbial technology for enhanced oil recovery and sulfide prevention and reduction. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 17–20 April 1994. [Google Scholar] [CrossRef]
- Johnson, R.J.; Folwell, B.D.; Wirekoh, A.; Frenzel, M.; Skovhus, T.L. Reservoir Souring—Latest developments for application and mitigation. J. Biotechnol. 2017, 256, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Prajapat, G.; Rellegadla, S.; Jain, S.; Agrawal, A. Reservoir souring control using benzalkonium chloride and nitrate in bioreactors simulating oil fields of western India. Int. Biodeterior. Biodegradation 2018, 132, 30–39. [Google Scholar] [CrossRef]
- Veshareh, M.J.; Nick, H.M. A sulfur and nitrogen cycle informed model to simulate nitrate treatment of reservoir souring. Sci. Rep. 2019, 9, 7546. [Google Scholar] [CrossRef]
- Myhr, B.-L.L.S.; Myhr, S.; Lillebø, B.-L.; Sunde, E.; Beeder, J.; Torsvik, T. Inhibition of microbial H 2 S production in an oil reservoir model column by nitrate injection. Appl. Microbiol. Biotechnol. 2002, 58, 400–408. [Google Scholar] [CrossRef]
- Kuijvenhoven, C.; Noirot, J.C.; Hubbard, P.; Oduola, L. One year experience with the injection of nitrate to control souring in Bonga Deepwater Development Offshore Nigeria. In Proceedings of the SPE International Conference on Oilfield Chemistry, Houston, TX, USA, 28 February–2 March 2007. [Google Scholar] [CrossRef]
- Veshareh, M.J.; Nick, H.M. Learnings from Reservoir Souring Treatment by Nitrate Injection in the Halfdan Oil Field. In Proceedings of the 80th EAGE Conference and Exhibition 2018, Copenhagen, Denmark, 11–14 June 2018. [Google Scholar] [CrossRef]
- Voordouw, G.; Hubert, C.; Nemati, M.; Jenneman, G.E. Is souring and corrosion by sulfate-reducing bacteria in oil fields reduced more efficiently by nitrate or by nitrite? In Proceedings of the CORROSION 2004, New Orleans, LA, USA, 28 March–1 April 2004; Available online: https://onepetro.org/NACECORR/proceedings/CORR04/All-CORR04/NACE-04762/115094 (accessed on 2 July 2016).
- Vik, E.A.; Janbu, A.O.; Garshol, F.; Henninge, L.B.; Engebretsen, S.; Kuijvenhoven, C.; Oliphant, D.; Hendriks, W.P. Nitrate-Based Souring Mitigation of Produced Water—Side Effects and Challenges from the Draugen Produced-Water ReInjection Pilot. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 28 February–2 March 2007. [Google Scholar] [CrossRef]
- Immanuel, O.M.; Abu, G.O.; Stanley, H.O. Mitigation of biogenic sulphide production by sulphate reducing bacteria in petroleum reservoir souring. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 4–6 August 2015. [Google Scholar] [CrossRef]
- Kögler, F.; Hartmann, F.S.; Schulze-Makuch, D.; Herold, A.; Alkan, H.; Dopffel, N. Inhibition of microbial souring with molybdate and its application under reservoir conditions. Int. Biodeterior. Biodegradation 2020, 157, 105158. [Google Scholar] [CrossRef]
- Qu, M.; Liang, T.; Hou, J.; Wu, W.; Wen, Y.; Xiao, L. Ultralow Concentration of Amphiphilic Molybdenum Disulfide Nanosheets for Enhanced Oil Recovery-Research and Field Application. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dubai, United Arab Emirates, 21–23 September 2021. [Google Scholar] [CrossRef]
- Tang, K.; Baskaran, V.; Nemati, M. Bacteria of the sulphur cycle: An overview of microbiology, biokinetics and their role in petroleum and mining industries. Biochem. Eng. J. 2009, 44, 73–94. [Google Scholar] [CrossRef]
- Basafa, M.; Hawboldt, K. Reservoir souring: Sulfur chemistry in offshore oil and gas reservoir fluids. J. Pet. Explor. Prod. Technol. 2018, 9, 1105–1118. [Google Scholar] [CrossRef]
- Jorgensen, B.B.; Findlay, A.J.; Pellerin, A. The biogeochemical sulfur cycle of marine sediments. Front. Microbiol. 2019, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A. Effect of Temperature on Souring in the Low and High Temperature Oil Fields. In Proceedings of the 16th Reservoir Microbiology Forum, London, UK, 30 November 2010. [Google Scholar]
- Larsen, J.; Skovhus, T.L.; Saunders, A.M.; Højris, B.; Agerbæk, M. Molecular Identification of Mic Bacteria from Scale and Produced Water: Similarities and Differences. In Proceedings of the CORROSION 2008, New Orleans, LA, USA, 16–20 March 2008. [Google Scholar]
- Gieg, L.M.; Jack, T.R.; Foght, J.M. Biological souring and mitigation in oil reservoirs. Appl. Microbiol. Biotechnol. 2011, 92, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Monod, J. The growth of bacterial cultures. Annu. Rev. Microbiol. 1949, 3, 371–394. [Google Scholar] [CrossRef]
- Button, D.K. Kinetics of nutrient-limited transport and microbial growth. Microbiol. Rev. 1985, 49, 270–297. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Levenspiel, O. Extended monod kinetics for substrate, product, and cell inhibition. Biotechnol. Bioeng. 1988, 32, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Merchuk, J.C.; Asenjo, J.A. The Monod equation and mass transfer. Biotechnol. Bioeng. 1995, 45, 91–94. [Google Scholar] [CrossRef]
- Eden, B.; Laycock, P.J.; Fielder, M. OTH 92385, Oilfield Reservoir Souring; HSE Books: Suffolk, UK, 1993; ISBN 0717606376, 9780717606375. [Google Scholar]
- Vale, T.O.D.; de Magalhaes, R.S.; de Almeida, P.F.; Matos, J.B.T.L.; Chinalia, F.A. The impact of alkyl polyglycoside surfactant on oil yields and its potential effect on the biogenic souring during enhanced oil recovery (EOR). Fuel 2020, 280, 118512. [Google Scholar] [CrossRef]
- Burger, E.D.; Jenneman, G.E.; Gao, X. The impact of dissolved Organic-Carbon type on the extent of reservoir souring. In Proceedings of the SPE International Conference on Oilfield Chemistry, The Woodlands, TX, USA, 8–10 April 2013. [Google Scholar] [CrossRef]
- Prausnitz, J.M.; Lichtenthaler, R.N.; De Azevedo, E.G. Molecular Thermodynamics of Fluid-Phase Equilibria, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1998. [Google Scholar]
- Duan, Z.; Sun, R.; Liu, R.; Zhu, C. Accurate thermodynamic model for the calculation of H2S solubility in pure water and brines. Energy Fuels 2007, 21, 2056–2065. [Google Scholar] [CrossRef]
- Feng, G.-X.; Mather, A.E. Solubility of H2S in n-dodecane. Fluid Phase Equilibria 1993, 87, 341–346. [Google Scholar] [CrossRef]
- Calsep. PVTSim 2021. Available online: https://www.calsep.com/pvtsim-nova (accessed on 23 May 2024).
- Zirrahi, M.; Azin, R.; Hassanzadeh, H.; Moshfeghian, M. Mutual solubility of CH4, CO2, H2S, and their mixtures in brine under subsurface disposal conditions. Fluid Phase Equilibria 2012, 324, 80–93. [Google Scholar] [CrossRef]
- Graham, A.; Singleton, M.; Salleh, I.K.; Khairuddin, K.; Ibrahim, J.; Sorbie, K. Experimental investigation of hydrogen sulfide scavenging capacities and mechanisms in iron-bearing minerals. In Proceedings of the First EAGE/IFPEN Conference on Sulfur Risk Management in Exploration and Production, Rueil-Malmaison, France, 18–20 September 2018. [Google Scholar] [CrossRef]
- Sunde, E.; Thorstenson, T.; Torsvik, T.; Vaag, J.E.; Espedal, M.S. Field-Related Mathematical Model to predict and reduce reservoir souring. In Proceedings of the SPE International Conference on Oilfield Chemistry, New Orleans, LA, USA, 2–5 March 1993. [Google Scholar] [CrossRef]
- Al-Kindi, A.; Prince-Wright, R.; Walsh, J.M.; Kuijvenhoven, C.; Morgenthaler, L.N.; Moore, W.R. Challenges for waterflooding in a deepwater environment. SPE Prod. Oper. 2008, 23, 404–410. [Google Scholar] [CrossRef]
- Gruesbeck, C.; Collins, R.E. Entrainment and deposition of fine particles in porous media. Soc. Pet. Eng. J. 1982, 22, 847–856. [Google Scholar] [CrossRef]
- Davis, J.B.; Updegraff, D.M. Microbiology in the petroleum industry. Bacteriol. Rev. 1954, 18, 215–238. [Google Scholar] [CrossRef] [PubMed]
- Tufenkji, N. Modeling microbial transport in porous media: Traditional approaches and recent developments. Adv. Water Resour. 2007, 30, 1455–1469. [Google Scholar] [CrossRef]
- Jensen, J.L.; Currie, I.D. A new method for estimating the Dykstra-Parsons coefficient to characterize reservoir heterogeneity. SPE Reserv. Eng. 1990, 5, 369–374. [Google Scholar] [CrossRef]
- Ligthelm, D.J.; De Boer, R.B.; Brint, J.F.; Schulte, W.M. Reservoir souring: An analytical model for H2S generation and transportation in an oil reservoir owing to bacterial activity. In Proceedings of the SPE Offshore Europe Conference and Exhibition, Aberdeen, UK, 3–6 September 1991. [Google Scholar] [CrossRef]
- Maxwell, S.; Spark, I. Souring of reservoirs by bacterial activity during seawater waterflooding. In Proceedings of the SPE International Conference on Oilfield Chemistry, The Woodlands, TX, USA, 2–4 February 2005. [Google Scholar] [CrossRef]
- Mitchell, A.F.; Skjevrak, I.; Jone, W. A Re-Evaluation of Reservoir Souring Patterns and Effect of Mitigation in a Mature North Sea Field. In Proceedings of the SPE International Conference on Oilfield Chemistry, Montgomery, TX, USA, 3–5 April 2017. [Google Scholar] [CrossRef]
- De Blanc, P.C.; McKINNEY, D.C.; Speitel, G.E., Jr. Modeling subsurface biodegradation of non-aqueous phase liquids. In Advances in Porous Media; Elsevier: Amsterdam, The Netherlands, 1996; Chapter 1; pp. 1–86. [Google Scholar] [CrossRef]
- Hosseininoosheri, P.; Lashgari, H.; Sepehrnoori, K. Numerical Prediction of Reservoir Souring under the Effect of Temperature, Ph, and Salinity on the Kinetics of Sulfate-Reducing Bacteria. In Proceedings of the SPE International Conference on Oilfield Chemistry, Montgomery, TX, USA, 3–5 April 2017. [Google Scholar] [CrossRef]
- Cheng, Y.; Hubbard, C.G.; Zheng, L.; Arora, B.; Li, L.; Karaoz, U.; Ajo-Franklin, J.; Bouskill, N.J. Next generation modeling of microbial souring—Parameterization through genomic information. Int. Biodeterior. Biodegradation 2018, 126, 189–203. [Google Scholar] [CrossRef]
- Al-Refai, S.R.; Al-Ajmi, M.; Oduola, L.; Carlos, C.M. Souring Prediction on Mature Waterflooded Reservoirs in North Kuwait. In Proceedings of the SPE Europec Featured at 81st EAGE Conference and Exhibition, London, UK, 3–6 June 2019. [Google Scholar] [CrossRef]
- PETEX. 2021. Available online: https://www.petex.com/products/ipm-suite/reveal/ (accessed on 23 May 2024).
- Snippe, J.; Lingli, W. An Efficient and Internally Consistent Reactive Transport Modelling Scheme for Sour Gas Injection Simulations. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 10–13 November 2014. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Liu, L.; Shabani, A. Impact of rock mineralogy on reservoir souring: A geochemical modeling study. Chem. Geol. 2020, 555, 119811. [Google Scholar] [CrossRef]
- Kathrada, M. Combining Sparse Data with Reaction Kinetics Using Fuzzy Logic to Predict Reservoir Souring. In Proceedings of the International Petroleum Technology Conference, Virtual, 23 March–1 April 2021. [Google Scholar] [CrossRef]
- Coombe, D.; Hubert, C.; Voordou, G. Mechanistic modelling of H2S souring treatments by application of nitrate or nitrite. Canadian International Petroleum Conference. In Proceedings of the PETSOC Canadian International Petroleum Conference, Calgary, AB, Canada, 8–10 June 2004. [Google Scholar] [CrossRef]
- Coombe, D.A.; Jack, T.; Voordouw, G.; Zhang, F.; Clay, B.; Miner, K. Simulation of bacterial souring control in an Alberta Heavy-Oil reservoir. J. Can. Pet. Technol. 2010, 49, 19–26. [Google Scholar] [CrossRef]
- Alkan, H.; Mukherjee, S.; Kögler, F. Microbial Enhanced Oil Recovery in Book Recovery Improvement, 1st ed.; Gulf Professional Publishing: Houston, TX, USA, 2022; Chapter 8. [Google Scholar] [CrossRef]
- De Siqueira, A.G.; Araujo, C.H.V.; Reksidler, R.; Pereira, M.D.C. Uncertainty analysis applied to biogenic reservoir souring simulation. In Proceedings of the SPE Europec Featured at EAGE Conference and Exhibition, Amsterdam, The Netherlands, 8–11 June 2009. [Google Scholar] [CrossRef]
- Ness, G.; Sorbie, K.; Lugo, N.; de Rezende, J.R.; Shi, X. Predicting Reservoir Souring in the Alba Field Using Produced Water Compositions—A Study of Biogenic Sulfate Loss. In Proceedings of the SPE International Conference on Oilfield Chemistry, The Woodlands, TX, USA, 28–29 June 2023. [Google Scholar] [CrossRef]
- Fathy, A.; Hassan, A.M.; Abdullah, M.B.; Al-Shalabi, E.W.; Rego, F.B.; Delshad, M.; Sepehrnoori, K. Numerical Study on Tackling Microbial Reservoir Souring during Engineered Water Injection. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 22–25 April 2024. [Google Scholar]
- Farhadinia, M.A.; Bryant, S.L.; Sepehrnoori, K.; Delshad, M. Development of a reservoir simulator for souring predictions. In Proceedings of the SPE Reservoir Simulation Conference, The Woodlands, TX, USA, 2–4 February 2009. [Google Scholar] [CrossRef]
- Farhadinia, M.A.; Bryant, S.L.; Sepehrnoori, K.; Delshad, M. Application of a 3D Reservoir Simulator with Biodegradation Capability to Evaluate Reservoir Souring Predictive Models. Pet. Sci. Technol. 2010, 28, 382–392. [Google Scholar] [CrossRef]
- Skjælaaen, I.; Ebigbo, A.; Espedal, M.; Helmig, R. A model for transport of hydrogen sulfide in oil- and water-saturated porous media. Comput. Vis. Sci. 2010, 13, 265–273. [Google Scholar] [CrossRef]
- Haghshenas, M.; Sepehrnoori, K.; Bryant, S.L.; Farhadinia, M.A. Modeling and simulation of nitrate injection for reservoir souring remediation. SPE J. 2012, 17, 817–827. [Google Scholar] [CrossRef]
- Evans, P. Reservoir Souring Modelling, Prediction and Mitigation. In Proceedings of the ASME 2008 27th International Conference on Offshore Mechanics and Arctic Engineering, Estoril, Portugal, 15–20 June 2009; ASME: New York, NY, USA, 2009; Volume 5: Materials Technology; CFD and VIV, pp. 67–73. [Google Scholar] [CrossRef]
- Evans, P.; Ambruss, J.; Maliska, C.R.; Kabche, J.P. Simulation of Reservoir Souring Considering Multiple Carbon Sources and Environmental Aspects. In Proceedings of the 4th International Symposium on Applied Microbiology and Molecular Biology in Oil Systems, Rio de Janeiro, Brazil, 28–30 August 2013. [Google Scholar] [CrossRef]
- Sugai, Y.; Owaki, Y.; Sasaki, K.; Kaneko, F.; Sakai, T. Numerical prediction of reservoir souring based on the growth kinetics of sulfate-reducing bacteria indigenous to an oilfield. In Proceedings of the SPE International Oilfield Corrosion Conference and Exhibition, Aberdeen, UK, 12–13 May 2014. [Google Scholar] [CrossRef]
- Cheng, Y.; Hubbard, C.G.; Li, L.; Bouskill, N.J.; Molins, S.; Zheng, L.; Sonnenthal, E.; Conrad, M.E.; Engelbrektson, A.; Coates, J.D.; et al. Reactive Transport Model of Sulfur Cycling as Impacted by Perchlorate and Nitrate Treatments. Environ. Sci. Technol. 2016, 50, 7010–7018. [Google Scholar] [CrossRef] [PubMed]
- Chaban, F.; Garduno, J.; Osorio, N.; Lee, J. Multi-Domain Integrated Workflow for Reservoir Souring Modeling and Prediction to Effectively Define and Mitigate H2S Production Risk in Offshore Developments Undertaking Waterflooding. In Proceedings of the SPE Annual Technical Conference and Exhibition, Virtual, 26–29 October 2020. [Google Scholar] [CrossRef]
- Mahmoodi, A.; Nick, H. Large-Scale Modelling of Microbial Activities Underground. In Proceedings of the 4th EAGE Global Energy Transition Conference & Exhibition, Paris, France, 14–17 November 2023. [Google Scholar] [CrossRef]
- Kane, R.D.; Surinach, P.P. A field study of microbiological growth and reservoir souring. In Proceedings of the Corrosion97, New Orleans, LA, USA, 9–14 March 1997; Available online: https://onepetro.org/NACECORR/proceedings/CORR97/All-CORR97/NACE-97208/113386 (accessed on 1 July 2016).
- Hubert, C.; Voordouw, G. Oil Field Souring Control by Nitrate-Reducing Sulfurospirillum spp. That Outcompete Sulfate-Reducing Bacteria for Organic Electron Donors. Appl. Environ. Microbiol. 2007, 73, 2644–2652. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, I.T.; Brown, M.H.; Skurray, R.A. Proton-dependent multidrug efflux systems. Microbiol. Rev. 1996, 60, 575–608. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.L.B.; Soares, H.M.; Furigo, A.; Schmidell, W.; Corseuil, H.X. Effects of nitrate injection on microbial enhanced oil recovery and oilfield reservoir souring. Appl. Biochem. Biotechnol. 2014, 174, 1810–1821. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.S.; Almeida, P.F.; dos Santos, J.N.; Sampaio, I.C.; Figueiredo, L.F.; Tereska, D.; Chinalia, F.A. Bioreactor for accurately assessing biocide effectiveness in controlling biogenic souring in mature oil wells. SPE J. 2018, 23, 1809–1816. [Google Scholar] [CrossRef]
- Hubbard, C.G.; Cheng, Y.; Engelbrekston, A.; Druhan, J.L.; Li, L.; Ajo-Franklin, J.B.; Coates, J.D.; Conrad, M.E. Isotopic insights into microbial sulfur cycling in oil reservoirs. Front. Microbiol. 2014, 5, 480. [Google Scholar] [CrossRef]
- Cannon, S. Petrophysics: A Practical Guide; Wiley-Blackwell: Oxford, UK, 2015; Available online: https://openlibrary.org/books/OL27518758M/Petrophysics (accessed on 1 July 2016).
- Tiab, D.; Donaldson, E.C. Petrophysics: Theory and Practice of Measuring Reservoir Rock and Fluid Transport Properties; Gulf Professional Publishing: Houston, TX, USA, 1996; ISBN 9780128031889. [Google Scholar]
- Ma, Y.Z. Petrophysical Data Analytics for Reservoir Characterization. In Quantitative Geosciences: Data Analytics, Geostatistics, Reservoir Characterization and Modeling; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Gunaltun, Y.M.; De Reus, H.; Nyborg, R. The reliability of laboratory and field parameters used in the prediction models. In Proceedings of the CORROSION 2003, San Diego, CA, USA, 16–20 March 2003; Available online: https://onepetro.org/NACECORR/proceedings/CORR03/All-CORR03/NACE-03622/114464 (accessed on 1 July 2016).
- Cavallaro, A.N.; Martinez, M.G.; Ostera, H.; Panarello, H.; Cordero, R.R. Oilfield Reservoir Souring during Waterflooding: A Case Study with Low Sulphate Concentration in Formation and Injection Waters. In Proceedings of the SPE International Conference on Oilfield Chemistry, The Woodlands, TX, USA, 2–4 February 2005. [Google Scholar] [CrossRef]
- CMG Computer Modelling Group Ltd. Reservoir Simulation Software (cmgl.ca); CMG Computer Modelling Group Ltd.: Calgary, AB, USA, 2021. [Google Scholar]
- Alkan, H.; Namazova, G.; Hatscher, S.; Dopffel, N. Modelling approaches to assess biogenic souring during waterflooding and EOR operations. In Proceedings of the First EAGE/IFPEN Conference on Sulfur Risk Management in Exploration and Production, Rueil-Malmaison, France, 18–20 September 2018. [Google Scholar] [CrossRef]
- Alkan, H.; Klueglein, N.; Mahler, E.; Kögler, F.; Beier, K.; Jelinek, W.; Herold, A.; Hatscher, S.; Leonhardt, B. An integrated German MEOR project update: Risk management and Huff’n Puff design. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar] [CrossRef]
- Bülltemeier, H.; Alkan, H.; Moh’d, A. A New Modeling Approach to MEOR calibrated by Bacterial Growth and Metabolite Curves. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 31 March–2 April 2014. [Google Scholar] [CrossRef]
- Voordouw, G.; Grigoryan, A.A.; Lambo, A.; Lin, S.; Park, H.S.; Jack, T.R.; Coombe, D.; Clay, B.; Zhang, F.; Ertmoed, R.; et al. Sulfide Remediation by Pulsed Injection of Nitrate into a Low Temperature Canadian Heavy Oil Reservoir. Environ. Sci. Technol. 2009, 43, 9512–9518. [Google Scholar] [CrossRef] [PubMed]
- Veshareh, M.J.; Kjeldsen, K.U.; Findlay, A.J.; Nick, H.M.; Røy, H.; Marietou, A. Nitrite is a more efficient inhibitor of microbial sulfate reduction in oil reservoirs compared to nitrate and perchlorate: A laboratory and field-scale simulation study. Int. Biodeterior. Biodegradation 2020, 157, 105154. [Google Scholar] [CrossRef]
- Engelbrektson, A.; Hubbard, C.G.; Tom, L.M.; Boussina, A.; Jin, Y.T.; Wong, H.; Piceno, Y.M.; Carlson, H.K.; Conrad, M.E.; Anderson, G.; et al. Inhibition of microbial sulfate reduction in a flow-through column system by (per)chlorate treatment. Front. Microbiol. 2014, 5, 315. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cheng, Y.; Hubbard, C.G.; Hubbard, S.; Ajo-Franklin, J.B. Biogenic sulfide control by nitrate and (per)chlorate—A monitoring and modeling investigation. Chem. Geol. 2018, 476, 180–190. [Google Scholar] [CrossRef]
- Maxwell, S.; MacKenzie, G.; Kuijvenhoven, C.; Lomans, B.; Granli, T. Laboratory studies of microbicide and nitrate strategies for mic and reservoir souring mitigation. In Proceedings of the Corrosion, New Orleans, LA, USA, 16–20 March 2008; Available online: https://onepetro.org/NACECORR/proceedings/CORR08/All-CORR08/NACE-08663/119128 (accessed on 1 July 2020).
- Greene, E.A.; Brunelle, V.; Jenneman, G.E.; Voordouw, G. Synergistic inhibition of microbial sulfide production by combinations of the metabolic inhibitor nitrite and biocides. Appl. Environ. Microbiol. 2006, 72, 7897–7901. [Google Scholar] [CrossRef] [PubMed]
- Jenneman, G.E.; McInerney, M.J.; Knapp, R.M. Effect of nitrate on biogenic sulfide production. Appl. Environ. Microbiol. 1986, 51, 1205–1211. [Google Scholar] [CrossRef]
- Reinsel, M.A.; Sears, J.T.; Stewart, P.S.; McInerney, M.J. Control of microbial souring by nitrate, nitrite or glutaraldehyde injection in a sandstone column. J. Ind. Microbiol. Biotechnol. 1996, 17, 128–136. [Google Scholar] [CrossRef]
- Jenneman, G.E.; Moffitt, P.D.; Bala, G.A.; Webb, R.H. Sulfide removal in reservoir brine by indigenous bacteria. SPE Prod. Facil. 1999, 14, 219–225. [Google Scholar] [CrossRef]
- Prajapat, G.; Jain, S.; Lal, B.; Lavania, M.; Agrawal, A. Control of reservoir souring by incomplete nitrate reduction in Indian oil fields. Bioresour. Technol. Rep. 2023, 21, 101302. [Google Scholar] [CrossRef]
- Mahmoodi, A.; Kiapi, M.R.A.; Nick, H.M. When nitrate treatment wins the battle against microbial reservoir souring but loses the war. Ecol. Model. 2023, 481, 110329. [Google Scholar] [CrossRef]
- Yin, B.; Wunch, K. Combined effects of microbes and nitrate on SRB growth, souring and corrosion. In Proceedings of the CORROSION 2017, New Orleans, LA, USA, 26–30 March 2017; Available online: https://onepetro.org/NACECORR/proceedings/CORR17/All-CORR17/NACE-2017-9425/125625 (accessed on 1 July 2018).
- Dopffel, N.; Koegler, F.; Hartmann, H.; Costea, P.I.; Mahler, E.; Herold, A.; Alkan, H. Microbial induced mineral precipitations caused by nitrate treatment for souring control during microbial enhanced oil recovery (MEOR). Int. Biodeterior. Biodegradation 2018, 135, 71–79. [Google Scholar] [CrossRef]
- Martin, R.L. Corrosion Consequences of Nitrate/Nitrite additions to oilfield brines. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008. [Google Scholar] [CrossRef]
- Peck, H. The Role of Adenosine-5′-phosphosulfate in the Reduction of Sulfate to Sulfite by Desulfovibrio desulfuricans. J. Biol. Chem. 1962, 237, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Tenti, P.; Roman, S.; Storelli, N. Molybdate to Prevent the Formation of Sulfide during the Process of Biogas Production. bioRxiv 2019. [Google Scholar] [CrossRef]
- Wilson, L.G.; Bandurski, R.S. An enzymatic reaction involving adenosine triphosphate and selenate. Arch. Biochem. Biophys. 1956, 62, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A.; Huttner, W.B. Chlorate—A potent inhibitor of protein sulfation in intact cells. Biochem. Biophys. Res. Commun. 1986, 141, 870–877. [Google Scholar] [CrossRef]
- Klueglein, N.; Mahler, E.; Sonwa, R.; Herold, A.; Hatscher, S.; Alkan, H. Testing of H2S Inhibitors for Application in a MEOR Field Pilot in Germany. In Proceedings of the SPE International Oilfield Corrosion Conference and Exhibition, Aberdeen, UK, 9–10 May 2016. [Google Scholar] [CrossRef]
- Kögler, F.; Dopffel, N.; Mahler, E.; Hartmann, F.S.F.; Schulze-Makuch, D.; Visser, F.; Frommherz, B.; Herold, A.; Alkan, H. Influence of Surface Mineralogy on the Activity of Halanaerobium Sp. during Microbial Enhanced Oil Recovery (MEOR). Fuel 2021, 290, 119973. [Google Scholar] [CrossRef]
- Kalpakci, B.; Magri, N.F.; Ravenscroft, P.D.; McTeir, M.D.K.; Arf, G.T. Mitigation of Reservoir Souring—Decision Process. In Proceedings of the SPE International Conference on Oilfield Chemistry, San Antonio, TX, USA, 14–17 February 1995. [Google Scholar] [CrossRef]


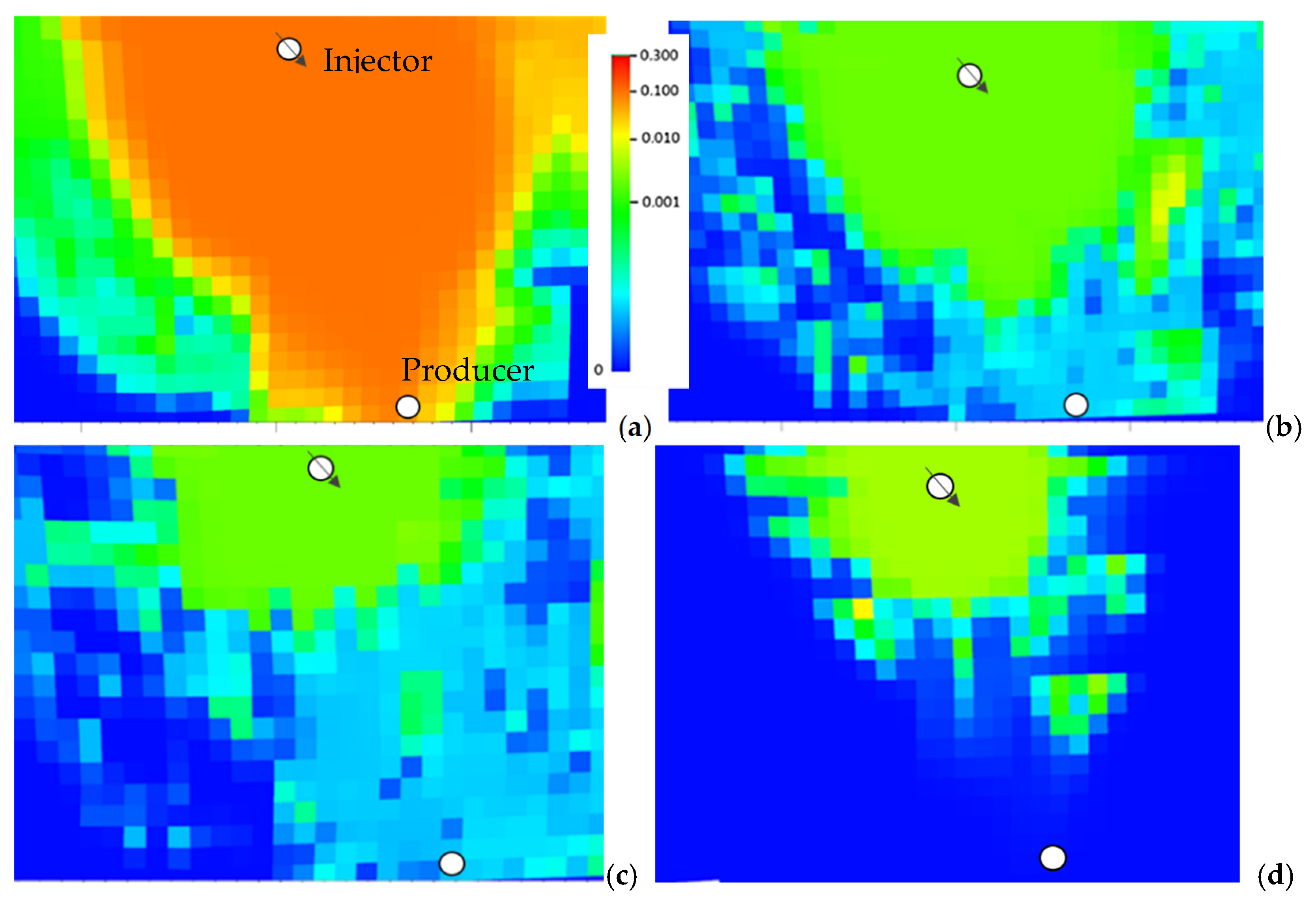

| Type of Evaluation | Rock Type | H2S Retention [mg/g] | Reference |
|---|---|---|---|
| Dynamic | Cores from various reservoirs, k = 5200 mD | 0.014 | [48] |
| Dynamic | Cores from various reservoirs, k = 0.4 mD | 0.0196 | [48] |
| Dynamic | Cores from various reservoirs, k = 8200 mD | 0.55 | [48] |
| Dynamic | Cores from various reservoirs, k = 740 mD | 1.95 | [48] |
| Static | Crushed cores with siderite content (<814 μm) | 0.24 | [49] |
| Dynamic | Cores with siderite content; from the available data | 0.003 | [49] |
| Static | Crushed rock (100–315 mm) with 20% wt. siderite | 0.5–3.7 | [47] |
| Static | Crushed rock (<100 mm) with 20% wt. siderite | 2.08–9.70 | [47] |
| Dynamic | Sand packs filled with sand with 4% wt. siderite | 1.03–1.74 | [47] |
| Dynamic | Sand packs filled with formation rock with 10% wt. siderite | 0.14 | [27] |
| Theoretical | Based on stoichiometry for 1% wt. siderite content | 0.34 | This study |
| Parameter | Range, Influence |
|---|---|
| Solubility H2S | Higher in oil than in water; higher in complex oil compositions containing asphaltenes and aromatics; lower in brines with increasing salinities |
| Partitioning, Kow, Kog, Kwg | Depending on the H2S solubility in oil and water phases, salinity, temperature, pressure, pH |
| pH | Over reservoir typical ranges, not relevant for the activation of SRB in oil reservoirs. The higher the pH, the higher the solubility in water, thus the lower the Kow |
| Water chemistry | Divalent cations in the formation water may precipitate with both sulfate (Ba2+, Sr2+, and Ca2+) and sulfide (Fe2+, Zn2+), acting as scavengers |
| Rock mineralogy | The presence of iron-bearing minerals is crucial for the in situ retention of H2S via precipitation |
| Temperature | Most SRB are not active at temperatures higher than 80 °C |
| Pressure | Not relevant effect on SRB activity for most common pressures in oilfields, therefore no relevant effect on H2S generation |
| Sulfate | One of the main components of biogenic souring, concentration has a strong impact on the possible H2S concentration generated |
| Salinity | Certain SRB species live in higher-salinity brines up to ca. 180 g/L; H2S solubility in water decreases as the salinity increases (salting-out effect) |
| Presence of SRB | If no indigenous SRB exist in the reservoir no biogenic souring is expected; however, SRB can be introduced with injection water |
| Permeability | Low permeability, small pore radius limits the transport of microbes therefore their planktonic movement; no relevant effect on H2S transport once generated |
| Heterogeneity | Important for the transport of SRB and H2S; if high, less retention in the reservoir and rapid breakthrough of H2S to the producers can be expected |
| Carbon sources | Completes the souring reaction; VFA can be an important source; oil components may also be a nutrient source for SRB |
| Year | Model Title | Overview | Ref. |
|---|---|---|---|
| 2004 | Modelling using commericial reservoir simulators, CMG-STARS | Kinetic models for microbial growth and metabolite production to simulate up-flow packed bioreactor tests | [65] |
| 2009 | A souring model in a 3D finite difference compositional non-isothermal reservoir simulator | The partition coefficient depending mainly on temperature and only weakly on pressure, to determine the H2S concentration in the two phases | [72] |
| 2009 | Uncertainty analysis using CMG-CMOST | H2S concentrations presented as a probabilistic range | [68] |
| 2010 | Modelling biosouring mitigation using commercial reservoir simulator CMG-STARS | Designing and optimizing nitrate injection programs as part of an overall management strategy of microbial souring in produced water recycle injection operations | [66] |
| 2010 | 3D modelling applied in numerical simulator MUFTE; not compatible with reservoir simulation | A model for the transport of H2S in an oil- and water-saturated porous medium with different retention mechanisms | [73] |
| 2012 | Using UTCHEM, a reactive flow simulator | The simulator is used to predict the onset of reservoir souring and the effectiveness of nitrate injection | [72,74] |
| 2013 | SourSim®RL, a reservoir souring code to be coupled with commercial reservoir simulators | Considers temperature distribution, SRB growth, H2S partitioning and transport mitigation with nitrate module and oil biodegradation also implemented; | [75,76] |
| 2014 | Souring simulator coupled with geochemical code PHREEQC is used | The coupling scheme to the geochemical solver is explicit, the partitioning of components between the phases including evaporation of H2O is handled fully implicitly | [62] |
| 2014 | A numerical simulator including the growth rate equation for the SRB by modifying a simulator of MEOR | The simulation suggested that SRB generates H2S only around the injection well because of a temperature drop there; souring can be prevented more surely by heating up to 50 °C, or reducing ethanol in the injection water | [77] |
| 2016 | TOUGHREACT for isotopic and biogeochemical systems; kinetics with Monod expression | With data from column experiments as constraints, a first reactive transport model of a new candidate inhibitor, perchlorate; compared with nitrate | [78] |
| 2017 | To simulate multiphase flow and bio-chemical reactions combining TMVOC and TOUGHREACT | An approach to utilize genomic information to constrain the biological parameters needed for modeling souring using data derived from oil reservoir studies | [59] |
| 2017 | Attempt to model reservoir souring by using the multi purpose 3D reservoir simulator UTCHEM | The work differs from earlier works by explicitly determining parametric values required for Monod kinetic model as function of salinity, temperature and pH | [58] |
| 2019 | Field simulation based on the use of the reservoir model in combination with SourSim®RL based on measured data | The initial phase consisted of the history match to define the most likely souring mechanism in the field. The forecast considered various scenarios with a range of sensitivities on carbon nutrient and sulphate levels | [60] |
| 2020 | A souring multi-domain workflow considering dynamic reservoir characteristics | An integrated approach from reservoir to facilities, and presents a case study by using commercial reservoir and souring simulators | [79] |
| 2020 | A coupled reactive transport model using PHREEQC to study the effect of mineralogical composition | The results show that chalk containing anhydrite produces more H2S in comparison to limestone; rock containing iron minerals can inhibit the H2S generation in porous media | [63] |
| 2021 | Use of fuzzy logic to combine basic principles with sparse data for practical uses | A fuzzy logic model built around the reaction kinetics and then conditioned with field data. It is reported that the model matches the published field data fairly well | [64] |
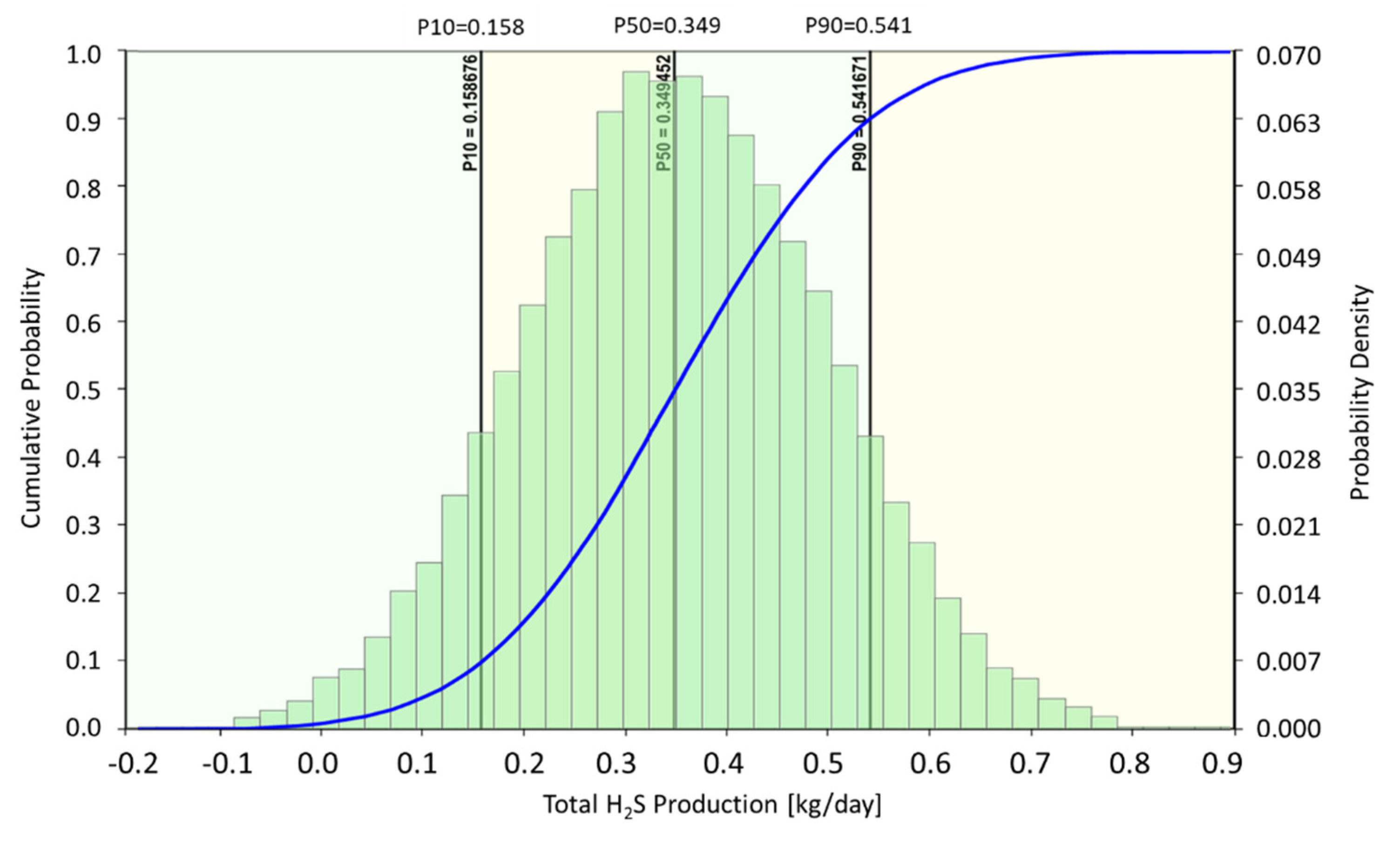
| 2022 | Use of commercial simulator CMG-STARS in combination with uncertainty analysis (CMOST) | Experimental data were used for the calibration of the model; the model was applied to predict potential souring using Monte-Carlo analysis | [67], this study |
| 2023 | A Thermo-Hydro-bio-Chemical (THbC) model coupled with industry-standard simulations | The model has been tested and successfully tunned using the history of the field data for predicting microbial sulfate reduction in an oil field in the Danish North Sea | [80] |
| Type | Why, How | Methods, Analytics | |
|---|---|---|---|
| Microbiological analyses | Analyses for: Microbial activity and composition; presence of SRMs, incubation;to growth; microbial community composition | Enumeration by MPN, ATP, OD600, DAPI, PCR, qFISH, DNA extraction and qPCR, batch incubations amplification of 16S rRNA | [7,9,33,55,81,82] |
| Bioreactors Sandpacks, Corefloods Micromodels | Souring and its mitigation under dynamic conditions, biofilm formation; transport of H2S under simulated reservoir conditions | Sandpack experiments, columns filled with formation materials; flow in micromodels, corefloods with analogue outcrops and reservoir cores | [17,27,29,83,84,85] |
| Chemical analyses | To determine the chemical composition of injected and produced water and oil; sulfide, sulfate, nitrate, VFA, cations, etc. Gas compositions; H2S, other gases | Spectrophotometric determination HPLC, GC, isotopic analysis | [9,30,86] |
| Petrophysical measurements | The permeability and pore radius/ throat as well as the porosity for the transport; model; mineralogy of the reservoir rock for reactivity; heterogeneity for transport in the reservoir; oil-water saturations for H2S partitioning | Routine and special core analysis, X-Ray measurements, well tests for heterogeneity, DP coeff. solubility of H2S in water and oil | [87,88,89] |
| Field-well measurements | Water cut for H2S partitioning, gas compositions for detecting H2S; corrosion monitoring; geology and production history for reservoir modelling production history for reservoir modelling | Routine field measurements and analyses, Draeger tubes corrosion tests; well tests | [12,56,90,91] |
| Property | Data |
|---|---|
| Formation salinity | Injection water: highly saline, up to 160 g/L, sulfate content around 70 mg/L. Produced water: highly saline up to 180 g/L, no detectable sulfate |
| Formation rock mineralogy | Sandstone with siderite content of 2–10% wt. |
| SRB | Microbiological analysis of the samples from the field confirmed the presence of Desulfovibrionales (Desulfohalobiaceae) in the produced water as well as in the injection water; the field is under PWRI |
| Organic acids, VFA | Organic acids (e.g., acetate, lactate, formate) are products of the MEOR process. Their amount is high enough for complete reduction of the available sulfate by SRB |
| Pressure | 30–70 bar under reservoir conditions; no detrimental effect on the SRB |
| Temperature | Injection water calculated 20–25 °C at the bottom hole during injection, reservoir temperature between 37° and 41 °C. Since SRB are present in the reservoir already, they can be active in a range valid for the reservoir studied |
| pH | Formation water pH~6.5, VFA due to MEOR can reduce the pH down to 5 (not considering pH-buffering effects of the formation rock); pH is in the range still allowing the activity of SRB |
| Permeability | Average permeability and the pore diameter are kavr 300 mD and 20 μm, respectively. This is high enough for the free movement of the SRB throughout the reservoir. On the other hand, the reservoir is highly heterogeneous |
| Injection rate | 150 m3/day, including a defined concentration of nutrients for in situ MEOR |
| Partitioning coefficient | Based on high salinity formation water and with the assumption of a pH of 6, Kow is taken as 30 in the base case |
| Grid | Corner Point |
|---|---|
| Number of cells | 36(x) × 31(y) × 16(z) |
| Cell size Refinement | 50 × 50 × 1.3 m 10 × 10 × 1.3 |
| Porosity | 0.018–0.291 |
| Permeability | 1–6130 mD (5 rock types) |
| Chemical/Application Mode | Conclusion | Ref. |
|---|---|---|
| Nitrate | ||
| Draugen North Sea oilfield (~70 °C): The seawater used for injection is treated by filtration, de-oxygenated using the scavenger sodium bisulfite, and sterilized using sodium hypochlorite plus biocide | 70–80 mg/L in injection water; the dosage could be reduced to 50 mg/L, was successful during the first 3 months. Stopping the treatment for 6 weeks caused quick rebound of H2S, resuming nitrate addition brought the souring under control again | [25] |
| Injection of nitrate in Nigeria Bonga field; tested first in laboratory experiments e.g., sandpacks | One year after the start of the project, there was only limited amount of water production and not yet H2S | [22] |
| Up-flow packed bioreactors inoculated with water produced from an oil field and injected with lactate | Nitrate gives less SRB inhibition, under experimental conditions is concluded that use of nitrite is more favorable than use of nitrate | [82] |
| Amendment of nitrate (5 mM NaNO3) in a water-oil separation tank in an offshore platform in Brazil | The conclusion was that nitrate treatment was effective in this surface facility, but continuous application would be necessary to suppress SRB | [13] |
| The injection water with low sulfate concentration (~1 mM). Nitrate (2.4 mM, 150 ppm) was added at the water plants continuously or with week-long pulses of 14 mM | For low temperature fields, with low sulfate concentration injected, the increase in souring can be stopped by nitrate injection. A pulsing strategy appears to give better results than continuous injection | [96] |
| Ca(NO3)2 continuously applied at 150 ppm field wide. A biocide, acrolein, was also applied in weekly batches to treat the injection lines and prevent nitrate-fed biofilm | After one month of treatment, H2S in the produced gas from one gathering system decreased from an average of 170 ppmv to 110 ppmv. A corresponding decrease in H2S in the produced water was observed | [14] |
| Column experiments to investigate the effects of nitrate injection on SRB inhibition and MEOR | Resulted in significant sulfide control (40–100% reduction) within few days | [84] |
| Nitrite | ||
| Using up-flow packed bioreactors both nitrate and nitrite are effective sulfide removers | Nitrite is a stronger SRB inhibitor. Nitrate gives less SRB inhibition, because it is only partially converted to nitrite | [82] |
| Testing various inhibitors among them nitrite in full field numerical simulations | 1 mM of nitrite can reduce souring by 92%, while perchlorate (1 mM) or nitrate (1 mM) reduce sulfide accumulation by 57% and 80% respectively | [97] |
| Molybdate | ||
| Batch/dynamic experiments and field test as a part of a MEOR project; a Na2MoO4 concentration of 0.5 mM was found as optimum | Molybdate inhibited the activity of SRB efficiently, while still enabling MEOR due to its specific inhibition mechanism. Adsorption is an issue! | [27] |
| Perchlorate | ||
| Columns with marine sediment flushed with coastal water amended with yeast extract and nitrate, chlorate, or perchlorate | The study indicates that (per)chlorate show great promise as inhibitors of sulfidogenesis in natural communities and provides insight into which organisms and respiratory processes are involved | [98] |
| With data from sandpack experiments, a reactive transport model compared perchlorate with nitrate. | Model runs previously matched with experimental data suggest that perchlorate is more effective than nitrate on a per mole of inhibitor basis. | [78] |
| Experiments under different matrix and inoculation conditions to evaluate treatment efficiency | Advantages of souring control with (per)chlorate treatments, and the application of galvanic signal as an economic, in-situ monitoring approach | [99] |
| A combination of laboratory experiments and numerical models to compare sulfide inhibitors | Perchlorate inhibits sulfate reduction by promoting sulfide oxidation | [97] |
| Others | ||
| A high-throughput screening strategy to identify inhibitors of SRMs, quantitatively ranked the selectivity and potency of compounds and synergistic interactions | Zinc pyrithione is the most potent inhibitor of H2S generation and is several orders of magnitude more potent than commonly used industrial biocides. | [4] |
| Some novel mitigation agents are cited | Adjuvants, anthraquinone, bacteriophage, diphenyliodonium salts | [18] |
| Combined | ||
| Microbiocide and nitrate are applied, either alone or in combination, in many seawater injection systems as controls to mitigate MIC or reservoir souring or both | Souring experiments, does not reflect the field data in that the dramatic decreases in H2S production seen in the model are not typical of the much less dramatic changes observed in the field | [100] |
| MICs of six broad-spectrum biocides and two specific metabolic inhibitors and fractional inhibitory concentration indexes (FICIs) for controlling a SRB | Nitrite was synergistic (FICI < 1) with all but one biocide due to its specific inhibition of dissimilatory sulfite reductase. Hence, combining nitrite with biocides allows more efficient and cost-effective control of SRB | [101] |
| Four new biocides and one commonly used biocide were tested in bioreactors operated for 591 days | SRB activity could recover within a period varied from 15 to 60 days. Neem-oil (NO) (1.5% vol/vol) and Dazomet (0.5% vol/vol) were the most efficient in controlling SRB | [85] |
| Sandpacked bioreactors simulating moderately high temperature oil reservoir for souring control | Co-injection of 2 mM nitrate and 0.75 mM BAC completely ceased H2S production and controlled souring. | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkan, H.; Kögler, F.; Namazova, G.; Hatscher, S.; Jelinek, W.; Amro, M. Assessment of the Biogenic Souring in Oil Reservoirs under Secondary and Tertiary Oil Recovery. Energies 2024, 17, 2681. https://doi.org/10.3390/en17112681
Alkan H, Kögler F, Namazova G, Hatscher S, Jelinek W, Amro M. Assessment of the Biogenic Souring in Oil Reservoirs under Secondary and Tertiary Oil Recovery. Energies. 2024; 17(11):2681. https://doi.org/10.3390/en17112681
Chicago/Turabian StyleAlkan, Hakan, Felix Kögler, Gyunay Namazova, Stephan Hatscher, Wolfgang Jelinek, and Mohd Amro. 2024. "Assessment of the Biogenic Souring in Oil Reservoirs under Secondary and Tertiary Oil Recovery" Energies 17, no. 11: 2681. https://doi.org/10.3390/en17112681
APA StyleAlkan, H., Kögler, F., Namazova, G., Hatscher, S., Jelinek, W., & Amro, M. (2024). Assessment of the Biogenic Souring in Oil Reservoirs under Secondary and Tertiary Oil Recovery. Energies, 17(11), 2681. https://doi.org/10.3390/en17112681








