Proposal and Verification of the Application of an Expert Inference Method to Present the Probability of Lithium-Ion Battery Thermal Runaway Risk
Abstract
1. Introduction
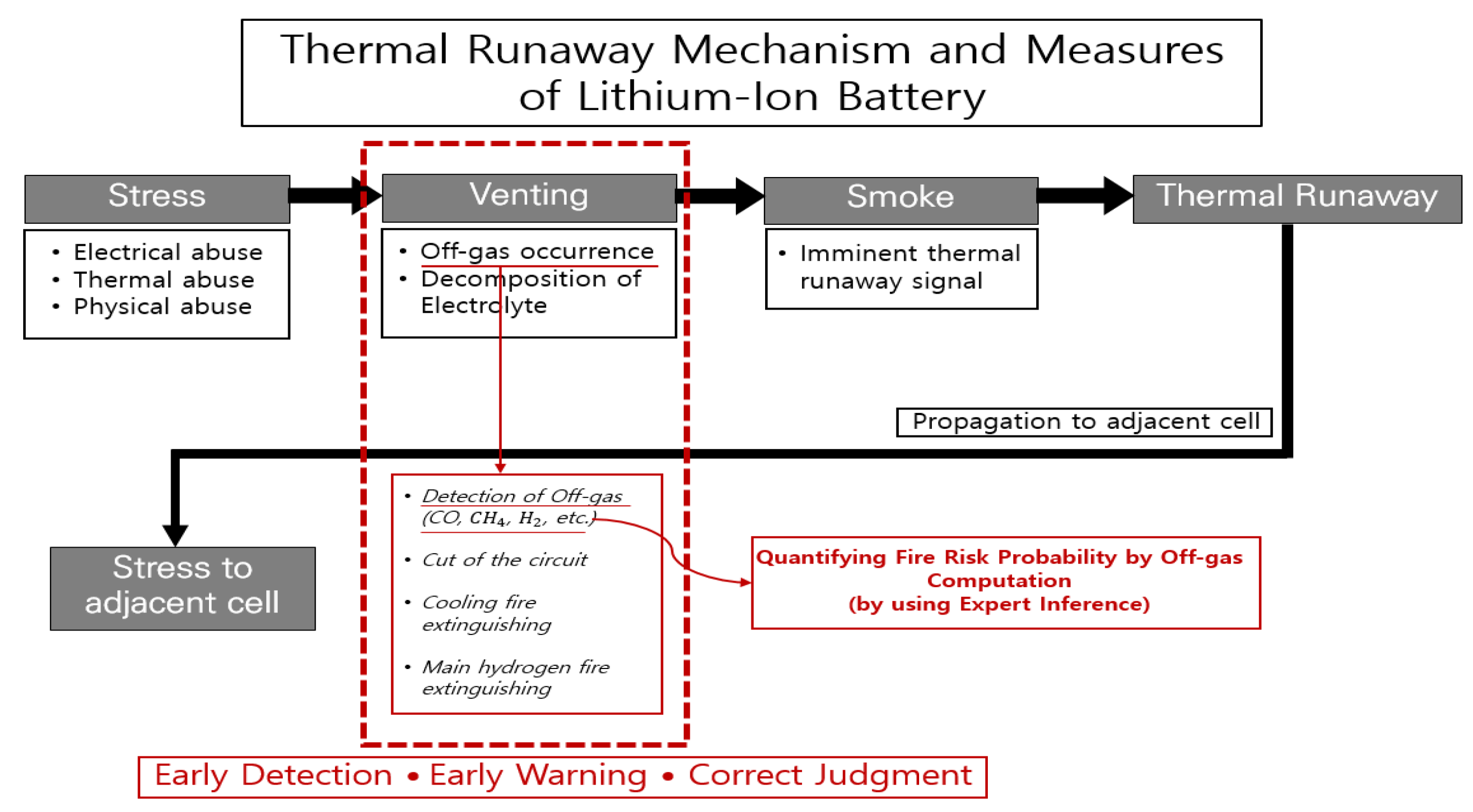
2. LIB Structure and Thermal Runaway Fire Mechanism and Compositional Reasoning Design Applying the Fuzzy Logic
2.1. LIB Structure and Thermal Runaway Fire Mechanism
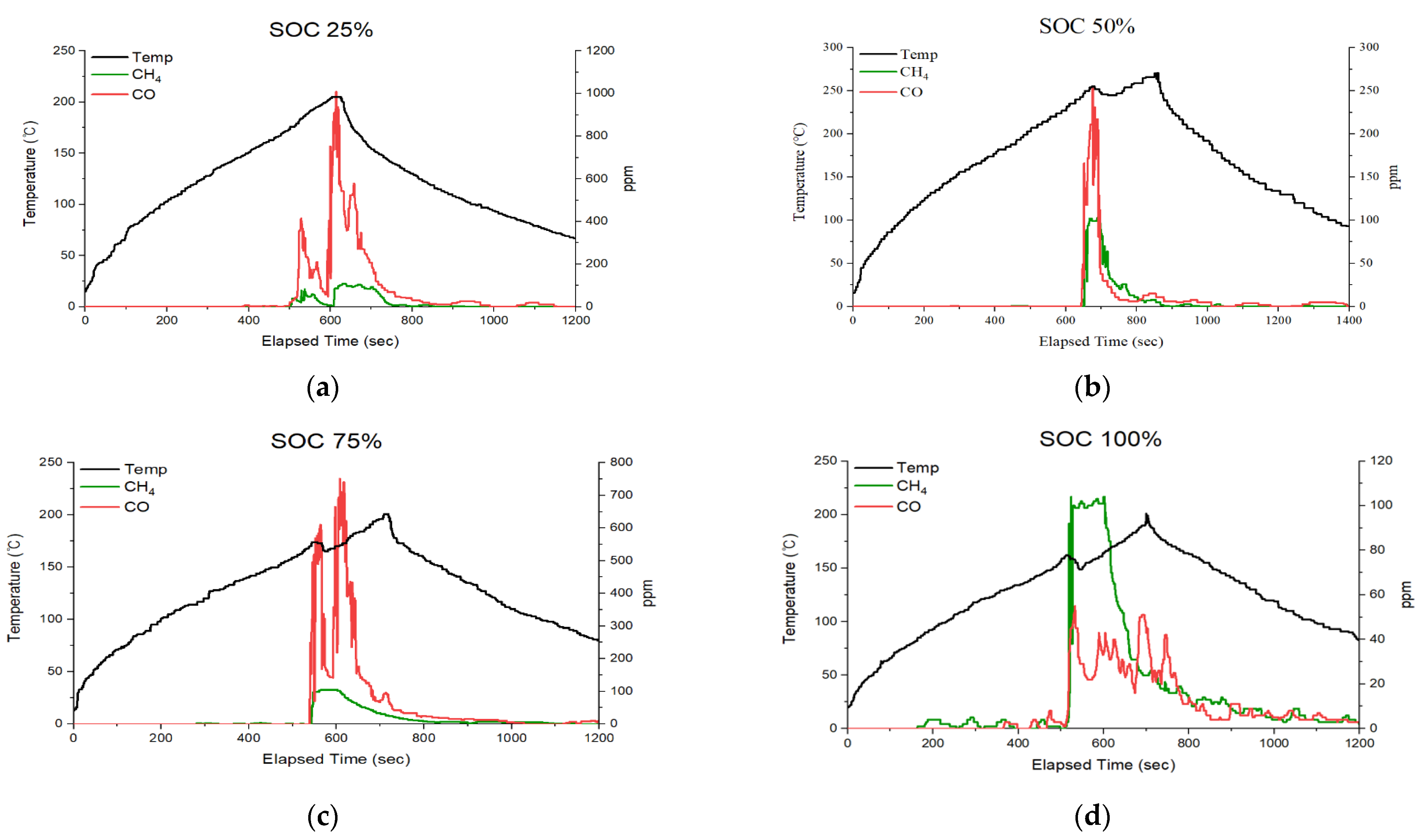
2.2. Problems in the Quantification of Thermal Runaway Probability Using Off-Gases
3. Application of the Expert Inference Method and Trend Analysis and Effectiveness Verification According to the Thermal Runaway Risk Calculation Method
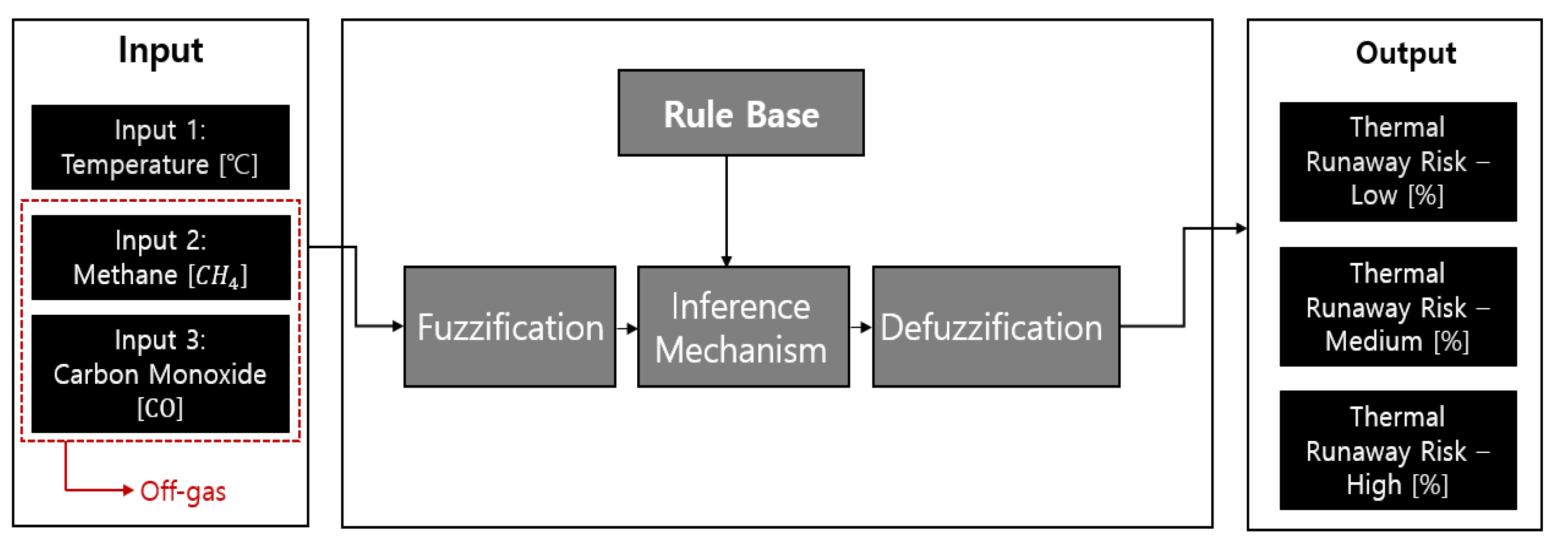
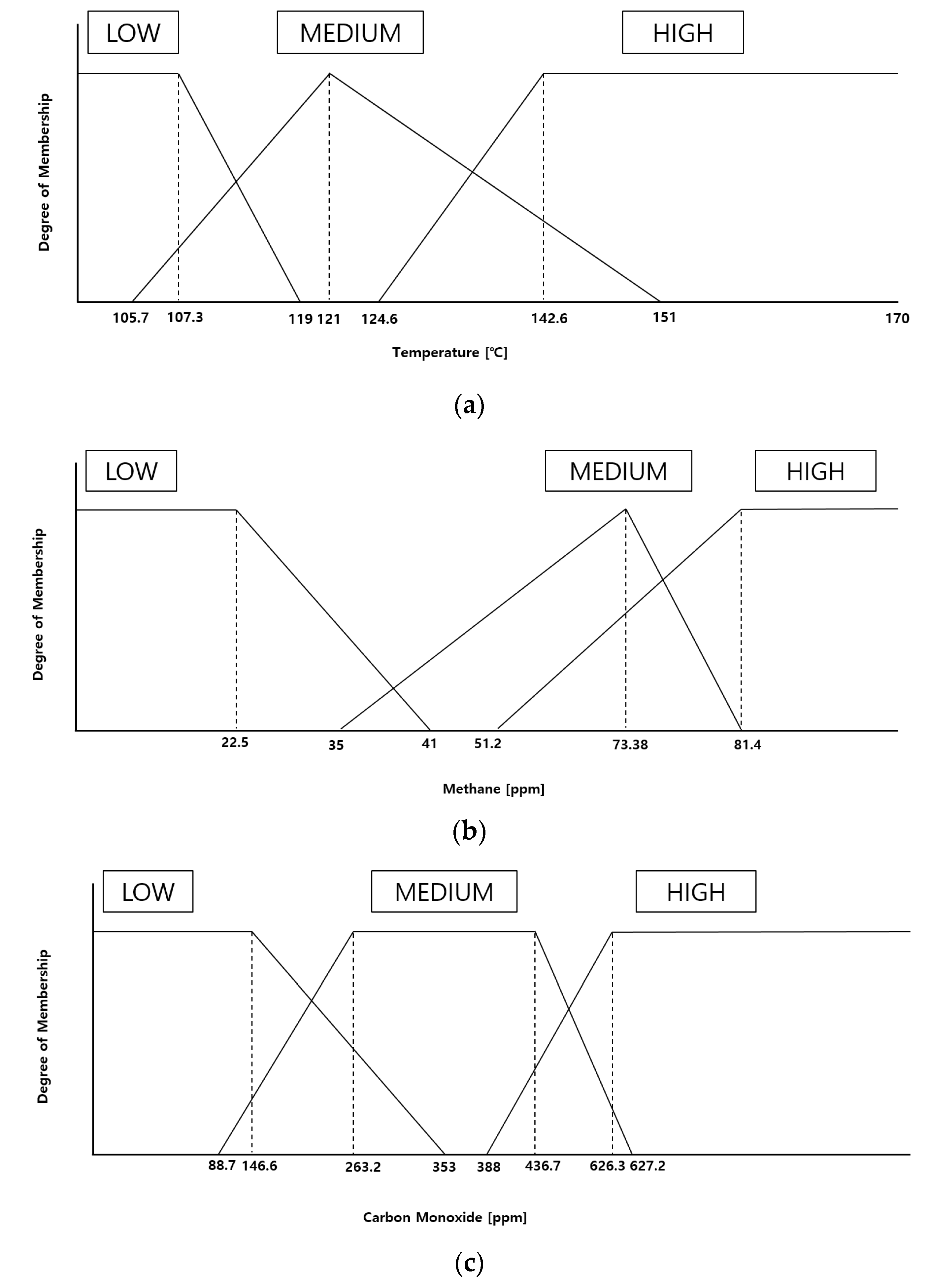
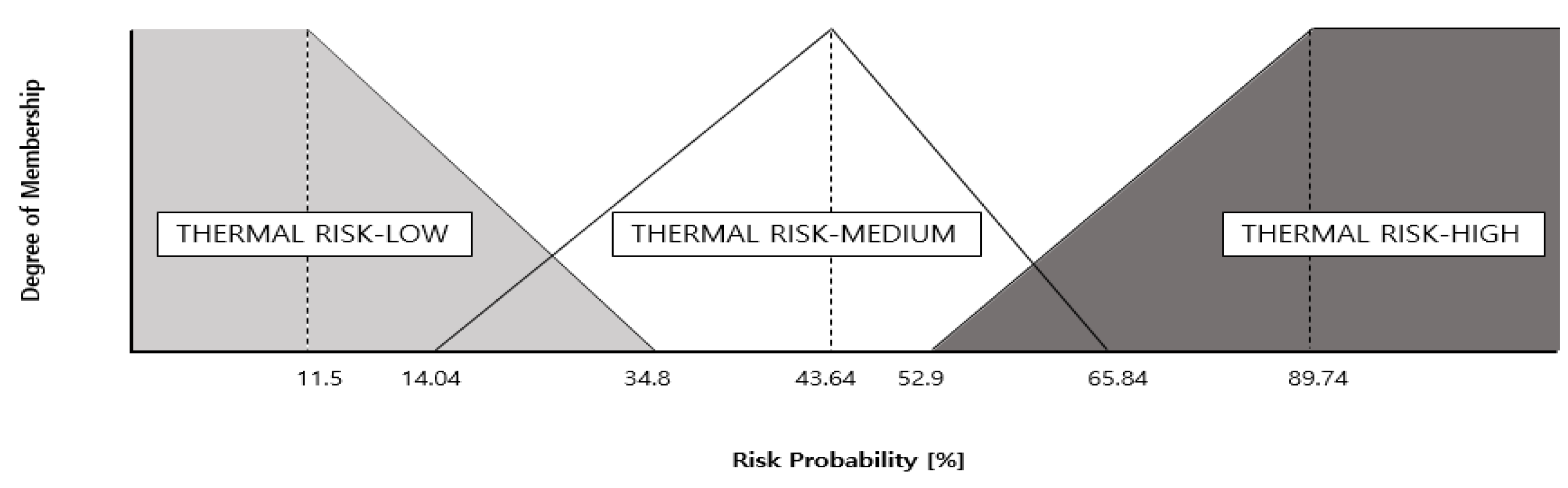
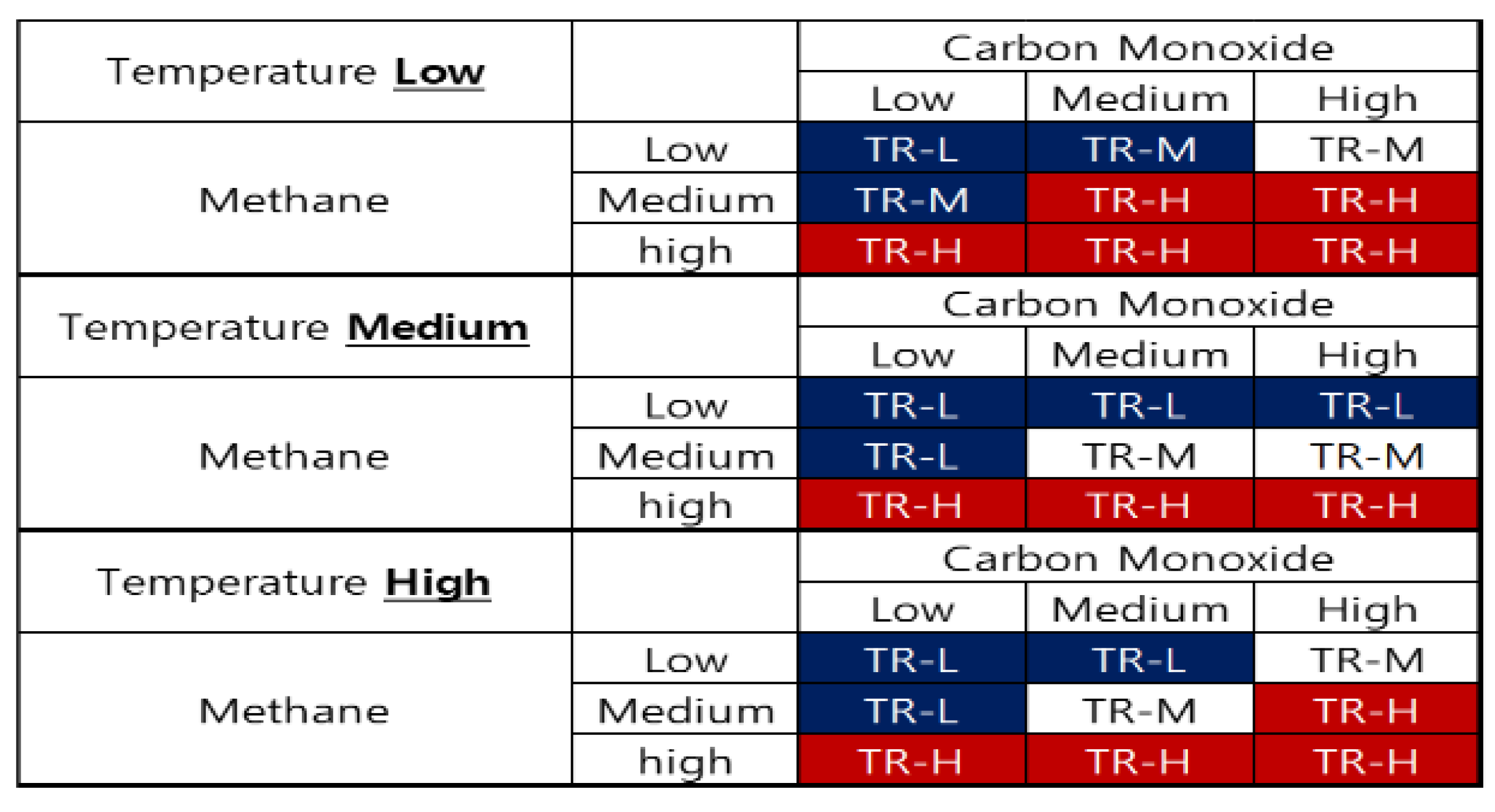
3.1. Proposal of Compositional Reasoning Design Utilizing Expert Inference Methods through Off-Gas Detection
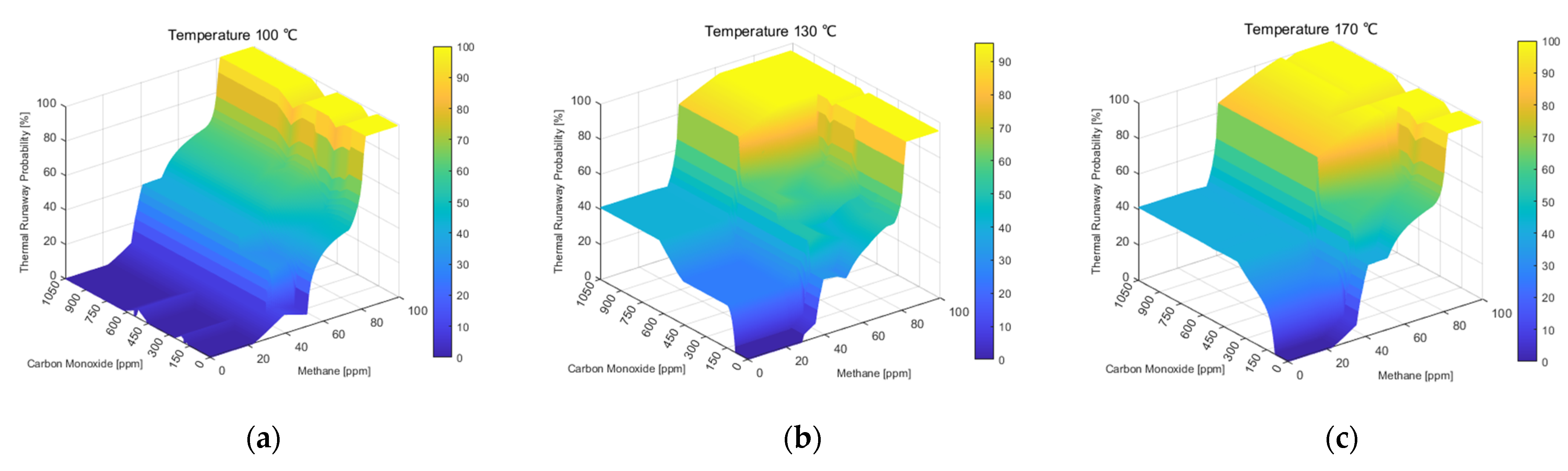
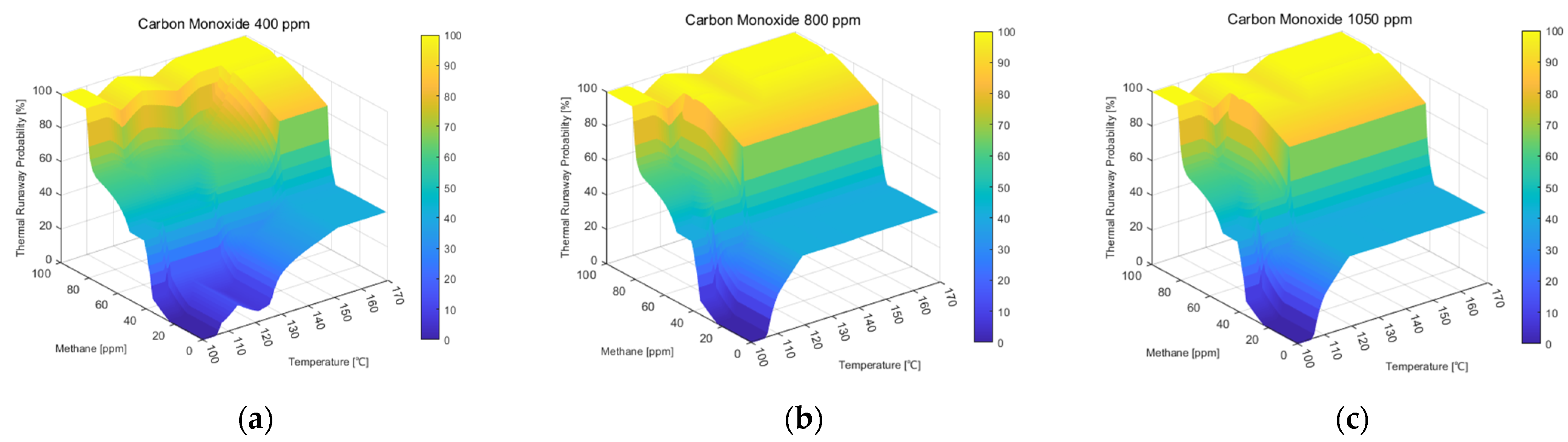
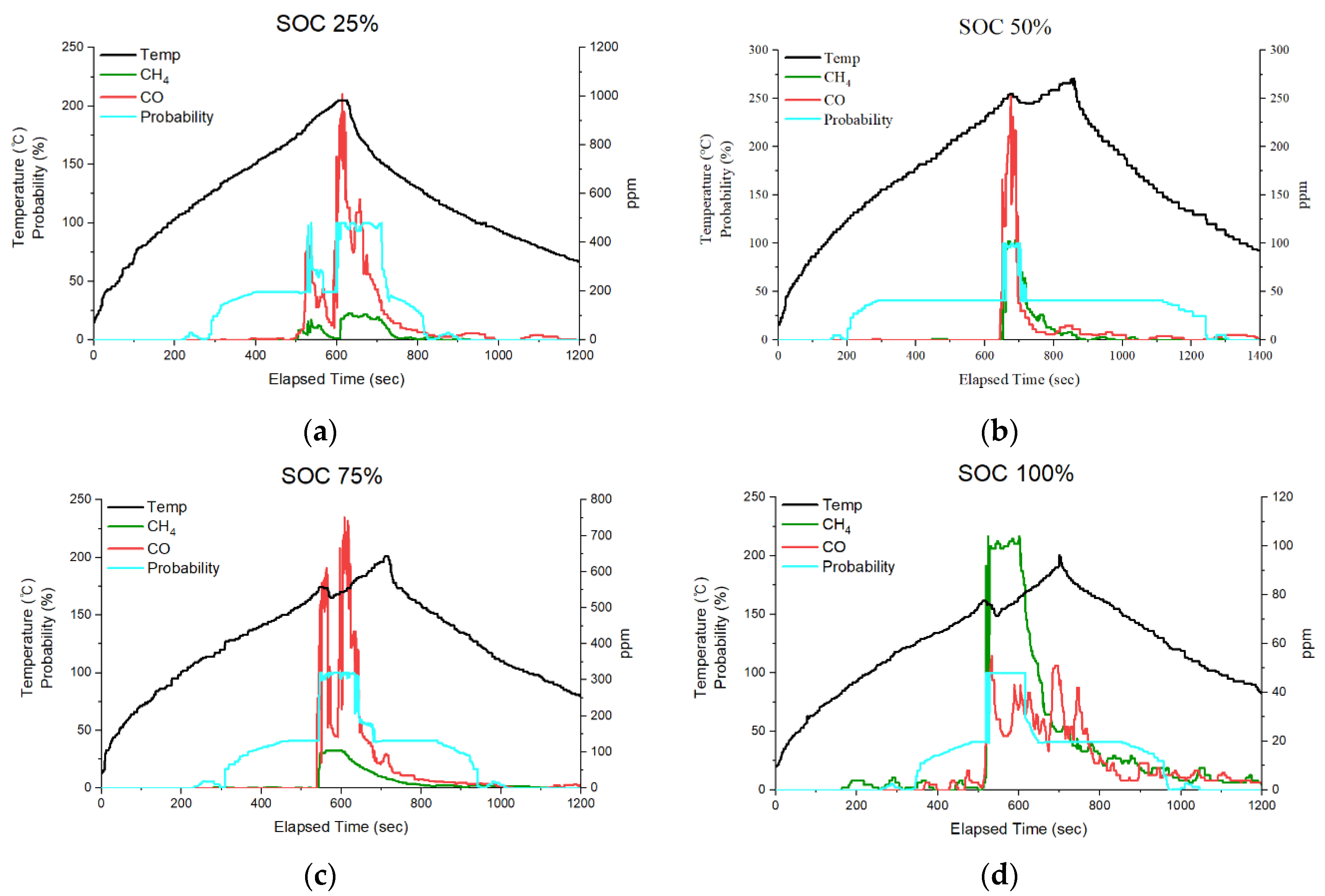
3.2. Trend Analysis According to the Proposed Thermal Runaway Risk Calculation Method
3.3. Verification of the Effectiveness of the Proposed Thermal Runaway Risk Calculation Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seoul National University. A Development on the Electrode Materials for Li Ion Secondary Batteries; Seoul National University: Seoul, Republic of Korea, 1999. [Google Scholar]
- Ioakimidis, C.S.; Murillo-Marrodán, A.; Bagheri, A.; Thomas, D.; Genikomsakis, K.N. Life Cycle Assessment of a Lithium Iron Phosphate (LFP) Electric Vehicle Battery in Second Life Application Scenarios. Sustainability 2019, 11, 2527. [Google Scholar] [CrossRef]
- Gang, M.B.; Kim, W.Y.; Kim, N.J. Numerical Comparative Study on the Thermal Runaway of NCM/LFP Batteries of the Same Geometry. J. Korean Soc. Geotherm. Hydrothermal. 2022, 18, 1–11. [Google Scholar]
- Song, Y.W. Manufacturing of All-Solid-State Lithium Secondary Batteries with Gel Polymer Electrolytes and Evaluation of Electrochemical Properties. Master’s Thesis, Chonnam National University, Gwangju, Republic of Korea, 2021. [Google Scholar]
- Lowe, M.; Tokuoka, S.; Trigg, T.; Gereffi, G. Lithium-ion Batteries for Electric Vehicles: The U.S. Value Chain; Duke University Center on Globalization, Governance & Competitiveness: Durham, NC, USA, 2010. [Google Scholar] [CrossRef]
- Batteries, L.I.R. Lithium Ion Rechargeable Batteries Technical Handbook; Panasonic: Tokyo, Japan, 2013; pp. 27–29. [Google Scholar]
- Kim, D.H. A Study on the Explosive Hazard of Lithium-ion Batteries and the Separation Distance for Storage and Handling of Spent Lithium Secondary Batteries. Master’s Thesis, Inha University Graduate School, Incheon, Republic of Korea, 2021. [Google Scholar]
- Kang, K.S. Analysis of Factors Increasing Thermal Runaway Hazard in Lithium-ion Battery Hi-nickel NCM Cathode Materials. Unpublished. Doctoral Dissertation, Seoul Metropolitan University Graduate School, Seoul, Republic of Korea, 2024. [Google Scholar]
- Lee, M.A. A Study on the Establishment of Response Systems for Thermal Runaway Fires in Electric Vehicle Charging Infrastructure: Focused on Comparison with Chinese Standards. Master’s Thesis, Gachon University Graduate School of Industrial Environment, Seongnam, Republic of Korea, 2023. [Google Scholar]
- Oh, E.Y.; Min, D.S.; Han, J.Y.; Jung, S.H.; Kang, T.S. Consequence Analysis of Toxic Gases Generated by Fire of Lithium Ion Batteries in Electric Vehicles. J. Korean Inst. Gas 2019, 23, 54–61. [Google Scholar] [CrossRef]
- Lee, S.; Park, J. Early Detection of Lithium-Ion Battery Thermal Runaway for Enhanced Safety in Electric Vehicle Applications. Energies 2022, 15, 1234–1256. [Google Scholar]
- Ahn, S.; Kim, H. Real-time Gas and Temperature Monitoring System for Lithium-ion Battery Safety. Energies 2021, 14, 2468. [Google Scholar]
- Hasoun, M.; Afia, A.E.; Chikh, K.; Khafallah, M.; Benkirane, K. Early Detection of Thermal Runaway in Lithium-ion Batteries by Coupling Differential Scanning Calorimetry with Acoustic Emission. In Proceedings of the 2019 International Conference of Computer Science and Renewable Energies (ICCSRE), Agadir, Morocco, 22–24 July 2019; pp. 1–6. [Google Scholar]
- Hong, S.H.; Choi, K.O.; Park, M.O.; Park, J.M.; Lee, S.H. An Experimental Study on the Fire Hazards of Cylindrical Lithium-ion Battery. Korean J. Hazard. Mater. 2019, 7, 64–69. [Google Scholar] [CrossRef]
- Lim, B.J.; Cho, S.H.; Lee, G.R.; Choi, S.M.; Park, C.D. Characteristics Analysis of Measurement Variables for Detecting Anomaly Signs of Thermal Runaway in Lithium-Ion Batteries. Trans. Korean Hydrog. New Energy Soc. 2022, 33, 85–94. [Google Scholar] [CrossRef]
- KFPA. Final Report on Fire Safety Measures for Energy Storage Devices. Fire Research Institute Policy Research Data. 2019. Available online: https://www.nfa.go.kr/nfa/publicrelations/policyarchive/policyresearch/?boardId=bbs_0000000000000613&mode=view&cntId=17&category=&pageIdx=&searchCondition=&searchKeyword= (accessed on 29 February 2024).
- Hwang, S.Y.; Choi, S.M.; Jian, S.; Choi, H.S.; Rh, D.S. A Study on the Detection Algorithm of Off-gas to Prevent Thermal runaway of Li-ion Battery for ESS. Trans. Korean Inst. Electr. Eng. 2022, 71, 1787–1795. [Google Scholar] [CrossRef]
- Lai, X.; Yao, J.; Jin, C.; Feng, X.; Wang, H.; Xu, C.; Zheng, Y. A Review of Lithium-Ion Battery Failure Hazards: Test Standards, Accident Analysis, and Safety Suggestions. Batteries 2022, 8, 248. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, J.M.; Lee, M.G.; Lim, M.G.; Rho, D.S. A Study on the Assessment Algorithm for Fire Stages in Li-ion based ESS. Korea Acad.-Ind. Coop. Soc. 2023, 339–342. Available online: https://www.kais99.org/jkais/springNfall/spring2023/oral/2023_spring_104.pdf (accessed on 29 February 2024).
- Chen, M.; Liu, J.; Ouyang, D.; Weng, J.; Wu, X.; Cao, S.; Wang, J. A large-scale experimental study on the thermal failure propagation behaviors of primary lithium batteries. J. Energy Storage 2020, 31, 101657. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, J.; Zhai, H.; Wang, Q. Experimental investigation on the characteristics of thermal runaway and its propagation of large-format lithium ion batteries under overcharging and overheating conditions. Energy 2021, 233, 121103. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, C.; Li, H.; Peng, W.; Zhang, Z.; Wang, Q. Experimental study on thermal runaway and its propagation in the large format lithium-ion battery module with two electrical connection modes. Energy 2020, 205, 117906. [Google Scholar] [CrossRef]
- Jia, Y.; Uddin, M.; Li, Y.; Xu, J. Thermal runaway propagation behavior within 18,650 lithium-ion battery packs: A modeling study. J. Energy Storage 2020, 31, 101668. [Google Scholar] [CrossRef]
- Kong, D.; Wang, G.; Ping, P.; Wen, J. A coupled conjugate heat transfer and CFD model for the thermal runaway evolution and jet fire of 18650 lithium-ion battery under thermal abuse. eTransportation 2022, 12, 100157. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, F.; Feng, X.; Jiang, F.; Ren, D.; Lu, L.; Yang, Y.; Liu, G.; Han, X.; Friess, B.; et al. Experimental study on thermal runaway propagation of lithium-ion battery modules with different parallel-series hybrid connections. J. Clean. Prod. 2021, 284, 124749. [Google Scholar] [CrossRef]
- Yang, M.; Rong, M.; Pan, J.; Ye, Y.; Yang, A.; Chu, J.; Yuan, H.; Wang, X. Thermal runaway behavior analysis during overheating for commercial LiFePO4 batteries under various state of charges. Appl. Therm. Eng. 2023, 230 Pt B, 120816. [Google Scholar] [CrossRef]
- Mamdani, E.H.; Assilian, S. An experiment in linguistic synthesis with a fuzzy logic controller. Int. J. Man-Mach. Stud. 1975, 7, 1–13. [Google Scholar] [CrossRef]
- Tobi, T.; Hanafusa, T. A practical application of fuzzy control for an air-conditioning system. Int. J. Approx. Reason. 1991, 5, 331–348. [Google Scholar] [CrossRef]
- Omrane, H.; Masmoudi, M.S.; Masmoudi, M. Fuzzy Logic Based Control for Autonomous Mobile Robot Navigation. Comput. Intell. Neurosci. 2016, 2016, 9548482. [Google Scholar] [CrossRef]
- Rubio, F.R.; Berenguel, M.; Camacho, E.F. Fuzzy logic control of a solar power plant. IEEE Trans. Fuzzy Syst. 1995, 3, 459–468. [Google Scholar] [CrossRef]
- Mert, A. Defuzzification of Non-Linear Pentagonal Intuitionistic Fuzzy Numbers and Application in the Minimum Spanning Tree Problem. Symmetry 2023, 15, 1853. [Google Scholar] [CrossRef]
- Chakraborty, A.; Mondal, S.P.; Alam, S.; Ahmadian, A.; Senu, N.; De, D.; Salahshour, S. The Pentagonal Fuzzy Number: Its Different Representations, Properties, Ranking, Defuzzification and Application in Game Problems. Symmetry 2019, 11, 248. [Google Scholar] [CrossRef]
- Hofmann, P. Defuzzification Strategies for Fuzzy Classifications of Remote Sensing Data. Remote Sens. 2016, 8, 467. [Google Scholar] [CrossRef]
- Kwak, S.; Choi, B.-J. Defuzzification Scheme and Its Numerical Example for Fuzzy Logic based Control System. J. Korean Inst. Intell. Syst. 2018, 28, 350–354. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shon, J.W.; Choi, D.; Lee, H.; Son, S.-Y. Proposal and Verification of the Application of an Expert Inference Method to Present the Probability of Lithium-Ion Battery Thermal Runaway Risk. Energies 2024, 17, 2566. https://doi.org/10.3390/en17112566
Shon JW, Choi D, Lee H, Son S-Y. Proposal and Verification of the Application of an Expert Inference Method to Present the Probability of Lithium-Ion Battery Thermal Runaway Risk. Energies. 2024; 17(11):2566. https://doi.org/10.3390/en17112566
Chicago/Turabian StyleShon, Jong Won, Donmook Choi, Hyunjae Lee, and Sung-Yong Son. 2024. "Proposal and Verification of the Application of an Expert Inference Method to Present the Probability of Lithium-Ion Battery Thermal Runaway Risk" Energies 17, no. 11: 2566. https://doi.org/10.3390/en17112566
APA StyleShon, J. W., Choi, D., Lee, H., & Son, S.-Y. (2024). Proposal and Verification of the Application of an Expert Inference Method to Present the Probability of Lithium-Ion Battery Thermal Runaway Risk. Energies, 17(11), 2566. https://doi.org/10.3390/en17112566





