Combustion Characteristics of Sinusoidal-Shaped Walls with Catalyst Segmentation in Micro-Combustors for Micro-Thermophotovoltaic Application
Abstract
1. Introduction
- Heat recirculation
- 2.
- Bluff body
- 3.
- Porous medium
- 4.
- Nonplanar walls
- 5.
- Catalytic combustion
2. Numerical Procedure
2.1. Physical Model
2.2. Numerical Model
2.3. Chemical Kinetic Model
2.4. Grid Independence
2.5. Model Validation
3. Results
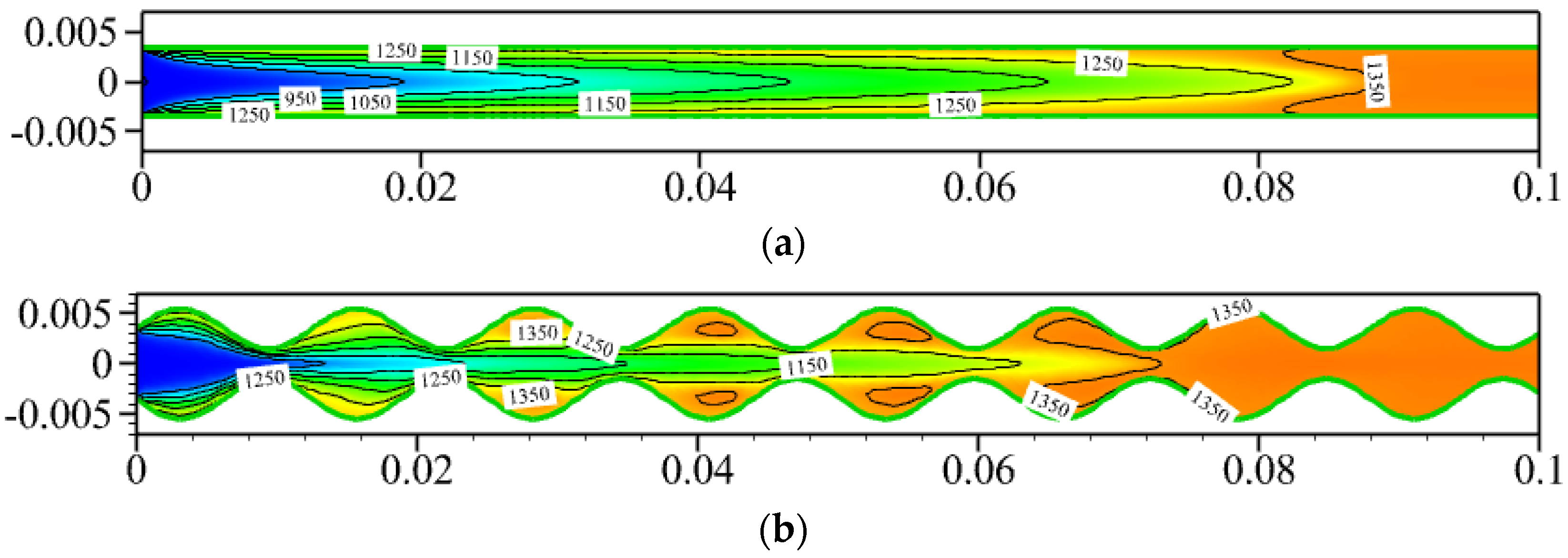
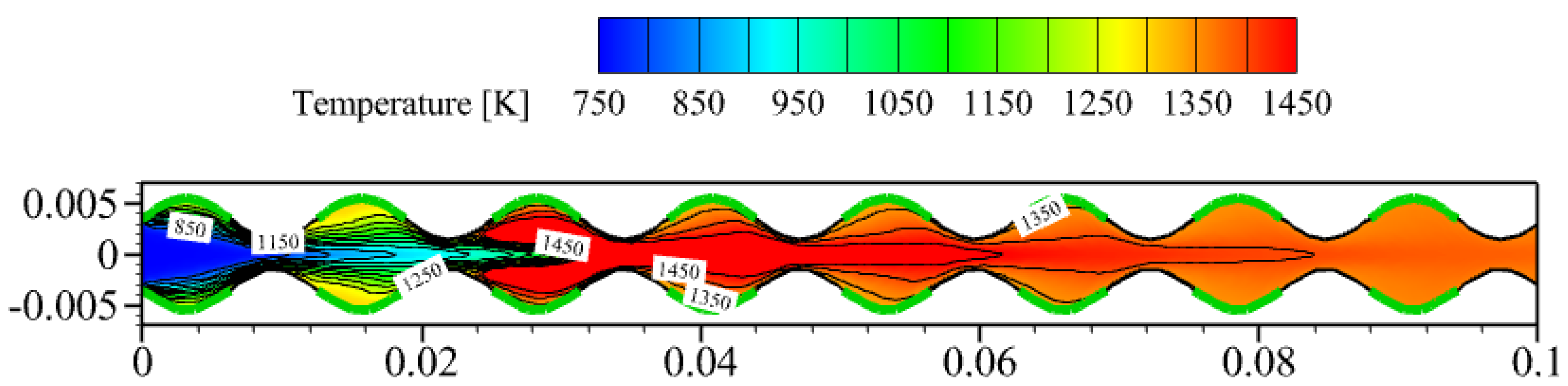
3.1. Effect of Sinusoidal-Shaped Wall
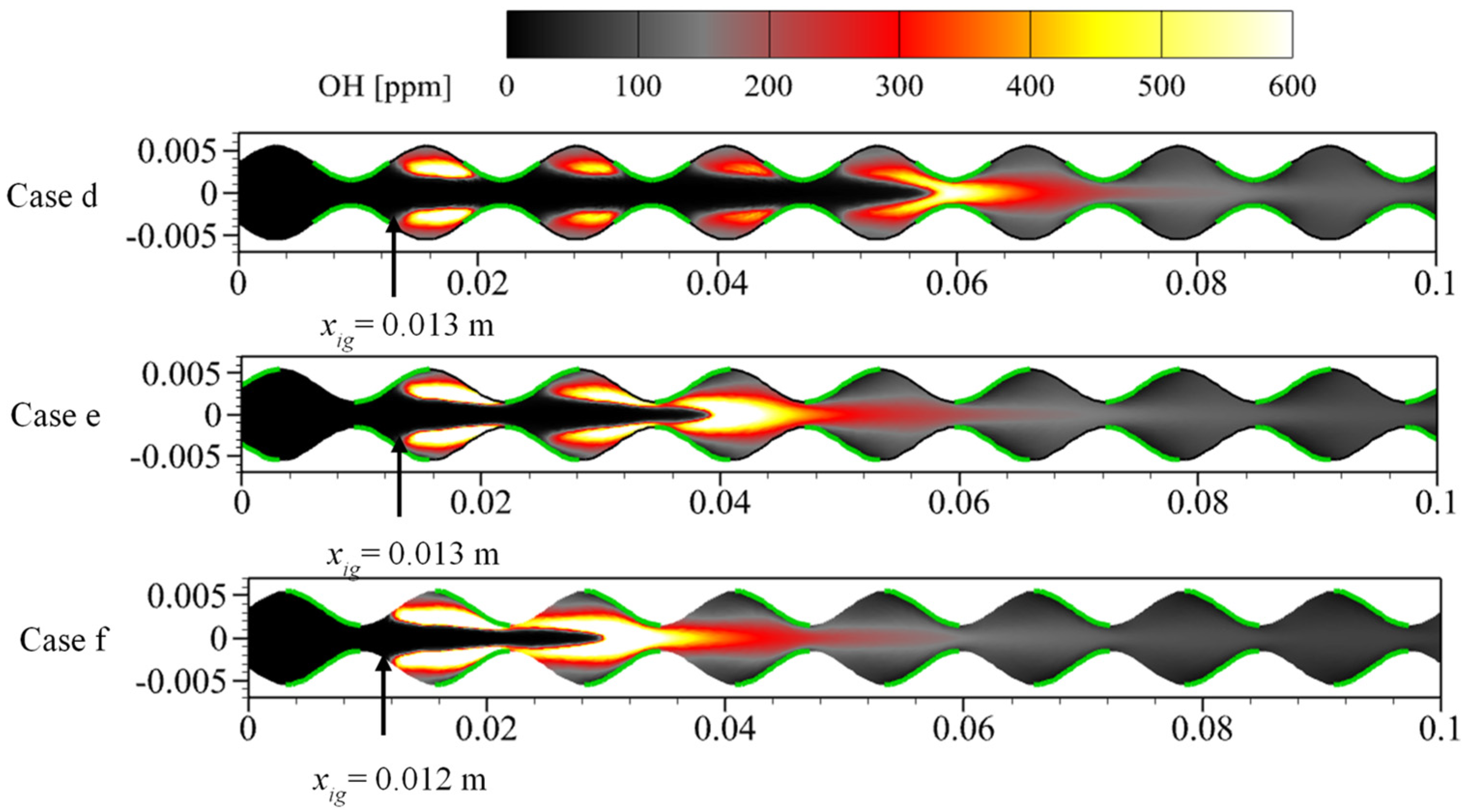
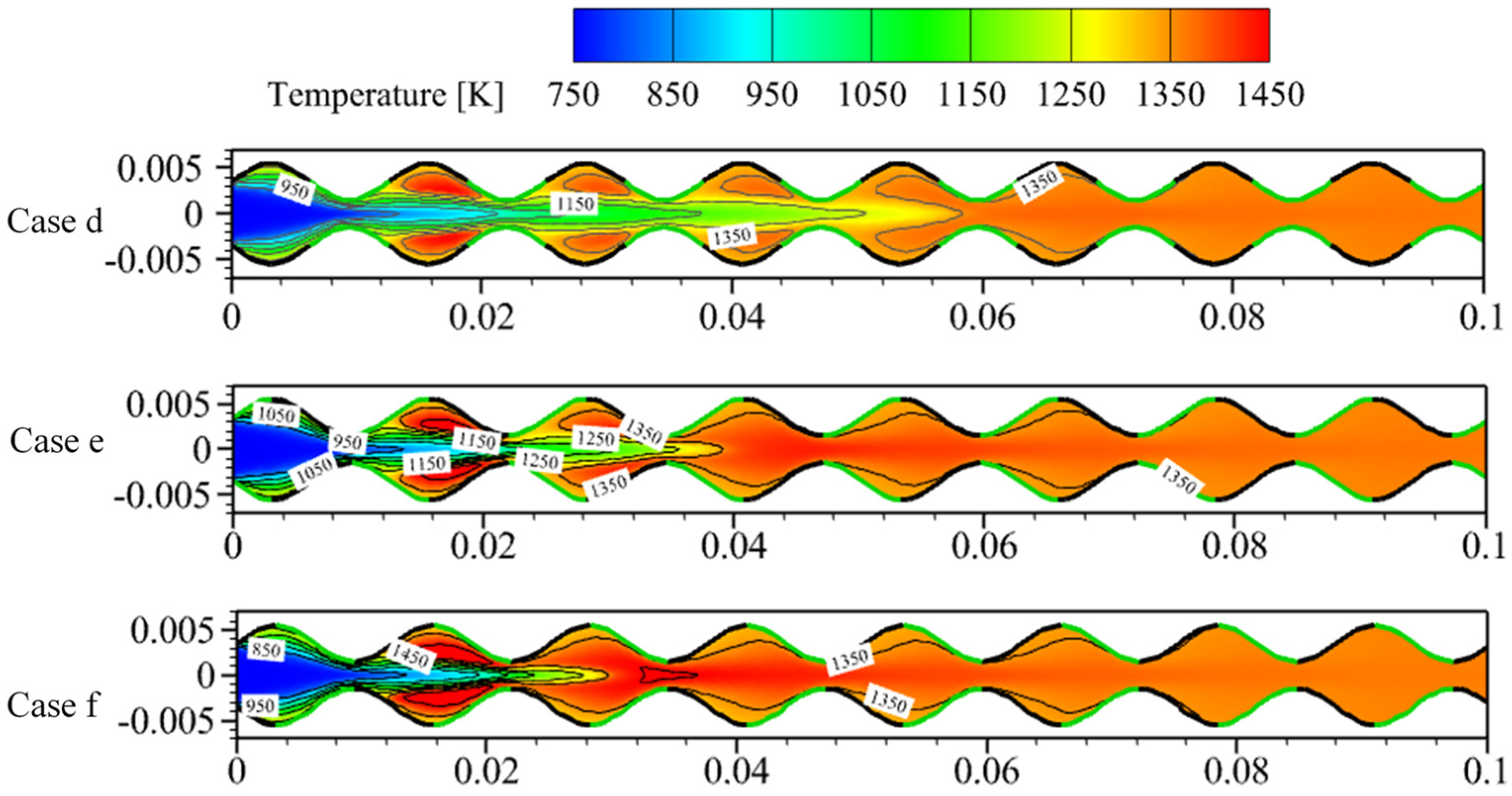
3.2. Effect of Catalyst Segmentation
3.3. Effect of Catalyst Segmentation Layout
4. Conclusions
- The cavities formed by the sinusoidal-shaped walls are highly favorable for the gas-phase reaction, as combustible mixtures in these cavities ignite more easily and are anchored, shifting the ignition position closer to the inlet by 0.050 m. However, the sinusoidal-shaped walls accelerate the flow velocity near the axis, leading to flame elongation and, consequently, increasing the combustor volume needed for complete methane combustion.
- Catalyst segments coated on the crest of the sinusoidal-shaped walls enhance the intensity of the gas-phase reaction, shorten the flame length, and increase the internal average temperature by a maximum of 62 K compared to the planar-wall combustor. However, the surface reaction is weaker, leading to the ignition position being far from the inlet. On the other hand, the catalyst segment coated on the trough enhances the surface reaction intensity and shortens the distance between the ignition position and the inlet of the combustor by around 0.064 m, but reduces the intensity of the gas-phase reaction, resulting in flame elongation.
- Catalyst segments coated on half of the crest-trough not only bring the ignition position closer to the inlet by a maximum of 0.065 m but also shorten the flame length, improving the internal temperature while substantially reducing the length of the combustor required for complete fuel conversion by more than 60%.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dunn-Rankin, D.; Leal, E.M.; Walther, D.C. Personal Power Systems. Prog. Energy Combust. Sci. 2005, 31, 422–465. [Google Scholar] [CrossRef]
- Walther, D.C.; Ahn, J. Advances and Challenges in the Development of Power-Generation Systems at Small Scales. Prog. Energy Combust. Sci. 2011, 37, 583–610. [Google Scholar] [CrossRef]
- Lloyd, S.A.; Weinberg, F.J. A Burner for Mixtures of Very Low Heat Content. Nature 1974, 251, 47–49. [Google Scholar] [CrossRef]
- Sitzki, L.; Borer, K.; Wussow, S.; Maruta, E.; Ronney, P. Combustion in Microscale Heat-Recirculating Burners. In 39th Aerospace Sciences Meeting and Exhibit; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2012. [Google Scholar]
- Tang, A.; Cai, T.; Huang, Q.; Deng, J.; Pan, J. Numerical Study on Energy Conversion Performance of Micro-Thermophotovoltaic System Adopting a Heat Recirculation Micro-Combustor. Fuel Process. Technol. 2018, 180, 23–31. [Google Scholar] [CrossRef]
- Taywade, U.W.; Deshpande, A.A.; Kumar, S. Thermal Performance of a Micro Combustor with Heat Recirculation. Fuel Process. Technol. 2013, 109, 179–188. [Google Scholar] [CrossRef]
- Wan, J.; Fan, A.; Maruta, K.; Yao, H.; Liu, W. Experimental and Numerical Investigation on Combustion Characteristics of Premixed Hydrogen/Air Flame in a Micro-Combustor with a Bluff Body. Int. J. Hydrogen Energy 2012, 37, 19190–19197. [Google Scholar] [CrossRef]
- Fan, A.; Wan, J.; Liu, Y.; Pi, B.; Yao, H.; Maruta, K.; Liu, W. The Effect of the Blockage Ratio on the Blow-off Limit of a Hydrogen/Air Flame in a Planar Micro-Combustor with a Bluff Body. Int. J. Hydrogen Energy 2013, 38, 11438–11445. [Google Scholar] [CrossRef]
- Peng, Q.; E, J.; Chen, J.; Zuo, W.; Zhao, X.; Zhang, Z. Investigation on the Effects of Wall Thickness and Porous Media on the Thermal Performance of a Non-Premixed Hydrogen Fueled Cylindrical Micro Combustor. Energy Convers. Manag. 2018, 155, 276–286. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, W.; E, J.; Xu, H.; Li, Z.; Tay, K.; Zeng, G.; Yu, W. Investigation on Premixed H2/C3H8/Air Combustion in Porous Medium Combustor for the Micro Thermophotovoltaic Application. Appl. Energy 2020, 260, 114352. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, W.; E, J.; Xu, H.; Li, Z.; Yu, W.; Tu, Y.; Wu, Y. Experimental Investigation on Premixed Hydrogen/Air Combustion in Varied Size Combustors Inserted with Porous Medium for Thermophotovoltaic System Applications. Energy Convers. Manag. 2019, 200, 112086. [Google Scholar] [CrossRef]
- Yang, W.M.; Chou, S.K.; Chua, K.J.; Li, J.; Zhao, X. Research on Modular Micro Combustor-Radiator with and without Porous Media. Chem. Eng. J. 2011, 168, 799–802. [Google Scholar] [CrossRef]
- Chabane, A.M.; Truffin, K.; Angelberger, C. Direct Numerical Simulation of Catalytic Combustion in a Meso-Scale Channel with Non-Planar Walls. Combust. Flame 2020, 222, 85–102. [Google Scholar] [CrossRef]
- Peng, Q.; E, J.; Zhang, Z.; Hu, W.; Zhao, X. Investigation on the Effects of Front-Cavity on Flame Location and Thermal Performance of a Cylindrical Micro Combustor. Appl. Therm. Eng. 2018, 130, 541–551. [Google Scholar] [CrossRef]
- Wan, J.; Fan, A.; Yao, H.; Liu, W. Flame-Anchoring Mechanisms of a Micro Cavity-Combustor for Premixed H2/Air Flame. Chem. Eng. J. 2015, 275, 17–26. [Google Scholar] [CrossRef]
- Yang, W.M.; Chou, S.K.; Shu, C.; Li, Z.W.; Xue, H. Combustion in Micro-Cylindrical Combustors with and without a Backward Facing Step. Appl. Therm. Eng. 2002, 22, 1777–1787. [Google Scholar] [CrossRef]
- Pan, J.F.; Huang, J.; Li, D.T.; Yang, W.M.; Tang, W.X.; Xue, H. Effects of Major Parameters on Micro-Combustion for Thermophotovoltaic Energy Conversion. Appl. Therm. Eng. 2007, 27, 1089–1095. [Google Scholar] [CrossRef]
- Khandelwal, B.; Sahota, G.P.S.; Kumar, S. Investigations into the Flame Stability Limits in a Backward Step Micro Scale Combustor with Premixed Methane–Air Mixtures. J. Micromech. Microeng. 2010, 20, 095030. [Google Scholar] [CrossRef]
- Bahaidarah, H.M.S.; Anand, N.K.; Chen, H.C. Numerical Study of Heat and Momentum Transfer in Channels with Wavy Walls. Numer. Heat Transf. Part A Appl. 2005, 47, 417–439. [Google Scholar] [CrossRef]
- Mansouri, Z. Combustion in Wavy Micro-Channels for Thermo-Photovoltaic Applications—Part I: Effects of Wavy Wall Geometry, Wall Temperature Profile and Reaction Mechanism. Energy Convers. Manag. 2019, 198, 111155. [Google Scholar] [CrossRef]
- Mansouri, Z. A Novel Wavy Micro-Combustor for Micro-Thermophotovoltaic Applications. Chem. Eng. Process.-Process Intensif. 2021, 163, 108371. [Google Scholar] [CrossRef]
- Han, L.; Li, J.; Zhao, D.; Xi, Y.; Gu, X.; Wang, N. Effect Analysis on Energy Conversion Enhancement and NOx Emission Reduction of Ammonia/Hydrogen Fuelled Wavy Micro-Combustor for Micro-Thermophotovoltaic Application. Fuel 2021, 289, 119755. [Google Scholar] [CrossRef]
- Kaisare, N.S.; Deshmukh, S.R.; Vlachos, D.G. Stability and Performance of Catalytic Microreactors: Simulations of Propane Catalytic Combustion on Pt. Chem. Eng. Sci. 2008, 63, 1098–1116. [Google Scholar] [CrossRef]
- Maruta, K.; Takeda, K.; Ahn, J.; Borer, K.; Sitzki, L.; Ronney, P.D.; Deutschmann, O. Extinction Limits of Catalytic Combustion in Microchannels. Proc. Combust. Inst. 2002, 29, 957–963. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Di Sarli, V.; Russo, G. A Novel Catalytic-Homogenous Micro-Combustor. Catal. Today 2009, 147, S156–S161. [Google Scholar] [CrossRef]
- Li, J.; Im, H.G. Effects of Dilution on the Extinction Characteristics of Strained Lean Premixed Flames Assisted by Catalytic Reaction. Proc. Combust. Inst. 2007, 31, 1189–1195. [Google Scholar] [CrossRef]
- Karagiannidis, S.; Mantzaras, J.; Jackson, G.; Boulouchos, K. Hetero-/Homogeneous Combustion and Stability Maps in Methane-Fueled Catalytic Microreactors. Proc. Combust. Inst. 2007, 31, 3309–3317. [Google Scholar] [CrossRef]
- Li, Y.-H.; Chen, G.-B.; Hsu, H.-W.; Chao, Y.-C. Enhancement of Methane Combustion in Microchannels: Effects of Catalyst Segmentation and Cavities. Chem. Eng. J. 2010, 160, 715–722. [Google Scholar] [CrossRef]
- Li, Y.-H.; Chen, G.-B.; Wu, F.-H.; Cheng, T.-S.; Chao, Y.-C. Effects of Catalyst Segmentation with Cavities on Combustion Enhancement of Blended Fuels in a Micro Channel. Combust. Flame 2012, 159, 1644–1651. [Google Scholar] [CrossRef]
- Li, Y.-H.; Chen, G.-B.; Wu, F.-H.; Cheng, T.-S.; Chao, Y.-C. Combustion Characteristics in a Small-Scale Reactor with Catalyst Segmentation and Cavities. Proc. Combust. Inst. 2013, 34, 2253–2259. [Google Scholar] [CrossRef]
- Ju, Y.; Maruta, K. Microscale Combustion: Technology Development and Fundamental Research. Prog. Energy Combust. Sci. 2011, 37, 669–715. [Google Scholar] [CrossRef]
- Dogwiler, U.; Mantzaras, J.; Benz, P.; Kaeppeli, B.; Bombach, R.; Arnold, A. Homogeneous Ignition of Methane-Air Mixtures over Platinum: Comparison of Measurements and Detailed Numerical Predictions. Symp. (Int.) Combust. 1998, 27, 2275–2282. [Google Scholar] [CrossRef]
- Reinke, M.; Mantzaras, J.; Schaeren, R.; Bombach, R.; Kreutner, W.; Inauen, A. Homogeneous Ignition in High-Pressure Combustion of Methane/Air over Platinum: Comparison of Measurements and Detailed Numerical Predictions. Proc. Combust. Inst. 2002, 29, 1021–1029. [Google Scholar] [CrossRef]
- Chen, G.-B.; Chen, C.-P.; Wu, C.-Y.; Chao, Y.-C. Effects of Catalytic Walls on Hydrogen/Air Combustion inside a Micro-Tube. Appl. Catal. A Gen. 2007, 332, 89–97. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Seo, S.; Broda, J.C.; Pal, S.; Santoro, R.J. An Experimental Estimation of Mean Reaction Rate and Flame Structure during Combustion Instability in a Lean Premixed Gas Turbine Combustor. Proc. Combust. Inst. 2000, 28, 775–782. [Google Scholar] [CrossRef]
- Warnatz, J.; Maas, U.; Dibble, R.W. Combustion; Springer: Berlin/Heidelberg, Germany, 1996; ISBN 978-3-642-97670-4. [Google Scholar]
- Deutschmann, O.; Maier, L.I.; Riedel, U.; Stroemman, A.H.; Dibble, R.W. Hydrogen Assisted Catalytic Combustion of Methane on Platinum. Catal. Today 2000, 59, 141–150. [Google Scholar] [CrossRef]
- Deutschmann, O.; Schmidt, R.; Behrendt, F.; Warnat, J. Numerical Modeling of Catalytic Ignition. Symp. (Int.) Combust. 1996, 26, 1747–1754. [Google Scholar] [CrossRef]
- Reinke, M.; Mantzaras, J.; Schaeren, R.; Bombach, R.; Inauen, A.; Schenker, S. High-Pressure Catalytic Combustion of Methane over Platinum: In Situ Experiments and Detailed Numerical Predictions. Combust. Flame 2004, 136, 217–240. [Google Scholar] [CrossRef]
- Chen, G.-B.; Chao, Y.-C.; Chen, C.-P. Enhancement of Hydrogen Reaction in a Micro-Channel by Catalyst Segmentation. Int. J. Hydrogen Energy 2008, 33, 2586–2595. [Google Scholar] [CrossRef]
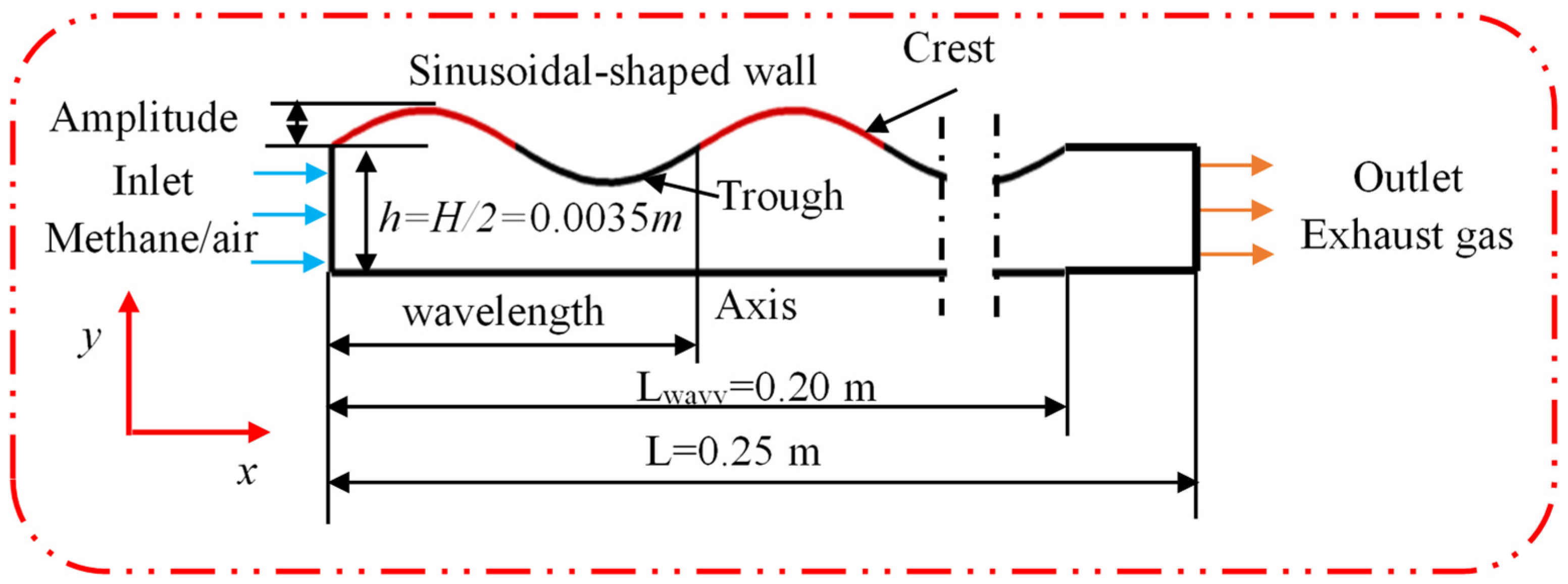

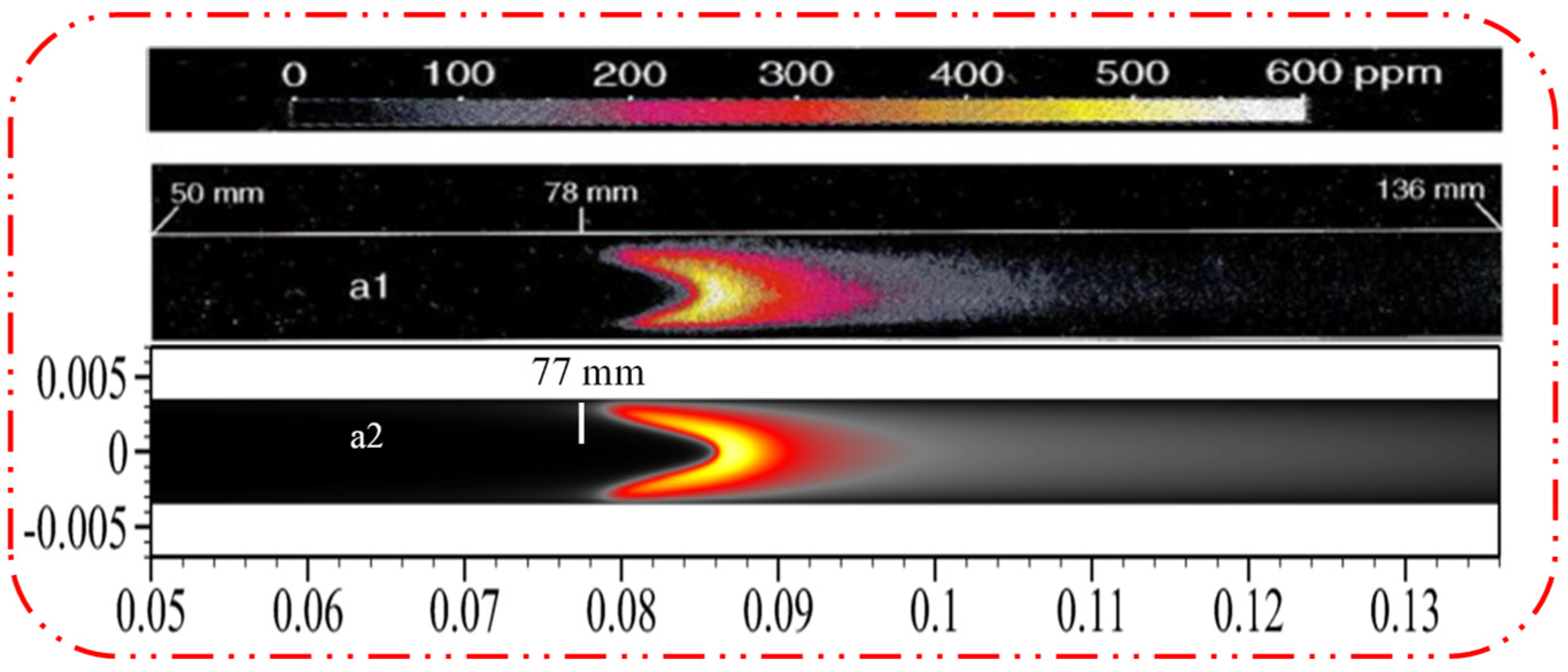
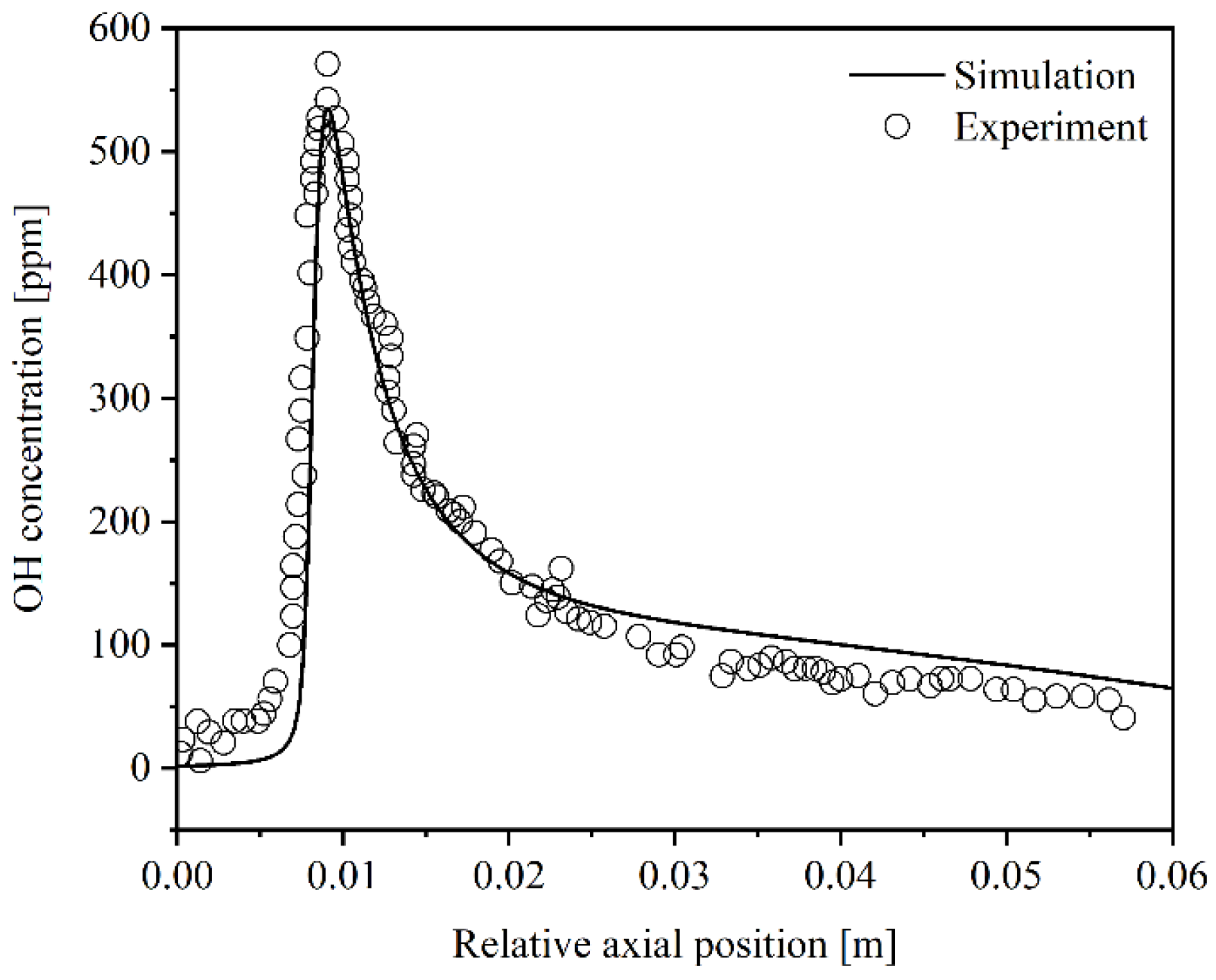
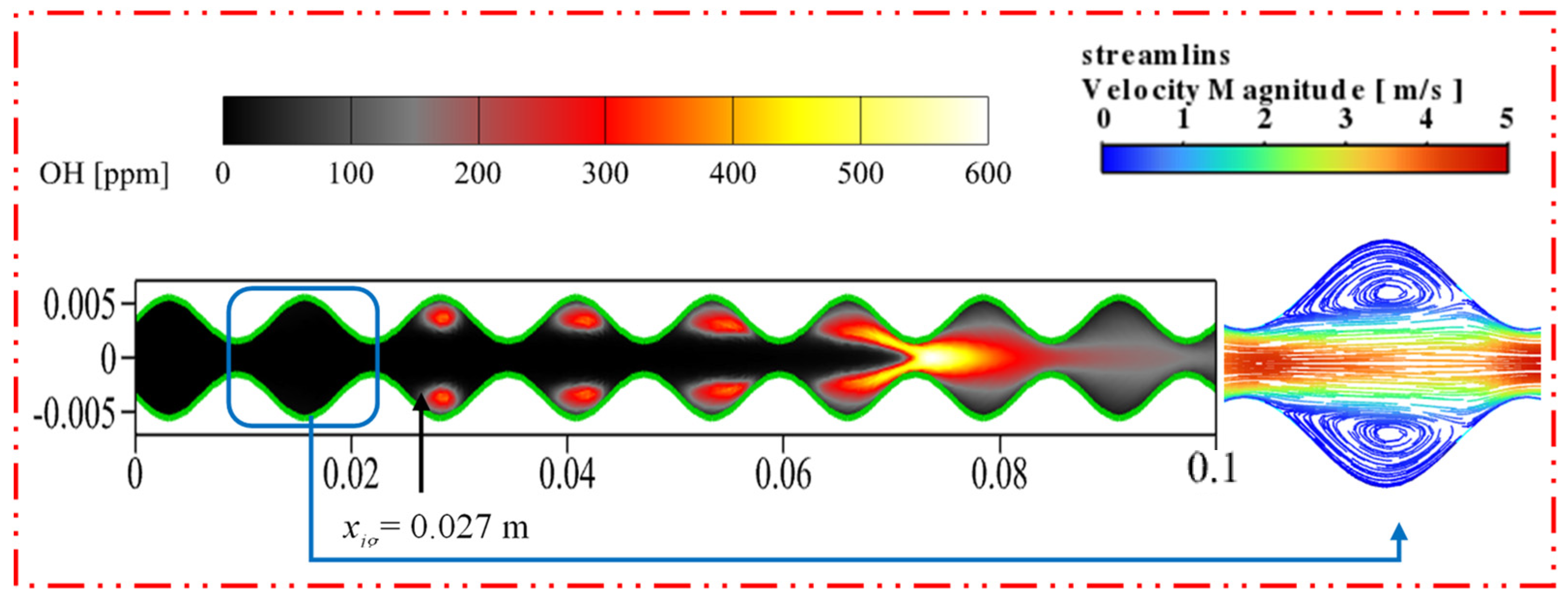


| Cases | Wall Shape | Catalyst Layout |
|---|---|---|
| a | Planar wall | Full coating |
| b | Sinusoidal-shaped wall | Full coating |
| c | Segmented coated on the crest | |
| d | Segmented coated on the trough | |
| e | Segmented coated on the half of trough-crest | |
| f | Segmented coated on the half of crest-trough |
| Parameters | Values |
|---|---|
| Inlet velocity | vinlet = 1 m/s |
| Equivalence ratio | Φ = 0.37 |
| Mass fraction of CH4 | |
| Mass fraction of O2 | |
| Inlet temperature | Tinlet = 750 K |
| Reynolds number | Re = 186 |
| Case | Maximum Mole Fraction of OH Radical [ppm] | Tavg [K] | Methane Complete Conversion Position xmcc [m] | Pmcc |
|---|---|---|---|---|
| a | 476.5 | 1280.2 | 0.089 | - |
| b | 510.6 | 1314.6 | 0.074 | 16.85% |
| c | 1909.4 | 1342.2 | 0.033 | 62.92% |
| d | 810.3 | 1327.0 | 0.061 | 31.46% |
| e | 1143.4 | 1335.6 | 0.041 | 53.93% |
| f | 1259.8 | 1338.0 | 0.031 | 65.17% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Q.; Guo, Z.; Li, Y. Combustion Characteristics of Sinusoidal-Shaped Walls with Catalyst Segmentation in Micro-Combustors for Micro-Thermophotovoltaic Application. Energies 2024, 17, 2560. https://doi.org/10.3390/en17112560
Yuan Q, Guo Z, Li Y. Combustion Characteristics of Sinusoidal-Shaped Walls with Catalyst Segmentation in Micro-Combustors for Micro-Thermophotovoltaic Application. Energies. 2024; 17(11):2560. https://doi.org/10.3390/en17112560
Chicago/Turabian StyleYuan, Qi, Zhiping Guo, and Yuan Li. 2024. "Combustion Characteristics of Sinusoidal-Shaped Walls with Catalyst Segmentation in Micro-Combustors for Micro-Thermophotovoltaic Application" Energies 17, no. 11: 2560. https://doi.org/10.3390/en17112560
APA StyleYuan, Q., Guo, Z., & Li, Y. (2024). Combustion Characteristics of Sinusoidal-Shaped Walls with Catalyst Segmentation in Micro-Combustors for Micro-Thermophotovoltaic Application. Energies, 17(11), 2560. https://doi.org/10.3390/en17112560






