Abstract
Waste heat recovery from exhaust gas is one of the most convenient methods to save energy in internal combustion engine-driven vehicles. This paper aims to investigate a reduction in waste heat from the exhaust gas of an internal combustion engine of a serial Diesel–electric hybrid bus by recovering part of the heat and converting it into useful power with the help of a split-flow supercritical CO2 (sCO2) recompression Brayton cycle. It can recover 17.01 kW of the total 33.47 kW of waste heat contained in exhaust gas from a 151 kW internal combustion engine. The thermal efficiency of the cycle is 38.51%, and the net power of the cycle is 6.55 kW. The variation in the sCO2 temperature at the shutdown of the internal combustion engine is analyzed, and a slow drop followed by a sudden and then a slow drop is observed. After 80 s from stopping the engine, the temperature drops by (23–33)% depending on the tube thickness of the recovery heat exchanger. The performances (net power, thermal efficiency, and waste heat recovery efficiency) of the split-flow sCO2 recompression Brayton cycle are clearly superior to those of the steam Rankine cycle and the organic Rankine cycle (ORC) with cyclopentane as a working fluid.
1. Introduction
The road transport sector is responsible for 1/3 of the final energy consumed in the EU and for 1/5 of the EU’s greenhouse gas emissions. Trucks and buses (heavy-duty vehicles) contribute 25% to road transport greenhouse gas emissions in the EU and 6% to total EU greenhouse gas emissions [1].
As emissions from road transport are increasing, the European Commission has proposed new and ambitious targets for new heavy-duty vehicles from 2030 in order for the EU to achieve climate neutrality in 2050. Currently, heavy vehicles in the EU are powered by internal combustion engines. The new CO2 standards will have the effect of both reducing the consumption of fossil fuels through energy efficiency increase and the electrification of vehicles, as well as increasing air quality and the health of EU citizens since heavy vehicles travel in urban areas [1].
Although the energy efficiency of heavy vehicles has increased significantly lately, there are many concerns about increasing the efficiency of internal combustion engines. One of these concerns relates to the recovery of waste heat discharged from the engine. The main technologies available for waste heat recovery are as follows: thermoelectric generators [2]; thermoacoustic generators [3]; turbocompound systems [4]; technologies using thermal cycles (steam Rankine [5]; organic Rankine [6]; Stirling [7] and Brayton with air [8] or with CO2 [9]) and refrigeration systems [10]). The purpose of waste heat recovery systems is to recover as much heat as possible to convert it into electricity or cold. The performance of these systems depends on the choice of the heat source and the working fluid, the cycle configuration, the performance of the components (heat exchangers, pumps, compressors, turbines), and the operating mode of internal combustion engines (variation in the operating regime as a result of the variation in road conditions determines the variation in temperature and the exhaust gas flow rate). Two solutions have been proposed to reduce the variation in engine exhaust gas characteristics: (i) the use of control strategies that monitor the engine’s operating status and adjust the waste heat recovery system and (ii) the use of heat storage technology [11]. The only internal combustion engines that operate at almost constant speed, regardless of road conditions, are those on series hybrid vehicles. In these vehicles, the internal combustion engine is completely mechanically decoupled from the driving wheels, and all the energy produced by it is converted into electricity by the generator, which supplies one or more electric traction motors or recharges the electric accumulator. Because the internal combustion engine is not directly connected to the wheels, it can be smaller than the engine should be for a vehicle of the same size; in addition, it runs at a constant speed when optimized for the lowest fuel consumption and the lowest CO2 emission for a certain period of time, followed by a period of inactivity (standstill). The average frequency of stops and starts is (60–70) stop/h, with an average duration of (15–50) min, which represents an average downtime of (25–40)% [12]. Thus, the fuel consumption of Diesel hybrid vehicles can be lower by about (18–29)% than that of conventional standard Diesel vehicles [13].
The efficiency of series hybrid vehicle engines can be further increased by applying a waste heat recovery system. Several studies [14,15,16] show that operation at a constant engine load is an advantage in the implementation of waste heat recovery systems. This paper thermodynamically analyses waste heat recovery from the Diesel engine exhaust gas of a series hybrid bus to increase engine power using the split-flow sCO2 recompression Brayton cycle. This thermodynamic cycle was chosen for engine exhaust temperatures of about 400 °C (after catalytic treatment and particulate filter) and power less than 1MW, where the most suitable cycles are the Brayton cycle with supercritical CO2 [17], the steam Rankine cycle [18,19] and the ORC with ethanol or cyclopentane [20,21].
All investigations related to the application of waste heat recovery technology using a thermal cycle for heavy-duty Diesel engine vehicles aimed at obtaining maximum thermal efficiency, reducing CO2 emissions, maximum heat recovery, minimizing the heat transfer area/net power output ratio or minimizing the period of investment recovery [22].
Depending on the objectives pursued, a cycle with a certain working fluid, a certain configuration, and a certain type of heat exchangers and expanders resulted. Because organic working fluids tend to decompose at high temperatures, limiting the inlet temperature to the expander, and the constant temperature evaporation process creates a large temperature difference between the working fluid and the heat source, resulting in reduced utilization of the heat source and an increase in the conversion efficiency of ORC systems is limited [23].
In the sCO2 Brayton cycle, the weak points of ORC are not encountered due to the thermophysical properties of CO2 (a non-toxic, stable, and non-flammable fluid) around the critical point that allow the introduction of heat into the cycle and its removal from the cycle with the avoidance of constant temperature evaporation and condensation with better coupling of the working fluid to the heat sources. In other words, all the processes undergone by the working fluid occur only in the supercritical phase, which combines the properties of gas with those of a liquid. In addition, the high density of CO2 leads to a relatively high-power density, which allows reducing the dimensions of the compressor and the expander, as well as reducing the energy consumed by the compressor [24].
Paper [24] states that there is no optimal sCO2 Brayton cycle configuration that covers all operating conditions, but split-flow, recompression, and double-expansion cycles are the best performers. Also, paper [25] states that the split-flow cycle with recompression has the highest efficiency over the cycle with a single cycle having a double expansion.
For real closed Brayton cycles, the thermodynamic efficiency depends on the pressure ratio but also on the minimum pressure in the cycle, with a maximum value of around 80 bar [26]. The recuperated cycle has the maximum efficiency at a much lower value of the pressure ratio than the simple cycle, and, in addition, the efficiency variation curve is relatively flatter, which allows a more stable operation [26]. For the recuperated Brayton cycle, the efficiency increases with the increasing turbine inlet temperature and turbine efficiency and decreases with increasing pressure drop, heat loss, and minimum recuperator temperature [26].
The Brayton cycle with split flow and recompression has a higher efficiency at moderate and high values of the turbine inlet temperature than the simple cycle [26]. For the hot source temperature (liquid Na from a nuclear reactor) of 545 °C, the cycle with recompression has the highest thermal efficiency (43.83%) compared to the other cycle configurations [25].
The higher efficiency of the recompression cycle compared to the simple recuperated cycle is due to the two-stage heat recovery and the use of the additional compressor to divert part of the working fluid from the main compressor. The recompressed fraction of the working fluid significantly influences the thermal efficiency of the cycle. The optimum value of the recompressed fraction (influenced by the high-pressure and low-pressure values of the cycle and the efficiency of the heat recuperators) is around 0.4 [27]. The only disadvantage of the sCO2 Brayton cycle is that the lack of phase change in the cycle leads to lower heat transfer coefficients than in the classic cycle, which increases the size of the heat exchange surfaces [28].
Thermodynamic cycles with CO2 as the working fluid are increasingly attracting the attention of specialists, especially in the field of electricity production with nuclear reactors, electricity production using geothermal and solar energy, and in the field of waste heat recovery from gas turbines and naval internal combustion engines and more recently in the field of waste heat recovery from road vehicle internal combustion engines.
A Brayton cycle with transcritical CO2 has the following turbine inlet parameters: pressure of 130 bar and temperature of 200 °C, which can convert about 20% of the waste heat of the gas discharged by an engine into electrical energy [29].
In paper [30], it is shown that a transcritical CO2 Rankine cycle system with a preheater and regenerator can increase the power of a 43.8 kW internal combustion engine by 9 kW (by about 20%) via recovering waste heat from the cooling liquid and exhaust gas from the engine. This system proved to be significantly more efficient in terms of the simultaneous recovery of heat from the coolant and exhaust gas from the engine than the organic Rankine cycle with R123 as the working fluid.
As the CO2 Brayton cycle can increase by (10–11)%, the efficiency of a naval gas turbine can increase by recovering the waste heat of the discharged gas [31]. The study of a split-flow sCO2 Brayton cycle with recompression and regeneration coupled to a marine gas turbine demonstrated an increase in turbine thermal efficiency of 12.38% [32].
The preheated transcritical CO2 Rankine cycle can improve the power and efficiency of an internal combustion engine through the combined recovery of waste heat from the coolant and exhaust gas, with improved dynamic performance at various engine operating conditions [33].
The analysis results of a sCO2 Brayton/Rankine cycle system with regeneration and recompression for waste heat recovery from a naval turbine showed that the thermal efficiency of the turbine increased by 10%, the power increased by 25%, and the efficiency of the system was highly dependent on the composition and the temperature of the exhaust gas [34].
When the cooling source cannot cool the CO2 to condensation, as is the case with the waste heat recovery system from the exhaust gas of the internal combustion engines of vehicles, the transcritical CO2 Rankine cycle becomes the sCO2 Brayton cycle.
The current study aims at to analyze the split-flow sCO2 recompression Brayton cycle for waste heat recovery from the exhaust gas of a Diesel engine for a series-hybrid bus. The cycle performance (thermal efficiency, efficiency of heat recovery, net power) is compared to those of the steam Rankine cycle and ORC with cyclopentane. Unlike other studies, here, the variation in the system performance to the variation in the operating conditions is evaluated (exhaust gas and cold source temperature). The variation in sCO2 temperature at the turbine inlet when the exhaust gas flow is interrupted as a result of stopping the engine is also analyzed.
The contribution of the paper is to analyze the advantage of the split-off sCO2 recompression Brayton cycle in terms of thermal efficiency over more mature technologies used to recover waste heat from the exhaust gas of an internal combustion engine.
2. System Analysis
The configuration of the heat recovery system based on the split-flow sCO2 recompression Brayton cycle and the cycle thermodynamic processes are shown in Figure 1. The system consists of a heater (HE) for sCO2 heating, a gas turbine (GT), a high-temperature heat recuperator (HTR), a low-temperature heat recuperator (LTR), a flow splitter, a cooler (C), a compressor for the flow passed through the cooler (MC), a compressor for the rest of the flow (RC) and an alternator (A). The distribution of the sCO2 flow between the two compressors depends on the operating conditions of the system (heat addition and environmental temperature) [35].
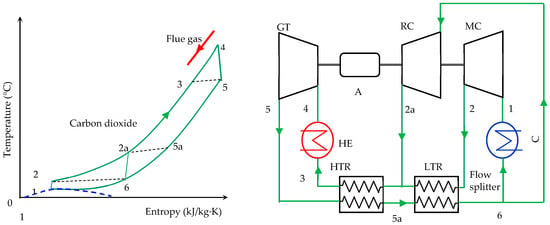
Figure 1.
Flow diagram and temperature–entropy diagram of the split-flow sCO2 recompression Brayton cycle.
3. Thermodynamic Model
The thermodynamic model of the split-flow sCO2 recompression Brayton cycle is given in Table 1.

Table 1.
Thermodynamic model of the split-flow sCO2 recompression Brayton cycle.
The enthalpy of the engine exhaust gas is calculated using the following equation:
where , , , —mass fraction of carbon dioxide, water vapor, and nitrogen and oxygen, respectively, in the engine exhaust gas;
hCO2(T), hN2(T), hO2(T), hH2O(T)—the specific enthalpy of carbon dioxide, water vapor, and nitrogen and oxygen corresponding to temperature T, kJ/kg (Table 2);

Table 2.
Specific enthalpy [36].
hl—latent heat of water vapor, hl = 2502 kJ/kg.
3.1. Model Validation
The thermodynamic model was implemented in a Matlab 9.10 program based on the CoolProp v6.4.1 [37] to simulate the operation of the waste heat recovery system in a steady state. To verify the proposed model, the split-flow sCO2 recompression Brayton cycle was simulated with the input data presented in [17], and the simulation results were compared. The cycle parameters and the results of the two simulations are given in Table 3.

Table 3.
Split-flow sCO2 recompression Brayton cycle.
It can be seen from Table 3 that there are small differences between the results of the two simulations (the highest at 4.925% in the case of the input heat flow and the lowest at 1.88% in the case of the power of the main compressor), which proves that the developed model is correct. These differences are due to the different values chosen for the isentropic efficiency of the turbine and compressors and for the effectiveness of the heat recuperators.
3.2. Simulation of Split-Flow sCO2 Recompression Brayton Cycle Applied to an Internal Combustion Engine of a Serial Diesel-Electric Hybrid Bus
The developed numerical model was applied to the split-flow sCO2 recompression Brayton cycle, which recovers heat from the exhaust gas of a Diesel engine from a series hybrid electric bus. The characteristics of a Diesel engine are given in Table 4.

Table 4.
Diesel engine characteristics.
Respecting the limits imposed by the thermal level of the heat source and the cooling source, the minimum temperature difference in the heat exchangers (especially in the heater and the cooler), and the optimal configuration of the heat recovery system based on split-flow sCO2 recompression Brayton cycle was chosen.
The data used for heat recovery system simulation are given in Table 5, and the simulation results for steady-state operation are presented in Table 6 and Table 7.

Table 5.
Input data for simulation of split-flow sCO2 recompression Brayton cycle.

Table 6.
Simulation results of split-flow sCO2 recompression Brayton cycle.

Table 7.
Parameters of simulated cycle.
The system can recover 17.01 kW of the total 33.47 kW contained in hot exhaust gases from the 151 kW Diesel engine and generates a net mechanical power of 9.6 kW, increasing the power of the engine by 6.33%.
As the Diesel engine of the series hybrid bus has periods of operation at constant load followed by periods of non-operation, it is desired to find out how the sCO2 temperature varies at the exit of the heater. When the engine is turned off, no more exhaust gas flows through the heater, and the CO2 heats up less, taking only the heat stored in the metal of the tubes through which it flows.
The variation over time in the average temperature of the tube Tt as a result of heat loss to the fluid flowing through it is described by the following equation:
where mt is tube mass, kg; ct is the specific heat of tube material, kJ/kg·K; is CO2 temperature, °C; Tt is the average tube temperature, °C; U is overall heat transfer coefficient, W/(m2·K):
in which di is the inner tube diameter, m; d0 is the outer tube diameter, m; λt is the tube thermal conductivity, W/(m·K); A is the heat transfer surface area, m2; αi is the heat transfer coefficient inside the tube, W/(m2·K).
To calculate the convection coefficient in the case of sCO2, it was assumed that the tube was horizontal and that the pressure in the tube was high. For these conditions, the following equation was adopted at the temperature of the wall (Tt) and the fluid (Tf) [38]:
where ; ; , kw is CO2 thermal conductivity at wall temperature is W/(m·K); ρw is CO2 density at wall temperature, kg/m3; ρf is CO2 density at fluid temperature, kg/m3; ρw is CO2 density at wall temperature, kg/m3; v is CO2 velocity inside the tube, m/s; cp,w is CO2-specific heat at wall temperature, J/(kg·K); cp,f is CO2-specific heat at fluid temperature, J/(kg·K); and μw is CO2 dynamic viscosity at fluid temperature, kg/(m·s).
The CO2 temperature variation as it flows through the tube is described by the following equation:
where is the mass of CO2 inside the tube, kg; is specific heat at a constant pressure of CO2, kJ/kg·K; and L is the tube length, m.
The two Equations (2) and (5) are linked by the following initial conditions:
A heat exchanger with tubes made of stainless steel (S34709 material) was chosen as a heat recovery device for CO2 heating. The characteristics of the heat exchanger are as follows: specific heat ct = 662 J/(kg·°C); thermal conductivity λt = 28.7 W/(m·°C); inner diameter of 21.7 mm, length of 2086.7 mm, and thickness of 4.9 mm [39]. The thermophysical properties (density, thermal conductivity, specific heat, and dynamic viscosity) of sCO2 were calculated using the data available in [40].
In Figure 2, a smooth decrease, followed by a sharp decrease, and again a smooth decrease can be seen in the sCO2 temperature at the exit of the heater. A total of 80 s after the engine stops, the temperature drops by 32.5% when the heat recovery tube thickness is 3 mm, by 27.5% when the thickness is 4 mm, and by 23.5% when the thickness is 5 mm.
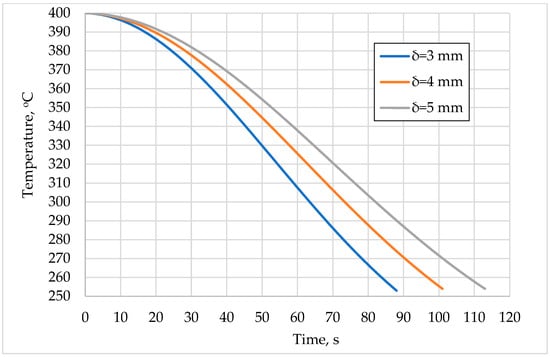
Figure 2.
Time variation in sCO2 temperature at heater outlet as function of non-operation time of engine and tube thickness (δ).
In addition to the variation in the characteristics of the heat source (engine exhaust gas), the cooling source can change its thermal level determined by the ambient temperature. Next, the variation in cycle thermal efficiency and heat recovery efficiency with the variation in the cooling source temperature, turbine inlet pressure, and temperature were analyzed.
Keeping the turbine inlet temperature and turbine outlet pressure unchanged, the variation in the thermal efficiency and the heat recovery efficiency with variation in turbine inlet pressure and the cooling source temperature was sought (Figure 3 and Figure 4).
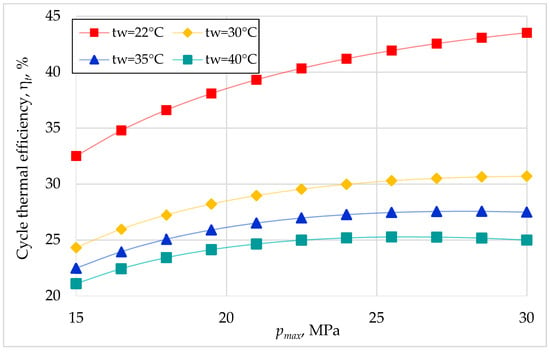
Figure 3.
Cycle thermal efficiency versus turbine inlet pressure for different cooling source temperatures (turbine inlet temperature 400 °C and turbine outlet pressure 7.4 MPa).
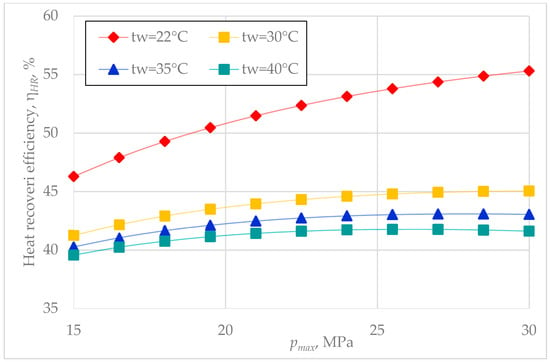
Figure 4.
Heat recovery efficiency versus turbine inlet pressure for different cooling water temperatures (turbine inlet temperature 400 °C and turbine outlet pressure 7.4 MPa).
Keeping the cooling source temperature and turbine outlet pressure unchanged, the variation in thermal efficiency and heat recovery efficiency was sought with the variation in turbine inlet pressure and temperature (Figure 5 and Figure 6).
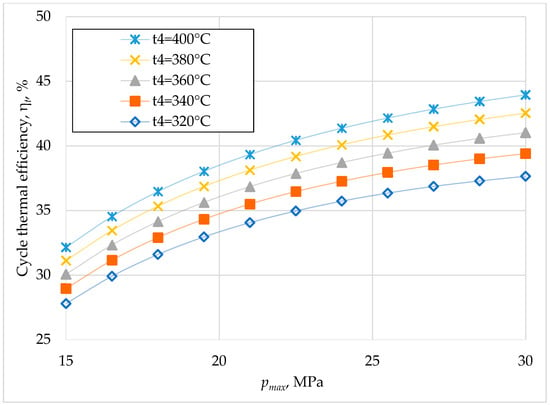
Figure 5.
Cycle thermal efficiency versus turbine inlet pressure for different turbine inlet temperatures (cooling water temperature 22 °C and turbine outlet pressure 7.4 MPa).
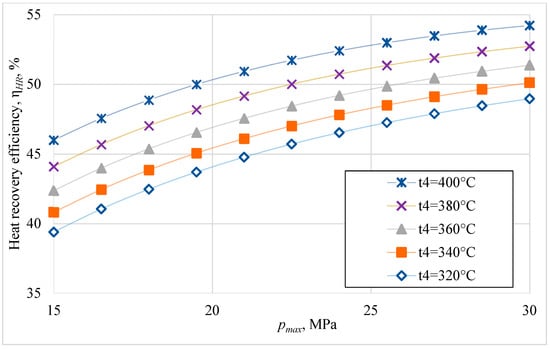
Figure 6.
Heat recovery efficiency versus turbine inlet pressure for different turbine inlet temperatures (cooling water temperature 22 °C and turbine outlet pressure 7.4 MPa).
It can be seen in Figure 3 that the cycle thermal efficiency has a greater variation with the turbine inlet pressure variation at lower cooling source temperatures. For high cooling source temperatures, the cycle efficiency increases with turbine inlet pressure up to a certain value, after which it remains constant.
The efficiency of waste heat recovery has similar variations to the thermal efficiency of the cycle (Figure 4). The thermal efficiency decreases with the decrease in the turbine inlet temperature, with this decrease being greater at higher values of the turbine inlet pressure (Figure 5). A decrease in the turbine inlet temperature of 60 °C for the turbine inlet pressure of 20 MPa causes a decrease in thermal efficiency by 15%. Considering the high rate of the temperature drop at the turbine inlet, when the internal combustion engine turns off (Figure 2), it is necessary to stop the heat recovery system when the internal combustion engine turns off. The waste heat recovery efficiency varies similarly to the thermal efficiency, with the difference that it decreases more at lower turbine inlet pressure values (Figure 6).
4. Performance Comparison of Split-Flow sCO2 Recompression Brayton Cycle with Other Cycles
To see which cycle performs better among those studied and presented in the literature, the performance of the split-flow sCO2 recompression Brayton cycle was compared with that of the steam Rankine cycle and the ORC with cyclopentane for the same characteristics of the hot source and the cold source.
The parameters of the steam Rankine cycle (Figure 7) were chosen in such a way that the steam quality at the turbine outlet was greater than 0.88, and the minimum temperature difference between the engine exhaust gas and the water in the Rankine cycle at the pinch point was at least 10 °C.
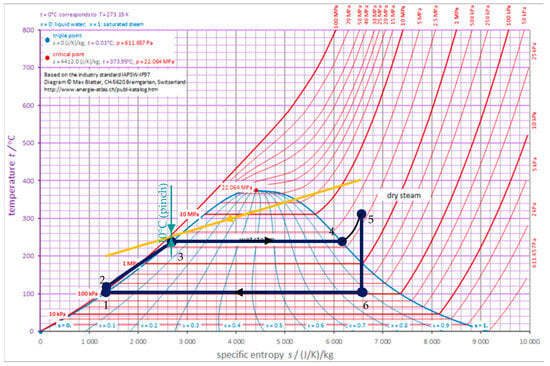
Figure 7.
Steam Rankine cycle.
The results of the cycle simulation using Matlab 9.10 and CoolProp v6.4.1 are presented in Table 8.

Table 8.
Simulation results of steam Rankine cycle.
Because cyclopentane decomposes at temperatures higher than 275 °C [41], was considered an intermediate circuit with thermal oil between the engine exhaust gas and the cyclopentane ORC, thus ensuring a safe operating temperature of 250 °C. The scheme and the T-s diagram of the cyclopentane ORC are given in Figure 8 and Figure 9.
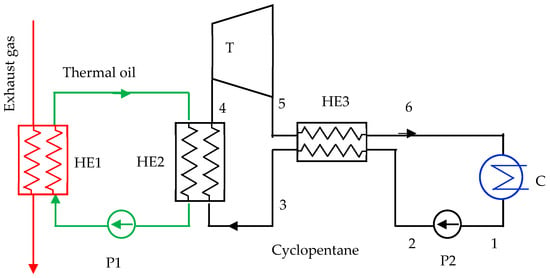
Figure 8.
Organic Rankine cycle with cyclopentane.
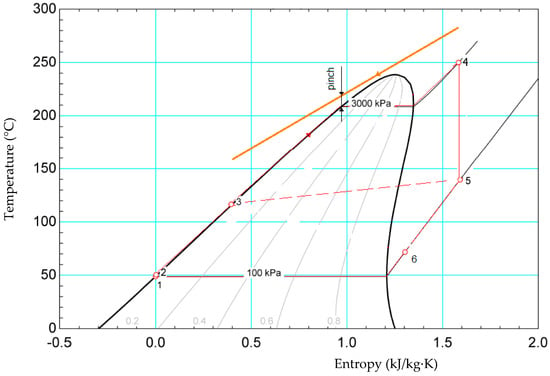
Figure 9.
Cyclopentane organic Rankine cycle.
The simulation results of cyclopentane ORC achieved using the Matlab 9.10 and CoolProp v6.4.1 are given in Table 9.

Table 9.
Simulation results of cyclopentane ORC.
In Table 10 and Figure 10, the performance of the three cycles for waste heat recovery of the engine exhaust gas is presented comparatively. It can be seen that the split-flow sCO2 recompression Brayton cycle has the highest net power, the highest thermal efficiency, and the highest heat recovery efficiency. This is explained by the fact that, in this cycle, the temperature variation curves of the heat source and the cycle working fluid are the closest compared to the other cycles. In the cyclopentane ORC and the steam Rankine cycle, with respect to the minimum pinch point temperature difference of 10 °C, the two curves were further apart.

Table 10.
Performance of split-flow sCO2 recompression Brayton cycle, steam Rankine cycle and cyclopentane ORC.
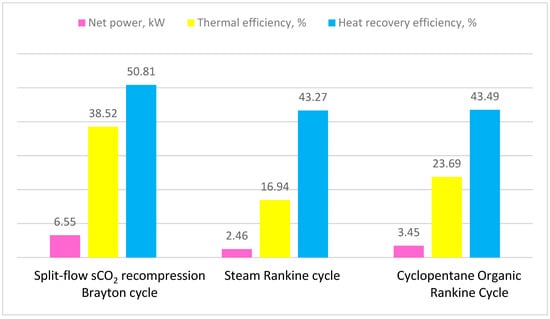
Figure 10.
Performance of the thermal cycles for waste heat recovery from engine exhaust gas.
The cyclopentane ORC cycle follows the split-flow sCO2 recompression Brayton cycle in terms of net power, thermal efficiency, and heat recovery efficiency. The higher performance compared to the steam Rankine cycle was due to the internal heat recovery.
Compared to technologies with a higher readiness level, only the turbocompound and combined cycles have a superior specific recoverable power (ratio between the electrical power produced by the recovery unit and the engine brake power) (3–20% and 3–7%, respectively [29]) and thermal efficiency (ratio between the output power and the inlet thermal power of the recovery unit) (55–70% and 8–40%, respectively [29]). The thermoelectric, ORC, Stirling engine, Inverted Brayton Cycle, and Trilateral Flash Cycle have lower performance (specific parameters) [29].
5. Conclusions
There are multiple studies related to waste heat recovery from the exhaust gas of internal combustion engines. This paper addresses the recovery of waste heat from the exhaust gas of the internal combustion engine of a series of hybrid electric buses using the split-flow sCO2 recompression Brayton cycle. A mathematical model of the heat exchange in the heat recuperator was developed, based on which the temperature variation in the CO2 was determined when the internal combustion engine was turned off. A mathematical model was developed, and the simulation of the thermal cycle was carried out for different operating conditions (turbine inlet temperature and pressure, cooling source temperature). It was observed that the performance of the cycle decreased greatly when the temperature at the turbine inlet was reduced, which led to the idea of stopping the cycle with the shutdown of the internal combustion engine or the use of a heat storage tank, which mitigated large variations in the temperature of the turbine inlet. For the same waste heat source characteristics, the net power, thermal efficiency, and heat recovery efficiency of the split-flow sCO2 recompression Brayton cycle are clearly superior to those of the steam Rankine cycle and cyclopentane ORC.
Author Contributions
Conceptualization, G.M. and I.V.I.; methodology, F.P.; software, C.I.; validation, G.M., M.F., and R.M.C.; formal analysis, F.P.; investigation, G.M.; resources, F.P.; data curation, C.I.; writing—original draft preparation, G.M.; writing—review and editing, I.V.I.; visualization, C.I.; supervision, F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Nomenclature
| Abbreviations or Symbol | |
| A | heat transfer surface area, m2; |
| cp | specific heat, kJ/(kg·K); |
| d | diameter, m; |
| h | enthalpy, kJ/kg |
| hl | latent heat of water vapor, kJ/kg |
| k | thermal conductivity, W/(m·K) |
| L | length, m |
| m | mass, kg |
| SR | flow split ratio |
| t | temperature, °C |
| T | temperature, K |
| U | overall heat transfer coefficient, W/(m2·K) |
| v | velocity, m/s |
| α | heat transfer coefficient, W/(m2·K) |
| δ | thickness, mm |
| ε | effectiveness |
| λ | thermal conductivity, W/(m·K) |
| η | efficiency |
| μ | dynamic viscosity, kg/(m·s). |
| ρ | density, kg/m3; |
| mass flow rate, kg/s | |
| power, kW | |
| Subscripts | |
| CO2 | carbon dioxide |
| env | environmental |
| exh | exhaust gas |
| f | fluid |
| GT | gas turbine |
| H2O | water |
| HR | heat recovery |
| HTR | higher temperature recuperator |
| i | inlet or inner |
| LTR | lower temperature recuperator |
| MC | main compressor |
| N2 | nitrogen |
| o | outlet/outer |
| O2 | oxygen |
| RC | recompressor |
| t | thermal or tube |
| w | cooling water |
References
- European Environment Agency. Available online: https://www.eea.europa.eu/en (accessed on 10 January 2024).
- Heber, L.; Schwab, J.; Knobelspies, T. 3 kW Thermoelectric Generator for Natural Gas-Powered Heavy-Duty Vehicles—Holistic Development, Optimization and Validation. Energies 2022, 15, 15. [Google Scholar] [CrossRef]
- Bou Nader, W.; Chamoun, J.; Dumand, C. Thermoacoustic engine as waste heat recovery system on extended range hybrid electric vehicles. Energy Convers. Manag. 2020, 215, 112912. [Google Scholar] [CrossRef]
- Aghaali, H.; Ångstrom, H.-E. A review of turbocompounding as a waste heat recovery system for internal combustion engines. Renew. Sustain. Energy Rev. 2015, 49, 813–824. [Google Scholar] [CrossRef]
- Freymann, R.; Ringler, J.; Seifert, M.; Horst, T. The second generation turbosteamer. MTZ Worldw. 2012, 73, 18–23. [Google Scholar] [CrossRef]
- Jelmer, R.; Olof, E.; Sven, B.A.; Karin, M. Exhaust waste heat recovery from a heavy-duty truck engine: Experiments and simulations. Energy 2022, 238 Pt B, 121698. [Google Scholar]
- Güven, M.; Bedir, H.; Anlas, G. Optimization and application of Stirling engine for waste heat recovery from a heavy-duty truck engine. Energy Convers. Manag. 2019, 180, 411–424. [Google Scholar] [CrossRef]
- Galindo, J.; Serrano, J.; Dolz, V.; Kleut, P. Brayton cycle for internal combustion engine exhaust gas waste heat recovery. Adv. Mech. Eng. 2015, 7, 1687814015590314. [Google Scholar] [CrossRef]
- Shi, L.; Shu, G.; Tian, H.; Chang, L.; Huang, G.; Chen, T. Experimental investigations on a CO2-based Transcritical Power Cycle (CTPC) for waste heat recovery of Diesel engine. Energy Procedia 2017, 129, 955–962. [Google Scholar] [CrossRef]
- Johnson, V. Heat-Generated Cooling Opportunities in Vehicles; SAE Technical Paper 2002-01-1969; SAE International: Warrendale, PA, USA, 2022. [Google Scholar] [CrossRef]
- Gequn, S.; Mingru, Z.; Hua, T.; Haiqiao, W.; Xingyu, L.; Yongzhan, H.; Weijie, Z. Experimental investigation on thermal OS/ORC (Oil Storage/Organic Rankine Cycle) system for waste heat recovery from Diesel engine. Energy 2016, 107, 693–706. [Google Scholar]
- Vyacheslav, R.; Timur, A.; Alexander, C.; Anatoly, V. Determination of the required power for bus hybrid engine. E3S Web Conf. 2020, 178, 01076. [Google Scholar] [CrossRef]
- Ruixiao, S.; Yuche, C.; Abhishek, D.; Philip, P. Hybrid electric buses fuel consumption prediction based on real-world driving data. Transp. Res. Part D Transp. Environ. 2021, 91, 102637. [Google Scholar]
- Jung, D.; Park, S.; Min, K. Study on the Application of the Waste Heat Recovery System to Heavy-Duty Series Hybrid Electric Vehicles; SAE Technical Paper 2013-01-1455; SAE International: Warrendale, PA, USA, 2013. [Google Scholar] [CrossRef]
- Arias, D.; Shedd, T.; Jester, R. Theoretical Analysis of Waste Heat Recovery from an Internal Combustion Engine in a HYBRID vehicle; SAE Technical Paper 2006-01-1605; SAE International: Warrendale, PA, USA, 2006. [Google Scholar] [CrossRef]
- Upendra, K.; Grauers, A. Synergy and Conflicts between Waste Heat Recovery System and Hybrid Electric Vehicle; Chalmers University of Technology: Gothenburg, Sweden, 2015. [Google Scholar]
- U.S. Department of Energy. 4R Supercritical Carbon Dioxide Brayton Cycle, Quadrennial Technology Review. 2015. Available online: www.energy.gov (accessed on 27 October 2023).
- Liu, L.; Yang, Q.; Cui, G. Supercritical Carbon Dioxide(s-CO2) Power Cycle for Waste Heat Recovery: A Review from Thermodynamic Perspective. Processes 2020, 8, 1461. [Google Scholar] [CrossRef]
- Rochau, G.E.; Pasch, J.J.; Carlson, M.D.; Fleming, D.D.; Kruizenga, A.M.; Sharpe, R.A.; Wilson, M.C. Supercritical CO2 Brayton Cycles, NP-NE Workshop #2–4 August 2014. Available online: https://www.osti.gov/biblio/1221819 (accessed on 21 March 2023).
- Da, L.; Qiang, S.; Ke, S.; Guodong, Z.; Shuzhan, B.; Guoxiang, L. Diesel engine waste heat recovery system comprehensive optimization based on system and heat exchanger simulation. Open Phys. 2021, 19, 331–340. [Google Scholar]
- Ion, V.I.; Popescu, F. Efficiency Improvement of a Biogas Engine-Driven CHP plant. Sci. Work. Univ. Food Technol. 2016, 63, 255–261. [Google Scholar]
- Wang, T.; Zhang, Y.; Zhang, J.; Peng, Z.; Shu, G. Comparisons of system benefits and thermo-economics for exhaust energy recovery applied on a heavy-duty Diesel engine and a light-duty vehicle gasoline engine. Energy Convers. Manag. 2014, 84, 97–107. [Google Scholar] [CrossRef]
- Dal, C.E.; Lazzaretto, A.; Toffolo, A. A novel extension of the SYNTHSEP methodology for the optimal synthesis and design of supercritical 2 cycles in waste heat recovery applications. Energy Convers. Manag. 2023, 276, 116535. [Google Scholar]
- White, C. Analysis of Brayton Cycles Utilizing Supercritical Carbon Dioxide. United States. 2014. Available online: https://www.osti.gov/biblio/1490264 (accessed on 12 March 2023).
- Seong, J.B.; Minseok, K.; Seong, K.C.; Seungjoon, B.; Jeong, I.L.; Jae, E.C. Review of supercritical CO2 power cycle technology and current status of research and development. Nucl. Eng. Technol. 2015, 47, 647–661. [Google Scholar]
- U.S. Department of Energy. Quadrennial Technology Review 2015, Chapter 4—Advancing Clean Electric Power Technologies. Available online: https://www.energy.gov/quadrennial-technology-review-2015-omnibus (accessed on 20 November 2023).
- Wang, R.; Wang, X.; Bian, X.; Zhang, X.; Cai, J.; Tian, H.; Shu, G.; Wang, M. An optimal split ratio in design and control of a recompression supercritical 2 Brayton system. Energy 2023, 277, 127676. [Google Scholar] [CrossRef]
- Mark, A. Optimization of the Closed Supercritical CO2 Brayton Cycle with the Detailed Simulation of Heat Exchangers; SoftInWay Inc.: Burlington, MA, USA, 2021. [Google Scholar]
- Battista, D.; Cipollone, D.R. Waste Energy Recovery and Valorization in Internal Combustion Engines for Transportation. Energies 2023, 16, 3503. [Google Scholar] [CrossRef]
- Chen, Y.; Lundqvist, P.; Platell, P. Theoretical research of carbon dioxide power cycle application in automobile industry to reduce vehicle’s fuel consumption. Appl. Therm. Eng. 2005, 25, 2041–2053. [Google Scholar] [CrossRef]
- Gequn, S.; Lingfeng, S.; Hua, T.; Xiaoya, L.; Guangdai, H.; Liwen, C. An improved CO2-based transcritical Rankine cycle (CTRC) used for engine waste heat recovery. Appl. Energy 2016, 176, 171–182. [Google Scholar]
- Walnum, H.T.; Neksa, P.; Nord, L.O.; Andresen, T. Modelling and simulation of CO2 (carbon dioxide) bottoming cycles for offshore oil and gas installations at design and off-design conditions. Energy 2013, 59, 513–520. [Google Scholar] [CrossRef]
- Hou, S.; Wu, Y.; Zhou, Y.; Yu, L. Performance analysis of the combined supercritical CO2 recompression and regenerative cycle used in waste heat recovery of marine gas turbine. Energy Convers. Manag. 2017, 151, 73–85. [Google Scholar] [CrossRef]
- Lingfeng, S.; Gequn, S.; Hua, T.; Tianyu, C.; Peng, L.; Ligeng, L. Dynamic tests of CO2-Based waste heat recovery system with preheating process. Energy 2019, 171, 270–283. [Google Scholar] [CrossRef]
- Correa, F.; Barraza, R.; Too, Y.C.S.; Padilla, R.V.; Cardemil, J.M. Optimized operation of recompression sCO2 Brayton cycle based on adjustable recompression fraction under variable conditions. Energy 2021, 227, 120334. [Google Scholar] [CrossRef]
- Yunus, A.Ç.; Boles, M.A. Thermodynamics: An Engineering Approach, 9th ed.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Bell, I.H.; Wronski, J.; Quoilin, S.; Lemort, V. Pure and Pseudo-pure Fluid Thermophysical Property Evaluation and the Open-Source Thermophysical Property Library CoolProp. Ind. Eng. Chem. Res. 2014, 53, 2498–2508. [Google Scholar] [CrossRef]
- Monjurul, M.E.; Guan, Z.; Klimenko, A.Y. A comprehensive review on heat transfer and pressure drop characteristics and correlations with supercritical CO2 under heating and cooling applications. Renew. Sustain. Energy Rev. 2018, 29, 658–675. [Google Scholar] [CrossRef]
- Han, S.; Cha, J.E.; Kim, J.; Sah, I.; Kim, Y.W. Design and Performance Analysis of a Supercritical Carbon Dioxide Heat Exchanger. Appl. Sci. 2020, 10, 4545. [Google Scholar] [CrossRef]
- Lemmon, E.W.; McLinden, M.O.; Friend, D.G. Thermophysical Properties of Fluid Systems. In NIST Chemistry WebBook, NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; NIST: Gaithersburg, MD, USA, 2015. Available online: https://webbook.nist.gov/chemistry/fluid (accessed on 15 April 2023).
- Ginosar, D.M.; Petkovic, L.M.; Guillen, D.P. Thermal Stability of Cyclopentane as an Organic Rankine Cycle Working Fluid. Energy Fuels 2011, 25, 4138–4144. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).