Abstract
Working fluids that incorporate solid microencapsulated phase change materials (MPCMs) can benefit from properties such as density and viscosity, which are crucial for improving heat capacity and transfer. However, limited data are available on these parameters for specific slurry and mass ratios. In this study, we present a comparative analysis of the experimental results on the viscosity of three different MPCM aqueous dispersions, namely MPCM 31-S50, MPCM 25-S50, and Micronal 5428X. Varying MPCM mass ratios of distilled water were used to obtain different mass concentrations of the phase change material (PCM), and the resulting slurries were analysed at temperatures ranging from 15 to 40 °C. Our findings showed that all slurries exhibited non-Newtonian characteristics at low shear rates, with viscosity stabilising at higher shear rates, resulting in the characteristics of a Newtonian fluid. The viscosity results were highly dependent on the type of MPCM base dispersion, particularly at high mass ratios, with the slurries having viscosities higher than those of water. Furthermore, we conducted density experiments as a function of temperature, using a flow test setup and a Coriolis flowmeter (Endress+Hauser, Reinach, Switzerland) to determine the density of two MPCMs, namely MPCM 25-S50 and Micronal 5428X. The test samples were prepared at mass concentrations of 10%, 15%, and 20% of the phase change material. We found significant differences in density and viscosity for different MPCM slurries as a result of both the PCM concentration and the material studied. Our results also revealed an apparent PCM phase change process, in which the slurry density significantly decreased in the temperature range of the phase transition from solid to liquid.
1. Introduction
1.1. MPCM
The transition towards a new green economy requires the development of technologies that improve energy efficiency. One such solution that is currently being explored is the use of phase change materials (PCMs) to enhance the properties of working fluids used in heat transfer systems and for thermal energy storage [1]. PCMs are substances that undergo a phase transition within a specific temperature range and possess the ability to store heat using the latent heat [2]. Of particular interest are microencapsulated phase change materials (MPCMs), where the phase change material is encapsulated within microcapsules, which can be added to a base liquid to form a slurry, resulting in an improved working fluid. This approach enables the creation of modern working fluids with enhanced properties based on natural liquids such as water [3]. Encapsulated PCMs in dispersion form micro/nanoencapsulated phase change material slurries (MPCMSs or NPCMSs), where the diameter of micro- and nanocapsules is typically within the micro- and nanometre range [4]. MPCMSs have potential applications in thermal energy storage systems and can serve as an alternative to traditional working fluids in heat transfer systems, such as a secondary refrigerant or coolant for solar collectors and PV panels [5,6,7].
There are three versions of phase change materials in working fluids, namely microencapsulated PCM slurries, PCM emulsions, and ice slurries [8,9]. The ice slurry is created by freezing ice crystals in a liquid using natural and inexpensive media such as water or alcohol. The correct mass ratio of liquid phase and ice allows the working fluid to be pumped [10]. PCM emulsions (PCEs), also known as phase change dispersions (PCDs), are formed by directly adding PCM to a base fluid such as water or alcohol [9,11,12]. To ensure the stability of such a liquid, additional ingredients are included in the formulation to prevent phase separation and ensure chemical stability [11,13]. However, such solutions have some drawbacks, such as causing corrosion as well as volume changes. The use of microcapsules for MPCM slurries reduces these negative effects [14,15]. Microencapsulation reduces the contact of the PCM with the surrounding fluid and increases heat transfer surfaces, which is essential due to the often-low thermal conductivity of PCM [16]. However, microencapsulation decreases the proportion of PCM in the slurry, affecting the energy transfer capacity, due to the reduction in the weight ratio of the phase change material [17]. The microcapsule form allows MPCMs to be used in combination with fabrics or building materials for thermoregulation. Other applications include medicine, automotive, or electronics [18,19,20,21]. The use of MPCM slurries in thermal systems allows us to reduce the volume of pumped fluid involved in heat transfer, because of an increase in heat capacity. The phase transformation temperature and the operating temperature range depend on the PCM [22]. The shell can be improved for heat transfer by adding effective conductive materials such as graphene, graphite, or metals [16,23].
To better use the benefits of MPCM slurries in working fluids, a deeper understanding of their physical properties is necessary. Although there are experimental results available on MPCM slurries in the literature [24,25,26,27,28], they are limited in scope due to the diversity of materials and applications. Researchers studying the heat transfer of working fluids using PCMs require knowledge of suspension parameters such as viscosity and density. Some papers can be found in the literature that focus on the heat transfer of flowing slurries for both MPCM and PCD (PCE) [29,30]. In all sources, the viscosity is a crucial parameter for understanding the flow regime, which is essential for heat transfer studies.
1.2. MPCM Slurry—Previous Viscosity Studies
Viscosity is among the most important properties of working fluids. It affects the flow parameters, which can be important for application in heat transfer systems and the economics of their operation. The viscosity of a fluid is closely linked to the flow regime and the dimensionless Reynolds number. Lower values of the Reynolds number, which is the ratio of inertia forces to viscosity forces, lead to a less turbulent flow behaviour and thus alter the heat transfer process.
MPCMS studies are conducted to obtain knowledge of how viscosity changes relative to the characteristics of the phase change material used and the parameters of the suspension that may affect it. The suspensions can use different types of PCM, and the microcapsule shells can be made of different materials. The mass concentration of the MPCM can be varied, and the suspensions can contain microcapsules of different diameters and shapes. It seems that the parameters mentioned can affect the value of dynamic viscosity of the fluids under study. This leads to the need to know the correlation of viscosity with these parameters, and it is required to see how individual suspensions behave during changes in temperature and shear rate values. Data of viscosity changes in relation to the parameters discussed are required for the development of thermal systems and heat exchangers in which the working fluid is an MPCMS.
Dutkowski et al. [27] reported the results of experimental testing of water–MPCM (Micronal DS 5039 X) slurries, with different concentrations of the mass ratio of microcapsules. Six samples were prepared with different mass ratios of the MPCM product to water; the tested samples had ratios of 10:90, 30:70, 50:50, 70:30, 90:10, and 100:0, respectively, where 100:0 represents the pure MPCM product. Viscosity tests were performed for shear rates of 0.0132–132.00 s−1 for temperatures of 10, 15, 17.5, 20, 22.5, 25, 27.5, 30, 40, and 50.0 °C, respectively. The second part of the study was the determination of the dynamic viscosity parameter during the non-steady state. Heating of the samples was carried out in the temperature range of 16–29 °C, with the process taking 17 min and the data collected at 15 s intervals. The main conclusions of the study are that the viscosity of suspensions is higher than that of water and increases with the mass concentration of MPCM, reaching values up to several hundred times higher, and that the phase transition of PCM does not affect the viscosity in the steady-state test. For small values of the shear rate, a highest viscosity value was observed, decreasing with an increasing shear rate until stabilisation was reached. Only for a concentration of 10% was the Newtonian fluid behaviour observed throughout the range. The visible effect of phase transition on viscosity during non-steady-state measurements was described. In another paper, the same authors [26] present a viscosity prediction model for a product with the trade name Micronal DS 5039 X. The model successfully predicts viscosity over a temperature range of 10 to 50 °C, a shear rate of 3–130 s−1, and to a maximum microcapsule mass concentration of 45%. The articles presented describe the behaviour of the viscosity parameter for one MPCM product; more experiments on other substances are needed.
Liu et al. [31] investigated the viscosity of a microencapsulated phase change slurry using a rheometer. The authors used MPCMS samples with mass ratio values of 5, 10, 20, 30, and 40%, respectively, and presented the results of the base fluid measurements, which comprised a mixture of water and alcohol. The viscosity gradually increased with the mass fraction; however, a sharp increase was observed for a 40% MPCM slurry.
Delgado et al. [24] used a rheometer to determine viscosity for an aqueous suspension of microencapsulated phase change material with three different values of the MPCM mass fraction, 14, 20, and 30%, respectively. The presented results showed a viscosity higher than that of the base liquid with a large increase in value between the levels of a 20% and 30% MPCM mass fraction. The fluid exhibited non-Newtonian behaviour.
Publications such as [25,32,33,34] provide additional information on viscosity. Typically, the authors report on viscosity experiments conducted on a single type of MPCM at varying PCM concentration levels. However, there is a lack of information on comparisons of different MPCMs at the same concentration. Conducting tests under identical conditions could potentially confirm whether the commonly reported properties of these suspensions exhibit significant differences as a result of the material used.
1.3. MPCM Slurry—Previous Density Studies
The density of a substance is a fundamental parameter that can be determined by the sum of the densities of its individual components and their respective mass fractions. For suspensions consisting of a base fluid, a phase change material, and a shell-forming material, the calculation of the expected density is outlined in [35]. The authors discuss the impact of the proportion of each component on the density of the fluid.
Dutkowski et al. [28] reported the results of density change measurements for a microencapsulated phase change material slurry with the trade name Micronal DS 5039. The slurry consisted of water and MPCM in four different mass proportions of 2.15, 4.30, 6.45, and 8.60%, respectively. The samples were heated using a thermostatic bath in the temperature range of 10 to 30 °C. The results obtained allowed them to conclude that the theoretical density calculations agreed with the experimental results and that the shell had no effect on the expansion of the PCM. It was also demonstrated that during the PCM phase change process, the process played a major role in density changes, despite the low concentration of PCM in the slurry.
Karaipekli et al. [36] described the results of density studies for very low concentrations of nanoencapsulated phase change slurry. The highest mass concentration of MPCM in the experiment was 2%. The density results obtained were close to the density of the base fluid, which was water. The authors showed that the density of the slurry decreased with increasing temperature. However, it increased with an increase in MPCM content in the slurry.
Allouche et al. [37] used a hydrometer to determine the density of an aqueous suspension of microencapsulated paraffin. The results presented showed a decrease in density values with temperature and an intensification of this process during the phase transition.
Density investigations have also been carried out on phase change emulsions (PCEs), which do not have a shell on PCM particles like MPCMs but often employ similar materials. In the study by Vasile et al. [29], experimental results of density variations during the cooling process of an aqueous paraffin dispersion were presented. They reported an increase in the density of the emulsion upon reaching the PCM phase change temperature. Studies of density changes as a function of temperature can also be found in the literature [14].
As in the case of viscosity, the authors focus on determining the density with respect to one type of MPCM. Typically, density tests are auxiliary tests during studies of a more extensive range of suspension properties. Few works in the literature are dedicated to the detailed analysis of density changes during the phase transformation of suspensions, especially suspensions that are involved in flow and heat transfer. However, this is the target use of the fluids under study.
1.4. Summary
The existing literature on MPCM suspensions reveals a significant lack of data related to their properties. Furthermore, there is an observable absence of comparative analyses of diverse MPCMs across varying mass concentrations. The characteristics associated with the non-Newtonian fluid properties of these suspensions remain ambiguous. A critical gap in the current understanding is the precise determination of the conditions under which these suspensions transition between non-Newtonian and Newtonian fluid behaviours. This highlights the need for further comprehensive investigation in this domain.
In this paper, we aim to address the existing knowledge deficit by reporting on new research findings that contribute to the understanding of MPCMS properties. Our study involved conducting dynamic viscosity experiments on three different MPCMs (MikroCaps PCM25-S50, MikroCaps PCM31-S50, and Micronal 5428X) and density measurements on two MPCMs (MikroCaps PCM25-S50 and Micronal 5428X). The acquired data provide new insights into the properties of microencapsulated phase change material suspensions and contribute substantially to the scientific understanding in this area.
2. Experimental Setup and Methodology
2.1. Equipment and Procedure for Viscosity Measurements
The viscosity determination experiment used a Brookfield DV-II + Pro rotational viscometer (Brookfield Co., Ltd., Toronto, ON, Canada). This device allows for dynamic viscosity measurements and the determination of viscosity shear rate curves. The device uses a ULA spindle with a corresponding adapter designed to work with the spindle. To provide an accurate sample temperature, a PolyScience thermostatic bath was used to supply water at the required temperature to the adapter’s water jacket. The temperature of the test sample was controlled by a sensor integrated into the viscosity measuring device, and there was also a measurement of the temperature of the water supplied by the thermostatic bath.
Viscosity measurements were carried out for rotational speeds from 0.3 to 100 rpm (18 different rotational speeds), corresponding to a shear rate of 0.367–122.300 s−1. Measurements were collected after the required temperature was reached and stabilized, after a subsequent 5 min wait. Measurements were taken for the following temperatures: 15, 20, 25, 28, 30, 35, and 40 °C. The temperature range was deliberately chosen to encompass viscosity measurements both before and after the phase change of the PCM, as well as during the phase change range. Tests were conducted for all temperature points for each MPCM, regardless of the phase change range specified. Each measurement point, consisting of a constant temperature and rotation speed, was measured three times to ensure accuracy. The results presented in this paper are the average for three measurements for each point. We took the measurements for a given temperature by changing the value of the shear rate from the lowest to the highest, then reversing the process from highest to lowest and back again. This method of measurement allowed us to determine if the shear rate was time dependent.
The measurement uncertainty for dynamic viscosity depends on the spindle used and the number of revolutions per minute. For the measurements presented in this paper, the accuracy for the maximum shear rate is ±0.64 mPas. The measurement of the temperature of the water bath is ±0.1 °C.
2.2. Test Setup and Procedure for Density Measurements
We utilized a custom-designed experimental setup to examine how MPCMS density changes in relation to temperature. The system, as shown in Figure 1, was designed to analyse the flow resistance, density, and heat transfer processes that occur during the flow of a microencapsulated phase change material slurry. A Tuthill Concord D model 0.68 pump enforced fluid flow through the setup. A Promass 80A Coriolis flowmeter (Endress+Hauser, Reinach, Switzerland) allowed the determination of density. The uncertainty of density measurements obtained by this flowmeter is ±0.5 kg/m3. The secondary fluid (water) was heated/cooled by the PolyScience thermostatic bath to regulate the temperature of the tested slurry. The main fluid (slurry) and the secondary fluid exchanged heat through a coaxial counter-current plate heat exchanger made by Wieland (Warwick, RI, USA). The temperature was measured by a flowmeter with an accuracy of ±0.5 °C, and temperatures in the test section were also measured using thermocouples. Parameters from the flowmeter and thermocouples were collected by an Endress-Hauser RSG45 recorder (Endress+Hauser, Reinach, Switzerland).
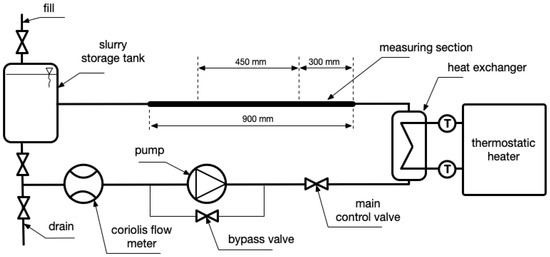
Figure 1.
Test setup for measuring density as a function of temperature.
To calibrate the Coriolis flowmeter, we used distilled water, whose physical properties are well-known. The results were compared with literature data [38] and used to perform error correction. The measurement error was less than 0.3%. During the experiment, the pump pumped the liquid at a constant load, and the thermostatic bath carried out a program that caused a linear increase in the temperature of the test liquid. The temperature ranged from 15 °C to 40 °C. The entire measurement cycle lasted two hours, and temperature and density measurements were taken every 5 min.
3. Materials
3.1. Slurry Properties
Experimental analysis was conducted on three different aqueous MPCM dispersions, sourced from two distinct suppliers, namely MikroCaps (Ljubljana, Slovenia) and Microtek Labs (Moraine, OH, USA), and identified by the trade names MPCM 25-S50, MPCM 31-S50, and Micronal 5428X. All slurries are white opaque liquids. Each MPCM used in the microcapsules has a unique phase transition temperature, with the melting range for MPCM 25-S50 being 23–27 °C, for MPCM 31-S50 being 29–33 °C, and for Micronal 5428X being 24–26 °C. The concentrates’ densities are lower than that of pure water, due to the use of materials with lower specific density. Inside the polymer shell is a paraffin wax. The MikroCaps utilize polyreutane for the shell, while polymethylmethacrylate is used for Micronal 5428X. The viscosity range of the concentrates includes values much higher than pure water; the viscosity for MPCMS is hundreds of times higher. To limit the impact of environmental conditions on the materials, all fluids used in this study were stored in sealed containers, and liquid was taken for sample preparation. Due to the stratification phenomenon of the aqueous dispersion, it was necessary to periodically shake the containers to maintain homogeneity and the mass concentration of MPCM in each portion of the liquid. Table 1 shows the technical data of the materials used. The average diameter of MPCM 25-S50 and MPCM 31-S50 microcapsules is 1–15 μm, while for Micronal 5428X it is equal to 1–5 μm.

Table 1.
MPCM specifications.
3.2. Materials for Viscosity Experiment
To measure the viscosity of various MPCM concentrations, each sample was created by diluting the MPCMS concentrate with distilled water (5 μS/cm). Additionally, tests were performed on pure concentrates. The series included the following mass ratios: 49.6%, 40%, 30%, 20%, 15%, and 10% for MPCM 25-S50, 50%, 40%, and 30% for MPCM 31-S50, and 40% and 30% for Micronal 5428X. To ensure precision, we utilized a high-precision laboratory scale with an accuracy of 0.001 kg. Sample preparation consisted of measuring the concentrate and adding the appropriate portion of distilled water; in the calculation, we considered the water contents of the concentrates according to technical data. The samples were then mixed mechanically until a homogeneous suspension was obtained. A single viscosity measurement requires a volume of 16 ml of liquid. The samples prepared for this purpose had a volume of 50 mL.
3.3. Materials for Density Experiment
For the experiment to determine density as a function of temperature, we used suspensions prepared from MPCM 25-S50 and Micronal 5428X concentrates. There were 10%, 15%, and 20% suspensions for both concentrates. The sample preparation process was the same as for the viscosity determination process. The preparation of the samples involved measuring the ingredients with a laboratory scale and mechanical mixing to obtain a homogeneous consistency. The volume of prepared samples was 2 litres, due to the volume of the test setup. Phase separation was not observed during the experiment; the suspension remained homogeneous.
4. Results and Discussion
4.1. Viscosity
The changes in viscosity as a function of the change in shear rate for temperatures of 15 °C, 20 °C, 25 °C, 28 °C, 30 °C, 35 °C, and 40 °C are shown as curves in Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8. Due to the accuracy of the measuring device and the mounted spindle with the adapter, it was possible to collect data in an incomplete range of shear rates for each MPCMS. Results for which the device was beyond the range of measurement were rejected. The charts also do not show MPCMSs with the highest concentrations above 40%, due to the readability of the data presented. For the mentioned cases, the dynamic viscosities for 15 °C were in the ranges of, respectively, MPCM 25-S50 49.6%—from 857 to 1137 mPas, and MPCM 31-S50 50%—from 266 to 483 mPas.
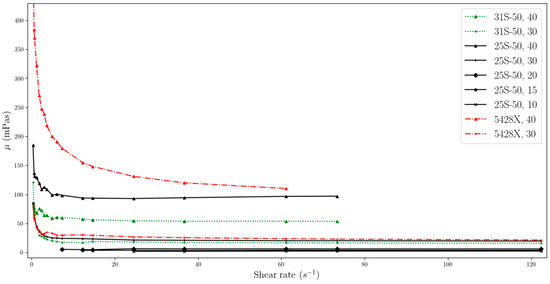
Figure 2.
Change in viscosity relative to shear rate, for temperature of 15 °C (two-column).
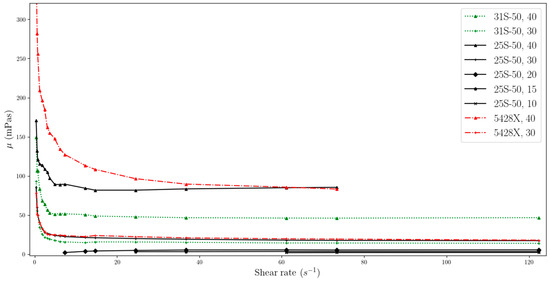
Figure 3.
Change in viscosity relative to shear rate, for temperature of 20 °C (two-column).
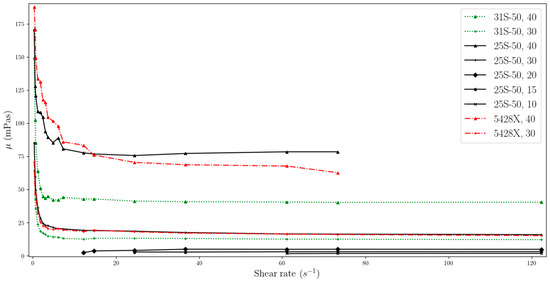
Figure 4.
Change in viscosity relative to shear rate, for temperature of 25 °C (two-column).
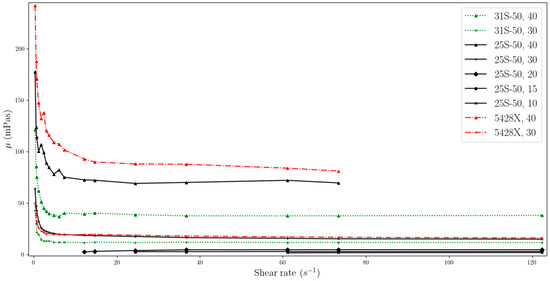
Figure 5.
Change in viscosity relative to shear rate, for temperature of 28 °C (two-column).

Figure 6.
Change in viscosity relative to shear rate, for temperature of 30 °C (two-column).
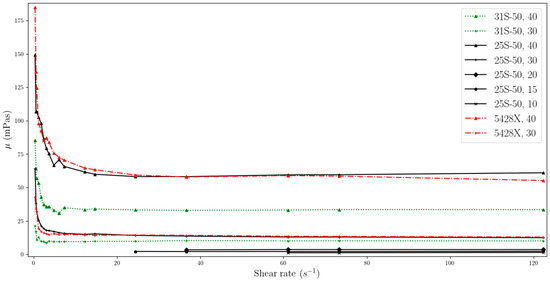
Figure 7.
Change in viscosity relative to shear rate, for temperature of 35 °C (two-column).
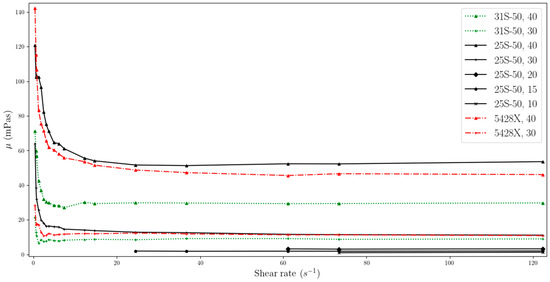
Figure 8.
Change in viscosity relative to shear rate, for temperature of 40 °C (two-column).
The data obtained indicate that the viscosity of slurries is strongly related to the shear rate especially at low values. MPCMSs exhibit non-Newtonian fluid behaviour in the range from 0 to about 20 s−1. Then, for measurements at a higher rpm of the measuring device and reaching higher shear rate values, the slurries stabilize, showing Newtonian fluid behaviour, which means that the viscosity value is independent of the shear rate. However, for dynamic viscosity measurements of fluids with MPCM mass concentrations of 20% and less, viscosity shows little dependence on shear rate throughout the range studied. But it should be noted that these ranges are narrower than for higher mass MPCM concentrations and include higher shear rate values.
The phenomenon regarding the non-Newtonian behaviour of working fluids with phase change material was already reported in the literature. According to Delgado et al. [24], the reason for the higher viscosity observed at lower shear rates can be explained by the random dispersion of stagnant microcapsules, which results in an increase in the apparent viscosity. On the other hand, at higher shear rates, the particles tend to arrange themselves in layers with small distances in the direction of flow, and larger distances between individual layers. This arrangement has the effect of lowering the viscosity.
For each of the working fluids tested, measurements were performed at temperatures before and after the phase transition, as well as in the PCM phase transition range. Experimental results showed that the phase change process had no effect on the dynamic viscosity values obtained. One possible explanation for this phenomenon is the encapsulation of the PCM, which appears to minimize its influence on the overall viscosity. However, as demonstrated in [27], viscosity does increase during the transition from a solid to a liquid PCM when the sample is simultaneously heated and measured. This suggests that the phase change process does have a temporary effect on viscosity. In our own experiment, we allowed the temperature to stabilize before taking measurements, which may have caused the PCM to transition from a solid to a liquid phase prior to measurement. Fisher et al. [13] reported viscosity data for PCMs, which took into account the temperature range of the phase change, indicating that the transition did indeed result in a change in dynamic viscosity for the tested dispersions.
Due to the reported hypothesis, a larger study of viscosity changes during the phase transition is recommended. MPCMS flowing through a heat exchange system is in a continuous process of heating in one exchanger and cooling in another, which means that the phase change of PCM occurs repeatedly. If this process affects the dynamic viscosity, it can imply changes in the flow regime and heat transfer, and this, in turn, can affect the economics of using MPCMS. The effect of the PCM phase on the flow resistance in an MPCMS was investigated in [39], but there were too few data to determine if there was an effect caused by a change in viscosity during the phase transition.
Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8 additionally reveal that MPCM 25-S50 and Micronal 5428X with concentrations of 30% and 40% have similar viscosities, while for MPCM 31-S50, the viscosity is lower. The values after stabilization of the dynamic viscosity are particularly close to each other, and for 30% mass ratios, they are almost identical. It is thus important to consider the potential for heat accumulation and resistance to flow in this context. The first two MPCMSs (MPCM 25-S50, Micronal 5428X) have a similar ability to accumulate energy, whereas MPCM 31-S50 has a lower capacity in this regard. A higher viscosity can create flow resistance, which may impede pump operation and increase energy transfer within a unit of fluid. Designing heat transfer systems requires careful attention to this aspect.
As can be seen from the data from our experiment, the viscosity stabilizes slightly faster for suspensions with lower MPCM mass concentrations, using the same MPCM. For example, a suspension with an MPCM mass concentration of 40% Micronal 5428X achieves stabilization at around the 14.6 s−1 point, while for a concentration of 30%, stabilization is achieved at around the 3 s−1 point.
Measurements on the device were taken at uneven intervals regarding the shear rate, and for this reason, the change in viscosity should be compared to the average rate per unit change in the shear rate parameter. Thus, we propose a new method to determine the stabilization point of the viscosity parameter by analysing the rate of change in viscosity with respect to the change in shear rate on the viscosity meter. Equation (1) reflects the change in viscosity between two measurement points divided by the change in shear rate over that range.
where RCV stands for the rate of change in viscosity, μi is the dynamic viscosity measured at the i-th measurement point, and sri is the value of the shear rate at the i-th measurement point. The resulting value is the average change in viscosity per 1 s−1. The proposed indicator of RCV shows that the rate of transition from non-Newtonian to Newtonian behaviour is concentration dependent and is faster the smaller the mass ratio of MPCM in the suspension. This is true for suspensions with the same microencapsulated phase change material as a component. For identical concentrations, but different microcapsule materials, there is no correlation (Figure 9).
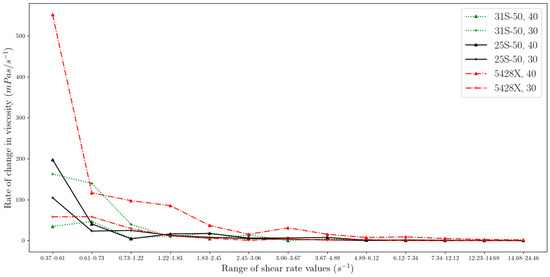
Figure 9.
The rate of change in viscosity in each range of shear rate for measurements taken at 15 °C (two-column).
Figure 10 shows the change in viscosity as a function of MPCM mass fraction (MPCM 25-S50), for a constant shear rate and temperature. The highest viscosity occurs for the highest MPCM mass ratio and decreases nonlinearly as the suspension is diluted by adding a base fluid such as water. MPCMS with a microcapsule content of 40% by weight shows a viscosity of 53.5 mPas, while a fluid with an MPCM weight percentage of 30% has a viscosity of 11.1 mPas. Further dilution to a level of 20% reduces the viscosity to 3.2 mPas.
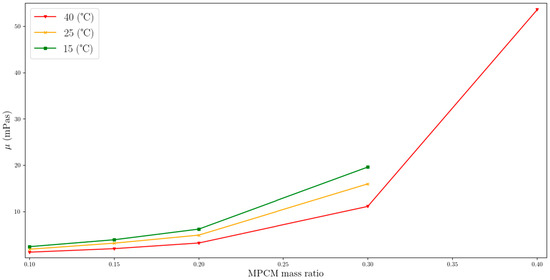
Figure 10.
Variation in dynamic viscosity for a constant shear rate relative to the MPCM mass ratio (single-column).
The viscosity changes in Figure 10 are more pronounced for suspensions with higher mass fractions of MPCM. These changes can have a significant impact on the economic feasibility of using MPCMs as working fluids, particularly in systems with large temperature differences. This influence may also be important if there are large temperature differences in the operating system. The flow regime is influenced by viscosity, and increasing the viscosity results in reduced flow turbulence, leading to lower heat exchanger efficiency.
4.2. Density
Our tests showed differences in the density parameter depending on the temperature. The MPCM named Micronal 5428X for certain concentrations showed a higher density than the MPCM 25-S50-based slurry. For both fluids, a clear visible phase transition was observed, which manifested as a rapid nonlinear decrease in density, which was nonlinear. The suspension with the highest concentration of MPCM displayed the minimum density due to the higher content of materials with a lower density than water. The results confirmed the dominant role of the PCM in the microcapsules and its impact on density. The shell material is not the determining factor, as the shell remains solid throughout the heating process.
Figure 11 illustrates the change in slurry’s density during heating. The heating process for the samples can be divided into three distinct stages. The first stage involves heating the PCM while it is in a solid state, during which its characteristics display a close-to-linear behaviour that changes as the phase transition range is approached. The second stage is marked by the actual phase transition, which is characterized by a rapid decrease in density. The final stage of the heating process is characterized by a decrease in density with increasing temperature and displays a near-linear behaviour. Notably, the transformation concludes rapidly with a clear boundary, whereas the beginning of the process is less clearly defined and starts smoothly.
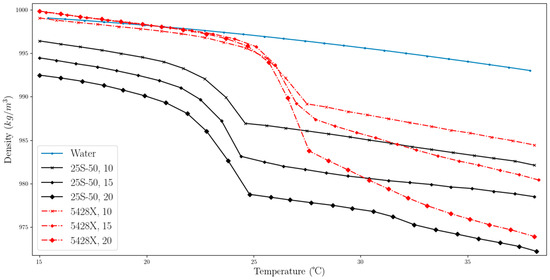
Figure 11.
Changes in density as a function of temperature for MPCM slurries and water (two-column).
To compare the properties of slurries with MPCM, water was used as a benchmark substance. Since water is also the base liquid in the suspensions, it is ideal for assessing how the addition of MPCM affects the density change properties of the liquids when heated. The density of water within the analysed temperature range was obtained using the REFPROP library [38]. As the materials used in the suspensions have a lower density than water (with the exception of T < 20 °C, for mass concentrations of 15% and 20%, for MPCM 5428X), the resulting suspensions have a lower density than the base liquid.
Each of the tested MPCMs has a distinct range of phase transformation (see Figure 11). For MPCM 25-S50, the declared range of phase change is between 24 °C and 26 °C; the results show that the rapid change starts just before the temperature of 24 °C and ends at around 25 °C. For suspensions from Micronal 5428X, the phase change behaviour of the fluid is similar in other temperature ranges.
The measurement results indicate that as the concentration of MPCM 25-S50 decreases, the density changes by a similar amount for every 5% change in concentration, although these values differ for temperatures before and after the phase change. Still, the lines remain parallel to each other. For Micronal 5428X, the density results are similar for each sample in the first stage and are close to the density of water. However, after the phase change, the differences in the density results increase. The lines are also not parallel, and the difference with each 5% decrease in MPCM mass concentration is not the same. The difference between the MPCMs used decreases with increasing temperature, reaching the closest values when approaching the highest temperature value in the experiment.
5. Conclusions
This paper has detailed the results of viscosity and density measurements performed on three distinct MPCM slurries (from two independent suppliers), with various mass concentrations of MPCM. Viscosity tests were conducted using a rotational viscometer for temperatures of 15, 20, 25, 28, 30, 35, and 40 °C for a range of shear rates spanning from 0.367 to 122.300 s−1. The custom build test setup with a Coriolis flowmeter allowed us to determine density changes as a function of temperature.
From the gathered results, several key observations and conclusions were derived. The viscosity of suspensions containing MPCM is higher than that of the base fluid (water), and it increases further with higher concentrations of phase change material. The phase change process does not affect the viscosity of the suspensions. Correlation between the viscosity and mass concentration of MPCM is not linear. Viscosity decreases rapidly with higher concentrations and decreases more slowly with lower concentrations. We introduced a new parameter, RCV (rate of change in viscosity), to demonstrate the rate of transition from non-Newtonian to Newtonian behaviour qualitatively. This parameter showed that the transition is dependent on concentration and progresses faster with smaller mass ratios of MPCM in the suspension. The density of suspensions containing MPCMs is lower than that of water due to the presence of materials with a lower density than the base fluid. The change in density during heating is nonlinear and can be divided into three stages: solid phase, phase change, and liquid phase. Differences in density behaviour during heating were exhibited by the MPCMs studied, despite having a constant mass concentration value. Micronal 5428X showed a higher density at lower temperatures than MPCM 25-S50, despite having a similar value of heat accumulation capacity in the dry content of the suspensions. During the phase transformation, Micronal 5428X-based suspensions exhibited a greater decrease in density, which was more dependent on mass concentration. This correlation was much less pronounced for working fluids with MPCM 25-S50.
Based on the results obtained with MCPMs from two different suppliers, further research is suggested to investigate the broader impact of the phase transition on the properties of the slurry. This is important in order to generalize regarding the impact of PCMs on the flow properties of the suspension and subsequently on heat transfer, due to differences between PCMs offered by various producers.
Another interesting research issue is the nonlinearity of viscosity changes with respect to changes in mass fractions. It is recommended that further research should be conducted to determine the optimal concentration values for MPCMs in suspension. Such a study should involve more samples of different concentrations and include a comparison of the viscosity parameter with the energy transport capability of MPCMs, the heat transfer characteristics for different mass concentrations, and flow resistance. A comprehensive study will help determine the most economical and efficient concentrations of MPCM.
The quality of a working fluid depends not only on the specific PCM and shell material used but also on the production method and stabilizing agent employed. Overall, these findings highlight the need for continued investigation to optimize the use of MPCM in heat transfer applications.
Author Contributions
Conceptualization, K.D., B.Z. and B.N.; methodology, K.D., M.K. and B.N.; software, B.N, M.K.; vali-dation, M.K., K.D.; formal analysis, B.N. and B.Z.; investigation, B.N., K.D, M.K.; resources, B.N., K.D. and B.Z.; data curation, K.D.; writing—original draft preparation, B.N., M.K. and B.B.; writing—review and editing, B.Z., K.D. and B.B.; visualization, B.N.; supervision, K.D., B.Z. and B.B.; project administration, K.D., B.Z. and B.B. funding acquisition, K.D. and B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declared that there are no conflicts of interest.
References
- Youssef, Z.; Delahaye, A.; Huang, L.; Trinquet, F.; Fournaison, L.; Pollerberg, C.; Doetsch, C. State of the art on phase change material slurries. Energy Convers Manag. 2013, 65, 120–132. [Google Scholar] [CrossRef]
- Faraj, K.; Khaled, M.; Faraj, J.; Hachem, F.; Castelain, C. Phase change material thermal energy storage systems for cooling applications in buildings: A review. Renew. Sustain. Energy Rev. 2020, 119, 109579. [Google Scholar] [CrossRef]
- Ran, F.; Chen, Y.; Cong, R.; Fang, G. Flow and heat transfer characteristics of microencapsulated phase change slurry in thermal energy systems: A review. Renew. Sustain. Energy Rev. 2020, 134, 110101. [Google Scholar] [CrossRef]
- Rodríguez-Cumplido, F.; Pabón-Gelves, E.; Chejne-Jana, F. Recent developments in the synthesis of microencapsulated and nanoencapsulated phase change materials. J. Energy Storage 2019, 24, 100821. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M.; Bohdal, T. Experimental studies of the influence of microencapsulated phase change material on thermal parameters of a flat liquid solar collector. Energies 2021, 14, 5135. [Google Scholar] [CrossRef]
- Ali, S.; Mustafa, M. Barriers facing Micro-encapsulated Phase Change Materials Slurry (MPCMS) in Photovoltaic Thermal (PV/T) application. Energy Rep. 2020, 6, 565–570. [Google Scholar] [CrossRef]
- Alvarado, J.L.; Marsh, C.; Sohn, C.; Phetteplace, G.; Newell, T. Thermal performance of microencapsulated phase change material slurry in turbulent flow under constant heat flux. Int. J. Heat Mass Transf. 2007, 50, 1938–1952. [Google Scholar] [CrossRef]
- Stamatiou, E.; Kawaji, M. Thermal and flow behaviour of ice slurries in a vertical rectangular channel. Part I: Local distribution measurements in adiabatic flow. Int. J. Heat Mass Transf. 2005, 48, 3527–3543. [Google Scholar] [CrossRef]
- Delgado, M.; Lázaro, A.; Mazo, J.; Zalba, B. Review on phase change material emulsions and microencapsulated phase change material slurries: Materials, heat transfer studies and applications. Renew. Sustain. Energy Rev. 2012, 16, 253–273. [Google Scholar] [CrossRef]
- Kumano, H.; Yamanada, Y.; Makino, Y.; Asaoka, T. Effect of initial aqueous solution concentration on rheological behaviour of ice slurry. Int. J. Refrig. 2016, 68, 218–225. [Google Scholar] [CrossRef]
- O’Neill, P.; Fischer, L.; Revellin, R.; Bonjour, J. Phase change dispersions: A literature review on their thermo-rheological performance for cooling applications. Appl. Therm. Eng. 2021, 192, 116920. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, P. Preparation and characterization of nano-sized phase change emulsions as thermal energy storage and transport media. Appl. Energy 2017, 190, 868–879. [Google Scholar] [CrossRef]
- Fischer, L.; Mura, E.; O’Neill, P.; von Arx, S.; Worlitschek, J.; Qiao, G.; Li, Q.; Ding, Y. Thermophysical properties of a phase change dispersion for cooling around 50 °C. Int. J. Refrig. 2020, 119, 410–419. [Google Scholar] [CrossRef]
- Delgado, M.; Lázaro, A.; Mazo, J.; Peñalosa, C.; Dolado, P.; Zalba, B. Experimental analysis of a low cost phase change material emulsion for its use as thermal storage system. Energy Convers. Manag. 2015, 106, 201–212. [Google Scholar] [CrossRef]
- Delgado, M.; Lázaro, A.; Mazo, J.; Marín, J.M.; Zalba, B. Experimental analysis of a microencapsulated PCM slurry as thermal storage system and as heat transfer fluid in laminar flow. Appl. Therm. Eng. 2012, 36, 370–377. [Google Scholar] [CrossRef]
- Liu, L.; Alva, G.; Huang, X.; Fang, G. Preparation, heat transfer and flow properties of microencapsulated phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2016, 66, 399–414. [Google Scholar] [CrossRef]
- Salunkhe, P.B.; Shembekar, P.S. A review on effect of phase change material encapsulation on the thermal performance of a system. Renew. Sustain. Energy Rev. 2012, 16, 5603–5616. [Google Scholar] [CrossRef]
- Jurkowska, M.; Szczygieł, I. Review on properties of microencapsulated phase change materials slurries (mPCMS). Appl. Therm Eng. 2016, 98, 365–373. [Google Scholar] [CrossRef]
- Mehling, H.; Brütting, M.; Haussmann, T. PCM products and their fields of application—An overview of the state in 2020/2021. J. Energy Storage 2022, 51, 104354. [Google Scholar] [CrossRef]
- Pathak, L.; Trivedi, G.V.N.; Parameshwaran, R.; Deshmukh, S.S. Microencapsulated phase change materials as slurries for thermal energy storage: A review. Mater. Today Proc. 2021, 44, 1960–1963. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, C.; Lin, Y.; Fang, G. Thermal properties and applications of microencapsulated PCM for thermal energy storage: A review. Appl. Therm. Eng. 2019, 147, 841–855. [Google Scholar] [CrossRef]
- Chai, L.; Shaukat, R.; Wang, L.; Wang, H.S. A review on heat transfer and hydrodynamic characteristics of nano/microencapsulated phase change slurry (N/MPCS) in mini/microchannel heat sinks. Appl. Therm. Eng. 2018, 135, 334–349. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Fang, X.; Zhang, Z. Preparation of graphite nanoparticles-modified phase change microcapsules and their dispersed slurry for direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2017, 159, 159–166. [Google Scholar] [CrossRef]
- Delgado, M.; Lázaro, A.; Peñalosa, C.; Zalba, B. Experimental analysis of the influence of microcapsule mass fraction on the thermal and rheological behaviour of a PCM slurry. Appl. Therm. Eng. 2014, 63, 11–22. [Google Scholar] [CrossRef]
- Zhang, G.H.; Zhao, C.Y. Thermal and rheological properties of microencapsulated phase change materials. Renew. Energy 2011, 36, 2959–2966. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M. Microencapsulated PCM slurries’ dynamic viscosity experimental investigation and temperature-dependent prediction model. Int. J. Heat Mass Transf. 2019, 145, 118741. [Google Scholar] [CrossRef]
- Dutkowski, K.; Fiuk, J.J. Experimental investigation of the effects of mass fraction and temperature on the viscosity of microencapsulated PCM slurry. Int. J. Heat Mass Transf. 2018, 126, 390–399. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M.; Zajączkowski, B.; Białko, B. The experimental investigation of mPCM slurries density at phase change temperature. Int. J. Heat Mass Transf. 2020, 159, 120083. [Google Scholar] [CrossRef]
- Vasile, V.; Necula, H.; Badea, A.; Revellin, R.; Bonjour, J.; Haberschill, P. Experimental study of the heat transfer characteristics of a paraffin-in-water emulsion used as a secondary refrigerant. Int. J. Refrig. 2018, 88, 1–7. [Google Scholar] [CrossRef]
- Zhang, G.; Cui, G.; Dou, B.; Wang, Z.; Goula, M.A. An experimental investigation of forced convection heat transfer with novel microencapsulated phase change material slurries in a circular tube under constant heat flux. Energy Convers. Manag. 2018, 171, 699–709. [Google Scholar] [CrossRef]
- Liu, C.; Ma, Z.; Wang, J.; Li, Y.; Rao, Z. Experimental research on flow and heat transfer characteristics of latent functional thermal fluid with microencapsulated phase change materials. Int. J. Heat Mass Transf. 2017, 115, 737–742. [Google Scholar] [CrossRef]
- Kong, M.; Alvarado, J.L.; Terrell, W.; Thies, C. Performance characteristics of microencapsulated phase change material slurry in a helically coiled tube. Int. J. Heat Mass Transf. 2016, 101, 901–914. [Google Scholar] [CrossRef]
- Ho, C.J.; Chang, P.C.; Yan, W.M.; Amani, M. Microencapsulated n-eicosane PCM suspensions: Thermophysical properties measurement and modeling. Int. J. Heat Mass Transf. 2018, 125, 792–800. [Google Scholar] [CrossRef]
- Trivedi, G.V.N.; Parameshwaran, R. Microencapsulated phase change material suspensions for cool thermal energy storage. Mater. Chem. Phys. 2020, 242, 122519. [Google Scholar] [CrossRef]
- Chen, L.; Wang, T.; Zhao, Y.; Zhang, X.R. Characterization of thermal and hydrodynamic properties for microencapsulated phase change slurry (MPCS). Energy Convers. Manag. 2014, 79, 317–333. [Google Scholar] [CrossRef]
- Karaipekli, A.; Erdoğan, T.; Barlak, S. The stability and thermophysical properties of a thermal fluid containing surface-functionalized nanoencapsulated PCM. Thermochim. Acta 2019, 682, 178406. [Google Scholar] [CrossRef]
- Allouche, Y.; Varga, S.; Bouden, C.; Oliveira, A.C. Experimental determination of the heat transfer and cold storage characteristics of a microencapsulated phase change material in a horizontal tank. Energy Convers. Manag. 2015, 94, 275–285. [Google Scholar] [CrossRef]
- Lemmon, E.W.; Bell, I.H.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 10.0; National Institute of Standards and Technology, Standard Reference Data Program: Gaithersburg, MD, USA, 2018. [Google Scholar]
- Dutkowski, K.; Kruzel, M.; Kaczmarek, D.; Nalepa, B.; Zajączkowski, B.; Valíček, J.; Harničárová, M. Influence of the Physical State of Microencapsulated PCM on the Pressure Drop of Slurry in a Circular Channel. Materials 2022, 15, 6719. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).