Ammonia Airship Cooling: An Option for Renewable Cooling in the Tropics
Abstract
:1. Introduction
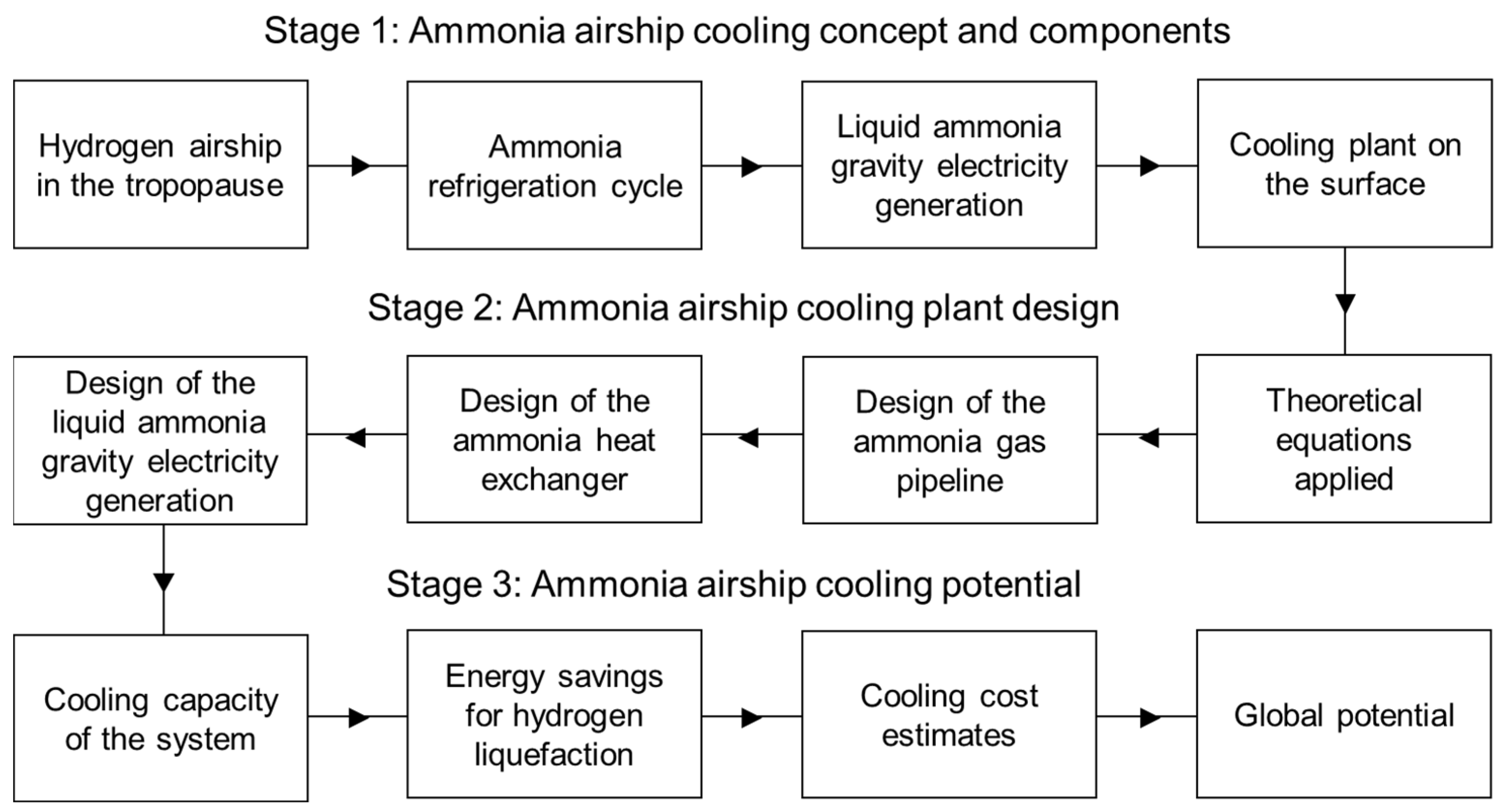
2. Materials and Methods
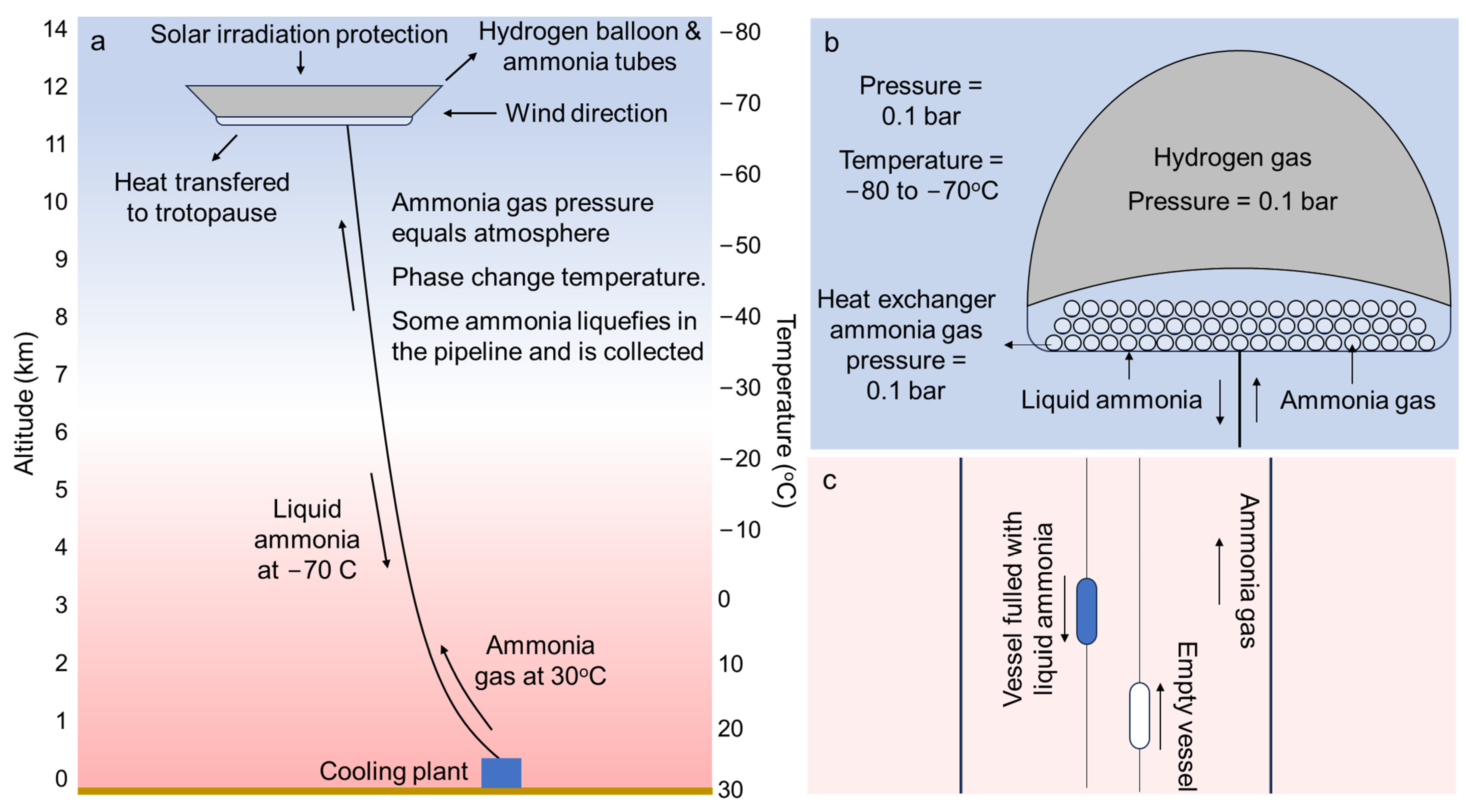
Ammonia Airship Cooling—AAC
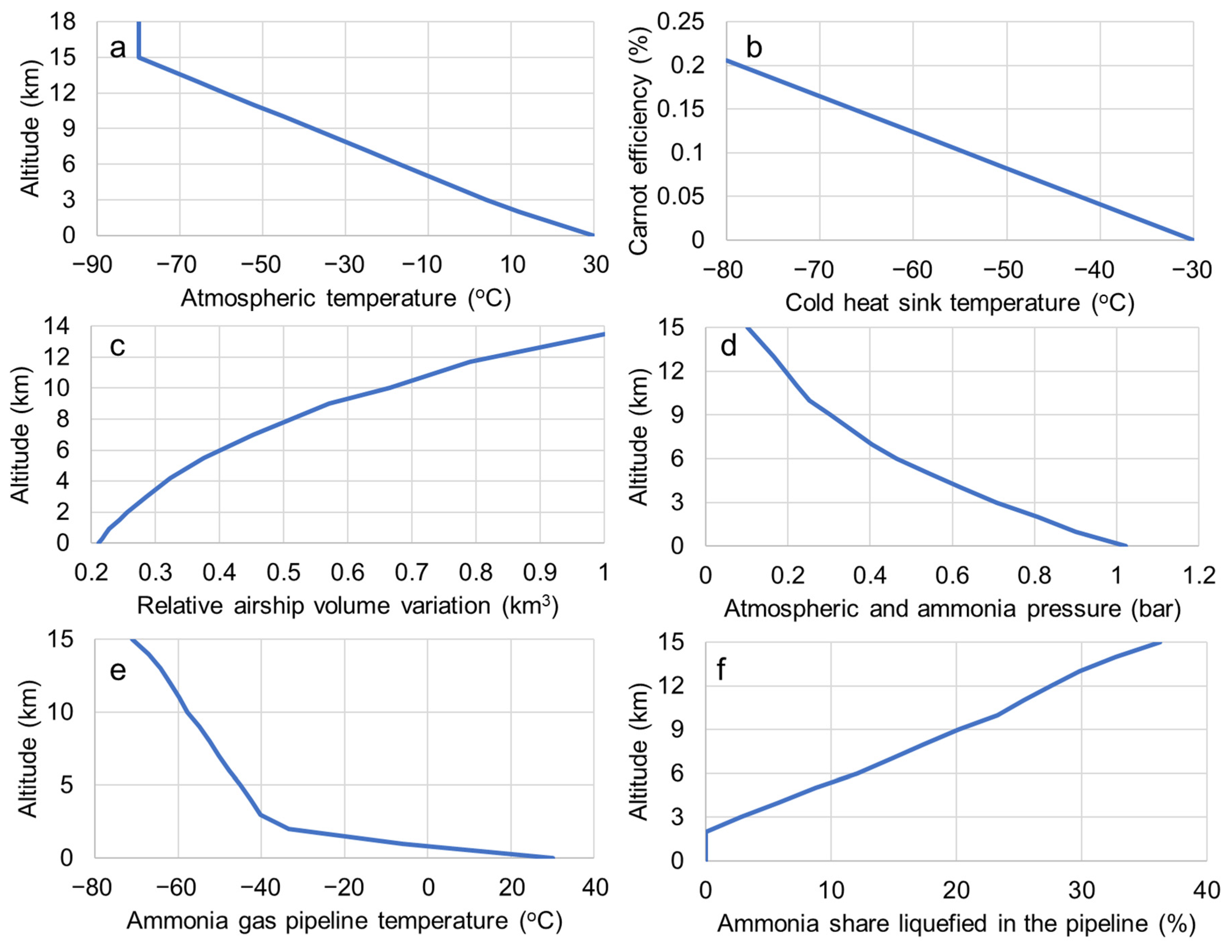
3. Results
Global Potential
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, K.; Tan, X.; Yan, Y.; Jiang, D.; Qi, S. Directing Energy Transition toward Decarbonization: The China Story. Energy 2022, 261, 124934. [Google Scholar] [CrossRef]
- Streimikiene, D.; Baležentis, T.; Volkov, A.; Morkūnas, M.; Žičkienė, A.; Streimikis, J. Barriers and Drivers of Renewable Energy Penetration in Rural Areas. Energies 2021, 14, 6452. [Google Scholar] [CrossRef]
- Marinakis, V. Big Data for Energy Management and Energy-Efficient Buildings. Energies 2020, 13, 1555. [Google Scholar] [CrossRef]
- Reich, M.; Oesterberg, M.; Regener, T.; Kent, D.; Nawfal, N.; Khames, S. New Performance Motors Set New Benchmarks in Drilling Performance. In Proceedings of the SPE/IADC Middle East Drilling Technology Conference and Exhibition, Abu Dhabi, United Arab Emirates, 20–22 October 2003. [Google Scholar] [CrossRef]
- Mihet-Popa, L.; Saponara, S. Toward Green Vehicles Digitalization for the Next Generation of Connected and Electrified Transport Systems. Energies 2018, 11, 3124. [Google Scholar] [CrossRef]
- Sarbu, I.; Mirza, M.; Muntean, D. Integration of Renewable Energy Sources into Low-Temperature District Heating Systems: A Review. Energies 2022, 15, 3124. [Google Scholar] [CrossRef]
- Namuq, M.; Reich, M.; Al-Zoubi, A. Numerical Simulation and Modeling of a Laboratory MWD Mud Siren Pressure Pulse Propagation in Fluid Filled Pipe. Oil Gas Eur. Mag. 2012, 38, 125–130. [Google Scholar]
- Sorknæs, P.; Johannsen, R.M.; Korberg, A.D.; Nielsen, T.B.; Petersen, U.R.; Mathiesen, B.V. Electrification of the Industrial Sector in 100% Renewable Energy Scenarios. Energy 2022, 254, 124339. [Google Scholar] [CrossRef]
- Nastasi, B.; Lo Basso, G. Hydrogen to Link Heat and Electricity in the Transition towards Future Smart Energy Systems. Energy 2016, 110, 5–22. [Google Scholar] [CrossRef]
- Zakeri, B.; Paulavets, K.; Barreto-Gomez, L.; Echeverri, L.G.; Pachauri, S.; Boza-Kiss, B.; Zimm, C.; Rogelj, J.; Creutzig, F.; Ürge-Vorsatz, D.; et al. Pandemic, War, and Global Energy Transitions. Energies 2022, 15, 6114. [Google Scholar] [CrossRef]
- Van de Graaf, T.; Overland, I.; Scholten, D.; Westphal, K. The New Oil? The Geopolitics and International Governance of Hydrogen. Energy Res. Soc. Sci. 2020, 70, 101667. [Google Scholar] [CrossRef]
- Hunt, J.D.; Nascimento, A.; Nascimento, N.; Vieira, L.; Romero, O. Possible Pathways for Oil and Gas Companies in a Sustainable Future: From the Perspective of a Hydrogen Economy. Renew. Sustain. Energy Rev. 2022, 160, 112291. [Google Scholar] [CrossRef]
- Ghafri, S.; Munro, S.; Cardella, U.; Funke, T.; Notardonato, W.; Trusler, J.; Leachman, J.; Span, R.; Kamiya, S.; Pearce, G.; et al. Hydrogen Liquefaction: A Review of the Fundamental Physics, Engineering Practice and Future Opportunities. Energy Environ. Sci. 2022, 15, 2690–2731. [Google Scholar] [CrossRef]
- Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- Kamran, M.S.; Ahmad, H.O.; Wang, H.S. Review on the Developments of Active Magnetic Regenerator Refrigerators–Evaluated by Performance. Renew. Sustain. Energy Rev. 2020, 133, 110247. [Google Scholar] [CrossRef]
- Eckener, H. Protecting Airships against Fire. Nature 1938, 142, 747. [Google Scholar] [CrossRef]
- Karataev, V.B.; Grosheva, P.Y.; Shkvarya, L.V. From the History of the Development of Controlled Aerostats (Airships) in the XIX-Early XX Centuries. Bylye Gody 2018, 49, 1159–1165. [Google Scholar] [CrossRef]
- Metlen, T.; Palazotto, A.N.; Cranston, B. Economic Optimization of Cargo Airships. CEAS Aeronaut. J. 2016, 7, 287–298. [Google Scholar] [CrossRef]
- Trancossi, M.; Dumas, A.; Madonia, M.; Pascoa, J.; Vucinic, D. Fire-Safe Airship System Design. SAE Int. J. Aerosp. 2012, 5, 11–21. [Google Scholar] [CrossRef]
- Dumas, A.; Trancossi, M.; Madonia, M. Hydrogen Airships: A Necessary Return Because of High Costs of Helium. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Proceedings (IMECE), Houston, TX, USA, 9–15 November 2012; Volume 6, pp. 533–540. [Google Scholar]
- Bonnici, M.; Tacchini, A.; Vucinic, D. Long Permanence High Altitude Airships: The Opportunity of Hydrogen. Eur. Transp. Res. Rev. 2014, 6, 253–266. [Google Scholar] [CrossRef]
- Provornaya, I.V.; Filimonova, I.V.; Eder, L.V.; Nemov, V.Y.; Zemnukhova, E.A. Prospects for the Global Helium Industry Development. Energy Rep. 2022, 8, 110–115. [Google Scholar] [CrossRef]
- Xu, J.; Lin, W. Integrated Hydrogen Liquefaction Processes with LNG Production by Two-Stage Helium Reverse Brayton Cycles Taking Industrial by-Products as Feedstock Gas. Energy 2021, 227, 120443. [Google Scholar] [CrossRef]
- Livingston, B. Blimps And Modern Warfare. Sci. Am. 1940, 162, 261–263. [Google Scholar] [CrossRef]
- Klemin, A. Future Leviathans of the Skies. Sci. Am. 1925, 132, 166–168. [Google Scholar] [CrossRef]
- Dolman, A.J. The Rediscovery of the Airship. Phys. Technol. 1983, 14, 169. [Google Scholar] [CrossRef]
- Hunt, J.D.; Nascimento, A.; Zakeri, B.; Barbosa, P.S.F.; Costalonga, L. Seawater Air-Conditioning and Ammonia District Cooling: A Solution for Warm Coastal Regions. Energy 2022, 254, 124359. [Google Scholar] [CrossRef]
- Hunt, J.D.; Zakeri, B.; Falchetta, G.; Nascimento, A.; Wada, Y.; Riahi, K. Mountain Gravity Energy Storage: A New Solution for Closing the Gap between Existing Short-and Long-Term Storage Technologies. Energy 2020, 190, 116419. [Google Scholar] [CrossRef]
- The Engineering ToolBox Convective Heat Transfer. Available online: https://www.engineeringtoolbox.com/convective-heat-transfer-d_430.html (accessed on 20 December 2023).
- The Engineering ToolBox Metals, Metallic Elements and Alloys-Thermal Conductivities. Available online: https://www.engineeringtoolbox.com/thermal-conductivity-metals-d_858.html (accessed on 20 December 2023).
- Incropera, F.P.; DeWitt, D.P.; Bergman, T.L.; Lavine, A.S. Fundamentals of Heat and Mass Transfer, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; Volume 6, ISBN 9780471457282. [Google Scholar]
- Wu, G.; Yan, Z.; Zhuang, D.; Ding, G.; Cao, F.; Meng, J. Design Method and Application Effects of Embedded-Clapboard Distributor on Refrigerant Distribution among Multi-Tubes of Micro-Channel Heat Exchangers. Int. J. Refrig. 2020, 119, 420–433. [Google Scholar] [CrossRef]
- Cao, H.S.; Vanapalli, S.; Holland, H.J.; Vermeer, C.H.; ter Brake, H.J.M. A Micromachined Joule–Thomson Cryogenic Cooler with Parallel Two-Stage Expansion. Int. J. Refrig. 2016, 69, 223–231. [Google Scholar] [CrossRef]
- Xu, J.H.; Zou, S.; Inaoka, K.; Xi, G.N. Effect of Reynolds Number on Flow and Heat Transfer in Incompressible Forced Convection over a 3D Backward-Facing Step. Int. J. Refrig. 2017, 79, 164–175. [Google Scholar] [CrossRef]
- Brkić, D. Can Pipes Be Actually Really That Smooth? Int. J. Refrig. 2012, 35, 209–215. [Google Scholar] [CrossRef]
- Gluesenkamp, K.R.; Nawaz, K. Separate Sensible and Latent Cooling: Carnot Limits and System Taxonomy. Int. J. Refrig. 2021, 127, 128–136. [Google Scholar] [CrossRef]
- Erdinc, M.T. Two-Evaporator Refrigeration System Integrated with Expander-Compressor Booster. Int. J. Refrig. 2023, 154, 349–363. [Google Scholar] [CrossRef]
- LMNO Engineering Gas Viscosity Calculator. Available online: https://www.lmnoeng.com/Flow/GasViscosity.php (accessed on 20 December 2023).
- The Engineering ToolBox Ammonia-Specific Heat, vs. Temperature and Pressure. Available online: https://www.engineeringtoolbox.com/ammonia-heat-capacity-specific-temperature-pressure-Cp-Cv-d_2016.html (accessed on 20 December 2023).
- Hunt, J.D.; Montanari, P.M.; Hummes, D.N.; Taghavi, M.; Zakeri, B.; Romero, O.J.; Zhou, W.; de Freitas, M.A.V.; José de Castro, N.; Schneider, P.S.; et al. Solid Air Hydrogen Liquefaction, the Missing Link of the Hydrogen Economy. Int. J. Hydrogen Energy 2023, 48, 29198–29208. [Google Scholar] [CrossRef]
- Jiaxing Inch Eco Materials Co., L. Airship Envelope Fabric TPU Coated Tear Resistant UHMWPE Fabric Inflatable. Available online: https://www.alibaba.com/product-detail/Airship-Envelope-Fabric-TPU-Coated-Tear_1600215218350.html?spm=a2700.galleryofferlist.normal_offer.d_title.21313aecya6E7U (accessed on 20 December 2023).
- Wan Star Aluminum Industry, Co. Thin Wall Aluminum Round Tube Customization Aluminum Pipe Fine Drawn Aluminum Tube for Manufacturing of Mechanical Parts. Available online: https://www.alibaba.com/product-detail/Thin-Wall-Aluminum-Round-Tube-Customization_11000007506803.html?spm=a2700.7735675.0.0.47f1WqZBWqZBf1&s=p (accessed on 20 December 2023).
- Hunt, J.D.; Zakeri, B.; Jurasz, J.; Tong, W.; Dąbek, P.B.; Brandão, R.; Patro, E.R.; Đurin, B.; Filho, W.L.; Wada, Y.; et al. Underground Gravity Energy Storage: A Solution for Long-Term Energy Storage. Energies 2023, 16, 825. [Google Scholar] [CrossRef]
- ECMWF ERA5 Monthly Averaged Data on Pressure Levels from 1940 to Present. Available online: https://cds.climate.copernicus.eu/cdsapp#!/dataset/reanalysis-era5-pressure-levels-monthly-means?tab=overview (accessed on 20 December 2023).
- Hunt, J.D.; Byers, E.; Balogun, A.-L.; Leal Filho, W.; Colling, A.V.; Nascimento, A.; Wada, Y. Using the Jet Stream for Sustainable Airship and Balloon Transportation of Cargo and Hydrogen. Energy Convers. Manag. X 2019, 3, 100016. [Google Scholar] [CrossRef]
- Hunt, J.D.; Zakeri, B.; Nascimento, A.; de Freitas, M.A.V.; Amorim, F.d.C.; Guo, F.; Witkamp, G.-J.; van Ruijven, B.; Wada, Y. Hydrogen Balloon Transportation: A Cheap and Efficient Mode to Transport Hydrogen. Int. J. Hydrogen Energy 2023, 53, 875–884. [Google Scholar] [CrossRef]



| Component | Role in the System |
|---|---|
| Hydrogen airship | Lift the ammonia tubes to the tropopause, where the temperature liquefies the ammonia. Its shape should minimize wind drag, provide additional lift with the wind and provide shade from the Sun to the ammonia tubes. |
| Ammonia aluminum tubes | Exchange heat between the tropopause and the ammonia gas to liquefy the ammonia. The pressure inside the tubes is similar to the pressure outside. The tubes are filled with ammonia, which is lighter than air and provides lift to the airship. |
| Flexible pipeline | The flexible pipeline transports ammonia gas from the cooling plant in the surface to the airship. It also provides lift as the ammonia inside the pipeline is lighter than air. |
| Liquid ammonia lifts | The lifts due to the liquid ammonia are used to transport the liquid ammonia in insolated tanks from the airship to the cooling plant in the surface. It also generates gravitational electricity with a generator in the cooling plant, similarly to how electricity is generated with Mountain Gravity Energy Storage (MGES) [28]. |
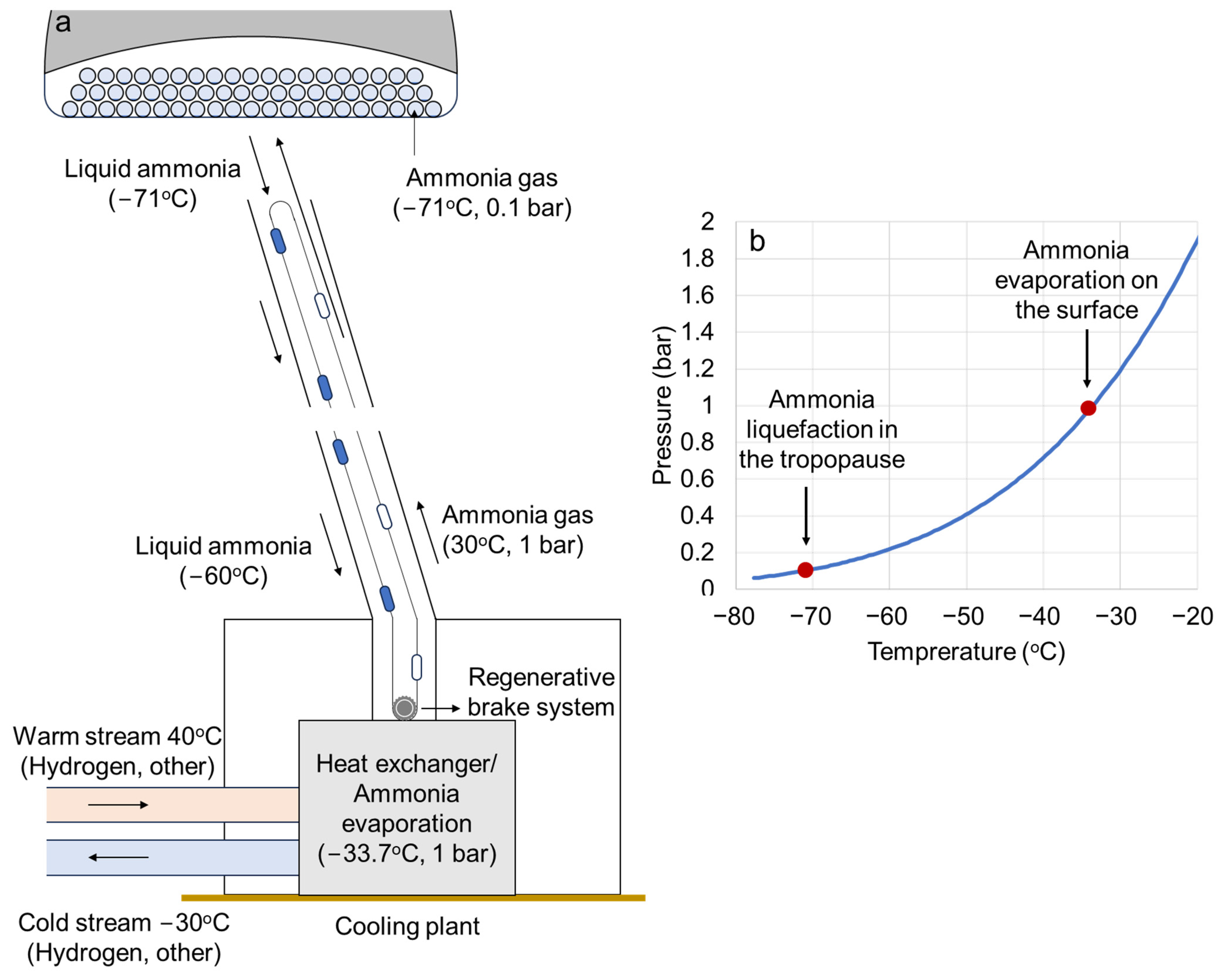
| Cooling plant | The cooling plant regasifies the liquid ammonia providing refrigeration services at −30 °C, which can be used to reduce the energy consumption to liquefy hydrogen. |
| Detail | Value |
|---|---|
| Ammonia gas pipeline length (m) | 15,000 |
| Ammonia gas pipeline inner diameter (m) | 6400–10,700 |
| Surface ammonia gas pressure (bar) | 1.02 |
| Temperature in the cooling plant (°C) | −33.7 |
| Total pressure drop in the pipeline (bar) | 0.92 |
| Airship ammonia gas pressure (bar) | 0.10 |
| Surface ammonia temperature (°C) | 30 |
| Airship ammonia temperature (°C) | −71.0 |
| Ammonia gas viscosity (μPa·s) | 7–10.3 [38] |
| Roughness of interior pipe wall (mm) | 0.1 |
| Ammonia gas velocity (m/s) | 24–58 |
| Ammonia flowrate (kg/s) | 750–478 |
| Ammonia flowrate (m3/s) | 1075–3770 |
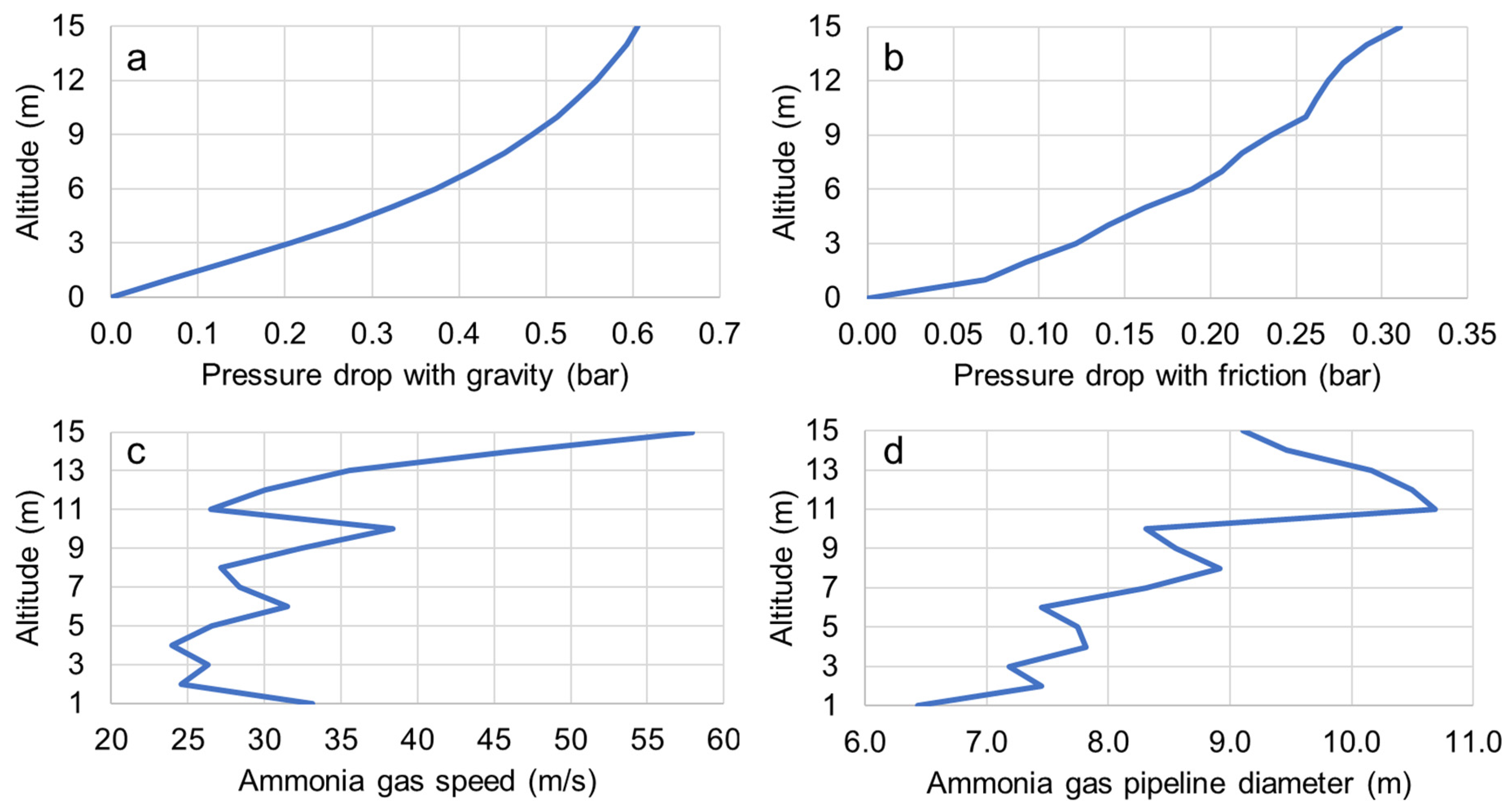
| Altitude (km) | Ammonia Pressure (bar) | Ammonia Temperature (°C) | Ammonia Density (kg/m3) | Ammonia Gas (%) | Horizontal Pressure Drop (bar) | Pressure Drop from Friction (bar) |
|---|---|---|---|---|---|---|
| 15 | 0.101 | −71.0 | 0.103 | 63.7 | 0.606 | 0.311 |
| 14 | 0.135 | −67.2 | 0.134 | 67.3 | 0.592 | 0.291 |
| 13 | 0.166 | −64.2 | 0.163 | 70.2 | 0.576 | 0.277 |
| 12 | 0.194 | −61.9 | 0.189 | 72.5 | 0.558 | 0.269 |
| 11 | 0.222 | −59.7 | 0.215 | 74.6 | 0.537 | 0.262 |
| 10 | 0.253 | −57.8 | 0.242 | 76.7 | 0.513 | 0.256 |
| 9 | 0.303 | −54.9 | 0.288 | 79.8 | 0.485 | 0.235 |
| 8 | 0.354 | −52.4 | 0.333 | 82.6 | 0.452 | 0.219 |
| 7 | 0.404 | −50.2 | 0.377 | 85.2 | 0.415 | 0.206 |
| 6 | 0.465 | −47.8 | 0.429 | 87.9 | 0.373 | 0.190 |
| 5 | 0.544 | −45.0 | 0.497 | 91.2 | 0.324 | 0.162 |
| 4 | 0.623 | −42.6 | 0.565 | 94.2 | 0.269 | 0.140 |
| 3 | 0.707 | −40.2 | 0.636 | 97.2 | 0.207 | 0.121 |
| 2 | 0.808 | −33.5 | 0.706 | 100.0 | 0.137 | 0.092 |
| 1 | 0.901 | −6.0 | 0.701 | 100.0 | 0.068 | 0.068 |
| 0 | 1.022 | 30.0 | 0.698 | 100.0 | - | - |
| Altitude (km) | Velocity (m/s) | Diameter (m) | Viscosity (μPa.s) | Reynolds Number (106) | Friction Factor |
|---|---|---|---|---|---|
| 15 | 57.9 | 9.1 | 7.11 | 9.9 | 0.039 |
| 14 | 45.9 | 9.5 | 7.19 | 9.8 | 0.039 |
| 13 | 35.6 | 10.2 | 7.25 | 9.4 | 0.038 |
| 12 | 30.1 | 10.5 | 7.31 | 9.3 | 0.037 |
| 11 | 26.5 | 10.7 | 7.37 | 9.3 | 0.037 |
| 10 | 38.4 | 8.3 | 7.45 | 12.3 | 0.040 |
| 9 | 32.4 | 8.6 | 7.52 | 12.3 | 0.040 |
| 8 | 27.1 | 8.9 | 7.58 | 12.0 | 0.039 |
| 7 | 28.4 | 8.3 | 7.65 | 13.2 | 0.040 |
| 6 | 31.5 | 7.5 | 7.73 | 15.1 | 0.042 |
| 5 | 26.6 | 7.7 | 7.8 | 14.9 | 0.041 |
| 4 | 24.0 | 7.8 | 7.86 | 15.2 | 0.041 |
| 3 | 26.3 | 7.2 | 8.08 | 16.5 | 0.043 |
| 2 | 24.5 | 7.5 | 8.99 | 14.3 | 0.042 |
| 1 | 33.1 | 6.4 | 10.3 | 14.4 | 0.044 |
| Details | Values |
|---|---|
| Heat transfer in the exchanger (MWt) | 654.4 |
| Temperature difference for heat transfer (°C) | 10 |
| Tube length (m) | 400 |
| Tube diameter (m) | 10 |
| Number of tubes | 62 |
| Number of tube layers | 3 |
| Airship dimensions (m × m × m) | 400 × 200 × 400 |
| Details | Values |
|---|---|
| Gravity electricity generation (MW) | 49.2 |
| Specific heat cooling potential (MWt) | 114.3 |
| Latent heat cooling potential (MWt) | 1026.8 |
| Total cooling capacity (MWt) | 1141.1 |
| Yearly cooling service (TWth) | 8.0 |
| Hydrogen liquefaction cold energy requirement (kWht/kg H2) | 3.7 |
| Hydrogen liquefaction electricity requirement (kWh/kg H2) | 9.3 |
| Hydrogen liquefaction electricity requirement with AAC (kWh/kg H2) | 6.5 |
| Details | Values | Description |
|---|---|---|
| Capital costs | ||
| Hydrogen airship envelope | 8,400,000 | The cost of the airship is mainly the envelope cost. The envelope proposed for the airship is ultra-high molecular weight polyethylene (UHMWPE) fabric. Assuming a 400 × 200 × 400 m dimension, the airship envelope area is 400,000 m2 for 21 USD/m2 [41]. |
| Flexible ammonia gas vertical pipeline envelope | 9,000,000 | The ammonia gas vertical pipeline has a length of 15 km and an average diameter of 9 m, which results in an area of 430,000 m2. The pipeline is also made of envelope fabric at a cost of 21 USD/m2 [41]. |
| Ammonia tubes | 18,500,000 | 62 aluminum tubes 400 m long, 10 m diameter, 3 mm thickness, 2640 kg/m3 density, and cost of 3 USD/kg [42]. |
| Gravity electricity generation system and anchor. | 98,400,000 | The cost for a gravity electricity generation system can be assumed to be 2000 USD/kW (Hunt et al., 2020; Hunt et al., 2023) [28,43], assuming an installed capacity of 49.2 MW. Apart from generating electricity with liquid ammonia, the system anchors the airship. It was assumed that the electricity generated is used to supply the electricity requirements by the AAC system. |
| Cooling plant | 20,000,000 | The cooling plant consists of a heat exchanger capable of exchanging 1141 MWt of cold with an average heat exchange temperature of 5 °C. |
| Total capital costs | 154,300,000 | - |
| Operation and maintenance costs | ||
| Airship hydrogen | 200,000 | The hydrogen volume in the airship is 16,000,000 m3 at a pressure of 0.1 and density of 0.01245 kg/m3, equivalent to 200,000. It needs to be refueled every year due to hydrogen leakage. Assuming a future cost of hydrogen of 1 USD/kg. The ammonia leakage is neglected. |
| Operation cost | 15,430,000 | The operation cost is low as most of the system will be automated, and it is assumed to be 10% of capital costs. |
| Maintenance cost | 30,860,000 | Due to the harsh operational conditions, the maintenance cost is assumed to be high and equal to 20% of the investment cost per year. |
| Cost parameters | ||
| Lifetime (years) | 10 | - |
| Levelized costs of AAC cooling (USD/MWht) | 8.25 | Assuming an interest rate of 5% per year, a 7.9 discount factor, |
| Levelized costs of mechanical cooling (USD/kWht) | 15 | Assuming the cooling provided at −30 °C, a hot source of 40 °C, a COP of 1, an electricity cost of 15 USD/MWh, and a negligible investment cost. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunt, J.D.; Zakeri, B.; Nascimento, A.; Guo, F.; Freitas, M.A.V.d.; Silva, C.V.; van Ruijven, B. Ammonia Airship Cooling: An Option for Renewable Cooling in the Tropics. Energies 2024, 17, 111. https://doi.org/10.3390/en17010111
Hunt JD, Zakeri B, Nascimento A, Guo F, Freitas MAVd, Silva CV, van Ruijven B. Ammonia Airship Cooling: An Option for Renewable Cooling in the Tropics. Energies. 2024; 17(1):111. https://doi.org/10.3390/en17010111
Chicago/Turabian StyleHunt, Julian David, Behnam Zakeri, Andreas Nascimento, Fei Guo, Marcos Aurélio Vasconcelos de Freitas, Cristiano Vitorino Silva, and Bas van Ruijven. 2024. "Ammonia Airship Cooling: An Option for Renewable Cooling in the Tropics" Energies 17, no. 1: 111. https://doi.org/10.3390/en17010111
APA StyleHunt, J. D., Zakeri, B., Nascimento, A., Guo, F., Freitas, M. A. V. d., Silva, C. V., & van Ruijven, B. (2024). Ammonia Airship Cooling: An Option for Renewable Cooling in the Tropics. Energies, 17(1), 111. https://doi.org/10.3390/en17010111








