From Academia to Industry: Criteria for Upscaling Ionic Liquid-Based Thermo-Electrochemical Cells for Large-Scale Applications
Abstract
:1. Introduction
2. Experimental
2.1. Design of the Thermoelectric Cell
2.2. Materials Selection
2.3. Thermoelectric Test Setup
3. Results and Discussion
4. Conclusions
- Ionic working fluids are able to resist at very high temperatures (>300 °C), enabling in this way the possibility of exploiting higher temperature gradients.
- Redox couples have high mobility in the working fluid and electrolyte–electrode interface.
- Electrodes with an increased electrochemical surface area could be obtained by tailoring the composition of the microporous layer and selecting active carbon with a pore distribution compatible with the size of the molecules composing the redox couple and the electrolyte.
- The improved system design is able to maximize the temperature gradient between the cold and hot sides of the generator.
- Optimized heat transfer between the heat source and the hot side of the generator (and conversely between the heat sink and the cold).
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Energy Investment 2023—Analysis. Available online: https://www.iea.org/reports/world-energy-investment-2023 (accessed on 7 August 2023).
- Bian, Q. Waste Heat: The Dominating Root Cause of Current Global Warming. Environ. Syst. Res. 2020, 9, 8. [Google Scholar] [CrossRef]
- Waste Heat: Innovators Turn to an Overlooked Renewable Resource. Available online: https://e360.yale.edu/features/waste-heat-innovators-turn-to-an-overlooked-renewable-resource (accessed on 7 August 2023).
- Jaziri, N.; Boughamoura, A.; Müller, J.; Mezghani, B.; Tounsi, F.; Ismail, M. A Comprehensive Review of Thermoelectric Generators: Technologies and Common Applications. Energy Rep. 2020, 6, 264–287. [Google Scholar] [CrossRef]
- Loni, R.; Najafi, G.; Bellos, E.; Rajaee, F.; Said, Z.; Mazlan, M. A Review of Industrial Waste Heat Recovery System for Power Generation with Organic Rankine Cycle: Recent Challenges and Future Outlook. J. Clean. Prod. 2021, 287, 125070. [Google Scholar] [CrossRef]
- Huang, P.; Copertaro, B.; Zhang, X.; Shen, J.; Löfgren, I.; Rönnelid, M.; Fahlen, J.; Andersson, D.; Svanfeldt, M. A Review of Data Centers as Prosumers in District Energy Systems: Renewable Energy Integration and Waste Heat Reuse for District Heating. Appl. Energy 2020, 258, 114109. [Google Scholar] [CrossRef]
- Zoui, M.A.; Bentouba, S.; Stocholm, J.G.; Bourouis, M. A Review on Thermoelectric Generators: Progress and Applications. Energies 2020, 13, 3606. [Google Scholar] [CrossRef]
- Yang, J.; Stabler, F.R. Automotive Applications of Thermoelectric Materials. J. Electron. Mater. 2009, 38, 1245–1251. [Google Scholar] [CrossRef]
- Dan, D.; Zhao, Y.; Wei, M.; Wang, X. Review of Thermal Management Technology for Electric Vehicles. Energies 2023, 16, 4693. [Google Scholar] [CrossRef]
- Massetti, M.; Jiao, F.; Ferguson, A.J.; Zhao, D.; Wijeratne, K.; Würger, A.; Blackburn, J.L.; Crispin, X.; Fabiano, S. Unconventional Thermoelectric Materials for Energy Harvesting and Sensing Applications. Chem. Rev. 2021, 121, 12465–12547. [Google Scholar] [CrossRef]
- Quickenden, T.I.; Vernon, C.F. Thermogalvanic Conversion of Heat to Electricity. Sol. Energy 1986, 36, 63–72. [Google Scholar] [CrossRef]
- Dupont, M.F.; MacFarlane, D.R.; Pringle, J.M. Thermo-Electrochemical Cells for Waste Heat Harvesting—Progress and Perspectives. Chem. Commun. Camb. Engl. 2017, 53, 6288–6302. [Google Scholar] [CrossRef]
- Salez, T.J.; Nakamae, S.; Perzynski, R.; Mériguet, G.; Cebers, A.; Roger, M. Thermoelectricity and Thermodiffusion in Magnetic Nanofluids: Entropic Analysis. Entropy 2018, 20, 405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kim, T.; Li, N.; Kang, T.J.; Chen, J.; Pringle, J.M.; Zhang, M.; Kazim, A.H.; Fang, S.; Haines, C.; et al. High Power Density Electrochemical Thermocells for Inexpensively Harvesting Low-Grade Thermal Energy. Adv. Mater. 2017, 29, 1605652. [Google Scholar] [CrossRef]
- Hupp, J.T.; Weaver, M.J. Solvent, Ligand, and Ionic Charge Effects on Reaction Entropies for Simple Transition-Metal Redox Couples. Inorg. Chem. 1984, 23, 3639–3644. [Google Scholar] [CrossRef]
- Gonçalves, W.D.G.; Caspers, C.; Dupont, J.; Migowski, P. Ionic Liquids for Thermoelectrochemical Energy Generation. Curr. Opin. Green Sustain. Chem. 2020, 26, 100404. [Google Scholar] [CrossRef]
- Abraham, T.J.; Macfarlane, D.R.; Baughman, R.H.; Jin, L.; Li, N.; Pringle, J.M. Towards Ionic Liquid-Based Thermoelectrochemical Cells for the Harvesting of Thermal Energy. Electrochim. Acta 2013, 113, 87–93. [Google Scholar] [CrossRef]
- Hu, R.; Cola, B.A.; Haram, N.; Barisci, J.N.; Lee, S.; Stoughton, S.; Wallace, G.; Too, C.; Thomas, M.; Gestos, A.; et al. Harvesting Waste Thermal Energy Using a Carbon-Nanotube-Based Thermo-Electrochemical Cell. Nano Lett. 2010, 10, 838–846. [Google Scholar] [CrossRef]
- Inagaki, M.; Konno, H.; Tanaike, O. Carbon Materials for Electrochemical Capacitors. J. Power Sources 2010, 195, 7880–7903. [Google Scholar] [CrossRef]
- Im, H.; Kim, T.; Song, H.; Choi, J.; Park, J.S.; Ovalle-Robles, R.; Yang, H.D.; Kihm, K.D.; Baughman, R.H.; Lee, H.H.; et al. High-Efficiency Electrochemical Thermal Energy Harvester Using Carbon Nanotube Aerogel Sheet Electrodes. Nat. Commun. 2016, 7, 10600. [Google Scholar] [CrossRef]
- Artyukhov, D.; Kiselev, N.; Gorshkov, N.; Kovyneva, N.; Ganzha, O.; Vikulova, M.; Gorokhovsky, A.; Offor, P.; Boychenko, E.; Burmistrov, I. Harvesting Waste Thermal Energy Using a Surface-Modified Carbon Fiber-Based Thermo-Electrochemical Cell. Sustainability 2021, 13, 1377. [Google Scholar] [CrossRef]
- Burmistrov, I.; Khanna, R.; Gorshkov, N.; Kiselev, N.; Artyukhov, D.; Boychenko, E.; Yudin, A.; Konyukhov, Y.; Kravchenko, M.; Gorokhovsky, A.; et al. Advances in Thermo-Electrochemical (TEC) Cell Performances for Harvesting Low-Grade Heat Energy: A Review. Sustainability 2022, 14, 9483. [Google Scholar] [CrossRef]
- Shi, X.-L.; Zou, J.; Chen, Z.-G. Advanced Thermoelectric Design: From Materials and Structures to Devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Kim, J.; Lim, J.-H.; Kim, M.-S.; Myung, N.V. Comprehensive Review on Thermoelectric Electrodeposits: Enhancing Thermoelectric Performance Through Nanoengineering. Front. Chem. 2021, 9, 762896. [Google Scholar] [CrossRef] [PubMed]
- Saleemi, M.; Toprak, M.S.; Li, S.; Johnsson, M.; Muhammed, M. Synthesis, Processing, and Thermoelectric Properties of Bulk Nanostructured Bismuth Telluride (Bi2Te3). J. Mater. Chem. 2011, 22, 725–730. [Google Scholar] [CrossRef]
- Kwon, H.-N.; Jang, S.-J.; Kang, Y.C.; Roh, K.C. The Effect of ILs as Co-Salts in Electrolytes for High Voltage Supercapacitors. Sci. Rep. 2019, 9, 1180. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Kim, B.; Ko, J.; You, D.-J.; Yin, Z.; Kim, H.; Shin, D.; Cho, S.; Yoo, J.; Kim, Y.S. All Solid State Flexible Supercapacitors Operating at 4 V with a Cross-Linked Polymer–Ionic Liquid Electrolyte. J. Mater. Chem. A 2016, 4, 4386–4391. [Google Scholar] [CrossRef]
- dos Santos Junior, G.A.; Fortunato, V.D.S.; Bastos, G.A.A.; Silva, G.G.; Ortega, P.F.R.; Lavall, R.L. High-Performance Lithium-Ion Hybrid Supercapacitors Based on Lithium Salt/Imidazolium Ionic Liquid Electrolytes and Ni-Doped LiMn2O4 Cathode Materials. ACS Appl. Energy Mater. 2020, 3, 9028–9039. [Google Scholar] [CrossRef]
- You, D.-J.; Yin, Z.; Ahn, Y.; Lee, S.-H.; Yoo, J.; Kim, Y.S. Redox-Active Ionic Liquid Electrolyte with Multi Energy Storage Mechanism for High Energy Density Supercapacitor. RSC Adv. 2017, 7, 55702–55708. [Google Scholar] [CrossRef]
- Laux, E.; Jeandupeux, L.; Uhl, S.; Keppner, H.; Pérez López, P.; Sanglard, P.; Vanoli, E.; Marti, R. Novel Ionic Liquids for Thermoelectric Generator Devices. Mater. Today Proc. 2019, 8, 672–679. [Google Scholar] [CrossRef]
- Al-Masri, D.; Dupont, M.; Yunis, R.; MacFarlane, D.R.; Pringle, J.M. The Electrochemistry and Performance of Cobalt-Based Redox Couples for Thermoelectrochemical Cells. Electrochim. Acta 2018, 269, 714–723. [Google Scholar] [CrossRef]
- Bozack, M.J.; Zhou, Y.; Worley, S.D. Structural Modifications in the Amino Acid Lysine Induced by Soft X-ray Irradiation. J. Chem. Phys. 1994, 100, 8392–8398. [Google Scholar] [CrossRef]
- Yu, X.-R.; Liu, F.; Wang, Z.-Y.; Chen, Y. Auger Parameters for Sulfur-Containing Compounds Using a Mixed Aluminum-Silver Excitation Source. J. Electron Spectrosc. Relat. Phenom. 1990, 50, 159–166. [Google Scholar] [CrossRef]
- Hao, L.H.; Hieu, D.T.T.; Danh, T.T.; Long, T.H.; Phuong, H.T.; Luan, L.Q.; Man, T.V.; Tuyen, L.A.; Ngoc, P.K.; Tap, T.D. Surface Features of Polymer Electrolyte Membranes for Fuel Cell Applications: An Approach Using S2p XPS Analysis. VNUHCM J. Sci. Technol. Dev. 2021, 24, 2100–2109. [Google Scholar] [CrossRef]
- Worley, C.M.; Vannet, M.D.; Ball, G.L.; Moddeman, W.E. Surface Chemistry of a Microcoated Energetic Material, Pentaerythritoltetranitrate (PETN). Surf. Interface Anal. 1987, 10, 273–279. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 2001; ISBN 978-0-471-04372-0. [Google Scholar]

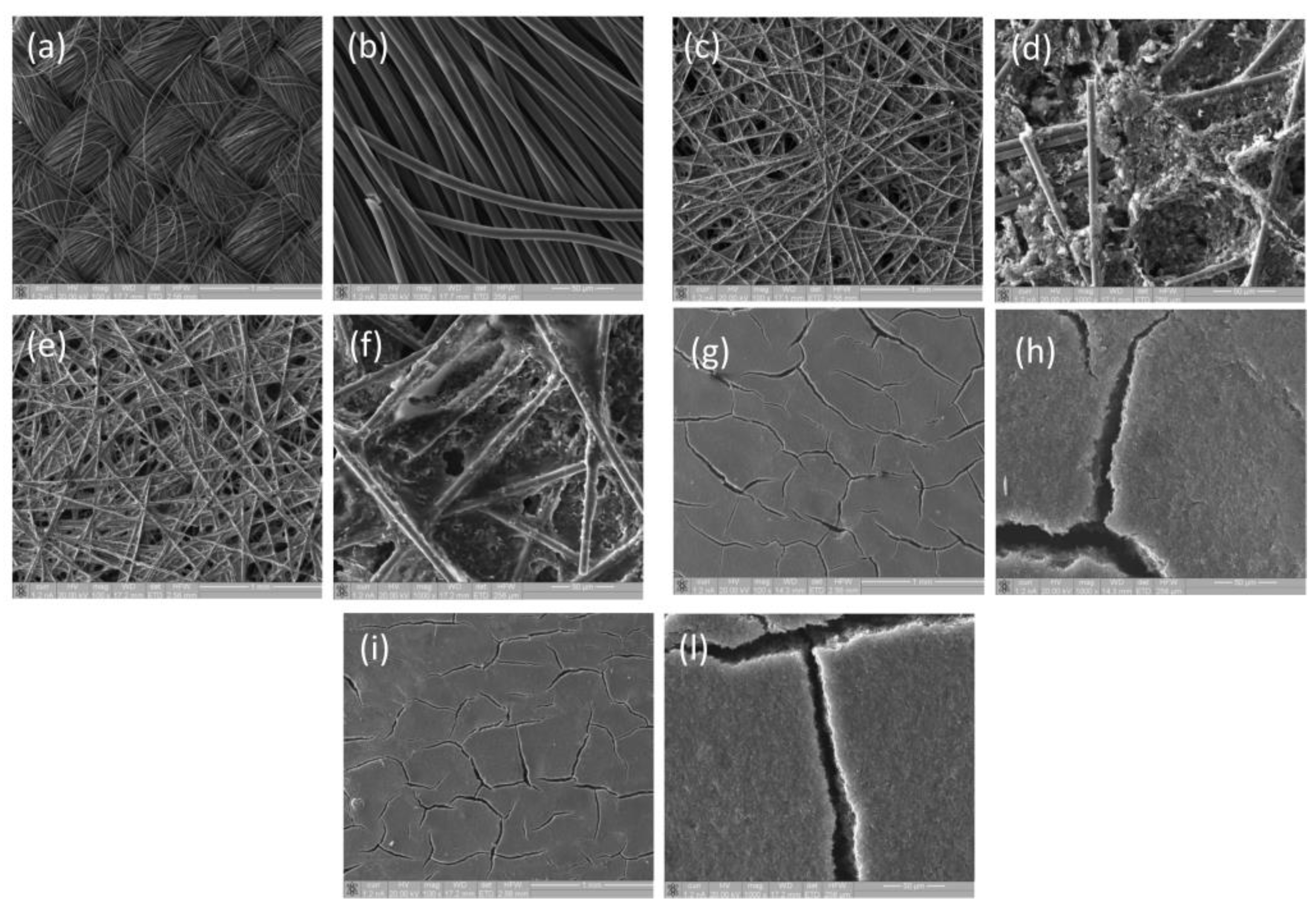

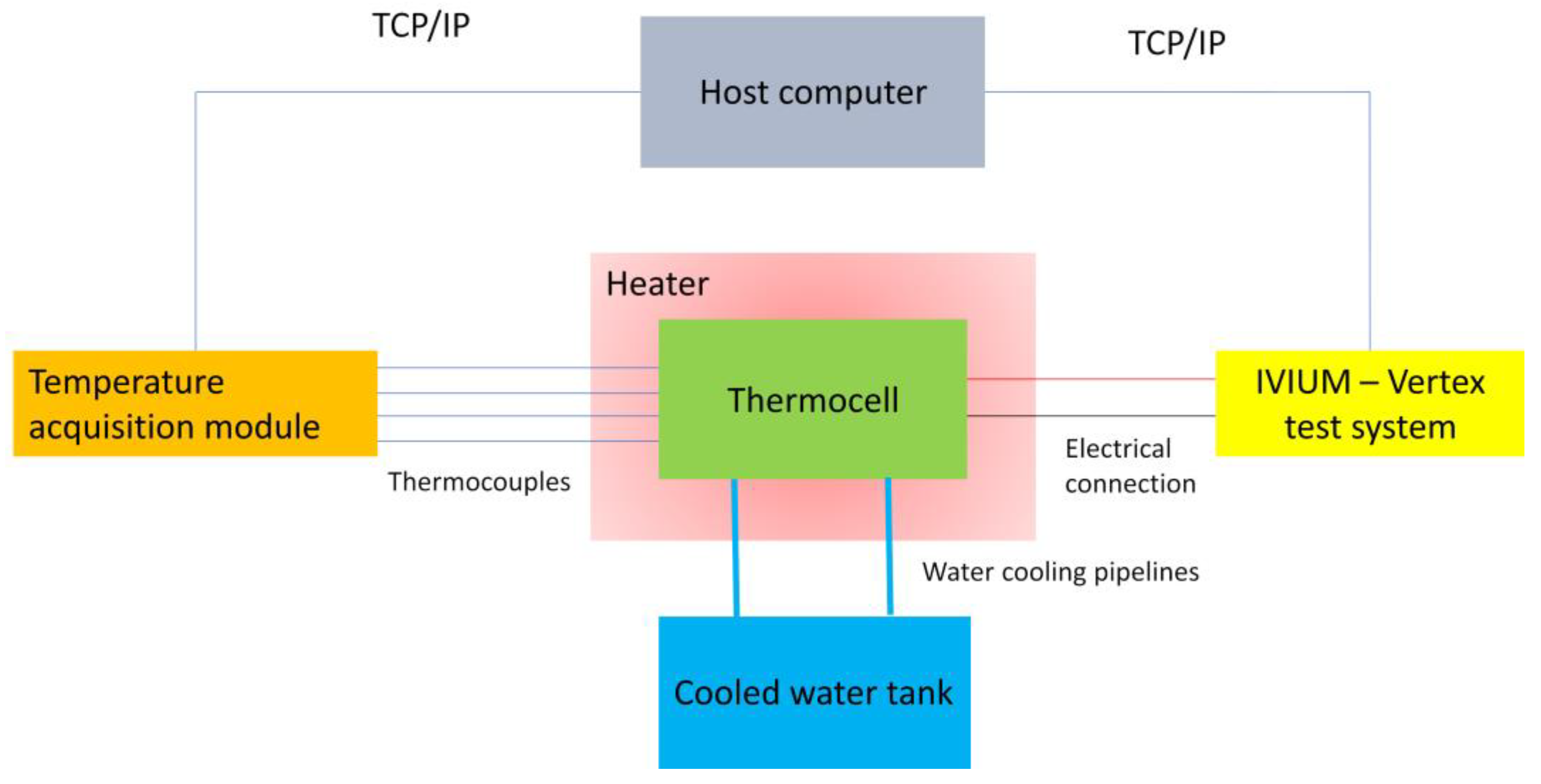
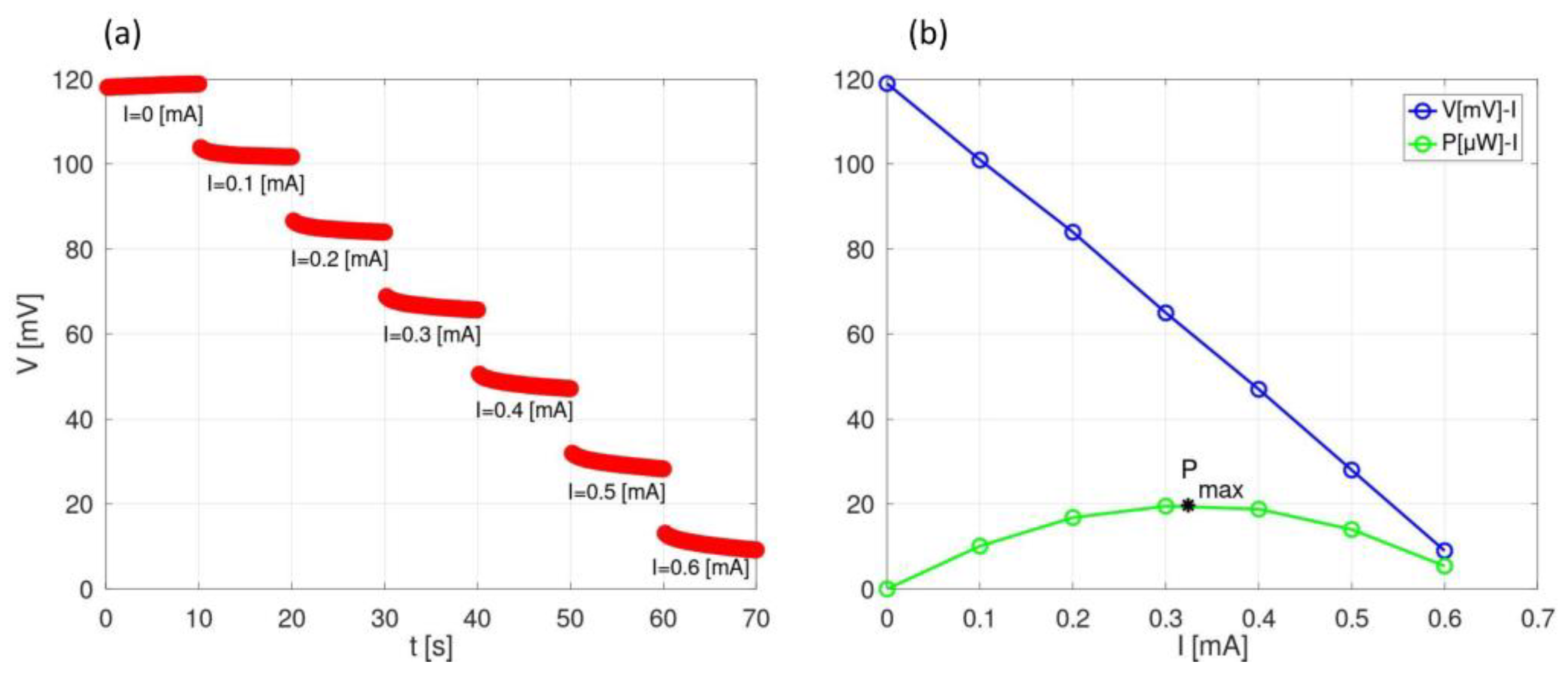
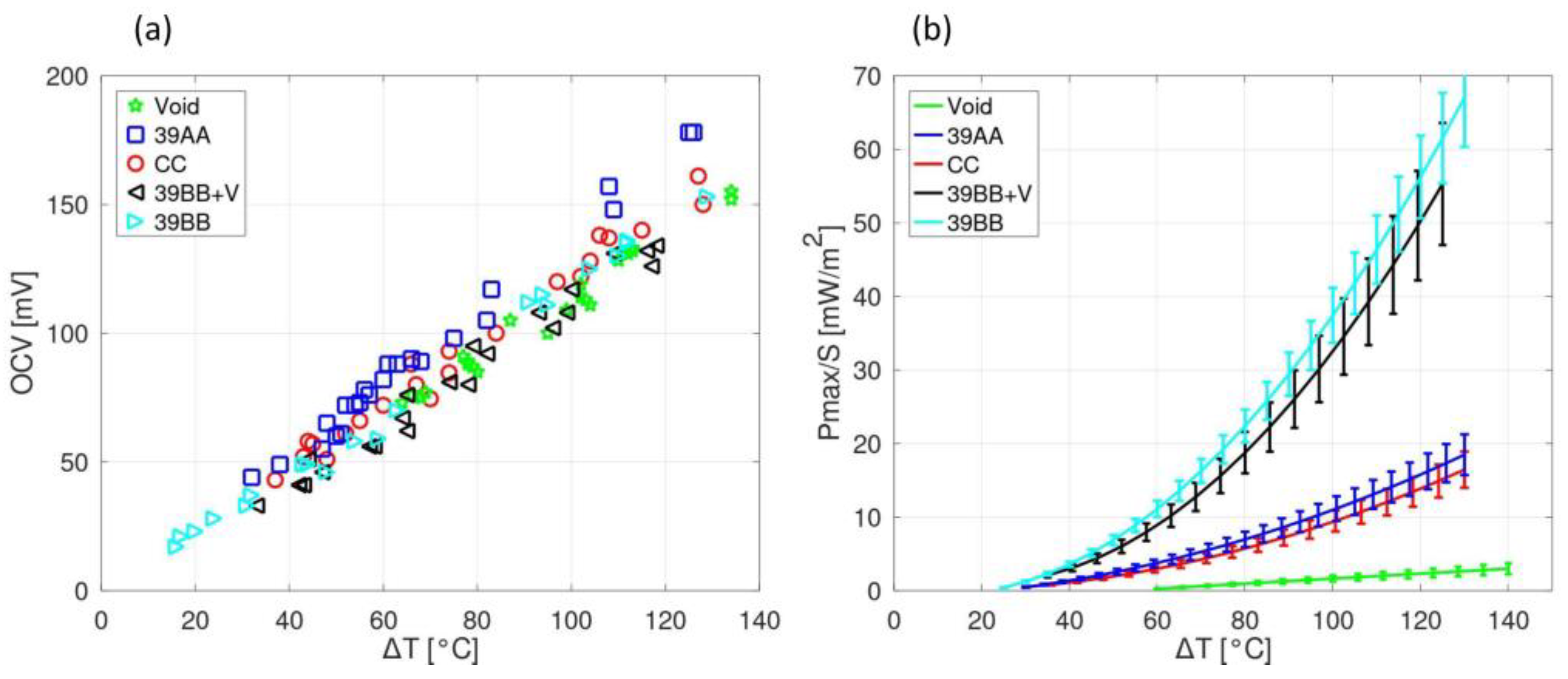
| ESW | σ @ 25 °C | Tmelting | Tcrystallization | Tdecomposition |
|---|---|---|---|---|
| 4.6 V | 9.4 mS/cm | 23 °C | −41 °C | >300 °C [29] |
| Material | MPL | Thickness (µm) | ECSA @ Tamb/T = 120 °C (1/cm2) | Electrical Resistivity (mOhm·m) |
|---|---|---|---|---|
| CC | No | 330 | 3.03/2.71 | 49 |
| 39AA | No | 280 | 2.71/1.47 | 5 |
| 39BB | Yes | 315 | 5.46/3.41 | 3 |
| 39BB + V | Yes | 330 | 4.33/3.04 | 46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiozzo, A.; Bertinetti, A.; Tommasi, A.; Nicol, G.; Rocca, R.; Nakamae, S.; Torres Bautista, B.E.; Campagna Zignani, S.; Laux, E.; Fantini, S.; et al. From Academia to Industry: Criteria for Upscaling Ionic Liquid-Based Thermo-Electrochemical Cells for Large-Scale Applications. Energies 2024, 17, 1. https://doi.org/10.3390/en17010001
Tiozzo A, Bertinetti A, Tommasi A, Nicol G, Rocca R, Nakamae S, Torres Bautista BE, Campagna Zignani S, Laux E, Fantini S, et al. From Academia to Industry: Criteria for Upscaling Ionic Liquid-Based Thermo-Electrochemical Cells for Large-Scale Applications. Energies. 2024; 17(1):1. https://doi.org/10.3390/en17010001
Chicago/Turabian StyleTiozzo, Arianna, Andrea Bertinetti, Alessio Tommasi, Giovanna Nicol, Riccardo Rocca, Sawako Nakamae, Blanca E. Torres Bautista, Sabrina Campagna Zignani, Edith Laux, Sebastien Fantini, and et al. 2024. "From Academia to Industry: Criteria for Upscaling Ionic Liquid-Based Thermo-Electrochemical Cells for Large-Scale Applications" Energies 17, no. 1: 1. https://doi.org/10.3390/en17010001
APA StyleTiozzo, A., Bertinetti, A., Tommasi, A., Nicol, G., Rocca, R., Nakamae, S., Torres Bautista, B. E., Campagna Zignani, S., Laux, E., Fantini, S., & Sgroi, M. F. (2024). From Academia to Industry: Criteria for Upscaling Ionic Liquid-Based Thermo-Electrochemical Cells for Large-Scale Applications. Energies, 17(1), 1. https://doi.org/10.3390/en17010001









