Research on the Ignition Process and Flame Stabilization of a Combination of Step and Strut: Experimental and Numerical Study
Abstract
1. Introduction
2. Methodology
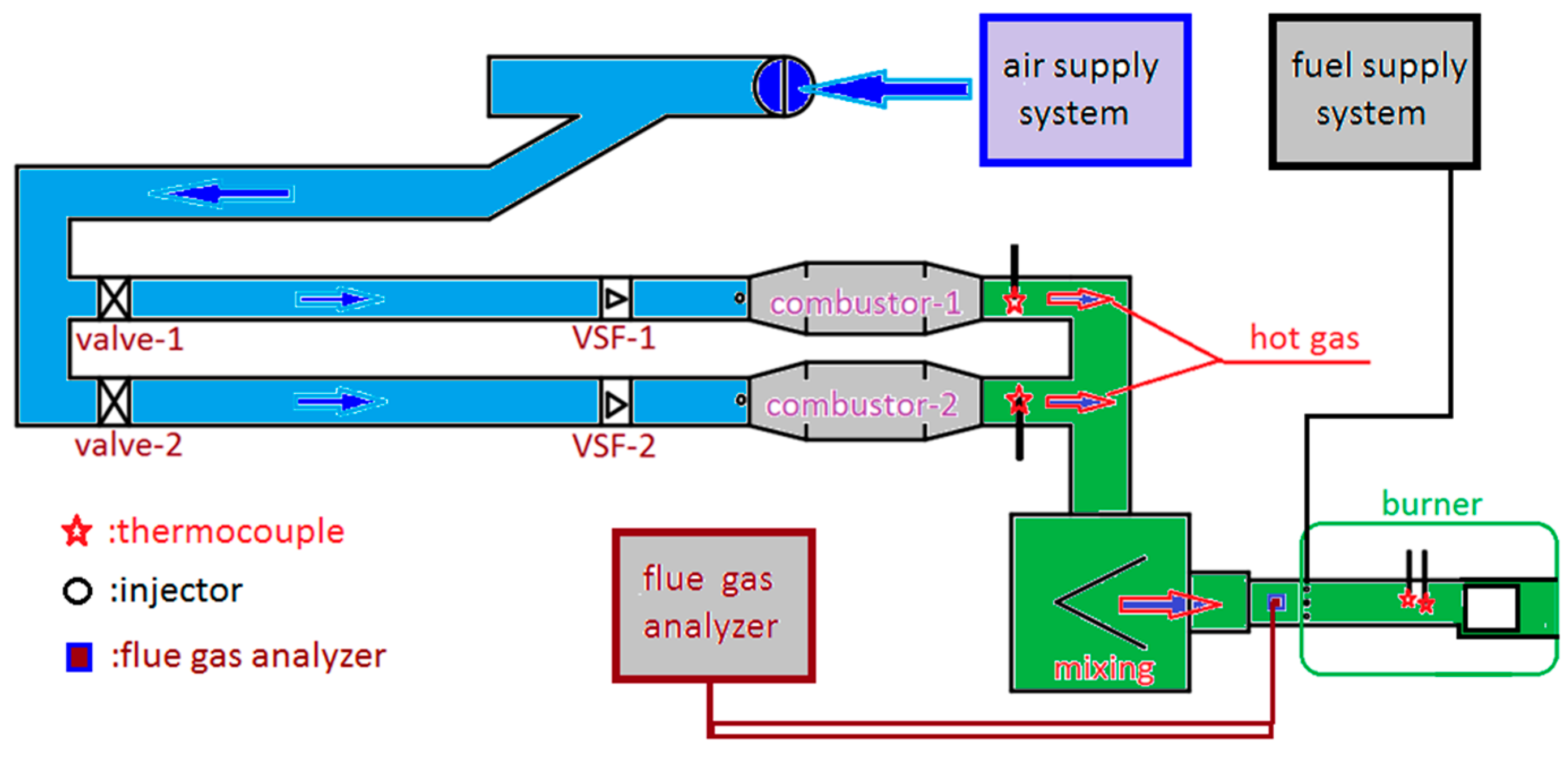
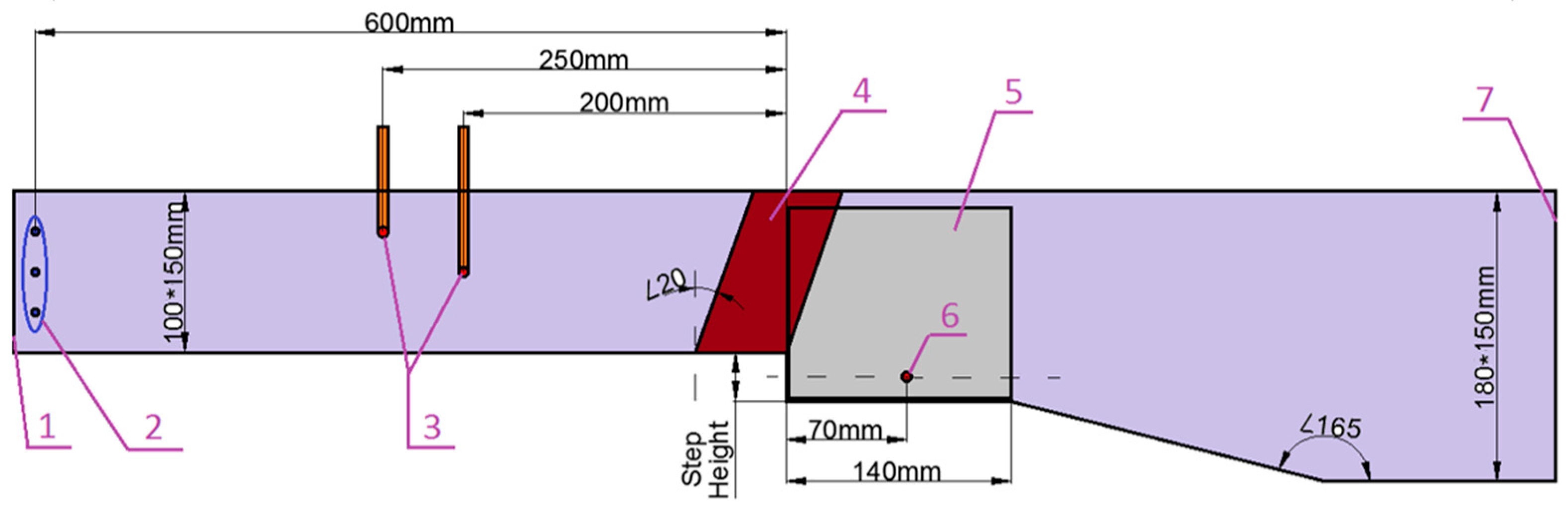
2.1. Experimental Set-Up
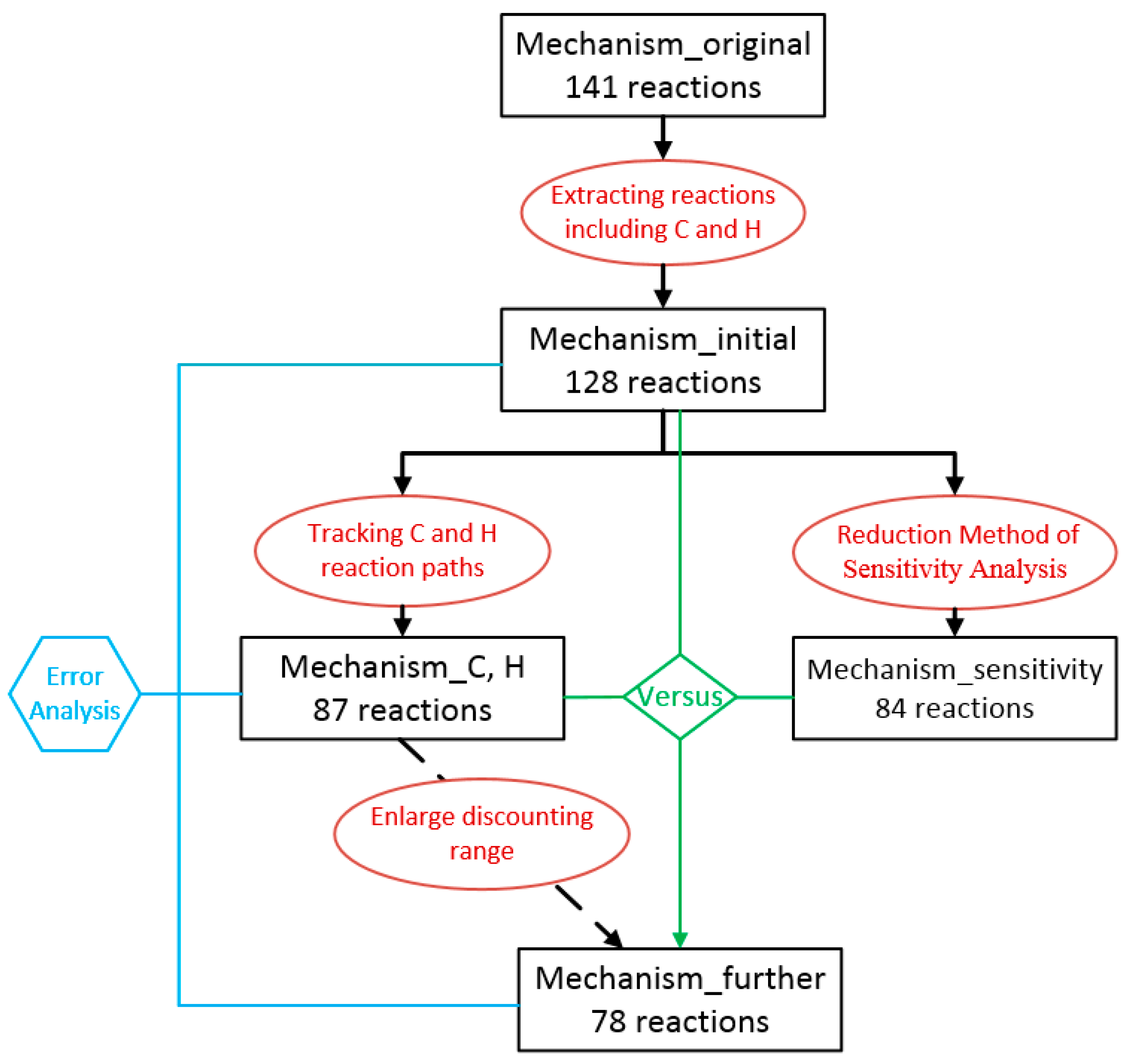
2.2. The Approach of Mechanism Reduction
2.3. Simulation Method Using CFD
3. Results and Discussion
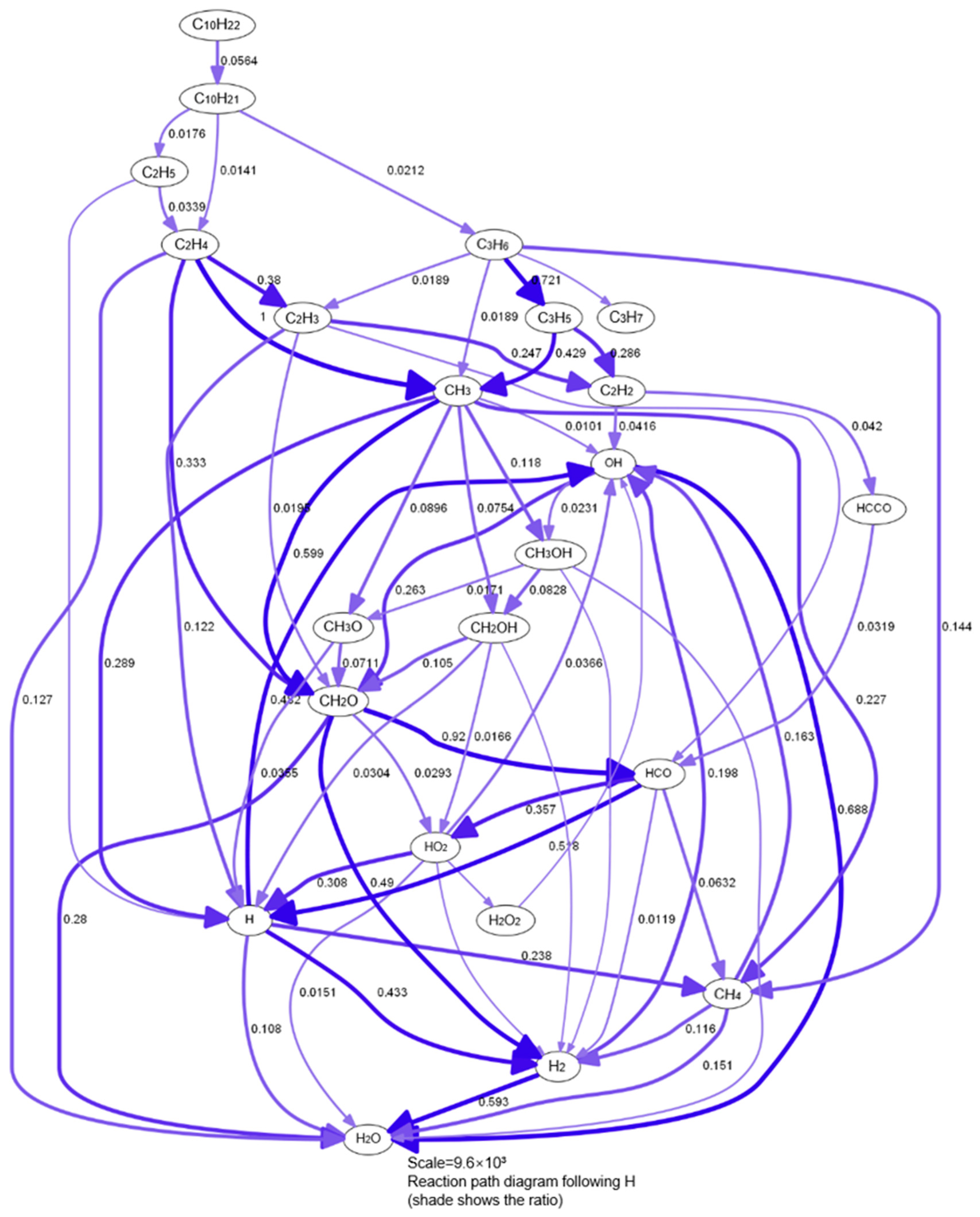
3.1. The Process of Mechanism Reduction
3.1.1. Selection of Original Mechanism
3.1.2. Preliminary Mechanism Reduction
- (1)
- Oxidation of alkane and alkyl radical: RH + O2 = R + HO2, R + O2 = QOO;
- (2)
- H-atom abstraction from alkane: RH + H = R + H2, RH + OH = R + H2O;
- (3)
- Isomerization of the alkyl radical: (ROO)1 = (ROO)2.
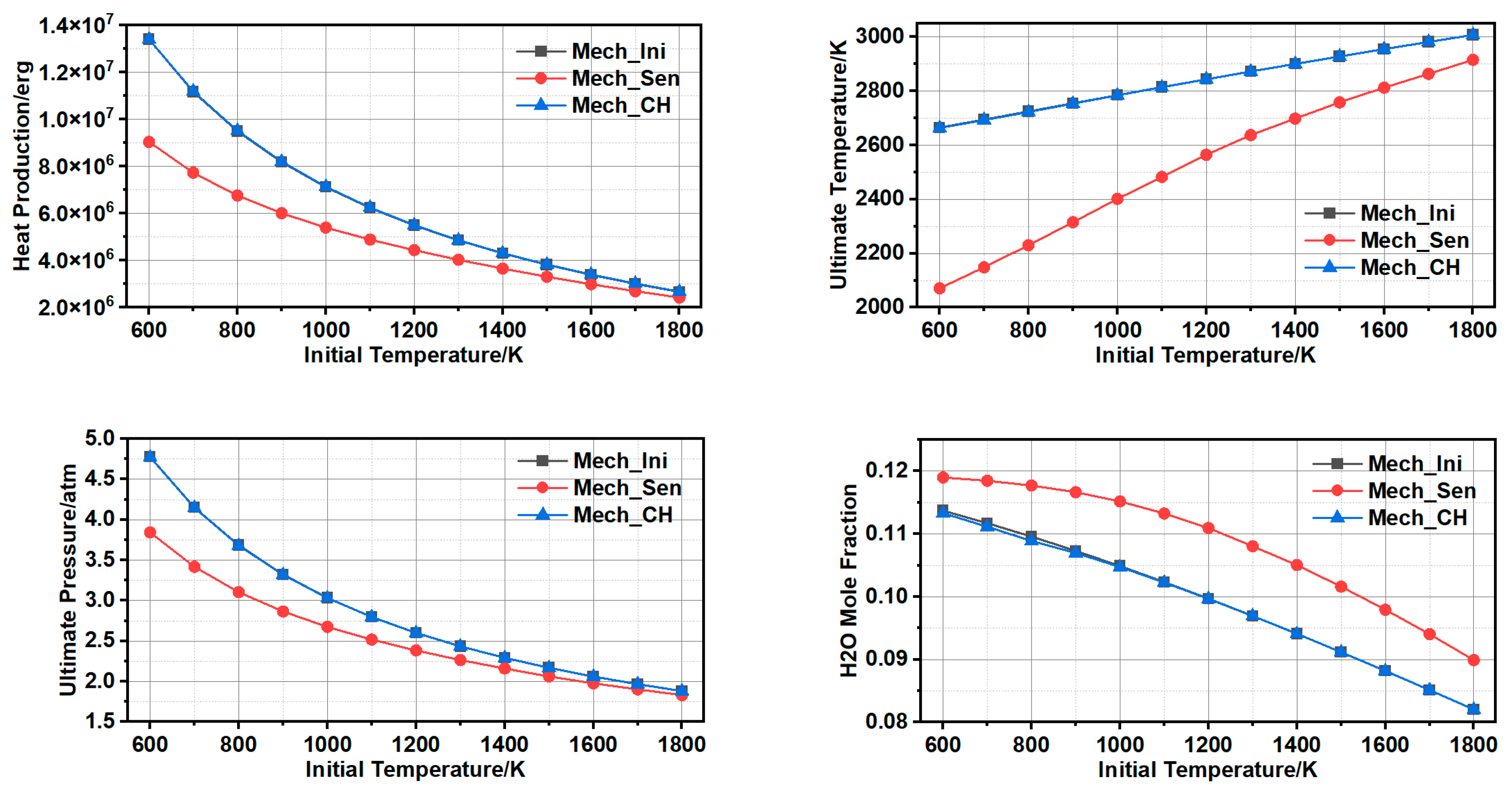
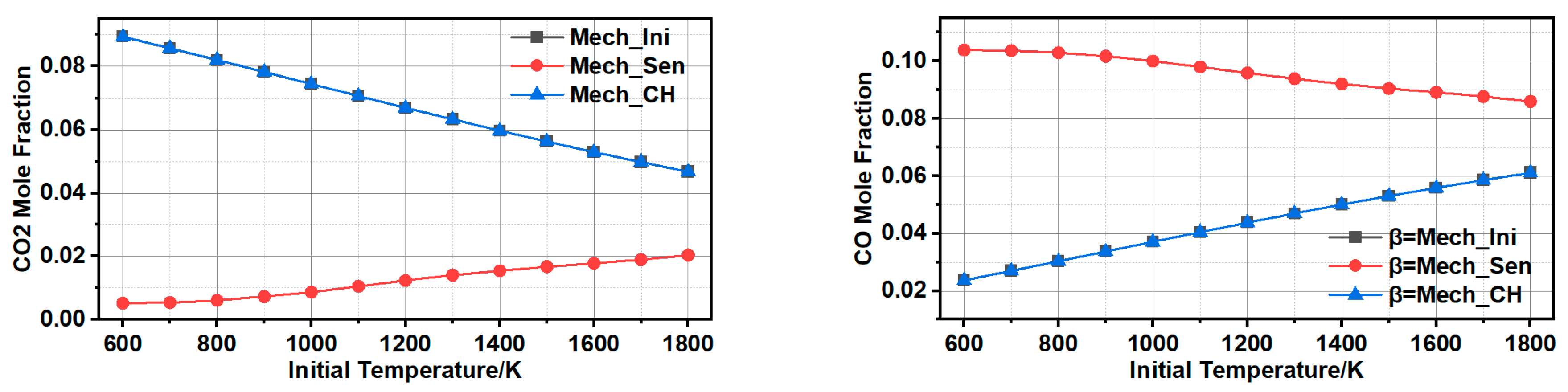
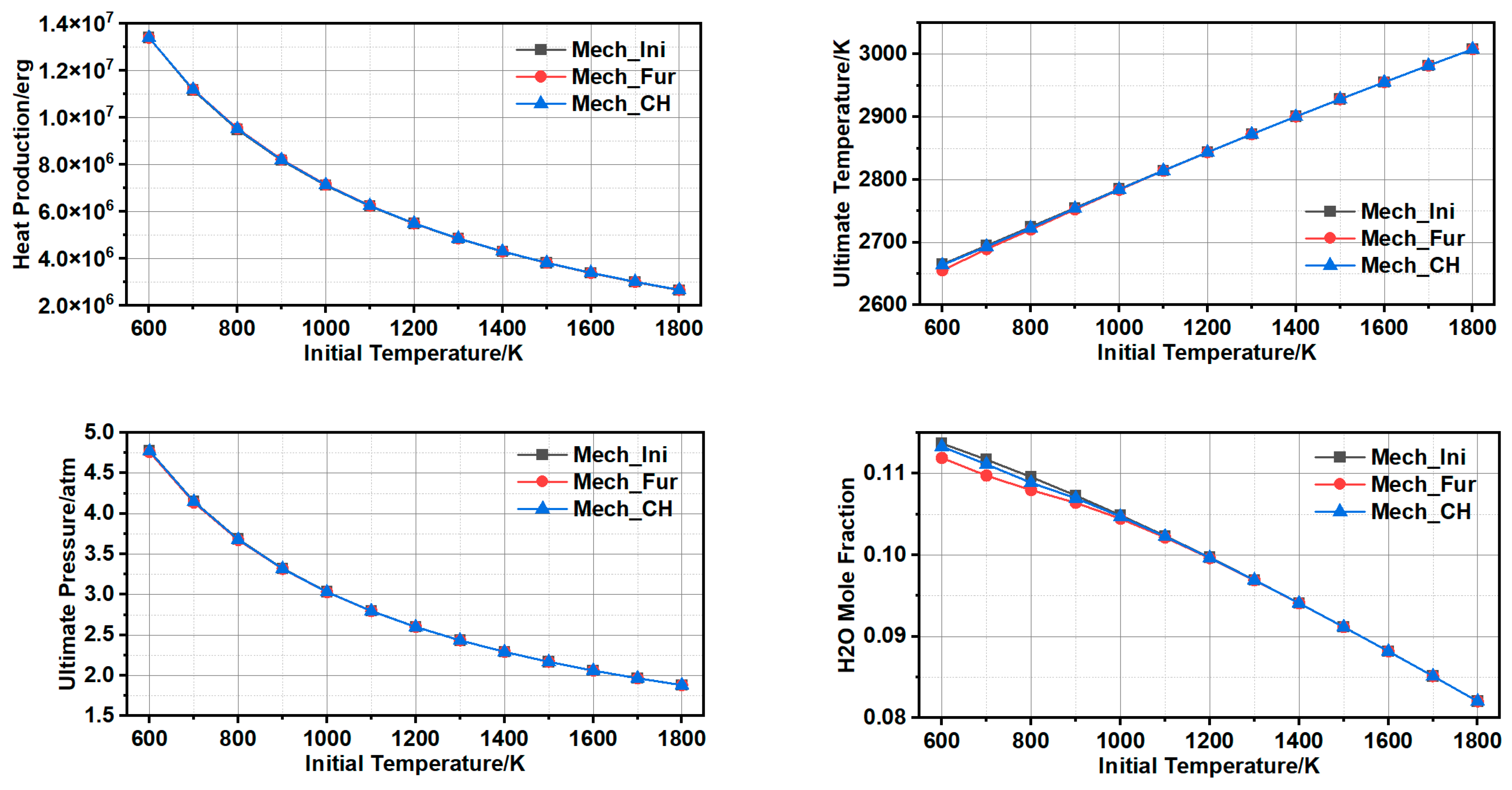
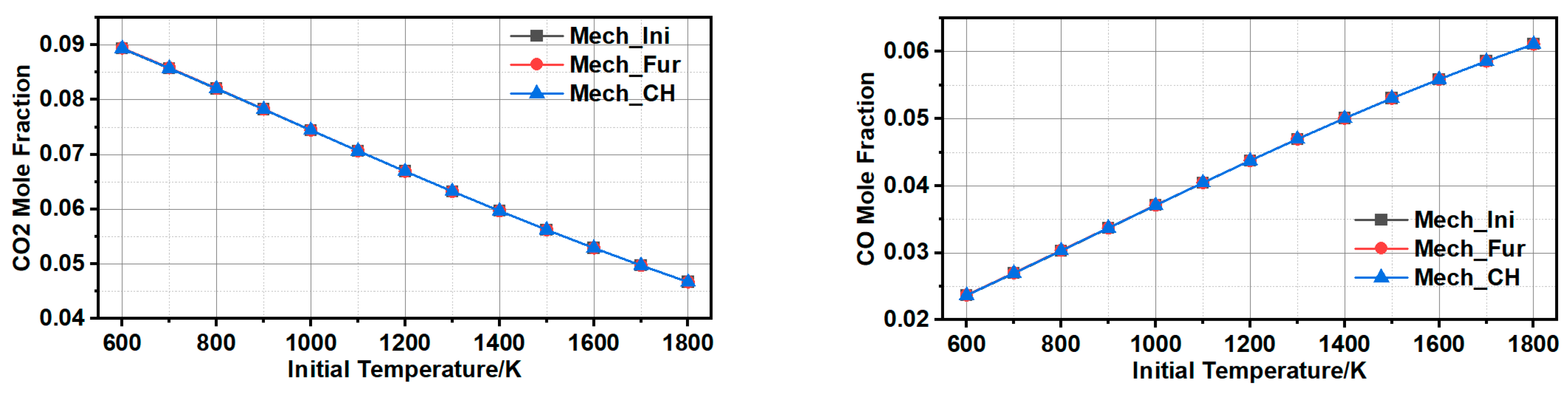
3.1.3. Comparison and Validation
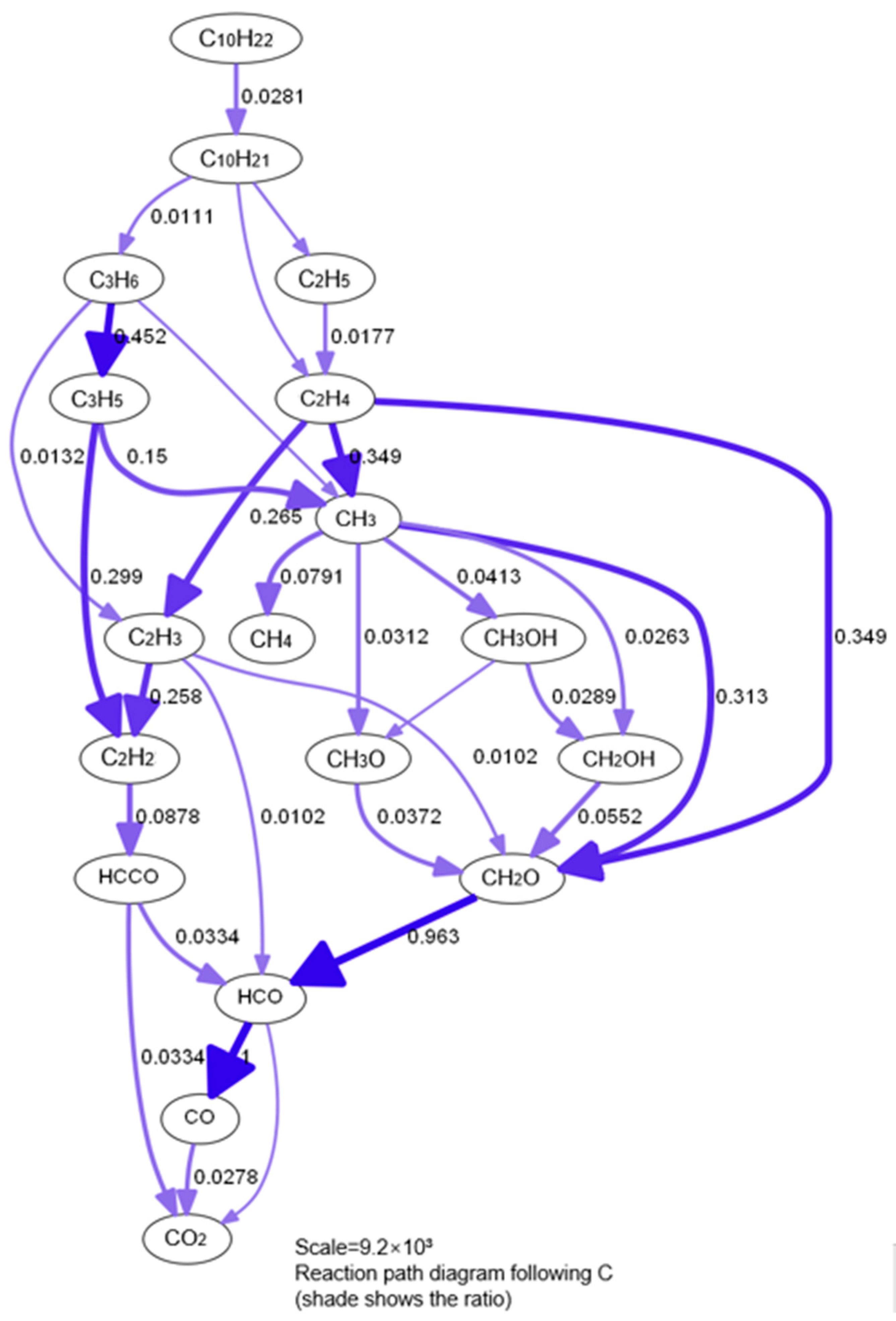
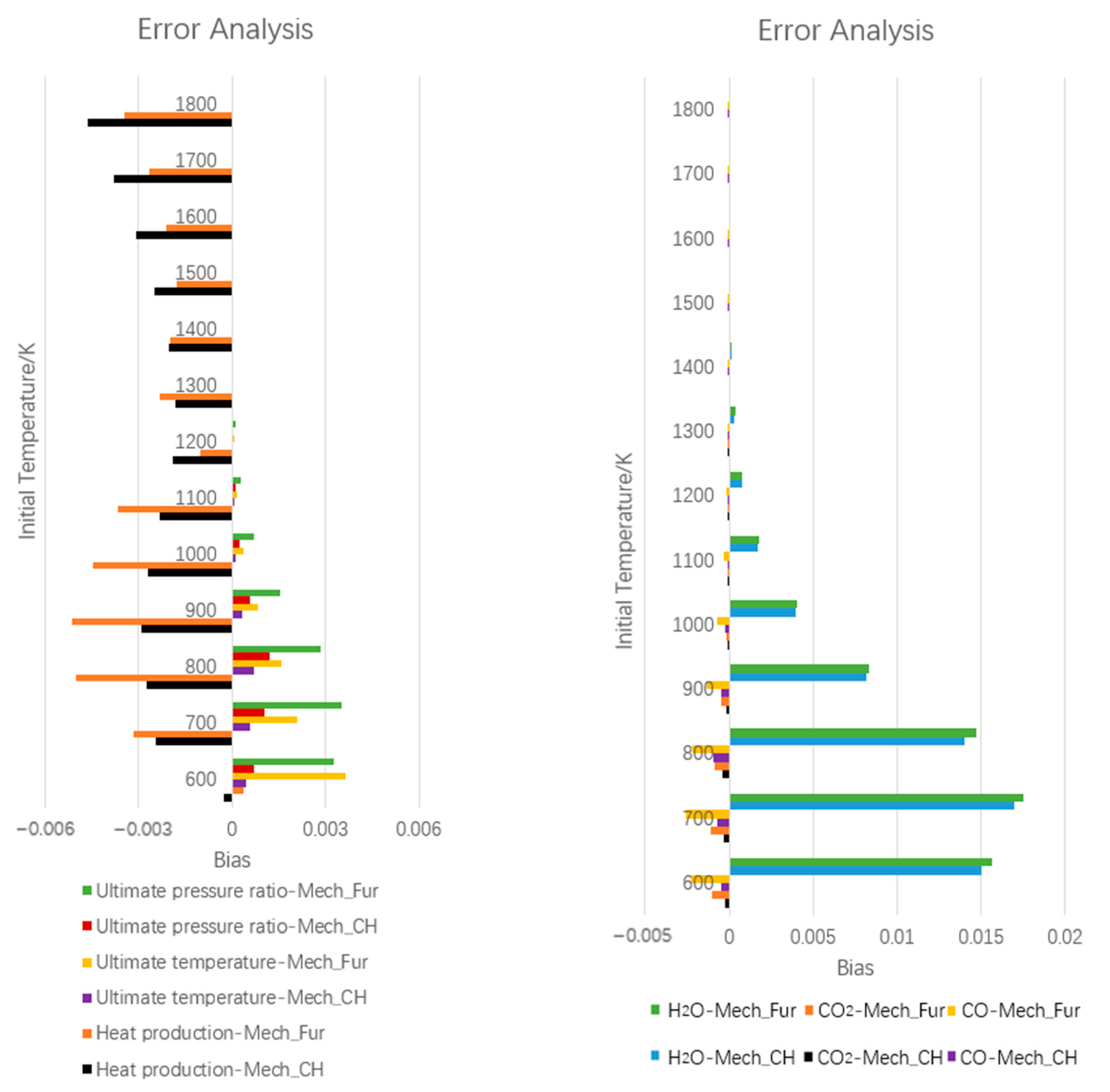
3.1.4. Further Simplification of Mechanism and Error Analysis
3.2. Experimental and Numerical Ignition Process
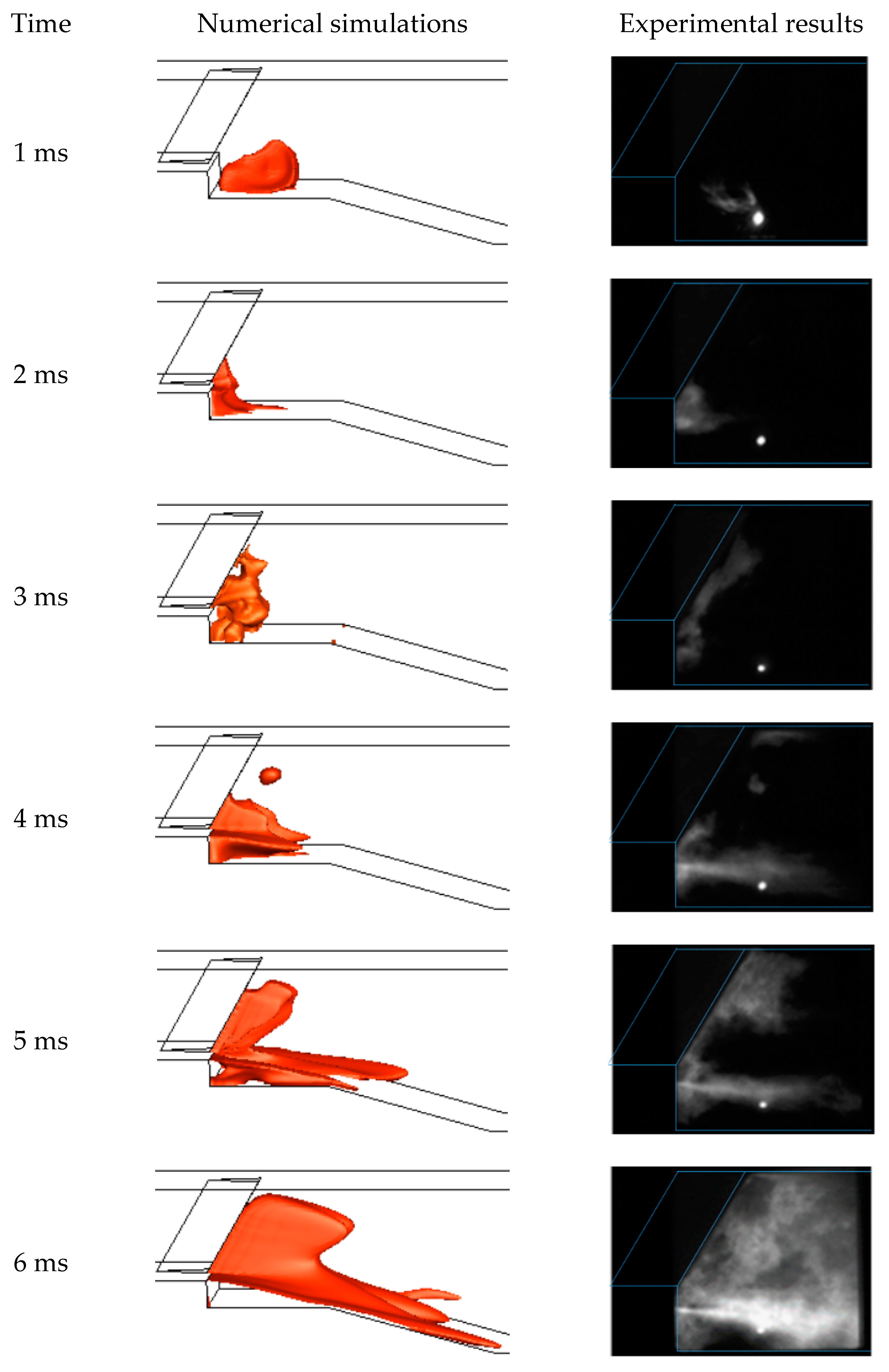
3.2.1. The Validation of Simulation Method
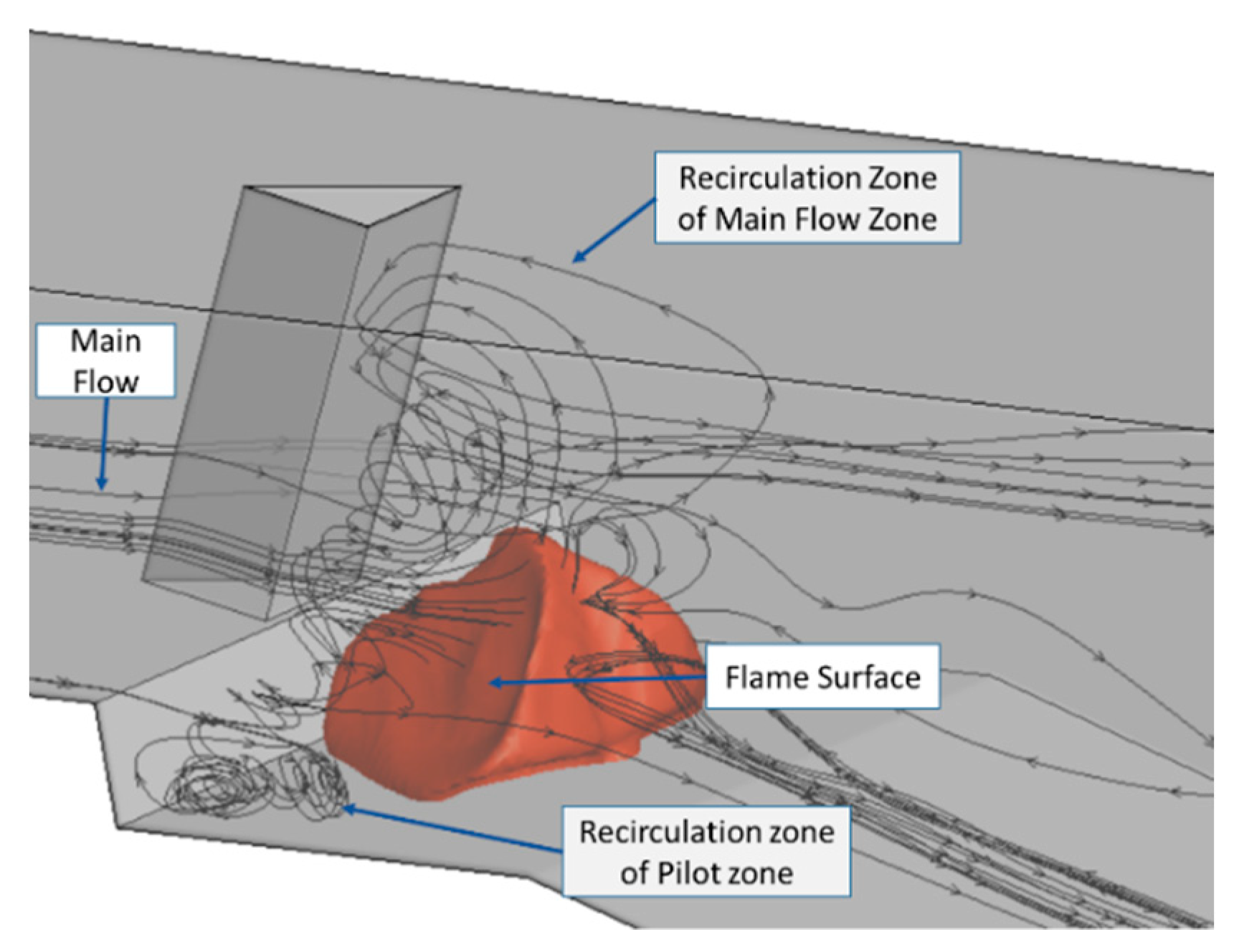
3.2.2. The Predictions of Ignition Process
3.3. Outlet Temperature Distribution
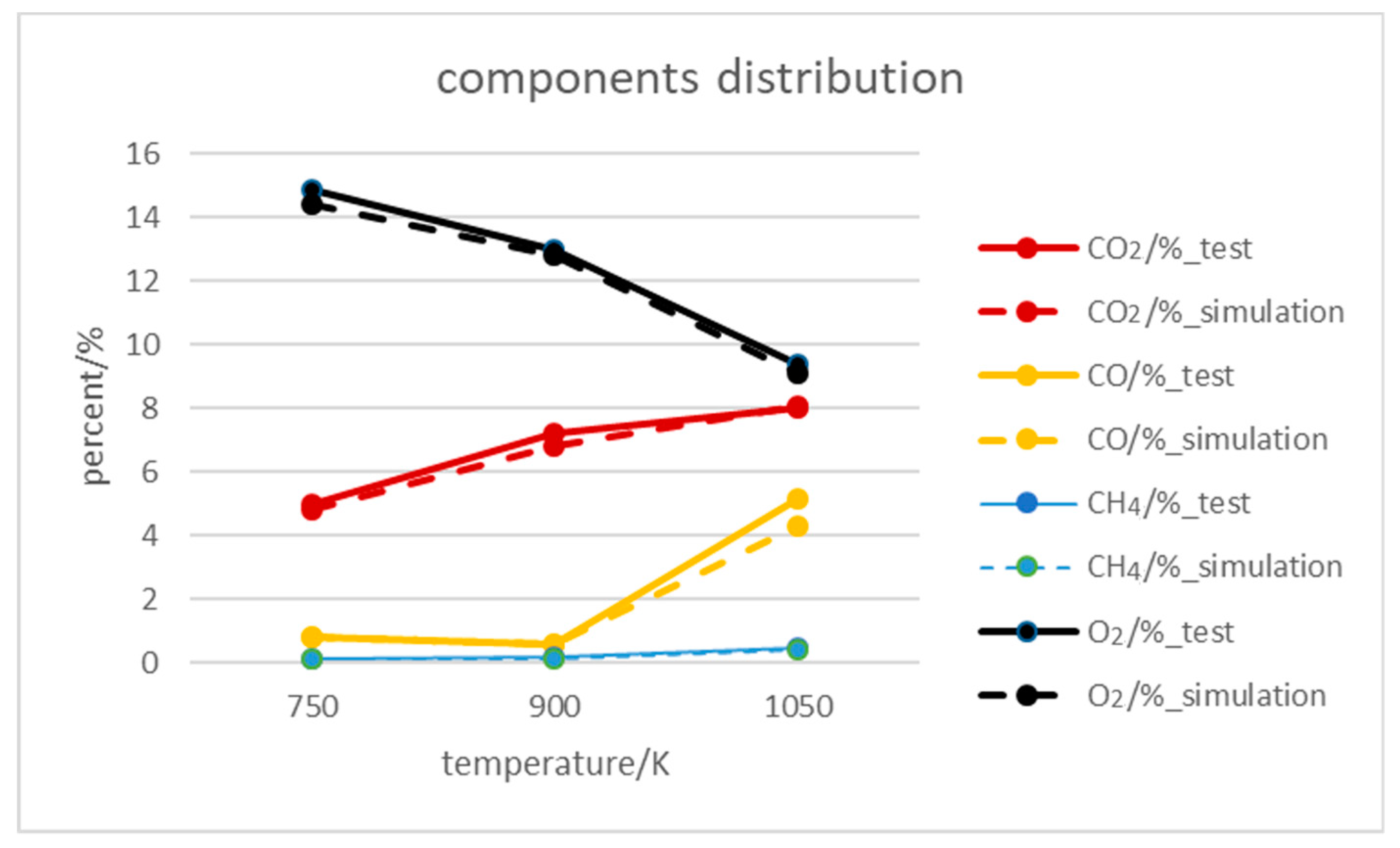
3.4. Outlet Components Distribution
4. Conclusions
- (1)
- A simplified mechanism was developed and verified using a reduction approach according to tracking C&H reaction paths, especially by tracking key elementary reactions more clearly such as correlate heat release. Compared with the sensitivity analysis method, the element tracing method based on probability density distribution proposed in this paper has stronger adaptability and higher accuracy. Mathematical equilibrium model and calculation were developed based on Gibbs principle of minimum free energy. And this simplified mechanism with half the size, but got similar accuracy and fidelity with its error controlled within 1.8%.
- (2)
- This simplified mechanism was well adapted to ignition simulation and prediction under complicated aerodynamic conditions, and its simulation was always consistent with experimental results. Base on the validated simulation method, flame details including kernel generation, flamelet breakup and flame propagation, were depicted and analysed; and the ignition process was predicted under the wider variation of velocity. Also, it provides possibilities for predicting ignition performance prediction beyond the designed boundary conditon.
- (3)
- The influence of inlet temperature on outlet temperature and component distribution was performed, the bias of experimental and numerical results was within 5%. The higher temperature accelerated side reactions, which caused the increasing coproducts of CO and CH4, also led to the decrease of volumetric heat production.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De Giorgi, M.G.; Sciolti, A.; Campilongo, S.; Ficarella, A. Image processing for the characterization of flame stability in a non-premixed liquid fuel burner near lean blowout. Aerosp. Sci. Technol. 2016, 49, 41–51. [Google Scholar] [CrossRef]
- Hernández, S.; Menga, E.; Moledo, S.; Romera, L.; Baldomir, A.; López, C.; Montoya, M.C. Optimization approach for identification of dynamic parameters of localized joints of aircraft assembled structures. Aerosp. Sci. Technol. 2017, 69, 538–549. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, X.; Han, H.; Huang, S.; Li, Z.; Zhong, H. Experiment on the influence of flow number of the pilot-stage centrifugal atomizer on ignition performance of internally staged combustor. Fuel 2022, 318, 123329. [Google Scholar] [CrossRef]
- Zhang, H.-L.; He, L.-M.; Chen, G.-C.; Qi, W.-T.; Yu, J.-L. Experimental study on ignition characteristics of kerosene–air mixtures in V-shaped burner with DC plasma jet igniter. Aerosp. Sci. Technol. 2018, 74, 56–62. [Google Scholar] [CrossRef]
- Huang, Y.; He, X.; Zhang, H.; Wei, J.; Sng, D.W.M. Spark ignition and stability limits of spray kerosene flames under subatmospheric pressure conditions. Aerosp. Sci. Technol. 2021, 114, 106734. [Google Scholar] [CrossRef]
- Vozka, P.; Kilaz, G. A review of aviation turbine fuel chemical composition-property relations. Fuel 2020, 268, 117391. [Google Scholar] [CrossRef]
- Miao, J.; Fan, Y.; Wu, W.; Zhao, S. Influence of air-entraining intensity on the afterburner ignition, flame-holding and combustion characteristics. Aerosp. Sci. Technol. 2020, 106, 106063. [Google Scholar] [CrossRef]
- Zhao, S.; Fan, Y. Experimental and numerical study on fuel distribution and flame expansion of the enhanced flame holding devices. Energy 2020, 203, 117850. [Google Scholar]
- Zettervall, N.; Fureby, C.; Nilsson, E. A reduced chemical kinetic reaction mechanism for kerosene-air combustion. Fuel 2020, 269, 117446. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, L.; Hua, J.; Liu, C.; Wei, H. Effects of pre-chamber jet ignition on knock and combustion characteristics in a spark ignition engine fueled with kerosene. Fuel 2021, 293, 120278. [Google Scholar] [CrossRef]
- Santana, E.R.; Weigand, B. Effect of the reaction mechanism on the numerical prediction of the performance of a scramjet combustor at cruise flight 8 Mach number. Aerosp. Sci. Technol. 2021, 112, 106595. [Google Scholar] [CrossRef]
- Dooley, S.; Won, S.H.; Heyne, J.; Farouk, T.I.; Ju, Y.; Dryer, F.L.; Kumar, K.; Hui, X.; Sung, C.-J.; Wang, H.; et al. The experimental evaluation of a methodology for surrogate fuel formulation to emulate gas phase combustion kinetic phenomena. Combust. Flame 2012, 159, 1444–1466. [Google Scholar] [CrossRef]
- Luo, Z.; Plomer, M.; Lu, T.; Som, S.; Longman, D.E. A reduced mechanism for biodiesel surrogates with low temperature chemistry for compression ignition engine applications. Combust. Theory Model. 2012, 16, 369–385. [Google Scholar] [CrossRef]
- Honnet, S.; Seshadri, K.; Niemann, U.; Peters, N. A surrogate fuel for kerosene. Proc. Combust. Inst. 2009, 32, 485–492. [Google Scholar] [CrossRef]
- Battin-Leclerc, F.; Fournet, R.; Glaude, P.; Judenherc, B.; Warth, V.; Côme, G.; Scacchi, G. Modeling of the gas-phase oxidation of n-decane from 550 to 1600 K. Proc. Combust. Inst. 2000, 28, 1597–1605. [Google Scholar] [CrossRef]
- Moréac, G.; Blurock, E.S.; Mauss, F. Automatic generation of a detailed mechanism for the oxidation of n-decane. Combust. Sci. Technol. 2006, 178, 2025–2038. [Google Scholar] [CrossRef]
- Bikas, G.; Peters, N. Kinetic modelling of n-decane combustion and autoignition: Modeling combustion of n-decanem. Combust. Flame 2001, 126, 1456–1475. [Google Scholar] [CrossRef]
- Ryu, J.I.; Kim, K.; Min, K.; Scarcelli, R.; Som, S.; Kim, K.S.; Temme, J.E.; Kweon, C.B.M.; Lee, T. Data-driven chemical kinetic reaction mechanism for F-24 jet fuel ignition—ScienceDirect. Fuel 2020, 290, 119508. [Google Scholar] [CrossRef]
- Dillstrom, T.; Violi, A. The effect of reaction mechanisms on the formation of soot precursors in flames. Combust. Theory Model. 2017, 21, 23–34. [Google Scholar] [CrossRef]
- Tang, Y.; Hassanaly, M.; Raman, V.; Sforzo, B.; Seitzman, J. Probabilistic modeling of forced ignition of alternative jet fuels. Proc. Combust. Inst. 2020, 38, 2589–2596. [Google Scholar] [CrossRef]
- Sun, W.; Chen, Z.; Gou, X.; Ju, Y. A path flux analysis method for the reduction of detailed chemical kinetic mechanisms. Combust. Flame 2010, 157, 1298–1307. [Google Scholar] [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W.C., Jr.; et al. GRI Mechanism. Available online: http://www.me.Berkeley.edu./gri-mech/ (accessed on 27 July 2010).
- Nadiri, S.; Agarwal, S.; He, X.; Kühne, U.; Fernandes, R.; Shu, B. Development of the chemical kinetic mechanism and modeling study on the ignition delay of liquefied natural gas (LNG) at intermediate to high temperatures and high pressures. Fuel 2021, 302, 121137. [Google Scholar] [CrossRef]
- Shu, J.; Fu, J.; Liu, J.; Ma, Y.; Wang, S.; Deng, B.; Zeng, D. Effects of injector spray angle on combustion and emissions characteristics of a natural gas (ng)-diesel dual fuel engine based on cfd coupled with reduced chemical kinetic model. Appl. Energy 2019, 233–234, 182–195. [Google Scholar] [CrossRef]
- Li, R.; Li, S.; Wang, F.; Li, X. Sensitivity analysis based on intersection approach for mechanism reduction of cyclohexane. Combust. Flame 2016, 166, 55–65. [Google Scholar] [CrossRef]
- Stagni, A.; Frassoldati, A.; Cuoci, A.; Faravelli, T.; Ranzi, E. Skeletal mechanism reduction through species-targeted sensitivity analysis. Combust. Flame 2016, 163, 382–393. [Google Scholar] [CrossRef]
- Koniavitis, P.; Rigopoulos, S.; Jones, W.P. Reduction of a detailed chemical mechanism for a kerosene surrogate via RCCE-CSP. Combust. Flame 2018, 194, 85–106. [Google Scholar] [CrossRef]
- Lu, T.; Ju, Y.; Law, C.K. Complex CSP for chemistry reduction and analysis. Combust. Flame 2001, 126, 1445–1455. [Google Scholar] [CrossRef]
- Correa, C.; Niemann, H.; Schramm, B.; Warnatz, J. Reaction mechanism reduction for higher hydrocarbons by the ILDM method. Proc. Combust. Inst. 2000, 28, 1607–1614. [Google Scholar] [CrossRef]
- Lu, T.; Law, C.K. A directed relation graph method for mechanism reduction. Proc. Combust. Inst. 2005, 30, 1333–1341. [Google Scholar] [CrossRef]
- Zhao, S.; Fan, Y. Analysis of flow resistance and combustion characteristics in the combined application of step and strut. Aerosp. Sci. Technol. 2020, 98, 105676. [Google Scholar]
- Le Dortz, R.; Bellenoue, M.; Bonneau, L.; Mazanchenko, E.; Sotton, J.; Strozzi, C. Laminar Burning Velocities and Markstein Lengths of Jet Fuel Surrogate/Air Mixtures in a Spherical Chamber. In Proceedings of the 8th European Combustion Meeting, Dubrovnik, Croatia, 18–21 April 2017. [Google Scholar]
- Chang, Y.; Jia, M.; Liu, Y.; Li, Y.; Xie, M. Development of a new skeletal mechanism for n-decane oxidation under engine-relevant conditions based on a decoupling methodology. Combust. Flame 2013, 160, 1315–1332. [Google Scholar] [CrossRef]
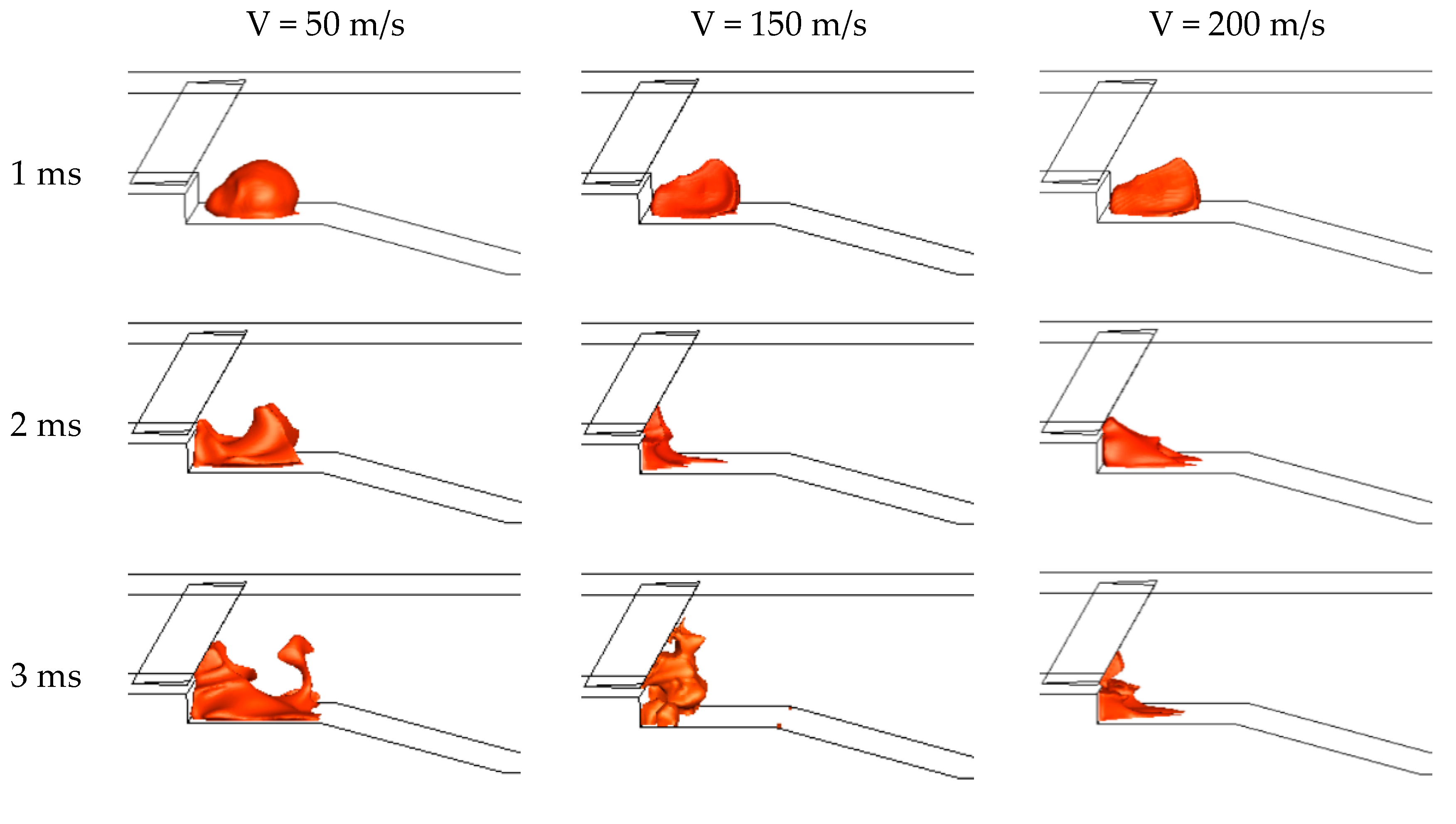
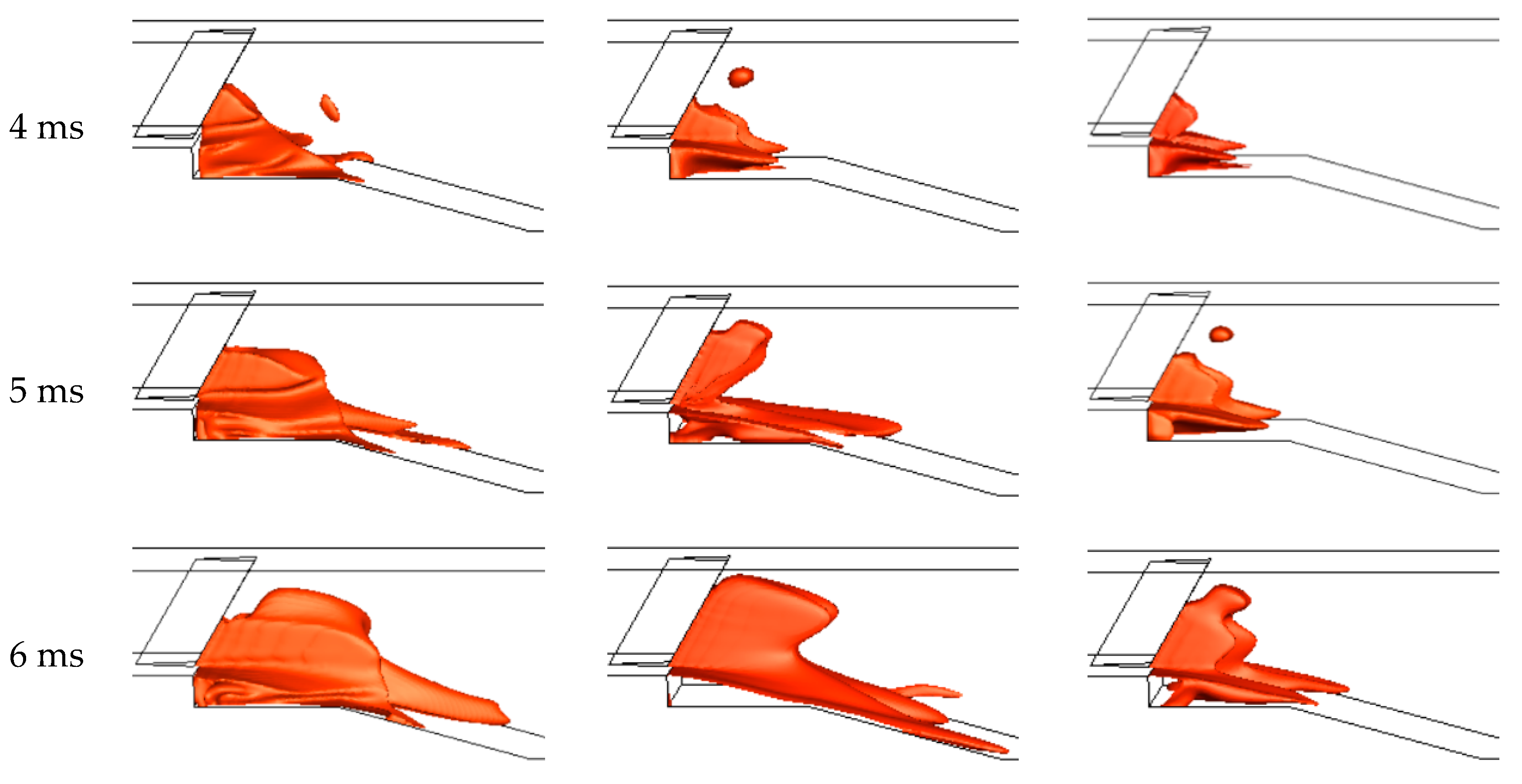

| Boundary Conditions | Boundary Location | Parameters |
|---|---|---|
| Velocity inlet | Inlet | V = 50, 100, 150, 200 m/s; T = 320 K |
| Outflow | Outlet | |
| Wall | Solid wall and liquid boundary | Stationary wall; no slip; no heat flux |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Xiao, H.; Li, Y. Research on the Ignition Process and Flame Stabilization of a Combination of Step and Strut: Experimental and Numerical Study. Energies 2023, 16, 2832. https://doi.org/10.3390/en16062832
Zhao S, Xiao H, Li Y. Research on the Ignition Process and Flame Stabilization of a Combination of Step and Strut: Experimental and Numerical Study. Energies. 2023; 16(6):2832. https://doi.org/10.3390/en16062832
Chicago/Turabian StyleZhao, Shilong, Hui Xiao, and Yafan Li. 2023. "Research on the Ignition Process and Flame Stabilization of a Combination of Step and Strut: Experimental and Numerical Study" Energies 16, no. 6: 2832. https://doi.org/10.3390/en16062832
APA StyleZhao, S., Xiao, H., & Li, Y. (2023). Research on the Ignition Process and Flame Stabilization of a Combination of Step and Strut: Experimental and Numerical Study. Energies, 16(6), 2832. https://doi.org/10.3390/en16062832





