Aromatic Clusters and Hydrogen Storage
Abstract
1. Introduction
2. Theoretical Background
3. Computational Details
4. Atomic Clusters
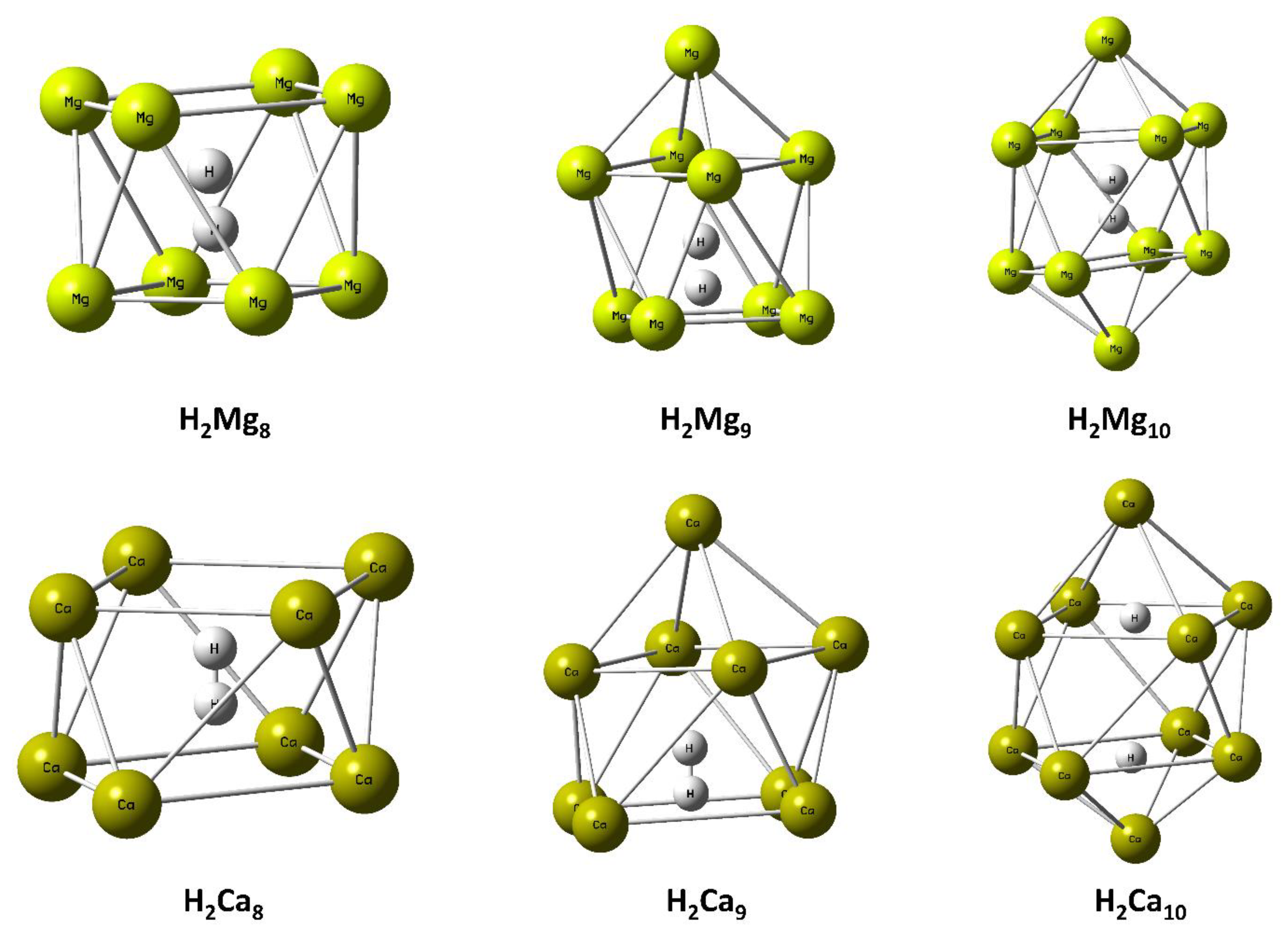
Mg and Ca Clusters
5. Ionic Clusters
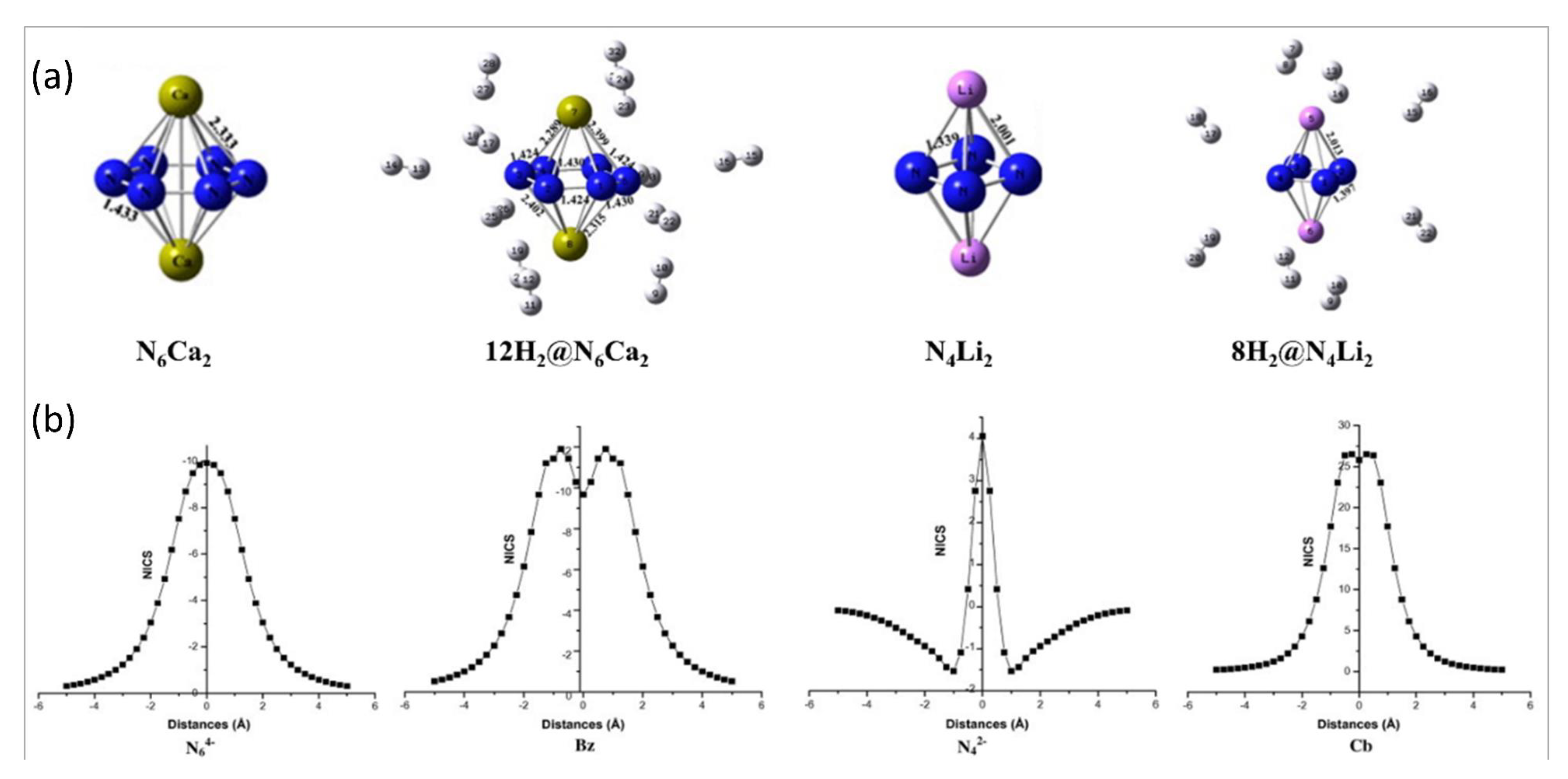
5.1. N4Li2 and N6Ca2 Clusters
5.2. Li3+ and Na3+ Ions
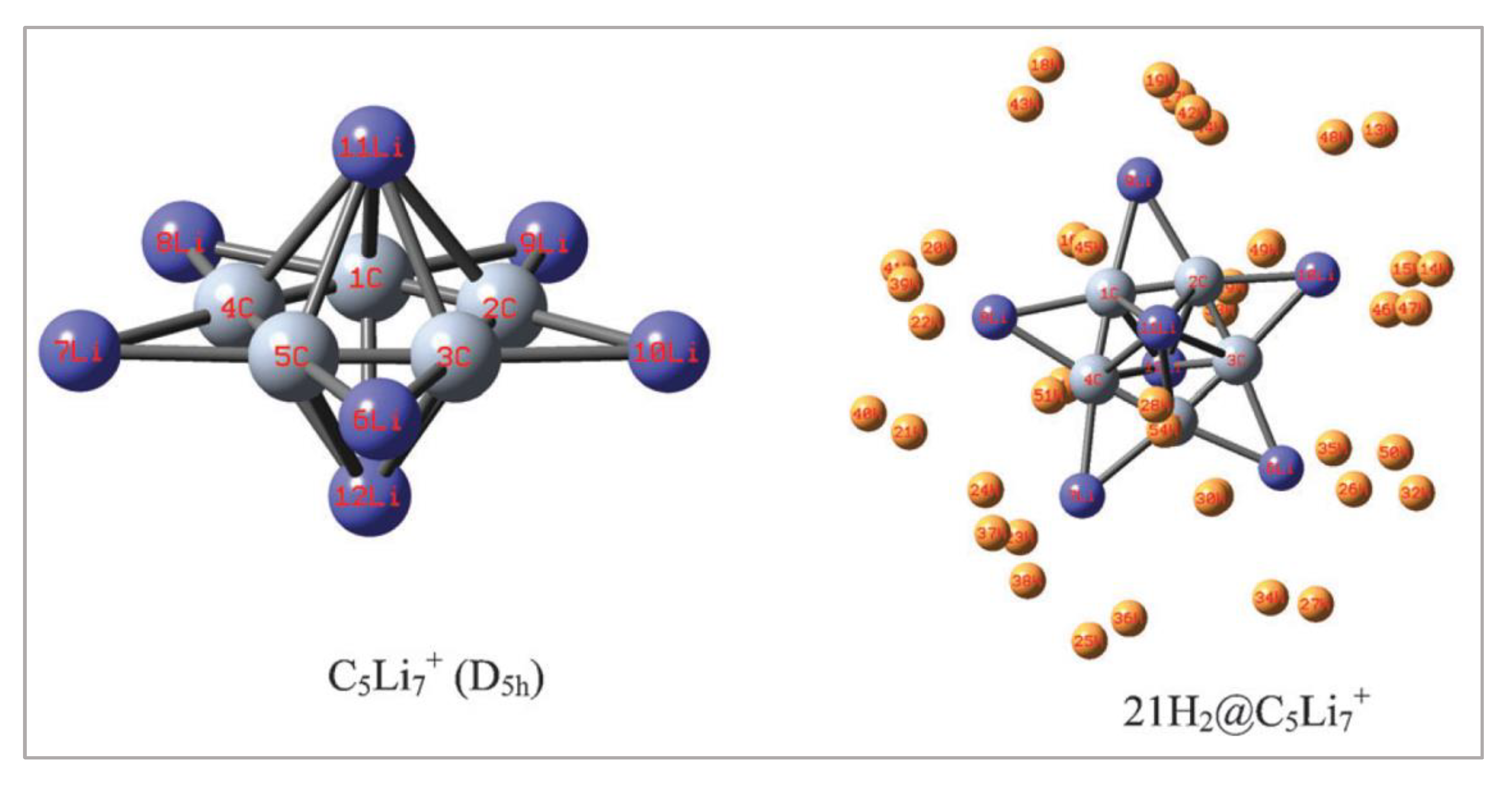
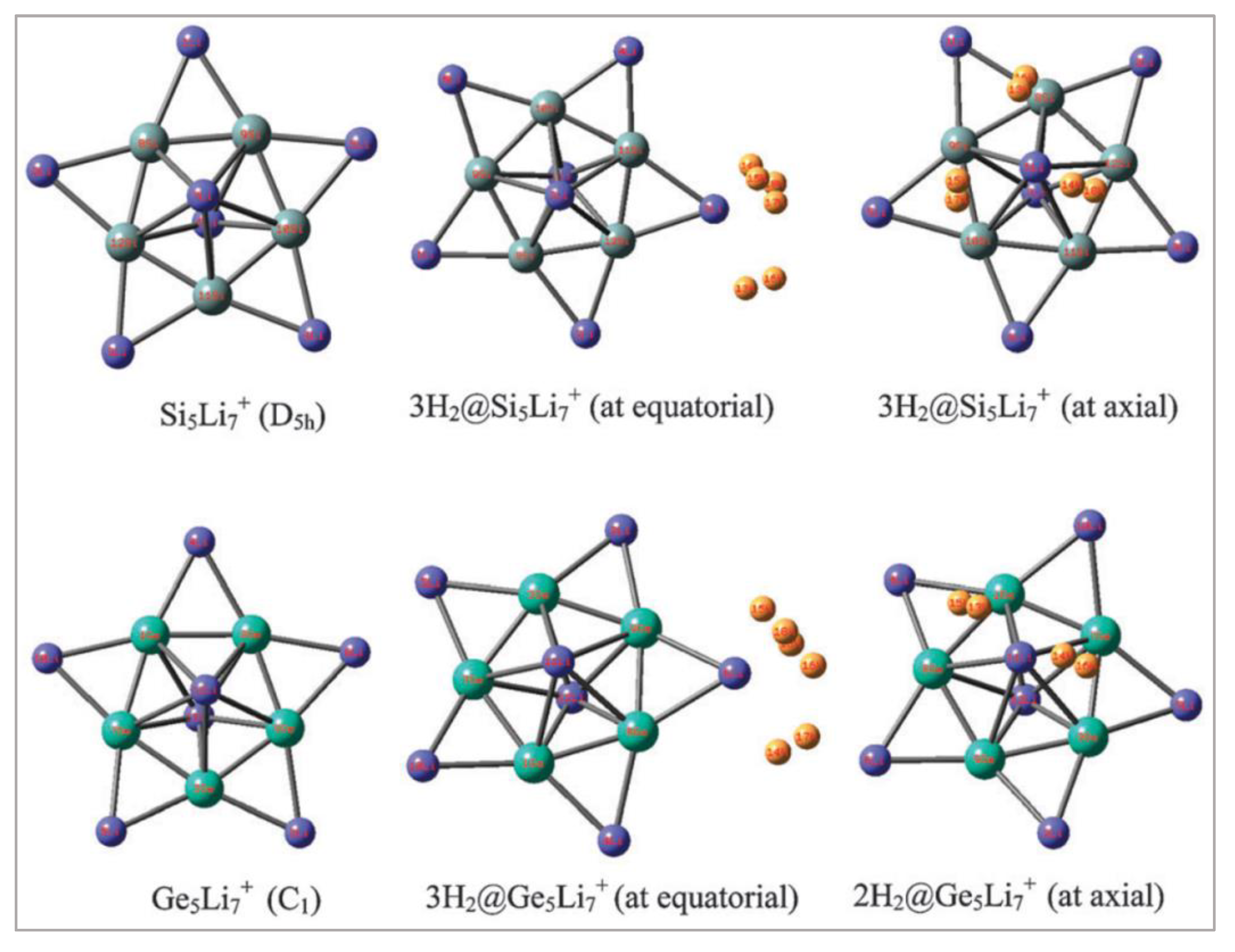
5.3. M5Li7+ (M = C, Si, Ge) Clusters
6. Cage-like Clusters
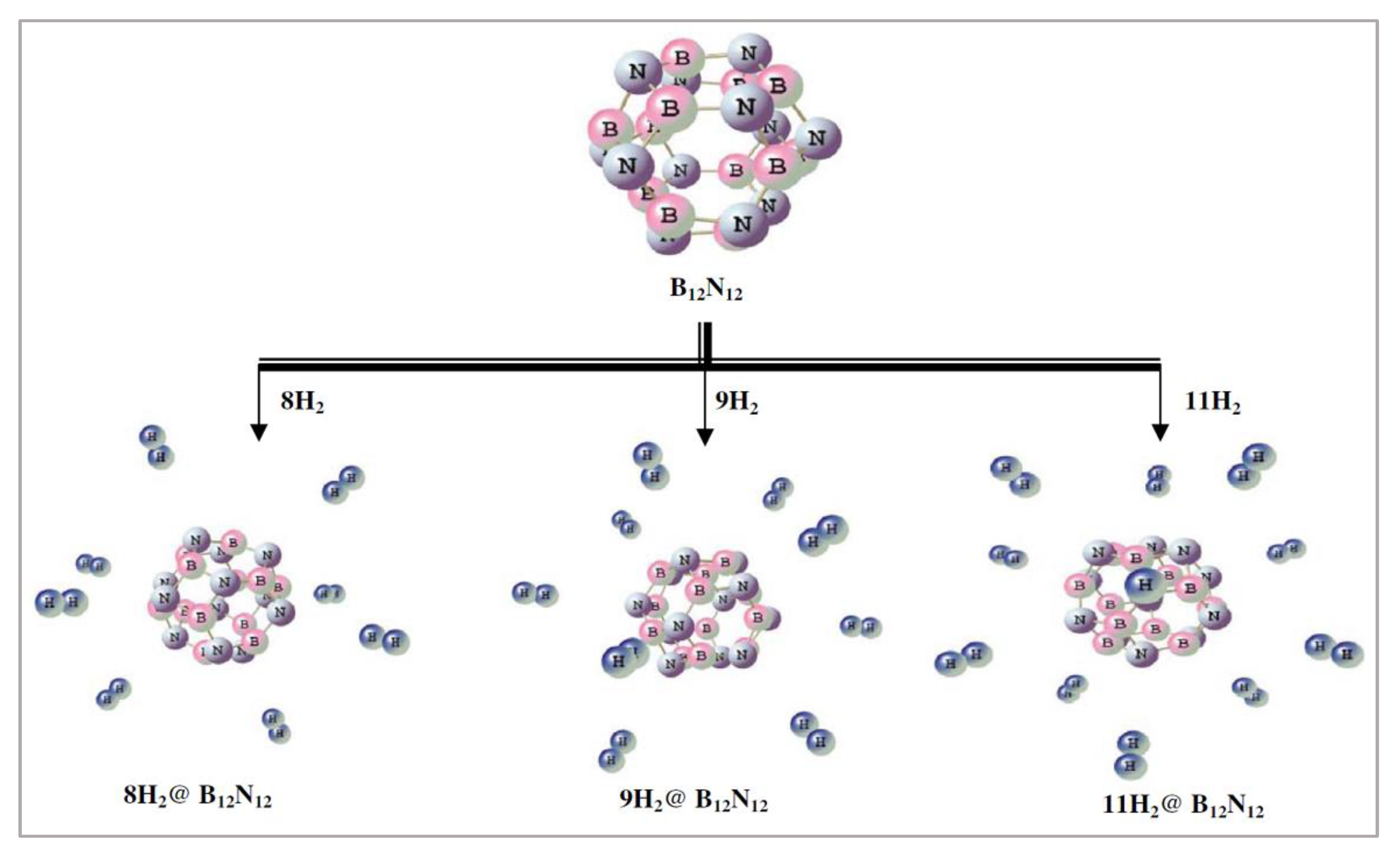
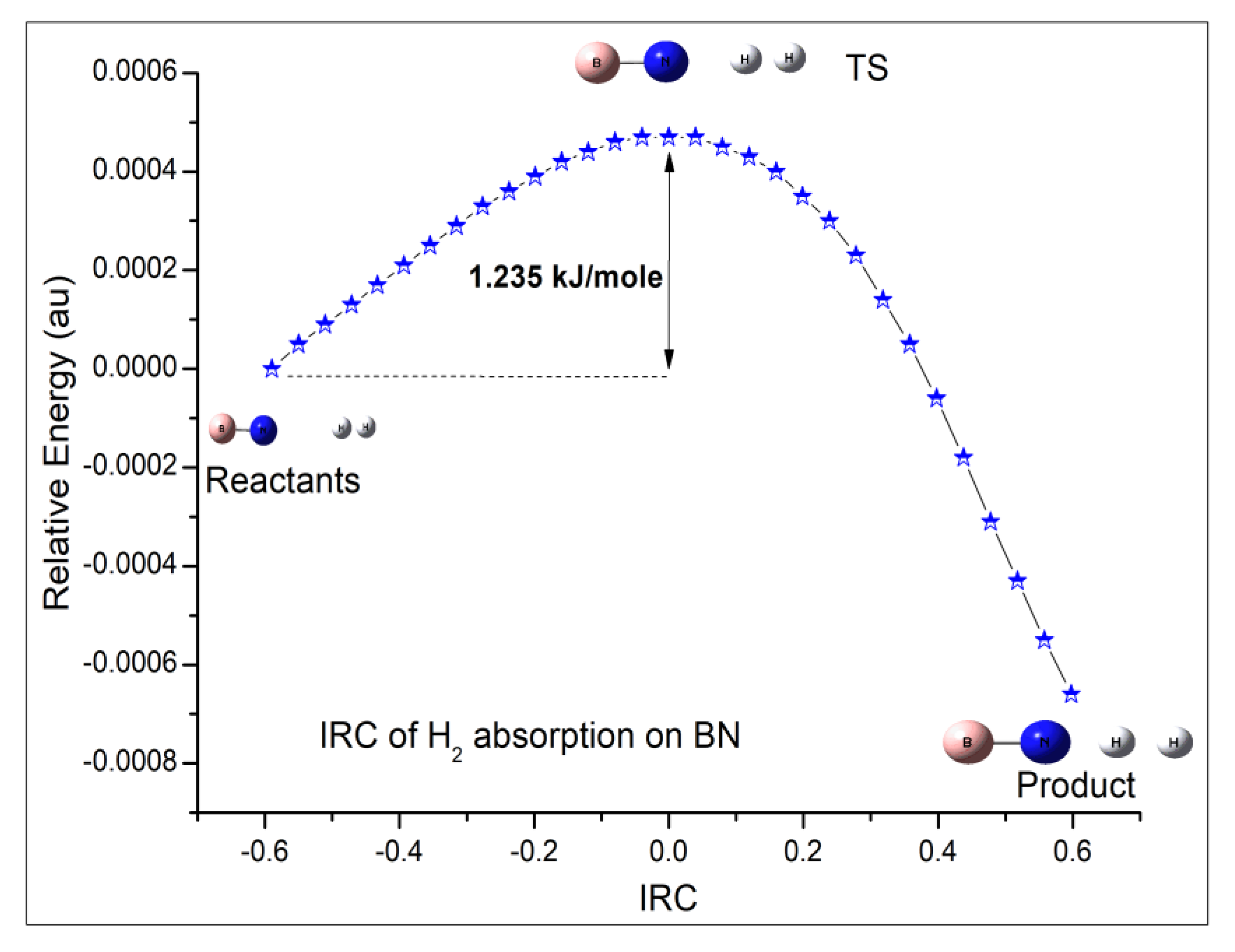
6.1. B12N12 Cage
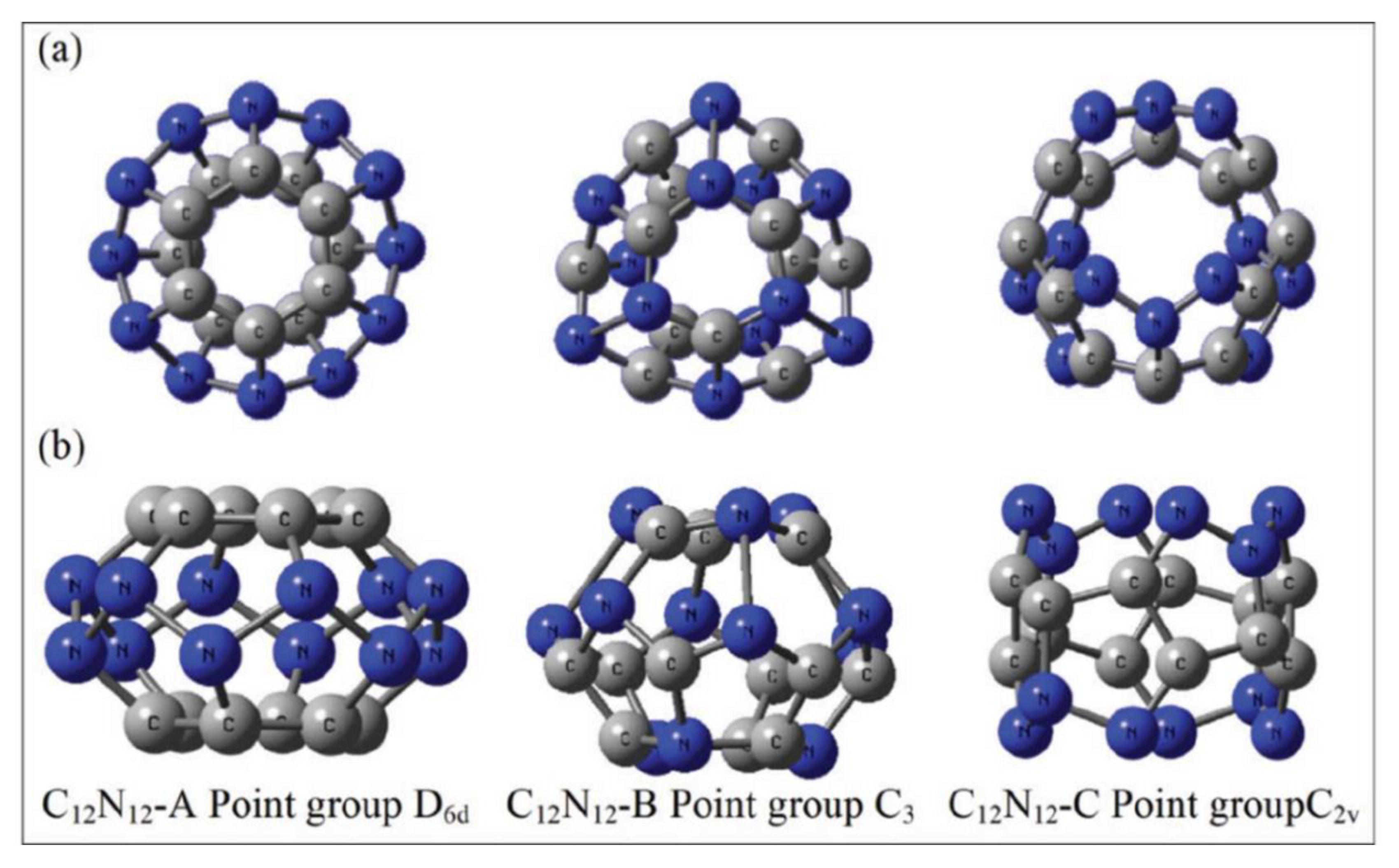
6.2. C12N12 Cage
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen storage for mobility: A review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. IPCC 2013: Summary for Policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Walsh, B.S.; Parratt, S.R.; Hoffmann, A.A.; Atkinson, D.; Snook, R.R.; Bretman, A.; Price, T.A.R. The impact of climate change on fertility. Trends Ecol. Evol. 2019, 34, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Raymundo, R.; Asseng, S.; Robertson, R.; Petsakos, A.; Hoogenboom, G.; Quiroz, R.; Hareau, G.; Wolf, J. Climate change impact on global potato production. Eur. J. Agron. 2018, 100, 87–98. [Google Scholar] [CrossRef]
- Mazdiyasni, O.; Kouchak, A.A. Substantial increase in concurrent droughts and heatwaves in the United States. Proc. Natl. Acad. Sci. USA 2015, 112, 11484–11489. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2015. 2017. Available online: https://www.epa.gov/sites/default/files/2017-02/documents/2017_complete_report.pdf (accessed on 8 February 2023).
- BP Statistical Review of World Energy. 2018. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2018-full-report.pdf (accessed on 8 February 2023).
- Zuttel, A. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, W.; Tumas, W. Hydrogen: An overview. Chem. Rev. 2007, 107, 3900–3903. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, O.A.; Latiff, A.A.A.; Saphira, M.R.; Daud, Z.; Ismail, N.; Ahsan, A.; Aziz, N.A.A.; Al-Gheethi, A.; Kumar, V.; Fadilat, A.; et al. Principles and mechanism of adsorption for the effective treatment of palm oil mill effluent for water reuse. In Nanotechnology in Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–33. [Google Scholar]
- Tarasov, B.P.; Lototskii, M.V.; Yartys, V.A. Problem of hydrogen storage and prospective uses of hydrides for hydrogen accumulation. Russ. J. Gen. Chem. 2007, 77, 694–711. [Google Scholar] [CrossRef]
- Mondal, S.; Das, P.; Giri, S. Hydrogen Trapping Potential of a Few Novel Molecular Clusters and Ions. In Atomic Clusters with Unusual Structure, Bonding and Reactivity: Theoretical Approaches, Computational Assessment and Applications; Chattaraj, P.K., Pan, S., Merino, G., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Hubner, O.; Glolss, A.; Fichtner, M.; Klopper, W. On the interaction of dihydrogen with aromatic systems. J. Phys. Chem. A 2004, 108, 3019–3023. [Google Scholar] [CrossRef]
- Srinivasu, K.; Chandrakumar, K.R.S.; Ghosh, S.K. Computational investigation of hydrogen adsorption by alkali metal doped organic molecules: Role of aromaticity. Chem. Phys. Chem. 2009, 10, 427–435. [Google Scholar] [CrossRef]
- Bodrenko, I.V.; Avdeenkov, A.V.; Bessarabov, D.G.; Bibikov, A.V.; Nikolaev, A.V.; Taran, M.D.; Tkalya, E.V. Hydrogen storage in aromatic carbon ring based molecular materials decorated with alkali or alkali-earth metals. J. Phys. Chem. C 2012, 116, 25286–25292. [Google Scholar] [CrossRef]
- Giri, S.; Chakraborty, A.; Chattaraj, P.K. Potential use of some metal clusters as hydrogen storage materials—A conceptual DFT approach. J. Mol. Model. 2011, 17, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Duley, S.; Giri, S.; Sathymurthy, N.; Islas, R.; Merino, G.; Chattaraj, P.K. Aromaticity and hydrogen storage capability of planar N64− and N42− rings. Chem. Phys. Lett. 2011, 506, 315–320. [Google Scholar] [CrossRef]
- Pan, S.; Merino, G.; Chattaraj, P.K. The hydrogen trapping potential of some Li-doped star-like clusters and super-alkali systems. Phys. Chem. Chem. Phys. 2012, 14, 10345–10350. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Chakraborty, A.; Chattaraj, P.K. Stability and aromaticity of nH2@B12N12 (n = 1–12) clusters. Nano Rev. 2011, 2, 5767. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Srinivasu, K.; Ghosh, S.K.; Chattaraj, P.K. Isomers of C12N12 as potential hydrogen storage materials and the effect of the electric field therein. RSC Adv. 2013, 3, 6991–7000. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual density functional theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef]
- Chattaraj, P.K. Chemical Reactivity Theory: A Density Functional View; Taylor and Francis/CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Cohen, A.J.; Mori-Sanchez, P.; Yang, W. Challenges for density functional theory. Chem. Rev. 2012, 112, 289–320. [Google Scholar] [CrossRef]
- Chakraborty, D.; Chattaraj, P.K. Conceptual density functional theory based electronic structure principles. Chem. Sci. 2021, 12, 6264–6279. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Chattaraj, P.K. Stability and structural dynamics of Be32−cluster. Chem. Phys. Lett. 2014, 593, 128–131. [Google Scholar] [CrossRef]
- Chattaraj, P.K. Electronegativity and hardness: A density functional treatment. J. Indian Chem. Soc. 1992, 69, 173–183. [Google Scholar]
- Chattaraj, P.K.; Roy, D.R. Update 1 of: Electrophilicity index. Chem. Rev. 2007, 107, PR46–PR74. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Sarkar, U.; Roy, D.R. Electrophilicity index. Chem. Rev. 2006, 106, 2065–2091. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic population analysis on LCAO–MO molecular wave functions I. J. Chem. Phys. 1955, 23, 1833. [Google Scholar] [CrossRef]
- Parr, R.G.; Donnelly, R.A.; Levy, M.; Palke, W.E. Electronegativity: The density functional viewpoint. J. Chem. Phys. 1978, 68, 3801. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density functional approach to the frontier-electron theory of chemical reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Pearson, R.G. Chemical Hardness: Applications from Molecules to Solids; Wiley-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Koopmans, T.A. Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1933, 1, 104–113. [Google Scholar] [CrossRef]
- Yang, W.; Mortier, W.J. The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines. J. Am. Chem. Soc. 1986, 108, 5708–5711. [Google Scholar] [CrossRef]
- Pearson, R.G. Recent advances in the concept of hard and soft acids and bases. J. Chem. Educ. 1999, 64, 561–567. [Google Scholar] [CrossRef]
- Pearson, R.G. The principle of maximum hardness. Acc. Chem. Res. 1993, 26, 250–255. [Google Scholar] [CrossRef]
- Parr, R.G.; Chattaraj, P.K. Principle of maximum hardness. J. Am. Chem. Soc. 1991, 113, 1854–1855. [Google Scholar] [CrossRef]
- Chamorro, E.; Chattaraj, P.K.; Fuentealba, P. Variation of the electrophilicity index along the reaction path. J. Phys. Chem. A 2003, 107, 7068–7072. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Elango, M.; Subramanian, V.; Chattaraj, P.K. Variation of electrophilicity during molecular vibrations and internal rotations. Theor. Chem. Acc. 2005, 113, 257–265. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Gutierrez-Oliva, S.; Jaque, P.; Toro-Labbé, A. Towards understanding the molecular internal rotations and vibrations and chemical reactions through the profiles of reactivity and selectivity indices: An ab initio SCF and DFT study. Mol. Phys. 2003, 101, 2841–2853. [Google Scholar] [CrossRef]
- Garza, J.; Vargas, R.; Cedillo, A.; Galván, M.; Chattaraj, P.K. Comparison between the frozen core and finite differences approximations for the generalized spin-dependent global and local reactivity descriptors in small molecules. Theor. Chem. Acc. 2006, 115, 257–266. [Google Scholar] [CrossRef]
- Faraday, M.X.X. On new compounds of carbon and hydrogen, and on certain other products obtained during the decomposition of oil by heat. Phil. Trans. R. Soc. 1825, 115, 440–466. [Google Scholar] [CrossRef]
- Kekulè, A. Sur la constitution des substances aromatiques. Bull. Soc. Chim. 1865, 3, 98–100. [Google Scholar]
- Kekulè, A. Untersuchungen uber aromatische verbindungen. Ann. Chem. Pharm. 1866, 137, 129–197. [Google Scholar]
- Kekulè, A. Lehrbuch der Organische Chemie Band 2; Verlag Ferdinand Enke: Erlangen, Germany, 1866; pp. 493–741. [Google Scholar]
- Kekulè, A. Ueber einige condensationsproducte des aldehyds. Liebigs Ann. Chem. 1872, 162, 77–124. [Google Scholar] [CrossRef]
- Giambiagi, M.; De Giambiagi, M.S.; Mundim, K.C. Definition of a multicenter bond index. Struct. Chem. 1990, 1, 423–427. [Google Scholar] [CrossRef]
- Sannigrahi, A.B.; Kar, T. Three-center bond index. Chem. Phys. Lett. 1990, 173, 569–572. [Google Scholar] [CrossRef]
- Matito, E.; Solà, M.; Salvador, P.; Duran, M. Electron sharing indexes at the correlated level. Application to aromaticity calculations. Faraday Discuss. 2007, 135, 325–345. [Google Scholar] [CrossRef]
- Fulton, R.L.; Mixon, S.T. Comparison of covalent bond indexes and sharing indexes. J. Phys. Chem. 1993, 97, 7530–7534. [Google Scholar] [CrossRef]
- Fulton, R.L. Sharing of electrons in molecules. J. Phys. Chem. 1993, 97, 7516–7529. [Google Scholar] [CrossRef]
- Mayer, I. Charge, Bond Order, and Valence in the ab initio SCF Theory. Chem. Phys. Lett. 1983, 97, 270–274. [Google Scholar] [CrossRef]
- Mayer, I. On bond orders and valences in the Ab initio quantum chemical theory. Int. J. Quantum Chem. 1986, 29, 73–84. [Google Scholar] [CrossRef]
- Mayer, I. Bond orders and valences from ab initio wave functions. Int. J. Quantum Chem. 1986, 29, 477–483. [Google Scholar] [CrossRef]
- Mayer, I. Bond order and valence: Relations to Mulliken’s population analysis. Int. J. Quantum Chem. 1984, 26, 151–154, Addendum Int. J. Quantum Chem. 1985, 28, 419–419. [Google Scholar] [CrossRef]
- Fradera, X.; Austen, M.A.; Bader, R.F.W. The Lewis Model and Beyond. J. Phys. Chem. A 1999, 103, 304–314. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Stephens, M.E. Spatial localization of the electronic pair and number distributions in molecules. J. Am. Chem. Soc. 1975, 97, 7391–7399. [Google Scholar] [CrossRef]
- Poater, J.; Fradera, X.; Duran, M.; Sola, M. An Insight into the Local Aromaticities of Polycyclic Aromatic Hydrocarbons and Fullerenes. Chem. Eur. J. 2003, 9, 1113–1122. [Google Scholar] [CrossRef]
- Matito, E.; Duran, M.; Solà, M. The aromatic fluctuation index (FLU): A new aromaticity index based on electron delocalization. J. Chem. Phys. 2005, 122, 014109, Erratum in J. Chem. Phys. 2006, 125, 059901. [Google Scholar] [CrossRef] [PubMed]
- Giambiagi, M.S.; Giambiagi, M.; Fortes, M.S. Multicenter bonds, bond valence and bond charge apportionment. J. Mol. Struct. Theochem 1997, 391, 141–150. [Google Scholar] [CrossRef]
- Giambiagi, M.; De Giambiagi, M.S.; Dos Santos Silva, C.D.; De Figuereido, A.P. Multicenter bond indices as a measure of aromaticity. Phys. Chem. Chem. Phys. 2000, 2, 3381–3392. [Google Scholar] [CrossRef]
- Bultinck, P.; Ponec, R.; Van Damme, S. Multicenter bond indices as a new measure of aromaticity in polycyclic aromatic hydrocarbons. J. Phys. Org. Chem. 2005, 18, 706–718. [Google Scholar] [CrossRef]
- Bultinck, P.; Fias, S.; Ponec, R. Local Aromaticity in Polycyclic Aromatic Hydrocarbons: Electron Delocalization versus Magnetic Indices. Chem.-Eur. J. 2006, 12, 8813–8818. [Google Scholar] [CrossRef]
- Bultinck, P.; Ponec, R.; Carbo-Dorca, R. Aromaticity in linear polyacenes: Generalized population analysis and molecular quantum similarity approach. J. Comput. Chem. 2007, 28, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Bultinck, P.; Rafat, M.; Ponec, R.; Gheluwe, B.V.; Carbo-Dorca, R.; Popelier, P.J. Electron Delocalization and Aromaticity in Linear Polyacenes: Atoms in Molecules Multicenter Delocalization Index. J. Phys. Chem. A 2006, 110, 7642–7648. [Google Scholar] [CrossRef]
- Ponec, R.; Bultinck, P.; Saliner, A.G. Multicenter Bond Indices as a New Means for the Quantitative Characterization of Homoaromaticity. J. Phys. Chem. A 2005, 109, 6606–6609. [Google Scholar] [CrossRef] [PubMed]
- Chattaraj, P.K.; Roy, D.R.; Elango, M.; Subramanian, V. Chemical reactivity descriptor based aromaticity indices applied to Al42− and Al44− systems. J. Mol. Struct. Theochem 2006, 759, 109–110. [Google Scholar] [CrossRef]
- Roy, D.R.; Bultinck, P.; Subramanian, V.; Chattaraj, P.K. Bonding, reactivity and aromaticity in the light of the multicenter indices. J. Mol. Struct. Theochem 2008, 854, 35–39. [Google Scholar] [CrossRef]
- Kruszewski, J.; Krygowski, T.M. Definition of aromaticity basing on the harmonic oscillator model. Tetrahedron Lett. 1972, 36, 3839–3842. [Google Scholar] [CrossRef]
- Krygowski, T.M. Crystallographic studies of inter- and intramolecular interactions reflected in aromatic character of π-electron systems. J. Chem. Inf. Comput. Sci. 1993, 33, 70–78. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Cyranski, M. Structural aspects of aromaticity. Chem. Rev. 2001, 101, 1385–1420. [Google Scholar] [CrossRef] [PubMed]
- Schleyer, P.v.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; Hommes, N.J.R.v.E. Nucleus-independent chemical shifts: A simple and efficient aromaticity probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef]
- Hirsch, A.; Chen, Z.; Jiao, H. Spherical aromaticity in Ih symmetrical fullerenes: The 2(N + 1)2 rule. Angew. Chem. Int. Ed. 2000, 39, 3915–3917. [Google Scholar] [CrossRef]
- Poater, J.; Solà, M. Open-shell spherical aromaticity: The 2N2 + 2N + 1 (with S = N + ½) rule. Chem. Commun. 2011, 47, 11647–11649. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 3.0, and 5.0.8; Semichem, Inc.: Shawnee, KS, USA, 2009. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.; Vreven, T., Jr.; Kudin, K.N.; Burant, J.C.N.; et al. Gaussian 03, Revision B.03; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Schlegel, H.B.; Scalmani, G.; Barone, V.; Mennucci, B.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251. [Google Scholar] [CrossRef] [PubMed]
- Kokalj, A. Computer graphics and graphical user interfaces as tools in simulations of matter at the atomic scale. Comp. Mater. Sci. 2003, 28, 155–168. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Q.; Sun, Q.; Jena, P.; Chen, X.S. Electric field enhanced hydrogen storage on polarizable materials substrates. Proc. Natl. Acad. Sci. USA 2010, 107, 2801–2806. [Google Scholar] [CrossRef]
- Sun, X.; Hwang, J.-Y.; Shi, S. Hydrogen storage in mesoporous metal oxides with catalyst and external electric field. J. Phys. Chem. C 2010, 114, 7178–7184. [Google Scholar] [CrossRef]
- Vogler, A.; Wright, R.E.; Kunkely, H. Photochemical reductive cis-elimination in cis-diazidobis(triphenylphosphane)platinum(II) evidence of the formation of bis(triphenylphosphane)platinum(0) and hexaazabenzene. Angew. Chem. Ind. Ed. Engl. 1980, 19, 717–718. [Google Scholar] [CrossRef]
- Chung, G.; Schmidt, M.W.; Gordon, M.S. An ab Initio study of potential energy surfaces for N8 isomers. J. Phys. Chem. A 2000, 104, 5647–5650. [Google Scholar] [CrossRef]
- Engelke, R. Ab initio correlated calculations of six nitrogen (N6) isomers. J. Phys. Chem. 1992, 96, 10789–10792. [Google Scholar] [CrossRef]
- Ha, T.-K.; Nguyen, M.T. The identity of the six nitrogen atoms (N6) species. Chem. Phys. Lett. 1992, 195, 179–183. [Google Scholar] [CrossRef]
- Strout, D.L. Acyclic N10 fails as a high energy density material. J. Phys. Chem. A 2002, 106, 816–818. [Google Scholar] [CrossRef]
- Perez-Peralta, N.; Contreras, M.; Tiznado, W.; Stewart, J.K.; Donald, J.; Merino, G. Stabilizing carbon-lithium stars. Phys. Chem. Chem. Phys. 2011, 13, 12975–12980. [Google Scholar] [CrossRef] [PubMed]
- Tiznado, W.; Perez-Peralta, N.; Islas, R.; Toro-Labbe, A.; Ugalde, J.M.; Merino, G. Designing 3-D Molecular Stars. J. Am. Chem. Soc. 2009, 131, 9426–9431. [Google Scholar] [CrossRef]
- Kuhn, A.; Sreeraj, P.; Pöttgen, R.; Wiemhöfer, H.-D.; Wilkening, M.; Heitjans, P. Li NMR Spectroscopy on Crystalline Li12Si7: Experimental Evidence for the Aromaticity of the Planar Cyclopentadienyl-Analogous Si56− Rings. Angew. Chem. Int. Ed. 2011, 50, 12099–12102. [Google Scholar] [CrossRef]
- Jena, N.K.; Srinivasu, K.; Ghosh, S.K. Computational investigation of hydrogen adsorption in silicon-lithium binary clusters. J. Chem. Sci. 2012, 124, 255–260. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Q.; Jena, P. Storage of molecular hydrogen in B-N cage: Energetics and thermal stability. Nano Lett. 2005, 5, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.-H.; Deng, W.-Q.; Han, K.-L. Endohedral BN metallofullerene M@B36N36 complex as promising hydrogen storage materials. J. Phys. Chem. C 2008, 112, 12195–12200. [Google Scholar] [CrossRef]
- Cui, X.-Y.; Yang, B.-S.; Wu, H.-S. Ab initio investigation of hydrogenation of (BN)16: A comparison with that of (BN)12. J. Mol. Struct. (Theochem) 2010, 941, 144–149. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, S.; Chattaraj, P.K. Aromatic Clusters and Hydrogen Storage. Energies 2023, 16, 2833. https://doi.org/10.3390/en16062833
Mondal S, Chattaraj PK. Aromatic Clusters and Hydrogen Storage. Energies. 2023; 16(6):2833. https://doi.org/10.3390/en16062833
Chicago/Turabian StyleMondal, Sukanta, and Pratim Kumar Chattaraj. 2023. "Aromatic Clusters and Hydrogen Storage" Energies 16, no. 6: 2833. https://doi.org/10.3390/en16062833
APA StyleMondal, S., & Chattaraj, P. K. (2023). Aromatic Clusters and Hydrogen Storage. Energies, 16(6), 2833. https://doi.org/10.3390/en16062833





