Effect of Ash from Salix viminalis on the Biomass and Heating Value of Zea mays and on the Biochemical and Physicochemical Properties of Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Soil and Composition of Ash from Salix viminalis, Compost, and Humic Acids
2.2. Study Design

2.3. Biochemical, Chemical, and Physicochemical Analyses of Soil
2.4. Computations and Statistical Analysis
3. Results
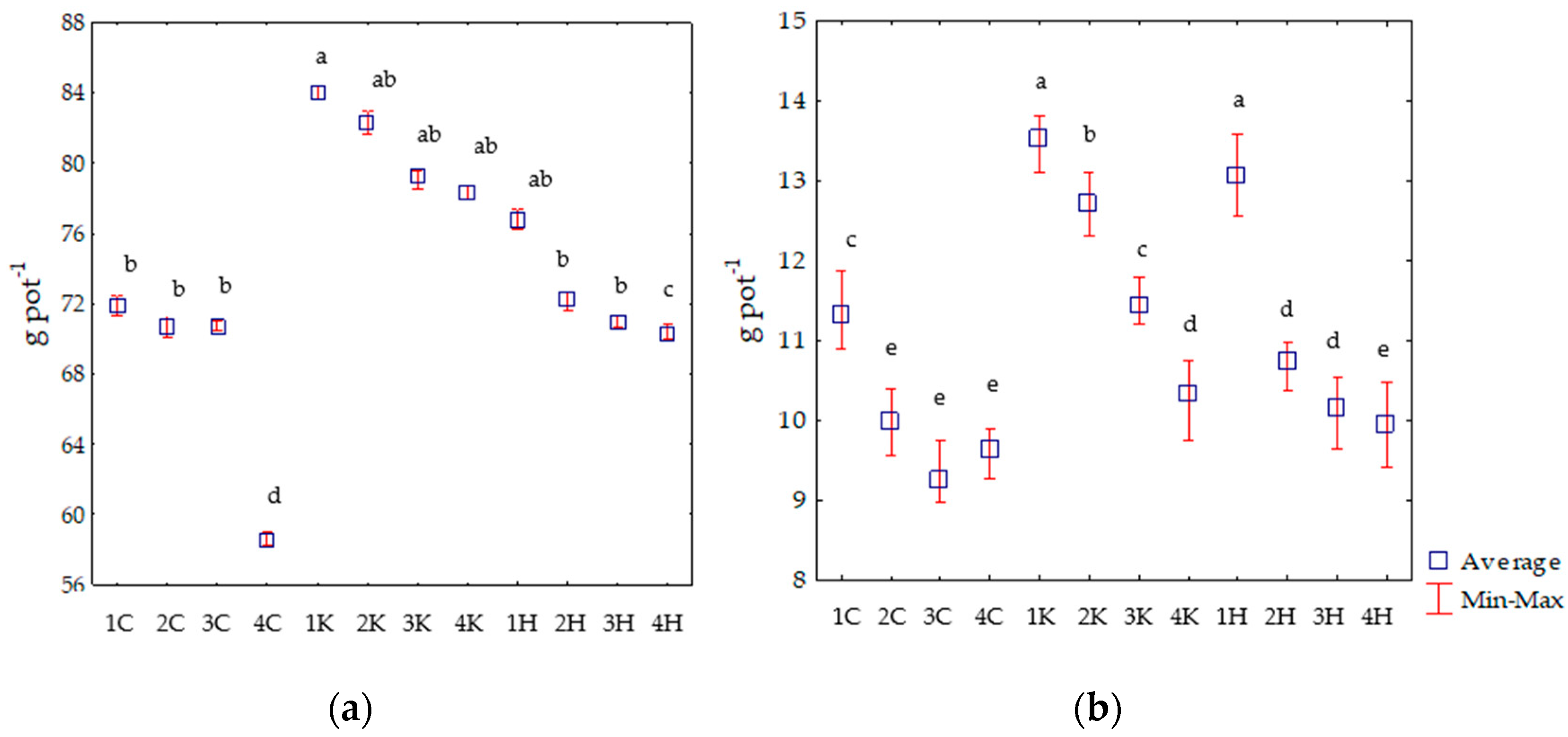
3.1. Biomass and Heating Value of Zea mays L. Cultivated in Soil with the Addition of Ash from Salix viminalis
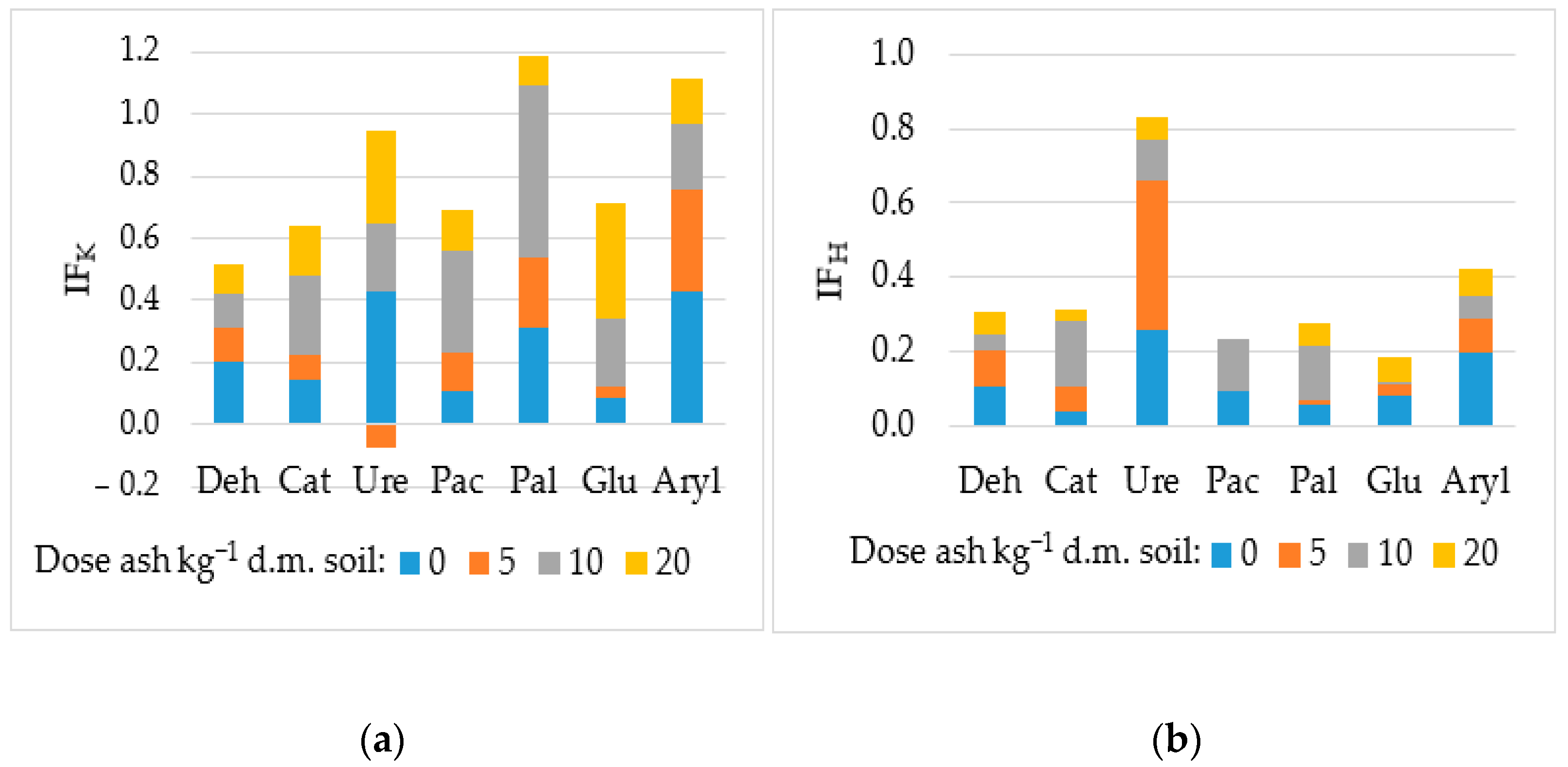
3.2. Biochemical and Physicochemical Properties of Soil
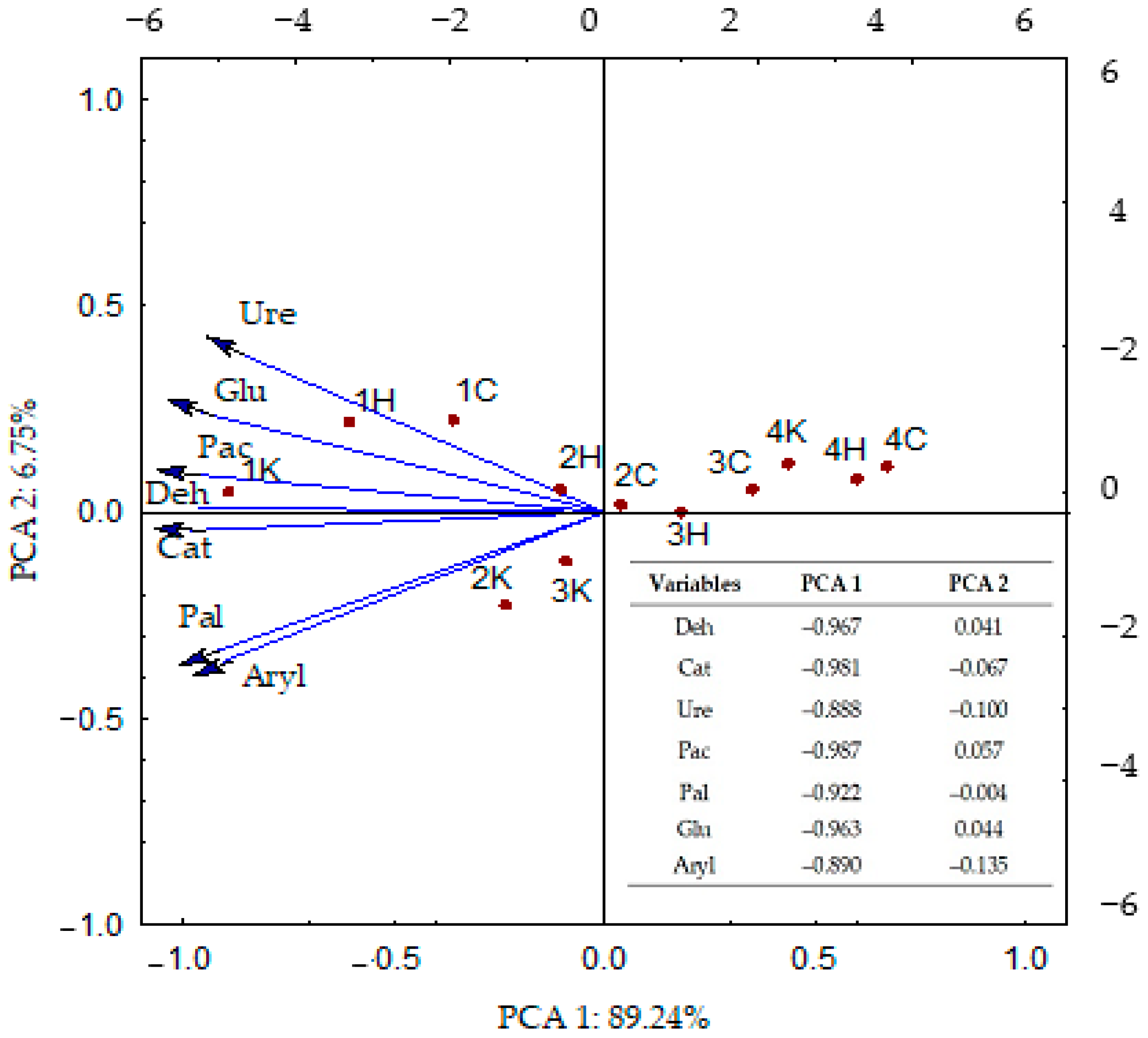
3.3. Correlations between the Analyzed Parameters
4. Discussion
4.1. Biomass and Heating Value of Zea mays L. Grown in Soil with the Addition of Ash from Salix viminalis
4.2. Biochemical and Physicochemical Properties of Soil
4.3. Correlations between the Analyzed Parameters
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| C | soil without compost and HumiAgra; |
| K | soil with compost; |
| H | soil with HumiAgra; |
| AP | yield of aboveground parts; |
| R | yield of roots; |
| Deh | dehydrogenases; |
| Cat | catalase; |
| Ure | urease; |
| Pac | acid phosphatase; |
| Pal | alkaline phosphatase; |
| Glu | β-glucosidase; |
| Aryl | arylsulfatase; |
| Corg | total organic carbon; |
| Ntotal | total nitrogen; |
| HAC | hydrolytic acidity; |
| EBC | total exchangeable base cations; |
| CEC | total cation exchange capacity of soil; |
| BS | basic cation saturation ratio in soil. |
References
- Liu, L.; Li, J.; Wu, G.; Shen, H.; Fu, G.; Wang, Y. Combined effects of biochar and chicken manure on maize (Zea mays L.) growth, lead uptake and soil enzyme activities under lead stress. Peer J. 2021, 9, e11754. [Google Scholar] [CrossRef]
- Mulyati; Baharuddin, A.B.; Tejowulan, R.S. Improving Maize (Zea mays L.) growth and yield by the application of inorganic and organic fertilizers plus. In Proceedings of the IOP Conference Series: Earth and Environmental Science, 3rd International Conference on Bioscience and Biotechnology, Lombok, Indonesia, 12–14 October 2020; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 712, p. 012027. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2008, 100, 5478–5484. [Google Scholar] [CrossRef]
- Oslaj, M.; Mursec, B.; Vindis, P. Biogas production from maize hybrids. Biomass Bioenerg. 2010, 34, 1538–1545. [Google Scholar] [CrossRef]
- Bubner, B.; Köhler, A.; Zaspel, I.; Zander, M.; Förster, N.; Gloger, J.-C.; Ulrichs, C.; Schneck, V. Breeding of multipurpose willows on the basis of Salix daphnoides Vill., Salix purpurea L. and Salix viminalis L. Appl. Agric. For. Res. 2018, 68, 53–66. [Google Scholar] [CrossRef]
- Maj, G.; Szyszlak-Bargłowicz, J.; Zajac, G.; Słowik, T.; Krzaczek, P.; Piekarski, W. Energy and emission characteristics of biowaste from the corn grain drying process. Energies 2019, 12, 4383. [Google Scholar] [CrossRef]
- Warmbier, K.; Wilczyński, A.; Danecki, L. Properties of one-layer experimental particleboards from willow (Salix viminalis) and industrial wood particles. Eur. J. Wood Prod. 2013, 71, 25–28. [Google Scholar] [CrossRef][Green Version]
- Zhai, F.-F.; Liu, J.-X.; Li, Z.-J.; Mao, J.-M.; Qian, Y.-Q.; Han, L.; Sun, Z.-Y. Assessing genetic diversity and population structure of Salix viminalis across Ergun and West Liao basin. Silva Fenn. 2017, 51, 7001. [Google Scholar] [CrossRef]
- Mojiri, A. The potential of corn (Zea mays) for phytoremediation of soil contaminated with cadmium and lead. J. Biol. Environ. Sci. 2011, 5, 17–22. [Google Scholar]
- Branquinho, C.; Serrano, H.C.; Pinto, M.J.; Martins-Loução, M.A. Revisiting the plant hyperaccumulation criteria to rare plants and earth abundant elements. Environ. Pollut. 2007, 246, 437–443. [Google Scholar] [CrossRef]
- Johan, P.D.; Ahmed, O.H.; Omar, L.; Hasbullah, N.A. Phosphorus transformation in soils following co-application of charcoal and wood ash. Agronomy 2021, 11, 2010. [Google Scholar] [CrossRef]
- Mandre, M.; Parn, H.; Ots, K. Short-term effects of wood ash on the soil and the lignin concentration and growth of Pinus sylvestris L. For. Ecol. Manag. 2006, 223, 349–357. [Google Scholar] [CrossRef]
- Yrjälä, K.; Katainen, R.; Jurgens, G.; Saarela, U.; Saano, A.; Romantschuk, M.; Fritze, H. Wood ash fertilization alters the forest humus Archea community. Soil Biol. Biochem. 2004, 36, 199–201. [Google Scholar] [CrossRef]
- Ondrasek, G.; Romic, D.; Rengel, Z. Interactions of humates and chlorides with cadmium drive soil cadmium chemistry and uptake by radish cultivars. Sci. Total Environ. 2020, 720, 134887. [Google Scholar] [CrossRef] [PubMed]
- Jansone, B.; Samariks, V.; Okmanis, M.; Kļaviņa, D.; Lazdiņa, D. Effect of High Concentrations of Wood Ash on Soil Properties and Development of Young Norway Spruce (Picea abies (L.) Karst) and Scots Pine (Pinus sylvestris L.). Sustainability 2020, 12, 9479. [Google Scholar] [CrossRef]
- Ozolincius, R.; Varnagiryte, I.; Armolaitis, K.; Karltun, E. Initial effects of wood ash fertilization on soil, needle and litterfall chemistry in a Scots pine (Pinus sylvestris L.). Stand Balt. For. 2005, 11, 59–67. [Google Scholar]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical characteristics of biomass. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Tosti, L.; van Zomeren, A.; Pels, J.R.; Dijkstra, J.J.; Comans, R.N.J. Assessment of biomass ash applications in soil and cement mortars. Chemosphere 2019, 223, 425–437. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical of biomass ash. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Park, J.-H.; Eom, J.-S.; Lee, S.-L.; Hwang, S.-W.; Kim, S.-H.; Kang, S.-W.; Yun, J.-J.; Cho, J.-S.; Lee, Y.-H.; Seo, D.-C. Exploration of the potential capacity of fly ash and bottom ash derived from wood pellet-based thermal power plant for heavy metal removal. Sci. Total Environ. 2020, 740, 140205. [Google Scholar] [CrossRef]
- Ondrasek, G.; Kranjčec, F.; Filipović, L.; Filipović, V.; Bubalo Kovačić, M.; Jelovica Badovinac, I.; Peter, R.; Petravić, M.; Macan, J.; Rengel, Z. Biomass bottom ash & dolomite similarly ameliorate an acidic low-nutrient soil, improve phytonutrition and growth, but increase Cd accumulation in radish. Sci. Total Environ. 2021, 753, 141902. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Ewees, S.A.; Sawsan, A.; Seaf, E.Y.; Dalia, M.S. Improving maize grain yield and its quality grown on a newly reclaimed sandy soil by applying micronutrients, organic manure and biological inoculation. Res. J. Agric. Biol. Sci. 2008, 4, 537–544. [Google Scholar]
- Perkiömäki, J.; Kiikkilä, O.; Moilanen, M.; Issakainen, J.; Tervahauta, A.; Fritze, H. Cadmium-containing wood ash in a pine forest: Effects on humus microflora and cadmium concentrations in mushrooms, berries, and needles. Can. J. For. Res. 2003, 33, 2443–2451. [Google Scholar] [CrossRef]
- Mercl, F.; Tejnecký, V.; Száková, J.; Tlustoš, P. Nutrient dynamics in soil solution and wheat response after biomass ash amendments. Agron. J. 2016, 108, 2222–2234. [Google Scholar] [CrossRef]
- Jagodzinski, L.S.; O’Donoghue, M.T.; Heffernan, L.B.; van Pelt, F.N.; O’Halloran, J.; Jansen, M.A. Wood ash residue causes a mixture of growth promotion and toxicity in Lemna minor. Sci. Total Environ. 2018, 625, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Levula, T.; Saarsalmi, A.; Rantavaara, A. Effects of ash fertilization and prescribed burning on macronutrient, heavy metal, sulphur and 137Cs concentrations in lingonberries (Vaccinium vitis-idaea). For. Ecol. Manag. 2000, 126, 269–279. [Google Scholar] [CrossRef]
- Demeyer, A.; Nkana, J.C.V.; Verloo, M.G. Characteristics of wood ash and influence on soil properties and nutrient uptake: An overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, A. Ecological and economical evaluation of biomass ash utilization–the Swedish approach. Ashes and particulate emissions from biomass combustion, Series Thermal Biomass Utilization. Inst. Chem. Engin. Techn. Univ. Graz. 1998, 3, 29–41. [Google Scholar]
- Zimmermann, S.; Frey, B. Soil respiration and microbial properties in an amid forest soil: Effects of wood ash. Soil Biol. Biochem. 2002, 34, 1727–1737. [Google Scholar] [CrossRef]
- Romdhane, L.; Ebinezer, L.B.; Panozzo, A.; Barion, G.; Cortivo, C.D.; Radhouane, L.; Vamerali, T. Effects of soil amendment with wood ash on transpiration, growth, and metal uptake in two contrasting maize (Zea mays L.) hybrids to drought tolerance. Front. Plant Sci. 2021, 12, 661909. [Google Scholar] [CrossRef]
- Pukalchik, M.; Mercl, F.; Terekhova, V.; Tlustoš, P. Biochar, wood ash and humic substances mitigating trace elements stress in contaminated sandy loam soil: Evidence from an integrative approach. Chemosphere 2018, 203, 228–238. [Google Scholar] [CrossRef]
- Cruz-Paredes, C.; Frøslev, T.G.; Michelsen, A.; Bang-Andreasen, T.; Hansen, M.; Ingerslev, M.; Skov, S.; Wallander, H.; Kjøller, R. Wood ash application in a managed Norway spruce plantation did not affect ectomycorrhizal diversity or N retention capacity. Fungal Ecol. 2019, 39, 1–11. [Google Scholar] [CrossRef]
- Małek, S.; Ważny, R.; Błońska, E.; Jasik, M.; Lasota, J. Soil fungal diversity and biological activity as indicators of fertilization strategies in a forest ecosystem after spruce disintegration in the Karpaty Mountains. Sci Total Environ. 2021, 751, 142335. [Google Scholar] [CrossRef]
- Nabeela, F.; Murad, W.; Khan, I.; Mian, I.A.; Rehman, H.; Adnan, M. Effect of wood ash application on the morphological, physiological and biochemical parameters of Brassica napus L. Plant Physiol. Biochem. 2015, 95, 15–25. [Google Scholar] [CrossRef]
- Błońska, E.; Prażuch, W.; Boroń, P.; Lasota, J. Effects of wood ash on the soil properties and fungal community structure in a beech forest in Poland. Geoderma Reg. 2023, 34, e00676. [Google Scholar] [CrossRef]
- Mundała, P.; Szwalec, A.; Kędzior, R. Accumulation of selected heavy metals in willow shoots (Salix viminalis L.) cultivated in the neighbourhood of a coal ash and slag landfill. Infrastrukt. Ecol. Rural Areas 2017, 3, 1043–1051. [Google Scholar] [CrossRef]
- Núñez-Delgado, A.; Quiroga-Lago, F.; Soto-González, B. Runoff characteristics in forest plots before and after wood ash fertilization. Maderas Cienc. Tecnol. 2011, 13, 267–284. [Google Scholar] [CrossRef]
- Someshwar, A.V. Wood and combination wood-fired boiler ash characterization. J. Environ. Qual. 1996, 25, 962–972. [Google Scholar] [CrossRef]
- Öhlinger, R. Dehydrogenase activity with the substrate TTC. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 241–243. [Google Scholar]
- Alef, K.; Nannipieri, P. (Eds.) Methods in Applied Soil Microbiology and Biochemistry, Enzyme Activities; Academic: London, UK, 1998; pp. 316–365. [Google Scholar]
- Carter, M.R. Soil Sampling and Methods of Analysis; Canadian Society of Soil Science; Lewis Publishers: London, UK, 1993. [Google Scholar]
- PN-EN. ISO 18125:2017-07; Solid Biofuels—Determination of Calorific Value. European Committee for Standardization: Brussels, Belgium, 2010. Available online: https://pkn.pl/pn-en-iso-18125-2017-07 (accessed on 10 October 2023).
- Kopetz, H.; Jossart, J.; Ragossnig, H.; Metschina, C. European Biomass Statistics 2007; European Biomass Association: Brussels, Belgium, 2007. [Google Scholar]
- PN-G-04584; Oznaczanie Zawartości Siarki Całkowitej i Popiołowej Automatycznymi Analizatorami. Determination of Total Sulphur and Ash Sulphur in Automatic Analyzers. National Standards Body in Poland: Warszaw, Poland, 2001. (In Polish)
- ISO 11261; Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 1995.
- Zaborowska, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Bisphenol A—A dangerous pollutant distorting the biological properties of soil. Int. J. Mol. Sci. 2021, 22, 12753. [Google Scholar] [CrossRef] [PubMed]
- Borowik, A.; Wyszkowska, J.; Wyszkowski, M. Resistance of aerobic microorganisms and soil enzyme response to soil contamination with Ekodiesel Ultra fuel. Environ. Sci. Poll. Res. 2017, 24, 24346–24363. [Google Scholar] [CrossRef] [PubMed]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Use of zeolite to neutralise nickel in a soil environment. Environ. Monit. Assess. 2018, 190, 54. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Sensitivity of Zea mays and soil microorganisms to the toxic effect of chromium (VI). Int. J. Mol. Sci. 2023, 24, 178. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Phytoremediation of soil contaminated with nickel, cadmium and cobalt. Int. J. Phytoremediation 2021, 23, 252–262. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Calorific value of Zea mays biomass derived from soil. Energies 2023, 16, 3788. [Google Scholar] [CrossRef]
- Dell Inc. Dell Statistica (Data Analysis Software System); Version 13.1; Dell Inc.: Tulsa, OK, USA, 2022. [Google Scholar]
- Varshney, A.; Dahiya, P.; Sharma, A.; Pandey, R.; Mohan, S. Fly ash application in soil for sustainable agriculture: An Indian overview. Energ. Ecol. Environ. 2022, 7, 340–357. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Energetic value of Elymus elongatus L. and Zea mays L. grown on soil polluted with Ni2+, Co2+, Cd2+, and sensitivity of rhizospheric bacteria to heavy metals. Energies 2021, 14, 4903. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Boros-Lajszner, E.; Kucharski, J. Calorific value of Festuca rubra biomass in the phytostabilization of soil contaminated with nickel, cobalt and cadmium which disrupt the microbiological and biochemical properties of soil. Energies 2022, 15, 3445. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Przybył, J.; Czajkowski, Ł.; Majka, J.; Pawłowski, A. Effects of Harvest Maturity on the Chemical and Energetic Properties of Corn Stover Biomass Combustion. Materials 2022, 15, 2831. [Google Scholar] [CrossRef]
- Miranda, M.T.; Sepúlveda, F.J.; Arranz, J.I.; Montero, I.; Rojas, C.V. Analysis of pelletizing from corn cob waste. J. Environ. Manag. 2018, 228, 303–311. [Google Scholar] [CrossRef]
- Jóvér, J.; Antal, K.; Zsembeli, J.; Blaskó, L.; Tamás, J. Assessment of gross calorific value of crop and bio-energy residues. Res. Agric. Eng. 2018, 64, 121–127. [Google Scholar] [CrossRef]
- Gusiatin, Z.M.; Kulikowska, D. Behaviors of heavy metals (Cd, Cu, Ni, Pb and Zn) in soil amended with composts. Environ. Technol. 2016, 37, 2337–2347. [Google Scholar] [CrossRef] [PubMed]
- Mindari, W.; Sasongko, P.E.; Kusuma, Z.; Syekhfani, S.; Ain, N. Efficiency of various sources and doses of humic acid on physical and chemical properties of saline soil and growth and yield of rice. In Proceedings of the AIP Conference Proceedings, 9th International Conference on Global Resource Conservation (ICGRC) and Aji from Ritsumeikan University, Malang City, Indonesia, 7–8 March 2018; AIP Publishing: Melville, NY, USA, 2019; p. 030001. [Google Scholar] [CrossRef]
- Tiwari, J.; Ramanathan, A.; Bauddh, K.; Korstad, J. Humic substances: Structure, function and benefits for agroecosystems—A review. Pedosphere 2023, 33, 237–249. [Google Scholar] [CrossRef]
- Baldrian, P. Microbial enzyme-catalyzed processes in soil and their analysis. Plant Soil Environ. 2009, 55, 370–378. [Google Scholar] [CrossRef]
- Ouyang, L.; Tang, Q.; Yu, L.Q.; Zhang, R.D. Effects of amendment of different biochars on soil enzyme activities related to carbon mineralisation. Soil Res. 2014, 52, 706–716. [Google Scholar] [CrossRef]
- Kong, L.; Chu, L.M. Subtropical urban turfs: Carbon and nitrogen pools and the role of enzyme activity. J. Environ. Sci. 2018, 65, 18–28. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Burns, R.G. Ecology of extracellular enzyme activities and organic matter degradation in soil: A complex community-driven process. In Methods of Soil Enzymology; Dick, R.P., Ed.; Soil Science Society of America: Madison, WI, USA, 2011; pp. 35–55. [Google Scholar] [CrossRef]
- Stone, M.M.; De Forest, J.L.; Plante, A.F. Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory. Soil Biol. Biochem. 2014, 75, 237–247. [Google Scholar] [CrossRef]
- Piotrowska-Długosz, A.; Kobierski, M.; Długosz, J. Enzymatic activity and physicochemical properties of soil profiles of luvisols. Materials 2021, 14, 6364. [Google Scholar] [CrossRef]
- Gianfreda, L.; Ruggiero, P. Enzyme activities in soil. In Nucleic Acids and Proteins in Soil; Nannipieri, P., Smalla, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 20–25. [Google Scholar] [CrossRef]
- Loeppmann, S.; Blagodatskaya, E.; Pausch, J.; Kuzyakov, Y. Enzyme properties down the soil profile—A matter of substrate quality in rhizosphere and detritusphere. Soil Biol. Biochem. 2016, 103, 274–283. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Baran, A. Effect of poultry litter biochar on soil enzymatic activity, ecotoxicity and plant growth. Appl. Soil Ecol. 2016, 105, 144–150. [Google Scholar] [CrossRef]
- Smenderovac, E.; Emilson, E.; Porter, C.T.; Morris, D.; Hazlett, P.; Diochon, A.; Basiliko, N.; Bélanger, N.; Markham, J.; Rutherford, P.M.; et al. Forest soil biotic communities show few responses to wood ash applications at multiple sites. Sci. Rep. 2022, 12, 4171. [Google Scholar] [CrossRef]
- Perucci, P.; Monaci, E.; Onofri, A.; Vischetti, C.; Casucci, C. Changes in physico-chemical and biochemical parameters of soil following addition of wood ash: A field experiment. Eur. J. Agron. 2008, 28, 155–161. [Google Scholar] [CrossRef]
- Abel, S.; Peters, A.; Trinks, S.; Schonsky, H.; Facklam, M.; Wessolek, G. Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 2013, 202–203, 183–191. [Google Scholar] [CrossRef]
- Basso, A.S.; Miguez, F.E.; Laird, D.A.; Horton, R.; Westgate, M. Assessing potential of biochar for increasing water-holding capacity of sandy soils. GCB Bioenergy 2013, 5, 132–143. [Google Scholar] [CrossRef]
- Foster, E.J.; Hansen, N.; Wallenstein, M.; Cotrufo, M.F. Biochar and manure amendments impact soil nutrients and microbial enzymatic activities in a semi-arid irrigated maize cropping system. Agric. Ecosyst. Environ. 2016, 233, 404–414. [Google Scholar] [CrossRef]
- Zornoza, R.F.; Moreno-Barriga, J.A.; Acosta, M.A.; Muñoz, A. Faz. Stability, nutrient availability and hydrophobicity of biochars derived from manure, crop residues, and municipal solid waste for their use as soil amendments. Chemosphere 2016, 144, 122–130. [Google Scholar] [CrossRef]
- Borchard, N.; Wolf, A.; Laabs, V.; Aeckersberg, R.; Scherer, H.W.; Moeller, A.W. Physical activation of biochar and its meaning for soil fertility and nutrient leaching—A greenhouse experiment. Soil Use Manag. 2012, 28, 177–184. [Google Scholar] [CrossRef]
- Brantley, K.E.; Savin, M.C.; Brye, K.R.; Longer, D.E. Pine woodchip biochar impact on soil nutrient concentrations and corn yield in a silt loam in the mid-Southern U.S. Agriculture 2015, 5, 30–47. [Google Scholar] [CrossRef]
- Lucchini, P.; Quilliam, R.S.; DeLuca, T.H.; Vamerali, T.; Jones, D.L. Increased bioavailability of metals in two contrasting agricultural soils treated with waste wood-derived biochar and ash. Environ. Sci. Pollut. Res. 2014, 21, 3230–3240. [Google Scholar] [CrossRef]
- Adekayode, F.O.; Olojugba, M.R. The utilization of wood ash as manure to reduce the use of mineral fertilizer for improved performance of maize (Zea mays L.) as measured in the chlorophyll content and grain yield. J. Soil Sci. Environ. Manag. 2010, 1, 40–45. [Google Scholar] [CrossRef]
- Arshad, M.A.; Soon, Y.K.; Azooz, R.H.; Lupwayi, N.Z.; Chang, S.X. Soil and crop response to wood ash and lime application in acidic soils. Agron. J. 2012, 104, 715–721. [Google Scholar] [CrossRef]
- Muhammad, I.; Puschenreiter, M.; Wenzel, W.W. Cadmium and Zn availability as affected by pH manipulation and its assessment by soil extraction, DGT and indicator plants. Bull. Environ. Contam. Toxicol. 2012, 416, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.S.; Chattopadhyay, G.N. Increasing bioavailability of phosphorus from fly ash through vermicomposting. J. Environ. Qual. 2002, 31, 2116–2119. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Tandon, P.K.; Khatoon, S. Effect of chromium on antioxidant potential of Catharanthus roseus varieties and production of their anticancer alkaloids: Vincristine and vinblastine. Biomed. Res. Int. 2014, 2014, 934182. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Kalaji, H.M.; Jajoo, A. Investigation of deleterious effects of chromium phytotoxicity and photosynthesis in wheat plant. Photosynthetica 2016, 54, 185–192. [Google Scholar] [CrossRef]
- Huang, S.; Peng, B.; Yang, Z.; Chai, L.; Zhou, L. Chromium accumulation, microorganism population and enzyme activities in soils around chromium-containing slag heap of steel alloy factory. Trans. Nonferrous Met. Soc. China 2009, 19, 241–248. [Google Scholar] [CrossRef]
- Boerner, R.E.J.; Brinkman, J.A. Fire frequency and soil enzyme activity in southern Ohio aok-hickory forests. Appl. Soil Ecol. 2003, 23, 137–146. [Google Scholar] [CrossRef]
- Peng, B.; Huang, S.H.; Yang, Z.H.; Chai, L.Y.; Xu, Y.Z.; Su, C.Q. Inhibitory effect of Cr(VI) on activities of soil enzymes. J. Cent. South. Univ. Technol. 2009, 16, 594–598. [Google Scholar] [CrossRef]
- Wu, G.; Kanga, H.; Zhang, X.; Shao, H.; Chu, L.; Ruand, C. A critical review on the bio-removal of hazardous heavy metals from contaminated soils: Issues, progress, eco-environmental concerns and opportunities. J. Hazard. Mater. 2010, 174, 1–8. [Google Scholar] [CrossRef]
- Lombard, N.; Prestat, E.; van Elsas, J.D.; Simonet, P. Soil-specific limitations for access and analysis of soil microbial communities by metagenomics. FEMS Microbiol. Ecol. 2011, 78, 31–49. [Google Scholar] [CrossRef]
- Belyaeva, O.N.; Haynes, R.J.; Birukova, O.A. Barley yield and soil microbial and enzyme activities as affected by contamination of two soils with lead, zinc or copper. Biol. Fertil. Soils 2005, 41, 85–94. [Google Scholar] [CrossRef]
- Pérez-Guzmán, L.; Lower, B.H.; Dick, R.P. Corn and hardwood biochars affected soil microbial community and enzyme activities. Agrosyst. Geosci. Environ. 2020, 3, e20082. [Google Scholar] [CrossRef]
| Type of Analysis | Pot Vegetation Experiment |
|---|---|
| Soil characteristics | Sandy clay: sand 69.41%, silt 27.71%, clay 2.88%; |
| Contents: organic carbon—6.18 g kg−1 d.m. soil, total nitrogen—1.27 g kg−1 d.m. soil; pHKCl—6.09; Hydrolytic acidity (HAC)—8.81 mmol(+) kg−1 d.m. soil; Sum of exchangeable base cations (EBC)—24.00 mmol(+) kg−1 d.m. soil; Cation exchange capacity (CEC)—32.81 mmol(+) kg−1 d.m. soil; Extent of saturation with base cations (BS)—73.14%. | |
| The mass of soil in the pot | 3.5 kg |
| Experimental plant | Zea mays L. variety LG 32.58 (4 plants in one pot) |
| Fertilization in mg kg−1 d.m. of soil | N—150 in the form of CO(NH2)2 P—70 in the form of KH2PO4 K—120 in the form of KCl + KH2PO4 Mg—15 in the form of MgSO4 × 7H2O |
| Dose of ash from Salix viminalis in g kg−1 d.m. of soil | 0, 5, 10, 20 |
| The content of elements in the ash (Figure 1a) in % d.m. | C—51.04, H—5.87, S—0.02, N—0.38; Cl—0.02; P—0.09; K—0.17; Mg—0.03; Ca—0.41; Na—0.009 |
| pH in KCl | 12.50 |
| Compost | Manufacturer: Ekokonsorcjum-Effekt company (Cracow, Poland; license no. 21/02) from green waste (grass, bushes) gathered in parks, squares, and home gardens; fruit and vegetables picked at market squares; and organic waste from food processing plants. Chemical composition in % of d.m.: Corg—23.20; NTotal—1.30; P—0.26; K—1.24; Mg—0.30; and Ca—1.43. |
| HumiAgra | Manufacturer: AgraPlant, Kielce, Poland. Chemical composition in % of d.m.: 90% humic acids (50% humins and 50% fulvic acids), 6% K, and 3% S. |
| Dose of compost and HumiAgra in g kg−1 d.m. of soil | 0 and 2.5 |
| Experiment duration in days | 60 |
| Number of replications | Four repetitions per combination |
| Conditions in the vegetation hall | The average temperature was 17.5 °C, air humidity reached 78.5%, and day length was from 13 h 4 min to 15 h 30 min. The soil moisture content—50% of the water capillary capacity. |
| Variable Factors | Plant Yield * | Enzymes ** | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AP | R | Deh | Cat | Ure | Pac | Pal | Glu | Aryl | |
| Ash dose | 26.23 | 50.51 | 93.47 | 80.84 | 87.01 | 95.64 | 68.42 | 92.92 | 57.13 |
| Additives | 54.83 | 31.47 | 2.64 | 15.85 | 5.00 | 3.15 | 21.99 | 4.69 | 36.22 |
| Ash * Additives | 7.95 | 5.50 | 1.07 | 2.72 | 7.89 | 0.93 | 6.04 | 2.19 | 6.55 |
| Error | 10.97 | 12.51 | 2.83 | 0.59 | 0.10 | 0.28 | 3.55 | 0.20 | 0.10 |
| Ash Dose, g kg−1 d.m. Soil | 14 Days | 28 Days | 42 Days |
|---|---|---|---|
| Without Additives | |||
| 0 | 43.02 a ± 1.73 | 36.71 bcd ± 2.47 | 26.03 f ± 2.23 |
| 5 | 42.37 a ± 1.47 | 35.12 cde ± 1.02 | 25.37 f ± 2.17 |
| 10 | 41.23 ab ± 0.60 | 35.07 cde ± 0.69 | 25.21 f ± 1.58 |
| 20 | 40.55 ab ± 0.40 | 31.43 e ± 2.56 | 24.78 f ± 2.08 |
| 41.80 B | 34.58 D | 25.35 F | |
| r | −0.96 * | −0.97 * | −0.96 * |
| Compost | |||
| 0 | 43.55 a ± 2.14 | 34.49 cde ± 1.98 | 24.58 f ± 3.58 |
| 5 | 43.43 a ± 1.57 | 34.07 cde ± 2.30 | 24.13 f ± 1.02 |
| 10 | 42.68 a ± 1.02 | 33.58 de ± 2.43 | 22.90 f ± 1.95 |
| 20 | 42.14 a ± 0.33 | 33.59 de ± 1.92 | 22.69 f ± 0.75 |
| 42.93 A | 33.950 E | 23.58 E | |
| r | −0.98 * | −0.87 * | −0.97 * |
| HumiAgra | |||
| 0 | 43.670 a ± 1.07 | 38.95 abc ± 0.63 | 26.31 f ± 0.91 |
| 5 | 43.07 a ± 1.08 | 35.56 cde ± 0.97 | 26.25 f ± 1.51 |
| 10 | 42.07 a ± 0.97 | 34.73 cde ± 1.56 | 25.99 f ± 0.61 |
| 20 | 42.14 a ± 1.29 | 34.55 cde ± 2.11 | 25.69 f ± 1.03 |
| 42.75 A | 35.95 C | 26.05 F | |
| r | −0.88 * | −0.80 | −1.00 * |
| Ash Dose, g kg−1 d.m. Soil | Heat of Combustion | Heating Value | Energy Production MJ kg−1 |
|---|---|---|---|
| MJ kg−1 Air-Dried Plant Matter | |||
| Without Additives | |||
| 0 | 18.42 a ± 0.04 | 16.50 a ± 0.03 | 0.340 b ± 0.02 |
| 20 | 17.91 c ± 0.03 | 15.93 c ± 0.02 | 0.27 c ± 0.02 |
| Compost | |||
| 0 | 18.29 b ± 0.02 | 16.44 a ± 0.03 | 0.40 a ± 0.03 |
| 20 | 17.94 c ± 0.02 | 16.13 b ± 0.03 | 0.36 ab ± 0.02 |
| HumiAgra | |||
| 0 | 17.90 c ± 0.05 | 16.17 b ± 0.02 | 0.36 ab ± 0.04 |
| 20 | 17.86 c ± 0.02 | 16.10 b ± 0.03 | 0.32 b ± 0.03 |
| Ash Dose, g kg−1 d.m. Soil | Deh | Cat | Ure | Pac | Pal | Glu | Aryl |
|---|---|---|---|---|---|---|---|
| Without Additives | |||||||
| 5 | −0.23 a ± 0.03 | −0.111 a ± 0.03 | −0.42 b ± 0.07 | −0.25 a ± 0.03 | −0.04 a ± 0.01 | −0.26 a ± 0.03 | −0.030 a ± 0.03 |
| 10 | −0.26 a ± 0.04 | −0.254 d ± 0.02 | −0.51 c ± 0.05 | −0.47 c ± 0.07 | −0.30 e ± 0.02 | −0.40 d ± 0.02 | −0.06 a ± 0.01 |
| 20 | −0.61 c ± 0.05 | −0.31 ef ± 0.02 | −0.56 d ± 0.02 | −0.60 d ± 0.05 | −0.39 f ± 0.04 | −0.53 f ± 0.04 | −0.23 e ± 0.02 |
| −0.37 A | −0.23 B | −0.50 B | −0.44 B | −0.24 B | −0.40 B | −0.11 A | |
| Compost | |||||||
| 5 | −0.29 b ± 0.08 | −0.15 b ± 0.01 | −0.62 f ± 0.08 | −0.23 a ± 0.02 | −0.10 b ± 0.02 | −0.29 ab ± 0.02 | −0.10 b ± 0.01 |
| 10 | −0.31 b ± 0.07 | −0.18 c ± 0.03 | −0.57 d ± 0.04 | −0.36 b ± 0.03 | −0.17 c ± 0.02 | −0.33 c ± 0.02 | −0.20 d ± 0.01 |
| 20 | −0.65 c ± 0.05 | −0.30 de ± 0.06 | −0.60 e ± 0.03 | −0.59 d ± 0.06 | −0.49 g ± 0.04 | −0.41 d ± 0.05 | −0.38 g ± 0.04 |
| −0.42 B | −0.21 B | −0.60 C | −0.40 A | −0.25 B | −0.34 A | −0.23 C | |
| HumiAgra | |||||||
| 5 | −0.24 a ± 0.06 | −0.09 a ± 0.05 | −0.35 a ± 0.03 | −0.31 b ± 0.01 | −0.08 b ± 0.05 | −0.30 ab ± 0.04 | −0.12 bc ± 0.01 |
| 10 | −0.30 b ± 0.04 | −0.16 b ± 0.02 | −0.56 d ± 0.02 | −0.45 c ± 0.03 | −0.24 d ± 0.02 | −0.44 e ± 0.02 | −0.17 c ± 0.02 |
| 20 | −0.63 c ± 0.09 | −0.31 f ± 0.03 | −0.63 f ± 0.06 | −0.64 e ± 0.06 | −0.39 f ± 0.03 | −0.54 f ± 0.01 | −0.31 f ± 0.03 |
| −0.39 A | −0.19 A | −0.39 A | −0.35 A | −0.18 A | −0.32A | −0.20 B | |
| Ash Dose, g kg−1 d.m. Soil | Total Organic Carbon (Corg) | Total Nitrogen (NTotal) | pHKCl | Hydrolytic Acidity (HAC) | Total Exchangeable Base Cations (EBC) | Total Cation Exchange Capacity of Soil (CEC) | Base Cations Saturation Ratio in Soil (BS) |
|---|---|---|---|---|---|---|---|
| g kg−1 | mmol(+) kg−1 Soil | % | |||||
| Without Additives | |||||||
| 0 | 7.87 g ± 0.18 | 1.68 d ± 0.01 | 4.35 f ± 0.05 | 15.71 c ± 0.04 | 44.10 k ± 0.05 | 59.81 j ± 0.26 | 73.73 i ± 0.31 |
| 5 | 8.10 fg ± 0.09 | 1.71 cd ± 0.02 | 6.85 d ± 0.04 | 5.81 d ± 0.04 | 122.00 h ± 1.00 | 127.81 g ± 0.04 | 95.45 f ± 0.01 |
| 10 | 8.27 fg ± 0.15 | 1.71 cd ± 0.01 | 7.00 c ± 0.03 | 3.71 f ± 0.04 | 123.00 h ± 0.10 | 126.71 g ± 1.04 | 97.07 d ± 0.01 |
| 20 | 10.34 b ± 0.11 | 1.73 bc ± 0.02 | 7.45 b ± 0.04 | 2.74 h ± 0.04 | 201.10 e ± 0.50 | 203.84 e ± 0.54 | 98.66 a ± 0.03 |
| 8.64 B | 1.708 A | 6.41 A | 6.99 B | 122.55 C | 129.54 C | 91.23 B | |
| r | 0.94 * | 0.923 * | 0.80 * | −0.81 * | 0.96 * | 0.96 * | 0.76 |
| Compost | |||||||
| 0 | 8.57 ef ± 0.10 | 1.72 bc ± 0.02 | 4.50 e ± 0.04 | 17.14 a ± 0.11 | 107.00 i ± 0.05 | 124.14 h ± 1.11 | 86.20 g ± 0.18 |
| 5 | 9.19 cd ± 0.13 | 1.74 bc ± 0.01 | 7.05 c ± 0.05 | 6.04 d ± 0.04 | 197.50 f ± 0.50 | 203.54 e ± 0.14 | 97.03 d ± 0.03 |
| 10 | 9.48 c ± 0.19 | 1.81 a ± 0.02 | 7.50 b ± 0.03 | 4.35 e ± 0.08 | 229.00 a ± 1.00 | 233.35 a ± 0.92 | 98.14 c ± 0.01 |
| 20 | 10.99 a ± 0.11 | 1.82 a ± 0.01 | 7.80 a ± 0.03 | 3.79 f ± 0.04 | 220.00 b ± 0.05 | 223.79 b ± 0.04 | 98.31 bc ± 0.01 |
| 9.56 A | 1.77 A | 6.71 A | 7.83 A | 188.38 A | 196.20 A | 94.92 A | |
| r | 0.99 * | 0.91 * | 0.81 * | −0.77 | 0.76 | 0.76 | 0.74 |
| HumiAgra | |||||||
| 0 | 8.06 g ± 0.08 | 1.71 cd ± 0.01 | 4.45 ef ± 0.05 | 16.43 b ± 0.07 | 50.30 j ± 0.05 | 66.73 i ± 0.23 | 75.38 h ± 0.03 |
| 5 | 8.16 fg ± 0.13 | 1.73 bc ± 0.03 | 6.85 d ± 0.05 | 6.00 d ± 0.07 | 136.50 g ± 0.30 | 142.50 f ± 0.38 | 95.79 e ± 0.02 |
| 10 | 8.82 de ± 0.08 | 1.76 b ± 0.01 | 7.05 c ± 0.05 | 3.98 f ± 0.07 | 204.00 d ± 0.30 | 207.985 d ± 0.08 | 98.09 c ± 0.04 |
| 20 | 10.34 b ± 0.09 | 1.81 a ± 0.02 | 7.55 b ± 0.05 | 3.26 g ± 0.08 | 215.00 c ± 0.30 | 218.26 c ± 1.26 | 98.51 ab ± 0.11 |
| 8.85 B | 1.75 A | 6.48 A | 7.42 A | 151.45 B | 158.87 B | 91.94 B | |
| r | 0.97 * | 1.00 * | 0.82 * | −0.80 | 0.89 * | 0.81 * | 0.75 |
| Variable Factors | R | Deh | Cat | Ure | Pac | Pal | Glu | Aryl | Corg | NTotal | pH | HAC | EBC | CEC | BS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AP | 0.66 * | 0.92 * | 0.91 * | 0.67 * | 0.80 * | 0.90 * | 0.85 * | 0.81 * | −0.84 * | −0.14 | −0.73 * | 0.70 * | −0.56 * | −0.55 * | −0.62 * |
| R | 1.00 | 0.65 * | 0.71 * | 0.65 * | 0.64 * | 0.60 * | 0.65 * | 0.68 * | −0.52 * | −0.03 | −0.57 * | 0.59 * | −0.40 * | −0.39 * | −0.51 * |
| Deh | 1.00 | 0.91 * | 0.66 * | 0.74 * | 0.95 * | 0.74 * | 0.78 * | −0.89 * | −0.15 | −0.65 * | 0.62 * | −0.53 * | −0.52 * | −0.52 * | |
| Cat | 1.00 | 0.78 * | 0.89 * | 0.91 * | 0.89 * | 0.86 * | −0.82 * | −0.10 | −0.79 * | 0.78 * | −0.56 * | −0.55 * | −0.70 * | ||
| Ure | 1.00 | 0.91 * | 0.67 * | 0.87 * | 0.67 * | −0.54 * | −0.15 | −0.77 * | 0.81 * | −0.55 * | −0.53 * | −0.69 * | |||
| Pac | 1.00 | 0.74 * | 0.97 * | 0.76 * | −0.68 * | −0.21 | −0.93 * | 0.94 * | −0.72 * | −0.70 * | −0.87 * | ||||
| Pal | 1.00 | 0.76 * | 0.83 * | −0.79 * | 0.01 | −0.60 * | 0.59 * | −0.41 * | −0.40 * | −0.48 * | |||||
| Glu | 1.00 | 0.79 * | −0.66 * | −0.15 | −0.90 * | 0.91 * | −0.67 * | −0.64 * | −0.83 * | ||||||
| Aryl | 1.00 | −0.58 * | 0.09 | −0.59 * | 0.59 * | −0.42 * | −0.41 * | −0.51 * | |||||||
| Corg | 1.00 | 0.49 * | 0.72 * | −0.64 * | 0.65 * | 0.65 * | 0.60 * | ||||||||
| NTotal | 1.00 | 0.41 * | −0.33 | 0.46 * | 0.46 * | 0.37 * | |||||||||
| pH | 1.00 | −0.99 * | 0.84 * | 0.82 * | 0.98 * | ||||||||||
| HAC | 1.00 | −0.81 * | −0.79 * | −0.98 * | |||||||||||
| EBC | 1.00 | 0.99 * | 0.83 * | ||||||||||||
| CEC | 1.00 | 0.81 * |
| Factor Tested in the Soil | Heating Value MJ kg−1 Air-Dried Plant Matter | Reference |
|---|---|---|
| Salix viminalis ash content | 15.93–16.50 | Our research |
| Maize variety | 7.62–10.79 | [57] |
| Corn grain drying process | 13.70–14.94 | [6] |
| Pellets from biomass | 15.68 | [58] |
| Cr (VI) content | 14.60–15.40 | [50] |
| Maize cultivation | 17.51 | [59] |
| Ni2+, Co2+, Cd2+ content | 14.79–14.97 | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Effect of Ash from Salix viminalis on the Biomass and Heating Value of Zea mays and on the Biochemical and Physicochemical Properties of Soils. Energies 2023, 16, 8037. https://doi.org/10.3390/en16248037
Boros-Lajszner E, Wyszkowska J, Kucharski J. Effect of Ash from Salix viminalis on the Biomass and Heating Value of Zea mays and on the Biochemical and Physicochemical Properties of Soils. Energies. 2023; 16(24):8037. https://doi.org/10.3390/en16248037
Chicago/Turabian StyleBoros-Lajszner, Edyta, Jadwiga Wyszkowska, and Jan Kucharski. 2023. "Effect of Ash from Salix viminalis on the Biomass and Heating Value of Zea mays and on the Biochemical and Physicochemical Properties of Soils" Energies 16, no. 24: 8037. https://doi.org/10.3390/en16248037
APA StyleBoros-Lajszner, E., Wyszkowska, J., & Kucharski, J. (2023). Effect of Ash from Salix viminalis on the Biomass and Heating Value of Zea mays and on the Biochemical and Physicochemical Properties of Soils. Energies, 16(24), 8037. https://doi.org/10.3390/en16248037







