Advances in Computational Fluid Dynamics Modeling for Biomass Pyrolysis: A Review
Abstract
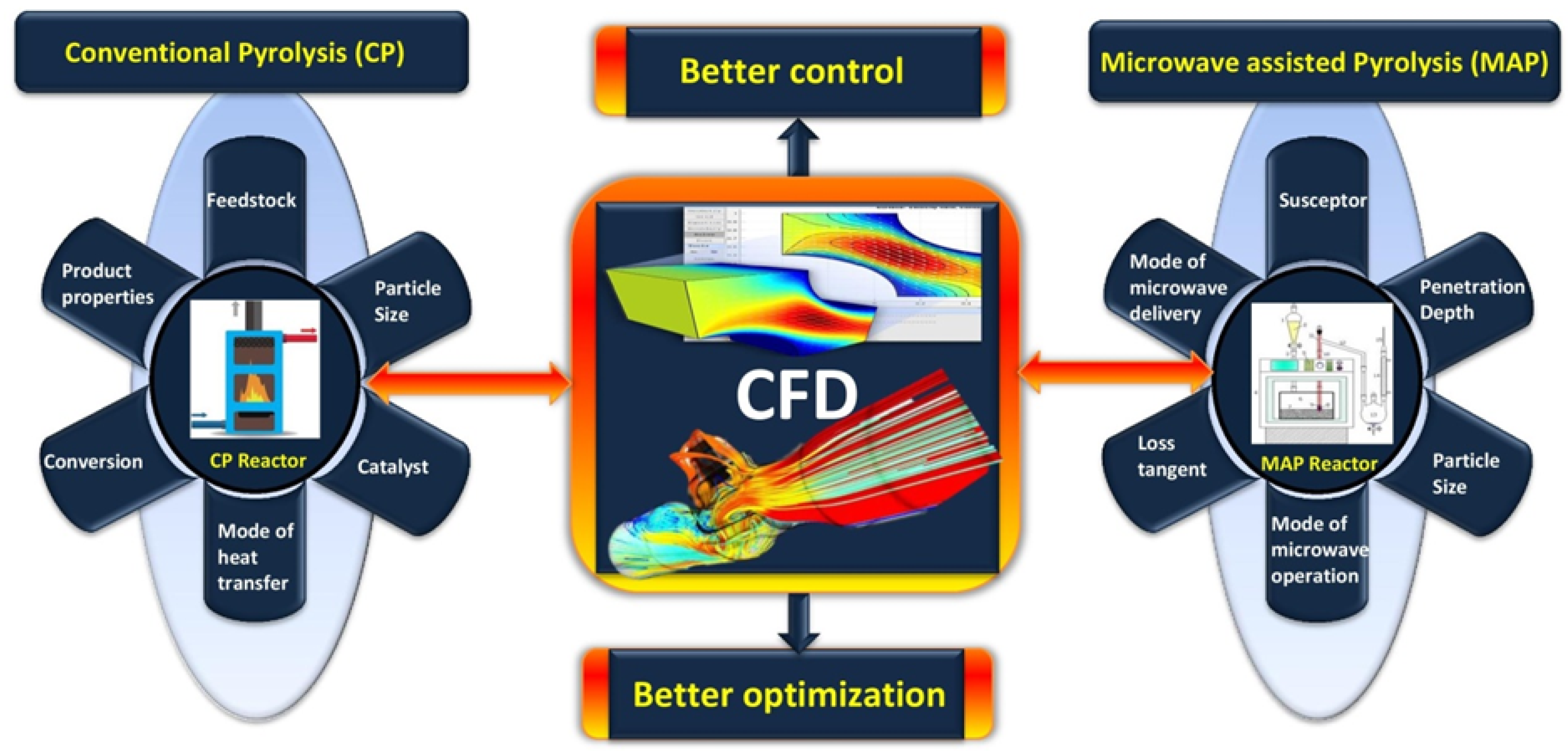
1. Introduction
1.1. Pyrolysis of Biomass and Waste Plastics
1.2. The Role of Modeling in Pyrolysis
2. The Fundamentals of CFD
2.1. Role of CFD in Pyrolysis
2.1.1. A Brief Overview of CFD Modeling Techniques for Pyrolysis
2.1.2. Sub Models for the Complex Physical and Chemical Processes during Biomass Pyrolysis
- (A)
- Reaction kinetics of pyrolysis
| Types of Kinetics | Reaction Kinetic Steps | Reference | |
|---|---|---|---|
| ine Single-component single-step reaction kinetics | Competitive | Wood → gas Wood → tar Wood → char | [54,55,56,57] |
| Non-competitive | Wood → gas + tar + char | ||
| Single-component multi-step reaction kinetics | Wood virgin→ Wood activated→ Gas + Tar + Char Wood → Tar; Tar → Gas | [58,59] | |
| Multi-component single-step reaction kinetics | Cellulose → Gas + Tar + Char Hemicellulose → Gas + Tar + Char Lignin → Gas + Tar + Char | [60,61] | |
| ine Multi-component multi-step reaction kinetics | Cellulose | 1. Biomass Activation | [62] |
| Hemicellulose | 2. Biomass decomposition to tar | ||
| Lignin | 3. Biomass decomposition to char and gas | ||
- (B)
- Conservation equations for the biomass components
- (C)
- Moisture evaporation
- (D)
- Variation in physical properties
- (E)
- The heat of pyrolysis
2.2. Research Gap and Objectives
3. CFD in Conventional Pyrolysis
3.1. Analysis of CFD Studies
3.2. Reaction Kinetics for CFD Simulation
3.3. Development of Numerical Models
3.4. CFD in Conventional Pyrolysis Reactors
4. CFD Models for Microwave-Assisted Pyrolysis
5. Challenges in CFD for Modeling Pyrolysis
- Complex chemical kinetics: Pyrolysis is characterized by a multifaceted array of chemical reactions, which encompass primary and secondary cracking, gasification, and char combustion. The intricacies of these reactions stem from their highly nonlinear nature and their strong dependency on variables such as temperature, pressure, and material composition. While CFD provides a robust framework for simulating fluid flow and heat transfer processes, it often struggles to accurately replicate the full complexity of pyrolysis reaction kinetics. This shortcoming is primarily due to the approximate nature of reaction models and the challenges in fully integrating detailed chemical kinetics within the CFD simulations. As highlighted in several studies [11,34,48,49,53], the inherent limitations of CFD in capturing the nuanced dynamics of these chemical processes underscore the need for the continual refinement of reaction models and computational strategies.
- High computational cost: One of the limitations of conventional CFD in modeling biomass pyrolysis is its high computational cost. The pyrolysis process occurs over a wide range of spatial and temporal scales, from the molecular scale of cellulose microfibrils to the meter scale of industrial reactors. This wide range of scales makes it challenging to accurately resolve all of the relevant physical processes using CFD. Additionally, the high temperatures and pressures involved in pyrolysis can result in highly transient and turbulent flow fields, thus further increasing the computational cost of the simulation. Therefore, conventional CFD models may not be able to capture all the relevant physical processes in biomass pyrolysis, and multi-scale CFD models are necessary to simulate the process accurately and efficiently [18,21].
- Uncertainty in input parameters: The complexity and variability in pyrolysis reactions are notably influenced by the characteristics of the biomass feedstock, such as particle size, composition, and moisture content. Vikram et al. [32] emphasized that reaction kinetics are significantly affected by factors like the heating rate and the initial temperature of the feedstock. Accurately capturing and integrating these parameters into CFD simulations is a critical challenge, thereby leading to uncertainty in modeling predictions. This is particularly pronounced in biomass pyrolysis due to the heterogeneity of the biomass material and the intricate nature of its thermal decomposition. The selection of the modeling approach, which includes kinetic, network, or mechanistic models, also impacts the input requirements and the ease of coupling with CFD simulations. For instance, network models, including the CPD model highlighted by Hameed et al. [11], often necessitate fewer input parameters, and they are more easily aligned with CFD, thus potentially reducing uncertainties. Nonetheless, despite these modeling advancements and their integration with CFD, the accurate prediction of pyrolysis product yields—like char, tar, and volatile matter—remains a complex task. This complexity is further heightened by the influence of inorganic species in the biomass and the dynamic interactions among its primary components (i.e., cellulose, hemicellulose, and lignin).
- Lack of experimental data: Despite significant advances in pyrolysis research, a notable gap persists in the availability of comprehensive experimental data for validating CFD models. References in key studies have highlighted specific shortcomings, such as insufficient data on biomass properties and the impacts of these properties on the pyrolysis process. Challenges in experimental setups, the scarcity of reference materials, and uncertainties in kinetic modeling further exacerbate the difficulty in accurately aligning CFD models with real-world phenomena. This deficiency in robust experimental validation not only underscores the complexities inherent in pyrolysis modeling, but it also poses a persistent hurdle in enhancing the precision and reliability of these models [49,129].
- Modeling of multi-physics phenomena: Pyrolysis presents a sophisticated amalgamation of heat transfer, mass transfer, and chemical reactions, each of which are governed by intricate physical and chemical principles. Successfully modeling these multi-physics phenomena demands the application of advanced numerical techniques, as well as precise, reliable models for each constituent process. The complexity is escalated by the necessity to accurately represent the transport phenomena of both the volatile and solid products that are generated during pyrolysis. This includes not only the accurate portrayal of the thermal degradation of biomass, but also the subsequent dynamics of evolved gases and residual solids within the reactor environment. Further, the modeling must account for the transient nature of these processes and their interdependence, i.e., how the evolution of gases influences the heat and mass transfer, as well as, in turn, the chemical reaction rates. The interaction of these variables creates a challenging scenario for CFD simulations, wherein the fidelity of the model to real-world phenomena is critical [13,19].
- Modeling of particle behavior: Pyrolysis predominantly revolves around the thermal decomposition of solid biomass particles. These particles, during the pyrolysis process, can exhibit intricate behaviors, which include agglomeration, fragmentation, and attrition. Such behaviors are influenced by the inherent heterogeneity of the biomass material and the dynamic interactions among its primary components [32]. Additionally, the hydrodynamic reactions of these particles in the turbulent flow, when coupled with the release of volatiles and the formation of char, contribute to the complexity. Accurately capturing these multi-faceted behaviors within the framework of CFD modeling is a daunting challenge. The integration of kinetic and network models [11] can provide insights into particle behavior. However, the precise simulation of particles in a turbulent reactor environment, when there are evolving physical and chemical properties, remains a pivotal challenge in ensuring the fidelity of CFD simulations of biomass pyrolysis.
- Optimization of reactor design: CFD plays a pivotal role in optimizing the design of pyrolysis reactors, thereby enabling the precise simulation and analysis of various operational scenarios [130]. The optimization process, however, is complicated by the extensive range of design parameters that must be considered. These parameters include the properties of the feedstock, the rate of heating, the specific geometry of the reactor, and the flow rates of gases and liquids within the system. Each of these factors can significantly influence the efficiency and effectiveness of the pyrolysis process, thereby presenting a considerable challenge in leveraging CFD to achieve an optimal reactor design [131]. The complexity arises from not only the sheer number of variables, but also their interdependencies and the impact they have on the reactor’s thermal and flow dynamics.
6. Potential Areas of Research and Development in the Future of CFD in Pyrolysis
- The design of the reactor is one of major outputs from the modeling, and it can be used to enhance the process efficiency and desired product yield.
- The operating conditions such as temperature, pressure, and the feedstock and susceptor characteristics can be investigated at multiple probabilities so as to optimize the product yield.
- It simplifies the design of scaled-up experiments for biomass pyrolysis from lab to pilot, as well as from the industrial scale transitions by the mitigation of energy consumption.
- Computational experiments such as multi-stage processes with parametric and sensitivity analysis implementation is easily conducted by the application of CFD modeling.
- Emission prediction and separation of the exhaust gases from the products to improve the product quality and to mitigate carbon emissions.
- The development of accurate and validated models: CFD models for pyrolysis need to be validated against experimental data to ensure their accuracy and reliability. Future research could focus on developing more accurate and validated models for the purpose of predicting the behavior of pyrolysis reactors.
- Multi-scale modeling: Pyrolysis is a multi-scale phenomenon that occurs at different spatial and temporal scales. Future research could focus on developing multi-scale CFD models that can capture the behavior of pyrolysis at different scales.
- Integration of CFD with other techniques: CFD can be combined with other techniques such as experimental measurements, optimization algorithms, and machine learning to improve the accuracy and efficiency of pyrolysis simulations.
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Yang, Q.; Ke, L.; Peng, Y.; Liu, Y.; Wu, Q.; Tian, X.; Dai, L.; Ruan, R.; Jiang, L. Review on the Catalytic Pyrolysis of Waste Oil for the Production of Renewable Hydrocarbon Fuels. Fuel 2021, 283, 119170. [Google Scholar] [CrossRef]
- Raheem, A.; Wan Azlina, W.A.K.G.; Taufiq Yap, Y.H.; Danquah, M.K.; Harun, R. Thermochemical Conversion of Microalgal Biomass for Biofuel Production. Renew. Sustain. Energy Rev. 2015, 49, 990–999. [Google Scholar] [CrossRef]
- Phanisankar, B.S.S.; Vasudeva Rao, N.; Manikanta, J.E. Conversion of Waste Plastic to Fuel Products. Mater. Today Proc. 2020, 33, 5190–5195. [Google Scholar] [CrossRef]
- Morgan, H.M.; Bu, Q.; Liang, J.; Liu, Y.; Mao, H.; Shi, A.; Lei, H.; Ruan, R. A Review of Catalytic Microwave Pyrolysis of Lignocellulosic Biomass for Value-Added Fuel and Chemicals. Bioresour. Technol. 2017, 230, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.J.; Andrew Lin, K.Y.; Hong, E.; Kwon, E.E.; Lee, J. The Valorization of Food Waste via Pyrolysis. J. Clean. Prod. 2020, 259, 120816. [Google Scholar] [CrossRef]
- Bahcivanji, L.; Gasc’o, G.; Paz-Ferreiro, J.; M’endez, A. The Effect of Post-Pyrolysis Treatment on Waste Biomass Derived Hydrochar. Waste Manag. 2020, 106, 55–61. [Google Scholar] [CrossRef]
- Yi, L.; Liu, H.; Li, S.; Li, M.; Wang, G.; Man, G.; Yao, H. Catalytic Pyrolysis of Biomass Wastes over Org-CaO/Nano-ZSM-5 to Produce Aromatics: Influence of Catalyst Properties. Bioresour. Technol. 2019, 294, 122186. [Google Scholar] [CrossRef]
- Singh, E.; Kumar, A.; Mishra, R.; You, S.; Singh, L.; Kumar, S.; Kumar, R. Pyrolysis of Waste Biomass and Plastics for Production of Biochar and Its Use for Removal of Heavy Metals from Aqueous Solution. Bioresour. Technol. 2021, 320, 124278. [Google Scholar] [CrossRef]
- Ephraim, A.; Pham Minh, D.; Lebonnois, D.; Peregrina, C.; Sharrock, P.; Nzihou, A. Co-pyrolysis of wood and plastics: Influence of plastic type and content on product yield, gas composition and quality. Fuel 2018, 231, 110–117. [Google Scholar] [CrossRef]
- Oyedun, A.O.; Gebreegziabher, T.; Ng, D.K.S.; Hui, C.W. Mixed-waste pyrolysis of biomass and plastics waste—A modelling approach to reduce energy usage. Energy 2014, 75, 127–135. [Google Scholar] [CrossRef]
- Hameed, S.; Sharma, A.; Pareek, V.; Wu, H.; Yu, Y. A Review on Biomass Pyrolysis Models: Kinetic, Network and Mechanistic Models. Biomass Bioenergy 2019, 123, 104–122. [Google Scholar] [CrossRef]
- Sharma, A.; Pareek, V.; Zhang, D. Biomass pyrolysis—A review of modelling, process parameters and catalytic studies. Renew. Sustain. Energy Rev. 2015, 50, 1081–1096. [Google Scholar] [CrossRef]
- Wang, G.; Dai, Y.; Yang, H.; Xiong, Q.; Wang, K.; Zhou, J.; Li, Y.; Wang, S. A Review of Recent Advances in Biomass Pyrolysis. Energy Fuels 2020, 34, 15557–15578. [Google Scholar] [CrossRef]
- Singh, R.I.; Brink, A.; Hupa, M. CFD modeling to study fluidized bed combustion and gasification. Appl. Therm. Eng. 2013, 52, 585–614. [Google Scholar] [CrossRef]
- Quiroz-P’erez, E.; Guti’errez-Antonio, C.; V’azquez-Rom’an, R. Modelling of production processes for liquid biofuels through CFD: A review of conventional and intensified technologies. Chem. Eng. Process.-Process Intensif. 2019, 143, 107629. [Google Scholar] [CrossRef]
- Wen, T.; Lu, L.; He, W.; Min, Y. Fundamentals and applications of CFD technology on analyzing falling film heat and mass exchangers: A comprehensive review. Appl. Energy 2020, 261, 114473. [Google Scholar] [CrossRef]
- Yadav, A.S.; Bhagoria, J.L. Heat transfer and fluid flow analysis of solar air heater: A review of CFD approach. Renew. Sustain. Energy Rev. 2013, 23, 60–79. [Google Scholar] [CrossRef]
- Zhong, H.; Xiong, Q.; Zhu, Y.; Liang, S.; Zhang, J.; Niu, B.; Zhang, X. CFD modeling of the effects of particle shrinkage and intra-particle heat conduction on biomass fast pyrolysis. Renew. Energy 2019, 141, 236–245. [Google Scholar] [CrossRef]
- Luo, H.; Wang, X.; Liu, X.; Wu, X.; Shi, X.; Xiong, Q. A review on CFD simulation of biomass pyrolysis in fluidized bed reactors with emphasis on particle-scale models. J. Anal. Appl. Pyrolysis 2022, 162, 105433. [Google Scholar] [CrossRef]
- Xiong, Q.; Choi, H.S.; Lan, X.; Wang, S. Recent advances in multiscale CFD simulation of pyrolysis. J. Anal. Appl. Pyrolysis 2022, 167, 105661. [Google Scholar] [CrossRef]
- Lu, L.; Liu, Z.; Xu, Z.; Lu, H. MFiX based multi-scale CFD simulations of biomass fast pyrolysis: A review. Chem. Eng. Sci. 2022, 248, 117131. [Google Scholar] [CrossRef]
- Ding, K.; Xiong, Q.; Zhong, Z.; Zhong, D.; Zhang, Y. CFD simulation of combustible solid waste pyrolysis in a fluidized bed reactor. Powder Technol. 2020, 362, 177–187. [Google Scholar] [CrossRef]
- Li, X.; Yin, C.; Kær, S.K.; Condra, T. A detailed pyrolysis model for a thermally large biomass particle. Fuel 2020, 278, 118397. [Google Scholar] [CrossRef]
- Rahbari, A.; Ebrahiminasab, F.; Bidabadi, M. Analytical model for predicting the heat loss effect on the pyrolysis of biomass particles. Chin. J. Chem. Eng. 2013, 21, 1114–1120. [Google Scholar] [CrossRef]
- Lu, L.; Brennan Pecha, M.; Wiggins, G.M.; Xu, Y.; Gao, X.; Hughes, B.; Shahnam, M.; Rogers, W.A.; Carpenter, D.; Parks, J.E. Multiscale CFD simulation of biomass fast pyrolysis with a machine learning derived intra-particle model and detailed pyrolysis kinetics. Chem. Eng. J. 2022, 431, 133853. [Google Scholar] [CrossRef]
- Hasse, C.; Debiagi, P.; Wen, X.; Hildebrandt, K.; Vascellari, M.; Faravelli, T. Advanced modeling approaches for CFD simulations of coal combustion and gasification. Prog. Energy Combust. Sci. 2021, 86, 100938. [Google Scholar] [CrossRef]
- Jayathilake, R.; Rudra, S. Numerical and experimental investigation of equivalence ratio (ER) and feedstock particle size on birchwood gasification. Energies 2017, 10, 1232. [Google Scholar] [CrossRef]
- Ortner, B.; Schmidberger, C.; Gerhardter, H.; Prieler, R.; Schröttner, H.; Hochenauer, C. Computationally inexpensive CFD approach for the combustion of sewage sludge powder, including the consideration of water content and limestone additive variations. Energies 2023, 16, 1798. [Google Scholar] [CrossRef]
- Mendecka, B.; Di Ilio, G.; Lombardi, L. Thermo-fluid dynamic and kinetic modeling of hydrothermal carbonization of olive pomace in a batch reactor. Energies 2020, 13, 4142. [Google Scholar] [CrossRef]
- Radmanesh, R.; Courbariaux, Y.; Chaouki, J.; Guy, C. A unified lumped approach in kinetic modeling of biomass pyrolysis. Fuel 2006, 85, 1211–1220. [Google Scholar] [CrossRef]
- Liu, Q.K.; Zhu, D.Q.; Tan, X.C.; Yuan, P.Q.; Cheng, Z.M.; Yuan, W.K.; Yang, J.Y. Lumped reaction kinetic models for pyrolysis of heavy oil in the presence of supercritical water. AIChE J. 2016, 62, 207–216. [Google Scholar] [CrossRef]
- Vikram, S.; Rosha, P.; Kumar, S. Recent modeling approaches to biomass pyrolysis: A review. Energy Fuels 2021, 35, 7406–7433. [Google Scholar] [CrossRef]
- Eser, S.; Uner, D. Application of computational fluid dynamics in chemical reaction engineering. Chem. Eng. Sci. 2007, 62, 5337–5354. [Google Scholar] [CrossRef]
- Salmi, T.; Warna, J.; Mikkola, J.P.; Murzin, D.Y. Kinetic modeling of catalytic reactions: A review. Chem. Eng. Sci. 2004, 59, 4723–4748. [Google Scholar] [CrossRef]
- Van Geem, K.M.; Reyniers, M.F.; Marin, G.B.; Froment, G.F. Kinetic modeling of chemical reactions in the presence of transport limitations. Chem. Eng. Sci. 2004, 59, 4903–4915. [Google Scholar] [CrossRef]
- Yang, Y.; El-Naas, M.H. Chemical kinetics modeling and computational fluid dynamics (CFD) simulation of wastewater treatment processes: A review. Chem. Eng. J. 2018, 344, 573–594. [Google Scholar] [CrossRef]
- Lu, J.; Dong, L.; Li, Y.; Sun, S.; Zhang, Y.; Li, W. A comprehensive review on biomass fast pyrolysis modeling. Renew. Sustain. Energy Rev. 2021, 137, 110623. [Google Scholar] [CrossRef]
- Xue, Y.; Yao, X.; Liu, Y.; Fang, M. Progress in biomass fast pyrolysis modeling and computations. J. Anal. Appl. Pyrolysis 2018, 131, 198–223. [Google Scholar] [CrossRef]
- Kulkarni, S.; Raman, S.; Gupta, A. A critical review of detailed kinetic models of biomass pyrolysis. Energy Fuels 2020, 34, 13139–13161. [Google Scholar]
- Zhang, H.; Wang, J. Numerical study of gas-solid flow and heat transfer in a multi-scale fluidized bed for biomass fast pyrolysis. Chem. Eng. J. 2020, 381, 122719. [Google Scholar] [CrossRef]
- Gómez, M.Á.; Martín, R.; Collazo, J.; Porteiro, J. CFD steady model applied to a biomass boiler operating in air enrichment conditions. Energies 2018, 11, 2513. [Google Scholar] [CrossRef]
- Chen, T.; Ku, X.; Lin, J.; Strom, H. CFD-DEM simulation of biomass pyrolysis in fluidized-bed reactor with a multistep kinetic scheme. Energies 2020, 13, 5358. [Google Scholar] [CrossRef]
- Luo, J.; Chen, J.; Yi, L. CFD-DEM Simulation of Particle Fluidization Behavior and Glycerol Gasification in a Supercritical Water Fluidized Bed. Energies 2022, 15, 7128. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, L.; Qiu, M.; Chen, M.; Liu, R.; Liu, M. CFD-DEM simulation of spouted bed dynamics under high temperature with an adhesive model. Energies 2021, 14, 2276. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.; Criado, J.; Pérez-Maqueda, L.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.; Favergeon, L.; Koga, N.; Moukhina, E.; Pérez-Maqueda, L.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochim. Acta 2020, 689, 178597. [Google Scholar] [CrossRef]
- Koga, N.; Vyazovkin, S.; Burnham, A.; Favergeon, L.; Muravyev, N.; Perez-Maqueda, L.; Saggese, C.; Sánchez-Jiménez, P. ICTAC Kinetics Committee recommendations for analysis of thermal decomposition kinetics. Thermochim. Acta 2022, 719, 179384. [Google Scholar] [CrossRef]
- Brown, M.; Maciejewski, M.; Vyazovkin, S.; Nomen, R.; Sempere, J.; Burnham, A.; Opfermann, J.; Strey, R.; Anderson, H.; Kemmler, A.; et al. Computational aspects of kinetic analysis: Part A: The ICTAC kinetics project-data, methods and results. Thermochim. Acta 2000, 355, 125–143. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling chemical and physical processes of wood and biomass pyrolysis. Prog. Energy Combust. Sci. 2008, 34, 47–90. [Google Scholar] [CrossRef]
- Bradbury, A.; Sakai, Y.; Shafizadeh, F. A kinetic model for pyrolysis of cellulose. J. Appl. Polym. Sci. 1979, 23, 3271–3280. [Google Scholar] [CrossRef]
- Jiang, G.; Wei, L. Analysis of pyrolysis kinetic model for processing of thermogravimetric analysis data. Phase Chang. Mater. Their Appl. 2018. [Google Scholar]
- Varhegyi, G.; Antal, M., Jr.; Jakab, E.; Szabó, P. Kinetic modeling of biomass pyrolysis. J. Anal. Appl. Pyrolysis 1997, 42, 73–87. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhang, J.; Xu, F.; Wiggins, G.; Daw, C. Coupling DAEM and CFD for simulating biomass fast pyrolysis in fluidized beds. J. Anal. Appl. Pyrolysis 2016, 117, 176–181. [Google Scholar] [CrossRef]
- Lamarche, P.; Tazerout, M.; Gelix, F.; Köhler, S.; Mati, K.; Paviet, F. Modelling of an indirectly heated fixed bed pyrolysis reactor of wood: Transition from batch to continuous staged gasification. Fuel 2013, 106, 118–128. [Google Scholar] [CrossRef]
- Ratte, J.; Fardet, E.; Mateos, D.; Héry, J. Mathematical modelling of a continuous biomass torrefaction reactor: Torspydtm column. Biomass Bioenergy 2011, 35, 3481–3495. [Google Scholar] [CrossRef]
- Liang, X. Numerical modeling of combustion and pyrolysis of cellulosic biomass in thermogravimetric systems. Fuel 2000, 79, 1477–1486. [Google Scholar] [CrossRef]
- Shafizadeh, F.; Chin, P.P.S. Thermal deterioration of wood. ACS Symp. Ser. 1977, 43, 57–81. [Google Scholar] [CrossRef]
- Han, J.; Kim, H. The reduction and control technology of tar during biomass gasification/pyrolysis: An overview. Renew. Sustain. Energy Rev. 2008, 12, 397–416. [Google Scholar] [CrossRef]
- Ghabi, C.; Benticha, H.; Sassi, M. Two-dimensional computational modeling and simulation of wood particles pyrolysis in a fixed bed reactor. Combust. Sci. Technol. 2008, 180, 833–853. [Google Scholar] [CrossRef]
- Romagnosi, L.; Gascoin, N.; El-Tabach, E.; Fedioun, I.; Bouchez, M.; Steelant, J. Pyrolysis in porous media: Part 1. Numerical model and parametric study. Energy Convers. Manag. 2013, 68, 63–73. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.; Liang, D. In-depth investigation of biomass pyrolysis based on three major components: Hemicellulose, cellulose and lignin. Energy Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Miller, R.; Bellan, J. A generalized biomass pyrolysis model based on superimposed cellulose, hemicelluloseand liqnin kinetics. Combust. Sci. Technol. 2010, 126, 97–137. [Google Scholar] [CrossRef]
- Kaczor, Z.; Buliński, Z.; Werle, S. Modelling approaches to waste biomass pyrolysis: A review. Renew. Energy 2020, 159, 427–443. [Google Scholar] [CrossRef]
- Lee, W. Pressure iteration scheme for two-phase flow modeling. In Multiphase Transport: Fundamentals, Reactor Safety, Applications; World Scientific: Singapore, 1980; pp. 407–432. [Google Scholar]
- Hobbs, M.; Radulovic, P.; Smoot, D. Combustion and gasification of coals in fixed-beds. Prog. Energy Combust. Sci. 1993, 19, 505–586. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling wood gasification in a countercurrent fixed-bed reactor. AIChE J. 2004, 50, 2306–2319. [Google Scholar] [CrossRef]
- Wickramaarachchi, W.; Narayana, M. Pyrolysis of single biomass particle using three-dimensional Computational Fluid Dynamics modelling. Renew. Energy 2020, 146, 1153–1165. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; Santoro, A.; Hernandez, E. Pyrolytic behavior and products of some wood varieties. Combust. Flame 2001, 124, 165–177. [Google Scholar] [CrossRef]
- Wijayanti, W.; Sasongko, M.; Kusumastuti, R. Modelling analysis of pyrolysis process with thermal effects by using Comsol Multiphysics. Case Stud. Therm. Eng. 2021, 28, 101625. [Google Scholar] [CrossRef]
- Grønli, M. A Theoretical and Experimental Study of the Thermal Degradation of Biomass. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 1996. [Google Scholar]
- Soria, J.; Zeng, K.; Asensio, D.; Gauthier, D.; Flamant, G.; Mazza, G. Comprehensive CFD modelling of solar fast pyrolysis of beech wood pellets. Fuel Process. Technol. 2017, 158, 226–237. [Google Scholar] [CrossRef]
- Chan, W.; Kelbon, M.; Krieger, B. Modelling and experimental verification of physical and chemical processes during pyrolysis of a large biomass particle. Fuel 1985, 64, 1505–1513. [Google Scholar] [CrossRef]
- Panton, R.; Rittmann, J. Pyrolysis of a Slab of Porous Material. In Proceedings of the Thirteenth International Symposium on Combustion, Salt Lake City, UT, USA, 23–29 August 1971; pp. 881–891. [Google Scholar]
- Thomson, R.; Kwong, P.; Ahmad, E.; Nigam, K. Clean syngas from small commercial biomass gasifiers; a review of gasifier development, recent advances and performance evaluation. Int. J. Hydrog. Energy 2020, 45, 21087–21111. [Google Scholar] [CrossRef]
- Tanoue, K.I.; Wijayanti, W.; Yamasaki, K.; Kawanaka, T.; Yoshida, A.; Nishimura, T.; Taniguchi, M.; Sasauchi, K.I. Numerical Simulation of the thermal conduction of packed bed of woody biomass particles accompanying volume reduction induced by pyrolysis. J. Jpn. Inst. Energy 2010, 89, 948–954. [Google Scholar] [CrossRef]
- Ruiza, S.; Gordilloa, G.; Mohamad, A. Numerical simulation of a simplified, transient, 2D, non-reactive heat transfer model of a lab-scale fixed-bed pyrolysis reactor. Appl. Therm. Eng. 2019, 150, 545–551. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Hoffmann, F.; Peters, B. Detailed numerical modeling of pyrolysis in a heterogeneous packedbed using XDEM. J. Anal. Appl. Pyrolysis 2014, 106, 9–20. [Google Scholar] [CrossRef]
- Borello, D.; Meloni, R.; Venturini, P.; De Filippis, P.; de Caprariis, B.; Di Carlo, A.; Frangioni, G. A 3D packed bed model for biomass pyrolysis: Mathematical formulation and experimental validation. Energy Procedia 2014, 61, 958–961. [Google Scholar] [CrossRef][Green Version]
- Feng, Y.; Zhang, Z.; Gao, J.; Feng, G.; Qiu, L. Research status of centrifugal granulation, physical heat recovery and resource utilization of blast furnace slags. J. Anal. Appl. Pyrolysis 2021, 157, 105220. [Google Scholar] [CrossRef]
- Maqsood, T.; Dai, J.; Zhang, Y.; Guang, M.; Li, B. Pyrolysis of plastic species: A review of resources and products. J. Anal. Appl. Pyrolysis 2021, 159, 105295. [Google Scholar] [CrossRef]
- Muelas, A.; Aranda, D.; Call’en, M.S.; Murillo, R.; Veses, A.; Asrardel, M.; Ballester, J. Properties and combustion characteristics of bio-oils from catalytic co-pyrolysis of grape seeds, polystyrene, and waste tires. Energy Fuels 2020, 34, 14190–14203. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Du, Y.; Liu, H.; Yang, C. Numerical investigations of the oxidative dehydrogenation of propane in a spouted bed reactor. Energy Fuels 2020, 34, 10858–10871. [Google Scholar] [CrossRef]
- Luo, H.; Lin, W.; Dam-Johansen, K.; Wu, H. Heat-Transfer-Corrected Isothermal Model for Devolatilization of Thermally Thick Biomass Particles. Energy Fuels 2020, 34, 9620–9631. [Google Scholar] [CrossRef]
- Yang, S.; Wang, H.; Wei, Y.; Hu, J.; Chew, J.W. Numerical investigation of bubble dynamics during biomass gasification in a bubbling fluidized bed. ACS Sustain. Chem. Eng. 2019, 7, 12288–12303. [Google Scholar] [CrossRef]
- Pecha, M.B.; Ramirez, E.; Wiggins, G.M.; Carpenter, D.; Kappes, B.; Daw, S.; Ciesielski, P.N. Integrated particle- and reactor-scale simulation of pine pyrolysis in a fluidized bed. Energy Fuels 2018, 32, 10683–10694. [Google Scholar] [CrossRef]
- Dong, Z.; Yang, Y.; Cai, W.; He, Y.; Chai, M.; Liu, B.; Yu, X.; Banks, S.W.; Zhang, X.; Bridgwater, A.V. Theoretical analysis of double logistic distributed activation energy model for thermal decomposition kinetics of solid fuels. Ind. Eng. Chem. Res. 2018, 57, 7817–7825. [Google Scholar] [CrossRef]
- Kumar, R.; Sarkar, A.; Halder, P. Solar pyrolysis of sawdust for bio-oil production: An experimental study. Renew. Energy 2019, 143, 431–437. [Google Scholar]
- Mujumdar, A.S.; Patil, P.; Gokhale, S. Solar pyrolysis of biomass for production of biochar. Renew. Energy 2019, 132, 865–872. [Google Scholar]
- Zhao, Y.; Feng, M.; Wu, M.; Wang, Z. Solar pyrolysis of corn stover for biochar production. Energy Convers. Manag. 2021, 227, 113724. [Google Scholar]
- Van de Velden, M.; Baeyens, J.; Brems, A.; Janssens, B.; Dewil, R. Fundamentals, kinetics and endothermicity of the biomass pyrolysis reaction. Renew. Energy 2010, 35, 232–242. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K. A review on the pyrolysis of woody biomass to bio-oil: Focus on kinetic models. Renew. Sustain. Energy Rev. 2015, 52, 1580–1595. [Google Scholar] [CrossRef]
- Xiong, Q.; Kong, S.; Passalacqua, A. Development of a generalized numerical framework for simulating biomass fast pyrolysis in fluidized-bed reactors. Chem. Eng. Sci. 2013, 99, 305–313. [Google Scholar] [CrossRef]
- Xue, Q.; Heindel, T.; Fox, R. A CFD model for biomass fast pyrolysis in fluidized-bed reactors. Chem. Eng. Sci. 2011, 66, 2440–2452. [Google Scholar] [CrossRef]
- Mellin, P.; Kantarelis, E.; Yang, W. Computational fluid dynamics modeling of biomass fast pyrolysis in a fluidized bed reactor, using a comprehensive chemistry scheme. Fuel 2014, 117, 704–715. [Google Scholar] [CrossRef]
- Lewis, A.D.; Fletcher, T.H. Prediction of sawdust pyrolysis yields from a flat-flame burner using the CPD model. Energy Fuels 2013, 27, 942–953. [Google Scholar] [CrossRef]
- Fletcher, T.H.; Pond, H.R.; Webster, J.; Wooters, J.; Baxter, L.L. Prediction of tar and light gas during pyrolysis of black liquor and biomass. Energy Fuels 2012, 26, 3381–3387. [Google Scholar] [CrossRef]
- Upadhyay, M.; Park, H.; Choi, H. Multiphase fluid dynamics coupled fast pyrolysis of biomass in a rectangular bubbling fluidized bed reactor: Process intensification. Chem. Eng. Process. Process. Intensif. 2018, 128, 180–187. [Google Scholar] [CrossRef]
- Park, H.; Choi, H. Fast pyrolysis of biomass in a spouted bed reactor: Hydrodynamics, heat transfer and chemical reaction. Renew. Energy 2019, 143, 1268–1284. [Google Scholar] [CrossRef]
- Pandey, B.; Prajapati, Y.; Sheth, P. CFD analysis of biomass gasification using downdraft gasifier. Mater. Today Proc. 2020, 44, 4107–4111. [Google Scholar] [CrossRef]
- Ranganathan, P.; Gu, S. Computational fluid dynamics modelling of biomass fast pyrolysis in fluidised bed reactors, focusing different kinetic schemes. Bioresour. Technol. 2016, 213, 333–341. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, J.; Zhu, Y.; Liang, S. Multi-fluid modeling biomass fast pryolysis with particle shrinkage model for complex reaction kinetics. Chem. Eng. Process. Process. Intensif. 2018, 128, 36–45. [Google Scholar] [CrossRef]
- Hooshdaran, B.; Haghshenasfard, M.; Hosseini, S.H.; Esfahany, M.N.; Lopez, G.; Olazar, M. CFD modeling and experimental validation of biomass fast pyrolysis in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis 2021, 154, 105011. [Google Scholar] [CrossRef]
- Wang, W.; Chen, G. Heat and Mass Transfer Model of Dielectric-Material-Assisted Microwave Freeze-Drying of Skim Milk with Hygroscopic Effect. Chem. Eng. Sci. 2005, 60, 6542–6550. [Google Scholar] [CrossRef]
- Rattanadecho, P. The simulation of microwave heating of wood using a rectangular wave guide: Influence of frequency and sample size. Chem. Eng. Sci. 2006, 61, 4798–4811. [Google Scholar] [CrossRef]
- Romano, V.; Marra, F.; Tammaro, U. Modelling of microwave heating of foodstuff: Study on the influence of sample dimensions with a FEM approach. J. Food Eng. 2005, 71, 233–241. [Google Scholar] [CrossRef]
- Geedipalli, S.; Rakesh, V.; Datta, A. Modeling the heating uniformity contributed by a rotating turntable in microwave ovens. J. Food Eng. 2007, 82, 359–368. [Google Scholar] [CrossRef]
- Chen, W.H.; Cheng, T.C.; Hung, C.I. Modeling and simulation of microwave double absorption on methanol steam reforming for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 333–344. [Google Scholar] [CrossRef]
- Clemens, J.; Saltiel, C. Numerical modeling of materials processing in microwave furnaces. Int. J. Heat Mass Transf. 1996, 39, 1665–1675. [Google Scholar] [CrossRef]
- Ciacci, T.; Galgano, A.; Di Blasi, C. Numerical simulation of the electromagnetic field and the heat and mass transfer processes during microwave-induced pyrolysis of a wood block. Chem. Eng. Sci. 2010, 65, 4117–4133. [Google Scholar] [CrossRef]
- Khraisheh, M.; Cooper, T.; Magee, T. Microwave and air drying I. Fundamental considerations and assumptions for the simplified thermal calculations of volumetric power absorption. J. Food Eng. 1997, 33, 207–219. [Google Scholar] [CrossRef]
- Sanga, E.C.; Mujumdar, A.S.; Raghavan, G. Simulation of Convection-Microwave Drying for a Shrinking Material. Chem. Eng. Process. Process. Intensif. 2002, 41, 487–499. [Google Scholar] [CrossRef]
- Malafronte, L.; Lamberti, G.; Barba, A.A.; Raaholt, B.; Holtz, E.; Ahrné, L. Combined convective and microwave assisted drying: Experiments and modeling. J. Food Eng. 2012, 112, 304–312. [Google Scholar] [CrossRef]
- Yang, S.; Dong, R.; Du, Y.; Wang, S.; Wang, H. Numerical study of the biomass pyrolysis process in a spouted bed reactor through computational fluid dynamics. Energy 2021, 214, 118839. [Google Scholar] [CrossRef]
- Romkes, S.; Dautzenberg, F.; van den Bleek, C.; Calis, H. CFD modelling and experimental validation of particle-to-fluid mass and heat transfer in a packed bed at very low channel to particle diameter ratio. Chem. Eng. J. 2003, 96, 3–13. [Google Scholar] [CrossRef]
- Guardo, A.; Coussirat, M.; Recasens, F.; Larrayoz, M.; Escaler, X. CFD study on particle-to-fluid heat transfer in fixed bed reactors: Convective heat transfer at low and high pressure. Chem. Eng. Sci. 2006, 61, 4341–4353. [Google Scholar] [CrossRef]
- Gao, X.; Yu, J.; Lu, L.; Rogers, W. Coupling particle scale model and superdem-CFD for multiscale simulation of biomass pyrolysis in a packed bed pyrolyzer. AIChE J. 2021, 67, e17139. [Google Scholar] [CrossRef]
- Choudhury, P. Dielectric materials and applications. J. Electrochem. Soc. 2019, 102, 3. [Google Scholar] [CrossRef]
- Motasemi, F.; Gerber, A. Multicomponent conjugate heat and mass transfer in biomass materials during microwave pyrolysis for biofuel production. Fuel 2018, 211, 649–660. [Google Scholar] [CrossRef]
- Pecate, S.; Kessas, S.; Morin, M.; Hemati, M. Beech wood gasification in a dense and fast internally circulating fluidized bed. Fuel 2019, 236, 554–573. [Google Scholar] [CrossRef]
- Parvez, A.; Wu, T.; Afzal, M.; Mareta, S.; He, T.; Zhai, M. Conventional and microwave-assisted pyrolysis of gumwood: A comparison study using thermodynamic evaluation and hydrogen production. Fuel Process. Technol. 2019, 184, 1–11. [Google Scholar] [CrossRef]
- White, F.M. Viscous Fluid Flow; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Crowe, C.; Sommerfeld, M.; Tsuji, Y. Multiphase Flows with Droplets and Particles; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- So, H.; Taube, A. Modelling and experimental investigation of microwave heating of adhesively bonded polypropylene joint. Int. J. Adhes. Adhes. 2004, 24, 307–312. [Google Scholar] [CrossRef]
- Bonnet, C.; Estel, L.; Ledoux, A.; Mazari, B.; Louis, A. Study of the thermal repartition in a microwave reactor: Application to the nitrobenzene hydrogenation. Chem. Eng. Process. Process. Intensif. 2004, 43, 1435–1440. [Google Scholar] [CrossRef]
- Chen, H.; Tang, J.; Liu, F. Simulation model for moving food packages in microwave heating processes using conformal FDTD method. J. Food Eng. 2008, 88, 294–305. [Google Scholar] [CrossRef]
- Modest, M.F. Radiative Heat Transfer; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Petrasch, J.; Coray, P.; Meier, A.; Brack, T.; Häberling, P.; Furrer, G.; Steinfeld, A. Numerical and experimental investigation of a PCM-based latent heat storage for solar parabolic trough power plants. Solar Energy 2007, 81, 968–976. [Google Scholar]
- Liu, H.; Cattolica, R.J.; Seiser, R.; Liao, C. Three-dimensional full-loop simulation of a dual fluidized-bed biomass gasifier. Appl. Energy 2015, 160, 489–501. [Google Scholar] [CrossRef]
- Pepiot, P.; Desjardins, O. Numerical analysis of the dynamics of two-and three-dimensional fluidized bed reactors using an Euler–Lagrange approach. Powder Technol. 2012, 220, 104–121. [Google Scholar] [CrossRef]
- Bi, X.T.; Masnadi, M.S. Multiphase Reactors for Biomass Processing and Thermochemical Conversions. In Multiphase Reactor Engineering for Clean and Low-Carbon Energy Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 331–376. [Google Scholar]

| Models | Advantages | Disadvantages |
|---|---|---|
| Discrete Particle Model | It is preferred for granular or particulate systems, as well as for granular flow. It is suitable for investigating individual particle interactions. It finds application in blending and inter particle interactions | It is not suitable for large-scale or a large number of particle systems, and it has limitations in chemical reaction systems and modeling fluid flow |
| Multi-scale Model | It is suitable for the investigation of hierarchical and complex systems that are to be captured with significant information at the molecular, meso, and macro scales | Implementation is computationally expensive and complicated since the comprehensive understanding of the multiple scales and models are necessary |
| Detailed Chemistry Models | It is suitable for the investigation of complex chemical reaction systems, such as pyrolysis and combustion | A large number of chemical species involved in the systems are computationally expensive. Also, it is not suitable for systems where chemical kinetics are less dominant |
| System | Model Type | Mass Conservation | Momentum Conservation | Heat Conservation | Kinetics | Remarks | References | |||
|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |||||
| Biomass bubbling fluidized bed | Multi-phase particle in cell framework | A | NA | A | A | A | A | Semi global kinetics | Model successfully captured the product yield at various operating conditions, and it found that the velocity and temperature of reaction were the most impacted parameters | [97] |
| Spouted bed reactor | Multi-phase particle in cell framework | A | A | A | A | A | A | Semi global kinetics | CPFD model with MSB-TGA results were not accurate due to the insignificant secondary tar decomposition kinetics | [97] |
| Imbert downdraft gasifier | Species transport along with k- for turbulence and the discrete phase model for particles | A | NA | A | A | A | A | Finite-rate reaction kinetics | CFD model applied to obtain producer gas composition to optimize the operating conditions and design parameters | [98] |
| Fluidized bed reactor | Eulerian–Eulerian CFD model | A | A | A | A | A | A | Lumped, multi-component, multi-stage kinetic mode | Fast pyrolysis major parameters predicted by CFD model with defined cellulose, hemicellulose and lignin | [99] |
| Fluidized bed reactor | Eulerian multi-phase flow model | A | A | A | A | A | A | Simple, global, and advanced kinetic scheme | Hydrodynamics predicted the mixing behaviors, and the advanced kinetic scheme improved the prediction | [93] |
| Fluidized bed reactor | Eulerian–Eulerian multi-fluid CFD model | A | A | A | A | A | A | Competitive reaction kinetics | Implementation of the particle shrinkage effects during pyrolysis improved the model prediction | [100] |
| Spouted bed reactor | Eulerian–Eulerian multi-phase CFD model | A | A | A | A | A | A | Competitive single-component single-step kinetics | The addition of kinetic theory granular flow to the CFD model enables one to predict pyrolysis yields better | [101] |
| Spouted bed reactor | Eulerian–Lagrangian particles in cell model | A | A | A | A | A | A | Two-step kinetic mechanism | The physical and thermo- chemical properties of biomass pyrolysis in spouted reactors are explored | [102] |
| Solver | Application or System | Law | Dimension | Variable | Reference |
|---|---|---|---|---|---|
| Finite Difference | Microwave furnace | Maxwell | Two | Temperature and electric field | [108] |
| Finite Difference | Freeze drying | - | One | Temperature | [103] |
| Finite Difference | Microwave heating | Maxwell | Three | Temperature and electric field | [104] |
| Finite Difference | Microwave-assisted pyrolysis of wood block | Maxwell | Two | Temperature and electric field | [109] |
| Calorimetric Technique | Microwave drying | Lambert | Zero | Power | [110] |
| Finite Element | Microwave drying | Lambert | Three | Moisture and temperature | [111] |
| Finite Element | Microwave drying | Lambert and Maxwell | Three | Temperature and absorbed power | [105] |
| Finite Element | Microwave heating with stationary and rotating objects | Maxwell | Three | Temperature and absorbed power | [106] |
| Finite Element | Steam methanol reforming by microwave heating | Maxwell | Three | Temperature, conversion, and yield | [107] |
| Finite Element | Convective or microwave-assisted drying | Maxwell | Two | Temperature and moisture | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, A.; Mishra, G.; Palla, S.; Ramesh, P.; Surya, D.V.; Basak, T. Advances in Computational Fluid Dynamics Modeling for Biomass Pyrolysis: A Review. Energies 2023, 16, 7839. https://doi.org/10.3390/en16237839
Kulkarni A, Mishra G, Palla S, Ramesh P, Surya DV, Basak T. Advances in Computational Fluid Dynamics Modeling for Biomass Pyrolysis: A Review. Energies. 2023; 16(23):7839. https://doi.org/10.3390/en16237839
Chicago/Turabian StyleKulkarni, Anirudh, Garima Mishra, Sridhar Palla, Potnuri Ramesh, Dadi Venkata Surya, and Tanmay Basak. 2023. "Advances in Computational Fluid Dynamics Modeling for Biomass Pyrolysis: A Review" Energies 16, no. 23: 7839. https://doi.org/10.3390/en16237839
APA StyleKulkarni, A., Mishra, G., Palla, S., Ramesh, P., Surya, D. V., & Basak, T. (2023). Advances in Computational Fluid Dynamics Modeling for Biomass Pyrolysis: A Review. Energies, 16(23), 7839. https://doi.org/10.3390/en16237839







