Solar-Powered Water Electrolysis Using Hybrid Solid Oxide Electrolyzer Cell (SOEC) for Green Hydrogen—A Review
Abstract
:1. Introduction
2. Electrolysis
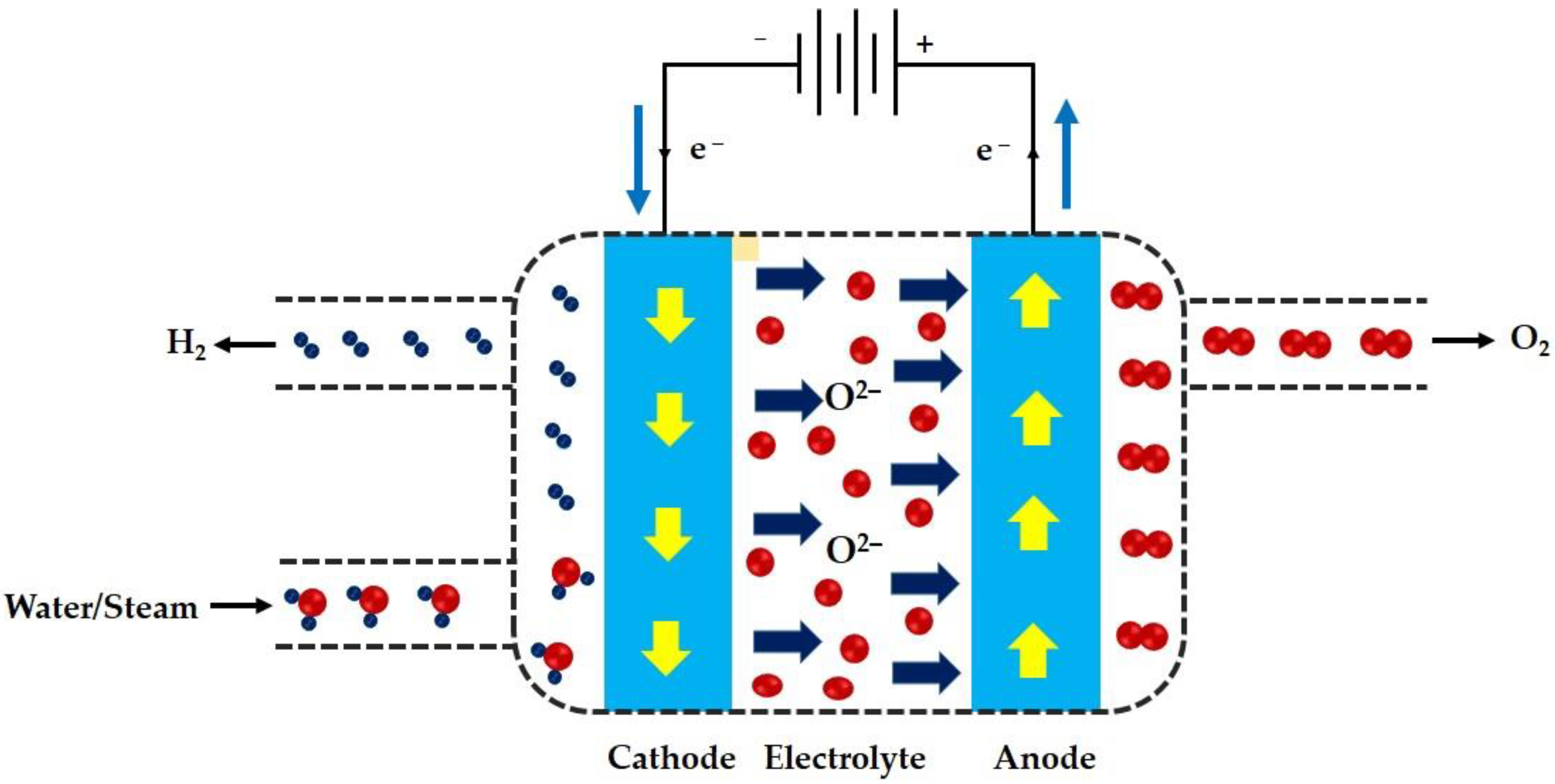
3. Solid Oxide Electrolysis
3.1. History of Solid Oxide Electrolysis
3.2. Solid Oxide Electrolysis Cells
4. Solar Hydrogen Generation System
4.1. State-of-the-Art of Electrolysis
- ➢
- To obtain high energy conversion efficiency, the dense electrolyte needs to be strongly ionic conductive, chemically stable, and have low electronic conductivity because electronic conduction reduces the ionic conductivity of the electrolyte and current efficiencies of the cell.
- ➢
- For the purpose of lowering the ohmic overpotential, the dense electrolyte should be as thin as feasible, but it must be gastight to completely rule out the possibility of H2 and O2 recombination.
- ➢
- Both electrodes must exhibit high electrical conductivity and chemical resistance under substantially reducing or oxidizing circumstances.
- ➢
- To permit gas movement between the electrode surface and the electrode–electrolyte interface and to establish a trustworthy electrolyte–electrode–gas triple-phase barrier (reaction sites), each electrode should have an appropriate amount of porosity and pore size.
- ➢
- To avoid the electrolyte failing owing to extremely high mechanical stress brought on by an imbalance between the two electrodes, the thermal expansion coefficient (TEC) of the two electrodes should be near to those of the electrolyte.
- ➢
- Materials are needed for interconnects in massive hydrogen production facilities. Since the connecting materials are simultaneously exposed to hydrogen/steam and oxygen, they must be chemically stable under reducing/oxidizing conditions.
- ➢
- The production procedure should be as inexpensive as feasible.
4.2. Solid Oxide Electrolysis Process
4.3. Solar-Powered Hybrid SOEC
4.4. Solar Plant Design
4.5. Hybrid SOEC
4.6. System Explanation
4.7. Hybrid Plant Scenarios
- Identify the most efficient location for steam extraction in the solar plant.
- Develop a method for utilizing the rejected hot streams from electrolysis to preheat the feed water for the CSP plant.
- Determine the optimal point for re-injecting the condensed steam into the Rankine cycle.
- Following that, a few concepts for the electrolysis process optimization were examined. These were concentrated on pressurizing the SOEC units to reduce parasitic losses brought on by the compression process [78].
4.7.1. Low-Pressure Circumstances
- Scenario 1: Low-pressure regulated steam was generated from the PS extraction received from the solar receiver. This steam can then be used to produce work through the high-pressure turbine stage. The aim is to reduce fines associated with CSP plants.
- Scenario 2: The low-pressure feedwater heater (LP-FWH) re-injected the PS return, which reduces the heat requirement and increases the volume of steam expanded in the final stage of the turbine. Identifying different situations is a new area of study.
- Scenario 3: As part of the strategy to decrease the need for LP-FWHs for heat and increase the capacity of our solar plant, we have installed a new FWH. This new FWH, called the heat recovery FWH (HR-FWH), was located between Pump 1 and the LP-FWH. It used rejected heat from the compressor inter-cooler system and exhaust sweep gas from the electrolysis process to preheat the feed water for the DSG-CRS plant.
- Scenario 4: To enhance the efficiency of the SOEC unit, it is suggested to circulate hydrogen at a high temperature after the HRS-1. This method helps to lower the power demand of the cathode electric heater while maintaining a high temperature after hydrogen recirculation to maximize the productivity of HRS-1.
- Scenario 5: In order to simplify the heat recovery system for the electrolyzer, the exhaust sweep gas stream was utilized to feed the economizer of the cathode loop instead of the exhaust cathode stream. This allows for a more centralized and compact heat recovery system for the preheating system of the solar plant by situating the condenser of the cathode loop near the compressor input.
4.7.2. High-Pressure Scenarios
4.7.3. Overall Hybrid Plant Performance
5. New Method for SOEC-Based Hydrogen Production
5.1. Solar Hydrogen Generation System Integrating PV/PETE and SOEC
5.2. Model and Performances
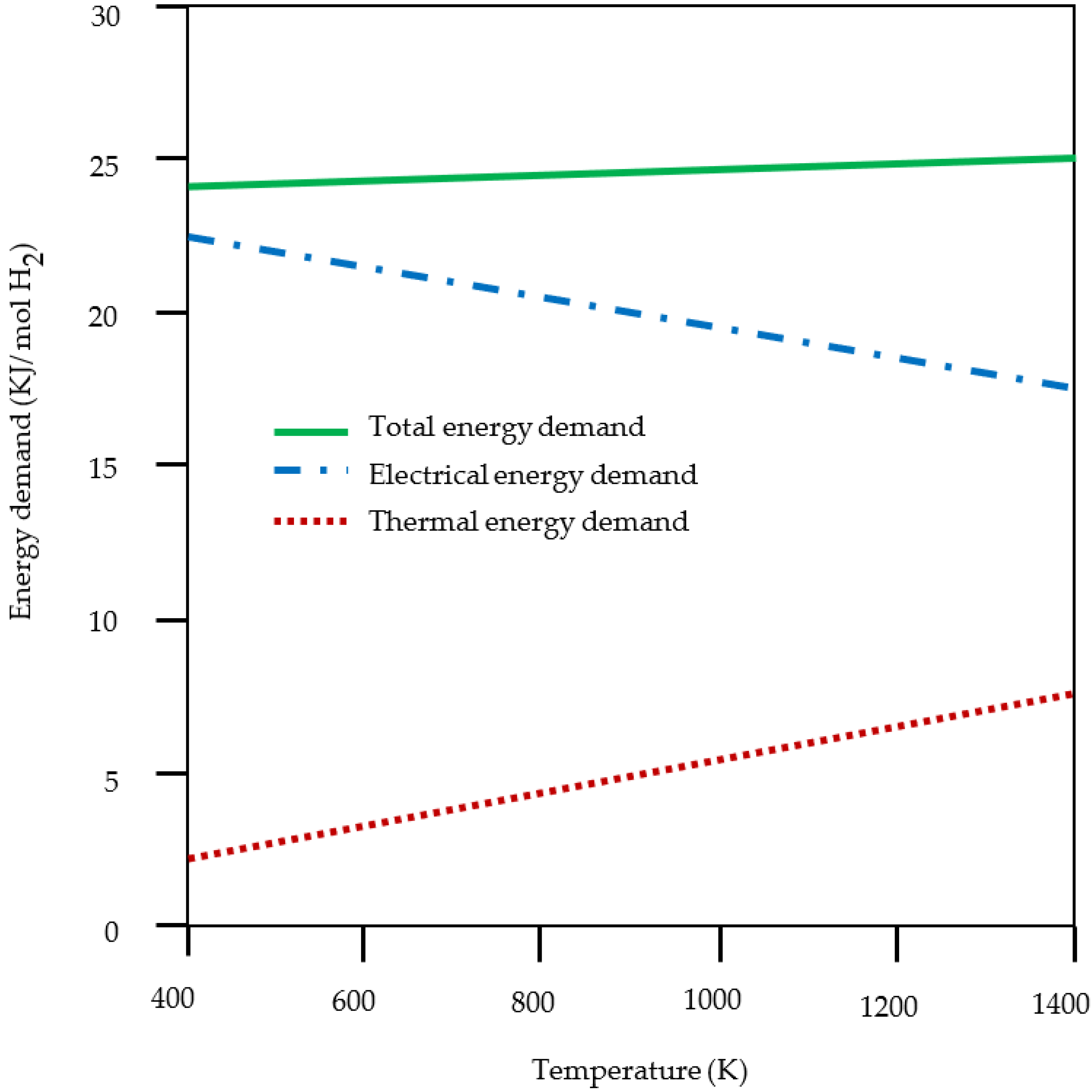
5.3. Efficiency of The System
6. Comparing and Contrasting Analysis Methods
7. Cost Evaluation for SOEC
8. Commercialization and Market Review
9. Conclusions and Recommendations
- Though hydrogen is an excellent fuel source that is clean and abundant, there are still a number of issues standing in the way of its mainstreaming despite the promising characteristics of SOECs; there is still a need to undertake further research into reducing degradation, and the successful designs for SOECs must be scaled up if they are to become an industry electrolyzer. Solid-state electrolyzers may pull energy from the heat they generate as they run at higher temperatures, but there is still an opportunity for advancement in SOECs. Oxygen SEOCs permit oxygen ions to pass through, and hydrogen SOECs only permit hydrogen ions to pass through. Nevertheless, the amount of hydrogen that can be produced was reduced in a single way. However, hybrid SOECs employ a mixed-ion conductor to simultaneously carry positively charged hydrogen ions (protons) and negatively charged oxygen ions.
- A viable method of storing solar energy and extracting hydrogen is the combination of solar photovoltaic (PV) cells with high-temperature electrolysis cells. The solar energy efficiency and solar-to-hydrogen efficiency (STH efficiency) might be as high as 77.05%, 55.99%, and 29.61%, respectively. These figures are anticipated to offer a theoretical foundation for the study and practical implementation of solar hydrogen generation. On the other hand, the atmospheric conditions that impact PV cell performance and STH production and storage are the challenges that stand in the way of solar hydrogen generation since solar energy fluctuates according to the season. However, there are fewer studies on the development of PV cells and PETE modules associated with the SOEC, and this deserves further research.
- The cost of producing hydrogen using SOECs now comes to about EUR 2000 per kilowatt-hour or USD 1933.02 per hour in USD. Further study is required to determine if the components in the SOEC will withstand long-term, high-temperature operation, even though it has better efficiency and low energy usage. The cost is still higher than other electrolysis, and further research is needed to reduce the overall system cost. Furthermore, to support the commercial use of hybrid SOECs, future research should concentrate on large-scale manufacturing technology and process simplification.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stern, N.; Valero, A. Innovation, Growth and the Transition to Net-Zero Emissions. Res. Policy 2021, 50, 104293. [Google Scholar] [CrossRef] [PubMed]
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R. A Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Baquero, J.E.G.; Monsalve, D.B. A Proposal for the Transformation of Fossil Fuel Energy Economies to Hydrogen Economies through Social Entrepreneurship. In Entrepreneurial Innovation for Securing Long-Term Growth in a Short-Term Economy; IGI Global: Hershey, PA, USA, 2021; pp. 48–70. [Google Scholar] [CrossRef]
- Reza, M.S.; Afroze, S.; Kuterbekov, K.; Kabyshev, A.; Bekmyrza, K.Z.; Haque, M.N.; Islam, S.N.; Hossain, M.A.; Hassan, M.; Roy, H.; et al. Advanced Applications of Carbonaceous Materials in Sustainable Water Treatment, Energy Storage, and CO2 Capture: A Comprehensive Review. Sustainability 2023, 15, 8815. [Google Scholar] [CrossRef]
- Reza, M.S.; Ahmad, N.B.H.; Afroze, S.; Taweekun, J.; Sharifpur, M.; Azad, A.K. Hydrogen Production from Water Splitting through Photocatalytic Activity of Carbon-Based Materials. Chem. Eng. Technol. 2023, 46, 420–434. [Google Scholar] [CrossRef]
- Wang, H.; Kong, H.; Pu, Z.; Li, Y.; Hu, X. Feasibility of High Efficient Solar Hydrogen Generation System Integrating Photovoltaic Cell/photon-Enhanced Thermionic Emission and High-Temperature Electrolysis Cell. Energy Convers. Manag. 2020, 210, 112699. [Google Scholar] [CrossRef]
- International Energy Agency. Net Zero by 2050: A Roadmap for the Global Energy Sector; IEA: Paris, France, 2021. [Google Scholar]
- Zaiter, I.; Ramadan, M.; Bouabid, A.; El-Fadel, M.; Mezher, T. Potential Utilization of Hydrogen in the UAE’s Industrial Sector. Energy 2023, 280, 128108. [Google Scholar] [CrossRef]
- Jayaprabakar, J.; Sri Hari, N.S.; Badreenath, M.; Anish, M.; Joy, N.; Prabhu, A.; Rajasimman, M.; Kumar, J.A. Nano Materials for Green Hydrogen Production: Technical Insights on Nano Material Selection, Properties, Production Routes and Commercial Applications. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Hassan, Q.; Abdulateef, A.M.; Hafedh, S.A.; Al-samari, A.; Abdulateef, J.; Sameen, A.Z.; Salman, H.M.; Al-Jiboory, A.K.; Wieteska, S.; Jaszczur, M. Renewable Energy-to-Green Hydrogen: A Review of Main Resources Routes, Processes and Evaluation. Int. J. Hydrogen Energy 2023, 48, 17383–17408. [Google Scholar] [CrossRef]
- AbouSeada, N.; Hatem, T.M. Climate Action: Prospects of Green Hydrogen in Africa. Energy Rep. 2022, 8, 3873–3890. [Google Scholar] [CrossRef]
- Yilanci, A.; Dincer, I.; Ozturk, H.K. A Review on Solar-Hydrogen/fuel Cell Hybrid Energy Systems for Stationary Applications. Prog. Energy Combust. Sci. 2009, 35, 231–244. [Google Scholar] [CrossRef]
- Espegren, K.; Damman, S.; Pisciella, P.; Graabak, I.; Tomasgard, A. The Role of Hydrogen in the Transition from a Petroleum Economy to a Low-Carbon Society. Int. J. Hydrogen Energy 2021, 46, 23125–23138. [Google Scholar] [CrossRef]
- Anika, O.C.; Nnabuife, S.G.; Bello, A.; Okoroafor, E.R.; Kuang, B.; Villa, R. Prospects of Low and Zero-Carbon Renewable Fuels in 1.5-Degree Net Zero Emission Actualisation by 2050: A Critical Review. Carbon Capture Sci. Technol. 2022, 5, 100072. [Google Scholar] [CrossRef]
- Hwang, H.T.; Varma, A. Hydrogen Storage for Fuel Cell Vehicles. Curr. Opin. Chem. Eng. 2014, 5, 42–48. [Google Scholar] [CrossRef]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H.-W. Hydrogen-A Sustainable Energy Carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Karimi, M.; Mehrpooya, M.; Pourfayaz, F. Proposal and Investigation of a Novel Hybrid Hydrogen Production and Liquefaction Process Using Solid Oxide Electrolyzer, Solar Energy, and Thermoelectric Generator. J. Clean. Prod. 2022, 331, 130001. [Google Scholar] [CrossRef]
- Hassan, Q.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. Large-Scale Green Hydrogen Production via Alkaline Water Electrolysis Using Solar and Wind Energy. Int. J. Hydrogen Energy 2023, 48, 34299–34315. [Google Scholar] [CrossRef]
- Tashie-Lewis, B.C.; Nnabuife, S.G. Hydrogen Production, Distribution, Storage and Power Conversion in a Hydrogen Economy-A Technology Review. Chem. Eng. J. Adv. 2021, 8, 100172. [Google Scholar] [CrossRef]
- Martínez de León, C.; Ríos, C.; Brey, J.J. Cost of Green Hydrogen: Limitations of Production from a Stand-Alone Photovoltaic System. Int. J. Hydrogen Energy 2023, 48, 11885–11898. [Google Scholar] [CrossRef]
- Pathak, S.K.; Tyagi, V.V.; Chopra, K.; Kalidasan, B.; Pandey, A.K.; Goel, V.; Saxena, A.; Ma, Z. Energy, Exergy, Economic and Environmental Analyses of Solar Air Heating Systems with and without Thermal Energy Storage for Sustainable Development: A Systematic Review. J. Energy Storage 2023, 59, 106521. [Google Scholar] [CrossRef]
- Li, X.; Pan, L.; Zhang, J. Development Status Evaluation and Path Analysis of Regional Clean Energy Power Generation in China. Energy Strateg. Rev. 2023, 49, 101139. [Google Scholar] [CrossRef]
- Sharma, S.; Jain, K.K.; Sharma, A. Solar Cells: In Research and Applications—A Review. Mater. Sci. Appl. 2015, 6, 1145–1155. [Google Scholar] [CrossRef]
- Keiner, D.; Salcedo-Puerto, O.; Immonen, E.; van Sark, W.G.J.H.M.; Nizam, Y.; Shadiya, F.; Duval, J.; Delahaye, T.; Gulagi, A.; Breyer, C. Powering an Island Energy System by Offshore Floating Technologies towards 100% Renewables: A Case for the Maldives. Appl. Energy 2022, 308, 118360. [Google Scholar] [CrossRef]
- Irish Energy Company EI-H2 Is Planning to Build a Green Hydrogen Facility in Cork. Available online: https://fuelcellsworks.com/news/irish-energy-company-ei-h2-is-planning-to-build-a-green-hydrogen-facility-in-cork/ (accessed on 21 August 2023).
- Zenith Energy and Ei-H2 Announce Joint Venture for Green Energy Facility at Bantry Bay. Available online: https://fuelcellsworks.com/news/zenith-energy-and-ei-h2-announce-joint-venture-for-green-energy-facility-at-bantry-bay/ (accessed on 21 August 2023).
- Bauer, F.; Tilsted, J.P.; Pfister, S.; Oberschelp, C.; Kulionis, V. Mapping GHG Emissions and Prospects for Renewable Energy in the Chemical Industry. Curr. Opin. Chem. Eng. 2023, 39, 100881. [Google Scholar] [CrossRef]
- Arsad, A.Z.; Hannan, M.A.; Al-Shetwi, A.Q.; Hossain, M.J.; Begum, R.A.; Ker, P.J.; Salehi, F.; Muttaqi, K.M. Hydrogen Electrolyser for Sustainable Energy Production: A Bibliometric Analysis and Future Directions. Int. J. Hydrogen Energy 2023, 48, 4960–4983. [Google Scholar] [CrossRef]
- El-Emam, R.S.; Zamfirescu, C.; Gabriel, K.S. Hydrogen Production Pathways for Generation-IV Reactors. In Handbook of Generation IV Nuclear Reactors: A Guidebook; Woodhead Publishing: Sawston, UK, 2023; pp. 665–680. ISBN 9780128205884. [Google Scholar]
- Naimi, Y.; Antar, A. Hydrogen Generation by Water Electrolysis. In Advances in Hydrogen Generation Technologies; IntechOpen: London, UK, 2018; ISBN 978-1-78923-535-7. [Google Scholar]
- Arcos, J.M.M.; Santos, D.M.F. The Hydrogen Color Spectrum: Techno-Economic Analysis of the Available Technologies for Hydrogen Production. Gases 2023, 3, 25–46. [Google Scholar] [CrossRef]
- Diab, J.; Fulcheri, L.; Hessel, V.; Rohani, V.; Frenklach, M. Why Turquoise Hydrogen Will Be a Game Changer for the Energy Transition. Int. J. Hydrogen Energy 2022, 47, 25831–25848. [Google Scholar] [CrossRef]
- Ajanovic, A.; Sayer, M.; Haas, R. The Economics and the Environmental Benignity of Different Colors of Hydrogen. Int. J. Hydrogen Energy 2022, 47, 24136–24154. [Google Scholar] [CrossRef]
- Difference between Grey Blue and Green Hydrogen|Compare the Difference between Similar Terms. Available online: https://www.differencebetween.com/difference-between-grey-blue-and-green-hydrogen/ (accessed on 20 October 2023).
- Sajna, M.S.; Zavahir, S.; Popelka, A.; Kasak, P.; Al-Sharshani, A.; Onwusogh, U.; Wang, M.; Park, H.; Han, D.S. Electrochemical System Design for CO2 Conversion: A Comprehensive Review. J. Environ. Chem. Eng. 2023, 11, 110467. [Google Scholar] [CrossRef]
- Dincer, I.; AlZahrani, A.A. Electrolyzers. In Comprehensive Energy Systems; Elsevier: Amsterdam, The Netherlands, 2018; Volume 4–5, pp. 985–1025. ISBN 9780128095973. [Google Scholar]
- Nasser, M.; Megahed, T.F.; Ookawara, S.; Hassan, H. A Review of Water Electrolysis–based Systems for Hydrogen Production Using Hybrid/solar/wind Energy Systems. Environ. Sci. Pollut. Res. 2022, 29, 86994–87018. [Google Scholar] [CrossRef]
- Vidas, L.; Castro, R. Recent Developments on Hydrogen Production Technologies: State-of-the-Art Review with a Focus on Green-Electrolysis. Appl. Sci. 2021, 11, 11363. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Gracia, J.M.; Fredriksson, H.O.; Niemantsverdriet, J.H. Electrocatalysts for the Generation of Hydrogen, Oxygen and Synthesis Gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef]
- Lo Basso, G.; Mojtahed, A.; Pastore, L.M.; De Santoli, L. High-Temperature Green Hydrogen Production: A Innovative—Application of SOEC Coupled with AEC through sCO2 HP. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Subotić, V.; Hochenauer, C. Analysis of Solid Oxide Fuel and Electrolysis Cells Operated in a Real-System Environment: State-of-the-Health Diagnostic, Failure Modes, Degradation Mitigation and Performance Regeneration. Prog. Energy Combust. Sci. 2022, 93, 101011. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, Y.; Li, D.; Xiong, Y. A Review on Recent Advances in Micro-Tubular Solid Oxide Fuel Cells. J. Power Source 2021, 506, 230135. [Google Scholar] [CrossRef]
- Afroze, S.; Reza, M.S.; Amin, M.R.; Taweekun, J.; Azad, A.K. Progress in Nanomaterials Fabrication and Their Prospects in Artificial Intelligence towards Solid Oxide Fuel Cells: A Review. Int. J. Hydrogen Energy 2022, in press. [Google Scholar] [CrossRef]
- Afroze, S.; Reza, M.S.; Cheok, Q.; Islam, S.N.; Abdalla, A.M.; Taweekun, J.; Azad, A.K.; Khalilpoor, N.; Issakhov, A. Advanced Applications of Fuel Cells during the COVID-19 Pandemic. Int. J. Chem. Eng. 2021, 2021, 5539048. [Google Scholar] [CrossRef]
- Afroze, S.; Absah, H.Q.H.H.; Reza, M.S.; Somalu, M.R.; Park, J.Y.; Nekoonam, S.; Issakhov, A.; Azad, A.K. Structural and Electrochemical Properties of Lanthanum Silicate Apatites La10Si6−X−0.2AlxZn0.2O27−δ for Solid Oxide Fuel Cells (SOFCs). Int. J. Chem. Eng. 2021, 2021, 6621373. [Google Scholar] [CrossRef]
- Wei, H.; Dai, J.; Maharik, I.; Ghasemi, A.; Mouldi, A.; Brahmia, A. Simultaneous Synthesis of H2, O2, and N2 via an Innovatory Energy System in Coronavirus Pandemic Time: Design, Techno-Economic Assessment, and Optimization Approaches. Int. J. Hydrogen Energy 2022, 47, 26038. [Google Scholar] [CrossRef]
- Benghanem, M.; Mellit, A.; Almohamadi, H.; Haddad, S.; Chettibi, N.; Alanazi, A.M.; Dasalla, D.; Alzahrani, A. Hydrogen Production Methods Based on Solar and Wind Energy: A Review. Energies 2023, 16, 757. [Google Scholar] [CrossRef]
- Jin, C.; Xiao, J.; Hou, J.; Wu, X.; Zhang, J.; Du, E. Flexibility Improvement Evaluation of Hydrogen Storage Based on Electricity–hydrogen Coupled Energy Model. Glob. Energy Interconnect. 2021, 4, 371–383. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, Z.; Fu, M.; Ren, T.; Li, X. Thermal and Electrochemical Performance Analysis of an Integrated Solar SOEC Reactor for Hydrogen Production. Appl. Therm. Eng. 2023, 229, 120603. [Google Scholar] [CrossRef]
- Kim, J.; Jun, A.; Gwon, O.; Yoo, S.; Liu, M.; Shin, J.; Lim, T.H.; Kim, G. Hybrid-Solid Oxide Electrolysis Cell: A New Strategy for Efficient Hydrogen Production. Nano Energy 2018, 44, 121–126. [Google Scholar] [CrossRef]
- Nasser, M.; Hassan, H. Assessment of Hydrogen Production from Waste Heat Using Hybrid Systems of Rankine Cycle with Proton Exchange Membrane/solid Oxide Electrolyzer. Int. J. Hydrogen Energy 2023, 48, 7135–7153. [Google Scholar] [CrossRef]
- El-Emam, R.S.; Ozcan, H.; Dincer, I. Comparative Cost Evaluation of Nuclear Hydrogen Production Methods with the Hydrogen Economy Evaluation Program (HEEP). Int. J. Hydrogen Energy 2014, 40, 11168–11177. [Google Scholar] [CrossRef]
- Jolaoso, L.A.; Bello, I.T.; Ojelade, O.A.; Yousuf, A.; Duan, C.; Kazempoor, P. Operational and Scaling-up Barriers of SOEC and Mitigation Strategies to Boost H2 Production- a Comprehensive Review. Int. J. Hydrogen Energy 2023, 48, 33017–33041. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Ou, Y.; Lei, X.; Yuan, J.; Liu, X.; Zhu, Y. Thermodynamic Analysis of Solid Oxide Electrolyzer Integration with Engine Waste Heat Recovery for Hydrogen Production. Case Stud. Therm. Eng. 2021, 27, 101240. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C. Technological Development of Hydrogen Production by Solid Oxide Electrolyzer Cell (SOEC). Int. J. Hydrogen Energy 2008, 33, 2337–2354. [Google Scholar] [CrossRef]
- Shen, M.; Ai, F.; Ma, H.; Xu, H.; Zhang, Y. Progress and Prospects of Reversible Solid Oxide Fuel Cell Materials. iScience 2021, 24, 103464. [Google Scholar] [CrossRef]
- Kojima, H.; Nagasawa, K.; Todoroki, N.; Ito, Y.; Matsui, T.; Nakajima, R. Influence of Renewable Energy Power Fluctuations on Water Electrolysis for Green Hydrogen Production. Int. J. Hydrogen Energy 2023, 48, 4572–4593. [Google Scholar] [CrossRef]
- Tucker, M.C. Progress in Metal-Supported Solid Oxide Electrolysis Cells: A Review. Int. J. Hydrogen Energy 2020, 45, 24203–24218. [Google Scholar] [CrossRef]
- Liang, J.; Han, M. Different Performance and Mechanisms of CO2 Electrolysis with CO and H2 as Protective Gases in Solid Oxide Electrolysis Cell. Int. J. Hydrogen Energy 2022, 47, 18606–18618. [Google Scholar] [CrossRef]
- Yan, Y.; Fang, Q.; Blum, L.; Lehnert, W. Performance and Degradation of an SOEC Stack with Different Cell Components. Electrochim. Acta 2017, 258, 1254–1261. [Google Scholar] [CrossRef]
- Xu, D.; Yan, A.; Yang, Y.; Xu, S.; Zhou, Y.; Yang, S.; Lin, W.F. Fast Ion-Conductive Electrolyte Based on a Doped LaAlO3 with an Amorphous Surface Layer for Low-Temperature Solid Oxide Fuel Cells. J. Power Source 2023, 561, 232723. [Google Scholar] [CrossRef]
- Kirchheim, R. On the Mixed Ionic and Electronic Conductivity in Polarized Yttria Stabilized Zirconia. Solid State Ion. 2018, 320, 239–258. [Google Scholar] [CrossRef]
- Gaikwad, P.S.; Mondal, K.; Shin, Y.K.; van Duin, A.C.T.; Pawar, G. Enhancing the Faradaic Efficiency of Solid Oxide Electrolysis Cells: Progress and Perspective. NPJ Comput. Mater. 2023, 9, 149. [Google Scholar] [CrossRef]
- Chen, Z.; Danilov, D.L.; Eichel, R.A.; Notten, P.H.L. Porous Electrode Modeling and Its Applications to Li-Ion Batteries. Adv. Energy Mater. 2022, 12, 2201506. [Google Scholar] [CrossRef]
- Li, X.; Shao, J.; Kim, S.K.; Yao, C.; Wang, J.; Miao, Y.R.; Zheng, Q.; Sun, P.; Zhang, R.; Braun, P.V. High Energy Flexible Supercapacitors Formed via Bottom-up Infilling of Gel Electrolytes into Thick Porous Electrodes. Nat. Commun. 2018, 9, 2578. [Google Scholar] [CrossRef]
- Rashidi, S.; Karimi, N.; Sunden, B.; Kim, K.C.; Olabi, A.G.; Mahian, O. Progress and Challenges on the Thermal Management of Electrochemical Energy Conversion and Storage Technologies: Fuel Cells, Electrolysers, and Supercapacitors. Prog. Energy Combust. Sci. 2022, 88, 100966. [Google Scholar] [CrossRef]
- Choi, Y.; Byun, S.; Seo, D.W.; Hwang, H.J.; Kim, T.W.; Kim, S.D. New Design and Performance Evaluation of 1 kW-Class Reversible Solid Oxide Electrolysis-Fuel Cell Stack Using Flat-Tubular Cells. J. Power Source 2022, 542, 231744. [Google Scholar] [CrossRef]
- Nechache, A.; Hody, S. Alternative and Innovative Solid Oxide Electrolysis Cell Materials: A Short Review. Renew. Sustain. Energy Rev. 2021, 149, 111322. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.W.; Ma, L.; Li, W.W.; Liu, X. Degradation of Solid Oxide Electrolysis Cells: Phenomena, Mechanisms, and Emerging Mitigation strategies—A Review. J. Mater. Sci. Technol. 2020, 55, 35–55. [Google Scholar] [CrossRef]
- Trini, M.; Hauch, A.; De Angelis, S.; Tong, X.; Hendriksen, P.V.; Chen, M. Comparison of Microstructural Evolution of Fuel Electrodes in Solid Oxide Fuel Cells and Electrolysis Cells. J. Power Source 2020, 450, 227599. [Google Scholar] [CrossRef]
- Deseure, J.; Aicart, J.; Deseure, J.; Aicart, J. Solid Oxide Steam Electrolyzer: Gas Diffusion Steers the Design of Electrodes. In Electrodialysis; IntechOpen: London, UK, 2019; ISBN 978-1-83968-383-1. [Google Scholar]
- Serhan, M.; Sprowls, M.; Jackemeyer, D.; Long, M.; Perez, I.D.; Maret, W.; Tao, N.; Forzani, E. Total Iron Measurement in Human Serum with a Smartphone. In Proceedings of the AIChE Annual Meeting, Orlando, FL, USA, 10–15 November 2019. [Google Scholar]
- Zarabi Golkhatmi, S.; Asghar, M.I.; Lund, P.D. A Review on Solid Oxide Fuel Cell Durability: Latest Progress, Mechanisms, and Study Tools. Renew. Sustain. Energy Rev. 2022, 161, 112339. [Google Scholar] [CrossRef]
- Elder, R.; Cumming, D.; Mogensen, M.B. High Temperature Electrolysis. In Carbon Dioxide Utilisation: Closing the Carbon Cycle, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2015; pp. 183–209. ISBN 9780444627483. [Google Scholar]
- Joubi, A.; Akimoto, Y.; Okajima, K. A Production and Delivery Model of Hydrogen from Solar Thermal Energy in the United Arab Emirates. Energies 2022, 15, 4000. [Google Scholar] [CrossRef]
- Shafiei Kaleibari, S.; Yanping, Z.; Abanades, S. Solar-Driven High Temperature Hydrogen Production via Integrated Spectrally Split Concentrated Photovoltaics (SSCPV) and Solar Power Tower. Int. J. Hydrogen Energy 2019, 44, 2519–2532. [Google Scholar] [CrossRef]
- Haussener, S. Solar Fuel Processing: Comparative Mini-Review on Research, Technology Development, and Scaling. Sol. Energy 2022, 246, 294–300. [Google Scholar] [CrossRef]
- Sanz-Bermejo, J.; Muñoz-Antón, J.; Gonzalez-Aguilar, J.; Romero, M. Optimal Integration of a Solid-Oxide Electrolyser Cell into a Direct Steam Generation Solar Tower Plant for Zero-Emission Hydrogen Production. Appl. Energy 2014, 131, 238–247. [Google Scholar] [CrossRef]
- Biswas, S.; Kaur, G.; Paul, G.; Giddey, S. A Critical Review on Cathode Materials for Steam Electrolysis in Solid Oxide Electrolysis. Int. J. Hydrogen Energy 2023, 48, 12541–12570. [Google Scholar] [CrossRef]
- Singh Aulakh, D.J.; Boulama, K.G.; Pharoah, J.G. On the Reduction of Electric Energy Consumption in Electrolysis: A Thermodynamic Study. Int. J. Hydrogen Energy 2021, 46, 17084–17096. [Google Scholar] [CrossRef]
- Kupecki, J.; Mastropasqua, L.; Motylinski, K.; Ferrero, D. Multilevel Modeling of Solid Oxide Electrolysis. In Solid Oxide-Based Electrochemical Devices: Advances, Smart Materials and Future Energy Applications; INC: New York, NY, USA, 2020; pp. 123–166. ISBN 9780128182857. [Google Scholar]
- Pitz-Paal, R. Concentrating Solar Power. In Future Energy: Improved, Sustainable and Clean Options for Our Planet; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 413–430. ISBN 9780081028865. [Google Scholar]
- Sarker, A.K.; Azad, A.K.; Rasul, M.G.; Doppalapudi, A.T. Prospect of Green Hydrogen Generation from Hybrid Renewable Energy Sources: A Review. Energies 2023, 16, 1556. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, N. Review on Integrated Green Hydrogen Polygeneration system——Electrolysers, Modelling, 4 E Analysis and Optimization. J. Clean. Prod. 2023, 414, 137631. [Google Scholar] [CrossRef]
- Maddaloni, M.; Marchionni, M.; Abbá, A.; Mascia, M.; Tola, V.; Carpanese, M.P.; Bertanza, G.; Artioli, N. Exploring the Viability of Utilizing Treated Wastewater as a Sustainable Water Resource for Green Hydrogen Generation Using Solid Oxide Electrolysis Cells (SOECs). Water 2023, 15, 2569. [Google Scholar] [CrossRef]
- Biswas, S.; Rathore, S.S.; Kulkarni, A.P.; Giddey, S.; Bhattacharya, S. A Theoretical Study on Reversible Solid Oxide Cells as Key Enablers of Cyclic Conversion between Electrical Energy and Fuel. Energies 2021, 14, 4517. [Google Scholar] [CrossRef]
- Omeiza, L.A.; Abdalla, A.M.; Wei, B.; Dhanasekaran, A.; Subramanian, Y.; Afroze, S.; Reza, M.S.; Bakar, S.A.; Azad, A.K. Nanostructured Electrocatalysts for Advanced Applications in Fuel Cells. Energies 2023, 16, 1876. [Google Scholar] [CrossRef]
- Saghafifar, M.; Mohammadi, K.; Powell, K. Design and Analysis of a Dual-Receiver Direct Steam Generator Solar Power Tower Plant with a Flexible Heliostat Field. Sustain. Energy Technol. Assess. 2020, 39, 100698. [Google Scholar] [CrossRef]
- Delgado-Torres, A.M.; García-Rodríguez, L. Solar Desalination Driven by Organic Rankine Cycles (Orc) and Supercritical CO2 Power Cycles: An Update. Processes 2022, 10, 153. [Google Scholar] [CrossRef]
- Jolaoso, L.A.; Duan, C.; Kazempoor, P. Life Cycle Analysis of a Hydrogen Production System Based on Solid Oxide Electrolysis Cells Integrated with Different Energy and Wastewater Sources. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Lin, X.; Song, H.; Liu, Y. Process Design and Comprehensive Comparison of Coal- and Biomass-Fired Oxy-Combustion Power Plant. Chem. Eng. Res. Des. 2022, 186, 568–586. [Google Scholar] [CrossRef]
- Biencinto, M.; González, L.; Valenzuela, L. Using Time-Windowed Solar Radiation Profiles to Assess the Daily Uncertainty of Solar Thermal Electricity Production Forecasts. J. Clean. Prod. 2022, 379, 134821. [Google Scholar] [CrossRef]
- Sánchez Ramos, J.; Toulou, A.; Guerrero Delgado, M.; Palomo Amores, T.R.; Castro Medina, D.; Álvarez Domínguez, S. Thermal Resilience of Citizens: Comparison between Thermal Sensation and Objective Estimation in Outdoor Spaces: A Case Study in Seville, Spain. Appl. Sci. 2022, 12, 11676. [Google Scholar] [CrossRef]
- Kopscick, G.M.; Vernon, M. Application and operating history of moderate-speed API 618 reciprocating compressors. In Proceedings of the Thirty-Third Turbomachinery Symposium, Houston, TX, USA, 19–24 April 2004; pp. 103–112. [Google Scholar]
- Yadav, D.; Banerjee, R. Net Energy and Carbon Footprint Analysis of Solar Hydrogen Production from the High-Temperature Electrolysis Process. Appl. Energy 2020, 262, 114503. [Google Scholar] [CrossRef]
- Lin, M.; Suter, C.; Diethelm, S.; Van Herle, J.; Haussener, S. Integrated Solar-Driven High-Temperature Electrolysis Operating with Concentrated Irradiation. Joule 2022, 6, 2102–2121. [Google Scholar] [CrossRef]
- Tang, S.; Xing, X.; Yu, W.; Sun, J.; Xuan, Y.; Wang, L.; Xu, Y.; Hong, H.; Jin, H. Synergizing Photo-Thermal H2 and Photovoltaics into a Concentrated Sunlight Use. iScience 2020, 23, 101012. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, J.; Li, Z.; Wang, J. Research Progress of Hydrogen Production Technology and Related Catalysts by Electrolysis of Water. Molecules 2023, 28, 5010. [Google Scholar] [CrossRef] [PubMed]
- Milewski, J.; Kupecki, J.; Szczęśniak, A.; Uzunow, N. Hydrogen Production in Solid Oxide Electrolyzers Coupled with Nuclear Reactors. Int. J. Hydrogen Energy 2021, 46, 35765–35776. [Google Scholar] [CrossRef]
- Brisse, A.; Schefold, J.; Léon, A. High-Temperature Steam Electrolysis. In Electrochemical Power Sources: Fundamentals, Systems, and Applications Hydrogen Production by Water Electrolysis; Elsevier B.V.: Amsterdam, The Netherlands, 2021; pp. 229–280. ISBN 9780128194249. [Google Scholar]
- El-Hadary, M.I.; Senthilraja, S.; Zayed, M.E. A Hybrid System Coupling Spiral Type Solar Photovoltaic Thermal Collector and Electrocatalytic Hydrogen Production Cell: Experimental Investigation and Numerical Modeling. Process Saf. Environ. Prot. 2023, 170, 1101–1120. [Google Scholar] [CrossRef]
- Wang, H.; Li, W.; Liu, T.; Liu, X.; Hu, X. Thermodynamic Analysis and Optimization of Photovoltaic/thermal Hybrid Hydrogen Generation System Based on Complementary Combination of Photovoltaic Cells and Proton Exchange Membrane Electrolyzer. Energy Convers. Manag. 2019, 183, 97–108. [Google Scholar] [CrossRef]
- Lv, S.; Ji, Y.; Qian, Z.; Pan, Y.; Zhang, Y.; He, W. Preliminary Experiment and Performance Evaluation of a Terrestrial Solar Thermoelectric Generators under Fluctuant Solar Radiation. Appl. Therm. Eng. 2021, 190, 116753. [Google Scholar] [CrossRef]
- Tijani, A.S.; Binti Kamarudin, N.A.; Binti Mazlan, F.A. Investigation of the Effect of Charge Transfer Coefficient (CTC) on the Operating Voltage of Polymer Electrolyte Membrane (PEM) Electrolyzer. Int. J. Hydrogen Energy 2018, 43, 9119–9132. [Google Scholar] [CrossRef]
- Next Level Solid Oxide Electrolysis. Available online: https://ispt.eu/projects/next-level-solid-oxide-electrolysis/ (accessed on 9 September 2023).
- Ursúa, A.; Gandía, L.M.; Sanchis, P. Hydrogen Production from Water Electrolysis: Current Status and Future Trends. Proc. IEEE 2012, 100, 410–426. [Google Scholar] [CrossRef]
- Herradon, C.; Le, L.; Meisel, C.; Huang, J.; Chmura, C.; Kim, Y.D.; Cadigan, C.; O’Hayre, R.; Sullivan, N.P. Proton-Conducting Ceramics for Water Electrolysis and Hydrogen Production at Elevated Pressure. Front. Energy Res. 2022, 10, 1020960. [Google Scholar] [CrossRef]
- Anghilante, R.; Colomar, D.; Brisse, A.; Marrony, M. Bottom-up Cost Evaluation of SOEC Systems in the Range of 10–100 MW. Int. J. Hydrogen Energy 2018, 43, 20309–20322. [Google Scholar] [CrossRef]
- Patonia, A.; Poudineh, R. Cost-Competitive Green Hydrogen: How to Lower the Cost of Electrolysers? OIES: Oxford, UK, 2022. [Google Scholar]
- Ghaebi Panah, P.; Cui, X.; Bornapour, M.; Hooshmand, R.A.; Guerrero, J.M. Marketability Analysis of Green Hydrogen Production in Denmark: Scale-up Effects on Grid-Connected Electrolysis. Int. J. Hydrogen Energy 2022, 47, 12443–12455. [Google Scholar] [CrossRef]
- Li, W.; Tian, H.; Ma, L.; Wang, Y.; Liu, X.; Gao, X. Low-Temperature Water Electrolysis: Fundamentals, Progress, and New Strategies. Mater. Adv. 2022, 3, 5598–5644. [Google Scholar] [CrossRef]
- Aricò, A.S.; Siracusano, S.; Briguglio, N.; Baglio, V.; Di Blasi, A.; Antonucci, V. Polymer Electrolyte Membrane Water Electrolysis: Status of Technologies and Potential Applications in Combination with Renewable Power Sources. J. Appl. Electrochem. 2013, 43, 107–118. [Google Scholar] [CrossRef]
- Wolf, S.E.; Winterhalder, F.E.; Vibhu, V.; de Haart, L.G.J.; Guillon, O.; Eichel, R.A.; Menzler, N.H. Solid Oxide Electrolysis Cells-Current Material Development and Industrial Application. J. Mater. Chem. A 2023, 11, 17977–18028. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen Production by PEM Water Electrolysis–A Review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Gallandat, N.; Romanowicz, K.; Züttel, A. An Analytical Model for the Electrolyser Performance Derived from Materials Parameters. J. Power Energy Eng. 2017, 5, 34–49. [Google Scholar] [CrossRef]
- Stypka, S.; Oberschachtsiek, B.; Radev, I.; Heinzel, A. Testing Field for PEM, Alkaline and Solid Oxide Electrolysis Technology. Chem. Ing. Tech. 2018, 90, 1443–1445. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent Progress in Alkaline Water Electrolysis for Hydrogen Production and Applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Ayodele, T.R.; Munda, J.L. Potential and Economic Viability of Green Hydrogen Production by Water Electrolysis Using Wind Energy Resources in South Africa. Int. J. Hydrogen Energy 2019, 44, 17669–17687. [Google Scholar] [CrossRef]
- Buttler, A.; Spliethoff, H. Current Status of Water Electrolysis for Energy Storage, Grid Balancing and Sector Coupling via Power-to-Gas and Power-to-Liquids: A Review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- David, M.; Ocampo-Martínez, C.; Sánchez-Peña, R. Advances in Alkaline Water Electrolyzers: A Review. J. Energy Storage 2019, 23, 392–403. [Google Scholar] [CrossRef]
- Lamagna, M.; Groppi, D.; Nastasi, B. Reversible Solid Oxide Cells Applications to the Building Sector. Int. J. Hydrogen Energy 2023, 48, 27033–27058. [Google Scholar] [CrossRef]
- Solid Oxide Electrolysis Cell (SOEC) Market Detailed Analysis Study. Available online: https://www.insightaceanalytic.com/report/solid-oxide-electrolysis-cell-soec-market/2076 (accessed on 26 October 2023).
- Green Fuels for Denmark Receives Danish IPCEI Funding. Available online: https://orsted.com/en/media/newsroom/news/2022/12/13666863 (accessed on 9 September 2023).
- Amelang, S. EU Report Says Making Green Hydrogen in Germany Uneconomical, Questioning Government Plans|Clean Energy Wire. Available online: https://www.cleanenergywire.org/news/eu-report-says-making-green-hydrogen-germany-uneconomical-questioning-government-plans (accessed on 9 September 2023).
- The National Hydrogen Strategy. One-Stop-Shop-Wasserstoff. Available online: https://www.bmwk.de/Redaktion/EN/Hydrogen/Dossiers/national-hydrogen-strategy.html (accessed on 9 September 2023).
- Rojas-Michaga, M.F.; Michailos, S.; Cardozo, E.; Akram, M.; Hughes, K.J.; Ingham, D.; Pourkashanian, M. Sustainable Aviation Fuel (SAF) Production through Power-to-Liquid (PtL): A Combined Techno-Economic and Life Cycle Assessment. Energy Convers. Manag. 2023, 292, 117427. [Google Scholar] [CrossRef]
- Qi, M.; Kim, M.; Dat Vo, N.; Yin, L.; Liu, Y.; Park, J.; Moon, I. Proposal and Surrogate-Based Cost-Optimal Design of an Innovative Green Ammonia and Electricity Co-Production System via Liquid Air Energy Storage. Appl. Energy 2022, 314, 118965. [Google Scholar] [CrossRef]
- POWER|A Decarbonizing World, Powered in Fresh New Ways|TOPSOE. Available online: https://www.topsoe.com/helping-you-decarbonize/power (accessed on 9 September 2023).
- Bloom Energy. Hydrogen Electrolyzers, Solid Oxide Electrolysis. Available online: https://www.bloomenergy.com/bloomelectrolyzer/ (accessed on 11 September 2023).




| Color | Gray | Blue | Turquoise | Yellow | Pink | Green |
|---|---|---|---|---|---|---|
| Process | SMR or gasification | SMR or gasification with carbon capture | Pyrolysis | Electrolysis specifically using solar power | Nuclear reactors powering electrolyzers | Electrolysis |
| Source | Methane or coal | Methane or coal | Methane | Solar energy | Nuclear energy | Renewable electricity |
| GHG emissions | Very high | Moderate to low | Comparatively low | Moderate | Zero | Zero |
| Cost (USD per kg) | 0.67 to 1.31 | 0.99 to 1.83 | 2 | 6.06 to 8.81 | 2.75 to 5.29 | 2.28 to 7.39 |
| Acceptance | Extremely unacceptable due to environmental damage | Acceptable | Acceptable | Acceptable | Acceptable | Highly acceptable |
| Variables | Symbol | Values |
|---|---|---|
| Irradiation intensity | I (W/m2) | 300–1000 |
| The optical efficiency of a concentrator | ηopt | 0.73 |
| Emissivity of a PV module | ε | 0.2 |
| PV panel effectiveness | ηmod | 0.9 |
| Temperature differential between a PV panel and water flow | ΔT (K) | 10 |
| Prevailing wind speed | vwind (m/s) | 4 |
| Prevailing temperature | T0 (K) | 293 |
| Coefficient of convective heat transfer | h (W/(m2 K)) | 8 |
| Temperature in the sky | Tsky (K) | 285 |
| Size of the heating portion | L (m) | 1–5 |
| The width of heating section | W (m) | 0.1 |
| The height of the heating section | H (m) | 0.001 |
| Thickness of the electrolyte | lelectrolyte (µm) | 1000 |
| Charge transfer coefficient or the symmetry factor | α | 0.5 |
| Amount of electrons generated by each reaction | z | 2 |
| Thickness of the anode | lanode (µm) | 100 |
| Cathode thickness | lcathode (µm) | 100 |
| Activation energy of anode | Eact,a (J/mol) | 1.2 × 105 |
| Activation energy of cathode | Eact,c (J/mol) | 1.0 × 105 |
| Density of anode exchange current | J0, a (A/m2) | 2000 |
| Density of cathode exchange current | J0, c (A/m2) | 5000 |
| Features | AE | PEM | SOEC | Ref. |
|---|---|---|---|---|
| Electrolyte | KOH/NaOH | Solid polymer electrolyte | Yttria-stabilized Zirconia (YSZ) | [111,112,113] |
| Electrode (H2 side) | Nickel-coated perforated stainless steel | Iridium oxide | Ni/YSZ | [111,112,113] |
| Electrode (O2 side) | Nickel-coated perforated stainless steel | Platinum carbon | Perovskites | [111,112,113] |
| Temperature (°C) | 40–90 | 20–100 | 600–900 | [111,113,114] |
| Voltage (V) | 1.8–2.4 | 1.8–2.2 | 0.7–1.5 | [111,113,114] |
| Pressure (bar) | <30 | <30 | <10 | [115] |
| Production (Nm3/h) | 10 | 5 | 5 | [116] |
| Output H2 pressure (bar) | 10 | 35 | 10 (after PSA) | [116] |
| Gas purity (%) | >99.5% | >99.995 | - | [117,118] |
| Stack energy consumption (kWh/Nm3) | 4.2–5.9 | 4.2–5.5 | >3 | [119] |
| System efficiency (% LHV) | 55—60 | 55–70 | 74–81 | [120,121] |
| Lifetime of stack/h | 55–120 | 60–100 | 8–20 | [120] |
| Degradation (%/a) | 0.25–1.5 | 0.5–2.5 | 3–50 | [119] |
| Maintenance cost (% of investment/year) | 2–3 | 3–5 | - | [119] |
| Capital cost (EUR/kW) | 880–1650 | 1540–2550 | >2000 | [120] |
| Technical sophistication | Omnipresent commercialization | Commercialization | Exploration and development phase | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afroze, S.; Sofri, A.N.S.B.; Reza, M.S.; Iskakova, Z.B.; Kabyshev, A.; Kuterbekov, K.A.; Bekmyrza, K.Z.; Taimuratova, L.; Uddin, M.R.; Azad, A.K. Solar-Powered Water Electrolysis Using Hybrid Solid Oxide Electrolyzer Cell (SOEC) for Green Hydrogen—A Review. Energies 2023, 16, 7794. https://doi.org/10.3390/en16237794
Afroze S, Sofri ANSB, Reza MS, Iskakova ZB, Kabyshev A, Kuterbekov KA, Bekmyrza KZ, Taimuratova L, Uddin MR, Azad AK. Solar-Powered Water Electrolysis Using Hybrid Solid Oxide Electrolyzer Cell (SOEC) for Green Hydrogen—A Review. Energies. 2023; 16(23):7794. https://doi.org/10.3390/en16237794
Chicago/Turabian StyleAfroze, Shammya, Amal Najeebah Shalihah Binti Sofri, Md Sumon Reza, Zhanar Baktybaevna Iskakova, Asset Kabyshev, Kairat A. Kuterbekov, Kenzhebatyr Z. Bekmyrza, Lidiya Taimuratova, Mohammad Rakib Uddin, and Abul K. Azad. 2023. "Solar-Powered Water Electrolysis Using Hybrid Solid Oxide Electrolyzer Cell (SOEC) for Green Hydrogen—A Review" Energies 16, no. 23: 7794. https://doi.org/10.3390/en16237794
APA StyleAfroze, S., Sofri, A. N. S. B., Reza, M. S., Iskakova, Z. B., Kabyshev, A., Kuterbekov, K. A., Bekmyrza, K. Z., Taimuratova, L., Uddin, M. R., & Azad, A. K. (2023). Solar-Powered Water Electrolysis Using Hybrid Solid Oxide Electrolyzer Cell (SOEC) for Green Hydrogen—A Review. Energies, 16(23), 7794. https://doi.org/10.3390/en16237794










