Kernel-Based Biodiesel Production from Non-Edible Oil Seeds: Techniques, Optimization, and Environmental Implications
Abstract
1. Introduction
Objective of the Paper
2. Kernel-Based Biomass Feedstock
2.1. Jatropha curcas L.
2.2. Pongamia pinnata
2.3. Calophyllum inophyllum
2.4. Madhuca indica
2.5. Sapium sebiferum
2.6. Azadirachta indica
2.7. Hevea brasiliensis
2.8. Sapindus mukorossi
2.9. Prunus Sibirica
2.10. Sterculia foetida
2.11. Prunus armeniaca
2.12. Camelia sinensis
2.13. Elaeis guineensis
2.14. Thevetia peruviana
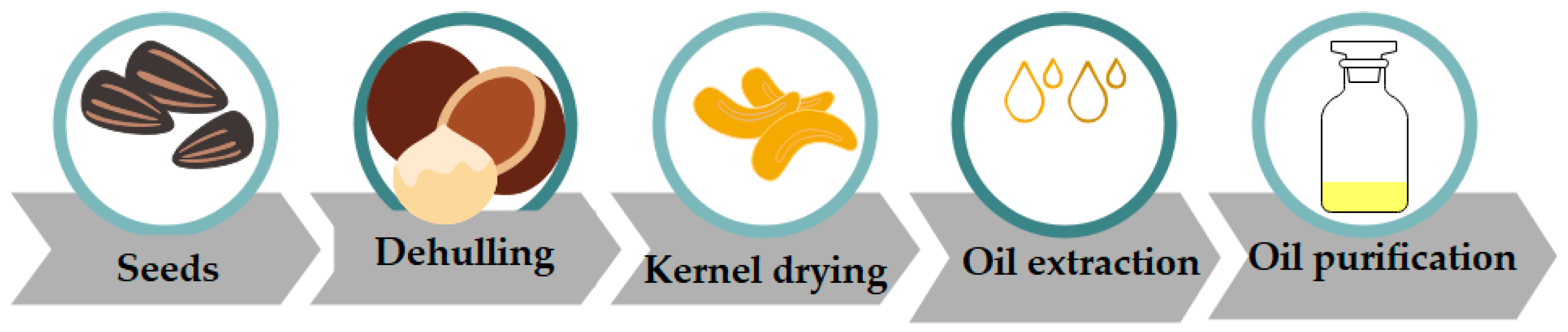
3. Preliminary Steps for Oil Extraction
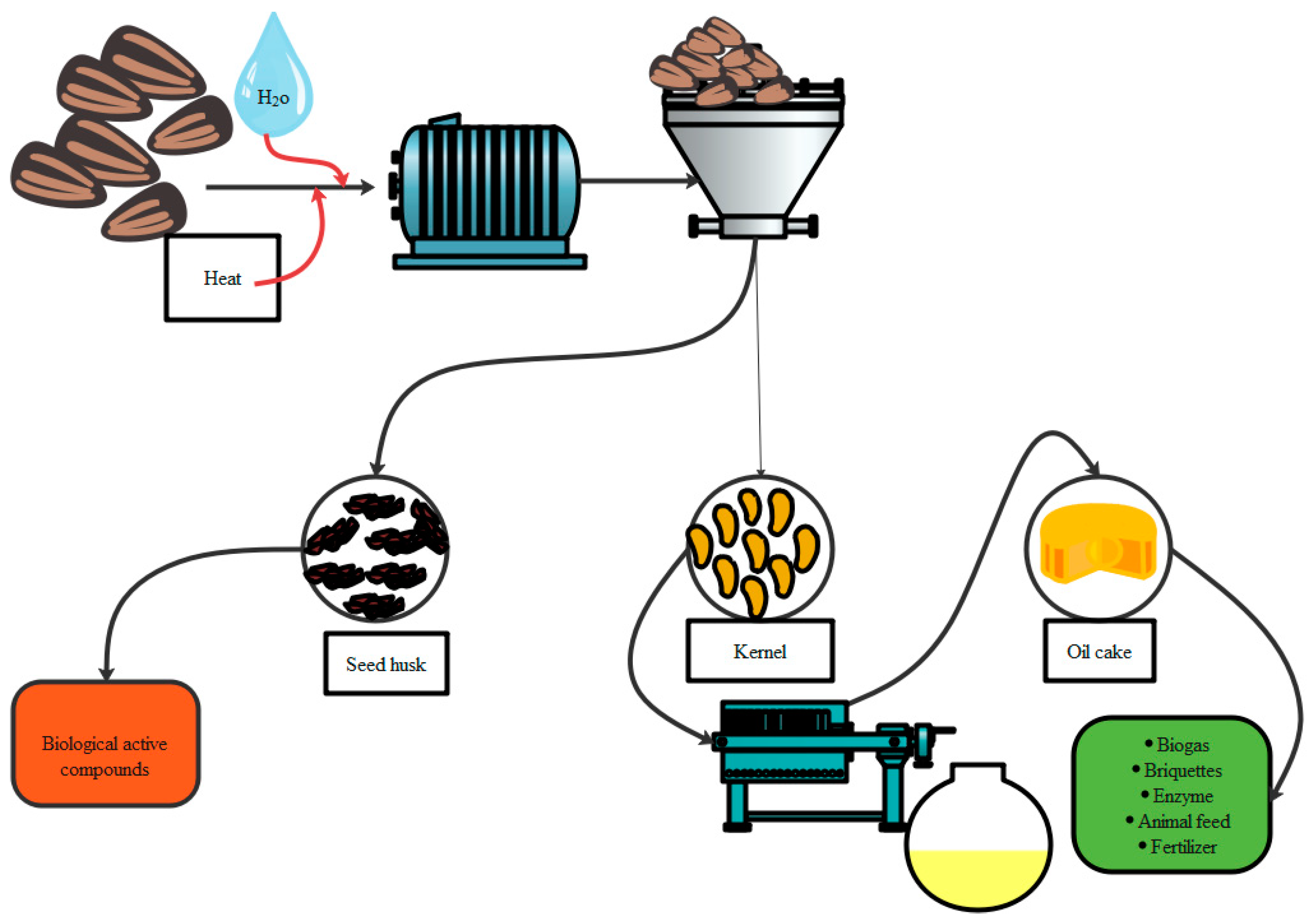
3.1. Kernel Processing
3.2. Decortication of Seeds
3.3. Processing of Kernel
3.4. Kernel Drying
3.5. Purification of Oil
3.6. Storage of Oil
3.7. Seed Cake
4. Current Status of Oil Extraction Techniques (Kernel Oil Extraction Techniques)
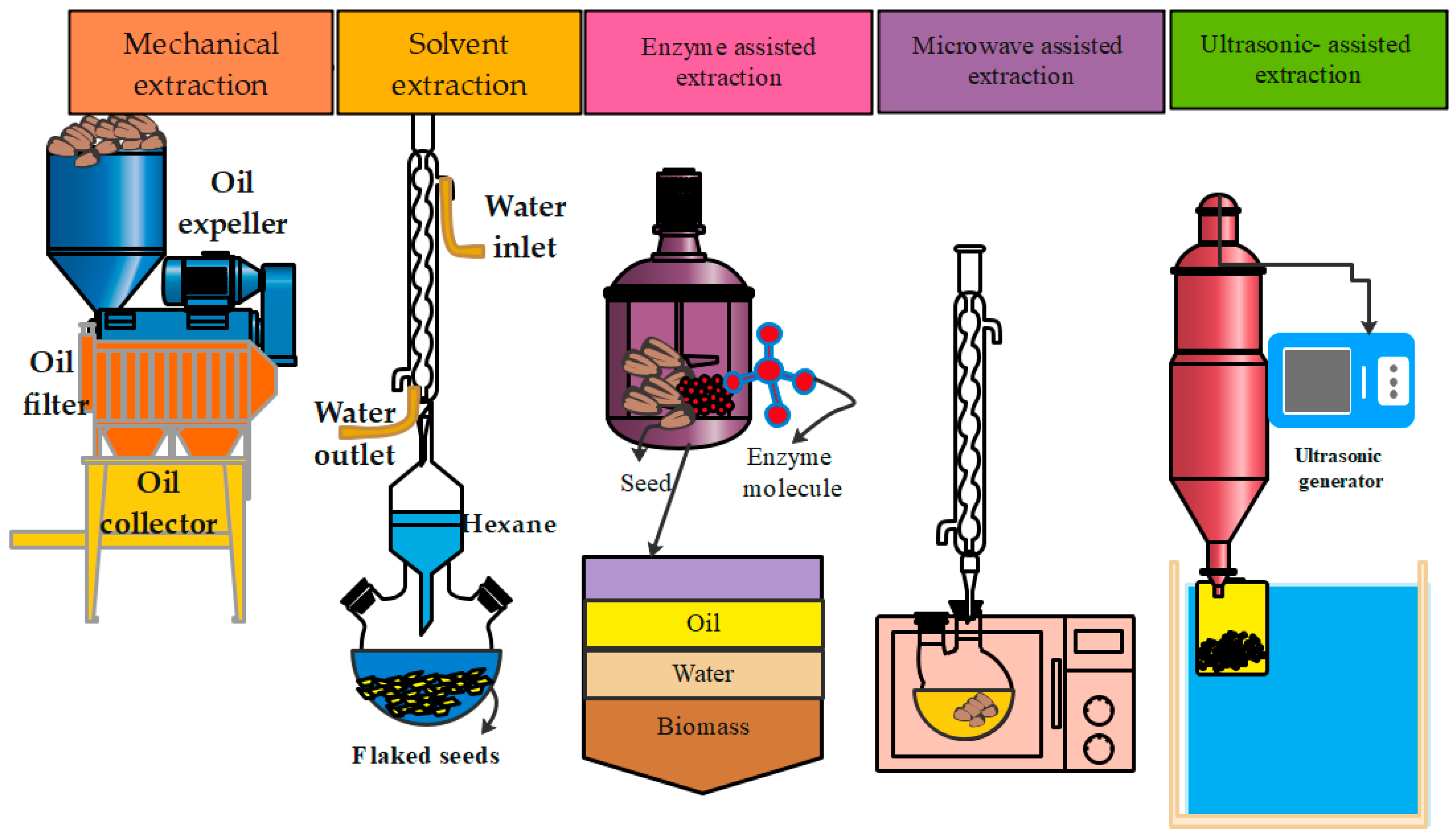
4.1. Oil Extraction Methods
4.2. Mechanical Extraction Methods
4.3. Solvent Extraction
4.4. Ultrasound-Assisted Extraction Method
4.5. Microwave-Assisted Extraction Method
4.6. Enzymatic Extraction
5. Fatty Acid Composition
6. Biodiesel Production Technologies
6.1. Homogenous Catalyst
6.2. Alkali-Catalyzed Transesterification
6.3. Acid Catalysts
6.4. Two-Stage Transesterification
6.5. Heterogeneous Catalysts
6.6. Solid Acid Catalysts
6.7. Natural Heterogeneous Catalysts
6.8. Enzyme-Based Transesterification
6.9. Transesterification Using Ultrasound
6.10. Microwave Transesterification
6.11. Direct Transesterification
7. Properties and Characteristics of Non-Edible Biodiesel
7.1. Kinematic Viscosity and Density
7.2. Flash Point
7.3. Cloud and Pour Point
7.4. Cetane Number
7.5. Acid Number
7.6. Calorific Value
7.7. Iodine Number
| Property | Unit | J. curcas [162] | M. indica [57] | P. pinnata [147] | A. indica [56] | H. brasiliensis [7] | S. sebifeum [8] | S. mukorossi oil [3] | P. armeniaca [1] | C. inophyllum [4] | P. sibirica [6] | C. sinensis [2] | E. guineensis [5] | S. foetida [9] | T. peruviana [86] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density at 15 °C | (kg/m3) | 918 | 920 | 931 | 912 | 0.920 | - | - | - | 943 | 879.7 | 907 | 874 | 955 | 890 |
| Kinematic viscosity at 40 °C | mm2s−1 | 37 | 24.58 | 46 | 20.5 | 38.10 | 46.92 | - | 34.82 | 26.642 | 4.34 | 27 | 4.03 | 42 | 36 |
| Free fatty acids (%) | % | - | - | - | - | 23.47 | - | - | - | - | - | 2.7 | - | 3.10 | - |
| Saponification number | mg KOH g−1 | - | - | - | - | - | 206.35 | 191.83 | 173 | 204.3 | - | 195.2 | - | - | - |
| Iodine value | g I 100 g−1 | 82 | 71 | - | - | - | 137.52 | 113.15 | 103 | 77 | - | 106.8 | 59 | - | - |
| Acid value | mg KOH g−1 | - | - | 30 | 42.2 | - | 4.12 | 1.65 | 40.4 | - | 0.206 | 0.2 | 6.171 | - | |
| Calorific value | MJ kg−1 | 37.5 | 36 | 39.122 | 32 | 39.72 | - | - | 38.69 | 37.81 | - | 37.14 | 41.3 | 36.446 | 39.8 |
| Flash point | °C | 238 | 232 | 226 | 214 | - | - | - | 238 | 175 | 119 | - | 190 | ||
| Pour point | °C | 4 | 10 | −2 | 10 | - | - | - | 6 | −8 | −6 | −3 | |||
| Cloud point | °C | 9 | 15 | 1 | 19 | - | - | - | 9 | - | 2 | 1 | - | ||
| Cetane number | - | 39 | 57 | 31 | 49.73 | - | - | - | - | 49.2 | 43.8 | 57.8 | - | - | |
| Oxidation stability | hour | - | - | - | 12.4 | - | - | - | - | 2.7 | - | - | - | 6 |
| Property | Unit | J. curcas [6] | M. indica [5] | P. pinnata [7] | A. indica [62] | H. brasiliensis [4] | S. sebifeum [8] | S. mukororsi [67] | P. armeniaca [1] | C. inophyllum [53] | P. sibirica [2] | C. sinensis [10] | E. guineensis [3] | S. foetida [9] | T. peruviana [91] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density at 15 °C | (kg/m3) | 880 | 916 | 890 | 820 | 860 | 900 | 876 | - | 868.6 | 878.2 | 884 | 875 | 875 | |
| Kinematic viscosity at 40 °C | mm2s−1 | 4.4 | 3.98 | 4.85 | 3.2 | 5.81 | 4.81 | 4.63 | 4.26 | 4.7 | 4.341 | 4.95 | 3.36 | 6 | 4.33 |
| Iodine value | % | 74.2 | 89 | 144 | 104.70 | - | - | 98 | 69.9 | ||||||
| Acid value | mg KOH g−1 | 0.48 | 0.42 | 0.9 | 0.7 | 0.14 | 0.25 | 0.25 | - | 0.14 | 0.057 | ||||
| Calorific value | MJ kg−1 | 41.17 | 39.4 | 35.56 | 39.6 | 40.02 | 39.04 | 39.38 | - | 37.5 | - | 42.279 | |||
| Flash point | °C | 163 | 129 | 180 | 120 | 130 | 137 | 140 | 105 | 141.5 | 173 | 165 | 100 | 162 | 75 |
| Pour point | °C | 6 | 2 | -8 | 0 | −4 | −8 | 8 | −5 | −3 | +3 | ||||
| Cloud point | °C | 4 | 5 | 9 | 4 | −1 | −4 | 10 | - | 3 | −13 | +12 | |||
| Cetane number | - | 57.1 | 51 | 58 | 48 | 36.5 | 50 | 56 | 50.45 | 48.8 | 51.1 | - | 54 | 61.5 | |
| Oxidation stability | h | 6 | 6 | 7.1 | 0.6 | 1.20 | 7.15 | 6.01 | 2.7 | - |
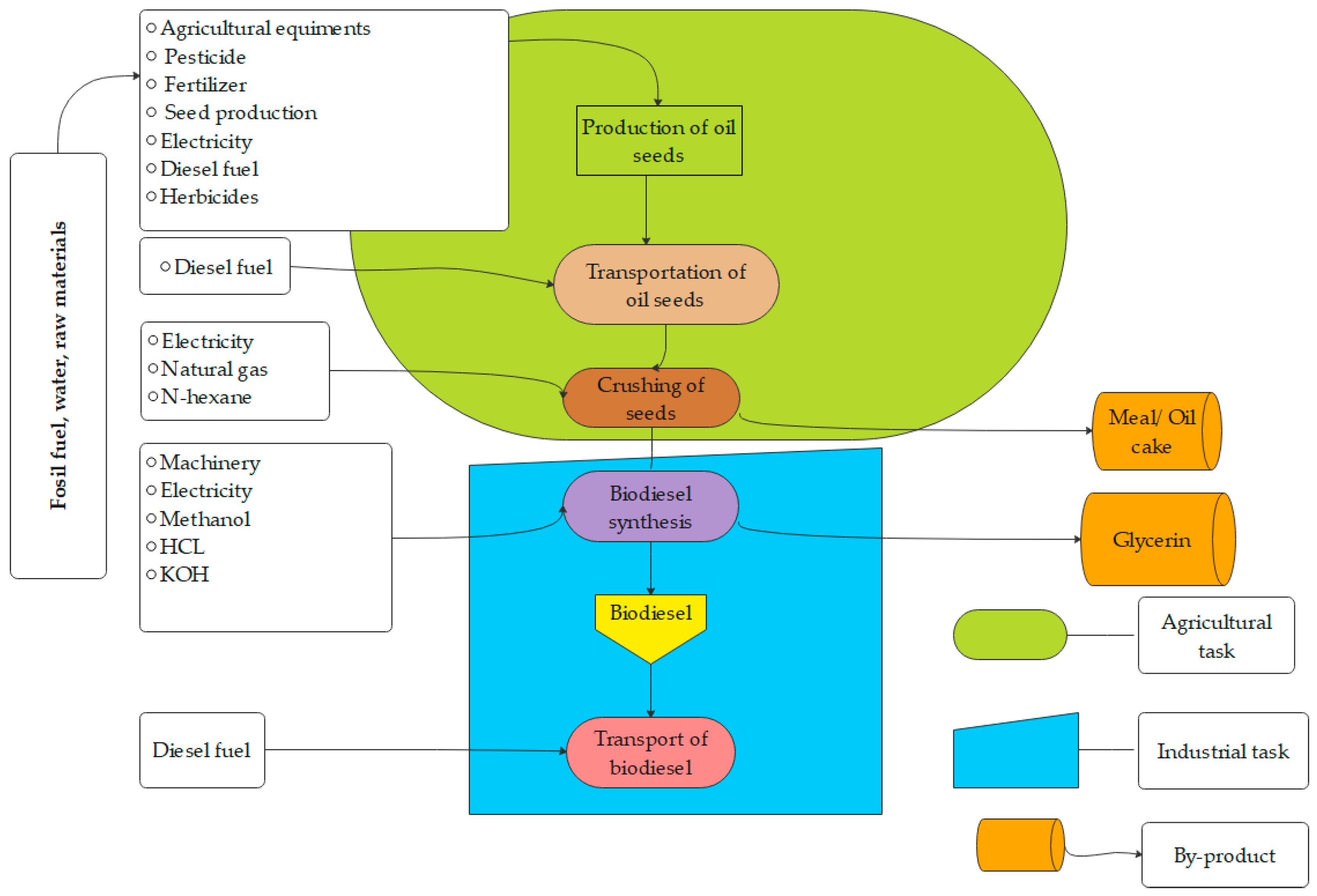
8. LCA of Biodiesel Production
9. Sustainable Biodiesel Production
- (1)
- Utilization of either green catalysts, biochar, or activated catalysts;
- (2)
- Application of enzyme catalysts produced from the oil cake of the feedstock;
- (3)
- Use of waste cooking oil as a feedstock for biodiesel synthesis;
- (4)
- Implementation of circular economy in biodiesel production.
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Silitonga, A.S.; Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I.; Chong, W.T.; Yusaf, T.F. Production of Biodiesel from Sterculia foetida and Its Process Optimization. Fuel 2013, 111, 478–484. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel Production: A Review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Foidl, N. Jatropha curcas L. as a source for the production of biofuel in Nicaragua. Bioresour. Technol. 1996, 58, 77–82. [Google Scholar] [CrossRef]
- Zullaikah, S.; Lai, C.-C.; Vali, S.R.; Ju, Y.-H. A Two-Step Acid-Catalyzed Process for the Production of Biodiesel from Rice Bran Oil. Bioresour. Technol. 2005, 96, 1889–1896. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, D. Biodiesel Production through the Use of Different Sources and Characterization of Oils and Their Esters as the Substitute of Diesel: A Review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Fayaz, H. Non-Edible Vegetable Oils: A Critical Evaluation of Oil Extraction, Fatty Acid Compositions, Biodiesel Production, Characteristics, Engine Performance and Emissions Production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- No, S. Inedible Vegetable Oils and Their Derivatives for Alternative Diesel Fuels in CI Engines: A Review. Renew. Sustain. Energy Rev. 2011, 15, 131–149. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Wei, D.; Zhang, X.; Chen, G. Biodiesel Production by Microalgal Biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Anjum, I.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A Comprehensive Review on Biodiesel as an Alternative Energy Resource and Its Characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Atabani, A.E.; Mahlia, T.M.I.; Masjuki, H.H.; Anjum, I.; Mekhilef, S. A Review on Prospect of Jatropha curcas for Biodiesel in Indonesia. Renew. Sustain. Energy Rev. 2011, 15, 3733–3756. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Biofuels and the Environment: Second Triennial Report to Congress; US Environmental Protection Agency: Washington, DC, USA, 2018.
- Ong, H.C.; Silitonga, A.S.; Masjuki, H.H.; Mahlia, T.M.I.; Chong, W.T.; Boosroh, M.H. Production and Comparative Fuel Properties of Biodiesel from Non-Edible Oils: Jatropha curcas, Sterculia foetida and Ceiba pentandra. Energy Convers. Manag. 2013, 73, 245–255. [Google Scholar] [CrossRef]
- Khan, L.M.; Hanna, M.A. Expression of Oil from Oilseeds—A Review. J. Agric. Eng. Res. 1983, 28, 495–503. [Google Scholar] [CrossRef]
- Singh, K.K.; Wiesenborn, D.P.; Tostenson, K.; Kangas, N. Influence of Moisture Content and Cooking on Screw Pressing of Crambe Seed. J. Am. Oil Chem. Soc. 2002, 79, 165–170. [Google Scholar] [CrossRef]
- Fadhlullah, M.; Widiyanto, S.N.B.; Restiawaty, E. The Potential of Nyamplung (Calophyllum inophyllum L.) Seed Oil as Biodiesel Feedstock: Effect of Seed Moisture Content and Particle Size on Oil Yield. Energy Procedia 2015, 68, 177–185. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Brown, R.J.; Senadeera, W.; Ashwath, N.; Rasul, M.G.; Rahman, M.M.; Hossain, F.M.; Moghaddam, L.; Islam, M.A.; O’Hara, I.M. Physio-Chemical Assessment of Beauty Leaf (Calophyllum inophyllum) as Second-Generation Biodiesel Feedstock. Energy Rep. 2015, 1, 204–215. [Google Scholar] [CrossRef]
- Khalaf, M.; Abdel-Fadeel, W.; Hashish, H.M.; Wapet, D.E.M.; Mahmoud, M.M.; Elhady, S.A.; Esmail, M.F.C. Experimental Investigation of Different Extraction Methods for Producing Biofuel from Jatropha Seeds and Castor Seeds. Int. J. Energy Res. 2023, 2023, 1780536. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Brown, J.R.; Senadeera, W.; Ashwath, N.; Laing, C.; Leski-Taylor, J.; Rasul, M.G. Optimisation of Bio-Oil Extraction Process from Beauty Leaf (Calophyllum inophyllum) Oil Seed as a Second Generation Biodiesel Source. Procedia Eng. 2013, 56, 619–624. [Google Scholar] [CrossRef]
- Kostić, M.D.; Joković, N.M.; Stamenković, O.S.; Rajković, K.M.; Milić, P.S.; Veljković, V.B. Optimization of Hempseed Oil Extraction by N-Hexane. Ind. Crops Prod. 2013, 48, 133–143. [Google Scholar] [CrossRef]
- Bhuiya, M.M.K.; Rasul, M.; Khan, M.; Ashwath, N.; Mofijur, M. Comparison of Oil Extraction between Screw Press and Solvent (n-Hexane) Extraction Technique from Beauty Leaf (Calophyllum inophyllum L.) Feedstock. Ind. Crops Prod. 2020, 144, 112024. [Google Scholar] [CrossRef]
- Chapuis, A.; Blin, J.; Carré, P.; Lecomte, D. Separation Efficiency and Energy Consumption of Oil Expression Using a Screw-Press: The Case of Jatropha curcas L. Seeds. Ind. Crops Prod. 2014, 52, 752–761. [Google Scholar] [CrossRef]
- Zheng, Y.; Wiesenborn, D.P.; Tostenson, K.; Kangas, N. Screw Pressing of Whole and Dehulled Flaxseed for Organic Oil. J. Am. Oil Chem. Soc. 2003, 80, 1039–1045. [Google Scholar] [CrossRef]
- Savoire, R. Screw Pressing Application to Oilseeds. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Willems, P.; Kuipers, N.J.M.; de Haan, A.B. Hydraulic Pressing of Oilseeds: Experimental Determination and Modeling of Yield and Pressing Rates. J. Food Eng. 2008, 89, 8–16. [Google Scholar] [CrossRef]
- Rombaut, N.; Savoire, R.; Thomasset, B.; Castello, J.; Van Hecke, E.; Lanoisellé, J.-L. Optimization of Oil Yield and Oil Total Phenolic Content during Grape Seed Cold Screw Pressing. Ind. Crops Prod. 2015, 63, 26–33. [Google Scholar] [CrossRef]
- Saraf, S.; Thomas, B. Influence of Feedstock and Process Chemistry on Biodiesel Quality. Process Saf. Environ. Prot. 2007, 85, 360–364. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B. Development of Biodiesel: Current Scenario. Renew. Sustain. Energy Rev. 2009, 13, 1646–1651. [Google Scholar] [CrossRef]
- Canakci, M. The Potential of Restaurant Waste Lipids as Biodiesel Feedstocks. Bioresour. Technol. 2007, 98, 183–190. [Google Scholar] [CrossRef]
- Muthukumaran, C.; Praniesh, R.; Navamani, P.; Swathi, R.; Sharmila, G.; Kumar, N.M. Process Optimization and Kinetic Modeling of Biodiesel Production Using Non-Edible Madhuca indica Oil. Fuel 2017, 195, 217–225. [Google Scholar] [CrossRef]
- Ng, J.-H.; Ng, H.K.; Gan, S. Recent Trends in Policies, Socioeconomy and Future Directions of the Biodiesel Industry. Clean Technol. Environ. Policy 2010, 12, 213–238. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.A. High Quality Biodiesel and Its Diesel Engine Application: A Review. Renew. Sustain. energy Rev. 2010, 14, 1999–2008. [Google Scholar] [CrossRef]
- De Souza, S.P.; Pacca, S.; de Ávila, M.T.; Borges, J.L.B. Greenhouse Gas Emissions and Energy Balance of Palm Oil Biofuel. Renew. Energy 2010, 35, 2552–2561. [Google Scholar] [CrossRef]
- Ali, M.; Ghobadian, B.; Safa, M. Energy Life-Cycle Assessment and CO2 Emissions Analysis of Soybean- Based Biodiesel: A Case Study. J. Clean. Prod. 2014, 66, 233–241. [Google Scholar] [CrossRef]
- Sheehan, J.; Camobreco, V.; Duffield, J.; Graboski, M.; Shapouri, H. Life Cycle Inventory of Biodiesel and Petroleum Diesel for Use in an Urban Bus; Final Report; National Renewable Energy Lab. (NREL): Golden, CO, USA, 1998.
- Piastrellini, R.; Arena, A.P.; Civit, B. Energy Life-Cycle Analysis of Soybean Biodiesel: Effects of Tillage and Water Management. Energy 2017, 126, 13–20. [Google Scholar] [CrossRef]
- Sajid, Z.; Khan, F.; Zhang, Y. Process Simulation and Life Cycle Analysis of Biodiesel Production. Renew. Energy 2016, 85, 945–952. [Google Scholar] [CrossRef]
- Neag, E.; Stupar, Z.; Maicaneanu, S.A.; Roman, C. Advances in Biodiesel Production from Microalgae. Energies 2023, 16, 1129. [Google Scholar] [CrossRef]
- Maroušek, J.; Maroušková, A.; Gavurová, B.; Tuček, D.; Strunecký, O. Competitive Algae Biodiesel Depends on Advances in Mass Algae Cultivation. Bioresour. Technol. 2023, 374, 128802. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Yusof, N.M.; Kamis, N.H.H.; Shaleh, S.R.M. Optimization of the Ionic Liquid-Microwave Assisted One-Step Biodiesel Production Process from Wet Microalgal Biomass. Energy Convers. Manag. 2018, 171, 1397–1404. [Google Scholar] [CrossRef]
- Roy, M.; Mohanty, K. Valorization of De-Oiled Microalgal Biomass as a Carbon-Based Heterogeneous Catalyst for a Sustainable Biodiesel Production. Bioresour. Technol. 2021, 337, 125424. [Google Scholar] [CrossRef]
- Openshaw, K. A Review of Jatropha Curcas: An Oil Plant of Unfulfilled Promise. Biomass Bioenergy 2000, 19, 1–15. [Google Scholar] [CrossRef]
- Balat, M. Potential Alternatives to Edible Oils for Biodiesel Production—A Review of Current Work. Energy Convers. Manag. 2011, 52, 1479–1492. [Google Scholar] [CrossRef]
- Achten, W.M.J.; Verchot, L.; Franken, Y.J.; Mathijs, E.; Singh, V.P.; Aerts, R.; Muys, B. Jatropha Bio-Diesel Production and Use. Biomass Bioenergy 2008, 32, 1063–1084. [Google Scholar] [CrossRef]
- Ãzam, M.M.; Waris, A.; Nahar, N.M. Prospects and Potential of Fatty Acid Methyl Esters of Some Non-Traditional Seed Oils for Use as Biodiesel in India. Biomass Bioenergy 2005, 29, 293–302. [Google Scholar] [CrossRef]
- Scott, P.T.; Pregelj, L.; Chen, N.; Hadler, J.S.; Djordjevic, M.A.; Gresshoff, P.M. Pongamia pinnata: An Untapped Resource for the Biofuels Industry of the Future. Bioenergy Res. 2008, 1, 2–11. [Google Scholar] [CrossRef]
- Karmee, S.K.; Chadha, A. Preparation of Biodiesel from Crude Oil of Pongamia pinnata. Bioresour. Technol. 2005, 96, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.M.; Lee, K.T.; Bhatia, S. Feasibility of Edible Oil vs. Non-Edible Oil vs. Waste Edible Oil as Biodiesel Feedstock. Energy 2008, 33, 1646–1653. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S. Potential Non-Edible Oil Resources as Biodiesel Feedstock: An Indian Perspective. Renew. Sustain. Energy Rev. 2011, 15, 1791–1800. [Google Scholar] [CrossRef]
- Sanford, S.D.; White, J.M.; Shah, P.S.; Wee, C.; Valverde, M.A.; Meier, G.R. Feedstock and Biodiesel Characteristics Report; Renewable Energy Group: Ames, IA, USA, 2009; Volume 416, pp. 1–136. [Google Scholar]
- Venkanna, B.K.; Reddy, C.V. Biodiesel Production and Optimization from Calophyllum inophyllum Linn Oil (Honne Oil)—A Three Stage Method. Bioresour. Technol. 2009, 100, 5122–5125. [Google Scholar] [CrossRef]
- Pinzi, S.; Garcia, I.L.; Lopez-Gimenez, F.J.; Luque de Castro, M.D.; Dorado, G.; Dorado, M.P. The Ideal Vegetable Oil-Based Biodiesel Composition: A Review of Social, Economical and Technical Implications. Energy Fuels 2009, 23, 2325–2341. [Google Scholar] [CrossRef]
- Atabani, A.E.; César, S. Calophyllum inophyllum L.—A Prospective Non-Edible Biodiesel Feedstock. Study of Biodiesel Production, Properties, Fatty Acid Composition, Blending and Engine Performance. Renew. Sustain. Energy Rev. 2014, 37, 644–655. [Google Scholar] [CrossRef]
- Fattah, I.M.R.; Kalam, M.A.; Masjuki, H.H.; Wakil, M.A. Biodiesel Production, Characterization, Engine Performance, and Emission Characteristics of Malaysian Alexandrian Laurel Oil. RSC Adv. 2014, 4, 17787–17796. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Progress in Biodiesel Processing. Appl. Energy 2010, 87, 1815–1835. [Google Scholar] [CrossRef]
- Jena, P.C.; Raheman, H.; Kumar, G.V.P.; Machavaram, R. Biodiesel Production from Mixture of Mahua and Simarouba Oils with High Free Fatty Acids. Biomass Bioenergy 2010, 34, 1108–1116. [Google Scholar] [CrossRef]
- Ghadge, S.V.; Raheman, H. Biodiesel Production from Mahua (Madhuca indica) Oil Having High Free Fatty Acids. Biomass Bioenergy 2005, 28, 601–605. [Google Scholar] [CrossRef]
- Acharya, N.; Nanda, P.; Panda, S.; Acharya, S. Analysis of properties and estimation of optimum blending ratio of blended mahua biodiesel. Eng. Sci. Technol. Int. J. 2017, 20, 511–517. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, H.; Yan, Y. Physicochemical Properties of Stillingia Oil: Feasibility for Biodiesel Production by Enzyme Transesterification. Ind. Crops Prod. 2009, 30, 431–436. [Google Scholar] [CrossRef]
- Wang, R.; Hanna, M.A.; Zhou, W.-W.; Bhadury, P.S.; Chen, Q.; Song, B.-A.; Yang, S. Production and Selected Fuel Properties of Biodiesel from Promising Non-Edible Oils: Euphorbia lathyris L., Sapium sebiferum L. and Jatropha curcas L. Bioresour. Technol. 2011, 102, 1194–1199. [Google Scholar] [CrossRef]
- Murillo, G.; He, Y.; Yan, Y.; Sun, J.; Bartocci, P.; Ali, S.S.; Fantozzi, F. Ultrasonics—Sonochemistry Scaled-up Biodiesel Synthesis from Chinese Tallow Kernel Oil Catalyzed by Burkholderia Cepacia Lipase through Ultrasonic Assisted Technology: A Non-Edible and Alternative Source of Bio Energy. Ultrason. Sonochem. 2019, 58, 104658. [Google Scholar] [CrossRef]
- Ragit, S.S.; Mohapatra, S.K.; Kundu, K.; Gill, P. Optimization of Neem Methyl Ester from Transesterification Process and Fuel Characterization as a Diesel Substitute. Biomass Bioenergy 2011, 35, 1138–1144. [Google Scholar] [CrossRef]
- Karmakar, A.; Karmakar, S.; Mukherjee, S. Biodiesel Production from Neem towards Feedstock Diversification: Indian Perspective. Renew. Sustain. Energy Rev. 2012, 16, 1050–1060. [Google Scholar] [CrossRef]
- Okieimen, F.E.; Bakare, O.I.; Okieimen, C.O. Studies on the Epoxidation of Rubber Seed Oil. Ind. Crop. Prod. 2002, 15, 139–144. [Google Scholar] [CrossRef]
- Ahmad, J.; Yusup, S.; Bokhari, A.; Kamil, R.N.M. Study of Fuel Properties of Rubber Seed Oil Based Biodiesel. Energy Convers. Manag. 2014, 78, 266–275. [Google Scholar] [CrossRef]
- Misra, R.D.; Murthy, M.S. Performance, Emission and Combustion Evaluation of Soapnut Oil–Diesel Blends in a Compression Ignition Engine. Fuel 2011, 90, 2514–2518. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Tango, M.S.; Budge, S.M.; Watts, K.C.; Islam, M.R. Non-Edible Plant Oils as New Sources for Biodiesel Production. Int. J. Mol. Sci. 2008, 9, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Baruah, D.C. Production and Characterization of Biodiesel Obtained from Sapindus mukorossi Kernel Oil. Energy 2013, 60, 159–167. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, S.; Liu, X.; Yu, H.; Yu, D.; Li, G.; Wang, L. Oil Content, Fatty Acid Composition and Biodiesel Properties among Natural Provenances of Siberian Apricot (Prunus sibirica L.) from China. Gcb Bioenergy 2021, 13, 112–132. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Zhao, G.; Binkoski, A.E.; Coval, S.M.; Etherton, T.D. The Effects of Nuts on Coronary Heart Disease Risk. Nutr. Rev. 2001, 59, 103–111. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Z.; Zhao, H. Determination of Ability of Several Arbor and Shrub Species to Endure and Survive Extreme Aridity with Limited-Areas Methods under Field Conditions in Horqin Sandy Land. Acta Ecol. Sin. 2006, 26, 467–474. [Google Scholar] [CrossRef]
- Kale, S.S.; Darade, V.; Thakur, H.A. Analysis of Fixed Oil from Sterculia foetida Linn. Int. J. Pharm. Sci. Res. 2011, 2, 2908. [Google Scholar]
- Silitonga, A.S.; Masjuki, H.H.; Mahlia, T.M.I.; Ong, H.C.; Chong, W.T.; Boosroh, M.H. Overview Properties of Biodiesel Diesel Blends from Edible and Non-Edible Feedstock. Renew. Sustain. Energy Rev. 2013, 22, 346–360. [Google Scholar] [CrossRef]
- Devan, P.K.; Mahalakshmi, N. V Performance, Emission and Combustion Characteristics of Poon Oil and Its Diesel Blends in a DI Diesel Engine. Fuel 2009, 88, 861–867. [Google Scholar] [CrossRef]
- Atabani, A.E.; Mahlia, T.M.I.; Anjum Badruddin, I.; Masjuki, H.H.; Chong, W.T.; Lee, K.T. Investigation of Physical and Chemical Properties of Potential Edible and Non-Edible Feedstocks for Biodiesel Production, a Comparative Analysis. Renew. Sustain. Energy Rev. 2013, 21, 749–755. [Google Scholar] [CrossRef]
- Vipunngeun, N.; Palanuvej, C. Fatty Acids of Sterculia foetida Seed Oil. J. Health Res. 2009, 23, 157. [Google Scholar]
- Salunkhe, D.K.; Kadam, S. Handbook of Fruit Science and Technology: Production, Composition, Storage, and Processing; CRC Press: Boca Raton, FL, USA, 1995; ISBN 1482273454. [Google Scholar]
- Anwar, M.; Rasul, M.; Ashwath, N. Optimization of Biodiesel Production from Stone Fruit Kernel Oil. Energy Procedia 2019, 160, 268–276. [Google Scholar] [CrossRef]
- Yadav, A.K.; Pal, A.; Dubey, A.M. Experimental Studies on Utilization of Prunus armeniaca L. (Wild Apricot) Biodiesel as an Alternative Fuel for CI Engine. Waste Biomass Valor. 2018, 9, 1961–1969. [Google Scholar] [CrossRef]
- Kate, A.E.; Lohani, U.C.; Pandey, J.P.; Shahi, N.C.; Sarkar, A. Traditional and Mechanical Method of the Oil Extraction from Wild Apricot Kernel: A Comparative Study. Res. J. Chem. Environ. Sci. 2014, 2, 54–60. [Google Scholar]
- Sharma, R.; Gupta, A.; Abrol, G.S.; Joshi, V.K. Value Addition of Wild Apricot Fruits Grown in North–West Himalayan Regions-a Review. J. Food Sci. Technol. 2014, 51, 2917–2924. [Google Scholar] [CrossRef] [PubMed]
- Yu-Shan, W.; Zhi-Shu, X.; Zhi-Bin, Z. Seed Deposition Patterns of Oil Tea Camellia Oleifera Influenced by Seed-Caching Rodents. J. Integr. Plant Biol. 2004, 46, 773. [Google Scholar]
- Demirbas, A.; Kinsara, R.A. Cost Analysis of Biodiesel from Kernel Oil of Tea Seed. Energy Sources Part B Econ. Plan. Policy 2017, 12, 480–486. [Google Scholar] [CrossRef]
- Singh, R. Energy Sufficiency Aspirations of India and the Role of Renewable Resources: Scenarios for Future. Renew. Sustain. Energy Rev. 2018, 81, 2783–2795. [Google Scholar] [CrossRef]
- Karmakar, B.; Halder, G. Progress and Future of Biodiesel Synthesis: Advancements in Oil Extraction and Conversion Technologies. Energy Convers. Manag. 2019, 182, 307–339. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Zhang, Z.; Doherty, W.O.S.; O’Hara, I.M. The Outlook of the Production of Advanced Fuels and Chemicals from Integrated Oil Palm Biomass Biorefinery. Renew. Sustain. Energy Rev. 2019, 109, 386–411. [Google Scholar] [CrossRef]
- Lam, M.K.; Tan, K.T.; Lee, K.T.; Mohamed, A.R. Malaysian Palm Oil: Surviving the Food versus Fuel Dispute for a Sustainable Future. Renew. Sustain. Energy Rev. 2009, 13, 1456–1464. [Google Scholar] [CrossRef]
- Mohammed, M.A.A.; Salmiaton, A.; Azlina, W.W.; Amran, M.S.M.; Fakhru’l-Razi, A.; Taufiq-Yap, Y.H. Hydrogen Rich Gas from Oil Palm Biomass as a Potential Source of Renewable Energy in Malaysia. Renew. Sustain. Energy Rev. 2011, 15, 1258–1270. [Google Scholar] [CrossRef]
- Murata, Y.; Tanaka, R.; Fujimoto, K.; Kosugi, A.; Arai, T.; Togawa, E.; Takano, T.; Ibrahim, W.A.; Elham, P.; Sulaiman, O. Development of Sap Compressing Systems from Oil Palm Trunk. Biomass Bioenergy 2013, 51, 8–16. [Google Scholar] [CrossRef]
- Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Norhasyima, R.S. Comparison of Palm Oil, Jatropha curcas and Calophyllum inophyllum for Biodiesel: A Review. Renew. Sustain. Energy Rev. 2011, 15, 3501–3515. [Google Scholar] [CrossRef]
- Sumathi, S.; Chai, S.P.; Mohamed, A.R. Utilization of Oil Palm as a Source of Renewable Energy in Malaysia. Renew. Sustain. Energy Rev. 2008, 12, 2404–2421. [Google Scholar] [CrossRef]
- Deka, D.C.; Basumatary, S. High Quality Biodiesel from Yellow Oleander (Thevetia peruviana) Seed Oil. Biomass Bioenergy 2011, 35, 1797–1803. [Google Scholar] [CrossRef]
- Yadav, A.K.; Khan, M.E.; Pal, A.; Dubey, A.M. Performance, Emission and Combustion Characteristics of an Indica Diesel Engine Operated with Yellow Oleander (Thevetia peruviana) Oil Biodiesel Produced through Hydrodynamic Cavitation Method. Int. J. Ambient Energy 2018, 39, 365–371. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Watts, K.C.; Rahman, M.S.; Islam, M.R. Soapnut Extract as a Natural Surfactant for Enhanced Oil Recovery. Energy Sources Part A Recover. Util. Environ. Eff. 2009, 31, 1893–1903. [Google Scholar] [CrossRef]
- Alamu, O.J. Effect of Ethanol—Palm Kernel Oil Ratio on Alkali-Catalyzed Biodiesel Yield. Fuel 2008, 87, 1529–1533. [Google Scholar] [CrossRef]
- Kavitha, M.S.; Murugavelh, S. Optimization and Transesteri Fi Cation of Sterculia Oil: Assessment of Engine Performance, Emission and Combustion Analysis. J. Clean. Prod. 2019, 234, 1192–1209. [Google Scholar] [CrossRef]
- Canoira, L.; Alcantara, R.; García-Martínez, M.J.; Carrasco, J. Biodiesel from Jojoba Oil-Wax: Transesterification with Methanol and Properties as a Fuel. Biomass Bioenergy 2006, 30, 76–81. [Google Scholar] [CrossRef]
- Wood, J.A.; Malcolmson, L.; Tiwari, B.; Gowen, A.; Mckenna, B. Pulse Foods: Processing, Quality and Nutraceutical Applications; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Shukla, B.D.; Srivastava, P.K.; Gupta, R.K. Oilseeds Processing Technology; Central Institute of Agricultural Engineering: Nabi Bagh, India, 1992.
- Carré, P.; Citeau, M.; Robin, G.; Estorges, M. Hull Content and Chemical Composition of Whole Seeds, Hulls and Germs in Cultivars of Rapeseed (Brassica napus). OCL 2016, 23, A302. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S. An Evaluation of Multipurpose Oil Seed Crop for Industrial Uses (Jatropha curcas L.): A Review. Ind. Crops Prod. 2008, 28, 1–10. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, H.; Xie, Y.; Liao, Q.; Lin, Y.; Liu, Y.; Liu, Y.; Xiao, H.; Gao, Z.; Hu, S. Postharvest Processing and Storage Methods for Camellia Oleifera Seeds. Food Rev. Int. 2020, 36, 319–339. [Google Scholar] [CrossRef]
- Martinez-Soberanes, E.E.; Mustafa, R.; Reaney, M.J.T.; Zhang, W.J. Seed Hull Utilization. In Food Wastes and By-Products: Nutraceutical and Health Potential; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 291–326. [Google Scholar] [CrossRef]
- Oomah, B.D.; Ward, S.; Balasubramanian, P. Dehulling and Selected Physical Characteristics of Canadian Dry Bean (Phaseolus vulgaris L.) Cultivars. Food Res. Int. 2010, 43, 1410–1415. [Google Scholar] [CrossRef]
- Ikebudu, J.A.; Sokhansanj, S.; Tyler, R.T.; Milne, B.J.; Thakor, N.S. Grain Conditioning for Dehulling of Canola. Can. Agric. Eng. 2000, 42, 27–32. [Google Scholar]
- Ashwath, N. Evaluating Biodiesel Potential of Australian Native and Naturalised Plant Species; CQUniversity: Sydney, Australia, 2010; ISBN 174254181X. [Google Scholar]
- Cao, L.; Zhang, S. Production and Characterization of Biodiesel Derived from Hodgsonia Macrocarpa Seed Oil. Appl. Energy 2015, 146, 135–140. [Google Scholar] [CrossRef]
- Indrasari, S.D.; Koswara, S.; Muchtadi, D.; Nagara, L.M. The Effect of Heating on the Physicochemical Characteristics of Rice Bran Oil. Indones. J. Agric. Sci. 2001, 2, 1–5. [Google Scholar] [CrossRef]
- Pradhan, R.C.; Mishra, S.; Naik, S.N.; Bhatnagar, N.; Vijay, V.K. Oil Expression from Jatropha Seeds Using a Screw Press Expeller. Biosyst. Eng. 2011, 109, 158–166. [Google Scholar] [CrossRef]
- Jaswant, S.; Bargale, P.C. Mechanical Expression of Oil from Linseed (Linum usitatissimum L.). J. Oilseeds Res. 1990, 7, 106–110. [Google Scholar]
- Lim, S.; Lee, K.T. Process Intensification for Biodiesel Production from Jatropha curcas L. Seeds: Supercritical Reactive Extraction Process Parameters Study. Appl. Energy 2013, 103, 712–720. [Google Scholar] [CrossRef]
- Franco, D.; Sineiro, J.; Núñez, M.J. Analysis of Variables and Modeling of Gevuina Avellana Oil Extraction with Ethanol near Azeotrope Conditions. J. Food Process Eng. 2009, 32, 664–681. [Google Scholar] [CrossRef]
- Karlovic, D.; Sovilj, M.; Turkulov, J. Kinetics of Oil Extraction from Corn Germ. J. Am. Oil Chem. Soc. 1992, 69, 471–476. [Google Scholar] [CrossRef]
- Nagy, B.; Simándi, B. Effects of Particle Size Distribution, Moisture Content, and Initial Oil Content on the Supercritical Fluid Extraction of Paprika. J. Supercrit. Fluids 2008, 46, 293–298. [Google Scholar] [CrossRef]
- Contran, N.; Chessa, L.; Lubino, M.; Bellavite, D.; Paolo, P.; Enne, G. State-of-the-Art of the Jatropha curcas Productive Chain: From Sowing to Biodiesel and by-Products. Ind. Crops Prod. 2015, 42, 202–215. [Google Scholar] [CrossRef]
- Koh, M.Y.; Idaty, T.; Ghazi, M. A Review of Biodiesel Production from Jatropha curcas L. Oil. Renew. Sustain. Energy Rev. 2011, 15, 2240–2251. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A Review on Biodiesel Production Using Catalyzed Transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Wilson, P. Biodiesel Production from Jatropha curcas: A Review. Sci. Res. Essays 2010, 5, 1796–1808. [Google Scholar]
- Ye, M.; Li, C.; Francis, G.; Makkar, H.P.S. Current Situation and Prospects of Jatropha curcas as a Multipurpose Tree in China. Agrofor. Syst. 2009, 76, 487–497. [Google Scholar] [CrossRef]
- Devappa, R.K.; Makkar, H.P.S.; Becker, K. Biodegradation of Jatropha curcas Phorbol Esters in Soil. J. Sci. Food Agric. 2010, 90, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Gunaseelan, V.N. Biomass Estimates, Characteristics, Biochemical Methane Potential, Kinetics and Energy Flow from Jatropha curcus on Dry Lands. Biomass Bioenergy 2009, 33, 589–596. [Google Scholar] [CrossRef]
- Mahanta, N.; Gupta, A.; Khare, S.K. Production of Protease and Lipase by Solvent Tolerant Pseudomonas Aeruginosa PseA in Solid-State Fermentation Using Jatropha curcas Seed Cake as Substrate. Bioresour. Technol. 2008, 99, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Francis, G.; Becker, K. Protein Concentrate from Jatropha curcas Screw-pressed Seed Cake and Toxic and Antinutritional Factors in Protein Concentrate. J. Sci. Food Agric. 2008, 88, 1542–1548. [Google Scholar] [CrossRef]
- Savoire, R.; Lanoisellé, J.-L.; Vorobiev, E. Mechanical Continuous Oil Expression from Oilseeds: A Review. Food Bioprocess Technol. 2013, 6, 1–16. [Google Scholar] [CrossRef]
- Anderson, D. A Primer on Oils Processing Technology. In Bailey’s Industrial Oil and Fat Products; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; Volume 5, pp. 1–56. [Google Scholar]
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The Antioxidant Activity and Oxidative Stability of Cold-pressed Oils. J. Am. Oil Chem. Soc. 2014, 91, 1291–1301. [Google Scholar] [CrossRef]
- Azadmard-Damirchi, S.; Alirezalu, K.; Achachlouei, B.F. Microwave Pretreatment of Seeds to Extract High Quality Vegetable Oil. World Acad Sci. Eng Technol 2011, 57, 72–75. [Google Scholar]
- Bhuiya, M.M.K.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Azad, A.K. Prospects of 2nd Generation Biodiesel as a Sustainable Fuel—Part: 1 Selection of Feedstocks, Oil Extraction Techniques and Conversion Technologies. Renew. Sustain. Energy Rev. 2016, 55, 1109–1128. [Google Scholar] [CrossRef]
- Sambasivam, K.M.; Murugavelh, S. Optimisation, Experimental Validation and Thermodynamic Study of the Sequential Oil Extraction and Biodiesel Production Processes from Seeds of Sterculia foetida. Environ. Sci. Pollut. Res. 2019, 26, 31301–31314. [Google Scholar] [CrossRef]
- Kou, D.; Mitra, S. Extraction of Semivolatile Organic Compounds from Solid Matrices. In Sample Preparation Techniques in Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003; Volume 162, pp. 139–182. [Google Scholar]
- Sivakumar, P.; Parthiban, K.S.; Sivakumar, P.; Vinoba, M.; Renganathan, S. Optimization of Extraction Process and Kinetics of Sterculia foetida Seed Oil and Its Process Augmentation for Biodiesel Production. Ind. Eng. Chem. Res. 2012, 51, 8992–8998. [Google Scholar] [CrossRef]
- Mahanta, P.; Shrivastava, A. Technology Development of Bio-Diesel as an Energy Alternative; Department of Mechanical Engineering, Indian Institute of Technology: Guwahati, India, 2004; pp. 1–19. Available online: https://www.newagepublishers.com/samplechapter/001305.pdf (accessed on 17 October 2023).
- Chemat, F.; Cravotto, G. Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 4, ISBN 1461448301. [Google Scholar]
- Chemat, F.; Khan, M.K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging Opportunities for the Effective Valorization of Wastes and By-Products Generated during Olive Oil Production Process: Non-Conventional Methods for the Recovery of High-Added Value Compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Luque-Garcıa, J.L.; de Castro, M.D.L. Ultrasound-Assisted Soxhlet Extraction: An Expeditive Approach for Solid Sample Treatment: Application to the Extraction of Total Fat from Oleaginous Seeds. J. Chromatogr. A 2004, 1034, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Koubaa, M.; Mhemdi, H.; Barba, F.J.; Roohinejad, S.; Greiner, R.; Vorobiev, E. Oilseed Treatment by Ultrasounds and Microwaves to Improve Oil Yield and Quality: An Overview. Food Res. Int. 2016, 85, 59–66. [Google Scholar] [CrossRef]
- Wei, F.; Gao, G.-Z.; Wang, X.-F.; Dong, X.-Y.; Li, P.-P.; Hua, W.; Wang, X.; Wu, X.-M.; Chen, H. Quantitative Determination of Oil Content in Small Quantity of Oilseed Rape by Ultrasound-Assisted Extraction Combined with Gas Chromatography. Ultrason. Sonochem. 2008, 15, 938–942. [Google Scholar] [CrossRef]
- Farahani, G.T.; Azari, P.Y. Improving the Oil Yield of Iranian Jatropha curcas Seeds by Optimising Ultrasound-Assisted Ethanolic Extraction Process: A Response Surface Method. Qual. Assur. Saf. Crops Foods 2016, 8, 95–104. [Google Scholar] [CrossRef]
- Koubaa, M.; Rosello-Soto, E.; Žlabur, J.; Rezek Jambrak, A.; Brncic, M.; Grimi, N.; Boussetta, N.; Barba, F.J. Current and New Insights in the Sustainable and Green Recovery of Nutritionally Valuable Compounds from Stevia Rebaudiana Bertoni. J. Agric. Food Chem. 2015, 63, 6835–6846. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Parniakov, O.; Deng, Q.; Patras, A.; Koubaa, M.; Grimi, N.; Boussetta, N.; Tiwari, B.K.; Vorobiev, E.; Lebovka, N. Application of Non-Conventional Extraction Methods: Toward a Sustainable and Green Production of Valuable Compounds from Mushrooms. Food Eng. Rev. 2016, 8, 214–234. [Google Scholar] [CrossRef]
- Ideris, F.; Nomanbhay, S.; Kusumo, F.; Silitonga, A.S.; Ong, M.Y.; Ong, H.C.; Mahlia, T.M.I. Optimization of Ultrasound-Assisted Oil Extraction from Canarium odontophyllum Kernel as a Novel Biodiesel Feedstock. J. Clean. Prod. 2021, 288, 125563. [Google Scholar] [CrossRef]
- Golmakani, M.-T.; Rezaei, K. Comparison of Microwave-Assisted Hydrodistillation Withthe Traditional Hydrodistillation Method in the Extractionof Essential Oils from Thymus vulgaris L. Food Chem. 2008, 109, 925–930. [Google Scholar] [CrossRef]
- Hao, J.; Han, W.; Xue, B.; Deng, X. Microwave-Assisted Extraction of Artemisinin from Artemisia annua L. Sep. Purif. Technol. 2002, 28, 191–196. [Google Scholar] [CrossRef]
- Letellier, M.; Budzinski, H.; Charrier, L.; Capes, S.; Dorthe, A.M. Optimization by Factorial Design of Focused Microwave Assisted Extraction of Polycyclic Aromatic Hydrocarbons from Marine Sediment. Fresenius’ J. Anal. Chem. 1999, 364, 228–237. [Google Scholar] [CrossRef]
- Pawelzik, E.; Irfan, I.; Luecke, W. Microwave Treatment of Rape to Ensure Seed Quality during Storage. In Proceedings of the 7th International Working Conference on Stored-Product Protection, Beijing, China, 14–19 October 1998; Sichuan Publishing House of Science and Technology: Chengdu, China, 1998; Volume 2, pp. 1671–1675. [Google Scholar]
- Azadmard-Damirchi, S.; Habibi-Nodeh, F.; Hesari, J.; Nemati, M.; Achachlouei, B.F. Effect of Pretreatment with Microwaves on Oxidative Stability and Nutraceuticals Content of Oil from Rapeseed. Food Chem. 2010, 121, 1211–1215. [Google Scholar] [CrossRef]
- Rosenthal, A.; Pyle, D.L.; Niranjan, K.; Gilmour, S.; Trinca, L. Combined Effect of Operational Variables and Enzyme Activity on Aqueous Enzymatic Extraction of Oil and Protein from Soybean. Enzyme Microb. Technol. 2001, 28, 499–509. [Google Scholar] [CrossRef]
- Shah, S.; Sharma, A.; Gupta, M.N. Extraction of Oil from Jatropha curcas L. Seed Kernels by Combination of Ultrasonication and Aqueous Enzymatic Oil Extraction. Bioresour. Technol. 2005, 96, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.; Pyle, D.L.; Niranjan, K. Aqueous and Enzymatic Processes for Edible Oil Extraction. Enzyme Microb. Technol. 1996, 19, 402–420. [Google Scholar] [CrossRef]
- Rao, T.V.; Rao, G.P.; Reddy, K.H.C. Experimental Investigation of Pongamia, Jatropha and Neem Methyl Esters as Biodiesel on CI Engine. Jordan J. Mech. Ind. Eng. 2008, 2, 117–122. [Google Scholar]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Biodiesel Production from High FFA Rubber Seed Oil. Fuel 2005, 84, 335–340. [Google Scholar] [CrossRef]
- Lamsal, B.P.; Johnson, L.A. Separating Oil from Aqueous Extraction Fractions of Soybean. J. Am. Oil Chem. Soc. 2007, 84, 785–792. [Google Scholar] [CrossRef]
- Liu, Z.; Gui, M.; Xu, T.; Zhang, L.; Kong, L.; Qin, L.; Zou, Z. Industrial Crops & Products Efficient Aqueous Enzymatic-Ultrasonication Extraction of Oil from Sapindus mukorossi Seed Kernels. Ind. Crops Prod. 2019, 134, 124–133. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, N.; Muk, H.; Chang, H. A Study on the Performance and Emission of a Diesel Engine Fueled with Karanja Biodiesel and Its Blends. Energy 2013, 56, 1–7. [Google Scholar] [CrossRef]
- Sanli, M.C.H. Biodiesel Production from Various Feedstocks and Their e V Ects on the Fuel Properties. J. Ind. Microbiol. Biotechnol. 2008, 35, 431–441. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Das, L.M. Process Optimization for Biodiesel Production from Jatropha, Karanja and Polanga Oils. Fuel 2009, 88, 1588–1594. [Google Scholar] [CrossRef]
- Olisakwe, H.C.; Tuleun, L.T.; Eloka-Eboka, A.C. Comparative Study of Thevetia peruviana and Jatropha curcas Seed Oils as Feedstock for Grease Production. Int. J. Eng. Res. Appl. 2011, 1, 793–806. [Google Scholar]
- Saravanan, N.; Nagarajan, G.; Puhan, S. Experimental Investigation on a DI Diesel Engine Fuelled with Madhuca indica Ester and Diesel Blend. Biomass Bioenergy 2010, 34, 838–843. [Google Scholar] [CrossRef]
- Wang, L.; Yu, H. Biodiesel from Siberian Apricot (Prunus sibirica L.) Seed Kernel Oil. Bioresour. Technol. 2012, 112, 355–358. [Google Scholar] [CrossRef]
- Devan, P.K.; Mahalakshmi, N. V Study of the Performance, Emission and Combustion Characteristics of a Diesel Engine Using Poon Oil-Based Fuels. Fuel Process. Technol. 2009, 90, 513–519. [Google Scholar] [CrossRef]
- Fukuda, H.; Kond, A.; Noda, H. Biodiesel Fuel Production by Transesterification. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, M.P. Prospects of Biodiesel from Jatropha in India: A Review. Renew. Sustain. Energy Rev. 2010, 14, 763–771. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel Production from Vegetable Oils via Catalytic and Non-Catalytic Supercritical Methanol Transesterification Methods. Prog. Energy Combust. Sci. 2005, 31, 466–487. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Zhao, C.; Ding, Y.; Xu, P. Bioresource Technology Biodiesel Production in Packed-Bed Reactors Using Lipase—Nanoparticle Biocomposite. Bioresour. Technol. 2011, 102, 6352–6355. [Google Scholar] [CrossRef] [PubMed]
- Barnwal, B.K.; Sharma, M.P. Prospects of Biodiesel Production from Vegetable Oils in India. Renew. Sustain. Energy Rev. 2005, 9, 363–378. [Google Scholar] [CrossRef]
- Gandhi, B.S.; Chelladurai, S.S.; Kumaran, D.S. Process Optimization for Biodiesel Synthesis from Jatropha curcas Oil. Distrib. Gener. Altern. Energy J. 2011, 26, 6–16. [Google Scholar]
- Ghadge, S.V.; Raheman, H. Process Optimization for Biodiesel Production from Mahua (Madhuca indica) Oil Using Response Surface Methodology. Bioresour. Technol. 2006, 97, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Sharon, H.; Karuppasamy, K.; Kumar, D.R.S.; Sundaresan, A. A Test on DI Diesel Engine Fueled with Methyl Esters of Used Palm Oil. Renew. Energy 2012, 47, 160–166. [Google Scholar] [CrossRef]
- Gurunathan, B.; Ravi, A. Biodiesel Production from Waste Cooking Oil Using Copper Doped Zinc Oxide Nanocomposite as Heterogeneous Catalyst. Bioresour. Technol. 2015, 188, 124–127. [Google Scholar] [CrossRef]
- Serin, H.; Ozcanli, M.; Kemal Gokce, M.; Tuccar, G. Biodiesel Production from Tea Seed (Camellia sinensis) Oil and Its Blends with Diesel Fuel. Int. J. Green Energy 2013, 10, 370–377. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B. A Hybrid Feedstock for a Very Efficient Preparation of Biodiesel. Fuel Process. Technol. 2010, 91, 1267–1273. [Google Scholar] [CrossRef]
- Alamu, O.J.; Akintola, T.A.; Enweremadu, C.C.; Adeleke, A.E. Characterization of Palm-Kernel Oil Biodiesel Produced through NaOH-Catalysed Transesterification Process. Sci. Res. Essay 2008, 3, 308–311. [Google Scholar]
- Schuchardt, U.; Sercheli, R.; Vargas, R.M. Transesterification of Vegetable Oils: A Review. J. Braz. Chem. Soc. 1998, 9, 199–210. [Google Scholar] [CrossRef]
- Demirbas, A. Comparison of Transesterification Methods for Production of Biodiesel from Vegetable Oils and Fats. Energy Convers. Manag. 2008, 49, 125–130. [Google Scholar] [CrossRef]
- Frascari, D.; Zuccaro, M.; Pinelli, D.; Paglianti, A. A Pilot-Scale Study of Alkali-Catalyzed Sunflower Oil Transesterification with Static Mixing and with Mechanical Agitation. Energy Fuels 2008, 22, 1493–1501. [Google Scholar] [CrossRef]
- Noureddini, H.; Gao, X.; Philkana, R.S. Immobilized Pseudomonas Cepacia Lipase for Biodiesel Fuel Production from Soybean Oil. Bioresour. Technol. 2005, 96, 769–777. [Google Scholar] [CrossRef]
- Cetinkaya, M.; Karaosmanoǧlu, F. Optimization of Base-Catalyzed Transesterification Reaction of Used Cooking Oil. Energy Fuels 2004, 18, 1888–1895. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.O.; Madzimbamuto, T.F. ScienceDirect A Review of Current Technology for Biodiesel Production: State of the Art. Biomass Bioenergy 2013, 61, 276–297. [Google Scholar] [CrossRef]
- Maçaira, J.; Santana, A.; Recasens, F.; Larrayoz, M.A. Biodiesel Production Using Supercritical Methanol/Carbon Dioxide Mixtures in a Continuous Reactor. Fuel 2011, 90, 2280–2288. [Google Scholar] [CrossRef]
- Kiss, A.A. Novel Process for Biodiesel by Reactive Absorption. Sep. Purif. Technol. 2009, 69, 280–287. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.R.A.; Sulaiman, N.M.N. Production of Biodiesel Using High Free Fatty Acid Feedstocks. Renew. Sustain. Energy Rev. 2012, 16, 3275–3285. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Betiku, E.; Ikhuomoregbe, D.I.O.; Ojumu, T.V. Production of Biodiesel from Crude Neem Oil Feedstock and Its Emissions from Internal Combustion Engines. African J. Biotechnol. 2012, 11, 6178–6186. [Google Scholar] [CrossRef]
- Aranda, D.A.G.; Santos, R.T.P.; Tapanes, N.C.O.; Ramos, A.L.D.; Antunes, O.A.C. Acid-Catalyzed Homogeneous Esterification Reaction for Biodiesel Production from Palm Fatty Acids. Catal. Letters 2008, 122, 20–25. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, L. Acid-Catalyzed Esterification of Zanthoxylum Bungeanum Seed Oil with High Free Fatty Acids for Biodiesel Production. Bioresour. Technol. 2008, 99, 8995–8998. [Google Scholar] [CrossRef]
- Berchmans, H.J.; Hirata, S. Biodiesel Production from Crude Jatropha curcas L. Seed Oil with a High Content of Free Fatty Acids. Bioresour. Technol. 2008, 99, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Fang, Z.; Liu, Y. Ultrasonic Transesterification of Jatropha curcas L. Oil to Biodiesel by a Two-Step Process. Energy Convers. Manag. 2010, 51, 2802–2807. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, S.; Liu, P.; Xue, F.; Tang, S. Comparison of Two Different Processes to Synthesize Biodiesel by Waste Cooking Oil. J. Mol. Catal. A Chem. 2006, 252, 107–112. [Google Scholar] [CrossRef]
- Çaylı, G.; Küsefoğlu, S. Increased Yields in Biodiesel Production from Used Cooking Oils by a Two Step Process: Comparison with One Step Process by Using TGA. Fuel Process. Technol. 2008, 89, 118–122. [Google Scholar] [CrossRef]
- Endalew, A.K.; Kiros, Y.; Zanzi, R. Heterogeneous Catalysis for Biodiesel Production from Jatropha curcas Oil (JCO). Energy 2011, 36, 2693–2700. [Google Scholar] [CrossRef]
- Yan, S.; DiMaggio, C.; Mohan, S.; Kim, M.; Salley, S.O.; Ng, K.Y. Advancements in Heterogeneous Catalysis for Biodiesel Synthesis. Top. Catal. 2010, 53, 721–736. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.R.; Aziz, N.; Fernando, W.J.N.; Kim, J. Technologies for Production of Biodiesel Focusing on Green Catalytic Techniques: A Review. Fuel Process. Technol. 2009, 90, 1502–1514. [Google Scholar] [CrossRef]
- Zhang, S.; Zu, Y.-G.; Fu, Y.-J.; Luo, M.; Zhang, D.-Y.; Efferth, T. Rapid Microwave-Assisted Transesterification of Yellow Horn Oil to Biodiesel Using a Heteropolyacid Solid Catalyst. Bioresour. Technol. 2010, 101, 931–936. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Yang, R.; Yan, Y. Biodiesel Production from Vegetable Oil Using Heterogenous Acid and Alkali Catalyst. Fuel 2010, 89, 2939–2944. [Google Scholar] [CrossRef]
- Arumugam, A. Production of Biodiesel from Non-Edible Sources: Technological Updates; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 032385897X. [Google Scholar]
- Parangi, T.; Mishra, M.K. Solid Acid Catalysts for Biodiesel Production. Comments Inorg. Chem. 2020, 40, 176–216. [Google Scholar] [CrossRef]
- Dhawane, S.H.; Kumar, T.; Halder, G. Parametric Effects and Optimization on Synthesis of Iron (II) Doped Carbonaceous Catalyst for the Production of Biodiesel. Energy Convers. Manag. 2016, 122, 310–320. [Google Scholar] [CrossRef]
- Vasić, K.; Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. Biodiesel Production Using Solid Acid Catalysts Based on Metal Oxides. Catalysts 2020, 10, 237. [Google Scholar] [CrossRef]
- Borah, M.J.; Devi, A.; Borah, R.; Deka, D. Synthesis and Application of Co Doped ZnO as Heterogeneous Nanocatalyst for Biodiesel Production from Non-Edible Oil. Renew. Energy 2019, 133, 512–519. [Google Scholar] [CrossRef]
- Patil, A.; Baral, S.S.; Dhanke, P.; Kore, V. Biodiesel Production Using Prepared Novel Surface Functionalised TiO2 Nano-Catalyst in Hydrodynamic Cavitation Reactor. Mater. Today Proc. 2020, 27, 198–203. [Google Scholar] [CrossRef]
- Babajide, O.; Petrik, L.; Musyoka, N.; Amigun, B.; Ameer, F. Use of Coal Fly Ash as a Catalyst in the Production of Biodiesel. Pet. Coal 2010, 52, 261–272. [Google Scholar]
- Sharma, Y.C.; Singh, B.; Korstad, J. Application of an Efficient Nonconventional Heterogeneous Catalyst for Biodiesel Synthesis from Pongamia pinnata Oil. Energy Fuels 2010, 24, 3223–3231. [Google Scholar] [CrossRef]
- Kotwal, M.S.; Niphadkar, P.S.; Deshpande, S.S.; Bokade, V.V.; Joshi, P.N. Transesterification of Sunflower Oil Catalyzed by Flyash-Based Solid Catalysts. Fuel 2009, 88, 1773–1778. [Google Scholar] [CrossRef]
- Ngamcharussrivichai, C.; Nunthasanti, P.; Tanachai, S.; Bunyakiat, K. Biodiesel Production through Transesterification over Natural Calciums. Fuel Process. Technol. 2010, 91, 1409–1415. [Google Scholar] [CrossRef]
- Gog, A.; Roman, M.; Toşa, M.; Paizs, C.; Irimie, F.D. Biodiesel Production Using Enzymatic Transesterification—Current State and Perspectives. Renew. Energy 2012, 39, 10–16. [Google Scholar] [CrossRef]
- Antczak, M.S.; Kubiak, A.; Antczak, T.; Bielecki, S. Enzymatic Biodiesel Synthesis—Key Factors Affecting Efficiency of the Process. Renew. Energy 2009, 34, 1185–1194. [Google Scholar] [CrossRef]
- Dizge, N.; Keskinler, B. Enzymatic Production of Biodiesel from Canola Oil Using Immobilized Lipase. Biomass Bioenergy 2008, 32, 1274–1278. [Google Scholar] [CrossRef]
- Shah, S.; Gupta, M.N. Lipase Catalyzed Preparation of Biodiesel from Jatropha Oil in a Solvent Free System. Process Biochem. 2007, 42, 409–414. [Google Scholar] [CrossRef]
- Lee, M.; Lee, J.; Lee, D.; Cho, J.; Kim, S.; Park, C. Improvement of Enzymatic Biodiesel Production by Controlled Substrate Feeding Using Silica Gel in Solvent Free System. Enzyme Microb. Technol. 2011, 49, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Kaieda, M.; Samukawa, T.; Matsumoto, T.; Ban, K.; Kondo, A.; Shimada, Y.; Noda, H.; Nomoto, F.; Ohtsuka, K.; Izumoto, E. Biodiesel Fuel Production from Plant Oil Catalyzed by Rhizopus oryzae Lipase in a Water-Containing System without an Organic Solvent. J. Biosci. Bioeng. 1999, 88, 627–631. [Google Scholar] [CrossRef]
- Chen, J.-W.; Wu, W.-T. Regeneration of Immobilized Candida antarctica Lipase for Transesterification. J. Biosci. Bioeng. 2003, 95, 466–469. [Google Scholar] [CrossRef]
- Nelson, L.A.; Foglia, T.A.; Marmer, W.N. Lipase-Catalyzed Production of Biodiesel. J. Am. Oil Chem. Soc. 1996, 73, 1191–1195. [Google Scholar] [CrossRef]
- Soumanou, M.M.; Bornscheuer, U.T. Improvement in Lipase-Catalyzed Synthesis of Fatty Acid Methyl Esters from Sunflower Oil. Enzyme Microb. Technol. 2003, 33, 97–103. [Google Scholar] [CrossRef]
- Meher, L.C.; Sagar, D.V.; Naik, S.N. Technical Aspects of Biodiesel Production by Transesterification—A Review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Vázquez, V.Á.; Flores, M.M.A.; Casas, L.F.H.; Castillo, N.A.M.; Uribe, A.R.; Aguado, H.C.C. Biodiesel Production Catalyzed by Lipase Extract Powder of Leonotis nepetifolia (Christmas Candlestick) Seed. Energies 2023, 16, 2848. [Google Scholar] [CrossRef]
- Santos, F.F.P.; Rodrigues, S.; Fernandes, F.A.N. Optimization of the Production of Biodiesel from Soybean Oil by Ultrasound Assisted Methanolysis. Fuel Process. Technol. 2009, 90, 312–316. [Google Scholar] [CrossRef]
- Fan, X.; Wang, X.; Chen, F. Ultrasonically Assisted Production of Biodiesel from Crude Cottonseed Oil. Int. J. Green Energy 2010, 7, 117–127. [Google Scholar] [CrossRef]
- Oliveira, P.A.; Baesso, R.M.; Morais, G.C.; Alvarenga, A.V.; Costa-Félix, R.P.B. Ultrasound-Assisted Transesterification of Soybean Oil Using Low Power and High Frequency and No External Heating Source. Ultrason. Sonochem. 2021, 78, 105709. [Google Scholar] [CrossRef] [PubMed]
- Sebayang, A.H.; Kusumo, F.; Milano, J.; Shamsuddin, A.H.; Silitonga, A.S.; Ideris, F.; Siswantoro, J.; Veza, I.; Mofijur, M.; Chia, S.R. Optimization of Biodiesel Production from Rice Bran Oil by Ultrasound and Infrared Radiation Using ANN-GWO. Fuel 2023, 346, 128404. [Google Scholar] [CrossRef]
- Azcan, N.; Danisman, A. Alkali Catalyzed Transesterification of Cottonseed Oil by Microwave Irradiation. Fuel 2007, 86, 2639–2644. [Google Scholar] [CrossRef]
- Groisman, Y.; Gedanken, A. Continuous Flow, Circulating Microwave System and Its Application in Nanoparticle Fabrication and Biodiesel Synthesis. J. Phys. Chem. C 2008, 112, 8802–8808. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V. V Biodiesel Production from Renewable Feedstocks: Status and Opportunities. Renew. Sustain. Energy Rev. 2012, 16, 4763–4784. [Google Scholar] [CrossRef]
- Marra, F.; de Bonis, M.V.; Ruocco, G. Combined Microwaves and Convection Heating: A Conjugate Approach. J. Food Eng. 2010, 97, 31–39. [Google Scholar] [CrossRef]
- Vyas, A.P.; Verma, J.L.; Subrahmanyam, N. A Review on FAME Production Processes. Fuel 2010, 89, 1–9. [Google Scholar] [CrossRef]
- Rokni, K.; Mostafaei, M.; Soufi, M.D.; Kahrizi, D. Microwave-Assisted Intensification of Transesterification Reaction for Biodiesel Production from Camelina Oil: Optimization by Box-Behnken Design. Bioresour. Technol. Rep. 2022, 17, 100928. [Google Scholar] [CrossRef]
- Kavitha, M. In Situ Acid Catalysed Transesterification of Biodiesel Production from Sterculia foetida Oil and Seed. Int. J. Green Energy 2019, 16, 1465–1474. [Google Scholar] [CrossRef]
- Kildiran, G.; Yücel, S.Ö.; Türkay, S. In-situ Alcoholysis of Soybean Oil. J. Am. Oil Chem. Soc. 1996, 73, 225–228. [Google Scholar] [CrossRef]
- Georgogianni, K.G.; Kontominas, M.G.; Pomonis, P.J.; Avlonitis, D.; Gergis, V. Conventional and in Situ Transesterification of Sunflower Seed Oil for the Production of Biodiesel. Fuel Process. Technol. 2008, 89, 503–509. [Google Scholar] [CrossRef]
- Shuit, S.H.; Lee, K.T.; Kamaruddin, A.H.; Yusup, S. Reactive Extraction and in Situ Esterification of Jatropha curcas L. Seeds for the Production of Biodiesel. Fuel 2010, 89, 527–530. [Google Scholar] [CrossRef]
- Choi, W.; Kim, G.; Lee, S.; Lee, H. Biodiesel Production from Scenedesmus sp. through Optimized in Situ Acidic Transesterification Process. Chem. Biochem. Eng. Q. 2014, 28, 367–374. [Google Scholar] [CrossRef]
- Koberg, M.; Gedanken, A. Direct Transesterification of Castor and Jatropha Seeds for FAME Production by Microwave and Ultrasound Radiation Using a SrO Catalyst. BioEnergy Res. 2012, 5, 958–968. [Google Scholar] [CrossRef]
- Koutsouki, A.A.; Tegou, E.; Kontakos, S.; Kontominas, M.G.; Pomonis, P.J.; Manos, G. In Situ Transesterification of Cynara Cardunculus L. Seed Oil via Direct Ultrasonication for the Production of Biodiesel. Fuel Process. Technol. 2015, 134, 122–129. [Google Scholar] [CrossRef]
- Kavitha, M.S.; Murugavelh, S. Biodiesel Production from Reactive Extraction of Sterculia and Waste Cooking Oil Blend Using an Acid Catalyst Biodiesel Production from Reactive Extraction of Sterculia and Waste Cooking Oil Blend Using an Acid. Int. J. Ambient. Energy 2019, 42, 1435–1440. [Google Scholar] [CrossRef]
- Tarigan, J.B.; Prakoso, H.T.; Siahaan, D.; Kaban, J. Rapid Biodiesel Production from Palm Kernel through in Situ Transesterification Reaction Using CaO as Catalyst. Int. J. Appl. Chem. 2017, 13, 631–646. [Google Scholar]
- Muhammad, A.B.; Obianke, M.; Gusau, L.H.; Aliero, A.A. Optimization of Process Variables in Acid Catalysed in Situ Transesterification of Hevea brasiliensis (Rubber Tree) Seed Oil into Biodiesel. Biofuels 2017, 8, 585–594. [Google Scholar] [CrossRef]
- Belagur, V.K.; Chitimi, V.R. Few Physical, Chemical and Fuel Related Properties of Calophyllum inophyllum Linn (Honne) Oil and Its Blends with Diesel Fuel for Their Use in Diesel Engine. Fuel 2013, 109, 356–361. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and Recent Trends in Biodiesel Fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Munarso, J. Plantation of Sterculia feotida L. as Vegetable Oil. Inf. Technol. Agric. Jakarta Indones. Agency Agric. Res. Dev. 2010, 1, 13–15. [Google Scholar]
- Barabás, I.; Todoruţ, I.-A. Biodiesel Quality, Standards and Properties. In Biodiesel-Quality, Emissions and By-Products; InTech: Rijeka, Croatia, 2011; Volume 2, pp. 3–28. [Google Scholar]
- Sureshkumar, K.; Velraj, R.; Ganesan, R. Performance and Exhaust Emission Characteristics of a CI Engine Fueled with Pongamia pinnata Methyl Ester (PPME) and Its Blends with Diesel. Renew. Energy 2008, 33, 2294–2302. [Google Scholar] [CrossRef]
- Demirbas, A. Tea Seed Upgrading Facilities and Economic Assessment of Biodiesel Production from Tea Seed Oil. Energy Convers. Manag. 2010, 51, 2595–2599. [Google Scholar] [CrossRef]
- Raj, F.R.M.S.; Sahayaraj, J.W. A Comparative Study over Alternative Fuel (Biodiesel) for Environmental Friendly Emission. In Proceedings of the Recent Advances in Space Technology Services and Climate Change 2010 (RSTS & CC-2010), Chennai, India, 13–15 November 2010; pp. 80–86. [Google Scholar]
- Fernando, S.; Karra, P.; Hernandez, R.; Jha, S.K. Effect of Incompletely Converted Soybean Oil on Biodiesel Quality. Energy 2007, 32, 844–851. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Muraleedharan, C.; Jayaraj, S. Performance and Emission Evaluation of a Diesel Engine Fueled with Methyl Esters of Rubber Seed Oil. Renew. Energy 2005, 30, 1789–1800. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Kumar, A.; Raheman, H. Biodiesel Production from Jatropha Oil (Jatropha curcas) with High Free Fatty Acids: An Optimized Process. Biomass Bioenergy 2007, 31, 569–575. [Google Scholar] [CrossRef]
- Omorogbe, S.O.; Ikhuoria, E.U.; Aigbodion, A.I.; Obazee, E.O.; Momodu, V.M. Production of Rubber Seed Oil Based Biodiesel Using Different Catalysts. Curr. Res. Chem. 2013, 5, 11–18. [Google Scholar] [CrossRef]
- Dufour, J.; Iribarren, D. Life Cycle Assessment of Biodiesel Production from Free Fatty Acid-Rich Wastes. Renew. Energy 2012, 38, 155–162. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Gao, Y.; Xi, B.; Chen, Z.; Chang, S.; Gao, S.; Zhao, G.; Weng, X.; Jia, L. Plantation Model of Soapberry (Sapindus mukorossi Gaertn.) in Southeast China in Relation to Environmental Impact Effect Based on a Life Cycle Assessment. BioEnergy Res. 2022, 15, 1342–1354. [Google Scholar] [CrossRef]
- Wagner, M.; Lippe, M.; Lewandowski, I.; Salzer, M.; Cadisch, G. CO2 Footprint of the Seeds of Rubber (Hevea brasiliensis) as a Biodiesel Feedstock Source. Forests 2018, 9, 548. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, Heterogeneous and Enzymatic Catalysis for Transesterification of High Free Fatty Acid Oil (Waste Cooking Oil) to Biodiesel: A Review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Luque, R.; Pineda, A.; Colmenares, J.C.; Campelo, J.M.; Romero, A.A.; Serrano-Riz, J.C.; Cabeza, L.F.; Cot-Gores, J. Carbonaceous Residues from Biomass Gasification as Catalysts for Biodiesel Production. J. Nat. Gas Chem. 2012, 21, 246–250. [Google Scholar] [CrossRef]
- Sanjay, B. Heterogeneous Catalyst Derived from Natural Resources for Biodiesel Production: A Review. Res. J. Chem. Sci. 2013, 3, 95–101. [Google Scholar]
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar Based Solid Acid Catalyst for Biodiesel Production. Appl. Catal. A Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Kastner, J.R.; Miller, J.; Geller, D.P.; Locklin, J.; Keith, L.H.; Johnson, T. Catalytic Esterification of Fatty Acids Using Solid Acid Catalysts Generated from Biochar and Activated Carbon. Catal. Today 2012, 190, 122–132. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Chen, Y.; Zhu, X. Biodiesel Production from Waste Cooking Oil Using a Heterogeneous Catalyst from Pyrolyzed Rice Husk. Bioresour. Technol. 2014, 154, 345–348. [Google Scholar] [CrossRef]
- Babadi, F.E.; Hosseini, S.; Soltani, S.M.; Aroua, M.K.; Shamiri, A.; Samadi, M. Sulfonated Beet Pulp as Solid Catalyst in One-Step Esterification of Industrial Palm Fatty Acid Distillate. J. Am. Oil Chem. Soc. 2016, 93, 319–327. [Google Scholar] [CrossRef]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil Cakes and Their Biotechnological Applications—A Review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef]
- Singhania, R.R.; Soccol, C.R.; Pandey, A. Application of Tropical Agro-Industrial Residues as Substrate for Solid-State Fermentation Processes. In Current Developments in Solid-State Fermentation; Springer: Berlin/Heidelberg, Germany, 2008; pp. 412–442. [Google Scholar]
- Salihu, A.; Bala, M.; Bala, S.M. Application of Plackett-Burman Experimental Design for Lipase Production by Aspergillus niger Using Shea Butter Cake. Int. Sch. Res. Not. 2013, 2013, 718352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fleuri, L.F.; de Oliveira, M.C.; de Lara Campos Arcuri, M.; Capoville, B.L.; Pereira, M.S.; Delgado, C.H.O.; Novelli, P.K. Production of Fungal Lipases Using Wheat Bran and Soybean Bran and Incorporation of Sugarcane Bagasse as a Co-Substrate in Solid-State Fermentation. Food Sci. Biotechnol. 2014, 23, 1199–1205. [Google Scholar] [CrossRef]
- Park, S.H.; Khan, N.; Lee, S.; Zimmermann, K.; DeRosa, M.; Hamilton, L.; Hudson, W.; Hyder, S.; Serratos, M.; Sheffield, E. Biodiesel Production from Locally Sourced Restaurant Waste Cooking Oil and Grease: Synthesis, Characterization, and Performance Evaluation. ACS Omega 2019, 4, 7775–7784. [Google Scholar] [CrossRef]
- Suzihaque, M.U.H.; Alwi, H.; Ibrahim, U.K.; Abdullah, S.; Haron, N. Biodiesel Production from Waste Cooking Oil: A Brief Review. Mater. Today Proc. 2022, 63, S490–S495. [Google Scholar] [CrossRef]
- Madadian, E.; Haelssig, J.B.; Mohebbi, M.; Pegg, M. From Biorefinery Landfills towards a Sustainable Circular Bioeconomy: A Techno-Economic and Environmental Analysis in Atlantic Canada. J. Clean. Prod. 2021, 296, 126590. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Zhang, L.; Chang, Y.; Hao, Y. Converting Waste Cooking Oil to Biodiesel in China: Environmental Impacts and Economic Feasibility. Renew. Sustain. Energy Rev. 2021, 140, 110661. [Google Scholar] [CrossRef]
- Ranjbari, M.; Esfandabadi, Z.S.; Ferraris, A.; Quatraro, F.; Rehan, M.; Nizami, A.-S.; Gupta, V.K.; Lam, S.S.; Aghbashlo, M.; Tabatabaei, M. Biofuel Supply Chain Management in the Circular Economy Transition: An Inclusive Knowledge Map of the Field. Chemosphere 2022, 296, 133968. [Google Scholar] [CrossRef] [PubMed]
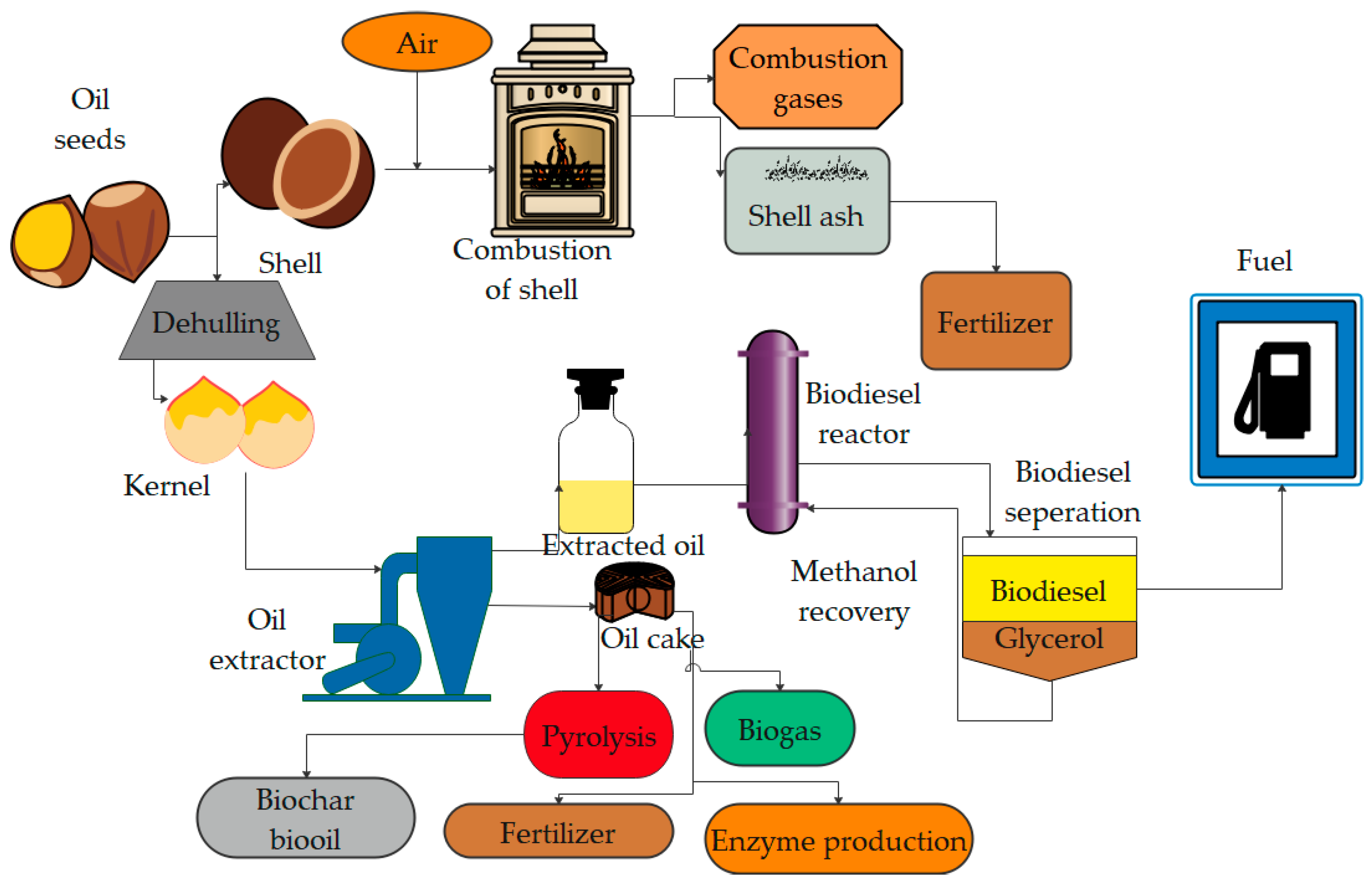
| S. No. | Common Name | Feedstock | Country | Oil Content (%) | Uses | Reference | |
|---|---|---|---|---|---|---|---|
| Seed (wt%) | Kernel (wt%) | ||||||
| 1 | Physic nut | Jatropha curcas | Indonesia, Thailand, Malaysia, Philipines, India, Pakistan, Nepal | 20–40 | 40–60 | Lighting, lubricant, soap making and biodiesel | [5,6,44] |
| 2 | Mahuva tree | Madhuca indica | India | 35–50 | 50 | Biodiesel, illuminant | [6,7,44] |
| 3 | Pongam oil tree | Pongamia pinnata | Western Ghats in India, Northern Australia, Fiji, and some regions of Eastern Asia | 25–50 | 30–50 | Biodiesel, fodder, green manure | [5,6,44] |
| 4 | Neem seed | Azardirachta indica | Native to India, Burma, Bangladesh, Sri Lanka, Malaysia, Pakistan, and Cuba | 20–30 | 25–45 | Oil illuminant, timber, firewood, biodiesel | [5,6,7] |
| 5 | Rubber seed | Hevea brasiliensis | Nigeria, India, Brazil, Southeast Asia, West Africa | 40–60 | 40–50 | Surface coatings including paints, printing inks, rubber/plastic processing, pharmaceuticals, lubricants, cosmetics, chemical intermediates, and diesel fuel substitute/extender | [6,7,51] |
| 6 | Chinese tallow tree | Sapium sebifeum | China, France, India, Sudan, Martinique, Algeria, and the southern area of the United States | 13–32 | 53–64 | Candles, soap, wood varnish | [6,7,58,60] |
| 7 | Soapnut | Sapindus mukorossi | Asia (India, Nepal, Bangladesh, Pakistan), America, Europe | 51.8 | 39 | Rural building construction, oil and sugar presses | [66,93] |
| 8 | Stone kernel fruit | Prunus armeniaca L. | Turkey, Iran, Uzbekistan | - | 54.2 | Oil | [77] |
| 9 | Calophyllum | Calophyllum inophyllum L. | Tropical regions of India, Malaysia, Indonesia, and the Philippines | 65 | 22 | Cosmetics, oil, timber | [89] |
| 10 | Siberian apricot | Prunus sibirica L. | Eastern Siberia, Russia, Monogolia, and China | - | 50.18 | Lubricant, surfactant, cosmetics, and medicinal uses | [68] |
| 11 | Tea seed | Camellia sinensis L. | Turkey, India, and China | - | 32.1 | Vitamins, polyphenols, and saponins | [82] |
| 12 | Palm kernel oil | Elaeis guineensis | Malaysia, Indonesia, Nigeria, Colombia, Thailand, Zaire, and Equador | - | 50 | Soap, oleochemicals, cosmetics, soaps, toothpaste, lubricants | [94] |
| 13 | Java olive | Sterculia foetida | Australia, Bangladesh, Djibouti, India, Eritrea, Ethiopia, Kenya, Malaysia | - | 50–60 | Biodiesel, medicinal, illuminant | [95] |
| 14 | Yellow oleander | Thevetia peruviana | Mexico, Brazil, America, and West indies | 67 | Oil | [48] | |
| Fatty Acids | J. curcas [157] (%) | M. indica [158] (%) | P. pinnata [51] (%) | A. indica [49] (%) | H. brasiliensis [63] (%) | S. sebifeum [59] (%) | S. mukorossi [153] (%) | P. armeniaca [77] (%) | C. inophyllum [20] (%) | P. sibirica [159] (%) | C. sinensis [82] (%) | E. guineensis [94] (%) | S. foetida [160] (%) | T. peruviana [157] (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caprylic | - | - | - | - | - | - | - | - | - | - | - | 3.3 | - | - |
| Capric | - | - | - | - | - | - | - | - | - | - | - | 3.4 | - | - |
| Lauric | - | - | - | - | - | 0.4 | - | - | - | 0.03 | - | 48.2 | - | - |
| Myristic | 1.4 | 1.0 | - | 0.26 | 2.2 | 0.1 | - | - | - | 0.03 | - | 16.2 | - | - |
| Palmitic | 12.7 | 17.8 | 3.7–7.9 | 14.9 | 10.2 | 7.5 | 4.75 | 5.85 | 13.60 | 3.79 | 11.4 | 8.4 | 22.4 | 15.6 |
| Palmitoleic | 0.7 | - | - | 0.1 | 3.71 | 0.35 | 0.2 | 0.67 | - | - | - | - | ||
| Stearic | 5.5 | 14.0 | 2.4–8.9 | 8.7 | 2 | 1.74 | 2.51 | 16.7 | 1.01 | 2.5 | 2.5 | 7.3 | 10.5 | |
| Oleic | 39.1 | 46.3 | 44.5–71.3 | 43.9 | 24 | 16.7 | 60.95 | 63.8 | 40.1 | 65.23 | 62.3 | 15.3 | 16.4 | 60.9 |
| Linoleic | 40.4 | 17.9 | 10.8–18.3 | 17.9 | 38.6 | 27.5 | 4.50 | 25.3 | 26.3 | 28.92 | 20.0 | 2.3 | 45.6 | 5.2 |
| Linolenic | 0.2 | - | - | 0.4 | 16.3 | 41.5 | 2.45 | 0.51 | 0.3 | 0.12 | 2.2 | - | - | 7.4 |
| Arachidic | - | 3.0 | 4.1 | 1.6 | - | - | 4.47 | - | 0.7 | 0.09 | - | 6.46 | 0.3 | |
| Eicosenoic | - | - | 2.4 | - | - | 0.59 | 18.84 | - | 0.3 | 0.11 | 0.8 | - | - | - |
| Behenic | - | - | 5.3 | 0.3 | - | - | 0.94 | 0.66 | 0.2 | - | - | - | - | 0.1 |
| Lignoceric Total | - 100 | - 100 | 1.–3.5 74.2 | - 79.36 | - 100 | - 100 | 1.01 100 | - 98.63 | - 98.4 | - 100 | - 99.2 | - 99.6 | - | - |
| Source | Catalyst Concentration (wt%) | Methanol to Oil Reaction (Ratio) | Reaction Temperature (°C) | Time (min) | Maximum Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| J. curcas | KOH—2.09 | 7.5:1 | 60 | 60 | 80.5 | [166] |
| M. indica | KOH—0.7 | 6:1 | 60 | 30 | 98 | [167] |
| P. pinnata | H2SO4—1 mL | 6:1 | 54.5–55.5 | 60 | 98.6 | [168] |
| A. indica | Cu-ZnO 10% | 10:1 | 55 | 60 | 97.18 | [169] |
| H. brasiliensis | KOH 1% | 6:1 | 55 | 67.5 | 96.8 | [64] |
| S. sebifeum | Lipase 5% | 5:1 | 40 | 125 | 55.22 | [60] |
| S. mukorossi | H2SO4 1% and KOH 1% | 6:1 and 8:1 | 60 | 30 | 92.5 | [59] |
| P. armeniaca L. | KOH—0.5% | 6:1 | 55 | 60 | 95.8 | [77] |
| C. inophyllum L. | H2SO4 0.5 mL | 8:1 | 45–65 | 30–150 | 89 | [50] |
| P. sibirica | KOH-1 | 5.5:1 | 60 | 60 | 88.7 | [94] |
| C. sinensis | NaOH—0.25 | 6:1 | 60 | 60 | 97.5 | [170] |
| E. guineensis | NaOH | 6:1 | 65 | 180 | 87 | [171] |
| S. foetida | KOH 1.5 | 12:1 | 55 | 60 | 90.2 | [95] |
| T. peruviana | KOH 1 | 6:1 | 55 | 35 | 97.5 | [172] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sambasivam, K.M.; Kuppan, P.; Laila, L.S.; Shashirekha, V.; Tamilarasan, K.; Abinandan, S. Kernel-Based Biodiesel Production from Non-Edible Oil Seeds: Techniques, Optimization, and Environmental Implications. Energies 2023, 16, 7589. https://doi.org/10.3390/en16227589
Sambasivam KM, Kuppan P, Laila LS, Shashirekha V, Tamilarasan K, Abinandan S. Kernel-Based Biodiesel Production from Non-Edible Oil Seeds: Techniques, Optimization, and Environmental Implications. Energies. 2023; 16(22):7589. https://doi.org/10.3390/en16227589
Chicago/Turabian StyleSambasivam, Kavitha Munisamy, Praveen Kuppan, Lafiya Shanavas Laila, Viswanaathan Shashirekha, Krishnamurthi Tamilarasan, and Sudharsanam Abinandan. 2023. "Kernel-Based Biodiesel Production from Non-Edible Oil Seeds: Techniques, Optimization, and Environmental Implications" Energies 16, no. 22: 7589. https://doi.org/10.3390/en16227589
APA StyleSambasivam, K. M., Kuppan, P., Laila, L. S., Shashirekha, V., Tamilarasan, K., & Abinandan, S. (2023). Kernel-Based Biodiesel Production from Non-Edible Oil Seeds: Techniques, Optimization, and Environmental Implications. Energies, 16(22), 7589. https://doi.org/10.3390/en16227589






