Characterization and Comparison of DSSCs Fabricated with Black Natural Dyes Extracted from Jamun, Black Plum, and Blackberry
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization Techniques
2.3. Natural Dye Extraction
2.4. Fabrication of Jamun, Black Plum, and Blackberry DSSC
2.5. Simulation Methods
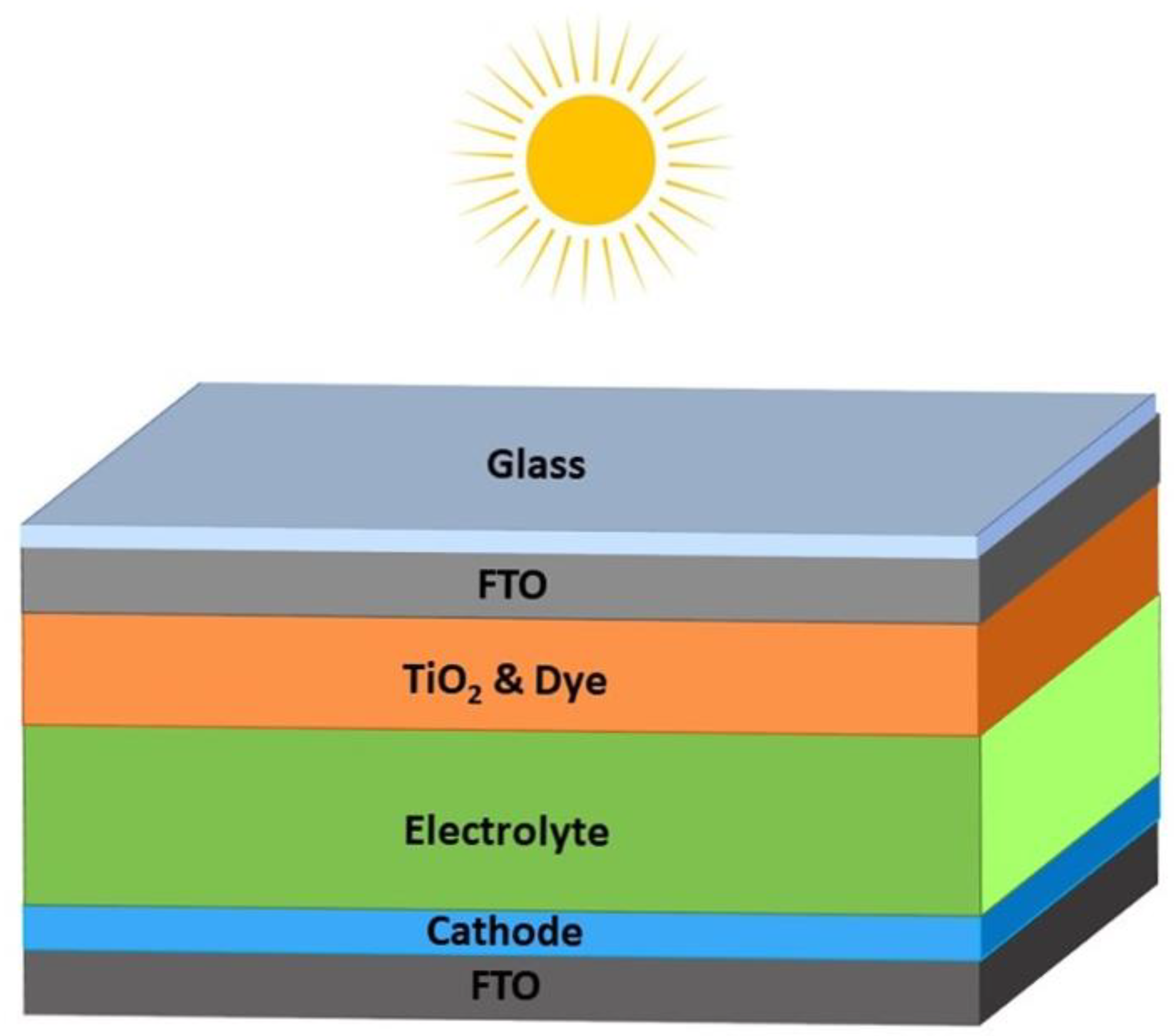
2.6. Device Architecture
2.7. Simulation Parameters
3. Results and Discussion
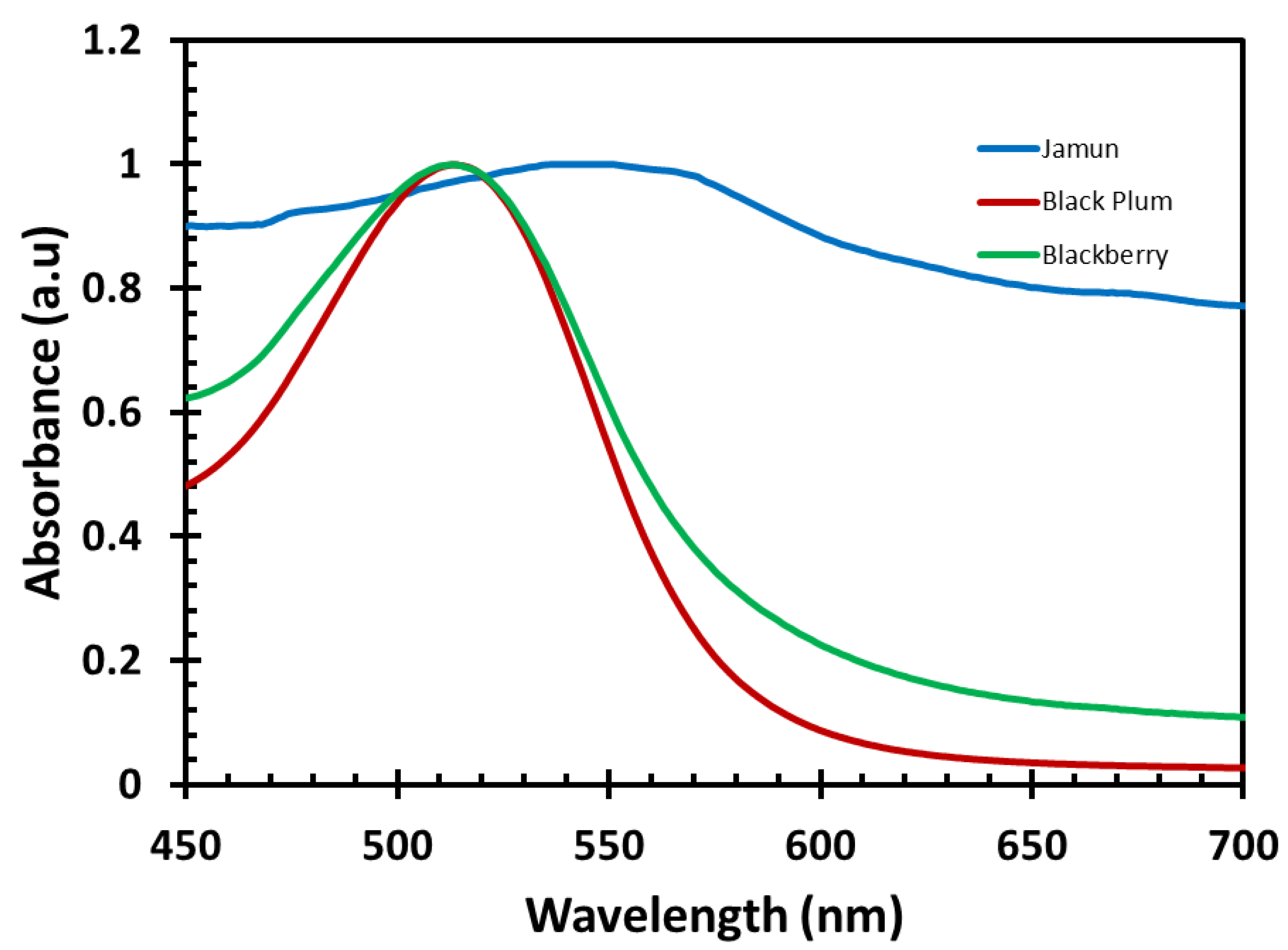
3.1. Absorption Spectroscopy Measurements
3.2. Emission Studies
3.3. Fourier Transformed Infrared (FTIR) Analysis
3.4. Raman Spectroscopy Analysis
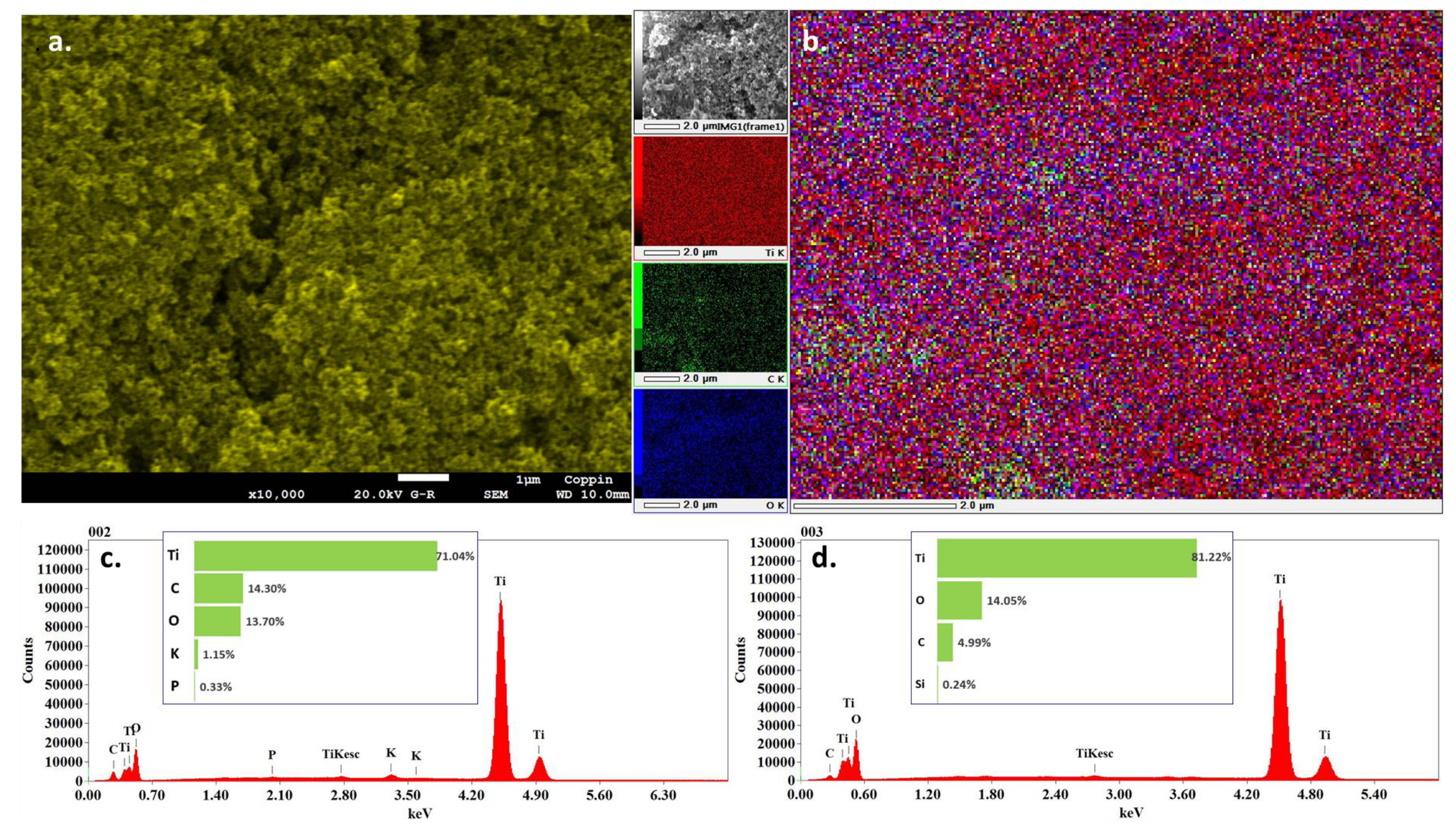
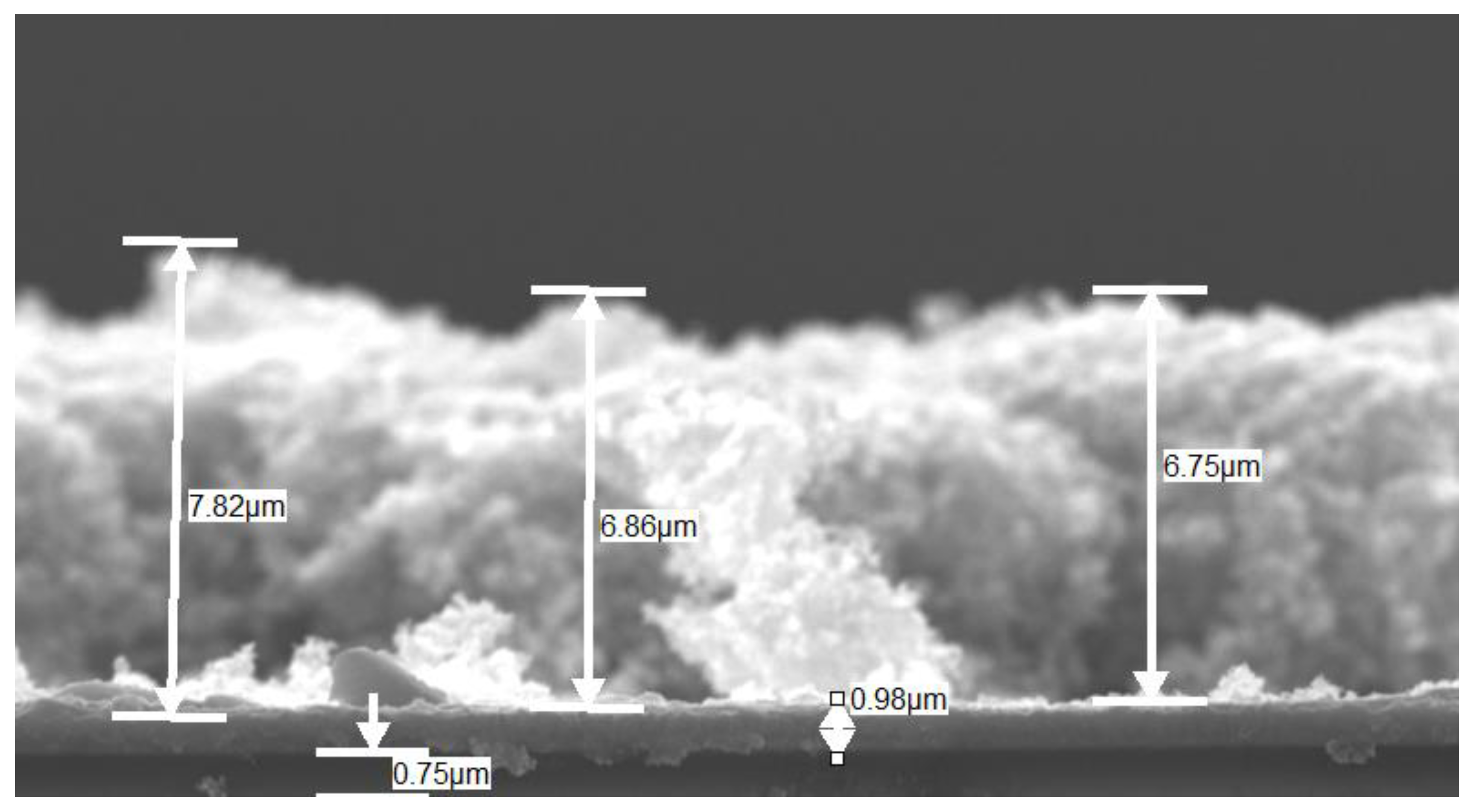
3.5. Field Emission Scanning Electron Microscopy (FESEM) and Energy Dispersive X-ray Spectroscopy (EDS)
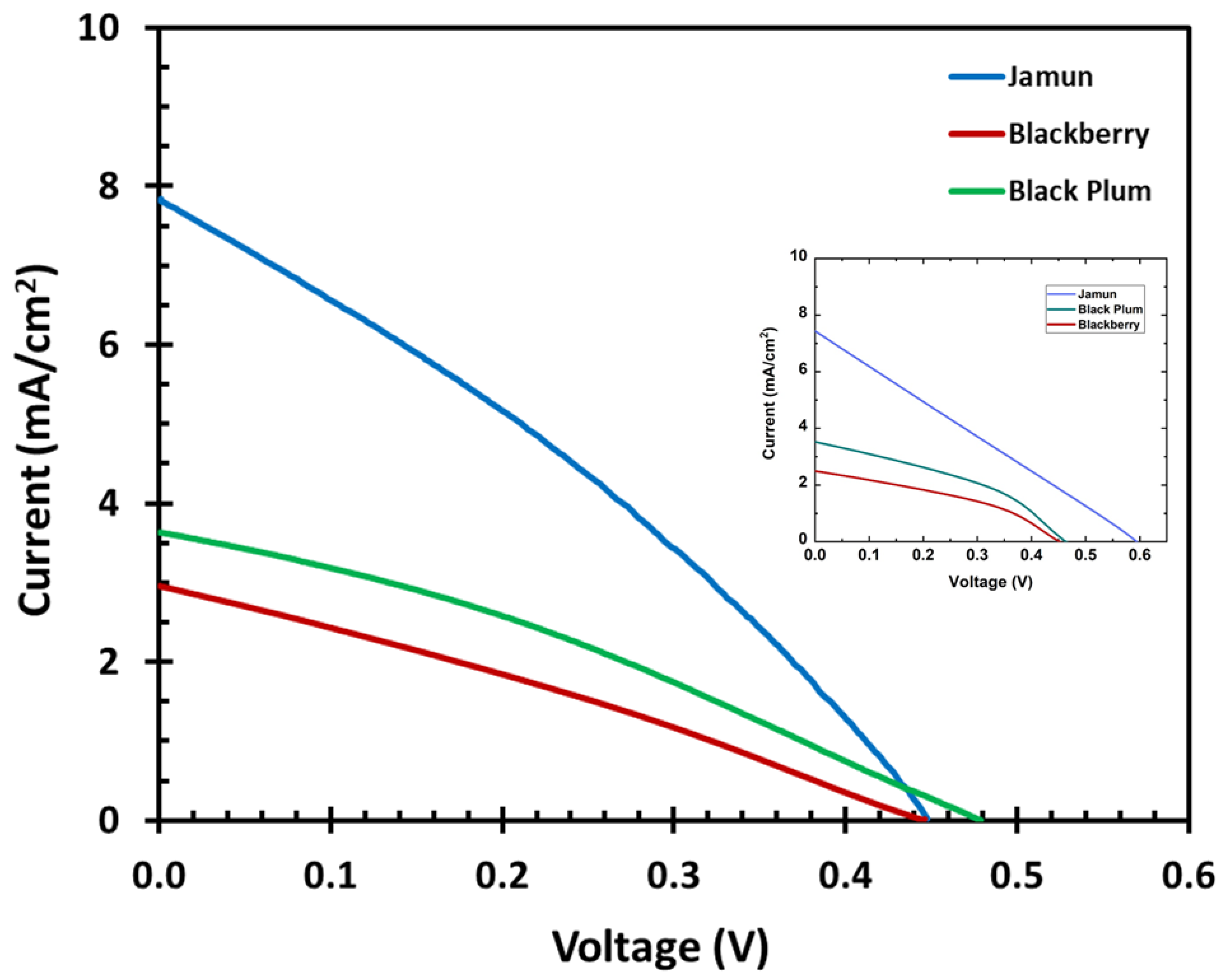
3.6. Current and Voltage Characteristics
3.7. Electrochemical Impedance Spectroscopic (EIS) Study
3.8. Correlations between Simulation and Experimental Data
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohtasham, J. Review Article-Renewable Energies. Energy Procedia 2015, 74, 1289–1297. [Google Scholar] [CrossRef]
- Vershinina, K.Y.; Dorokhov, V.V.; Nyashina, G.S.; Romanov, D.S. Environmental Aspects and Energy Characteristics of the Combustion of Composite Fuels Based on Peat, Oil, and Water. Solid Fuel Chem. 2019, 53, 294–302. [Google Scholar] [CrossRef]
- Cséfalvay, E.; Horváth, I.T. Sustainability Assessment of Renewable Energy in the United States, Canada, the European Union, China, and the Russian Federation. ACS Sustain. Chem. Eng. 2018, 6, 8868–8874. [Google Scholar] [CrossRef]
- Richhariya, G.; Kumar, A.; Tekasakul, P.; Gupta, B. Natural dyes for dye sensitized solar cell: A review. Renew. Sustain. Energy Rev. 2017, 69, 705–718. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Baranoff, E.; Grätzel, M. Dye-sensitized solar cells: A brief overview. Sol. Energy 2011, 85, 1172–1178. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, D.; Cao, Y.; Tsao, H.N.; Yuan, Y.; Zakeeruddin, S.M.; Wang, P.; Grätzel, M. A Stable Blue Photosensitizer for Color Palette of Dye-Sensitized Solar Cells Reaching 12.6% Efficiency. J. Am. Chem. Soc. 2018, 140, 2405–2408. [Google Scholar] [CrossRef]
- Bhand, S.; Salunke-Gawali, S. Amphiphilic photosensitizers in Dye Sensitized Solar Cells. Inorganica Chim. Acta 2019, 495, 118955. [Google Scholar] [CrossRef]
- Ludin, N.A.; Mahmoud, A.A.-A.; Mohamad, A.B.; Kadhum, A.A.H.; Sopian, K.; Karim, N.S.A. Review on the development of natural dye photosensitizer for dye-sensitized solar cells. Renew. Sustain. Energy Rev. 2014, 31, 386–396. [Google Scholar] [CrossRef]
- Wang, T.-H.; Huang, T.-W.; Tsai, Y.-C.; Chang, Y.-W.; Liao, C.-S. A photoluminescent layer for improving the performance of dye-sensitized solar cells. Chem. Commun. 2015, 51, 7253–7256. [Google Scholar] [CrossRef] [PubMed]
- Stathatos, E. Dye sensitized solar cells: A new prospective to the solar to electrical energy conversion Issues to be solved for efficient energy harvesting. J. Eng. Sci. Technol. Rev. 2012, 4, 9–13. [Google Scholar] [CrossRef]
- Anantharaj, G.; Lakshminarasimhan, N. Interfacial Modification of Photoanode|Electrolyte Interface Using Oleic Acid Enhancing the Efficiency of Dye-Sensitized Solar Cells. ACS Omega 2018, 3, 18285–18294. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, R.; Attia, F.M.; Su, R.; Anees, P.; El-Shafei, A.; Adhikari, A.V. Asymmetric Dual Anchoring Sensitizers/Cosensitizers for Dye Sensitized Solar Cell Application: An Insight into Various Fundamental Processes inside the Cell. J. Phys. Chem. C 2019, 123, 24383–24395. [Google Scholar] [CrossRef]
- Khamrang, T.; Seetharaman, A.; Kumar, M.D.; Velusamy, M.; Jaccob, M.; Ramesh, M.; Kathiresan, M.; Kathiravan, A. New D–D′–A Configured Dye for Efficient Dye-Sensitized Solar Cells. J. Phys. Chem. C 2018, 122, 22241–22251. [Google Scholar] [CrossRef]
- Sharmoukh, W.; Cong, J.; Gao, J.; Liu, P.; Daniel, Q.; Kloo, L. Molecular Engineering of D–D−π–A-Based Organic Sensitizers for Enhanced Dye-Sensitized Solar Cell Performance. ACS Omega 2018, 3, 3819–3829. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Shit, A.; Nandi, S. Carbon Dot Assisted Synthesis of Nanostructured Polyaniline for Dye Sensitized Solar Cells. Energy Fuels 2017, 31, 7364–7371. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.-J.; Xia, Y.-Y. A comparative theoretical investigation of ruthenium dyes in dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2007, 185, 283–288. [Google Scholar] [CrossRef]
- Ali, M.M.; Pervez, W.; Ghann, W.; Uddin, J. Photophysical Studies of Ruthenium-Based Complexes and the Performance of Nanostructured TiO2 Based Dye Sensitized Solar Cells. J. Nanomed. Nanotechnol. 2019, 10, 1–5. [Google Scholar] [CrossRef][Green Version]
- Oh, J.; Ghann, W.; Kang, H.; Nesbitt, F.; Providence, S.; Uddin, J. Comparison of the performance of dye sensitized solar cells fabricated with ruthenium-based dye sensitizers: Di-tetrabutylammonium cis-bis(isothiocyanato)bis(2,2′-bipyridyl-4,4′- di-carboxylato) ruthenium(II) (N719) and tris(bipyridine)ruthenium(II) chloride (Ru-BPY). Inorganica Chim. Acta 2018, 482, 935–943. [Google Scholar]
- Aghazada, S.; Nazeeruddin, M.K. Ruthenium Complexes as Sensitizers in Dye-Sensitized Solar Cells. Inorganics 2018, 6, 52. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Lu, Y.; Tang, W.; Zhao, S.; Liu, Q.; Zhu, W.; Tian, H.; Xie, Y. Efficient solar cells sensitized by a promising new type of porphyrin: Dye-aggregation suppressed by double strapping. Chem. Sci. 2018, 10, 2186–2192. [Google Scholar] [CrossRef]
- Birel, Ö.; Nadeem, S.; Duman, H. Porphyrin-Based Dye-Sensitized Solar Cells (DSSCs): A Review. J. Fluoresc. 2017, 27, 1075–1085. [Google Scholar] [CrossRef]
- Ghann, W.; Kang, H.; Emerson, E.; Oh, J.; Chavez-Gil, T.; Nesbitt, F.; Williams, R.; Uddin, J. Photophysical properties of near-IR cyanine dyes and their application as photosensitizers in dye sensitized solar cells. Inorganica Chim. Acta 2017, 467, 123–131. [Google Scholar] [CrossRef]
- Pepe, G.; Cole, J.M.; Waddell, P.G.; McKechnie, S. Molecular engineering of cyanine dyes to design a panchromatic response in co-sensitized dye-sensitized solar cells. Mol. Syst. Des. Eng. 2016, 1, 86–98. [Google Scholar] [CrossRef]
- Phinjaturus, K.; Maiaugree, W.; Suriharn, B.; Pimanpaeng, S.; Amornkitbamrung, V.; Swatsitang, E. Dye-sensitized solar cells based on purple corn sensitizers. Appl. Surf. Sci. 2016, 380, 101–107. [Google Scholar] [CrossRef]
- Lim, A.; Ekanayake, P.; Lim, L.B.L.; Bandara, J.S. Co-dominant effect of selected natural dye sensitizers in DSSC performance. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 167, 26–31. [Google Scholar] [CrossRef]
- Aung, S.H.; Hao, Y.; Oo, T.Z.; Boschloo, G. Kinetic study of carminic acid and santalin natural dyes in dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2016, 325, 1–8. [Google Scholar] [CrossRef]
- Dai, Q.; Rabani, J. Photosensitization of nanocrystalline TiO2 films by pomegranate pigments with unusually high effi-ciency in aqueous medium. Chem Commun. 2001, 20, 2142–2143. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Kaneyuki, T.; Mori, A.; Packer, L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Del-phinidin, cyanidin, and pelargonidin. J. Agric. Food Chem. 2002, 50, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.-X.; Fujii, M.; Terahara, N.; Yoshimoto, M. Molecular Mechanisms behind the Chemopreventive Effects of Antho-cyanidins. J. Biomed. Biotechnol. 2004, 2004, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Iwuji, O.; Ghann, W.; Iwuji, C.; Uddin, J. Dragon Fruit Dye as a Sensitizer for Dye-Sensitized Solar Cells. Nanosci. J. 2018, 1, 5–8. [Google Scholar]
- Sanjay, P.; Isaivani, I.; Deepa, K.; Madhavan, J.; Senthil, S. The preparation of dye sensitized solar cells using natural dyes extracted from Phytolacca icosandra and Phyllanthus reticulatus with ZnO as photoanode. Mater. Lett. 2019, 244, 142–146. [Google Scholar] [CrossRef]
- Das, S.K.; Ganguli, S.; Kabir, H.; Khandaker, J.I.; Ahmed, F. Performance of Natural Dyes in Dye-Sensitized Solar Cell as Photosensitizer. Trans. Electr. Electron. Mater. 2019, 21, 105–116. [Google Scholar] [CrossRef]
- Ghann, W.; Kang, H.; Sheikh, T.; Yadav, S.; Chavez-Gil, T.; Nesbitt, F.; Uddin, J. Fabrication, Optimization and Characterization of Natural Dye Sensitized Solar Cell. Sci. Rep. 2017, 7, 41470. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.L.; Yates, J.T. TiO2-based Photocatalysis: Surface Defects, Oxygen and Charge Transfer. Top. Catal. 2005, 35, 197–210. [Google Scholar] [CrossRef]
- Lu, G.; Linsebigler, A.; Yates, J.T. Ti3+ Defect Sites on TiO2(110): Production and Chemical Detection of Active Sites. J. Phys. Chem. 1994, 98, 11733–11738. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of Nature and Location of Defects on Bandgap Narrowing in Black TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef]
- Nowotny, M.K.; Bak, T.; Nowotny, J. Electrical Properties and Defect Chemistry of TiO2 Single Crystal. I. Electrical Conductivity. J. Phys. Chem. B 2006, 110, 16270–16282. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I.; Negishi, N.; Kutsuna, S.; Ihara, T.; Sugihara, S.; Takeuchi, K. Role of oxygen vacancy in the plasma-treated TiO2 photocatalyst with visible light activity for NO removal. J. Mol. Catal. A: Chem. 2000, 161, 205–212. [Google Scholar] [CrossRef]
- Pacchioni, G. Oxygen Vacancy: The Invisible Agent on Oxide Surfaces. Chemphyschem 2003, 4, 1041–1047. [Google Scholar] [CrossRef]
- Cheng, H.; Selloni, A. Surface and subsurface oxygen vacancies in anataseTiO2 and differences with rutile. Phys. Rev. B 2009, 79, 092101. [Google Scholar] [CrossRef]
- Setvin, M.; Schmid, M.; Diebold, U. Aggregation and electronically induced migration of oxygen vacancies inTiO2 anatase. Phys. Rev. B 2015, 91, 195403. [Google Scholar] [CrossRef]
- Ako, R.T.; Ekanayake, P.; Young, D.J.; Hobley, J.; Chellappan, V.; Tan, A.L.; Gorelik, S.; Subramanian, G.S.; Lim, C.M. Evaluation of surface energy state distribution and bulk defect concentration in DSSC photoanodes based on Sn, Fe, and Cu doped TiO. Appl. Surf. Sci. 2015, 351, 950–961. [Google Scholar] [CrossRef]
- Kabir, D.; Forhad, T.; Ghann, W.; Richards, B.; Rahman, M.M.; Uddin, N.; Rakib, R.J.; Shariare, M.H.; Chowdhury, F.I.; Rabbani, M.M.; et al. Dye-sensitized solar cell with plasmonic gold nanoparticles modified photoanode. Nano-Structures Nano-Objects 2021, 26, 100698. [Google Scholar] [CrossRef]
- Saadmim, F.; Forhad, T.; Sikder, A.; Ghann, W.; Ali, M.M.; Sitther, V.; Ahammad, A.J.S.; Subhan, A.; Uddin, J. Enhancing the Performance of Dye Sensitized Solar Cells Using Silver Nanoparticles Modified Photoanode. Molecules 2020, 25, 4021. [Google Scholar] [CrossRef] [PubMed]
- Karim, F.; Sikder, A.; Ghann, W.; Green, K.; Ozturk, B.; Ali, M.M.; Uddin, J. Nanostructured Dye Sensitized Solar Cells with Different Counter Electrodes. Am. J. Phys. Chem. 2020, 9, 1. [Google Scholar] [CrossRef]
- Ghann, W.E.; Kang, W.H.; Uddin, J.; Chowdhury, F.A.; Khondaker, S.I.; Moniruzzaman, M.; Kabir, M.H.; Rahman, M.M. Syn-thesis and Characterization of Reduced Graphene Oxide and Their Application in Dye-Sensitized Solar Cells. ChemEngineering 2019, 3, 7. [Google Scholar] [CrossRef]
- Ghann, W.; Kang, H.; Uddin, J.; Gonawala, S.J.; Mahatabuddin, S.; Ali, M.M. Dendrimer-based Nanoparticle for Dye Sensitized Solar Cells with Improved Efficiency. J. Nanomed. Nanotechnol. 2018, 9, 1000496. [Google Scholar] [CrossRef] [PubMed]
- Decock, K.; Zabierowski, P.; Burgelman, M. Modeling metastabilities in chalcopyrite-based thin film solar cells. J. Appl. Phys. 2012, 111, 3686651. [Google Scholar] [CrossRef]
- Decock, K.; Khelifi, S.; Burgelman, M. Modelling multivalent defects in thin film solar cells. Thin Solid Film. 2011, 519, 7481–7484. [Google Scholar] [CrossRef]
- Burgelman, M.; Nollet, P.; Degrave, S. Modelling polycrystalline semiconductor solar cells. Thin Solid Film. 2000, 361–362, 527–532. [Google Scholar] [CrossRef]
- Bak, T.; Nowotny, M.K.; Sheppard, L.R.; Nowotny, J. Mobility of electronic charge carriers in titanium dioxide. J. Phys. Chem. C 2008, 112, 12981–12987. [Google Scholar] [CrossRef]
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 Anatase with a Bandgap in the Visible Region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef] [PubMed]
- Earnshaw, A.; Greenwood, N.N. Chemistry of the Elements; Butterworth-Heinemann: Oxford, UK, 1997. [Google Scholar]
- Enright, B.; Fitzmaurice, D. Spectroscopic determination of electron and hole effective masses in a nanocrystalline semiconductor film. J. Phys. Chem. 1996, 100, 1027–1035. [Google Scholar] [CrossRef]
- Lakhdar, N.; Hima, A. Electron transport material effect on performance of perovskite solar cells based on CH3NH3GeI. Opt. Mater. 2019, 99, 109517. [Google Scholar] [CrossRef]
- Fitton, B. The mobilities of holes and electrons in iodine single crystals. J. Phys. Chem. Solids 1969, 30, 211–215. [Google Scholar] [CrossRef]
- Jahantigh, F.; Safikhani, M.J. The effect of HTM on the performance of solid-state dye-sanitized solar cells (SDSSCs): A SCAPS-1D simulation study. Appl. Phys. A 2019, 125, 276. [Google Scholar] [CrossRef]
- Simhony, M. Measurements on the dielectric constant of iodine single crystals in various crystallographic directions. J. Phys. Chem. Solids 1963, 24, 1297–1300. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Smestad, G.P.; Grätzel, M.; Zhang, J.Z. Ultrafast Electron Injection: Implications for a Photoelectrochemical Cell Utilizing an Anthocyanin Dye-Sensitized TiO2 Nanocrystalline Electrode. J. Phys. Chem. B 1997, 101, 9342–9351. [Google Scholar] [CrossRef]
- Aboulouard, A.; Gultekin, B.; Can, M.; Erol, M.; Jouaiti, A.; Elhadadi, B.; Zafer, C.; Demic, S. Dye sensitized solar cells based on titanium dioxide nanoparticles synthesized by flame spray pyrolysis and hydrothermal sol-gel methods: A comparative study on photovoltaic performances. J. Mater. Res. Technol. 2019, 9, 1569–1577. [Google Scholar] [CrossRef]
- Kao, M.; Chen, H.; Young, S.; Kung, C.; Lin, C. The effects of the thickness of TiO2 films on the performance of dye-sensitized solar cells. Thin Solid Film. 2009, 517, 5096–5099. [Google Scholar] [CrossRef]
- Favaro, L.; Balcão, V.; Rocha, L.; Silva, E.; Oliveira, J., Jr.; Vila, M.; Tubino, M.; Favaro, L.I.L.; Balcão, V.M.; Rocha, L.K.H.; et al. Physicochemical Characterization of a Crude Anthocyanin Extract from the Fruits of Jussara (Euterpe edulis Martius): Potential for Food and Pharmaceutical Applications. J. Braz. Chem. Soc. 2018, 29, 2072–2088. [Google Scholar] [CrossRef]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fang, L.; Luo, G. Electrolytes in Dye-Sensitized Solar Cells. Chem. Rev. 2015, 115, 2136–2173. [Google Scholar] [CrossRef]
- Sarker, S.; Ahammad, A.J.S.; Seo, H.W.; Kim, D.M. Electrochemical Impedance Spectra of Dye-Sensitized Solar Cells: Fundamentals and Spreadsheet Calculation. Int. J. Photoenergy 2014, 2014, 851705. [Google Scholar] [CrossRef]
- Wang, Q.; Moser, J.-E.; Grätzel, M. Electrochemical Impedance Spectroscopic Analysis of Dye-Sensitized Solar Cells. J. Phys. Chem. B 2005, 109, 14945–14953. [Google Scholar] [CrossRef]
- von Hauff, E. Impedance Spectroscopy for Emerging Photovoltaics. J. Phys. Chem. C 2019, 123, 11329–11346. [Google Scholar] [CrossRef]
- Das, T.K.; Ilaiyaraja, P.; Sudakar, C. Template assisted nanoporous TiO2 nanoparticles: The effect of oxygen vacancy defects on photovoltaic performance of DSSC and QDSSC. Sol. Energy 2018, 159, 920–929. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Z.; Shi, J.; Jia, G.; Shen, S.; Zhou, J.; Li, C. Trap states and carrier dynamics of TiO2 studied by photoluminescence spectroscopy under weak excitation condition. Phys. Chem. Chem. Phys. 2010, 12, 7083–7090. [Google Scholar] [CrossRef]
- Hao, Y.-N.; Chen, T.; Zhang, X.; Zhou, H.; Ma, Y. Ti-Ti σ bond at oxygen vacancy inducing the deep defect level in anatase TiO2 (101) surface. J. Chem. Phys. 2019, 150, 224702. [Google Scholar] [CrossRef] [PubMed]
- Porto, S.P.S.; Fleury, P.A.; Damen, T.C. Raman Spectra of TiO2, MgF2, ZnF2, FeF2, and MnF. Phys. Rev. B 1967, 154, 522–526. [Google Scholar] [CrossRef]
- Clifford, J.N.; Martínez-Ferrero, E.; Viterisi, A.; Palomares, E. Sensitizer molecular structure-device efficiency relationship in dye sensitized solar cells. Chem. Soc. Rev. 2010, 40, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Prima, E.C.; Nugroho, H.S.; Nugraha; Refantero, G.; Panatarani, C.; Yuliarto, B. Performance of the dye-sensitized quasi-solid state solar cell with combined anthocyanin-ruthenium photosensitizer. RSC Adv. 2020, 10, 36873–36886. [Google Scholar] [CrossRef] [PubMed]







| Parameters/Layers | FTO | TiO2 and Dye | I−/I3− (Electrolyte) | Reference |
|---|---|---|---|---|
| Relative permittivity, εr | 9 | 10 | 30 | [58,60,61] |
| Bandgap energy (eV) | 3.5 | 3.2 | 1.3 | [55,56,58,60] |
| Electron affinity (eV) | 4 | 4.2 | 2.22 | [58,60] |
| Electron mobility (cm2/V.s) | 33 | 200 | 200 | [54,58,59,60] |
| Hole mobility (cm2/V.s) | 8 | 50 | 100 | [54,58,59,60] |
| Donor concentration, Nd (cm−3) | 2.0 × 1016 | 1.35 × 1011 | 0 | [58,60] |
| Acceptor concentration, Na (cm−3) | 0 | 0 | 1.35 × 1011 | [58,60] |
| Conduction band density of states/Nc (cm−3) | 2.2 × 1018 | 1.8 × 1019 | 1.0 × 1018 | [57,58,59,60] |
| Valence band density of states/Nv (cm−3) | 1.8 × 1019 | 3.5 × 1019 | 1.0 × 1019 | [57,58,59,60] |
| Radiative recombination (cm3/s) | 2.3 × 10−5 | 2.3 × 10−5 | 5.0 × 10−6 |
| Parameters/Dyes | Jamun | Black Plum | Blackberry |
|---|---|---|---|
| Defect type | Neutral | Single donor | Single donor |
| Capture cross-section electrons (cm2) | 1.0 × 10−15 | 1.0 × 10−15 | 1.0 × 10−15 |
| Capture cross-section holes (cm2) | 1.0 × 10−15 | 1.0 × 10−15 | 1.0 × 10−15 |
| Energetic distribution | Single | Single | Single |
| Reference for defect energy level Et | Above Ev | Below Ec | Below Ec |
| Energy level with respect to reference (eV) | 2.2 | 0.2 | 0.1 |
| Characteristic energy (eV) | 0.1 | 0.1 | 0.1 |
| Defect density, Nt (cm−3) | 2.5 × 1017 | 1.0 × 1020 | 1.0 × 1022 |
| Voc (V) | Isc (mA/cm2) | Vmp (V) | Imp (mA/cm2) | Fill Factor | Efficiency (%) | |
|---|---|---|---|---|---|---|
| Jamun | 0.45 | 7.84 | 0.26 | 4.25 | 0.31 | 1.09 |
| Black plum | 0.48 | 3.63 | 0.25 | 2.16 | 0.32 | 0.55 |
| Blackberry | 0.45 | 2.96 | 0.24 | 1.58 | 0.29 | 0.38 |
| Dye | Voc (V) | Jsc (mA/cm2) | Vmp (V) | Imp (mA/cm2) | Fill Factor | Efficiency (%) |
|---|---|---|---|---|---|---|
| Jamun (experimental) | 0.45 | 7.84 | 0.26 | 4.25 | 0.31 | 1.09 |
| Jamun (simulation) | 0.59 | 7.42 | 0.3 | 3.7 | 0.25 | 1.11 |
| Black plum (experimental) | 0.48 | 3.63 | 0.25 | 2.16 | 0.32 | 0.55 |
| Black plum (simulation) | 0.46 | 3.54 | 0.3 | 2.08 | 0.38 | 0.62 |
| Blackberry (experimental) | 0.45 | 2.96 | 0.24 | 1.58 | 0.29 | 0.38 |
| Blackberry (simulation) | 0.45 | 2.5 | 0.3 | 1.42 | 0.38 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikder, A.; Ghann, W.; Jani, M.R.; Islam, M.T.; Ahmed, S.; Rahman, M.M.; Patwary, M.A.M.; Kazi, M.; Islam, J.; Chowdhury, F.I.; et al. Characterization and Comparison of DSSCs Fabricated with Black Natural Dyes Extracted from Jamun, Black Plum, and Blackberry. Energies 2023, 16, 7187. https://doi.org/10.3390/en16207187
Sikder A, Ghann W, Jani MR, Islam MT, Ahmed S, Rahman MM, Patwary MAM, Kazi M, Islam J, Chowdhury FI, et al. Characterization and Comparison of DSSCs Fabricated with Black Natural Dyes Extracted from Jamun, Black Plum, and Blackberry. Energies. 2023; 16(20):7187. https://doi.org/10.3390/en16207187
Chicago/Turabian StyleSikder, Ahmed, William Ghann, Md Rafsun Jani, Md Tohidul Islam, Saquib Ahmed, Mohammed M. Rahman, Md Abdul Majed Patwary, Mohsin Kazi, Jahidul Islam, Faisal I. Chowdhury, and et al. 2023. "Characterization and Comparison of DSSCs Fabricated with Black Natural Dyes Extracted from Jamun, Black Plum, and Blackberry" Energies 16, no. 20: 7187. https://doi.org/10.3390/en16207187
APA StyleSikder, A., Ghann, W., Jani, M. R., Islam, M. T., Ahmed, S., Rahman, M. M., Patwary, M. A. M., Kazi, M., Islam, J., Chowdhury, F. I., Yousuf, M. A., Rabbani, M. M., Shariare, M. H., & Uddin, J. (2023). Characterization and Comparison of DSSCs Fabricated with Black Natural Dyes Extracted from Jamun, Black Plum, and Blackberry. Energies, 16(20), 7187. https://doi.org/10.3390/en16207187









