A Review on Liquid Electrolyte Stability Issues for Commercialization of Dye-Sensitized Solar Cells (DSSC)
Abstract
1. Introduction
2. Dye-Sensitized Solar Cell (DSSC)
Design and Working Principles
3. Volatile Organic Solvents
3.1. Common Issues
3.1.1. Volatility and Thermal Decomposition
3.1.2. Leakage Issues and Sealing Methods
3.1.3. Light Absorption
3.1.4. Instability of Redox Mediator
4. Ionic Liquids
5. Other Alternatives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bett, A.; Burger, B.; Friedrich, L.; Kost, C.; Nold, S. Photovoltaics Report; Fraunhofer Institute for Solar Energy Systems; ISE: Freiburg, Germany, 2022. [Google Scholar]
- Płaczek-Popko, E. Top PV Market Solar Cells 2016. Opto-Electron. Rev. 2017, 25, 55–64. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Ho, K.-C. Dye-Sensitized Solar Cells. Encyclopedia of Modern Optics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 270–281. [Google Scholar] [CrossRef]
- Muñoz-García, A.B.; Benesperi, I.; Boschloo, G.; Concepcion, J.J.; Delcamp, J.H.; Gibson, E.A.; Meyer, G.J.; Pavone, M.; Pettersson, H.; Hagfeldt, A.; et al. Dye-sensitized solar cells strike back. Chem. Soc. Rev. 2021, 50, 12450–12550. [Google Scholar] [CrossRef] [PubMed]
- Mathews, I.; Kantareddy, S.N.R.; Buonassisi, T.; Peters, I.M. Technology and Market Perspective for Indoor Photovoltaic Cells. Joule 2019, 3, 1415–1426. [Google Scholar] [CrossRef]
- Michaels, H.; Rinderle, M.; Freitag, R.; Benesperi, I.; Edvinsson, T.; Socher, R.; Gagliardi, A.; Freitag, M. Dye-Sensitized Solar Cells under Ambient Light Powering Machine Learning: Towards Autonomous Smart Sensors for the Internet of Things. Chem. Sci. 2020, 11, 2895–2906. [Google Scholar] [CrossRef]
- Customized Solar Cells. GCell. Dye Sensitized Solar Cells. Available online: https://gcell.com/gcell-products/custom-solar-cell (accessed on 30 May 2023).
- Complete Solid-State Dye-Sensitized Solar Cell. Available online: https://www.ricoh.com/technology/tech/066_dssc (accessed on 30 May 2023).
- Schoden, F.; Dotter, M.; Knefelkamp, D.; Blachowicz, T.; Schwenzfeier Hellkamp, E. Review of State of the Art Recycling Methods in the Context of Dye Sensitized Solar Cells. Energies 2021, 14, 3741. [Google Scholar] [CrossRef]
- Montalvo, C.; Rietveld, E.; Peck, D. A Longer Lifetime for Products: Benefits for Consumers and Companies; European Union: Luxembourg, 2016. [Google Scholar]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, D.; Suo, J.; Cao, Y.; Eickemeyer, F.T.; Vlachopoulos, N.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Hydroxamic Acid Preadsorption Raises Efficiency of Cosensitized Solar Cells. Nature 2023, 613, 60–65. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Jose, R.; Brown, T.M.; Fabregat-Santiago, F.; Bisquert, J. A Perspective on the Production of Dye-Sensitized Solar Modules. Energy Environ. Sci. 2014, 7, 3952–3981. [Google Scholar] [CrossRef]
- Ganta, D.; Combrink, K.; Villanueva, R. Natural Dye-Sensitized Solar Cells: Fabrication, Characterization, and Challenges. In Energy, Environment, and Sustainability; Springer: Berlin/Heidelberg, Germany, 2018; pp. 129–155. [Google Scholar] [CrossRef]
- Djhé. File: Operating Diagram of DSC.svg. Available online: https://upload.wikimedia.org/wikipedia/commons/a/a7/Operating_diagram_of_DSC.svg (accessed on 14 January 2023).
- Pal, A.K.; Potter, H.C. Advances in Solar Energy: Solar Cells and Their Applications. In Energy, Environment, and Sustainability; Springer: Berlin/Heidelberg, Germany, 2018; pp. 75–127. [Google Scholar] [CrossRef]
- Jordan, D.C.; Kurtz, S.R. Photovoltaic Degradation Rates—An Analytical Review. Prog. Photovolt. 2011, 21, 12–29. [Google Scholar] [CrossRef]
- Harikisun, R.; Desilvestro, H. Long-term stability of dye solar cells. Sol. Energy 2011, 85, 1179–1188. [Google Scholar] [CrossRef]
- Gossen, K.; Dotter, M.; Brockhagen, B.; Storck, J.L.; Ehrmann, A. Long-term investigation of unsealed DSSCs with glycerol-based electrolytes of different compositions. AIMS Mater. Sci. 2022, 9, 283–296. [Google Scholar] [CrossRef]
- Fang, H.; Ma, J.; Wilhelm, M.J.; DeLacy, B.G.; Dai, H. Influence of Solvent on Dye-sensitized Solar Cell Efficiency: What Is so Special about Acetonitrile? Part. Part. Syst. Charact. 2021, 38, 2000220. [Google Scholar] [CrossRef]
- Mosconi, E.; Selloni, A.; De Angelis, F. Solvent Effects on the Adsorption Geometry and Electronic Structure of Dye-Sensitized TiO2: A First-Principles Investigation. J. Phys. Chem. C 2012, 116, 5932–5940. [Google Scholar] [CrossRef]
- Lun Pang, C.; Lindsay, R.; Thornton, G. Chemical Reactions on Rutile TIO2(110). Chem. Soc. Rev. 2008, 37, 2328. [Google Scholar] [CrossRef]
- Pan, X.; Dai, S. Chapter 4 Electrolyte Used in Dye-Sensitized Solar Cells. Dye-Sensitized Solar Cells; Walter de Gruyter GmbH: Berlin, Germany, 2022; pp. 205–268. [Google Scholar] [CrossRef]
- Foley, J.K.; Korzeniewski, C.; Pons, S. Anodic and Cathodic Reactions in Acetonitrile/Tetra-n-Butylammonium Tetrafluoroborate: An Electrochemical and Infrared Spectroelectrochemical Study. Can. J. Chem. 1988, 66, 201–206. [Google Scholar] [CrossRef]
- Electrochemical Window. Available online: https://en.wikipedia.org/wiki/Electrochemical_window (accessed on 5 February 2023).
- Galanov, S.I.; Sidorova, O.I.; Gavrilenko, M.A. The Process of Acetonitrile Synthesis over γ-Al2O3 Promoted by Phosphoric Acid Catalysts. Procedia Chem. 2014, 10, 108–113. [Google Scholar] [CrossRef]
- Niir Project Consultancy Services (NPCS). Acetonitrile—Manufacturing Plant, Detailed Project Report, Profile, Business Plan, Industry Trends, Market Research, Survey, Manufacturing Process, Machinery, Raw Materials, Feasibility Study, Investment Opportunities, Cost and Revenue, Plant Economics in Project Reports & Profiles. Available online: https://www.niir.org/profile-project-reports/profile/1654/acetonitrile-manufacturing-plant-detailed-project-report-profile-business-plan-industry-trends-market-research-survey-manufacturing-process-machinery-raw-materials-feasibility-study-investment-opportunities-cost-revenue-plant-economics.html (accessed on 14 February 2023).
- IMARC Group. Acetonitrile Market Price Trends, Report and Forecast 2023–2028. Available online: https://www.imarcgroup.com/acetonitrile-technical-material-market-report (accessed on 17 February 2023).
- Kim, J.-Y.; Kim, J.Y.; Lee, D.-K.; Kim, B.; Kim, H.; Ko, M.J. Importance of 4-Tert-Butylpyridine in Electrolyte for Dye-Sensitized Solar Cells Employing SnO2 Electrode. J. Phys. Chem. C 2012, 116, 22759–22766. [Google Scholar] [CrossRef]
- Venkatesan, S.; Hidayati, N.; Liu, I.-P.; Lee, Y.-L. Highly efficient gel-state dye-sensitized solar cells prepared using propionitrile and poly(vinylidene fluoride-co-hexafluoropropylene). Journal of Power Sources 2016, 336, 385–390. [Google Scholar] [CrossRef]
- O’Neil, M.J. (Ed.) The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals; Royal Society of Chemistry: Cambridge, UK, 2013; p. 14. [Google Scholar]
- Hinsch, A.; Kroon, J.M.; Kern, R.; Uhlendorf, I.; Holzbock, J.; Meyer, A.; Ferber, J. Long-Term Stability of Dye-Sensitised Solar Cells. Prog. Photovolt. Res. Appl. 2001, 9, 425–438. [Google Scholar] [CrossRef]
- Lewis, R.J., Sr. (Ed.) Sax’s Dangerous Properties of Industrial Materials, 11th ed.; Wiley-Interscience, Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; p. 24. [Google Scholar]
- Yoon, S.; Tak, S.; Kim, J.; Jun, Y.; Kang, K.; Park, J. Application of Transparent Dye-Sensitized Solar Cells to Building Integrated Photovoltaic Systems. Build. Environ. 2011, 46, 1899–1904. [Google Scholar] [CrossRef]
- Nagygyörgy, V.; Stathatos, E.; Pokol, G.; Madarász, J. In Situ Evolved Gas Analysis of TMOS-Based Gel Electrolytes Containing Guanidinium Thiocyanate for Quasi-Solid-State Dye-Sensitized Solar Cells by TG-FTIR and TG-MS. Period. Polytech. Chem. Eng. 2018, 62, 533–545. [Google Scholar] [CrossRef]
- Szindler, M.; Szindler, M.; Drygała, A.; Lukaszkowicz, K.; Kaim, P.; Pietruszka, R. Dye-Sensitized Solar Cell for Building-Integrated Photovoltaic (BIPV). Appl. Mater. 2021, 14, 3743. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Valeronitrile. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Valeronitrile#section=Boiling-Point (accessed on 2 March 2023).
- De Rossi, F.; Mincuzzi, G.; Di Giacomo, F.; Fahlteich, J.; Amberg-Schwab, S.; Noller, K.; Brown, T.M. A Systematic Investigation of Permeation Barriers for Flexible Dye-Sensitized Solar Cells. Energy Technol. 2016, 4, 1455–1462. [Google Scholar] [CrossRef]
- Chiang, T.H.; Chen, C.H.; Liu, C.Y. Effect of Sealing with Ultraviolet-Curable Adhesives on the Performance of Dye-Sensitized Solar Cells. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Nursam, N.M.; Hidayat, J.; Muliani, L.; Anggraeni, P.N.; Retnaningsih, L.; Idayanti, N. From Cell to Module: Fabrication and Long-Term Stability of Dye-Sensitized Solar Cells. IOP Conference Series: Mater. Sci. Eng. 2017, 214, 012007. [Google Scholar] [CrossRef]
- Martins, J.; Emami, S.; Ivanou, D.; Mendes, A. Ultralow Temperature Glass Frit Encapsulation for Stable Dye-Sensitized Solar Cells. ACS Appl. Energy Mater. 2022, 5, 14185–14192. [Google Scholar] [CrossRef]
- Capitão, J.; Martins, J.; Emami, S.; Ivanou, D.K.; Mendes, A. Fully Glass Frit Encapsulated Dye-Sensitized Solar Cells: Chal-lenges for Hermetical Sealing of Electrolyte Injection Holes. Sol. Energy 2023, 249, 476–484. [Google Scholar] [CrossRef]
- Kato, N.; Tanaka, H.; Takeda, Y.; Higuchi, K.; Nakajima, J. Demonstration of 12 Years Outdoor Working of Highly Durable Dye-Sensitized Solar Cell Modules Employing Hydrophobic Surface Passivation and Suppression of Biased Distribution of Iodine Ions. ACS Sustain. Chem. Eng. 2023, 11, 5014–5022. [Google Scholar] [CrossRef]
- Poskela, A.; Miettunen, K.; Tiihonen, A.; Lund, P.D. Extreme Sensitivity of Dye Solar Cells to UV-Induced Degradation. Energy Sci. Eng. 2020, 9, 19–26. [Google Scholar] [CrossRef]
- Flarup Jensen, K.; Brandt, H.; Im, C.; Wilde, J.; Hinsch, A. Stability of UV Illuminated Dye Sensitized Solar Cells (DSC) Studied by Photoinduced Absorption in the Second Range. In Proceedings of the 28th European PV Solar Energy Conference and Exhibition, Paris, France, 30 September–4 October 2013. [Google Scholar]
- Cong, J.; Yang, X.; Kloo, L.; Sun, L. Iodine/Iodide-Free Redox Shuttles for Liquid Electrolyte-Based Dye-Sensitized Solar Cells. Energy Environ. Sci. 2012, 5, 9180. [Google Scholar] [CrossRef]
- Brown, R.A.; Swift, E.H. The formal potential of the antimonous-antimonic half cell in hydrochloric acid solutions. J. Am. Chem. Soc. 1949, 71, 2719–2723. [Google Scholar] [CrossRef]
- Masud, N.; Kim, H.K. Redox Shuttle-Based Electrolytes for Dye-Sensitized Solar Cells: Comprehensive Guidance, Recent Progress, and Future Perspective. ACS Omega 2023, 8, 6139–6163. [Google Scholar] [CrossRef] [PubMed]
- Oskam, G.; Bergeron, B.P.; Meyer, G.J.; Searson, P.C. Pseudohalogens for Dye-Sensitized TiO2 Photoelectrochemical Cells. J. Phys. Chem. B 2001, 105, 6867–6873. [Google Scholar] [CrossRef]
- Boschloo, G.; Hagfeldt, A. Characteristics of the Iodide/Triiodide Redox Mediator in Dye-Sensitized Solar Cells. Acc. Chem. Res. 2009, 42, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Nusbaumer, H.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M. An Alternative Efficient Redox Couple for the Dye-Sensitized Solar Cell System. Chem. A Eur. J. 2003, 9, 3756–3763. [Google Scholar] [CrossRef]
- Asgher, M.; Miettunen, K.; Halme, J.; Vahermaa, P.; Toivola, M.; Aitola, K.; Lund, P. Review of Stability for Advanced Dye Solar Cells. Energy Environ. Sci. 2010, 3, 418. [Google Scholar] [CrossRef]
- Feldt, S.M.; Gibson, E.A.; Gabrielsson, E.; Sun, L.; Boschloo, G.; Hagfeldt, A. Design of Organic Dyes and Cobalt Polypyridine Redox Mediators for High-Efficiency Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2010, 132, 16714–16724. [Google Scholar] [CrossRef]
- Ji, J.M.; Zhou, H.; Eom, Y.K.; Kim, C.H.; Kim, H.K. 14.2% Efficiency Dye-Sensitized Solar Cells by Co-Sensitizing Novel Thieno[3,2-b]Indole-Based Organic Dyes with a Promising Porphyrin Sensitizer. Adv. Energy Mater. 2020, 10, 2000124. [Google Scholar] [CrossRef]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M.; et al. Dye-Sensitized Solar Cells for Efficient Power Generation under Ambient Lighting. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Yamanaka, N.; Kawano, R.; Kubo, W.; Masaki, N.; Kitamura, T.; Wada, Y.; Watanabe, M.; Yanagida, S. Dye-Sensitized TiO2 Solar Cells Using Imidazolium-Type Ionic Liquid Crystal Systems as Effective Electrolytes. J. Phys. Chem. B 2007, 111, 4763–4769. [Google Scholar] [CrossRef] [PubMed]
- Kawano, R.; Nazeeruddin, M.K.; Sato, A.; Grätzel, M.; Watanabe, M. Amphiphilic ruthenium dye as an ideal sensitizer in conversion of light to electricity using Ionic liquid crystal electrolyte. Electrochemistry Communications 2007, 9, 1134–1138. [Google Scholar] [CrossRef]
- Lau, G.P.S.; Décoppet, J.-D.; Moehl, T.; Zakeeruddin, S.M.; Grätzel, M.; Dyson, P.J. Robust High-Performance Dye-Sensitized Solar Cells Based on Ionic Liquid-Sulfolane Composite Electrolytes. Sci. Rep. 2015, 5, 18158. [Google Scholar] [CrossRef]
- Tedla, A.; Mu, Y.-T.; Sharma, J.; Tai, Y. Shelf-Life Studies on an Ionic-Liquid-Stabilized Dye-Sensitized Solar Cell. IEEE J. Photovolt. 2017, 7, 177–183. [Google Scholar] [CrossRef]
- Yu, Z.; Vlachopoulos, N.; Hagfeldt, A.; Kloo, L. Incompletely Solvated Ionic Liquid Mixtures as Electrolyte Solvents for Highly Stable Dye-Sensitized Solar Cells. RSC Adv. 2013, 3, 1896–1901. [Google Scholar] [CrossRef]
- Yamanaka, N.; Kawano, R.; Kubo, W.; Kitamura, T.; Wada, Y.; Watanabe, M.; Yanagida, S. Ionic Liquid Crystal as a Hole Transport Layer of Dye-Sensitized Solar Cells. Chem. Commun. 2005, 115, 740–742. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Shi, D.; Zhang, J.; Wang, M.; Jing, X.; Humphry-Baker, R.; Wang, P.; Zakeeruddin, S.M.; Grätzel, M. Enhance the Optical Absorptivity of Nanocrystalline TiO2 Film with High Molar Extinction Coefficient Ruthenium Sensitizers for High Performance Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2008, 130, 10720–10728. [Google Scholar] [CrossRef]
- Abu Talip, R.A.; Yahya, W.Z.N.; Bustam, M.A. Ionic Liquids Roles and Perspectives in Electrolyte for Dye-Sensitized Solar Cells. Sustainability 2020, 12, 7598. [Google Scholar] [CrossRef]
- Tedla, A.; Tai, Y. Influence of Binary Solvent System on the Stability and Efficiency of Liquid Dye Sensitized Solar Cells. J. Photochem. Photobiol. A-Chem. 2018, 358, 70–75. [Google Scholar] [CrossRef]
- Wang, P.; Yang, L.; Wu, H.; Cao, Y.; Zhang, J.; Xu, N.; Chen, S.; Decoppet, J.-D.; Zakeeruddin, S.M.; Grätzel, M. Stable and Efficient Organic Dye-Sensitized Solar Cell Based on Ionic Liquid Electrolyte. Joule 2018, 2, 2145–2153. [Google Scholar] [CrossRef]
- Wang, N.; Hu, J.; Gao, L.; Ma, T. Current Progress in Solid-State Electrolytes for Dye-Sensitized Solar Cells: A Mini-Review. J. Electron. Mater. 2020, 49, 7085–7097. [Google Scholar] [CrossRef]
- Bach, U.; Lupo, D.W.; Moser, J.-E.; Weissörtel, F.; Salbeck, J.; Spreitzer, H.; Grätzel, M. Solid-State Dye-Sensitized Mesoporous TiO2 Solar Cells with High Photon-to-Electron Conversion Efficiencies. Nature 1998, 395, 583–585. [Google Scholar] [CrossRef]
- Ursu, D.; Vajda, M.; Miclau, M. Investigation of the P-Type Dye-Sensitized Solar Cell Based on Full Cu2O Electrodes. J. Alloys Compd. 2019, 802, 86–92. [Google Scholar] [CrossRef]
- Higashino, Y.; Erten-Ela, S.; Kubo, Y. π-Expanded Dibenzo-BODIPY with near-Infrared Light Absorption: Investigation of Photosensitizing Properties of NiO-Based p-Type Dye-Sensitized Solar Cells. Dye. Pigment. 2019, 170, 107613. [Google Scholar] [CrossRef]
- Kim, J.H.; Koo, S.-J.; Cho, H.; Choi, J.W.; Ryu, S.Y.; Kang, J.-W.; Jin, S.-H.; Ahn, C.; Song, M. 6.16% Efficiency of Solid-State Fiber Dye-Sensitized Solar Cells Based on LiTFSI Electrolytes with Novel TEMPOL Derivatives. ACS Sustain. Chem. Eng. 2020, 8, 15065–15071. [Google Scholar] [CrossRef]
- Rokesh, K.; Anandan, S.; Jothivenkatachalam, K. Polymer Electrolytes in Dye Sensitized Solar Cells. Mater. Focus 2015, 4, 262–271. [Google Scholar] [CrossRef]
- Storck, J.; Dotter, M.; Adabra, S.; Surjawidjaja, M.; Brockhagen, B.; Grothe, T. Long-Term Stability Improvement of Non-Toxic Dye-Sensitized Solar Cells via Poly(Ethylene Oxide) Gel Electrolytes for Future Textile-Based Solar Cells. Polymers 2020, 12, 3035. [Google Scholar] [CrossRef]
- Raut, P.; Kishnani, V.; Mondal, K.; Gupta, A.K.; Jana, S.C. A Review on Gel Polymer Electrolytes for Dye-Sensitized Solar Cells. Micromachines 2022, 13, 680. [Google Scholar] [CrossRef]
- Yang, H.; Huang, M.; Wu, J.; Lan, Z.; Hao, S.; Lin, J. The polymer gel electrolyte based on poly(methyl methacrylate) and its application in quasi-solid-state dye-sensitized solar cells. Mater. Chem. Phys. 2008, 110, 38–42. [Google Scholar] [CrossRef]
- Wortmann, M.; Layland, A.S.; Frese, N.; Kahmann, U.; Grothe, T.; Storck, J.L.; Blachowicz, T.; Grzybowski, J.; Hüsgen, B.; Ehrmann, A. On the reliability of highly magnified micrographs for structural analysis in materials science. Sci. Rep. 2020, 10, 14708. [Google Scholar] [CrossRef]
- Santos, F.; Ivanou, D.; Mendes, A. The renaissance of monolithic dye-sensitized solar cells. Mater. Today Commun. 2022, 32, 104030. [Google Scholar] [CrossRef]
- Li, D.; Qin, D.-B.; Deng, M.; Luo, Y.; Meng, Q. Optimization the Solid-State Electrolytes for Dye-Sensitized Solar Cells. Energy Environ. Sci. 2009, 2, 283–291. [Google Scholar] [CrossRef]
- Han, H.; Bach, U.; Cheng, Y.; Caruso, R.A.; MacRae, C.M. A design for monolithic all-solid-state dye-sensitized solar cells with a platinized carbon counterelectrode. Appl. Phys. Lett. 2009, 94, 103102. [Google Scholar] [CrossRef]
- Kay, A.; Grätzel, M. Low cost photovoltaic modules based on dye sensitized nanocrystalline titanium dioxide and carbon powder. Sol. Energy Mater. Sol. Cells 1996, 44, 99–117. [Google Scholar] [CrossRef]
- Rong, Y.; Liu, G.; Wang, H.; Li, X.; Han, H. Monolithic all-solid-state dye-sensitized solar cells. Front. Optoelectron. 2013, 6, 359–372. [Google Scholar] [CrossRef]
- Transparent Conducting Oxide (TCO) Glass Market Size 2023 To 2030. Available online: https://www.businessresearchinsights.com/market-reports/transparent-conducting-oxide-tco-glass-market-103811 (accessed on 30 May 2023).
- Tripodi, A.; Bahadori, E.; Cespi, D.; Passarini, F.; Cavani, F.; Tabanelli, T.; Rossetti, I. Acetonitrile from Bioethanol Ammoxidation: Process design from the grass-roots and life cycle analysis. ACS Sustain. Chem. Eng. 2018, 6, 5441–5451. [Google Scholar] [CrossRef]

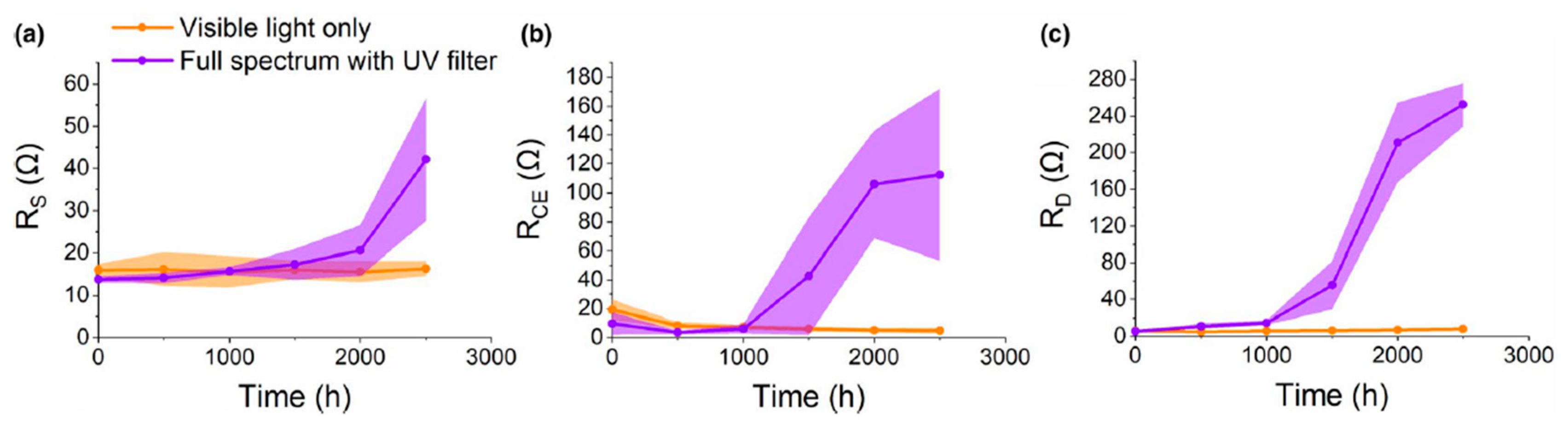
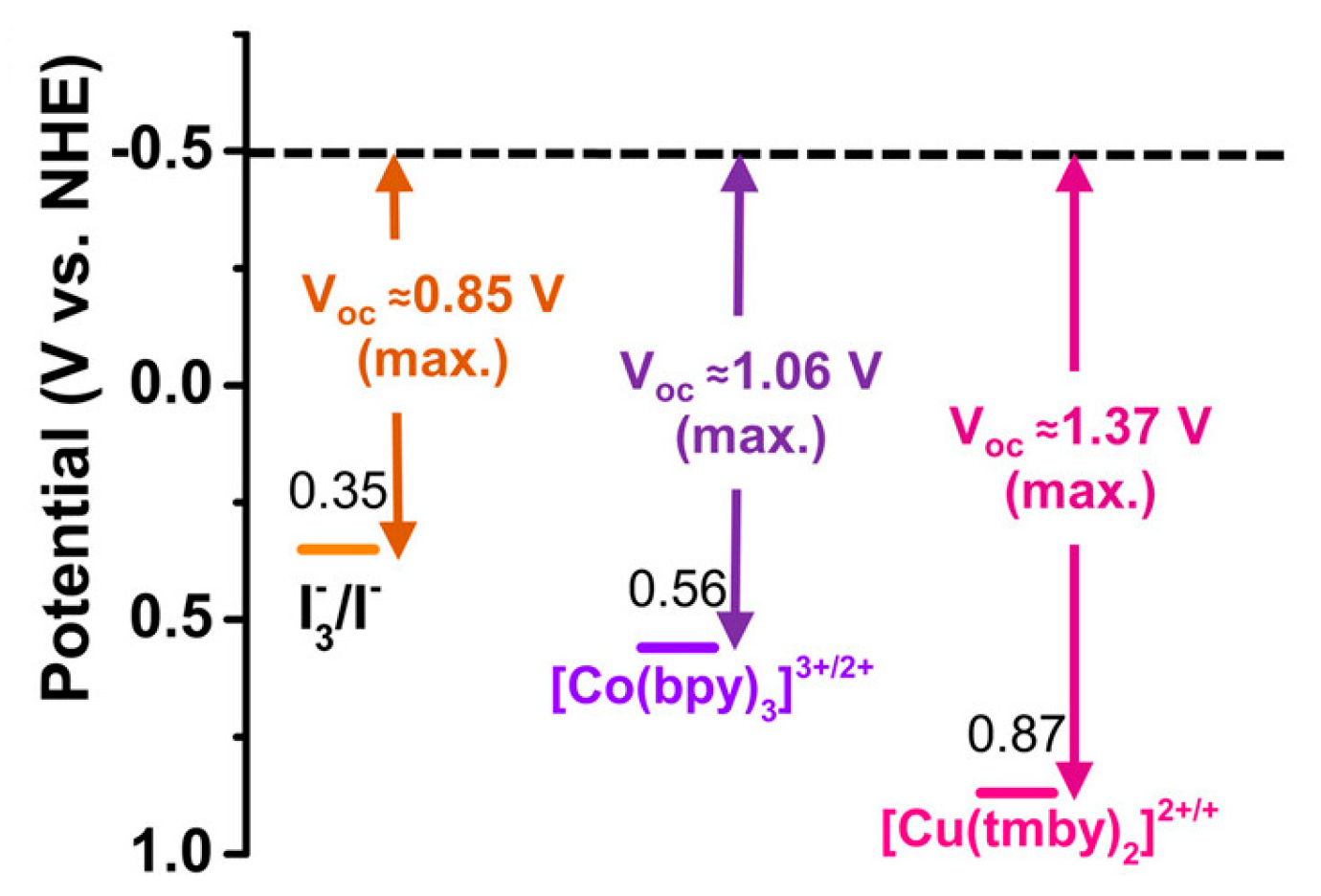

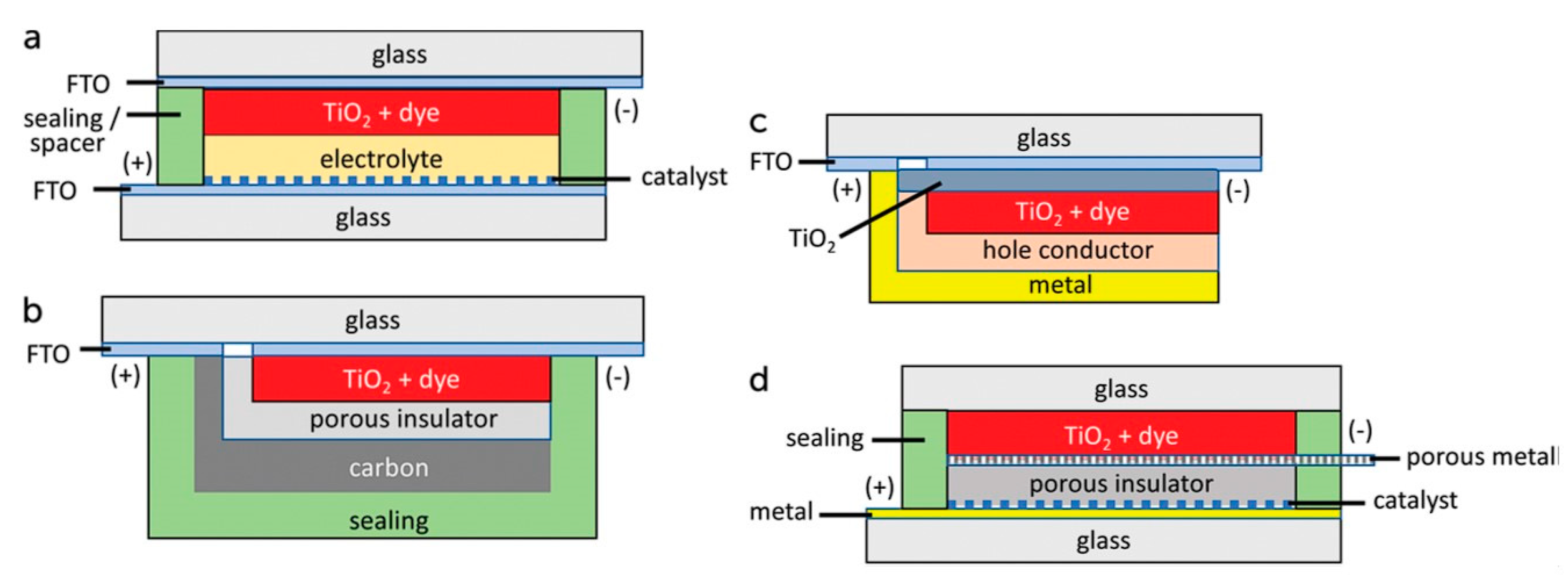
| Electrolyte Used | Short Circuit Current Density | Open Circuit Voltage | Fill Factor FF | Efficiency |
|---|---|---|---|---|
| Acetonitrile-based | 18.62 | 744 | 0.755 | 10.5 |
| Methoxypropionitrile-based | 17.98 | 746 | 0.737 | 9.7 |
| Binary ionic liquid | 14.77 | 681 | 0.737 | 7.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebenezer Anitha, A.; Dotter, M. A Review on Liquid Electrolyte Stability Issues for Commercialization of Dye-Sensitized Solar Cells (DSSC). Energies 2023, 16, 5129. https://doi.org/10.3390/en16135129
Ebenezer Anitha A, Dotter M. A Review on Liquid Electrolyte Stability Issues for Commercialization of Dye-Sensitized Solar Cells (DSSC). Energies. 2023; 16(13):5129. https://doi.org/10.3390/en16135129
Chicago/Turabian StyleEbenezer Anitha, Angellina, and Marius Dotter. 2023. "A Review on Liquid Electrolyte Stability Issues for Commercialization of Dye-Sensitized Solar Cells (DSSC)" Energies 16, no. 13: 5129. https://doi.org/10.3390/en16135129
APA StyleEbenezer Anitha, A., & Dotter, M. (2023). A Review on Liquid Electrolyte Stability Issues for Commercialization of Dye-Sensitized Solar Cells (DSSC). Energies, 16(13), 5129. https://doi.org/10.3390/en16135129





