3.1. Effect of the Applied Bias Potential
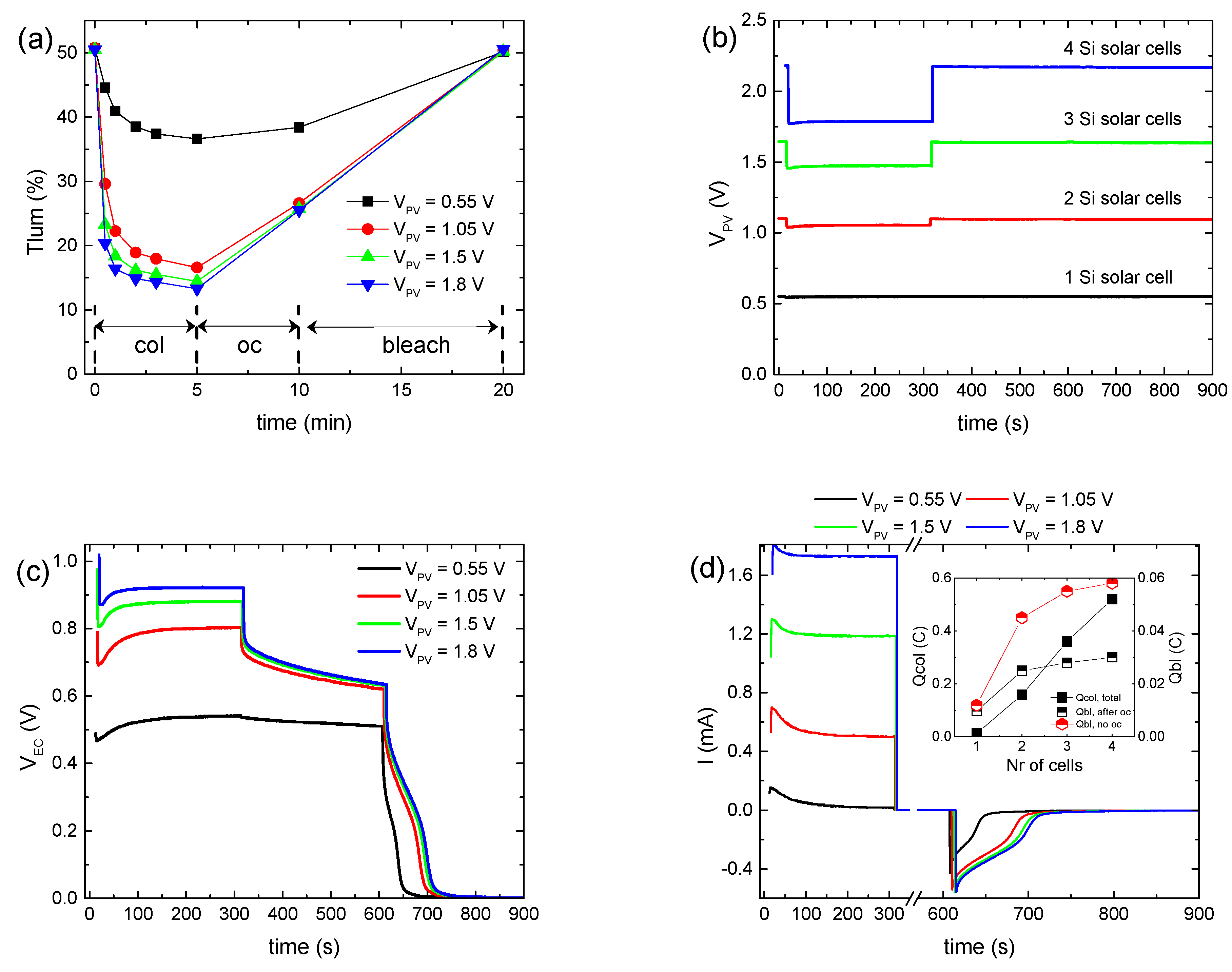
Figure 1a shows the variation in the luminous transmittance (
Tlum) during a testing procedure of a hybrid ECD for the different values of the applied bias potential (
VPV), while the corresponding full transmittance spectra in the visible and near-IR regions appear in
Figure S1. To vary
VPV, up to four BPW34 mini silicon solar cells were connected in series. A typical IPCE spectrum and characteristic I–V curves of BPW34 mini silicon solar cells connected in series appear in
Figure S2, and their characteristic photovoltaic parameters appear in
Table S2.
We observe that with
VPV increasing from 0.55 to 1.05 V (
Figure 1b), the coloration depth increased significantly from 36.6 to 16.6% (
Figure 1a), while the coloration time decreased slightly from less than 3 min to 1–2 min. Note that the coloration time was defined as the time needed for the ECD to attain 90% of its maximum optical modulation (Δ
T).
Subsequently, a minor improvement was observed in the coloration depth for
VPV ~1.5 V, while it remained nearly constant when
VPV increased further (~1.8 V) (
Figure 1a,b), even though the coloration time for
VPV ~1.8 V decreased even further to less than 1 min. At the same time, the voltage at the ECD terminals (
VEC) exhibited a sub-linear increment with
VPV (
Figure 1c). In particular, its value at the end of the coloration step tends to an upper bound value of nearly 1 V (
Figure S3a). Meanwhile, the total current passing through the ECD during coloration (
Itotal) increased linearly with
VPV (
Figure 1d and
Figure S3b).
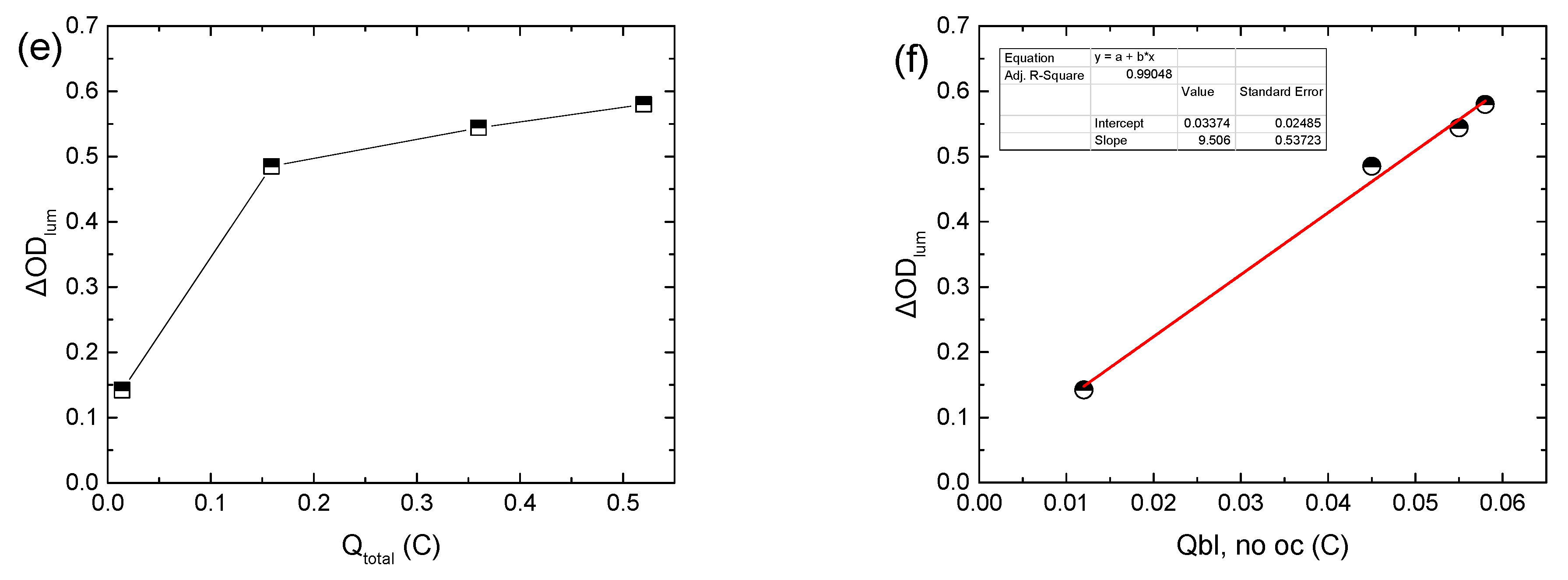
Even though Δ
ODlum increased linearly with
VEC, as expected [
10,
14] (
Figure S3c), its variation with the total charge during coloration (
Qtotal) was sub-linear (
Figure 1e). Due to the fact that both Δ
ODlum and
VEC tend to an upper bound value when
VPV is increased above ~1 V, we can assume that initially,
Icol will also show the same behavior with
VPV. Finally, for a certain
VPV,
Itotal attains a maximum value, after an initial increment, and then a lower steady-state one (
Figure 1d), as observed also in [
12]. The time needed for the steady state is approximately the same as the coloration time. Therefore, after
Itotal has reached its steady-state value, we can assume that
Itotal ≈
Iloss [
28], and thus, the major part of the increment in
Itotal with
VPV can be attributed to the increment in
Iloss, showing enhanced interfacial losses with an increase in
VPV [
14]. As a result,
Iloss increased from 7.65 to 194, 464, and 676 μA/cm
2 as
VPV increased from 0.55 to 1.05, 1.5, and 1.8 V, respectively. In other words, when the ECD reaches its final colored (charged) state, then all the current passing through the ECD is due to the interfacial loss reactions (Reaction 3) and
Icol tends to zero. If this mechanism is not possible, for example, due to the presence of a barrier layer, then the total current should fall to zero, resembling the behavior of a battery during charging.
Under open circuit conditions, partial bleaching of the ECD (
Figure 1a) and a significant voltage drop at the ECD terminals (
Figure 1c) were observed, due to the loss reactions at the EC layer/electrolyte interface (Reaction 3). Moreover, the initial voltage drop was more pronounced as
VPV increased, due to the higher difference between the electrochemical potential of
LixWO3 and the redox potential of the electrolyte. This is due to the lower value (more negative) of the electrochemical potential of
LixWO3 vs NHE since its value becomes more negative as the optical density of the EC layer increases [
14]. However, after 5 min under open circuit conditions, both
Tlum and
VEC converge when
VPV ≥ 1.05 V. Finally, after the ECD terminals were connected with a 1 kOhm resistor, the ECD was bleached (discharged) (
Figure 1c), returning in all cases to its initial optical state (
Figure 1a). As a result, an opposite bleaching current (
Figure 1d) was measured, from which the charge released during bleaching (
Qbl, after oc) was calculated.
As expected, the values of
Qbl, after oc when
VPV ≥ 1.05 V are nearly the same (
Figure 1d, inset). Moreover, a large difference was observed between the values of
Qtotal and
Qbl, after oc for the same
VPV value (
Figure 1d, inset) for two reasons: firstly, as explained above,
Qtotal includes also the charge responsible for triiodide reduction (Reaction 3) at the EC layer/electrolyte interface (
Qloss), and secondly, part of the stored charge in the
WO3 film is lost during the open circuit step, as explained above. By skipping the open circuit step in the testing procedure (
Figure S4a–c), a more accurate estimation of the overall stored charge in the
WO3 film (
Qbl, no oc) was possible (
Figure 1d, inset). As a result, Δ
ODlum now varies linearly with
Qbl, no oc (
Figure 1f), and from the linear regression analysis, the coloration efficiency could be calculated, being 24.3 cm
2 C
−1, considering that the area of the ECD was 1.6 × 1.6 cm
2. In this manner, a better estimation of the coloration efficiency is possible, since when using
Qtotal, as is common in the relevant literature [
11,
12,
13], an underestimation takes place.
Finally, since the ECD returns to its initial optical state after bleaching, we can assume that
Qcol ≈
Qbl, no oc. Therefore,
Qloss can be calculated indirectly from Equation (2):
and as appears in
Figure S4d,
Qloss improved significantly with
VPV, as assumed above. It is characteristic that the
Qloss/
Qtotal ratio varies from 0.14 to 0.72, 0.85, and 0.89 when
VPV increases from 0.55 to 1.05, 1.5, and 1.8, respectively. Alternatively,
Iloss (or equally
Qloss) can be calculated by the difference between
Itotal and the coloration or optical current (
Iopt), as proposed by Bogati et al. in [
14,
16]. However, a previously known and constant coloration efficiency is a prerequisite.
3.3. Stability of the Hybrid ECD
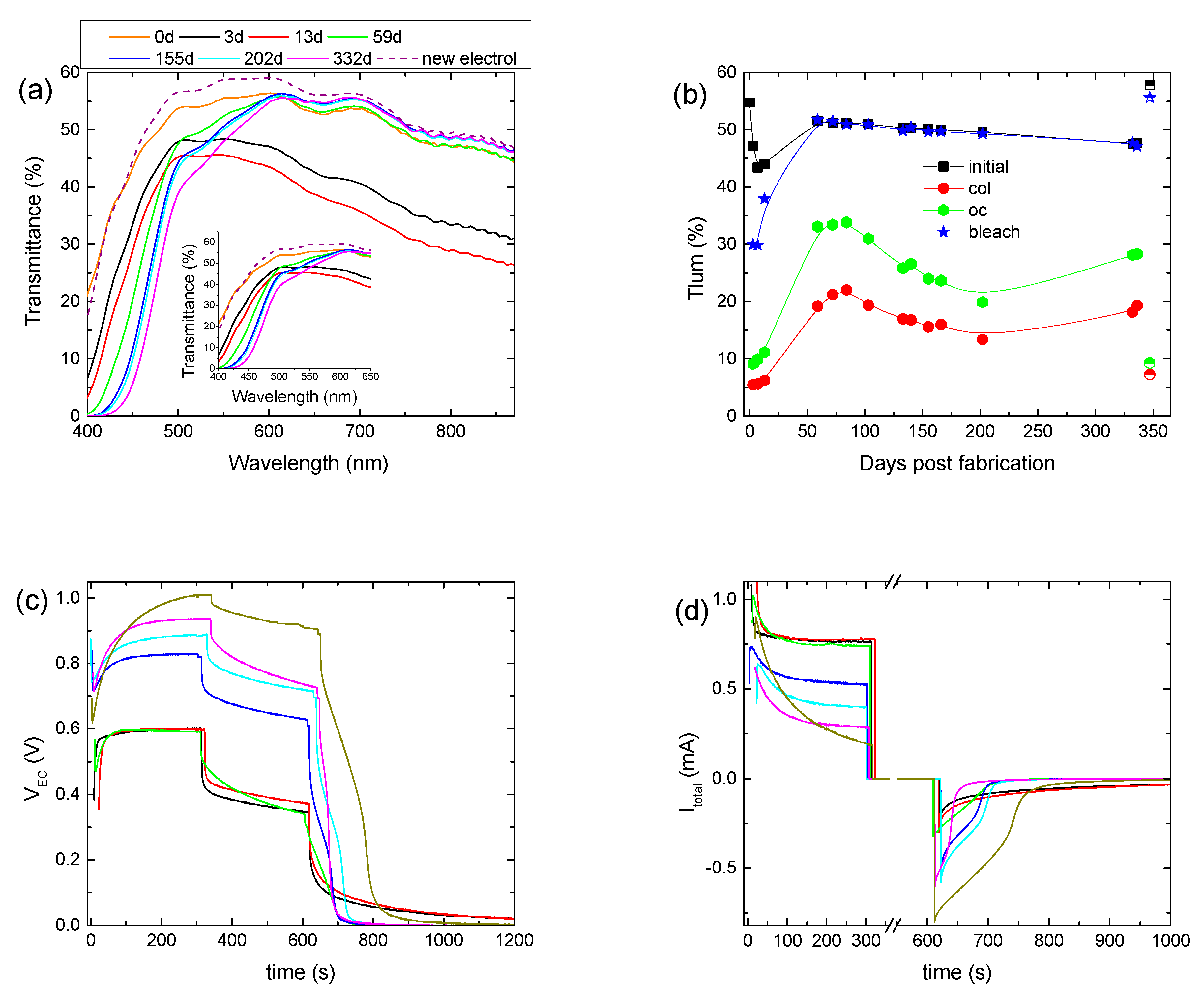
The stability of the hybrid ECD was also examined by performing a prolonged aging test for at least one year. Initially, the hybrid ECD remained under open circuit conditions in the dark for 3 days to examine if self-coloration takes place. Indeed, an overall reduction in the transmittance was observed (
Figure 3a), with the luminous transmittance in the colored state (
Tlum, col) decreasing from 55 to 47.2% (
Figure 3b). This fact shows that the electrochemical potential (vs NHE) of the as-prepared
WO3 layer is higher (more positive) than that of the redox electrolyte. As a result, the transfer of electrons from the redox electrolyte to the
WO3 film is possible, inducing self-coloration [
14].
Then, for the following 10 days, we observed an intense coloration, where the device exhibited a
Tlum, col of 5–6% and a contrast ratio above 7:1 (
Figure 3b,
Table 2). Moreover, the electrical properties of the ECD did not show any significant changes (
Figure 3c,d). Optical and electrical losses during the open circuit step were also observed, as expected (
Figure 3c,d). Finally, it is worth mentioning that the ECD could not return to its initial optical state and the values of both
VEC and
Itotal were not equal to zero at the end of the bleaching step (
Figure 3c,d), showing a remaining coloration of the ECD.
Afterwards, the ECD was stored under open circuit conditions for nearly 45 days, and for the following 150 days, the ECD was tested regularly. As appears in
Figure 3a, a significant change in the transmittance spectra occurred. More specifically, a gradual reduction in the transmittance of the ECD up to 600 nm was observed, as compared with the transmittance measured immediately after assembling the device (day 0), possibly due to the formation of free iodine (
I2). Similarly, a reduction in the transmittance in the case of an
I−/
I3− redox electrolyte in the 400–700 nm region after 50 consecutive voltammetry scans was observed in [
11]. As the authors stated, the absorption in the blue region resulted initially from the production of
I3− ions, having an absorption maximum at 360 nm, and then from their further oxidation to iodine, which shows a strong absorption for
λ > 430 nm [
18].
During this period,
Tlum, col varied within the range of 22–13%, showing a trend of improving the coloration depth and the Δ
ODlum, as the device was cycled (
Figure 3b) (
Table 2). Moreover, during coloration,
VEC increased considerably. More specifically, its value at the end of the coloration step increased from 0.6 V to nearly 0.9 V (
Figure 3c and
Figure S6a), while at the same time, only a minor increment in the
VPV value occurred, from 0.98 V to 1.1 V (
Figure S6b), depicting a small change in the operating point of the mini-Si solar cells. Therefore, the increment in the
VEC value was not due to the increment in the
VPV value.
A decrement in
Itotal could explain the
VEC increment, since the voltage drop at the terminals of the 0.5 kOhm resistor would decrease, leaving a larger part of
VPV to be applied at the terminals of the ECD. Indeed, both the maximum value of
Itotal and its steady-state value at the end of the coloration step, which correlates well with
Iloss, decreased (
Figure 3d). For example, the steady-state value of
Itotal was 0.73 mA after 59 days and decreased to 0.4 mA after 200 days. As a result,
Qtotal showed a clear trend for reduction (
Figure S6c) (
Table 2), and the improvement in the optical performance can be attributed to the reduced losses and the training effect of the
WO3 film due to the continuous operation.
Moreover, the increment percentage of
Tlum during the open circuit step varied from 73% (day 55) to 50% (day 200), and the voltage drop percentage varied from 45% (59th day) to nearly 20% (200th day) (
Figure S6a), both showing reduced optical and electrical losses. As a result,
Qbl, after oc showed an increment from 17.8 mC (59th day) to 28.8 mC (200th day) (
Figure S6b) (
Table 2). Finally, the device could return to its initial optical state, and both
VEC and
Itotal were equal to zero after nearly 100 s of bleaching (
Figure 3c,d), showing a complete bleaching (discharge) of the ECD.
A second and longer storage period under open circuit conditions followed for 130 days. After that, the initial transmittance of the ECD decreased further (
Figure 3a) for
λ < 600 nm,
Tlum, col increased to 18.2% (
Figure 3b), and in general, the optical performance deteriorated (
Table 2). A further drop in
Itotal or equally in
Qtotal was also observed (
Figure 3d and
Figure S6c). During the open circuit step, the increment percentage of
Tlum and the voltage drop percentage were 55% and 26%, respectively (
Figure 3b and
Figure S6a) (
Table 2), and the value of
Qbl, after oc was 16.7 mC, less than that before the storage period. It seems that, as after the first storage period, the performance of the ECD was affected negatively by storage under open circuit conditions. Nevertheless, the performance drop was not so intense after the second storage period. The exact influence of storage under open circuit conditions is not clear and needs to be further examined.
Nevertheless, by comparing days, before and after the second storage period, where the ECD exhibited the same optical density modulation (Δ
ODlum) (i.e., for days 100 and 330), reduced values of Δ
Tlum, Δ
VEC, and
Qtotal and an improved value of
Qbl, after oc were observed (
Table 2). That is another indication regarding the reduction in losses at the EC layer/electrolyte interface, after a prolonged testing period.
We believe that one of the reasons behind the reduction in losses, after a prolonged testing period, is the instability of the redox electrolyte. More specifically, the reduction in the initial transmittance of the ECD for
λ < 600 nm (
Figure 3a), as discussed above, indicates the formation of free iodine [
11,
18,
20]. Initially, the concentration of free
I2 is very low, since iodine is almost transformed to triiodide according to Reaction 4 [
29]:
After prolonged testing, the direction of Reaction 4 is reversed, leading to the consumption of
I3− ions and the formation of free
I2. Another possibility is the oxidation of triiodide ions to iodine according to Reaction 5 [
11]:
Reaction 5 takes place at more positive potentials than Reaction 2; therefore, it can be avoided by properly selecting the applied bias potential [
10]. The presence of
I2 can also negatively affect the conductivity of the electrolyte [
20]. Therefore, due to the reduced availability of
I3− ions, the losses at the
WO3/electrolyte interface are reduced. At the same time, a slight variation in the redox potential of the electrolyte to lower values is expected [
29].
To verify our above assumptions regarding the role of the electrolyte, the electrolyte was replaced with a fresh one. First, we observed that the transmittance of the ECD returned to its initial state (day 0) (
Figure 3a,b) and the device exhibited optical performance (optical density modulation, coloration depth, contrast ratio) similar to that during its first cycle (3rd day). However, a significantly reduced value of
Itotal was measured during coloration, leading to a reduced value of
Qtotal (
Figure 3c and
Figure S6c). Moreover, reduced optical and electrical losses during the open circuit step (
Table 2) were also observed. All the above resulted in a
Qbl, after oc value 2.5 times larger than that in the first cycle (3rd day) (
Table 2). Finally, the device could return to its initial optical state after bleaching. Therefore, we can assume that under prolonged testing, changes occur in not only the concentration of the electrolyte but also the properties of the EC layer/electrolyte interface. An increment in the charge transfer resistance at the
WO3/electrolyte interface, due to possible adsorption of electrolyte species, could explain the above results well.
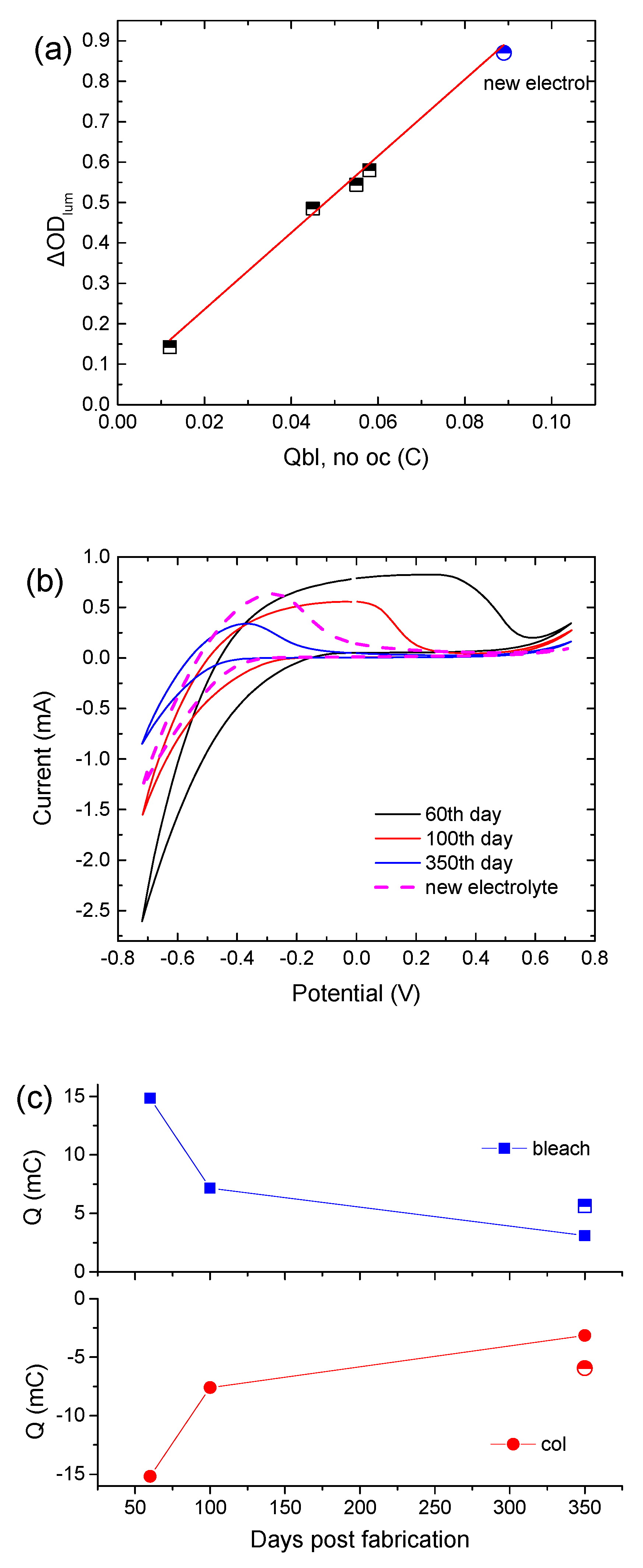
It is interesting to note that the CE is not affected by the replacement of the electrolyte with a fresh one, since the slope in the Δ
ODlum vs.
Qbl, no oc graph remains the same (
Figure 4a). Moreover,
Figure 4b shows cyclic voltammograms of the above ECD for different days post-fabrication. Their shape is typical in the case where an amorphous
WO3 film is used as the EC layer [
18]. Moreover,
WO3 is a well-known cathodic electrochromic material, meaning that it is colored during the cathodic scan. During the cathodic scan, a negative shift of the voltage value where coloration begins (the point where current changes sign from positive to negative) was observed, showing that a higher applied bias potential was necessary for coloration. The voltammogram area during the cathodic pulse decreases considerably, revealing an overall decrement in charge density exchanged during coloration (
Figure 4c). Replacement of the electrolyte causes a slight positive shift of the voltage value where coloration begins, and an increment in
Qcol by almost 80% (
Figure 4c). Also, the ratio of
Qcol/
Qbl is equal to 1, showing a reversible coloration–bleaching procedure. Finally, the potential value, where the current tends to zero during the anodic pulse, was found to decrease, showing that the deintercalation of Li
+ ions was made easier due to the training of the
WO3 film.
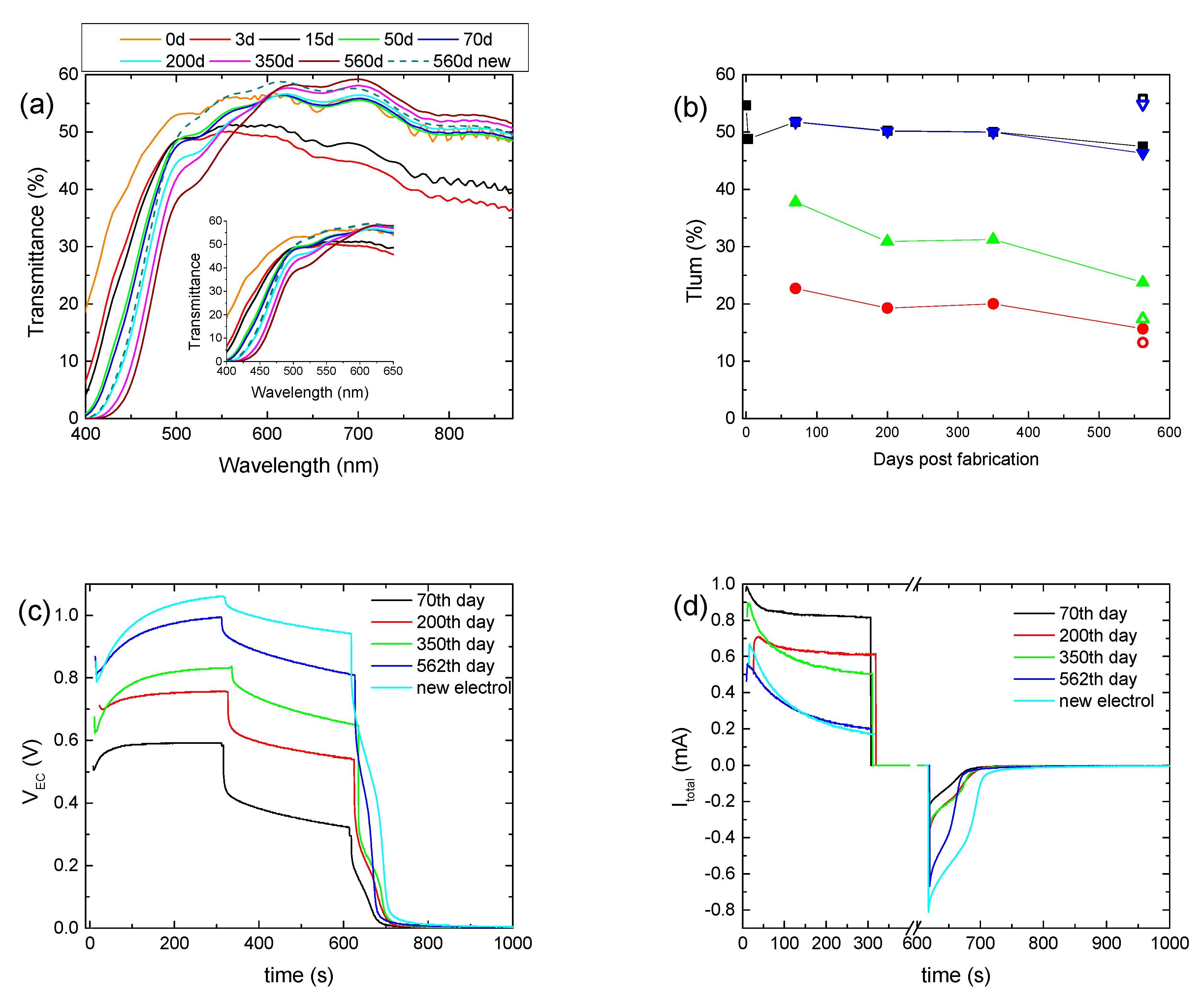
To examine further the effect of the prolonged testing on the overall performance of a hybrid ECD, the first coloration–bleaching cycle was performed 70 days after its fabrication, followed by only four more coloration–bleaching cycles, for a total period of nearly 18 months. In the meantime, only the transmittance was measured, and the ECD remained under open circuit conditions. Initially, due to self-coloration, an overall decrement in the transmittance was observed, while after 50 days, the transmittance of the ECD for wavelengths above 600 nm returned to its initial value (day 0), showing that self-coloration was no longer possible (
Figure 5a). Nevertheless, the transmittance of the ECD at short wavelengths reduced continuously, due to the absorption of the electrolyte, even before the first coloration–bleaching cycle was performed. For example, the transmittance at 500 nm was reduced from its initial value of 53.1% to 47.8% and then to 38.4% after 70 days and 560 days, respectively. Therefore, the formation of free I
2 can take place even without the application of a bias potential, due to Reaction 4.
During the first operation cycle, an intense coloration and optical irreversibility were not observed, as before. In general, the optical performance of the device was comparable to that of the previously aged ECD, showing a small improvement after 560 days (
Figure 5b and
Figure S7,
Table 3). Again, an increment in
VEC values during coloration occurred; i.e., the
VEC value at the end of the coloration step increased from 0.59 V to 0.99 V (
Figure 5c and
Figure S6a), whereas both the maximum value of
Itotal and its value after 5 min of coloration decreased (
Figure 5d), with a small exception for
Itotal, max of 350 days. Accordingly,
Qtotal showed a clear trend for reduction (
Figure S7b and
Table 3). Optical and electrical losses during the open circuit step also showed a trend for reduction (
Figure 5b,c and
Figure S7b). All the above resulted in an improved value of
Qbl, after oc, being 3 times larger after 560 days, compared with its value after the first coloration–bleaching cycle (70th day) (
Figure S7b and
Table 3). Finally, the substitution of the electrolyte with a fresh one remarkably improves the performance of the ECD, where optical and electrical losses were minimized. Note that the fresh electrolyte used here was the EL-HPE high-performance electrolyte, supplied by GreatSellSolar materials.
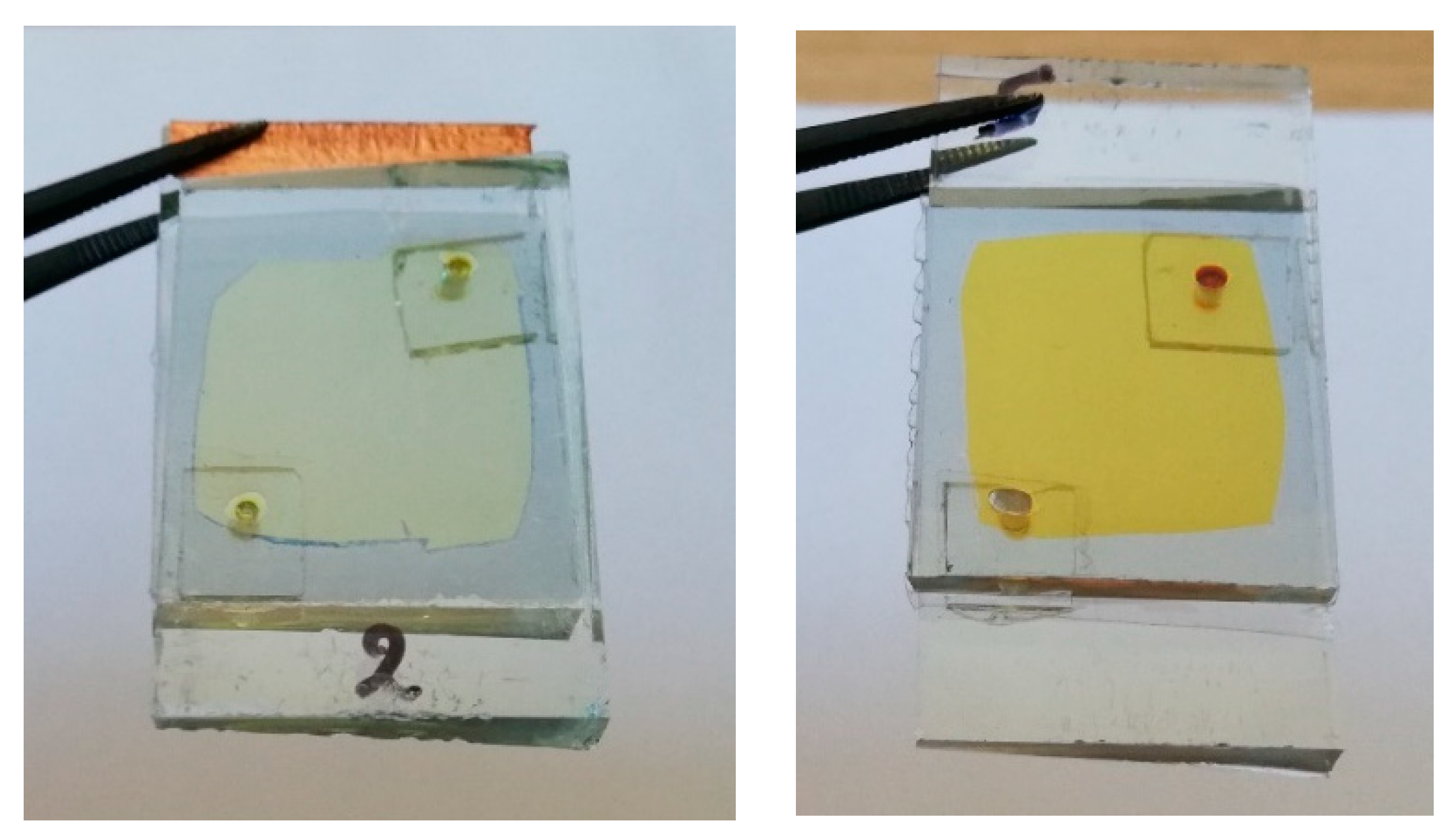
Finally,
Figure 6 shows images of hybrid ECDs, immediately after assembling and after one year, where the more intense yellowish tint is obvious.
In summary, the above-described mechanisms that are responsible for the performance variation of hybrid ECDs, and especially the instability of the redox electrolyte, take place irrespective of whether the device is cycled or not. Therefore, this is a significant limitation imposed using an iodide/triiodide redox electrolyte.