Abstract
In light of the growing interest in hydrogen as an energy carrier and reducing agent, various industries, including the iron and steel sector, are considering the increased adoption of hydrogen. To meet the rising demand in energy-intensive industries, the production of hydrogen must be significantly expanded and further developed. However, current hydrogen production heavily relies on fossil-fuel-based methods, resulting in a considerable environmental burden, with approximately 10 tons of CO2 emissions per ton of hydrogen. To address this challenge, methane pyrolysis offers a promising approach for producing clean hydrogen with reduced CO2 emissions. This process involves converting methane (CH4) into hydrogen and solid carbon, significantly lowering the carbon footprint. This work aims to enhance and broaden the understanding of methane pyrolysis in a liquid metal bubble column reactor (LMBCR) by utilizing an expanded and improved experimental setup based on the reactor concept previously proposed by authors from Montanuniversitaet in 2022 and 2023. The focus is on investigating the process parameters’ temperature and methane input rate with regard to their impact on methane conversion. The liquid metal temperature exhibits a strong influence, increasing methane conversion from 35% at 1150 °C to 74% at 1250 °C. In contrast, the effect of the methane flow rate remains relatively small in the investigated range. Moreover, an investigation is conducted to assess the impact of carbon layers covering the surface of the liquid metal column. Additionally, a comparative analysis between the LMBCR and a blank tube reactor (BTR) is presented.
1. Introduction
Hydrogen is a crucial industrial chemical with a global production rate of approximately 90 million metric tons per year. Its primary applications are found in the chemical industry, particularly in refining processes for fossil fuel production and in the production of ammonia (NH3) for agricultural fertilizers. Although hydrogen’s potential for mobility is often highlighted, its current usage in this field is not significant compared to the aforementioned applications. However, to achieve the desired energy transition, it is necessary to convert certain industrial processes, which are challenging to electrify, from fossil energy sources (such as coal and natural gas) to hydrogen [1].
Among the energy-intensive industries, the production of metals, especially iron and steel, stands out. To shift the global steel production of about two Gt/a from carbon-based to hydrogen-based processes, a doubling of the global hydrogen capacity is required. According to the International Energy Agency’s (IEA) report [2], annual hydrogen production is projected to increase sixfold by 2050, potentially covering about 10% of the global energy demand.
The primary method employed for hydrogen production at present is steam methane reforming (SMR), a process in which natural gas undergoes chemical conversion with steam at temperatures ranging from 800 to 1000 °C, resulting in the formation of carbon monoxide (CO) and hydrogen (H2) (cf. Equation (1)). This reforming reaction is accompanied by the slightly exothermic water–gas shift reaction, which enhances the yield of hydrogen but also leads to the production of carbon dioxide (CO2) (cf. Equation (2)) [1,2,3].
The overall forward reaction (cf. Equation (3)) is endothermic (ΔH0 = +165 kJ/mol CH4, or related to one mole of hydrogen ΔH0 = +41.25 kJ/mol H2) and requires a certain amount of energy which is provided via the combustion of natural gas, producing additional CO2 [4].
Electrolysis is a process in which water is decomposed into hydrogen and oxygen using electrical energy, providing the advantage of a CO2-free H2-production pathway (cf. Equation (4)) in case renewable electricity is used. However, due to the considerably higher standard reaction enthalpy of +286 kJ/mol H2, the energy demand associated with electrolysis is significantly higher compared to steam methane reforming [5,6,7].
A promising alternative is the non-oxidative thermal decomposition of methane, also known as methane pyrolysis or methane cracking, which could serve as a technology, providing a missing link between the cheap but highly CO2-emitting, fossil-fuel-driven processes and the possibly clean yet energy-intensive water-electrolysis technologies. The pyrolysis reaction of methane (cf. Equation (5)) is endothermic and can be written as follows [8]:
Methane cracking necessitates a certain energy input into the gas. There are two primary categories of methane pyrolysis, catalytic and non-catalytic, employing various heat sources such as solar thermal power [9], plasma reactors [10,11], microwave heating [12,13,14], or reactor designs utilizing liquid media in bubble columns [9,15,16]. Additionally, conventional processes like fixed bed, moving bed, or fluidized bed reactors have been explored, as summarized in table form by Keipi et al. [17].
According to literature sources [18,19,20,21], non-catalytic methane pyrolysis theoretically commences between 530 and 700 °C. However, in practical applications, temperatures above 1200 °C are necessary to ensure adequate methane decomposition, while temperatures below 1000 °C can suffice when a catalyst is employed [22]. Catalytic methane pyrolysis occurs within a temperature range of 600–900 °C, which is comparable to the operating temperature of steam methane reforming. Extensive research has been conducted on various catalysts, including carbonaceous materials and transition metals like Ni, Co, or Fe. These transition metals possess partially filled 3d orbitals, enabling them to efficiently facilitate the decomposition mechanism by absorbing electrons from the C-H bond and lowering so the activation energy for the pyrolysis reaction. Notably, solid metallic catalysts demonstrate superior performance compared to carbon-based catalysts; however, their effectiveness diminishes at temperatures exceeding 600 °C as pyrolysis carbon deposits at active sites during the reaction [18,19,20,21,22,23,24].
Previous attempts to develop methane decomposition processes have faced a notable challenge associated with the deposition of solid carbon on the surfaces of the reactor walls, non-catalytic solid fillings, and the catalyst material itself. This unwanted carbon deposition phenomenon hampers the functionality of the catalyst, resulting in its deactivation. In severe cases, it can even lead to the complete blockage of the reactor [25,26].
The liquid metal bubble column reactor (LMBCR) offers an alternative approach to prevent carbon deposition on reactor walls and catalyst deactivation. In this method, pure methane or natural gas is injected into a liquid metal at elevated temperatures [9]. The high energy input causes methane gas to decompose within the rising bubbles in the liquid metal bubble column. Upon reaching the surface, the bubbles burst, releasing carbon, hydrogen, unconverted methane, and gaseous intermediates from the pyrolysis reaction. Since carbon remains in a solid state, direct CO2 emissions are prevented and carbon can be utilized as a process by-product. Possible fields of application are the agricultural sector, where high amounts of carbon could be used for soil enhancement, or classic applications like rubber products, additives in lubricants, casting powders, or anode material for the metallurgical industry. Additionally, the liquid metal can serve as a reaction catalyst by incorporating catalytically active metals into the molten alloy [27,28].
The development of liquid metal bubble column reactors for methane pyrolysis dates back to the early 20th century, with the first proposals created by Tyrer et al. [29] in 1931 and Oblad et al. [30] in 1956. Steinberg et al. [31] introduced the concept of methane pyrolysis in liquid tin for hydrogen production, while Martynov et al. [32] proposed a process using a lead–bismuth alloy for hydrogen production via counter-current methane feeding.
Serban et al. [33] investigated the use of a stainless steel tube reactor with different heat-transfer media, including blank tube experiments without any fillings, low melting point liquid metals (e.g., tin or lead), granular materials (e.g., silicon carbide), and mixtures of liquid tin with solid media. They observed carbon formation during the experiments and achieved a methane conversion of 51% using a 0.5 µm porous mott sparger at a temperature of 750 °C. Paxman et al. [34,35,36] compared methane pyrolysis in an empty reactor of Al2O3 with a LMBCR filled with liquid tin and found lower methane conversions in the LMBCR experiments in contrast to Serban et al. [33]. They attributed this to the catalytic effect of stainless steel in the experiments of Serban et al. [33] and the dispersed small bubbles generated by the mott sparger. Plevan et al. [9] conducted experiments in a stainless steel tubular reactor filled with liquid tin, investigating the effects of temperature, methane flow rate, and reactor height. They found that methane conversion increased with higher temperatures and lower flow rates. The use of an empty reactor resulted in higher conversions compared to the LMBCR experiments. Geißler et al. [16,37] used a bubble column reactor with liquid tin and a packed-bed area with liquid tin and quartz glass fragments, achieving methane conversions of up to 73% at 1175 °C and a CH4 feed rate of 0.200 SLM. Subsequent studies conducted by Hofberger et al. [38] involved scaling up the experimental setup originally developed by Geißler et al. [16,37] by a factor of 3.75 referred to as the reactor volume. Despite an increase in the methane throughput from 0.200 to 0.500 SLM, the resulting conversion rate was not significantly impacted.
Upham et al. [39] investigated the effectiveness of various alloying systems to augment catalytic activity and achieved a methane conversion rate of 86% utilizing a liquid metal bubble column comprising Ni27Bi73. Similarly, Palmer et al. [40] employed Ni27Bi73 as a liquid catalytic medium and explored the use of different hydrocarbon feedstocks, including methane, propane, benzene, and crude oil. Perez et al. [41] conducted experiments using liquid gallium in a LMBCR, attaining a methane conversion rate of 80% with the aid of a porous plate for the introduction of feed gas. Based on the feasibility study by Ebner et al. [42], Scheiblehner et al. [43] conducted experiments with the highest CH4 flow rate to date of 0.500 SLM in a LMBCR applying lance sparging and compared different binary copper alloys to investigate the chemical and physical effects of liquid metal on methane pyrolysis. Their experimental results demonstrated that specific alloys exert a positive influence on methane conversion, not limited to catalytically active metals like nickel but also encompassing surface tension lowering metals such as tin or bismuth. The presence of these metals resulted in smaller bubble diameters, which in turn increased the overall interface between liquid metal and gas, enabling a more efficient heat transfer.
Kang et al. [15] investigated methane pyrolysis in different mixtures of KCl with MnCl2, while Rahimi et al. [44] explored the use of a liquid metal bubble column reactor containing Ni27Bi73 with a cover layer of liquid salt to reduce metal contamination in the carbon product. Patzschke et al. [45] investigated the performance of different solid catalysts dispersed in an eutectic molten salt NaBr/KBr (48.7:51.3 mol%). They found that the Co-Mn catalyst demonstrated the most favourable outcomes, exhibiting high resistance against deactivation.
Table 1 presents a comprehensive summary of the main findings from the aforementioned authors. The key parameters that exhibit significant influence on methane conversion include temperature, methane flow rate, bath height, composition of the liquid metal, and the type of bubble generator used. Based on the relatively small reactor dimensions and methane flow rates, the current technological readiness level (TRL) of methane pyrolysis falls within the TRL3 range, an assumption that is consistent with the conclusions made by Hermesmann et al. [46].

Table 1.
Performance of reactor concepts of previous works concerning LMBCRs in ascending order of liquid metal temperature (Øi = inner diameter of lances; PS = pore size of porous bubble generators, (*) studies from Karlsruhe Institute of Technology (KIT)).
Figure 1 provides a graphical comparison of the results from the literature regarding methane pyrolysis. CH4 conversion is considered the main evaluation criterion, with individual studies arranged by ascending temperature. Furthermore, the methane flow rate, the type of gas injection, and the applied bath height are given for each study. The analysis reveals that temperature exerts a substantial influence on CH4 conversion, as demonstrated by Rahimi et al. [and Upham et al. [39], where a temperature increase of merely 40 °C led to a doubling of methane conversion under comparable bath height and flow rate conditions. The flow rate range (0.005–0.500 SLM) and bath height range (100–1100 mm) investigated in the literature appear to have a relatively minor impact in this comparison. This is further corroborated by the findings of Perez et al. [41], who attained nearly the same conversion as Upham et al. [39] within the temperature range of 1000–1050 °C, despite using a CH4 flow rate that was 30 times higher and a bath height that constituted only 14% of Upham et al.’s [39] setup. Notably, the primary differentiator was the type of bubble generator employed, with the porous plate showing a significant influence on CH4 conversion alongside temperature. Serban et al. [33] also achieved a conversion exceeding 50% using a porous sparger, despite employing a relatively low bath height (102 mm) and a temperature of 750 °C.
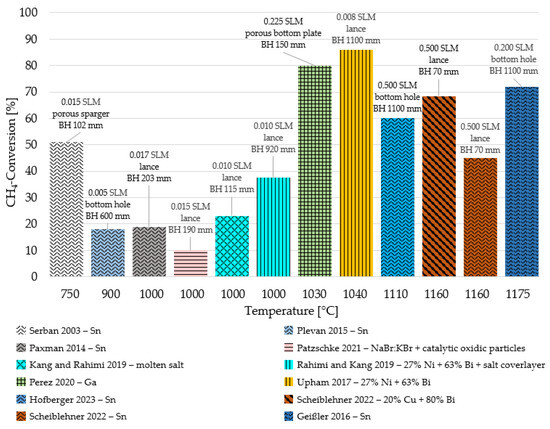
Figure 1.
Results from different studies concerning methane conversion in liquid metal bubble column reactors (CH4 feed rate in SLM = standard litres per minute; BH = bath height; different patterns indicate the used liquid media) [9,15,33,34,38,39,41,43,44,45].
This work aims to investigate methane pyrolysis in an LMBCR using an expanded and improved experimental setup based on the reactor concept previously proposed by Scheiblehner et al. [43,47]. Methane pyrolysis is conducted in an inductively heated liquid metal bubble column reactor employing molten tin as the heat transfer medium. Further, this study utilizes a substantially larger reactor vessel to examine the effects of temperatures between 900 and 1250 °C and methane input rates of up to four standard litres per minute (4.000 SLM). In contrast to the experimental configurations outlined in the existing literature, this study’s reactor concept additionally incorporates advancements in the carbon discharge system. This is facilitated by the substantially augmented methane flow rates and corresponding design modifications (cf. Figure 2). Within this framework, carbon discharge is achieved partially through entrainment within the gas flow, with the remaining carbon portion being deposited on top of the liquid metal column. Temperature profiles within the reactor freeboard serve as indicators for deducing the presence of carbon layers at distinct experimental stages. To comprehensively investigate the impact of these carbon layers, a series of experiments were executed wherein diverse carbon cover layers were initially applied onto the surface of the liquid metal column. Furthermore, by using the developed reactor as a blank tube reactor (BTR) by excluding the liquid metal fill, a comparative analysis was conducted about the influence of flow rate variations. This approach not only highlights certain disparities between the developed reactor concept (cf. Section 2.1) and the BTR but also allows for a comprehensive assessment against the prior literature findings concerning blank tube reactors.
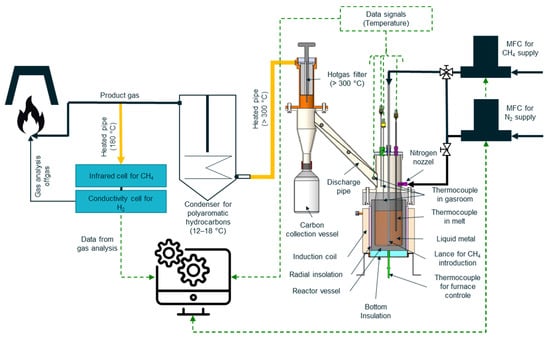
Figure 2.
Overview of the experimental setup used in this study, which comprises two mass flow controllers, the liquid metal bubble column reactor, thermocouples, the discharge system including a hot gas filter, a condenser to cool the product gas, as well as the gas analysis system.
2. Materials and Methods
2.1. Experimental Setup
In this study, pure tin served as the liquid metal. All experimental procedures took place within a reactor fabricated from isostatic pressed graphite, making it highly suitable for induction furnace heating. The reactor vessel’s inner diameter is 110 mm, and its height is 280 mm, which is the sum of 185 mm of graphite crucible and about 95 mm of steel casing up to the entrance to the discharge tube. Throughout the experiments, the reactor was partially filled with liquid tin, with a filling height of 140 mm. The chosen filling level results in the coexistence of two distinct reactor compartments: a 140 mm liquid metal bubble column and a 140 mm empty tubular reactor (reactor freeboard), which exhibit different temperature profiles, gas residence times, and heat transfer conditions to the gas throughout the experiment. Methane was introduced using a 700 mm long alumina lance with an outer diameter of 8 mm and six capillaries, each having an inner diameter of 0.8 mm. Unlike the experiments conducted by Scheiblehner et al. [43], no supplementary purging with nitrogen was necessary in this study to ensure adequate product discharge. This was due to the significantly higher input flow rates ranging from 1.000 to 4.000 SLM.
Figure 2 illustrates the experimental setup used. The graphite reactor was equipped with an external thread M140, facilitating its connection to a graphite flange. This flange, in turn, was connected to a stainless steel construction comprising the tubular reactor compartment and the discharge system of the experimental setup through four M12 steel screws, ensuring an airtight system to prevent any oxidation inside the graphite vessel. The reactor was positioned within the induction coil of the furnace and externally insulated at the circumference and the bottom. To ensure accurate monitoring and control of the furnace power and temperature, a type K thermocouple was positioned within the insulation at the outer bottom of the crucible. The Al2O3 lance for methane introduction into the liquid metal was positioned three millimetres above the reactor bottom. The inlet gas feed was regulated using Bronkhorst thermal mass flow controllers.
In the gas space above the liquid metal bath, three type K thermocouples were installed to measure temperatures at different heights in the tubular reactor compartment. The first thermocouple was positioned at a height of 185 mm, the second at 240 mm, and the third was placed at the entrance to the discharge tube, at a height of 280 mm. Additionally, a separate type K thermocouple, protected by an alumina tube, was utilized to measure the temperature of the melt at a height of 60 mm.
As a result of the bubble bursting at the melt surface, liquid metal droplets can be ejected. Previous studies [48,49] have shown that macroscopic jet droplets (0.1–2 mm) and film droplets (<50 µm) can be produced in liquid steel at 1580 °C. The selected bath height of 140 mm has been determined as the optimum value for the experimental setup to control metal ejection in the carbon product within acceptable limits. To achieve this, the height of the tubular reactor compartment above the liquid metal column must exhibit a certain value to ensure that a majority of the ejected macroscopic metal droplets can descend back into the metal bath, thereby preventing their entry into the discharge system. However, the discharge pipe, angled at 45°, facilitated the gravimetric separation of residual macroscopic metal particles from the product stream. This separation was achievable owing to the significantly higher density of the metal particles in comparison to the carbon content carried by the gas stream. Within the discharge system, a hot gas filter was installed and heated to approximately 350 °C to prevent condensation of specific pyrolysis intermediates. The hot gas filter effectively separated the carbon from the gas stream, with the carbon collected in the designated vessel. The particle-free product gas exited the discharge system through the outlet of the hot gas filter. Additionally, to support the carbon discharge into the collection vessel, it was possible to flush the system with nitrogen at regular intervals via a nozzle.
A water-cooled condenser was connected downstream of the hot gas filter to condense any intermediate products from the CH4 decomposition with boiling points above room temperature. Such intermediates are, for example, polyaromatic hydrocarbons like pyrene which could contaminate the gas analyser. From the product gas, a partial stream was fed to the analysing system. The hydrogen content was determined using the Caldos27 thermal conductivity analyser from ABB. The content of non-decomposed methane was measured with the Uras26 infrared photometer from ABB. The product gas was subsequently flared under a hood.
2.2. Calculations
To assess the efficiency of the methane cracking process, a series of experiments were conducted under various operating conditions, with methane conversion being the key evaluation criterion. Calculations concerning the methane conversion (XCH4) were based on the measured residual methane volume fraction υCH4 out in the product gas, which can be expressed by Equations (6) and (7), where H2 out and CH4 out are the respective volume flows of hydrogen and undecomposed methane.
Equation (8) is formulated by expressing H2 out and CH4 out as functions of the input volume flow of methane, CH4 in, and the conversion rate of methane, XCH4 [%].
Transcribing Equation (8) results in Equation (9) allows the calculation of methane conversion only from the measured value of residual methane volume fraction in the product gas.
Using a similar procedure, the hydrogen yield can be calculated starting from Formula (6) and resulting in Equation (10).
If the methane decomposition solely follows the given reaction Equation (5), resulting in the formation of solid carbon and hydrogen, the methane conversion and hydrogen yield should be equivalent. However, in practical experiments, the calculated methane conversion (cf. Equation (9)) may differ by 0.1% to 3% from the hydrogen yield (cf. Equation (10)). This discrepancy arises from the fact that the product gas analysed using the instruments may contain traces of gaseous intermediate products produced during methane decomposition, which are not condensable at room temperature. Consequently, the total sum of hydrogen and methane in the product gas may not precisely add up to 100%. Notably, the literature mentions important intermediate products of methane decomposition, such as ethane, ethylene, acetylene, and benzene [16,27,50]. Geißler et al. [16] observed an intermediate content of approximately 1.5 atomic percent (at%), which is consistent with the order of magnitude derived from the discrepancies between CH4 conversion and H2 yield in this study. Due to the limitations in analysing intermediate components during the experiments, all conversion calculations rely on the assumption that the resulting product gas consists solely of H2 and undecomposed CH4.
3. Results and Discussion
Experiments were conducted using the aforementioned reactor under various temperatures and methane flow rates. In each LMBCR experiment, the crucible was filled with a specific mass of solid tin, leading to the formation of a 140 mm liquid tin column at 1100 °C. The density of liquid tin was calculated using the formula suggested by Gale et al. [51] for liquid pure metals. Additionally, experiments were carried out in a blank reactor to assess both the efficacy of the LMBCR technology and to provide further comparisons with the literature. To prevent oxidation of the internal surfaces of the carbon crucible, the experimental setup was purged with nitrogen during melting and heating. Once the reactor temperatures stabilized, the nitrogen purge was halted, and CH4 injection commenced. The input rate was gradually increased to the desired level to protect the alumina lance from potential breakage due to thermal stresses caused by the rapid introduction of methane. Subsequently, depending on the specific pyrolysis experiments performed, either the temperature or CH4 flow rate was adjusted, and these parameters were maintained until methane conversion reached a stable state for at least 15 min.
3.1. Temperature Dependency
As documented in previous studies [9,16,33,34,37], methane pyrolysis exhibits a significant temperature dependence. Figure 3 presents the temperature impact on methane conversion at a constant CH4 flow rate of 2.000 SLM using the experimental setup described above. Three experiments (trial 1, trial 2, and trial 3) were conducted for comparison. Trials 1 and 3 applied the same temperature control, starting pyrolysis at 900 °C and increasing it in 50 °C increments up to 1250 °C, with 20 min of holding time after stabilization of conversion values at each temperature. Conversely, trial 2 began at 1250 °C and was subsequently decreased to 900 °C following the same procedure. The maximum temperature limit of 1250 °C is a result of constraints associated with both the induction furnace and the thermocouples, as they approach their operational limits within this temperature range.
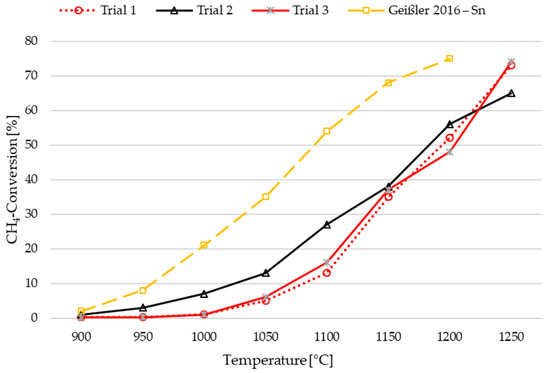
Figure 3.
Temperature dependency of methane pyrolysis in the presented LMBCR at an SGV of 3.5 mm/s compared to Geißler et al. [16] who applied 2.6 mm/s. For trial 1 and trial 3, ascending temperature was applied, while descending temperature was used for trial 2.
In trials 1 and 3, CH4 conversion did not occur until approximately 1000 °C and then exhibited a gradual increase with temperature. Consequently, carbon formation was minimal during the initial stages of these two experiments. As described by Scheiblehner et al. [43], carbon deposition on top of the liquid metal column and in the discharge system positively influenced the conversion. An explanation for this tendency can be found in the possible catalytic effect of solid carbon on the thermal decomposition of CH4 as demonstrated by Muradov et al. [20] and Serrano et al. [52]. The described effect is clearly shown in trial 2 at the starting temperature of 1250 °C, where the conversion is lower compared to trial 1, but due to the high starting temperature considerable carbon production commenced immediately, leading to a positive impact on conversion between 1200 °C and 900 °C.
Geißler et al. [16] conducted experiments within a comparable temperature range (820–1175 °C) using a tin bubble column partially filled with quartz rings, with a reactor diameter of 40.6 mm and a bath height of 1100 mm. Notably, the main differences between their study and the present work lie in the CH4 flow rate, respectively, in the superficial gas velocity (SGV), which is 2.6 mm/s in Geißler et al. [16] versus 3.5 mm/s in this study, and the bath height (seven times higher in Geißler et al. [16]). These variations, combined with the packed bed reactor concept, resulted in a significantly longer residence time in the hot section of the reactor, ultimately contributing to higher methane conversions in Geißler et al.’s [16] experiments when compared to the results of this study.
In initial tests, the temperature in the melt was determined at different immersion depths of the thermocouple within the alumina protection tube. As a result, a relatively constant melt temperature profile over the bad height was obtained, with a deviation of approximately ±3 °C, leading us to assume its constancy.
In Figure 4, temperature profiles of the whole reactor length are presented with measurements taken at three distinct points in the tubular reactor segment (T1 at 185 mm, T2 at 240 mm, and T3 at 280 mm). The position of T3, where the temperature remained below 200 °C in all experiments, was defined as the end of the reactor. Notably, an abrupt temperature drop occurs immediately after the gas exits the liquid metal bubble column. Due to the significantly longer residence time within the tubular reactor compartment, it is presumed to contribute to the conversion at temperatures above 1000 °C. Nevertheless, even at the highest applied melt temperature of 1250 °C, the gas temperature descends below this threshold after traversing approximately 50 mm in the reactor freeboard (equivalent to a reactor height of 190 mm).
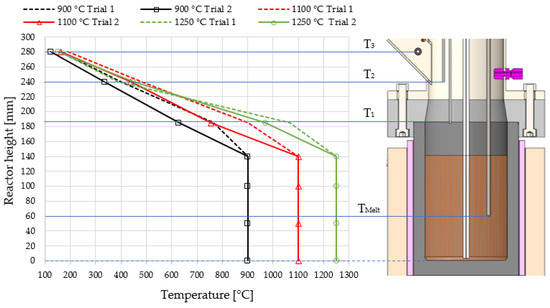
Figure 4.
Temperature profile along the entire reactor length comprising a 140 mm liquid metal bubble column reactor (LMBCR) section, where the temperature is assumed to be relatively constant and a subsequent 140 mm tubular reactor compartment exhibiting a sharp drop in temperature.
Figure 4 further highlights the observations from trial 2 in Figure 3, wherein a decreasing temperature program of 1250–900 °C was applied. Notably, a considerably faster cooling of the product gas within the tubular reactor compartment was observed. This phenomenon is attributed to the significant initial carbon production resulting from the high starting temperature. Consequently, less heat is transferred from the molten bath to the tubular reactor compartment due to the produced carbon which partially deposits on the liquid metal. Furthermore, this carbon layer increases the gas velocity in this region. When the experimental setup was opened at the end of the trial, it was evident that the tubular reactor not only fills with carbon but also forms channels through this carbon layer, enabling a much faster gas flow to the discharge pipe.
The impact of the carbon layer formation can be succinctly described as follows: it reduces the temperature in the tubular reactor section by mitigating heat radiation emanating from the liquid metal. Additionally, it induces a higher gas flow velocity due to the formation of channels created by the streaming gas, resulting in a decreased residence time within the tubular reactor compartment. However, despite these changes, there is an observed increase in the conversion of CH4. This phenomenon is attributed to the catalytic influence of the freshly formed solid carbon on the dissociation reactions involved in methane pyrolysis.
3.2. Carbon Cover Layers
In order to explore the beneficial impact of carbon on the methane pyrolysis process within a LMBCR, a series of experiments were conducted. A defined initial carbon layer was introduced atop the liquid metal column before the commencement of the experiments. Four distinct carbon layers were examined, comprising 100 mm of activated carbon with a grain size of 3–5 mm, as well as 25 mm, 100 mm and 150 mm of carbon derived from previous methane pyrolysis experiments carried out in the LMBCR. Figure 5 illustrates the impact of these initial cover layers on the temporal evolution of methane conversion. In all these experiments, a temperature of 1160 °C and a CH4 flow rate of 2.000 SLM were employed.
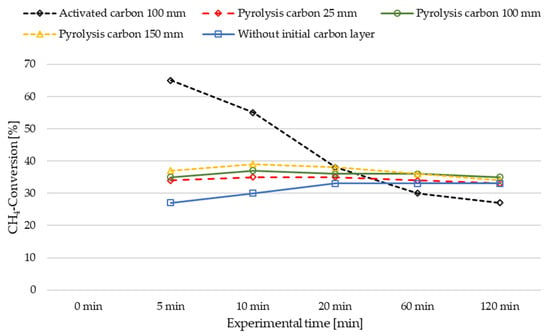
Figure 5.
Influence of initial carbon cover layers on the methane pyrolysis performance in a LMBCR at 1160 °C melt temperature with a methane flow rate of 2.000 SLM.
For the three different layer heights of pyrolysis carbon from previous experiments (25 mm, 100 mm, 150 mm), higher methane conversions were observed at the beginning compared to the experiment without a carbon layer (comparison experiment). Notably, the experiment with the highest cover layer of 150 mm exhibited a 10% higher conversion at the outset than the comparison experiment. The other two trials with pyrolysis carbon (100 mm and 25 mm) showed similar trends with slightly lower conversions. However, with progressing time, the conversion in the experiments with pyrolysis carbon slightly decreased, while the comparison experiment without an initial carbon layer exhibited a continuous, slight increase in conversion. After two hours, all four experiments reached approximately the same conversion level. This suggests that the height of the layer impacts the conversion only at the beginning of the pyrolysis process.
Regarding temperature, the trial without a carbon starter layer exhibited a lower temperature at thermocouple T1 (185 mm) after two hours of experimental time (cf. Figure 6). We postulate that this can be explained by the gradual build-up of a C layer over the two-hour test period, as only a portion of the produced carbon entered the discharge system. This build-up of carbon provided some shielding of heat radiation emitted by the melt, leading to a steady decline in the gas chamber’s temperature over the entire duration of the experiment. Therefore, the increase in conversion in the experiment without an initial carbon layer may be attributed, with some probability, to the catalytic effect of the slowly formed carbon layer (cf. Figure 5). In contrast, the experiments with pyrolysis carbon as a cover layer initially exhibited a higher CH4 conversion, which subsequently decreased slightly. This can be attributed to the channels formed through the carbon layer by the gas flow, resulting in higher flow velocities and reduced gas–carbon contact area and time, thereby diminishing the catalytic effect of the relatively high carbon layer. Another possible explanation could be that the freshly formed carbon in the case of the comparison experiment exhibits better catalytic activity compared to the pyrolysis carbon used as an initial cover layer due to catalyst aging of the latter.
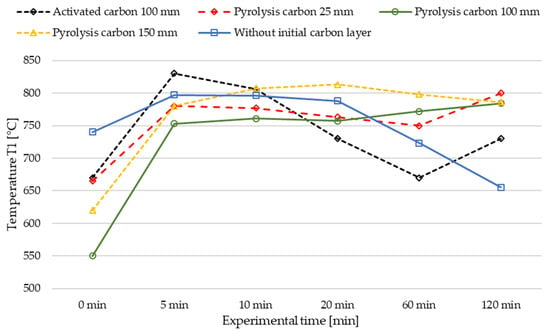
Figure 6.
Influence of initial carbon cover layers on the temperature T1 in the described LMBCR (cf. Figure 4) at a liquid metal temperature of 1160 °C and a methane flow rate of 2.000 SLM.
When activated carbon was used as a cover layer, the conversion was relatively high (65%) at the start of the experiment but continuously declined throughout the test duration. This behaviour may be attributed to two potential effects. Firstly, according to specifications, the used activated carbon possesses a large specific surface area of 1050 m2/g, enhancing the potential for any carbon-related catalytic effect on the decomposition reaction. However, once the activated carbon particles were covered with newly formed pyrolysis carbon, gas penetration into the pores was hindered, limiting the large inner surface area’s catalytic effect. The second effect is also described in literature by Moliner et al. [53] who state that the behaviour of carbonaceous materials is influenced not only by their surface area but also significantly by their surface chemistry. Specifically, the presence of oxygenated groups on the surface plays a crucial role. These oxygenated groups exhibit high reactivity and can have a pivotal impact during the initial stage of the reaction. There are two potential reaction mechanisms:
- (1)
- The surface oxygen groups react directly with CH4 molecules, akin to a partial oxidation reaction.
- (2)
- Alternatively, these oxygen groups may detach from the surface in the form of CO or CO2, creating active reaction sites. In both cases, the extent of methane conversion is closely linked to the concentration of surface oxygenated groups. The formation of CO or CO2 comes along with a substantial heat release. This temporary rise in temperature could also enhance the conversion in the area filled with activated carbon providing better conditions for methane dissociation.
As depicted in Figure 6, an initial temperature rise is observed in the curve of the activated carbon experiment, but the other experiments also exhibit similar behaviour. Thus, we attribute this initial temperature rise to the increase in convective heat transport due to the start of methane flow into the system, raising the temperature in the gas space above the liquid metal column in all experiments.
Using the Uras26 infrared photometer from ABB also, the content of CO and CO2 was measured during the experiment. However, for the experiment with activated carbon, an initial peak of 36% CO and 4% CO2 was observed, which gradually decreased following the temperature trend of this specific trial. After 60 min of experimental time, both CO and CO2 concentrations reached 0%. These observations imply that both of the effects described above contribute to some extent to influencing the pyrolysis performance.
Although an initial cover layer of activated carbon exhibited highly favourable effects at the onset of the experiment, it later became a significant drawback. Unlike the trials with initial layers of pyrolysis carbon, where a partial discharge of the newly produced carbon was observed throughout the entire experiment, the activated carbon trial encountered blockages in the tubular reactor segment due to the particle size of 3–5 mm.
3.3. Flow Rate Dependence
Serban et al. [33], Paxman et al. [34], and Geißler et al. [16,37] have conducted studies on the influence of methane flow rate on CH4 conversion. Their investigations covered a flow rate range of 0.005–0.200 SLM and temperatures between 750 and 1175 °C. Geißler et al. [16] specifically demonstrated that at temperatures above 1000 °C, increasing the flow rate from 0.050 to 0.200 SLM led to a reduction in CH4 conversion of approximately 5–9% (absolute). Furthermore, Hofberger et al. [38] studied flow rate variations of 0.300–0.500 SLM within a similar temperature range, and their findings revealed that the largest difference in methane conversion resulting from changing the flow rate was only 5% (absolute).
Similar findings were also obtained in the present work where a very wide flow rate range, compared to the literature, of 1.000–4.000 SLM was investigated. The melt temperature was maintained at approximately 1160 °C while varying the flow rate. Each new flow rate setting was allowed to stabilize, and the system was monitored for 15 to 20 min to ensure steady conditions before proceeding with the experiments.
Figure 7 presents the outcomes of the flow rate variation, and a comparative analysis is made using data derived from previous studies conducted at the KIT [16,38]. Despite differences in temperature ranging from 1110 °C to 1175 °C, as well as variations in bath heights and reactor diameters, exponential trend lines are drawn to demonstrate the agreement between the KIT study data and the results obtained in this investigation. The graph indicates a diminishing impact of the flow rate on methane conversion as flow rates increase. Specifically, at flow rates below 0.500 SLM the methane conversion experiences a decline of approximately 3–4% (absolute) per 0.100 SLM increase in flow rate, whereas above 1.500 SLM this decline is reduced to only 0.2% (absolute) per 0.100 SLM increase in flow rate.
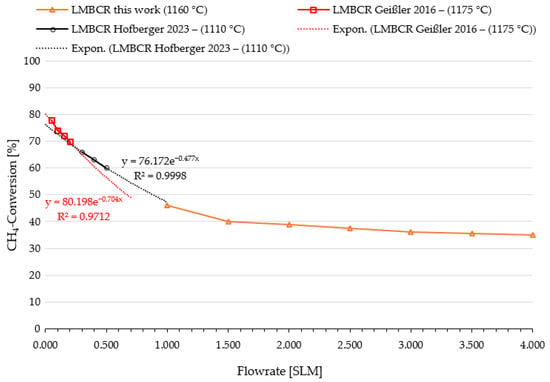
Figure 7.
Influence of flow rate on methane conversion in a LMBCR. Comparison of studies from KIT [16,38] with the experimental results derived from this work.
The observed minimal impact of flow rate variation on methane conversion in this study indicates that lance sparging within the examined flow rate range generates bubbles that closely approach the maximum bubble diameter achievable with the utilized liquid media and lance characteristics. Consequently, elevating the flow rate beyond a certain threshold does not necessarily lead to an increase in bubble diameter, as large bubbles generated through a sparging lance tend to disintegrate into smaller bubbles once they surpass a critical diameter [54].
As depicted in Figure 8, the tubular reactor compartment displays diverse temperature profiles corresponding to the applied methane flow rate. Higher flow rates correspond to elevated temperatures in this reactor segment, attributed to augmented convective heat transport. Consequently, the residence time within this segment notably diminishes with increasing flow rate and temperature.
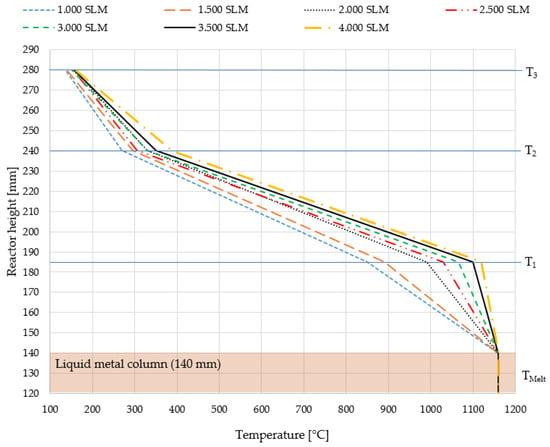
Figure 8.
Temperature profiles in the tubular reactor segment for various flow rates of methane, considering a constant bath height of 140 mm of liquid tin and a melt temperature of 1160 °C which is assumed to be constant. Temperature measurement was conducted at the same positions as depicted in Figure 4.
The obtained results lead us to hypothesize that a comparable bubble size, close to the critical diameter, is achieved for flow rates ranging from 1.500 to 4.000 SLM in the LMBCR segment. If gas injection conditions in this section yield similar bubble sizes, ascent rates, and, consequently, residence times, despite varying flow rates, the marginal decrease in conversion within the investigated flow rate range could be attributed to differences in temperatures and residence times in the tubular reactor segment.
3.4. Blank Tube Reactor
In addition to the described reactor setup, which includes a 140 mm liquid metal bubble column reactor (LMBCR) and a 140 mm tubular reactor section, experiments were also conducted without any liquid metal in a 280 mm blank tube reactor (BTR). The purpose of these experiments was to investigate the influence of flow rate on methane conversion in the absence of liquid metal. The temperature of the graphite reactor vessel was carefully controlled to maintain proximity to 1160 °C, while the methane flow rate was intentionally varied.
Various investigations in the literature have presented diverse results regarding the performance of blank tube reactors in contrast to liquid metal bubble column reactors [9,33,34,41]. Figure 9 illustrates a comparison between experimental results from the literature and the findings of this study. The yield of hydrogen and carbon in thermal methane decomposition primarily depends on the residence time of methane in the reactor’s hot zone and the prevailing heat exchange surfaces and heat transfer conditions.
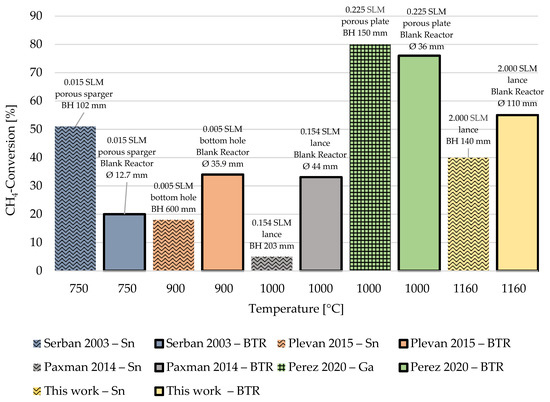
Figure 9.
Methane pyrolysis in liquid metal bubble column reactors (LMBCRs) versus blank tube reactors (BTRs)—results from different authors. (BH = bath height; SLM = standard litre per minute; different patterns indicate the used metal alloy) [9,33,34,41].
In LMBCRs, the residence time involves the time taken by bubbles to rise through the molten metal and flow through the tubular reactor compartment above the liquid metal column. Conversely, in BTRs, methane solely passes through a relatively long tubular reactor segment, which, in terms of heat transfer conditions, is similar to the reactor freeboard in a LMBCR. Considering residence time alone, the BTR appears to provide more favourable conditions for achieving high methane conversion. However, the LMBCR has the potential to outperform the BTR in terms of heat exchange surface area and heat transfer conditions, provided a sufficiently large heat exchange surface is available. Bubble size plays a crucial role in this regard, as smaller bubbles can enhance heat transfer conditions and lead to improved methane conversion. This is evident in the results of Serban et al. [33] and Perez et al. [41], who achieved notably better conversions using gas injection through porous spargers in the LMBCR (cf. Figure 9). On the other hand, the work of Plevan et al. [9] and Paxman et al. [34], who utilized lances and single bottom holes for methane input, respectively, favoured the blank reactor. This phenomenon was also observed in the experimental configuration employed in this study, coupled with the specific CH4-flow rates applied. It is attributed to the potentially prolonged residence time within the BTR and the less favourable gas introduction method through lances in the case of the LMBCR, resulting in the formation of large gas bubbles. Consequently, the conversion levels achieved in the BTR experiments conducted in this work were consistently 10 to 15% higher than those observed in the LMBCR experiments.
As depicted in Figure 10, the decrease in methane conversion between flow rates of 1.500 SLM and 4.000 SLM in the BTR was twice as high compared to the experiments with varying CH4 flow rates in the LMBCR (cf. Figure 7). This observation can be attributed to the substantially longer tubular reactor segment, where the gas residence time exhibits a strong dependence on the volumetric flow rate. This stands in contrast to a liquid metal bubble column, where the maximum bubble diameter plays a significant role in determining the gas residence time up to a certain extent. This correlation results in a more pronounced decrease in methane conversion with increasing flow rates during the BTR experiments.
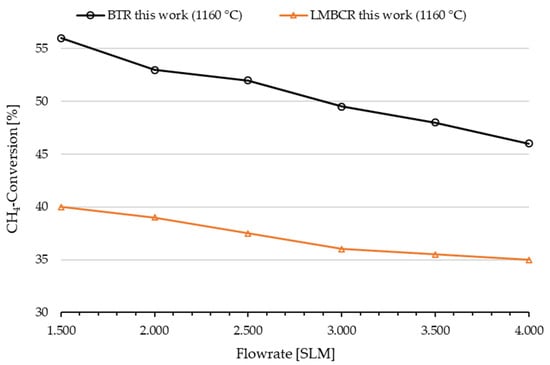
Figure 10.
Influence of flow rate on methane conversion at a temperature of 1160 °C in a blank tube reactor (BTR) compared to the LMBCR results.
While methane pyrolysis in liquid metal reactors exhibits the potential to outperform other reactor types like the blank reactor when employing appropriate sparging technology, the advantages of LMBCR technology extend beyond methane conversion to carbon discharge as well. In the experimental setup used, LMBCR experiments yielded fine, almost flocculent carbon, easily discharged via the inclined discharge pipe into the collection bottle. In contrast, the carbon formed in blank reactor experiments accumulated on the hot crucible walls, forming a dense layer requiring considerable mechanical effort for removal, rendering continuous operation unfeasible.
4. Conclusions
This study investigates methane pyrolysis in a liquid metal bubble column reactor, which offers a promising route for hydrogen production with reduced CO2 emissions and lower energy consumption compared to water electrolysis and steam methane reforming. In this work, pyrolysis of methane was achieved by introducing pure CH4 into an inductively heated graphite reactor via a sparging lance. The reactor configuration comprises a 140 mm liquid tin bubble column coupled with a 140 mm tubular reactor compartment, facilitating methane decomposition into hydrogen and carbon at elevated temperatures.
At a liquid metal temperature of 1160 °C and a methane flow rate of 2.000 SLM, the achieved methane conversion was approximately 40%. By increasing the temperature to 1250 °C, the conversion could be significantly improved, reaching up to 75%, highlighting the crucial role of temperature in influencing methane conversion.
Moreover, for methane flow rates between 1.500 SLM and 4.000 SLM, there was only a relatively small decrease in the pyrolysis yield. This suggests that the flow rate variation in the applied range has a limited impact on methane conversion, indicating that the used lance sparging results in similar bubble sizes, close to the maximum bubble diameter for the liquid media utilized. Therefore, small differences in CH4 conversion may be attributed to different residence times in the tubular reactor section which is strongly dependent on the prevalent volumetric flow.
The formation of a carbon layer on the liquid metal bubble column exhibited a positive influence on methane conversion which is attributed to a catalytic effect on the dissociation reactions. The produced pyrolysis carbon, as well as activated carbon, was also used as an initial cover layer on top of the liquid metal to improve methane conversion right from the onset of an experiment. However, based on the presented results, we do not recommend the utilization of any initial carbon cover layer, since no sustained and substantial advantage could be achieved compared to trials without a starting layer.
Despite achieving higher conversion rates in experiments conducted within a blank tube reactor in contrast to those carried out in a 140 mm liquid metal bubble column coupled with a 140 mm tubular reactor segment, the literature sources [33,41] propose that the employment of appropriate sparging methodologies may surmount this drawback of the LMBCR due to the generation of smaller bubbles providing a larger heat exchange surface. Moreover, the feasibility of a continuous process utilizing a BTR appears limited, as the carbon product tends to accumulate as a compact layer on the reactor walls, impeding its discharge.
5. Outlook
In the ongoing development of the LMBCR technology at Montanuniversitaet Leoben (MUL), an extensive investigation pertaining to the introduction of gas through porous spargers is planned for examination. This endeavour will encompass the systematic evaluation of various sparger configurations and types.
In order to obtain deeper insights into the methane decomposition mechanisms, the application of an online gas analysis capable of characterizing not only H2 and CH4 but also all possible gaseous intermediates of CH4 pyrolysis is deemed necessary. This analysis could be achieved through techniques such as gas chromatography-mass spectrometry (GC-MS) or Fourier-transform infrared (FTIR) spectrometry. Thus, a better understanding of the thermodynamics, reaction mechanisms, and kinetics involved in the methane decomposition process could be gained.
Furthermore, a detailed assessment of the carbon’s physical and chemical properties, along with its purity, is of utmost importance. Consequently, investigations into metal content and polyaromatic hydrocarbon (PAH) contamination will be conducted.
In addition to the aforementioned aspects, the investigation of the most efficient catalyst composition plays a vital role in enhancing the methane decomposition process in the liquid metal bubble column reactor.
Moreover, the development of a further-advanced process and reactor design is a key focus in the laboratory-scale experiments. A comprehensive understanding of the interplay between various process parameters at different reactor sizes can be achieved using a stepwise scale-up of the reactor dimensions from a laboratory to a pilot plant scale. Through these combined efforts, a more complete comprehension of the LMBCR technology can be attained, leading to enhanced hydrogen production efficiency and facilitating its practical implementation in large-scale industrial applications.
Author Contributions
Methodology, D.N.; Validation, D.N.; Investigation, D.N., D.S. and A.S.; Resources, A.S.; Writing—original draft, D.N.; Writing—review and editing, H.A., D.S. and S.W.; Supervision, H.A. and S.W.; Project administration, H.A. and S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- International Energy Agency. Global Hydrogen Review 2021. Available online: https://www.iea.org/reports/global-hydrogen-review-2021 (accessed on 25 August 2023).
- International Energy Agency. Net Zero by 2050. Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 25 August 2023).
- Parkinson, B.; Matthews, J.W.; McConnaughy, T.B.; Upham, D.C.; McFarland, E.W. Techno-Economic Analysis of Methane Pyrolysis in Molten Metals: Decarbonizing Natural Gas. Chem. Eng. Technol. 2017, 40, 1022–1030. [Google Scholar] [CrossRef]
- Chai, S.; Zhang, G.; Li, G.; Zhang, Y. Industrial hydrogen production technology and development status in China: A review. Clean. Technol. Environ. Policy 2021, 23, 1931–1946. [Google Scholar] [CrossRef]
- Parkinson, B.; Tabatabaei, M.; Upham, D.C.; Ballinger, B.; Greig, C.; Smart, S.; McFarland, E. Hydrogen production using methane: Techno-economics of decarbonizing fuels and chemicals. Int. J. Hydrogen Energy 2018, 43, 2540–2555. [Google Scholar] [CrossRef]
- Machhammer, O.; Bode, A.; Hormuth, W. Financial and Ecological Evaluation of Hydrogen Production Processes on Large Scale. Chem. Eng. Technol. 2016, 39, 1185–1193. [Google Scholar] [CrossRef]
- Romagnoli, F.; Blumberga, D.; Pilicka, I. Life cycle assessment of biohydrogen production in photosynthetic processes. Int. J. Hydrogen Energy 2011, 36, 7866–7871. [Google Scholar] [CrossRef]
- Patlolla, S.R.; Katsu, K.; Sharafian, A.; Wei, K.; Herrera, O.E.; Mérida, W. A review of methane pyrolysis technologies for hydrogen production. Renew. Sustain. Energy Rev. 2023, 181, 113323. [Google Scholar] [CrossRef]
- Plevan, M.; Geißler, T.; Abánades, A.; Mehravaran, K.; Rathnam, R.K.; Rubbia, C.; Salmieri, D.; Stoppel, L.; Stückrad, S.; Wetzel, T. Thermal cracking of methane in a liquid metal bubble column reactor: Experiments and kinetic analysis. Int. J. Hydrogen Energy 2015, 40, 8020–8033. [Google Scholar] [CrossRef]
- Fincke, J.R.; Anderson, R.P.; Hyde, T.A.; Detering, B.A. Plasma Pyrolysis of Methane to Hydrogen and Carbon Black. Ind. Eng. Chem. Res. 2002, 41, 1425–1435. [Google Scholar] [CrossRef]
- Mašláni, A.; Hrabovský, M.; Křenek, P.; Hlína, M.; Raman, S.; Sikarwar, V.S.; Jeremiáš, M. Pyrolysis of methane via thermal steam plasma for the production of hydrogen and carbon black. Int. J. Hydrogen Energy 2021, 46, 1605–1614. [Google Scholar] [CrossRef]
- Chen, W.-H.; Liou, H.-J.; Hung, C.-I. A numerical approach of interaction of methane thermocatalytic decomposition and microwave irradiation. Int. J. Hydrogen Energy 2013, 38, 13260–13271. [Google Scholar] [CrossRef]
- Domínguez, A.; Fidalgo, B.; Fernandez, Y.; Pis, J.; Menendez, J. Microwave-assisted catalytic decomposition of methane over activated carbon for CO2-free hydrogen production. Int. J. Hydrogen Energy 2007, 32, 4792–4799. [Google Scholar] [CrossRef]
- Fidalgo, B.; Fernández, Y.; Domínguez, A.; Pis, J.J.; Menéndez, J.A. Microwave-assisted pyrolysis of CH4/N2 mixtures over activated carbon. J. Anal. Appl. Pyrolysis 2008, 82, 158–162. [Google Scholar] [CrossRef]
- Kang, D.; Rahimi, N.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Catalytic methane pyrolysis in molten MnCl2-KCl. Appl. Catal. B Environ. 2019, 254, 659–666. [Google Scholar] [CrossRef]
- Geißler, T.; Abánades, A.; Heinzel, A.; Mehravaran, K.; Müller, G.; Rathnam, R.K.; Rubbia, C.; Salmieri, D.; Stoppel, L.; Stückrad, S.; et al. Hydrogen production via methane pyrolysis in a liquid metal bubble column reactor with a packed bed. Chem. Eng. J. 2016, 299, 192–200. [Google Scholar] [CrossRef]
- Keipi, T.; Tolvanen, K.E.; Tolvanen, H.; Konttinen, J. Thermo-catalytic decomposition of methane: The effect of reaction parameters on process design and the utilization possibilities of the produced carbon. Energy Convers. Manag. 2016, 126, 923–934. [Google Scholar] [CrossRef]
- Dunker, A.; Kumar, S.; Mulawa, P. Production of hydrogen by thermal decomposition of methane in a fluidized-bed reactor—Effects of catalyst, temperature, and residence time. Int. J. Hydrogen Energy 2006, 31, 473–484. [Google Scholar] [CrossRef]
- Msheik, M.; Rodat, S.; Abanades, S. Methane Cracking for Hydrogen Production: A Review of Catalytic and Molten Media Pyrolysis. Energies 2021, 14, 3107. [Google Scholar] [CrossRef]
- Muradov, N.; Vezirolu, T. From hydrocarbon to hydrogen–carbon to hydrogen economy. Int. J. Hydrogen Energy 2005, 30, 225–237. [Google Scholar] [CrossRef]
- Parfenov, V.E.; Nikitchenko, N.V.; Pimenov, A.A.; Kuz’min, A.E.; Kulikova, M.V.; Chupichev, O.B.; Maksimov, A.L. Methane Pyrolysis for Hydrogen Production: Specific Features of Using Molten Metals. Russ. J. Appl. Chem. 2020, 93, 625–632. [Google Scholar] [CrossRef]
- Abbas, H.F.; Wan Daud, W. Hydrogen production by methane decomposition: A review. Int. J. Hydrogen Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Muradov, N.; Smith, F.; Huang, C.; T-Raissi, A. Autothermal catalytic pyrolysis of methane as a new route to hydrogen production with reduced CO2 emissions. Catal. Today 2006, 116, 281–288. [Google Scholar] [CrossRef]
- Ayillath Kutteri, D.; Wang, I.-W.; Samanta, A.; Li, L.; Hu, J. Methane decomposition to tip and base grown carbon nanotubes and COx-free H2 over mono- and bimetallic 3d transition metal catalysts. Catal. Sci. Technol. 2018, 8, 858–869. [Google Scholar] [CrossRef]
- Abánades, A.; Ruiz, E.; Ferruelo, E.M.; Hernández, F.; Cabanillas, A.; Martínez-Val, J.M.; Rubio, J.A.; López, C.; Gavela, R.; Barrera, G.; et al. Experimental analysis of direct thermal methane cracking. Int. J. Hydrogen Energy 2011, 36, 12877–12886. [Google Scholar] [CrossRef]
- Amin, A.M.; Croiset, E.; Malaibari, Z.; Epling, W. Hydrogen production by methane cracking using Ni-supported catalysts in a fluidized bed. Int. J. Hydrogen Energy 2012, 37, 10690–10701. [Google Scholar] [CrossRef]
- Hu, C.; Shen, H.; Zhang, S.; Li, H. Methane pyrolysis in preparation of pyrolytic carbon: Thermodynamic and kinetic analysis by density functional theory. Chin. J. Aeronaut. 2020, 33, 1064–1073. [Google Scholar] [CrossRef]
- Palmer, C.; Tarazkar, M.; Kristoffersen, H.H.; Gelinas, J.; Gordon, M.J.; McFarland, E.W.; Metiu, H. Methane Pyrolysis with a Molten Cu–Bi Alloy Catalyst. ACS Catal. 2019, 9, 8337–8345. [Google Scholar] [CrossRef]
- Tyrer, D. Production of Hydrogen. 1,803,221, USA (28.04.1931). Available online: https://www.freepatentsonline.com/1803221.html (accessed on 16 May 2022).
- Oblad, A.G. Production of Hydrogen and Carbon. 2,760,847, USA (28.08.1956). Available online: https://patents.google.com/patent/US2760847A/en (accessed on 16 May 2022).
- Steinberg, M. Fossil fuel decarbonization technology for mitigating global warming. Int. J. Hydrogen Energy 1999, 24, 771–777. [Google Scholar] [CrossRef]
- Martynov, P.N.; Gulevich, A.V.; Orlov, Y.; Gulevsky, V.A. Water and hydrogen in heavy liquid metal coolant technology. Prog. Nucl. Energy 2005, 47, 604–615. [Google Scholar] [CrossRef]
- Serban, M.; Lewis, M.A.; Marshall, C.L.; Doctor, R.D. Hydrogen Production by Direct Contact Pyrolysis of Natural Gas. Energy Fuels 2003, 17, 705–713. [Google Scholar] [CrossRef]
- Paxman, D. Experimental and Theoretical Investigation of Solar Molten Media Methane Cracking for Hydrogen Production. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2014. [Google Scholar]
- Paxman, D.; Trottier, S.; Nikoo, M.; Secanell, M.; Ordorica-Garcia, G. Initial Experimental and Theoretical Investigation of Solar Molten Media Methane Cracking for Hydrogen Production. Energy Procedia 2014, 49, 2027–2036. [Google Scholar] [CrossRef]
- Paxman, D.; Trottier, S.; Flynn, M.R.; Kostiuk, L.; Secanell, M. Experimental and numerical analysis of a methane thermal decomposition reactor. Int. J. Hydrogen Energy 2017, 42, 25166–25184. [Google Scholar] [CrossRef]
- Geißler, T.; Plevan, M.; Abánades, A.; Heinzel, A.; Mehravaran, K.; Rathnam, R.K.; Rubbia, C.; Salmieri, D.; Stoppel, L.; Stückrad, S.; et al. Experimental investigation and thermo-chemical modeling of methane pyrolysis in a liquid metal bubble column reactor with a packed bed. Int. J. Hydrogen Energy 2015, 40, 14134–14146. [Google Scholar] [CrossRef]
- Hofberger, C.M.; Dietrich, B.; Durán Vera, I.; Krumholz, R.; Stoppel, L.; Uhlenbruck, N.; Wetzel, T. Natural Gas Pyrolysis in a Liquid Metal Bubble Column Reaction System—Part I: Experimental Setup and Methods. Hydrogen 2023, 4, 295–306. [Google Scholar] [CrossRef]
- Upham, D.C.; Agarwal, V.; Khechfe, A.; Snodgrass, Z.R.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon. Science 2017, 358, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Bunyan, E.; Gelinas, J.; Gordon, M.J.; Metiu, H.; McFarland, E.W. CO2-Free Hydrogen Production by Catalytic Pyrolysis of Hydrocarbon Feedstocks in Molten Ni–Bi. Energy Fuels 2020, 34, 16073–16080. [Google Scholar] [CrossRef]
- Pérez, B.J.L.; Jiménez, J.A.M.; Bhardwaj, R.; Goetheer, E.; van Sint Annaland, M.; Gallucci, F. Methane pyrolysis in a molten gallium bubble column reactor for sustainable hydrogen production: Proof of concept & techno-economic assessment. Int. J. Hydrogen Energy 2021, 46, 4917–4935. [Google Scholar] [CrossRef]
- Ebner, T.; Sprung, A.; Hochfellner, A.; Samberger, S.; Antrekowitsch, H. Preliminary Investigations on Methane Pyrolysis for the Production of H2 in a Molten Metal Bath. World Metall. ERZMETALL 2021, 74, 84–89. [Google Scholar]
- Scheiblehner, D.; Neuschitzer, D.; Wibner, S.; Sprung, A.; Antrekowitsch, H. Hydrogen production by methane pyrolysis in molten binary copper alloys. Int. J. Hydrogen Energy 2022, 48, 6233–6243. [Google Scholar] [CrossRef]
- Rahimi, N.; Kang, D.; Gelinas, J.; Menon, A.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Solid carbon production and recovery from high temperature methane pyrolysis in bubble columns containing molten metals and molten salts. Carbon 2019, 151, 181–191. [Google Scholar] [CrossRef]
- Patzschke, C.F.; Parkinson, B.; Willis, J.J.; Nandi, P.; Love, A.M.; Raman, S.; Hellgardt, K. Co-Mn catalysts for H2 production via methane pyrolysis in molten salts. Chem. Eng. J. 2021, 414, 128730. [Google Scholar] [CrossRef]
- Hermesmann, M.; Müller, T.E. Green, Turquoise, Blue, or Grey? Environmentally friendly Hydrogen Production in Transforming Energy Systems. Prog. Energy Combust. Sci. 2022, 90, 100996. [Google Scholar] [CrossRef]
- Scheiblehner, D.; Neuschitzer, D.; Antrekowitsch, H.; Wibner, S.; Sprung, A. Hydrogen Production by Methane Pyrolysis in Molten Cu-Ni-Sn Alloys. Metals 2023, 7, 1310. [Google Scholar] [CrossRef]
- Müller, K.T.; Holappa, L.; Neuschütz, D. Control of Ejections Caused by Bubble Bursting in Steelmaking Processes. Steel Res. Int. 2003, 74, 61–69. [Google Scholar] [CrossRef]
- Han, Z.; Holappa, L. Bubble bursting phenomenon in Gas/Metal/Slag systems. Metall. Mater. Trans. B 2003, 34, 525–532. [Google Scholar] [CrossRef]
- Fau, G.; Gascoin, N.; Gillard, P.; Steelant, J. Methane pyrolysis: Literature survey and comparisons of available data for use in numerical simulations. J. Anal. Appl. Pyrolysis 2013, 104, 1–9. [Google Scholar] [CrossRef]
- Gale, W.F.; Totemeier, T.C.; Smithells, C.J. Smithells Metals Reference Book, 8th ed.; Elsevier Butterworth-Heinemann: Oxford, UK, 2004. [Google Scholar]
- Serrano, D.P.; Botas, J.A.; Guil-Lopez, R. H2 production from methane pyrolysis over commercial carbon catalysts: Kinetic and deactivation study. Int. J. Hydrogen Energy 2009, 34, 4488–4494. [Google Scholar] [CrossRef]
- Moliner, R.; Suelves, I.; Lazaro, M.; Moreno, O. Thermocatalytic decomposition of methane over activated carbons: Influence of textural properties and surface chemistry. Int. J. Hydrogen Energy 2005, 30, 293–300. [Google Scholar] [CrossRef]
- Thorat, B.N.; Joshi, J.B. Regime transition in bubble columns: Experimental and predictions. Exp. Therm. Fluid Sci. 2004, 28, 423–430. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).