Abstract
Recovery and reuse of valuable chemicals from hydrothermal carbonization process liquid (HTC-PL) from tomato plant biomass (TPB) was conducted. Different HTC-PLs were characterized with FTIR and Py-GC-MS analyses revealing the presence of low molecular weight linear, cyclic, and aromatics compounds in the HTC-PL. Separation of these valuable chemicals by fractional distillation resulted in eutectic constrains. Solvent extraction separation followed by solvent recovery and reuse provided encouraging results. The non-polar portion of HTC-PLs were extracted by using n-hexane (C6H14), and diethyl ether (C2H5)2O solvents with 8.5 and 4.3% recoveries, respectively. Characterization by FTIR and Py-GC-MS revealed petrol fuel like materials in the extracts of C6H14 and (C2H5)2O, irrespective of higher boiling components. Blends of both non-polar extracts were flame tested revealing good burning characteristics with minimal smoke and residue. Bench scale spirit lamp tests showed the blend would be very useful for greenhouse winter heating. The polar extracts using methylene chloride (CH2Cl2) resulted in about 55% recovery. Py-GC-MS analysis revealed acetic acid and 5-Hydroxymethyl furfural (5HMF) majors in the extract along with related derivatives. 5HMF is a valued chemical and demonstrated to be a useful building block for many industrial applications, and flatform chemical for various synthesis. Other identified minor components of HTC-PL were vanillin, divinyl terephthalate, and syringol. After the extractions of polar and non-polar components in three steps, the HTC-PL residue was applied as nutrient source after maintaining pH (5.6) and concentration (TOC, 100–200 mg/L) to typical greenhouse plants. Plant growth was encouraging. The paper discusses all the potential valued reuse applications of HTC-PL in greenhouses without discharges, which contributes to environmental protection and economic benefits.
1. Introduction
Conversion of different biomass into solid char products by hydrothermal carbonization (HTC) has been the target of many research efforts, focusing on the use of char products as fuel. However, HTC process liquid (HTC-PL) has been overlooked due to analytical complexities. HTC-PL is acidic (pH 3.5–4.5), contains high nutrients and a huge variety of organics originated from biomass materials. Discharge of this toxic HTC-PL is injurious to the environment, specifically nutrient discharge is strictly restricted in the study area (southern Ontario) to protect Lake Erie from algae bloom (eutrophication), a binational issue. The present study addressed the issue in a GH setting by recycle and reuse of HTC-PL originated from HTC of GH TPB, in an environmentally sustainable technique.
The literature review on valorization of HTC-PL revealed very limited information. A commercial company (Ingelia, Italy) proposed treatment by membrane filtration and subsequent use of the RO concentrate, which is rich in nutrients as organic fertilizer. The permeate is then recycled back to HTC process [1]. Kambo et al. [2] also recycled HTC-PL to HTC and indicated some benefits. Anaerobic digestion for production of biogas (CH4) are also observed [3,4,5,6]. Solvent extraction from cattle manure HTC-PL, and assessment to reuse as petroleum fuel were restricted due to the higher carbon compounds in HTC-PL compared to actual fuel [7]. Other than these general trials, further industrial valorization is restricted with the limiting factor of significantly variable physical and chemical characteristics of HTC-PL. Those variations are obvious and depend on origin, sources, and types of biomass as well as conditions of HTC operations. Ultimately, the area remained under theoretical and strategic thinking. Process development requires substantial analytical and engineering efforts, which are limitedly tied to the organic compounds present in HTC-PL, as the part of green chemistry and inorganic synthesis.
To proceed with HTC-PL research knowledge of biomass compositions is essential. In general, biomass is composed of cellulose (30–60%), hemicellulose (20–40%), and lignin (1–25%) components [8]. Concentrations and types of biomass constituents are completely dependent on the source and types of biomass as well as on the rates and types of nutrients applied for the plant growth, which transmits to plant and ultimately to biomass. A comparative assessment of TPB with other biomass is shown in Table 1 [9]. Compared to corn stover, hemicellulose, cellulose, and lignin in TPB are higher, but ash content is lower, which could be a benefit of lower fouling clinker formation during combustion.

Table 1.
Comparison of tomato plant biomass with other biomass.
The chemical transformations occur during the HTC pretreatment process include hydrolysis, dehydration, decarboxylation, decomposition, condensation, bond cleavage, formation of new bonds, and isomerization of hemicellulose, cellulose, and lignin derivatives [10,11]. These steps may be followed by re-polymerization of oligomers along with aromatization. Based on biomass material characteristics and HTC process conditions, the HTC-PL is supposed to contain some aromatics, phenol, and furan derivatives such as furfural and 5 -hydroxymethyl furfural (5HMF), and carboxylic acid along with other organic acids. Acids formed during the HTC process work as self-catalyst to improve hydrochar surface quality [12]. The rate of reactions in HTC depends upon the time, temperature, and medium of reaction. The water initiates hydrolysis reaction, lowering the activation energy level that transforms hemicellulose and cellulose producing oligomers and monomers [13]. However, water replaces the toxic and corrosive agents, which is a positive side of the use of water medium at this stage [14].
The literature review revealed that lignocellulosic biomass HTC conversion products are derivatives of phenol, furan, and furfural along with basic organic acids and lignin alcohol. Studies are limited in extraction and valorization of those useful chemicals. The present study covered possible extraction of group of organics from HTC-PL, assessed some of their applications, and proposed innovative processes for separation and applications of residue components. Analytical quantification identified and extracted fractions of HTC-PL chemicals applying conventional (TOC, IC, UV), and advanced (FTIR, and Py-GC-MS) techniques. The extraction, separation and reuse approach would resolve the concern with land disposal restrictions, and GH producers can manage biomass waste materials in an environmentally sustainable method. The extracts from HTC-PL can be used as heating fuel and as valued chemicals. The present study also conducted an evaluation of residue from HTC-PL in a soil spray amendment for tomato plant growth with encouraging results. Those achievements demonstrate multiple benefits to GH producers.
2. Methodologies
2.1. Hydrothermal Carbonization
The HTC process was operated at 200–260 °C under pressure (2–5 Mpa) for 5–60 min in absence of air. About 10–30 g of grinded tomato plant biomass (TPB) was mixed with deionized water at different ratios 1:6–1:12 (biomass-water) and loaded into the HTC chamber. System pressure remained isotropic, with vapor pressure of sub-critical water, that gradually increased along with temperature. Additional details are in [9]. Above the critical point (374 °C), the supercritical water initiates free radical reactions, while subcritical water promotes ionic reactions
To prepare samples, sub-critical water temperature ranges mentioned above was used. Under these conditions the dielectric constant (DEC) of water decreases with the increase of temperature. At a DEC lower than 80, self-dissociation of water into H+ (H3O+) and OH- ions occurs, which increases the Kw (dissociation constant) of water to about 10−11 mol2 dm−6 at 200–300 °C compared to 10−14 mol2 dm−6 at room temperature. Increased Kw decreases the polarity of water and thus can promote non-polar reactions acting as an organic solvent [15]. This is how the water at the sub-critical stage promotes solubilization of lignocellulosic biomass organics into the HTC-PL. To assess the behavior of sub-critical water that influences reaction dynamics, acidic and basic catalyzed experiments were conducted. Acetic acid (pH 3) and KOH (pH 10.5) were used.
2.2. Solvent Extraction of Organics from HTC-PL
To extract slightly polar compounds, methylene chloride (CH2Cl2, i.e., dichloromethane), boiling point (b.p.) 41 °C, was used as the solvent, while hexane (C6H14, b.p. 69 °C), and diethyl ether (C2H5)2O, b.p. 35 °C) solvents were used individually to extract paraffin and naphthalene group of compounds. Used solvents were recovered by distillation and condensation methods. Acetone extraction was also applied after CH2Cl2, hexane, and ether extractions to verify any other group of compounds may be recovered. The CH2Cl2 solubility (2.0 g/100 mL) is higher than chloroform (0.8 g/100 mL), thus CH2Cl2 was preferably used. Additionally, the b.p. of chloroform (61 °C) is higher than CH2Cl2., which requires higher energy to recover the solvent.
2.3. Fractionation Distillation for HTC-PL Chemicals
A bench scale fractional distillation and condensation system set-up was used. Fractionation is a primary refining process by which a liquid or vapor mixture can be separated into fractions by vaporization and condensation. Batch feeding was used in this research. The section above the feed (upper part) is the rectifying section, while below is the exhausting section or stripping section. Two streams (vapor and liquid) move counter currently through the assembly of individual stage trays or plates in the fractionating tower. For effective separation or rectification, proper number of fractionating plates and high reflux ratio are important. However, in bench scale, the number of trays were limited.
2.4. Characterization of HTC-PLs and Extracts
Different analytical techniques were applied. Ultraviolet visible (UV-Vis) spectroscopy was applied to estimate unsaturation in chemical compounds in the samples. HTC-PL samples were scanned with light wavelength from 200–500 nm, Hach DR 5000 UV-Vis Laboratory Spectrophotometer was used. Total Organic Carbon (TOC) and inorganic carbon (IC) contents in HTC-PL were analyzed using Shimadzu Corporation, Kyoto Japan (Model # 54215000555) TOC analyzer. The process water was diluted by DI water (1:100) and analyzed in triplicate, where average is reported. Fourier Transform Infrared (FTIR) spectroscopy was conducted using a Spotlight 200i FT-IR microscopy (PerkkinElmer, Massachusetts, USA) system. A total of 32 scans at a resolution of 4 cm−1 were averaged to give the spectra for analysis. OriginPro data analysis and graphing software (V 9, OriginLab corporation, MA, USA) were used for the analysis. Gas Chromatography—Mass Spectrometry and Pyrolysis–gas chromatography–mass spectrometry (GC-MS and PY-GC-MS) was conducted by using Agilent 5977B GC/MSD (Mississauga, ON), the latest and trusted single quadrupole GC-MS. To determine different substances within the HTC-PL samples, a single-shot mode was used to convert the biomass into hydrolyzates and inject it into the GCMS. The temperature of the PY was set at 300 °C, similar to the GC column temperature, so not to interfere with the performance of the GC. The conditions of pyrolysis were 500 °C for a minute, and GC at 300 °C. The analyzed data were integrated to obtain the peak areas by using F-search program. The areas were identified and sorted by area percent with the help of Agilent Qualitative Analysis Software 10.0. Chemical structures were prepared using ChemSketch Freeware for R&D, version 2021-2.0; (https://www.acdlabs.com, downloaded on 10 August 2022).
3. Results and Discussion
3.1. Identification and Assessment of Organic Functionalities in HTC-PL
To develop an overview of possible applications and valorization steps, organic functionalities in constituents of HTC-PL is essential. Figure 1 shows a typical analysis of 4 HTC-PLs at 200 °C, 220 °C and 260 °C, which reveal almost similar products type as the curves are superimposed on each other, with minimal shifts in some cases.
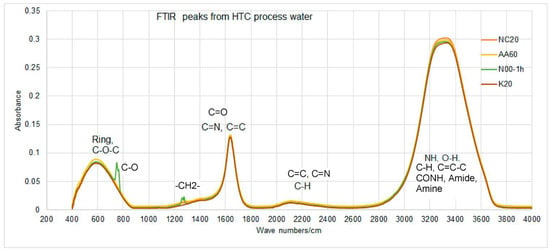
Figure 1.
FTIR micrographs from HTC-PLs at different HTC conditions.
In Figure 1, NC20 represents operation at 200°C without any chemical addition but with water, AA60 indicates with acetic acid (pH 3.5) at 260 °C, N00-1h is operation at 200°C for 1 h without any chemical but with water only, and K20 represents operation with KOH (pH 10.0). Except for N00-1h, a residence time of 30 min was maintained in all cases. Figure 1 reveals that all the runs provided almost similar type of chemicals in the process water except for a slight variation in the case run at 260 °C and 200 °C (1h) as well as reduction of pH with temperature. The pH values at 220 °C, 230 °C and 240 °C were 4.9, 4.5, and 4.23, respectively. This could be attributed to the higher acidic -OH (aromatic) and amide (CONH) functional along with ether functions as reveal at about 2300 and 600 cm−1 peak areas, respectively of Figure 1 (yellow peak). It can be mentioned here that after hemicellulose and cellulose degradation at 220 °C, lignin degradation is not expected to occur at this temperature as its degradation temperature is much higher. Thus the variations of functionals at this short temperature rage is not remarkable. All the peaks are interpreted based on basic concept of organic functional bond stretching/banding absorbance reported in the literature [10,16,17,18,19]. Table 2 shows a summarized list of functionals that are producing peaks due to bond stretching along with respective group of compounds to be assigned.

Table 2.
Absorbance range and possible functionals providing FTIR peaks.
Strong absorption peaks appeared at 1550–1750, 2100–2250, 2850–3700, and 700–750 cm−1, which were assigned to the stretching of C-O-C, C=C, C=O, C=N, C-H, O-H, C=C-H, and N-H bonds as labeled in Figure 2, respectively. The corresponding compound groups are also listed in Table 1. The band stretching of =CH-, -C=C- and -C=CH2 are assigned to alkenes, and that of -CH- and -C=C- are assigned to the cleavage of alkyl side chains and scission of alkenes double bond (=). Mild bending at 1290 cm−1 is for the -CH2- group in the chain, while a small peak at 720 cm−1 is assigned to the symmetric stretching of C-O bond [10,16,17]. Aromatic C-H stretching vibration at 3060 cm−1, C=C band vibration at 1588 cm−1, and C-O bands at 1280 cm−1 (green) in case of N00-1hr that may contain a hetero atom. As mentioned earlier in the case of C-O bond, stretching at 725 cm−1 could be similar for other hetero atoms (e.g., C-P) from organic phosphates [10]. The peak for C-O at 1288 cm−1 can also be assigned to C=O of carboxylic acids in HTC-PLs.
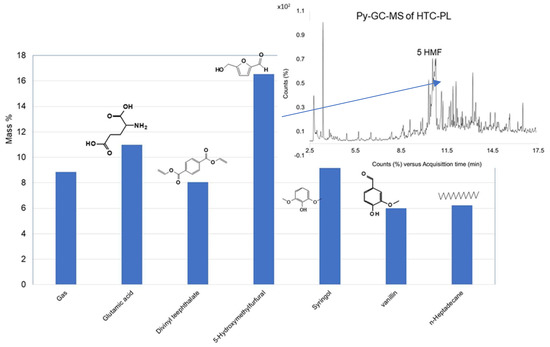
Figure 2.
Selectivity of major compounds in HTC-PL.
3.2. GC-MS Analysis of HTC-PLs and Reaction Model in HTC
Three HTC-PLs were analyzed by PY-GCMS, and the TIC of one of those HTC-PLs produced at 260 °C is presented along with structures of respective chemicals using the F-Search library. Out of 22 chemicals, only 7 chemicals including gas were found to be >4%, constituting about 75% of the total mass. The major chemicals and mass (%) present are summarized in Figure 2, along with TIC from Py-GC-MS. The highest concentration was revealed with 5-hydroxy methyl furfurals (5HMF). Compounds in Figure 2 were generated in the HTC reactor through transformations of TPB constituents at the temperature of 260 °C and pH 3.5 mostly by acidic hydrolysis.
Simplified paths of furfural and 5HMF formation from glucose and fructose, respectively are summarized in Figure 3a. Those transformations are also discussed in the literature [20,21,22]. Both acid and base catalysts support organic dissociation from biomass [23]. Lewis/Bronsted acid catalyzed dehydration of hexose sugar such as fructose (sometimes glucose) produces 5HMF. Glucose in presence of Lewis acid form fructose in the 1st stage subsequently dehydrated into fructose (catalyzed by Bronsted acid) forming 5HMF [24]. The C5-Xylose hydrolyzes to normal organic acids (acetic acid, formic acid, levulinic acid, glyconic acid, etc.) and remain in the process water.
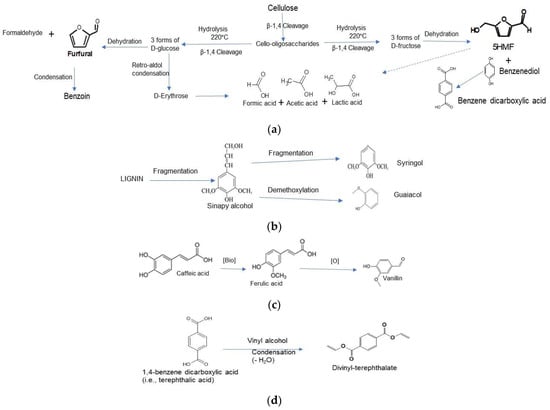
Figure 3.
(a). Cellulose degradation steps producing furfural, 5HMF and other products during HTC process. (b). Lignin transformation into syringol and guaicol. (c) Transformation of caffeic acid into vanillin via ferulic acid. (d). Condensation of Phthalic acid with vinyl alcohol into divinyl-tetephthalate.
The 2nd highest identified product is Glutamic acid (GA), an α amino acid, which is formed by the biosynthesis of proteins in living beings. Degradation of hemicellulose and cellulose may also produce GA. As synthesized within the body as needed, glutamic acid need not supply from outside.
The 3rd product is syringol, which is one of the intermediate products of lignin pyrolysis degradation into phenol product (dimethoxy). Syringol and guaiacol are derivatives of sinapy lignin alcohol by thermal degradation [25]. Lignin transformation steps providing syringol and guaiacol are as below, Figure 3b.
The 4th product Vanillin is an oxidation product from lignin along with 5 DMF [26]. Ferulic acid, which was identified in tomato plant stems is the source of vanillin in the present case. Biotransformation of caffeic acid may also produce vanillin via ferulic acid oxidation. The following reactions, (Figure 3c, show the formation of vanillin from ferulic acid.
The 5th product Divinyl terephthalate (DVT) is an aromatic compound that can be a condensation product of Phthalic acid (oxidation product of benzene diol) with vinyl alcohol, Figure 3d, producing an ester. Vinyl alcohol is expected to be produced from hemicellulose compounds in the TPB.
The 6th product n-Heptadecane is an unbranched alkane hydrocarbon, produced through splitting of long cellulose chain.
Transformations of TPB constituents in the HTC reactor may be summarized as:
| C5 Xylose- | Organic acids, aldehyde, alcohol, furfural |
| C6 glucose, fructose- | Furfural and furan derivatives |
| Lignin- | Alcohol derivatives from lignin alcohol |
| Cellulose- | Split into smaller chains and some derivatives |
It should be noted that the HTC process produces HC (hydrochar) that remains as a slurry in water. At this stage, all the generated chemicals are distributed between HTC-PL and HC, and respective proportions between HC and HTC-PL depend on hydrophobicity-based sorption and dissolution competitive characteristics of chemicals. Chemicals remain in equilibrium between HC and HTC-PL following the theory of sorption. It was observed that materials preferred to be in the aqueous phase, where 5HMF, syringol, palmitic acid, and guaiacol were observed to be the major in process water. There were other compounds identified both in HC and HTC-PL, but in lower concentrations. Hydrolysis of cellulose, hemicellulose, and lignin alcohols discussed earlier. The hydrolyzed products can undergo other reactions including condensation, dehydration, decarboxylation, aromatization, and polymerization. Lignin will transform into lignin alcohols (S, G, and H) as defined. Possible transformations were observed individually by different researchers with hemicellulose, cellulose, and lignin components in bench scale setting [20,21,27,28]. Present products were assessed based on findings in HTC-PL.
The identified end products from TPB were furan, furfural, 5HMF, pyran and their derivatives including formic, acetic acid, smaller cellulosic fractions and paraffins, which agree with some of the biomass [22,29]. As products from cellulose, hemicellulose, and lignin existed together in sub-critical water during TPB HTC, synergistic impacts make the reaction mechanisms and products identification very complicated. To make a more practical and research-based approach, a simplified model as shown in Figure 4 is established through the incorporation of HTC-PL analytical findings and interrelating degradative reactions of cellulose, hemicellulose and lignin components at subcritical conditions using actual chemicals identified in tomato plant.
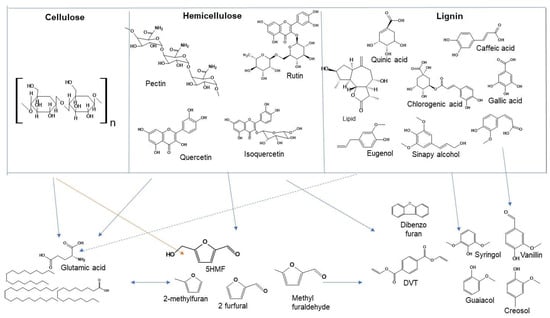
Figure 4.
Proposed TPB components transformation model producing chemicals as identified in HTC-PL.
To represent structures of hemicellulose and lignin, actual chemicals identified in different parts of tomato plants are incorporated. Quercetin, Ferulic acid, Gallic acid, Chlorogenic acid, Caffeic acid, Rutin, Linolenic acid, and Pectin as indicated in Figure 4 were identified and extracted from tomato plant of different areas [30,31,32]. Phenolic compounds such as gallic acid, chlorogenic acid, ferulic acid, caffeic acid, rutin, and quercetin were identified from leaf of tomato plant. Quercetin is a hemi-cellulosic sugar. Most of the products followed the 5HMF transformation path using 5HMF as a platform chemical.
3.3. Extraction of HTC-PL Chemicals by Fractional Distillation
A bench-scale fractional distillation column was used to separate possible fractions at lower temperature. In the 1st step, HTC-PL was heated to about 95 °C to evaporate the low boiler fraction, leaving water with the heavier part in the still. However, no significant condensate could be collected for use in the reflux as a lighter fraction. In the 2nd stage, HTC-PL was heated to about 110 °C, followed by 130 °C and 150 °C in the subsequent stages. A small quantity of condensate was collected in the 2nd stage of operation at about 110–120 °C, and no significant part could be collected at stages 3 and 4 due to very little feed. Analysis of the extract from the decanted upper part in 2nd stage revealed a common boiling point liquid mixer of water with similar boiler chemicals (seemed to be a eutectic). Probably it was not well separated in a short length with 2-trays bench-scale column, also there was feed limitation to run the distiller continuously. A long column with 15–20 trays or more and arrangement of top and internal refluxes is needed in a future study. A vacuum distiller can also be included.
3.4. Extraction of Chemicals from HTC-PL by Organic Solvents
3.4.1. Methylene Chloride Extraction
Methylene chloride was used for the extraction of slightly polar compounds from HTC-PL. Py-GC-MS analysis of extracts was conducted. The calculated respective mass ratio (%) of identified materials and chemicals present in HTC-PL above 4% mass ratio are shown in Figure 5. It reveals that acetic acid and 5HMF are the major products constituting about 90% of the total extract. Due to shortage of earlier HTC-PL, another version of HTC-PL at 220 °C showed lower amount of 5HMF at lower temperature. Considering the cost and versatility of 5HMF, a method of separation of 5HMF was the main focus. 5HMF was reported by US Department of Energy to be one of the top 10 value-added chemicals from lignocellulosic biomass [33].
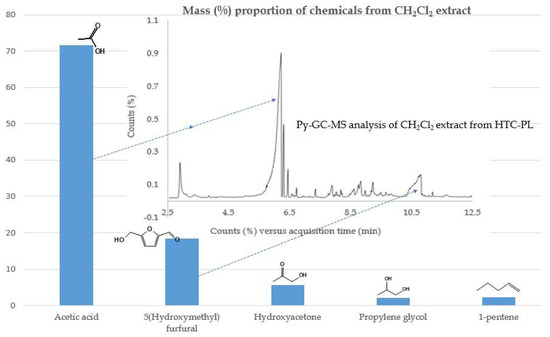
Figure 5.
Major chemicals in methylene chloride extracts from HTC-PL as identified by GC-MS.
Separation of 5HMF from acetic acid was a challenge. It was observed that acetic acid (b.p. 118 °C) and 5HMF (b.p. 116–118 °C) are co-boiling in the present distillation system. As such, distillation separation was not successful. Difficulty also appeared in solvent extraction separation, that attributed to free solubility of 5HMF in acetone and other solvents. Trial with tri-octylamine (C24H51N) solvent provided similar result of co-dissolution. Tri-octylamine is an extractant of organic acids and is not specific to 5HMF, so this solvent did not provide positive results in separation of 5HMF. Finally, catalytic (Co, Mn) oxidation of 5HMF at room temperature produced a white hard solid adherence to the experimental flask revealed to be furan dicarboxylic acid (FDCA) through transformation of 5HMF. It suggests that 5HMF can be separated via FDCA conversion path from acetic acid mixer. This technique by tuning catalyst selection and conducting the experiment in a crystallizer to crystallize FDCA is the best way of 5HMF separation. The added benefit of using FDCA route is that FDCA is a costly product, about $147.25/g [34], a white hard crystal having higher density (1.5 g/mL), boiling point (420 °C) and melting point (340 °C). Crystals of FDCA can be separated by filtration. About 98% recovery was reported by this technique [35]. At this stage, distillation separation of acetic acid (b.p. 118 °C) and FDCA is possible as the boiling point of FDCA is very high, and co-distillation will not happen. The boiling point of hydroxy acetone (146 °C) and propylene glycol (188 °C) are higher than acetic acid thus will remain in the residue with FDCA. Acetic acid may also be separated by water dissolution, a more cost-effective technique.
The reaction steps in Figure 6 show the separation of 5HMF through the oxidation-conversion path. Starting with 5HMF, two intermediate oxidation products di-formyl furan and HMF-carboxylic acid can be combined together to form the intermediate product named 5-formylfuran 2 carboxylic acid, which ultimately will convert into furan dicarboxylic acid (FDCA) [36].
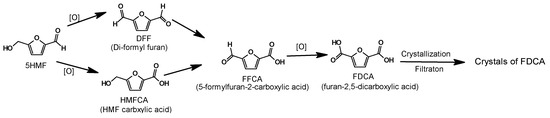
Figure 6.
Crystalized separation of 5HMF through oxidation-conversion into FDCA.
After solvent extraction of economic products, the residual part of HTC-PL can be used as bioreactor feed for production of methane gas as noted earlier. The present study investigated a newer area of HTC-PL residue application by using it as plant nutrient support in a GH setting (detail is given in Section 3.5).
3.4.2. Diethyl Ether and n Hexane Extracts
Diethyl ether (ether) and n hexane (hexane) solvents were used individually to extract non-polar paraffin and naphthalene hydrocarbon components from HTC-PL. Extracts were compared with commercial petroleum gasoline and diesel to establish the possibility of using paraffin and naphthalene extracts from HTC-PL as a substitute to petroleum fuels. Extracts were characterized by functional identification using FTIR analysis followed by PY-GC-MS analysis.
Commercial petrol and diesel were analyzed by Py-GC-MS in parallel to ether and hexane extracts. Figure 7a–c shows the Py-GC-MS TICs of petroleum fuel, ether extract, and hexane extract, respectively. Assessment of the chromatographs reveals mild differences between petrol and the extracts. Extracted products from HTC-PL seem to be slightly heavier than petrol products. Experimental assessment showed a gradual increased weight sequence of about 0.77 mg/mL < 0.80 mg/mL < 8.6 mg/mL for petrol, ether, and hexane extracts, respectively when same volume was weighted. Evaluating 50% fractions on both side of TICs in Figure 7, it reveals that low boilers are dominant in petrol, where ether extract is slightly lower in low boiler, but hexane extract is the least with low boiling components.
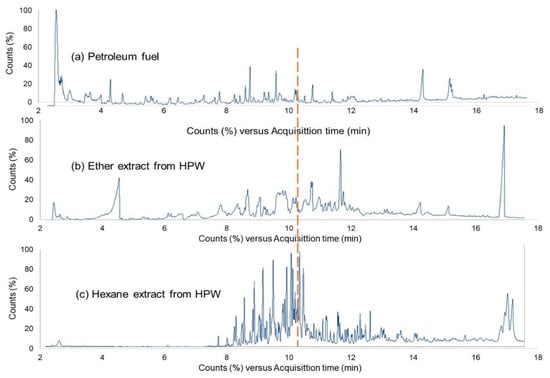
Figure 7.
GC-MS TICs of (a) petroleum fuel, (b) ether extract, and (c) hexane extract.
The reason for the less low boiler part in hexane extract is due to the higher boiling point of hexane (69 °C) compared to ether (34.5 °C). During the hexane recovery step, the extracted mixer is required to be heated above 69 °C compared to 34.5 °C in case of ether recovery. In general, car fuel boiling range is 40–200 °C (Shell Oil), while lighter boiling component between 40–70 °C is reasonably not present in hexane extract as it was heated above 70 °C. However, the result reveals the possibility of using hexane and ether extracts not as direct petrol fuel, but as a blending component with petrol-fuel precursor and co-pre-treat in the reforming unit producing automobile fuel (petroleum fuel). By this step high boiler parts of ether and hexane extracts will reform to match with petrol fuel. Burning tests of extracts also support the assumption as both the extracts were well-burned with minimal smoke and residue.
FTIR assessment of petrol, Figure 8a reveals cyclic (700–750 1/cm) and saturated alkane (2900–2950 1/cm) dominancy that provides higher octane number in petrol. Neither ether nor hexane extracts showed cyclic compounds. Extracts showed some saturated alkane, but are dominated with high O-H (alcohol) at 3200–3600 1/cm and >C=O stretching at 1500–1750 1/cm. Those are oxygen containing reactive functionals, which can reform into cyclic and branched saturated alkane or aromatic rings to increase octane number if a reforming treatment is applied.
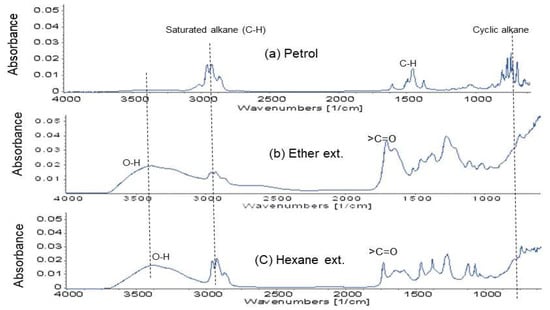
Figure 8.
Comparative FTIR micrographs of (a) petrol, along with (b) ether, and (c) hexane extracts (ext.) from HTC-PL.
Reforming treatment of petrol precursor is a vital operation in oil refinery for higher octane car fuel production where lighter fraction of crude oil is heated at about 530 °C mixing with recycled gas (off gases, H2) in the presence of Pt catalyst. This initiates the reforming reactions that transform different organic compounds into desired forms to increase octane number of fuel (Branched and aromatic hydrocarbon provide higher octane). Three reactors are used sequentially as most of the initial reactions are endothermic and need the feed stream to reheat prior to feed the subsequent reactors. In general, the following reactions occur in the reforming unit:
- Dehydrogenation of Naphthene and dehydrocyclization of Paraffins to produce aromatics
- Isomerization of Paraffins, Isomerization of Naphthene and Naphthene Dehydro-isomerization
- Hydrocracking of heavy Paraffins (takes place in 3rd reactor)
During reforming operations, the above reactions will homogenize the boiling range of ether and hexane extracts to match with petrol. Bulk extracts can be fed to reformer without blending to produce biofuel as well. To assess biofuel for automobile use, respective tests specific for automobile fuel such as kinematic viscosity, specific gravity, boiling point, cloud point, pour point, octane number, Sulphur content, and ASTM distillation including composition of reformed stock are essential. Those tests are not within the scope of present study and are recommended for future studies.
3.5. Soil Application of HTC-PL Residue as Nutrient Support
The residue from HTC-PL after three-step extractions were used as nutrient supply to tomato plants. Normal tap water along with HTC-PL residue was mixed together and analyzed for pH and conductivities to match with a recommended limit of 5.60 and 1.6 mS/cm, respectively. After about 45 days, seedling germinations were transplanted in two outdoor separate nursery plant-pots with similar composition of pot soil and HC mixer. yellowing appeared on both pot’s plants. At this stage, one of the pots was supplemented with HTC-PL residue. Within 20–30 days the pot with HTC-PL residue addition started to grow well compared to those without HTC-PL residue. Figure 9a,b reveals the differences of plants with and without HTC-PL residue addition. The rate of diluted HTC-PL application was 5–8 mL (depending on pot soil moistness) at every alternate day or every day. The observation suggests that soil water retention along with nutrients from HTC-PL residue improved the pot plant growth (Figure 9a). The amendment helped in supplying and retaining nutrients in soil, improving soil characteristics. Results reveal that HTC-PL residue can be a nutrient amendment for plants in greenhouse.
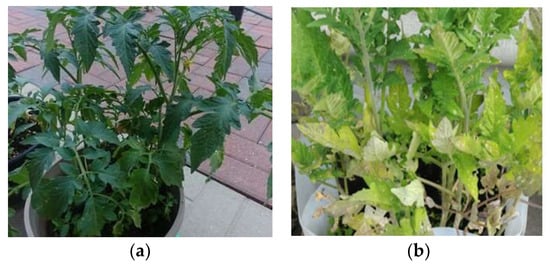
Figure 9.
Tomato planting nursery pots substituted with and without HTC-PL residue. (a) With HTC-PL residue addition; (b) Without HTC-PL residue addition.
Higher conductivity resists plant nutrients uptake due to osmotic pressure from soluble salts and reduces plant health including leaf edge burning from the accumulation of salts. Recommended target for tomato plant root zone is <8.0 mmol/L for K, Ca, and Na, and that for Mg is 4.5 mmol/L, respectively [37]. Higher than target level of metals (K, Ca) cause yellowing of plant leaves. The concentrations of Ca (15.3 mmol/L), Mg (11.5 mmol/L), and K (17.5 mmol/L) were above the target level, which were adjusted prior to application. Micronutrients (TP, TN) in HTC-PL were reduced after dilation to the recommended concentrations of 150–250 mg/L for N in diluted fertilizer solution for agricultural spray in a continuous feed basis, which was applied in present case. Original N concentration of about 412 mg/L in HTC-PL was reduced to about 215 mg/L after dilution, which was perfect as fertilizer. One beneficial aspect of HTC-PL application as fertilizer is that we can state the concentration of fertilizer solution independent of fertilizer analysis as all the concentrations (TN (412 mg/L), NO2/NO3 (381 mg/L), TKN (30 mg/L), and TP (70 mg/L)) are available in mg/L. Commercial NPK fertilizer sold in the market contains N, P, and K along with other impurities needs to be standardized by analyzing and determining the individual concentrations of N, P, and K in terms of N, P2O5, and K2O utilizing P × 1.2 = %P2O5, and K × 2.3 = %K2O rule. The analysis and calculations of impure fertilizers are not required for the present case. (Note: The NPK used in fertilizer standardization should not be confused with PNK used for waste nutrient water disposal management. The rule of calculations is completely different). Adverse impact, if any, is from the presence of organics in HTC-PL, which were not observed during the study. Organics may be metabolized in soil or could be evaluated along with phytotoxicity impacts operating the system for longer period. The Na and other heavy metals (Al, Fe, Mn) were below the target level, and no impact is expected.
4. Conclusions
Results demonstrate multiple benefits to GHs from hydrothermal process liquid recycle-reuses; (i) valued chemicals extraction as commercial commodities (5HMF, FDCA), (ii) use of nonpolar HTC-PL components as fuel for GH winter heating, (iii) HTC-PL residue after extractions can be used as plant nutrient supply (fertilizer), (iv) prospect of blending the nonpolar hydrocarbon fractions with commercial petrol just ahead of reforming would enhance auto fuel production. Other applications of HTC-PL residue after solvent extraction include use in bioreactor operation for methane gas (fuel) production. A reaction model based on reactor chemicals from tomato plant contents is developed for hydrothermal carbonization reactor. It reveals that the properties of HTC-PL depend on biomass type and processing conditions. Thus, the reaction mechanisms and model would also be material and method specific, which cannot be generalized, and should be assessed individually. Due to the relatively high residual carbon content, HTC-PL could be further used as an external carbon source in conventional nitrification and denitrification processes. However, this aspect needs to be investigated in future works, especially in relation to the inhibition of nitrifying and denitrifying bacteria.
Author Contributions
Conceptualization, R.G.Z. and A.D.; Methodology, A.-T.J.-U.; Software, A.-T.J.-U. and S.A.S.; Formal Analysis, A.-T.J.-U.; Investigation, A.-T.J.-U., A.D. and R.G.Z.; Resources, R.G.Z. and A.D.; Validation, A.D. and R.G.Z.; Writing Original Draft, A.-T.J.-U.; Reviewing and Editing, M.T.R., S.A.S., O.N. and R.G.Z.; Supervision, R.G.Z. and A.D.; Funding Acquisition, R.G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The University of Guelph funded the research through a general-purpose research account.
Data Availability Statement
Data are included within the manuscript.
Acknowledgments
Ontario Ministry of Agriculture Food and Rural Affairs (OMAFRA) supported with biomass supply; Joanne helped with metal and TOC analyses. All the supports are highly acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| DEC | dielectric constant |
| DSS | digested sewage sludge |
| FDCA | furan dicarboxylic acid |
| GH | greenhouse |
| HC | hydrochar |
| HHV | high heating value |
| HTC | hydrothermal carbonization |
| HTC-PL | HTC process liquid |
| 5HMF | 5-hydroxymethyl furfural |
| Kw | dissociation constant |
| NPK | Nitrogen phosphorous potassium |
| TIC | total ionic chromatogram |
| TPB | tomato plant biomass |
| TKN | Total Kjeldahl Nitrogen |
| USS | undigested sewage sludge |
References
- Langone, M.; Basso, D. Process Waters from Hydrothermal Carbonization of Sludge: Characteristics and Possible Valorization Pathways. Int. J. Environ. Res. Public Health 2020, 17, 6618. [Google Scholar] [CrossRef] [PubMed]
- Kambo, H.; Minaret, J.; Dutta, A. Process Water from the Hydrothermal Carbonization of Biomass: A Waste or a Valuable Product? Waste Biomass Valor. 2018, 9, 1181–1189. [Google Scholar] [CrossRef]
- Merzari, F.; Langone, M.; Andreottola, G.; Fiori, L. Methane production from process water of sewage sludge hydrothermalcarbonization. A review. Valorising sludge through hydrothermal carbonization. Crit. Rev. Environ. Sci. Technol. 2019, 49, 947–988. [Google Scholar] [CrossRef]
- Wirth, B.; Reza, T.; Mumme, J. Influence of digestion temperature and organic loading rate on the continuous anaerobic treatment of process liquor from hydrothermal carbonization of sewage sludge. Bioresour. Technol. 2015, 198, 215–222. [Google Scholar] [CrossRef]
- Wirth, B.; Reza, T. Continuous Anaerobic Degradation of Liquid Condensate from Steam-Derived Hydrothermal Carbonization of Sewage Sludge. ACS Sustain. Chem. Eng. 2016, 4, 1673–1678. [Google Scholar] [CrossRef]
- Qiao, W.; Peng, C.; Wang, W.; Zhang, Z. Biogas production from supernatant of hydrothermally treated municipal sludge by upflow anaerobic sludge blanket reactor. Bioresour. Technol. 2011, 102, 9904–9911. [Google Scholar] [CrossRef]
- Yin, S.; Ryan, R.; Harris, M.; Tan, Z. Subcritical hydrothermal liquefaction of cattle manure to bio-oil: Effects of conversion parameters on bio-oil yield and characterization of bio-oil. Bioresour. Technol. 2010, 101, 3657–3664. [Google Scholar] [CrossRef]
- Nanda, S.; Mohanty, P.; Pant, K.K. Characterization of North American lignocellulosic biomass and biochars in terms of their candidacy for alternate renewable fuels. Bioenergy Res. 2013, 6, 663–677. [Google Scholar] [CrossRef]
- Janal-Uddin, A.T.; Shakirudeen, A.; Salaudeen, S.A.; Dutta, A.; Zytner, R.G. Hydrothermal Conversion of Waste Biomass from Greenhouses into Hydrochar for Energy, Soil Amendment, and Wastewater Treatment Applications. Energies 2002, 15, 3663. [Google Scholar] [CrossRef]
- Nicolae, S.A.; Au, H.; Modugno, P.; Luo, H.; Szego, A.; Qiao, M.; Li, L.; Yin, W.; Heeres, H.; Berged, N.; et al. Recent advances in hydrothermal carbonisation: From tailored carbon materials and biochemicals to applications and bioenergy. Green Chem. 2020, 22, 4747. [Google Scholar] [CrossRef]
- Collard, F.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sust. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Titirici, M.; White, R.; Falcoa, C.; Sevillab, M. Black perspectives for a green future: Hydrothermal carbons for environment protection and energy storage. Energy Environ. Sci. 2012, 5, 6796. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuel. Bioprod. Bior. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Kruse, A.; Dahmen, N. Water—A magic solvent for biomass conversion J. Supercrit. Fluids 2015, 96, 36–45. [Google Scholar] [CrossRef]
- Harvey, A.H.; Friend, D.G. Physical Properties of Water, Aqueous Systems at Elevated Temperatures and Pressure: Physical Chemistry in Water, Steam and Hydrothermal Solutions; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Yang, B.; Cheng, M. Py-FTIR-GC/MS Analysis of Volatile Products of Automobile Shredder Residue Pyrolysis. Polymers 2020, 12, 2734. [Google Scholar] [CrossRef]
- Tarhan, S.Z.; Koçer, A.; Özçimen, D.; Gökalp, I. Utilization of hydrothermal process water for microalgal growth. Eurasian J. Biol. Chem. Sci. 2020, 3, 42–47. [Google Scholar]
- Fuertes, A.B.; Arberstain, M.C.; Sevilla, M.; Macia-Agullo, J.A.; Fiol, S.; Lopez, R.J.; Smernik, R.J.; Aitkenhead, W.P.; Arce, F.; Macias, F. Chemical and structural properties of carbonaceous products obtained by pyrolysis and hydrothermal carbonisation of corn stover. Aus. J. Soil Res. 2010, 48, 618–626. [Google Scholar] [CrossRef]
- Inoue, S.; Hanaoka, T.; Minowa, T. Hot compressed water treatment for production of charcoal from wood. J. Chem. Eng. Japan 2002, 35, 1020–1023. [Google Scholar] [CrossRef]
- Möller, M.; Schröder, U. Hydrothermal production of furfural from xylose and xylan as model compounds for hemicelluloses. RSC Adv. 2013, 3, 22253. [Google Scholar] [CrossRef]
- Yu, Y.; Lou, X.; Wu, H. Some Recent Advances in Hydrolysis of Biomass in Hot-Compressed Water and Its Comparisons with Other Hydrolysis Methods. Energy Fuels 2008, 22, 46–60. [Google Scholar] [CrossRef]
- Antal, M.; Mok, W.; Richards, G. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from D-fructose and sucrose. Carbohydr. Res. 1990, 199, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Karagöz, S.; Bhaskar, T.; Muto, A.; Sakata, Y. Catalytic Hydrothermal Treatment of Pine Wood Biomass: Effect of RbOH and CsOH on Product Distribution. J. Chem. Technol. Biotechnol. 2005, 80, 1097–1102. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dumont, M.; Raghavan, V. Sustainable production of hydroxymethyl furfural and levulinic acid: Challenges and opportunities. Biomass Bioenergy 2015, 72, 143–183. [Google Scholar] [CrossRef]
- Kibet, J.; Khachatryan, L.; Dellinger, B. Molecular Products and Radicals from Pyrolysis of Lignin. Environ. Sci. Technol. 2012, 46, 12994–13001. [Google Scholar] [CrossRef] [PubMed]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. Available online: www.elsevier.com/locate/ppolysci (accessed on 16 May 2022). [CrossRef]
- Buendia-Kandia, F.; Mauviel, G.; Guedon, E.; Rondags, E.; Petitjean, D.; Dufour, A. Decomposition of Cellulose in Hot-Compressed Water: Detailed Analysis of the Products and Effect of Operating Conditions. Energy Fuels 2018, 32, 4127−4138. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, H.; Junhong, J.; Peng, P.; Zhai, M.; She, D. Hydrothermal degradation of hemicelluloses from triploid poplar in hot compressed water at 180–340 °C. Polym. Degrad. Stab. 2016, 126, 179–187. [Google Scholar] [CrossRef]
- Peterson, A.; Vogel, F.; Lachance, R.; Fro¨ling, M.; Antal, M.J.; Tester, J. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Torres, A. Physical, chemical, and bioactive compounds of tree tomato (Cyphomandra betacea). Arch. Lat. Nutr. 2012, 62, 381–388. (In Spanish) [Google Scholar] [PubMed]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Chaidez, C.; Ornelas-Paz, J.D.; López-Mata, M.A.; Márquez-Ríos, E.; Estrada, M.I. Chemical constitution and effect of extracts of tomato plants by-products on the enteric viral surrogates. Int J. Environ. Health Res. 2015, 25, 299–311. [Google Scholar] [CrossRef]
- Kim, D.; Kwack, Y.; Lee, J.; Chun, C. Antimicrobial Activity of Various Parts of Tomato Plants Varied with Different Solvent Extracts; Plant Pathol. J. 2019, 35, 149–155; [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Chemicals from Biomass. In Energy Efficiency and Renewable Energy; U.S. Department of Energy: Washington, DC, USA, 2004; pp. 1–76. [Google Scholar]
- Fisher Scientific. Catalog Number AAH2871807, Furan-2,5-dicarboxalic Acid (96%), quantity 1g, price S147,25 Each. 2022. Available online: https://www.fishersci.com/shop/products/furan-2-5-dicarboxylic-acid-98-thermo-scientific/AAH2871803 (accessed on 15 September 2022).
- Triebl, C. Simulation and Economic Analysis of 5-Hydroxymethylfurfural Conversion to 2,5-Furandicarboxylic Acid. ‘Diplom-Ingenieur’ Thesis, Leoben University of Mining, Leoben, Austria, 2012. [Google Scholar]
- Xu, S.; Zhou, P.; Zhang, Z.; Yang, C.; Zhang, B.; Deng, K.; Steven Bottle, S.; Zhu, H. Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid Using O2 and a Photocatalyst of Co-thioporphyrazine Bonded to g-C3N4. J. Am. Chem. Soc. 2017, 139, 14775–14782. [Google Scholar] [CrossRef]
- De-Kreij, C.; Voogt, M.; Baas, R. Nutrient solutions and water quality for soilless cultures. In Applied Plant Research; Division Glasshouse: Naaldwijk, The Netherlands, 2003; Available online: https://edepot.wur.nl/456342 (accessed on 16 May 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).