Hydrothermal Carbonization of Sewage Sludge: New Improvements in Phosphatic Fertilizer Production and Process Water Treatment Using Freeze Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Chemicals
2.2. Hydrothermal Carbonization
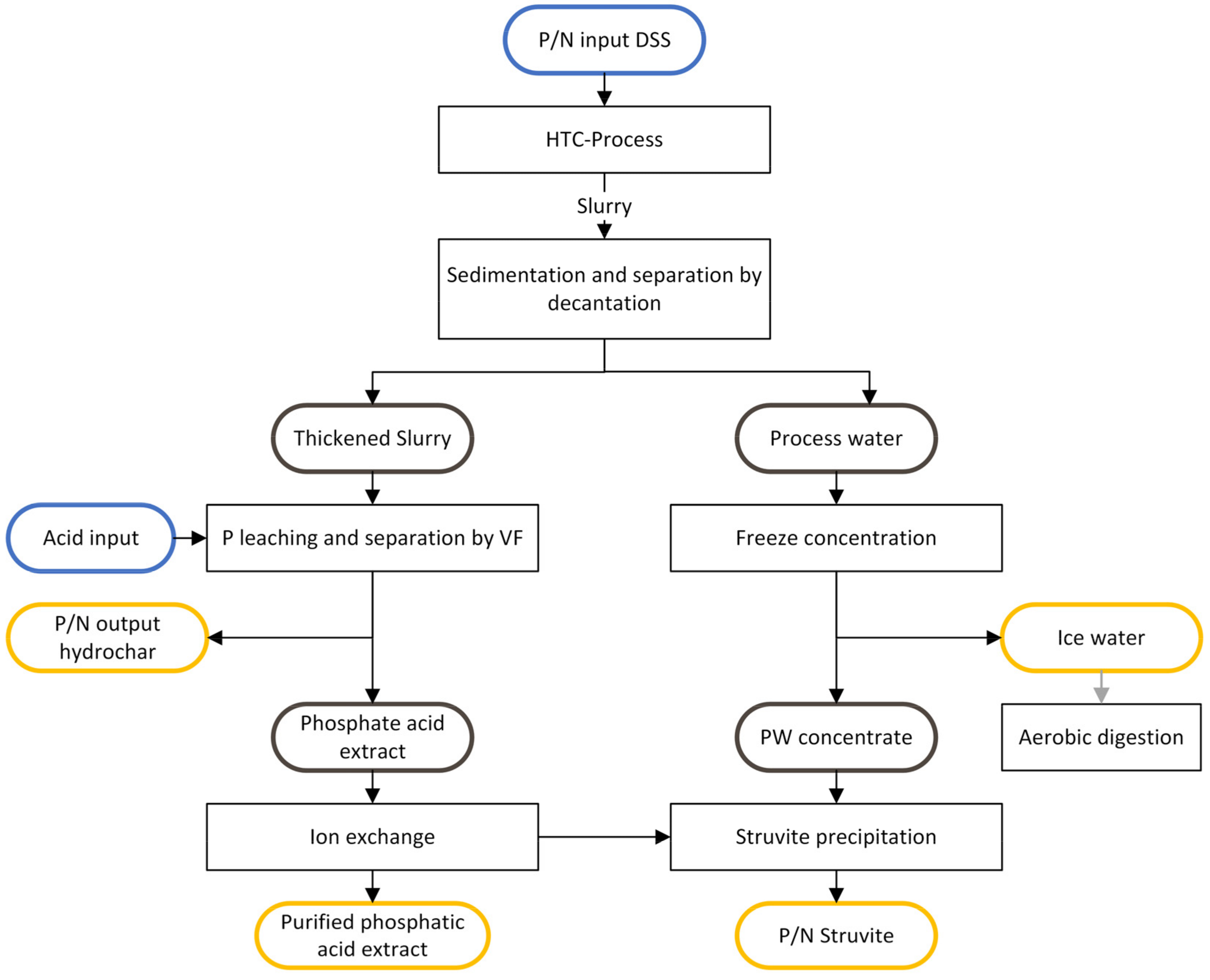
2.3. Separation and Phosphorus Extraction
2.4. Acid Extract Purification Using Ion-Exchange Resins
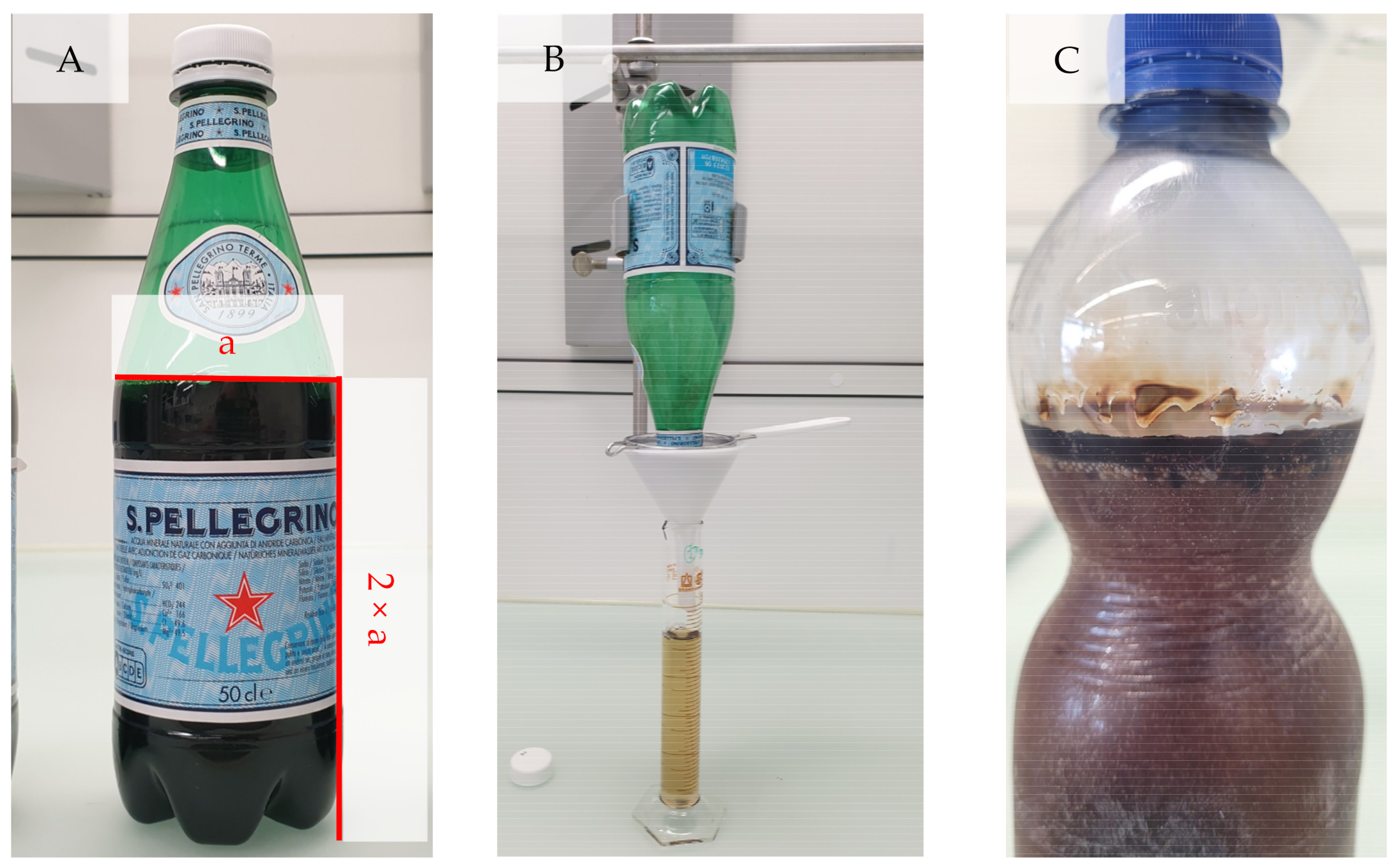
2.5. Freeze Concentration Bottle Test
2.6. Aerobic Digestion
2.7. Struvite Precipitation
2.8. Characterization of Feedstock and Final Products
3. Results and Discussion
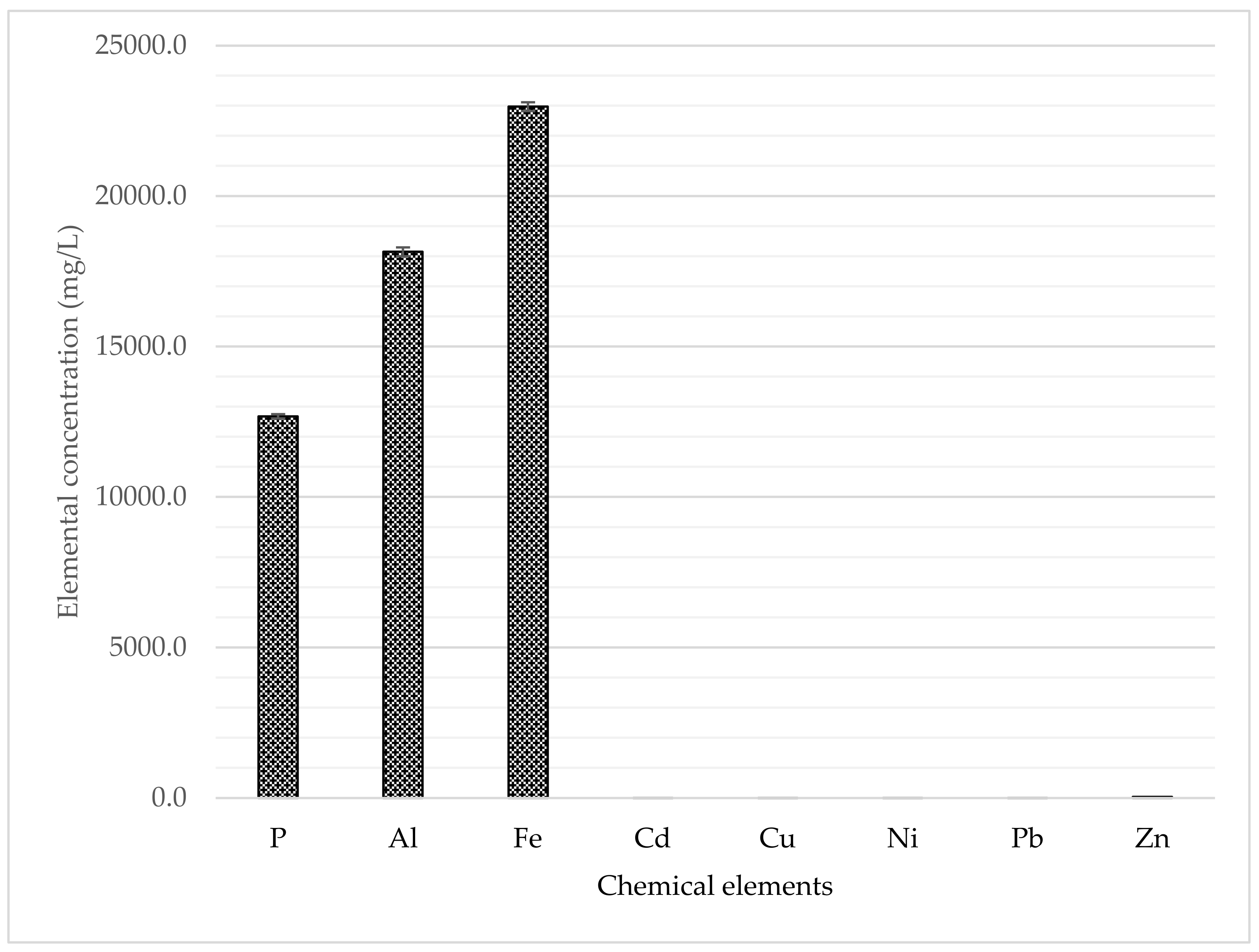
3.1. Hydrothermal Carbonization and P-Extraction
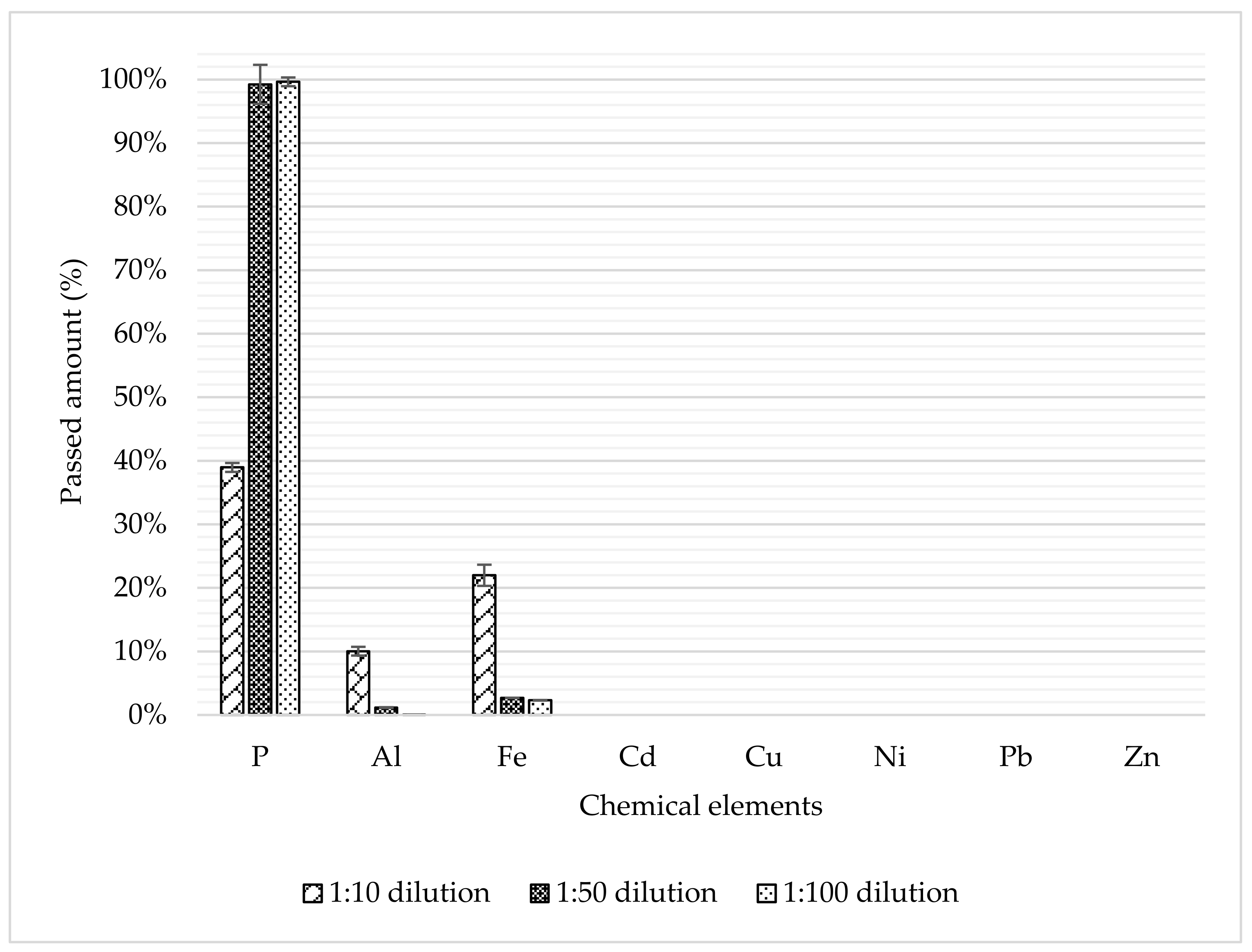
3.2. Purification Step via Ion Exchange
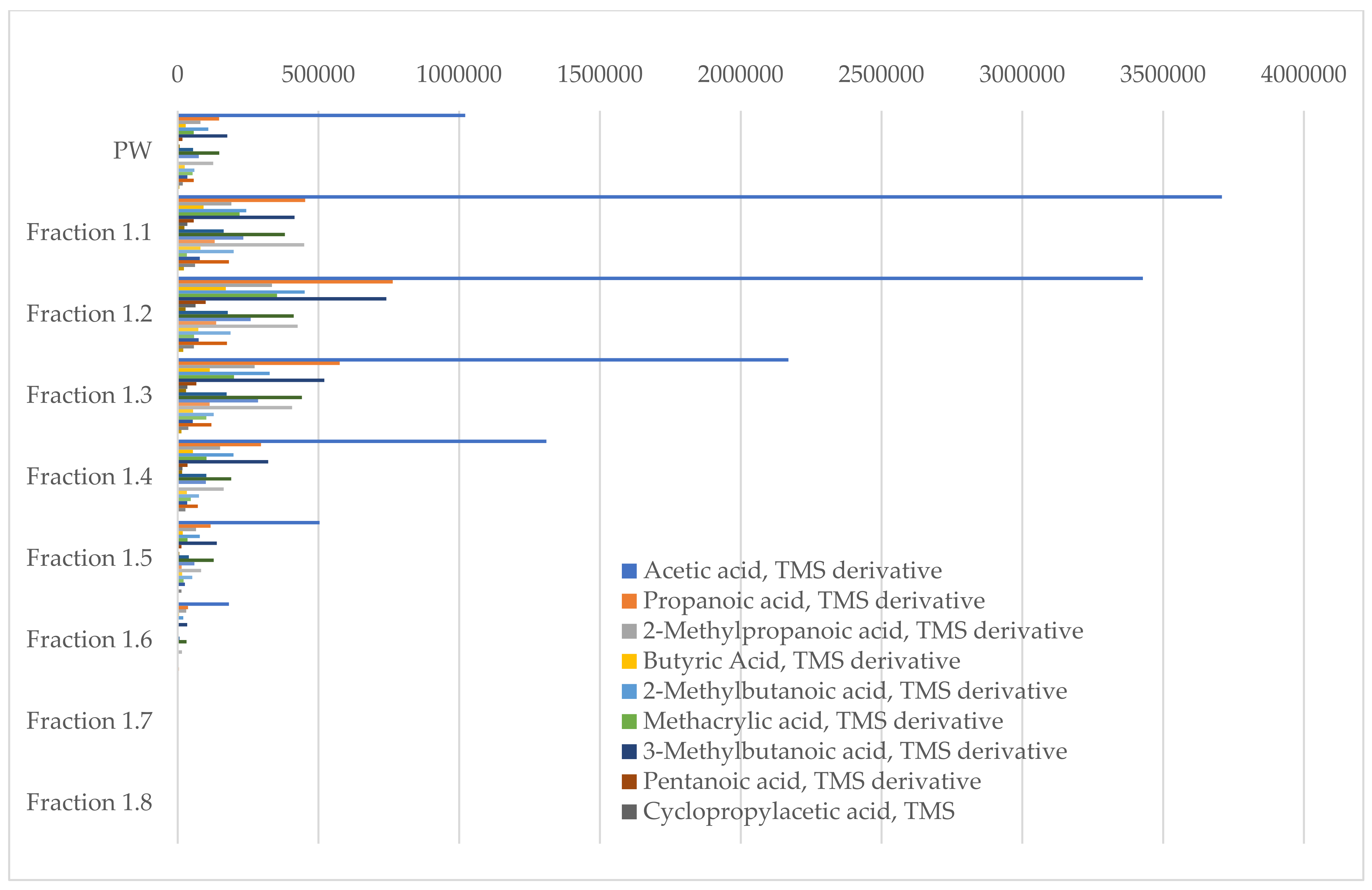
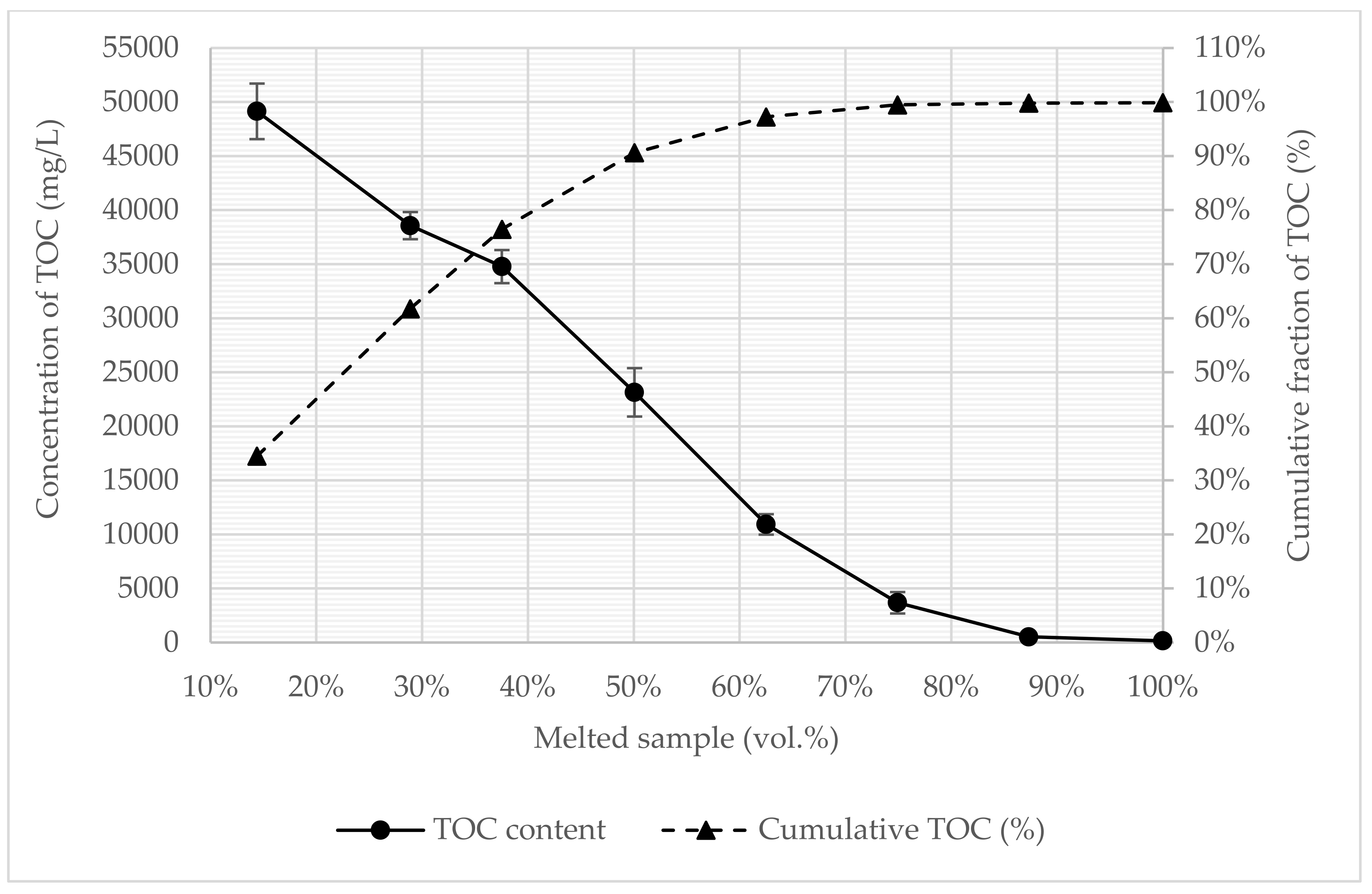
3.3. Process Water Treatment
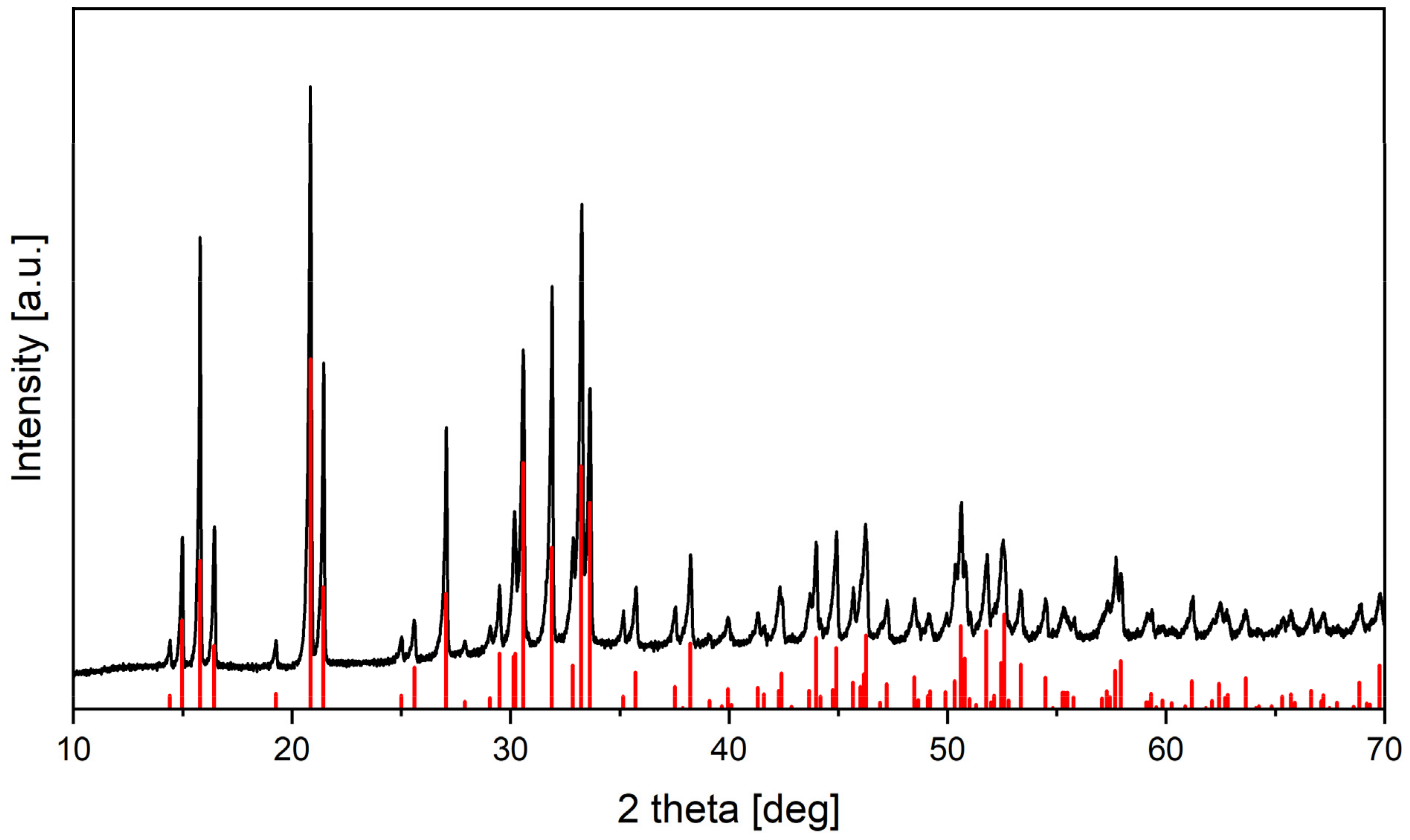
3.4. Struvite Fertilizer
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Functional group | Sulphonic acid |
| Ionic form as supplied | Sodium |
| Total exchange capacity | 2.0 meq/mL, minimum |
| Moisture holding capacity | 43–50% |
| Shipping weight | 830 kg/m3, approximately |
| Particle size range | 0.3–1.2 mm |
| >1.2 mm | 5.0%, maximum |
| <0.3 mm | 1.0%, maximum |
| Uniformity co-efficient | 1.7, maximum |
| Mean bead size | 0.45–0.55 mm |
| Treatment flow rate | 5–40 bv/h |
| Maximum operating temperature | 140 °C |
| Operating pH range | 0–14 |
| Resistance to reducing agents | Good |
| Resistance to oxidizing agents | Generally good, chlorine should be absent |
| Inorganic Pollutant | Mineral Recycling Fertilizer (MinRec) | Mineral Fertilizer | |
|---|---|---|---|
| Threshold Value in Grams | Threshold Value in Grams Per Ton of | ||
| Per Ton of Phosphorus (P) | Dry Matter (DM) | Phosphorus (P) | |
| Vanadium (V) | - | 4000 | - |
| Chromium (Cr) | 1000 | 2000 | - |
| Nickel (Ni) | 500 | - | - |
| Copper (Cu) | 3000 | - | - |
| Zinc (Zn) | 10,000 | - | - |
| Arsenic (As) | 100 | - | - |
| Cadmium (Cd) | 25 | - | 50 1 |
| Mercury (Hg) | 2 | - | - |
| Lead (Pb) | 500 | - | - |

References
- Cordell, D.; White, S. Peak Phosphorus: Clarifying the Key Issues of a Vigorous Debate about Long-Term Phosphorus Security. Sustainability 2011, 3, 2027–2049. [Google Scholar] [CrossRef]
- El Bamiki, R.; Raji, O.; Ouabid, M.; Elghali, A.; Khadiri Yazami, O.; Bodinier, J.-L. Phosphate Rocks: A Review of Sedimentary and Igneous Occurrences in Morocco. Minerals 2021, 11, 1137. [Google Scholar] [CrossRef]
- Pufahl, P.K.; Groat, L.A. Sedimentary and Igneous Phosphate Deposits: Formation and Exploration: An Invited Paper. Econ. Geol. 2017, 112, 483–516. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.-O.; White, S. The Story of Phosphorus: Global Food Security and Food for Thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- de Ridder, M. Risks and Opportunities in the Global Phosphate Rock Market: Robust Strategies in Times of Uncertainty; The Hague Centre for Strategic Studies: Den Haag, The Netherlands, 2012; ISBN 978-94-91040-69-6. [Google Scholar]
- Index Mundi Commodity Prices—Rock Phosphate Monthly Price—US Dollars per Metric Ton. Available online: https://www.indexmundi.com/commodities/?commodity=rock-phosphate&months=240 (accessed on 28 July 2022).
- Bertau, M.; Fröhlich, P.; Gellermann, C.; Maurer, A.; Vohrer, U.; Wendler, K. Phosphatrückgewinnung—Statuspapier der ProcessNet-Fachgruppe “Rohstoffe”; DECHEMA: Frankfurt am Main, Germany, 2017. [Google Scholar]
- Mehr, J.; Jedelhauser, M.; Binder, C. Transition of the Swiss Phosphorus System towards a Circular Economy—Part 1: Current State and Historical Developments. Sustainability 2018, 10, 1479. [Google Scholar] [CrossRef]
- Schenk, K.; Wächter, M. Phosphorreiche Abfälle. Ein Modul der Vollzugshilfe zur Verordnung über die Vermeidung und die Entsorgung von Abfällen (Abfallverordnung, VVEA); Umwelt-Vollzug: Bundesamt für Umwelt: Bern, Switzerland, 2020. [Google Scholar]
- Escala, M.; Zumbühl, T.; Koller, C.; Junge, R.; Krebs, R. Hydrothermal Carbonization as an Energy-Efficient Alternative to Established Drying Technologies for Sewage Sludge: A Feasibility Study on a Laboratory Scale. Energy Fuels 2013, 27, 454–460. [Google Scholar] [CrossRef]
- Lühmann, T.; Wirth, B. Sewage Sludge Valorization via Hydrothermal Carbonization: Optimizing Dewaterability and Phosphorus Release. Energies 2020, 13, 4417. [Google Scholar] [CrossRef]
- Volpe, M.; Fiori, L.; Merzari, F.; Messineo, A.; Andreottola, G. Hydrothermal Carbonization as an Efficient Tool for Sewage Sludge Valorization and Phosphorous Recovery. Chem. Eng. Trans. 2020, 80, 199–204. [Google Scholar] [CrossRef]
- Marin-Batista, J.D.; Mohedano, A.F.; Rodríguez, J.J.; De La Rubia, M.A. Energy and Phosphorous Recovery through Hydrothermal Carbonization of Digested Sewage Sludge. Waste Manag. 2020, 105, 566–574. [Google Scholar] [CrossRef]
- Becker, G.C.; Wüst, D.; Köhler, H.; Lautenbach, A.; Kruse, A. Novel Approach of Phosphate-Reclamation as Struvite from Sewage Sludge by Utilising Hydrothermal Carbonization. J. Environ. Manag. 2019, 238, 119–125. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.; Ross, A.B.; Camargo-Valero, M.A. Evaluation and Comparison of Product Yields and Bio-Methane Potential in Sewage Digestate Following Hydrothermal Treatment. Appl. Energy 2017, 208, 1357–1369. [Google Scholar] [CrossRef]
- Urbanowska, A.; Kabsch-Korbutowicz, M.; Wnukowski, M.; Seruga, P.; Baranowski, M.; Pawlak-Kruczek, H.; Serafin-Tkaczuk, M.; Krochmalny, K.; Niedzwiecki, L. Treatment of Liquid By-Products of Hydrothermal Carbonization (HTC) of Agricultural Digestate Using Membrane Separation. Energies 2020, 13, 262. [Google Scholar] [CrossRef]
- Wüst, D.; Arauzo, P.; Habicht, S.; Cazaña, F.; Fiori, L.; Kruse, A. Process Water Recirculation During Hydrothermal Carbonization as a Promising Process Step Towards the Production of Nitrogen-Doped Carbonaceous Materials. Waste Biomass Valorization 2022, 13, 2349–2373. [Google Scholar] [CrossRef]
- Stemann, J.; Putschew, A.; Ziegler, F. Hydrothermal Carbonization: Process Water Characterization and Effects of Water Recirculation. Bioresour. Technol. 2013, 143, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Blach, T.; Lechevallier, P.; Engelhart, M. Effect of Temperature during the Hydrothermal Carbonization of Sewage Sludge on the Aerobic Treatment of the Produced Process Waters. J. Water Process Eng. 2023, 51, 103368. [Google Scholar] [CrossRef]
- Villamil, J.A.; Mohedano, A.F.; Rodriguez, J.J.; De La Rubia, M.A. Valorisation of the Liquid Fraction from Hydrothermal Carbonisation of Sewage Sludge by Anaerobic Digestion: Anaerobic Digestion of the Liquid Fraction from HTC of Sewage Sludge. J. Chem. Technol. Biotechnol. 2018, 93, 450–456. [Google Scholar] [CrossRef]
- Gerner, G.; Meyer, L.; Wanner, R.; Keller, T.; Krebs, R. Sewage Sludge Treatment by Hydrothermal Carbonization: Feasibility Study for Sustainable Nutrient Recovery and Fuel Production. Energies 2021, 14, 2697. [Google Scholar] [CrossRef]
- John, M.; Häkkinen, A.; Louhi-Kultanen, M. Purification Efficiency of Natural Freeze Crystallization for Urban Wastewaters. Cold Reg. Sci. Technol. 2020, 170, 102953. [Google Scholar] [CrossRef]
- Petzold, G.; Orellana, P.; Moreno, J.J.; Junod, J.; Bugueño, G. Freeze Concentration as a Technique to Protect Valuable Heat-Labile Components of Foods. In Innovative Processing Technologies for Foods with Bioactive Compounds; Moreno, J.J., Ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-315-37127-6. [Google Scholar]
- Samsuri, S.; Rizan, N.A.N.; Hung, S.H.; Amran, N.A.; Sambudi, N.S. Progressive Freeze Concentration for Volume Reduction of Produced Water and Biodiesel Wastewater. Chem. Eng. Technol. 2019, 42, 1764–1770. [Google Scholar] [CrossRef]
- Uald-lamkaddam, I.; Dadrasnia, A.; Llenas, L.; Ponsá, S.; Colón, J.; Vega, E.; Mora, M. Application of Freeze Concentration Technologies to Valorize Nutrient-Rich Effluents Generated from the Anaerobic Digestion of Agro-Industrial Wastes. Sustainability 2021, 13, 13769. [Google Scholar] [CrossRef]
- Ulrich, J.; Stelzer, T. Melt Crystallization. In Crystallization: Basic Concepts and Industrial Applications; Beckmann, W., Ed.; Wiley-VCH: Weinheim, Germany, 2013; ISBN 978-3-527-65034-7. [Google Scholar]
- Williams, P.M.; Ahmad, M.; Connolly, B.S.; Oatley-Radcliffe, D.L. Technology for Freeze Concentration in the Desalination Industry. Desalination 2015, 356, 314–327. [Google Scholar] [CrossRef]
- Dadrasnia, A.; de Bona Muñoz, I.; Yáñez, E.H.; Lamkaddam, I.U.; Mora, M.; Ponsá, S.; Ahmed, M.; Argelaguet, L.L.; Williams, P.M.; Oatley-Radcliffe, D.L. Sustainable Nutrient Recovery from Animal Manure: A Review of Current Best Practice Technology and the Potential for Freeze Concentration. J. Clean. Prod. 2021, 315, 128106. [Google Scholar] [CrossRef]
- Prestes, A.A.; Helm, C.V.; Esmerino, E.A.; Silva, R.; da Cruz, A.G.; Prudencio, E.S. Freeze Concentration Techniques as Alternative Methods to Thermal Processing in Dairy Manufacturing: A Review. J. Food Sci. 2022, 87, 488–502. [Google Scholar] [CrossRef]
- Petzold, G.; Moreno, J.; Lastra, P.; Rojas, K.; Orellana, P. Block Freeze Concentration Assisted by Centrifugation Applied to Blueberry and Pineapple Juices. Innov. Food Sci. Emerg. Technol. 2015, 30, 192–197. [Google Scholar] [CrossRef]
- Valle, S.F.; Giroto, A.S.; Guimarães, G.G.F.; Nagel, K.A.; Galinski, A.; Cohnen, J.; Jablonowski, N.D.; Ribeiro, C. Co-Fertilization of Sulfur and Struvite-Phosphorus in a Slow-Release Fertilizer Improves Soybean Cultivation. Front. Plant Sci. 2022, 13, 861574. [Google Scholar] [CrossRef]
- Vogel, C.; Rivard, C.; Wilken, V.; Muskolus, A.; Adam, C. Performance of Secondary P-Fertilizers in Pot Experiments Analyzed by Phosphorus X-Ray Absorption near-Edge Structure (XANES) Spectroscopy. Ambio 2018, 47, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Gad, S.E.; Barbare, R. Phosphoric Acid. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2005; pp. 414–415. ISBN 978-0-12-369400-3. [Google Scholar]
- Kraus, F.; Zamzow, M.; Conzelmann, L.; Remy, C.; Kleyböcker, A.; Seis, W.; Miehe, U.; Hermann, L.; Hermann, R.; Kabbe, C. Ökobilanzieller Vergleich Der P-Rückgewinnung Aus Dem Abwasserstrom Mit Der Düngemittelproduktion Aus Rohphosphaten Unter Einbeziehung von Umweltfolgeschäden Und Deren Vermeidung; UBA-FB; Umweltbundesamt: Dessau-Rosslau, Germany, 2019; Volume 13. [Google Scholar]
- YARA Fertilizer Industry Handbook. 2022. Available online: https://www.yara.com/siteassets/investors/057-reports-and-presentations/other/2022/fertilizer-industry-handbook-2022-with-notes.pdf/ (accessed on 2 March 2023).
- White, P.J.; Brown, P.H. Plant Nutrition for Sustainable Development and Global Health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Samsuri, S.; Amran, N.A.; Jusoh, M. Optimization of progressive freeze concentration on apple juice via response surface methodology. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 358, p. 012042. [Google Scholar] [CrossRef]
- WTW. Manometric BOD Measuring Devices OxiTop IS 6 & IS 12—Instruction Manual; ba31107de05; WTW: Weilheim, Germany, 2017. [Google Scholar]
- Miller, F.M.; Young, G.L.; von Seebach, M. Formation and Techniques for Control of Sulfur Dioxide and Other Sulfur Compounds in Portland Cement Kiln Systems; R&D Serial No. 2460; Portland Cement Association: Skokie, IL, USA, 2002. [Google Scholar]
- Raj, A.; Ibrahim, S.; Jagannath, A. Combustion Kinetics of H2S and Other Sulfurous Species with Relevance to Industrial Processes. Prog. Energy Combust. Sci. 2020, 80, 100848. [Google Scholar] [CrossRef]
- Swiss Water Association. Infoblatt—Verfügbarkeit von Fällmitteln für ARA in der Schweiz, Herbst 2022; Verband Schweizer Abwasser- und Gewässerschutzfachleute: Glattbrugg, Switzerland, 2022. [Google Scholar]
- Chung, J.W.; Gerner, G.; Ovsyannikova, E.; Treichler, A.; Baier, U.; Libra, J.; Krebs, R. Hydrothermal Carbonization as an Alternative Sanitation Technology: Process Optimization and Development of Low-Cost Reactor. Open Res. Eur. 2022, 1, 139. [Google Scholar] [CrossRef]
- Kaiser, D.E.; Pagliari, P. Understanding Phosphorus Fertilizers. Available online: https://extension.umn.edu/phosphorus-and-potassium/understanding-phosphorus-fertilizers (accessed on 12 October 2022).
- Ion Exchange Ltd. Indion 225 Na F—Bulletin R047. Available online: https://ionresins.com/pdf/pds/225_Na_F_WQA_PDS.pdf (accessed on 20 August 2021).
- Swiss Federal Council. Chemical Risk Reduction Ordinance 2005; Swiss Federal Council: Bern, Switzerland, 2005. [Google Scholar]

| C | H | N | S | Odiff | Ash | H/C | O/C | HHV | LHV | |
|---|---|---|---|---|---|---|---|---|---|---|
| [wt.%] | [wt.%] | [wt.%] | [wt.%] | [wt.%] | [wt.%] | [MJ kg−1] | [MJ kg−1] | |||
| DSS | 30.4 | 4.3 | 4.2 | 1.4 | 17.5 | 42.2 | 1.7 | 0.4 | - | - |
| HTC-HC | 27.3 | 3.7 | 3.0 | 9.8 | 11.3 | 44.9 | 1.6 | 0.3 | 12.1 | 11.2 |
| Nutrient Content and Recovery | |||||
|---|---|---|---|---|---|
| P | N | K | Mg | Ca | |
| [mg kg−1] | [mg kg−1] | [mg kg−1] | [mg kg−1] | [mg kg−1] | |
| DSS | 33,613 (-) | 42,066 (-) | 1489 (-) | 3353 (-) | 33,064 (-) |
| HTC-HC | 17,798 (35.6) | 29,529 (47.1) | 1 1347 (60.7) | 1 2133 (42.7) | 1 45,856 (93.1) |
| [mg L−1] | [mg L−1] | [mg L−1] | [mg L−1] | [mg L−1] | |
| HTC-PW | 17.9 (0.1) | 6797 (25.6) | 146 (15.5) | 64 (3.0) | 536 (2.6) |
| HTC-PAE | 12,681 (72.2) | 7030 (32.0) | 199 (25.6) | 931 (53.1) | 556 (3.2) |
| Precipitants | Heavy Metal Content | ||||||
|---|---|---|---|---|---|---|---|
| Fe [mg kg−1] | Al [mg kg−1] | Ni [mg kg−1] | Cu [mg kg−1] | Zn [mg kg−1] | Cd [mg kg−1] | Pb [mg kg−1] | |
| DSS | 74,091 | 53,446 | 18 | 242 | 583 | <1 | 15 |
| HTC-HC | 49,863 | 32,649 | 1 23 | 360 | 783 | <1 | 23 |
| [mg L−1] | [mg L−1] | [mg L−1] | [mg L−1] | [mg L−1] | [mg L−1] | [mg L−1] | |
| HTC-PW | 367 | 16 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| HTC-PAE | 22,970 | 18,150 | 1.8 | <0.1 | 35.2 | <0.1 | <0.1 |
| Parameter | Unit | PW | Concentrate | Ice Water | ||||
|---|---|---|---|---|---|---|---|---|
| CODtot | mg/L | 56,300.0 | 102,666.7 | ±2886.8 | (91.3%) | 10,793.3 | ±864.9 | (9.6%) |
| TOC (NPOC) | mgC/L | 20,856.7 | 37,503.3 | ±811.9 | (90.0%) | 3768.0 | ±449.0 | (9.0%) |
| DOC | mgC/L | 20,826.7 | 37,710.0 | ±746.5 | (90.6%) | 3796.0 | ±398.3 | (9.1%) |
| TNtot | mgN/L | 6797.3 | 12,646.7 | ±222.8 | (93.1%) | 1262.0 | ±89.6 | (9.3%) |
| TNdis | mgN/L | 6773.0 | 12,530.0 | ±337.8 | (92.6%) | 1263.7 | ±90.6 | (9.3%) |
| Ammonium | mgN/L | 3070.0 | 5533.3 | ±111.5 | (90.2%) | 562.7 | ±61.2 | (9.2%) |
| Nitrite | mgN/L | 1.5 | 2.9 | ±0.03 | (98.2%) | 0.2 | ±0.0 | (6.2%) |
| Nitrate | mgN/L | 193.0 | 346.3 | ±3.1 | (89.8%) | 36.7 | ±3.5 | (9.5%) |
| Phosphatetot | mgP/L | 15.9 | 29.2 | ±0.8 | (91.8%) | BLD | - | |
| Phenoltot | mg/L | 688.0 | 1353.3 | ±87.4 | (98.5%) | 107.7 | ±23.8 | (7.8%) |
| pH | - | 7.59 | 7.96 | ±0.06 | 7.77 | ±0.02 | ||
| EC | mS/cm | 17.0 | 26.7 | ±0.1 | 3.9 | ±0.4 | ||
| Inorganic Contaminant | Mineral Recycling Fertilizer | Struvite Precipitate |
|---|---|---|
| Threshold Value in Grams Per Metric Ton Phosphorus | Value in Grams Per Metric Ton Phosphorus | |
| Nickel (Ni) | 500 | BLD |
| Copper (Cu) | 3000 | BLD |
| Zinc (Zn) | 10,000 | BLD |
| Cadmium (Cd) | 25 | BLD |
| Lead (Pb) | 500 | BLD |
| Nutrient | Value in Kilogram Per Metric Ton (%) |
|---|---|
| Phosphorus (P) | 137.1 (13.7) |
| Phosphorus converted to phosphorus pentoxide (P2O5) | 314.0 (31.4) |
| Nitrogen (Ncal.) | † 57.1 (5.7) |
| Potassium (K) | <1.4 (<0.14) |
| Calcium (Ca) | 5.0 (0.5) |
| Magnesium (Mg) | 95.9 (9.5) |
| Aluminum (Al) | 1.5 (0.15) |
| Iron (Fe) | 17.9 (1.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerner, G.; Chung, J.W.; Meyer, L.; Wanner, R.; Heiniger, S.; Seiler, D.; Krebs, R.; Treichler, A.; Kontic, R.; Kulli, B. Hydrothermal Carbonization of Sewage Sludge: New Improvements in Phosphatic Fertilizer Production and Process Water Treatment Using Freeze Concentration. Energies 2023, 16, 7027. https://doi.org/10.3390/en16207027
Gerner G, Chung JW, Meyer L, Wanner R, Heiniger S, Seiler D, Krebs R, Treichler A, Kontic R, Kulli B. Hydrothermal Carbonization of Sewage Sludge: New Improvements in Phosphatic Fertilizer Production and Process Water Treatment Using Freeze Concentration. Energies. 2023; 16(20):7027. https://doi.org/10.3390/en16207027
Chicago/Turabian StyleGerner, Gabriel, Jae Wook Chung, Luca Meyer, Rahel Wanner, Simon Heiniger, Daniel Seiler, Rolf Krebs, Alexander Treichler, Roman Kontic, and Beatrice Kulli. 2023. "Hydrothermal Carbonization of Sewage Sludge: New Improvements in Phosphatic Fertilizer Production and Process Water Treatment Using Freeze Concentration" Energies 16, no. 20: 7027. https://doi.org/10.3390/en16207027
APA StyleGerner, G., Chung, J. W., Meyer, L., Wanner, R., Heiniger, S., Seiler, D., Krebs, R., Treichler, A., Kontic, R., & Kulli, B. (2023). Hydrothermal Carbonization of Sewage Sludge: New Improvements in Phosphatic Fertilizer Production and Process Water Treatment Using Freeze Concentration. Energies, 16(20), 7027. https://doi.org/10.3390/en16207027








