Photovoltaic-Assisted Photo(electro)catalytic Hydrogen Production: A Review
Abstract
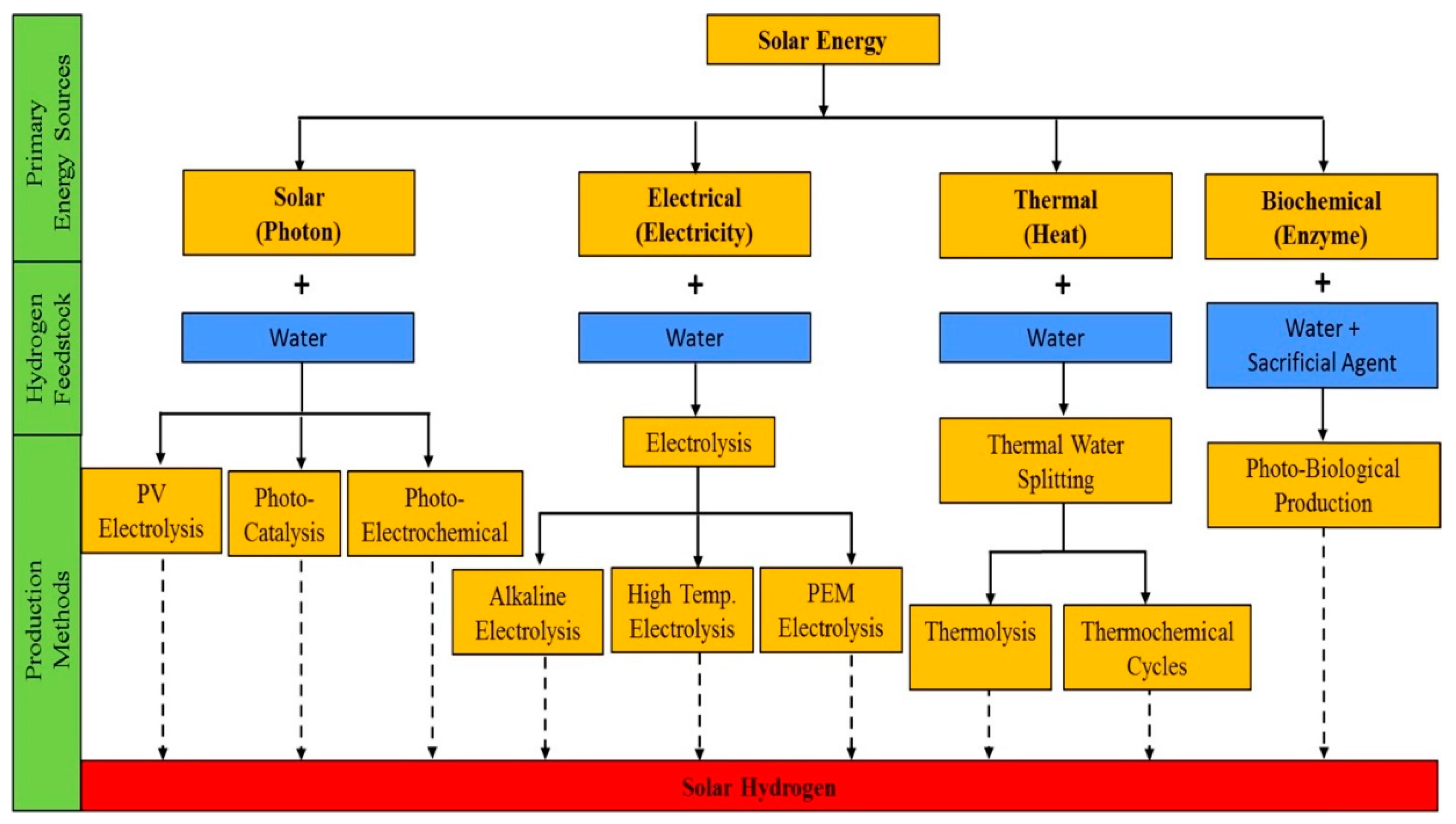
1. Introduction
2. Basic Principle of the PV-PEC Integrated System
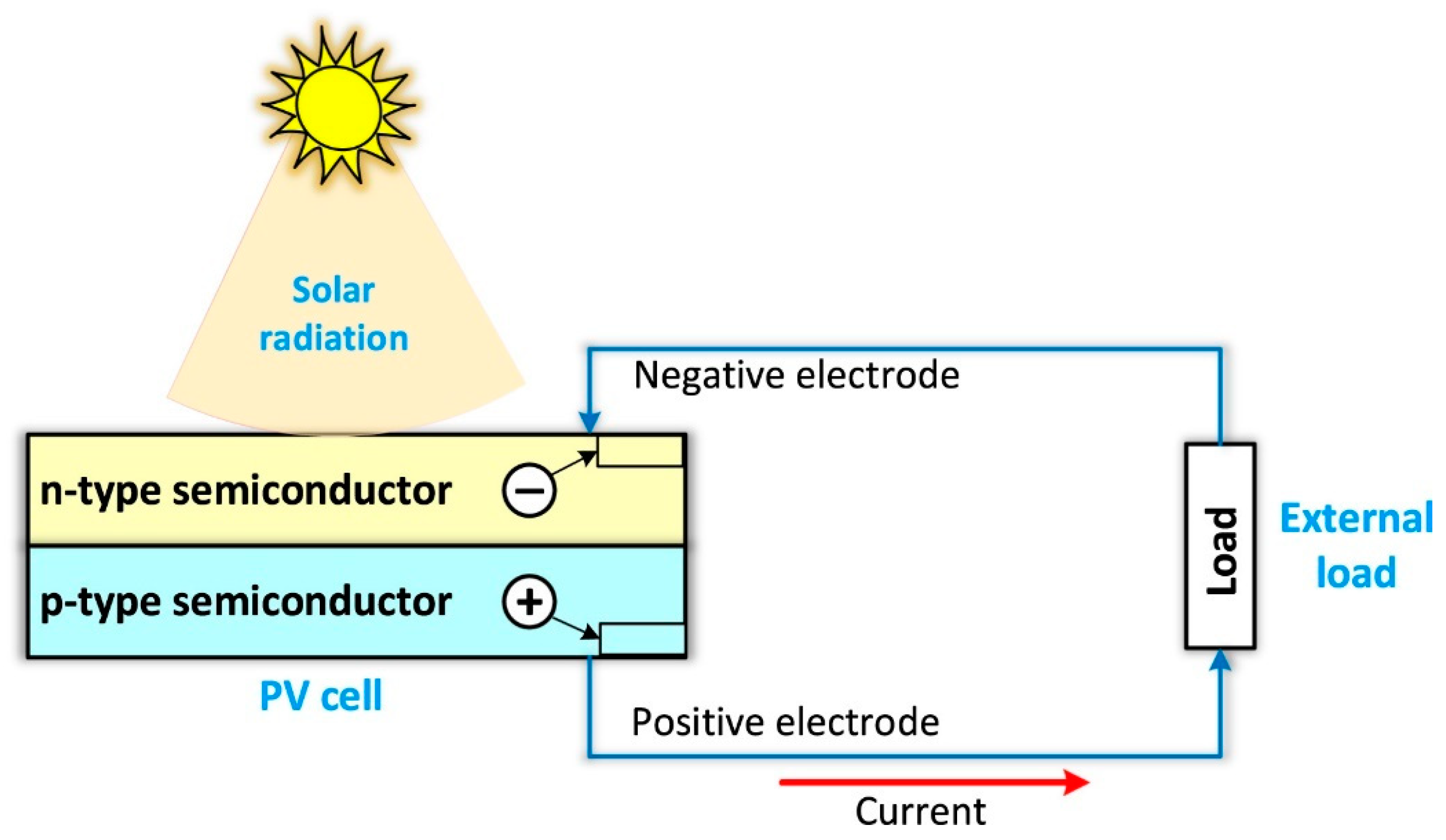
2.1. Basic Principle of the PV System
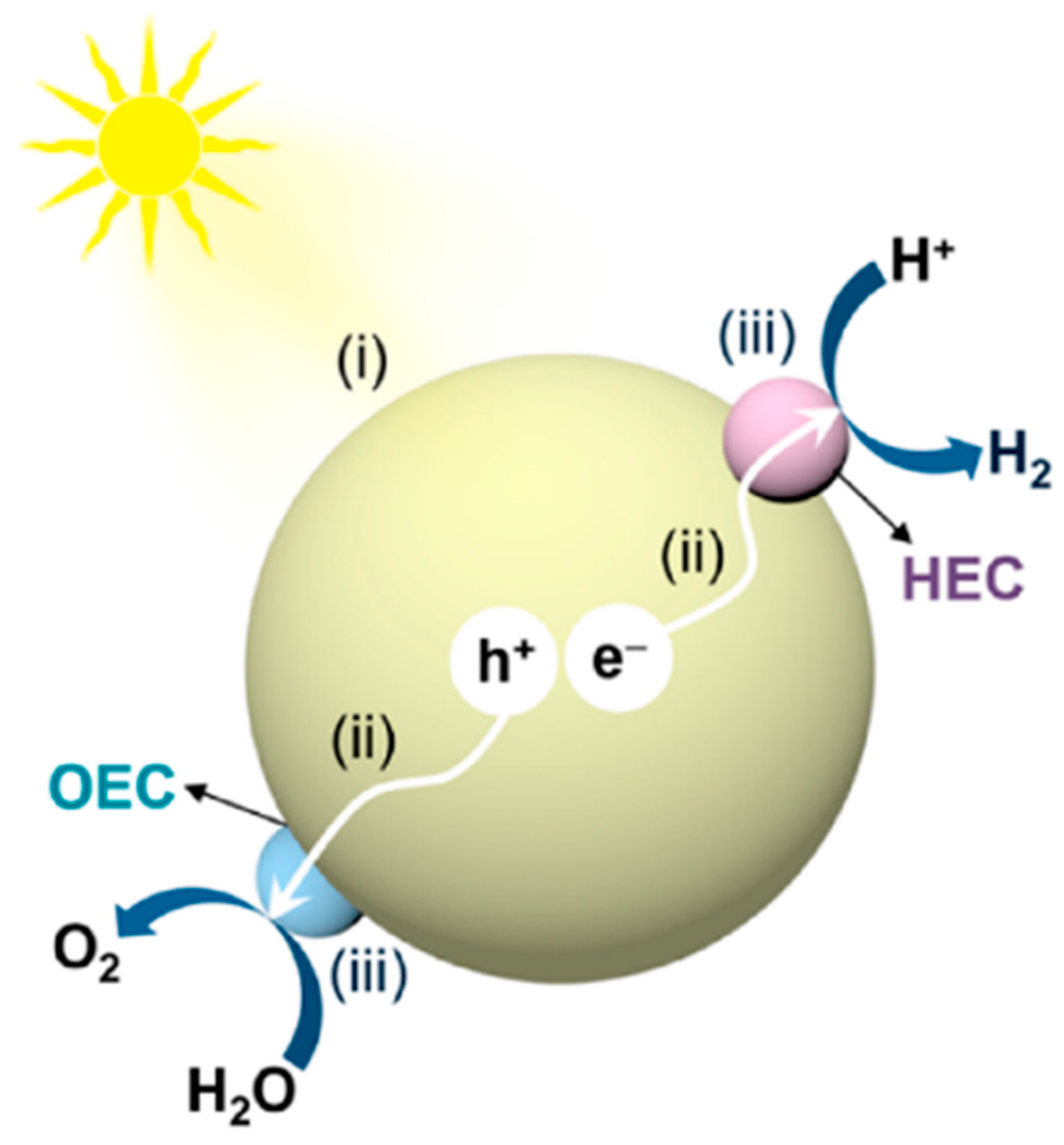
2.2. Basic Principle of the PEC System
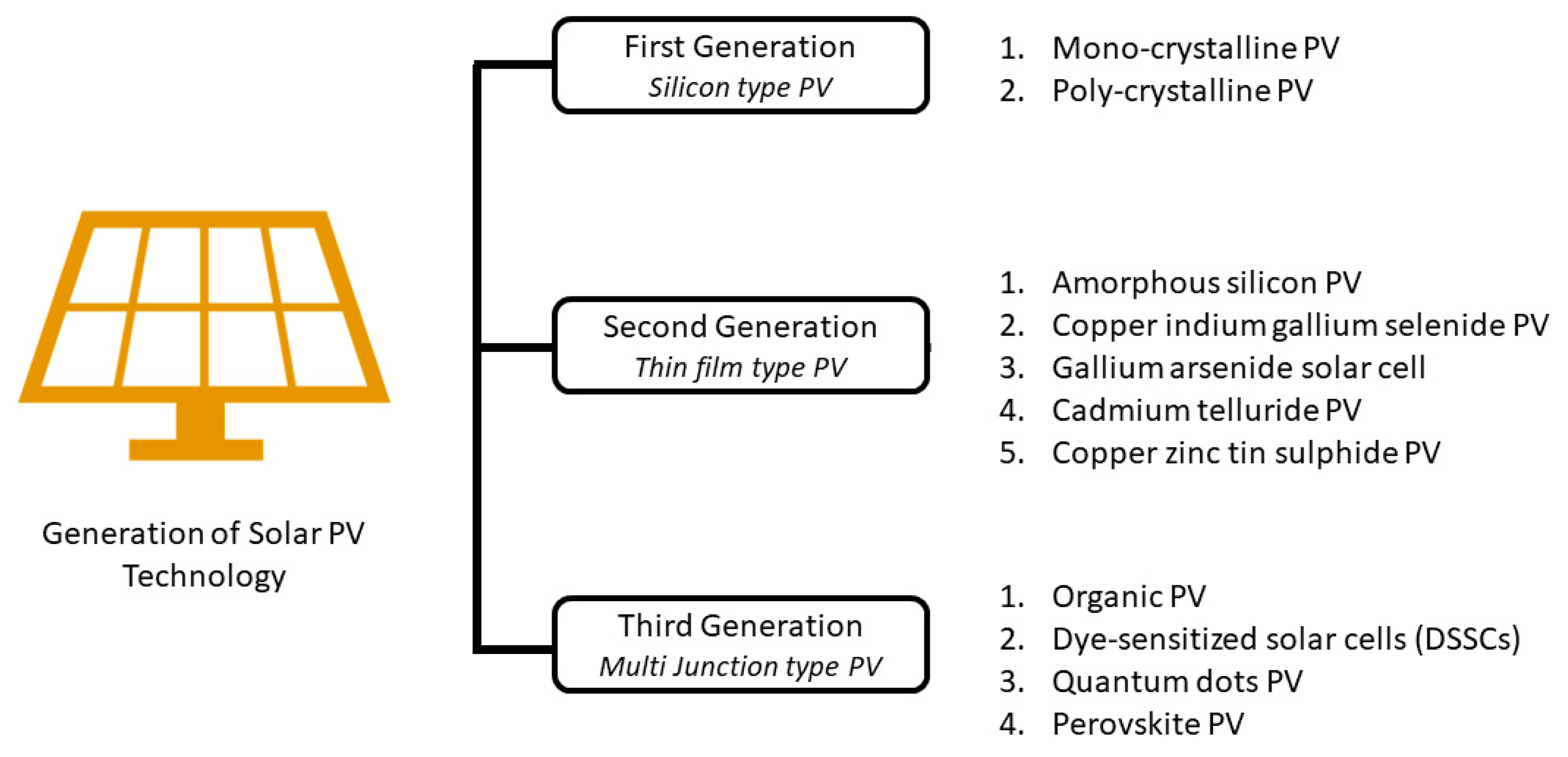
3. Types of Solar PV Technology
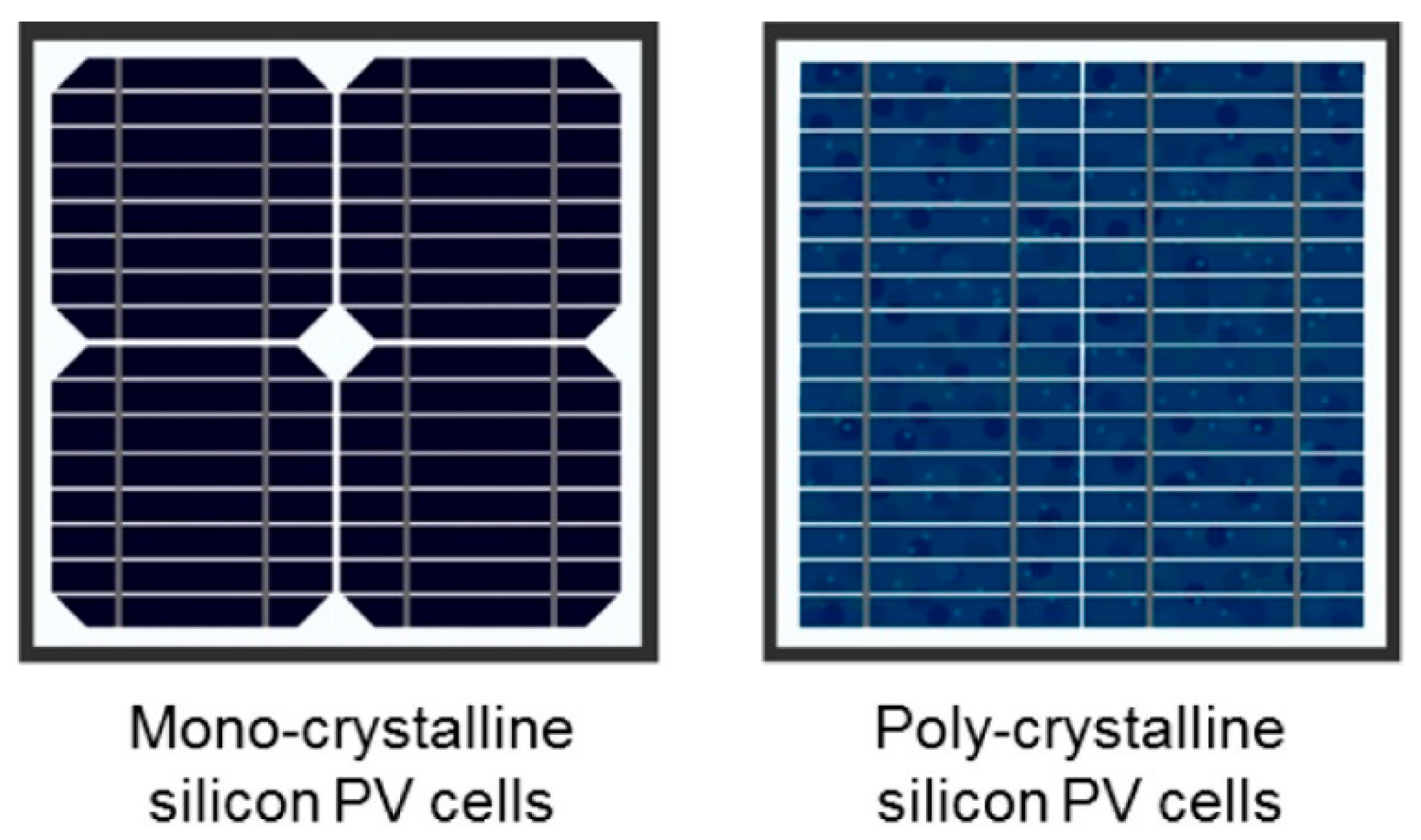
3.1. First Generation of PV Technology
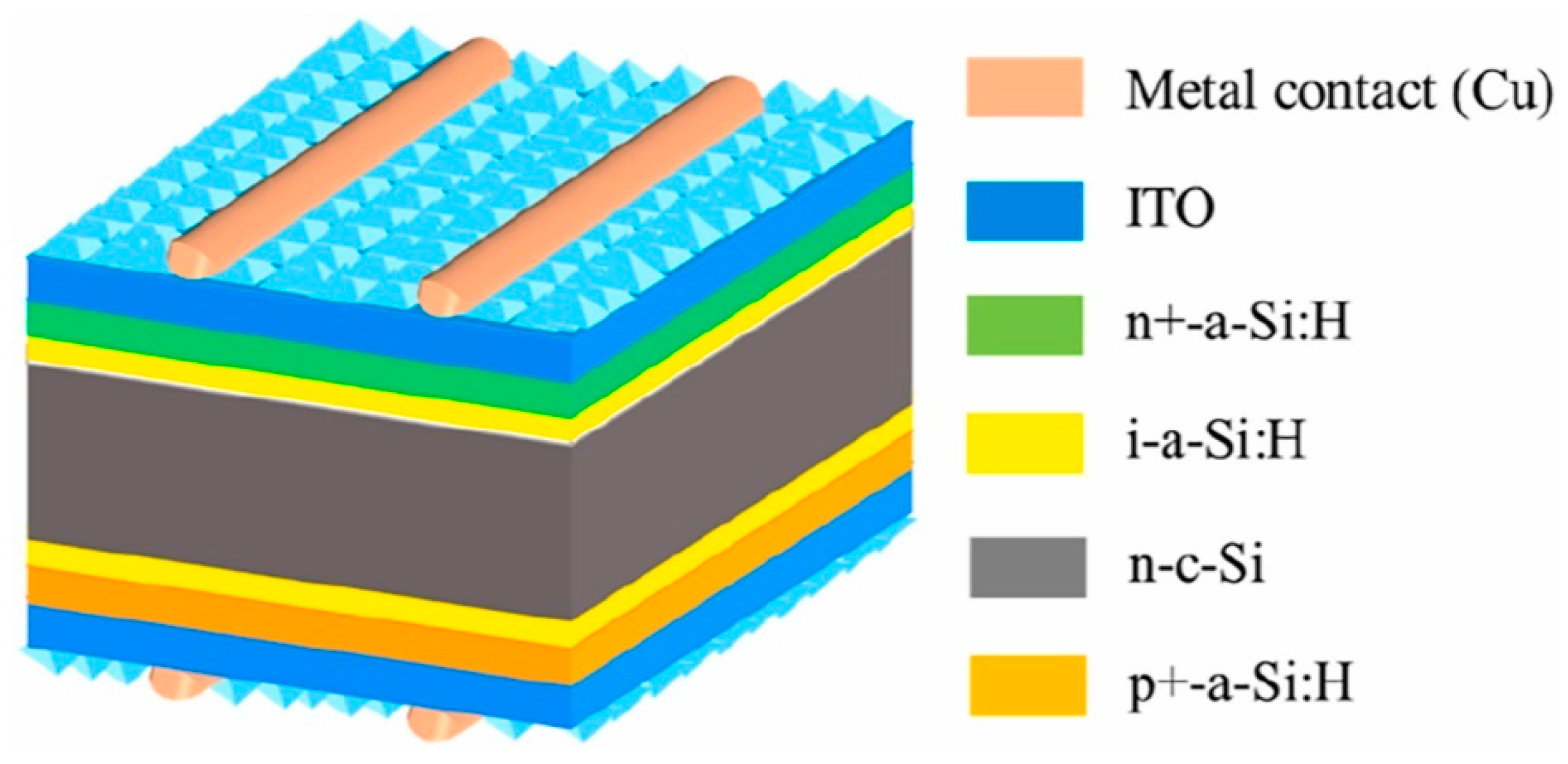
3.2. Second Generation of PV Technology
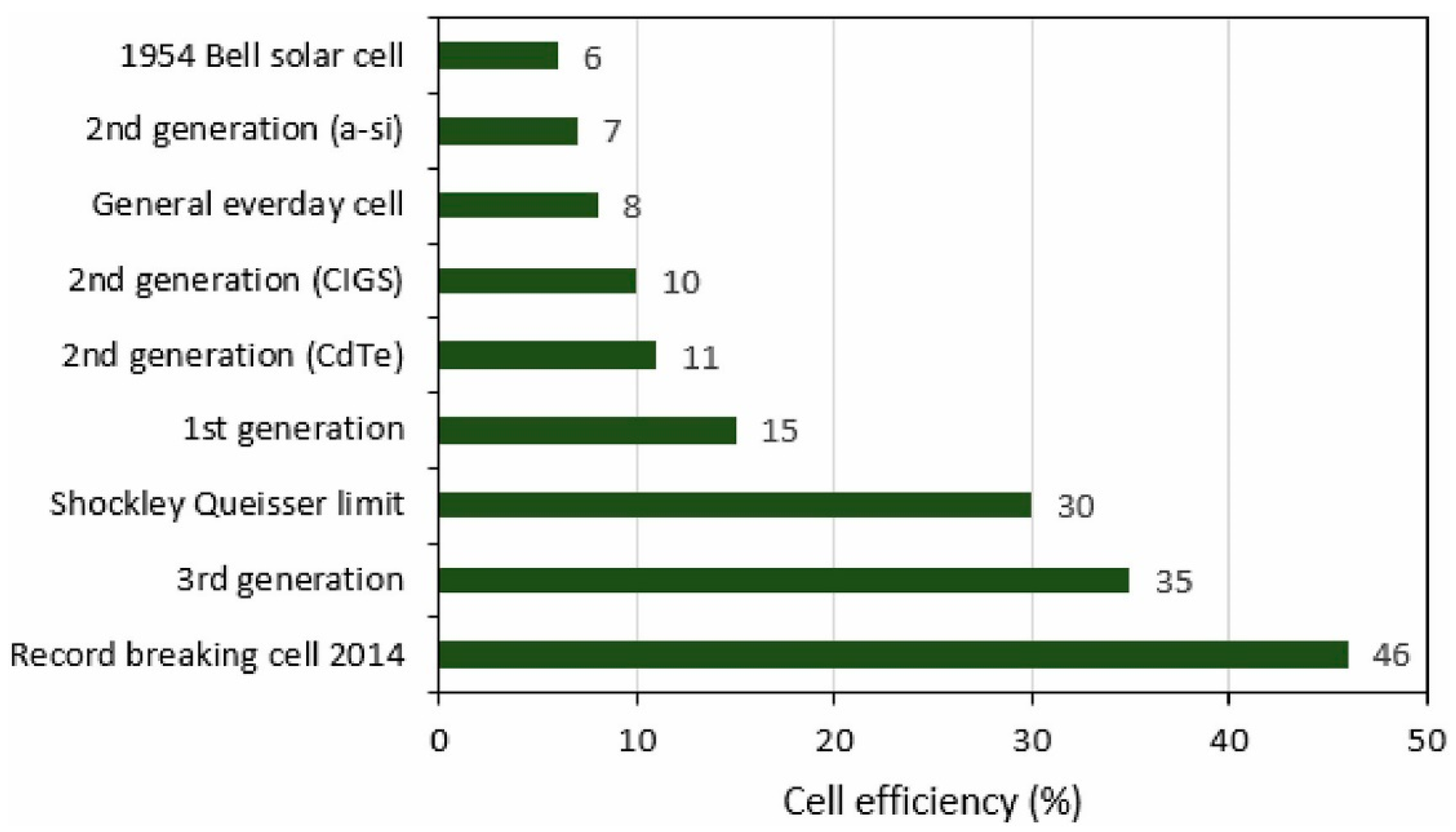
3.3. Third Generation of PV Technology
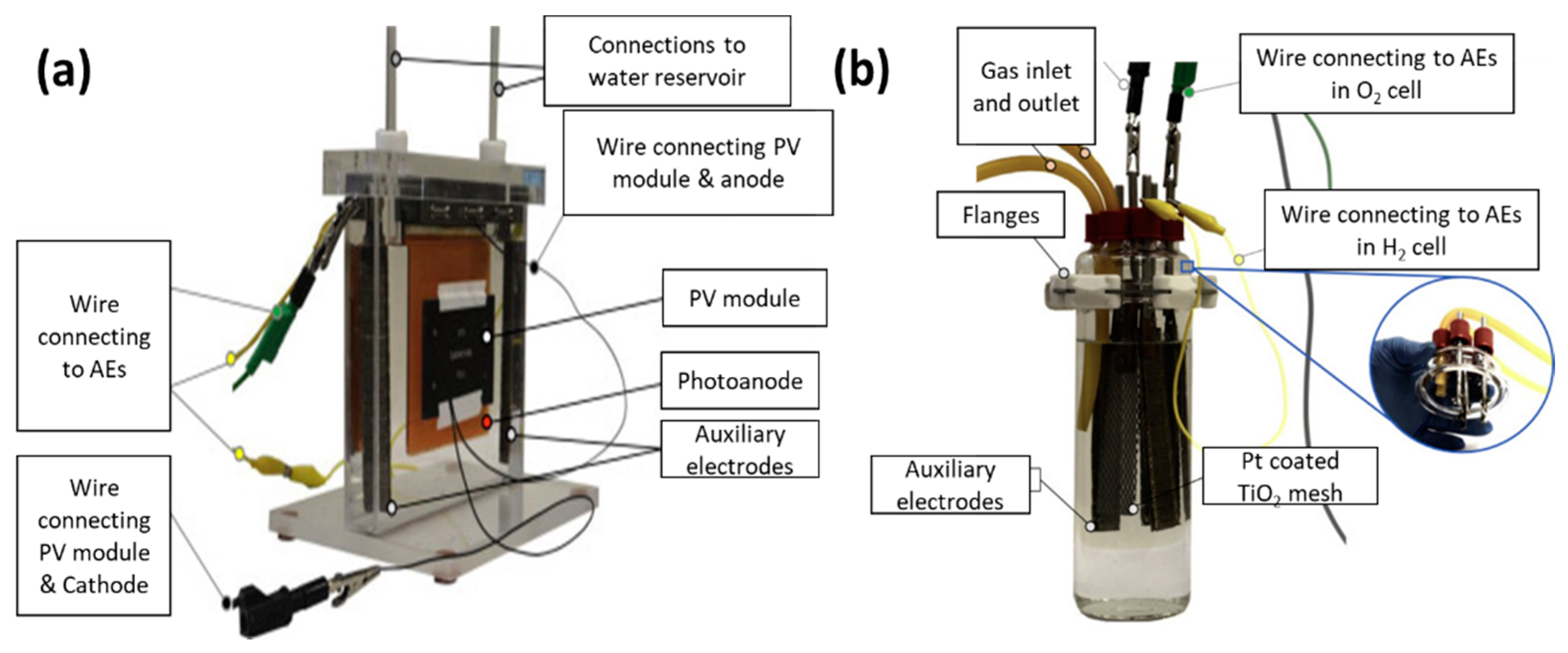
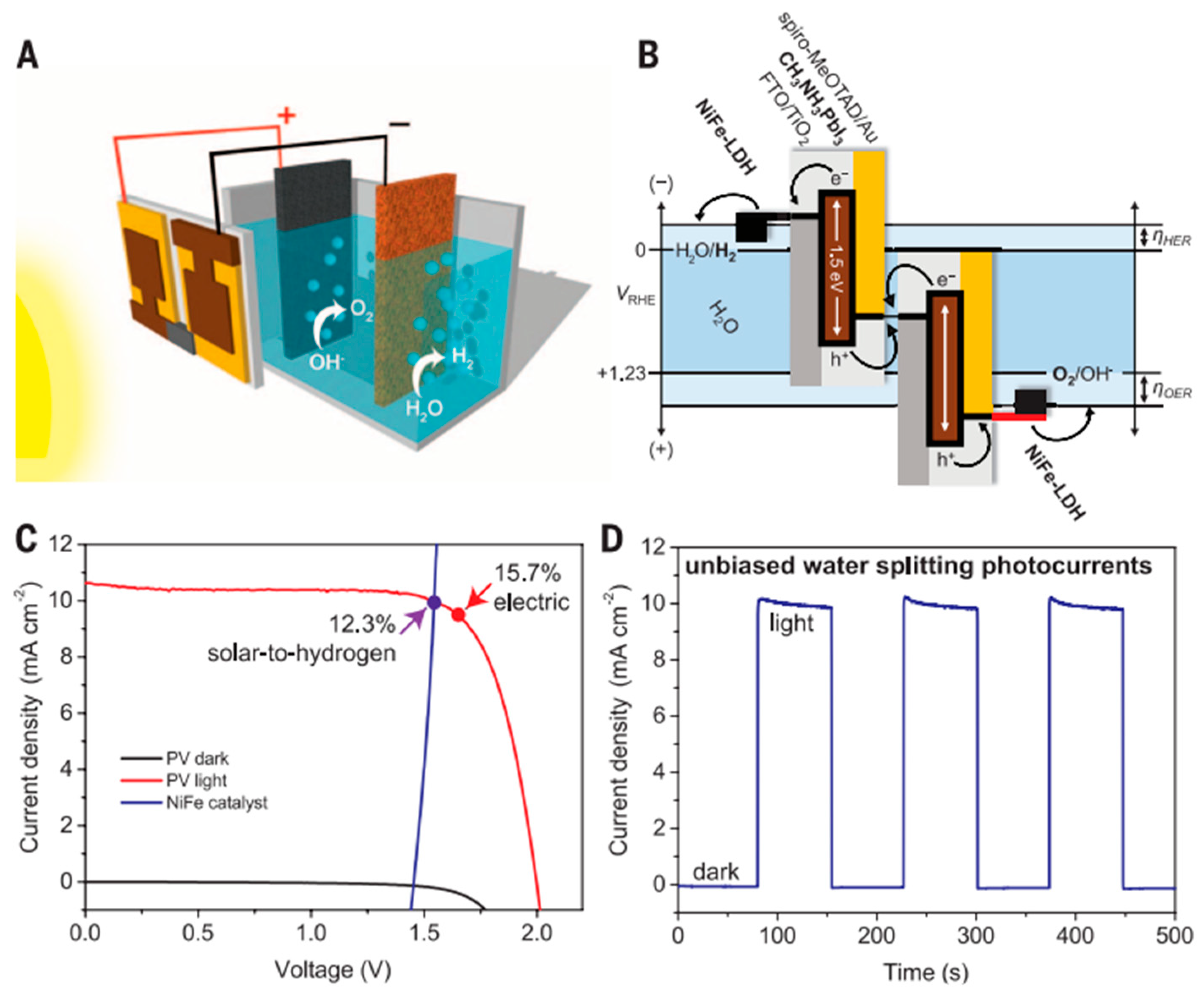
4. Recent Research on PV-PEC Systems
| Type of PV System | PEC Materials | Source of Light | Performance Activity | Ref./Year |
|---|---|---|---|---|
| Silicon PV | Hematite photocatalyst | Visible Light |
| [51]/2020 |
| Amorphous silicon carbide | Light intensity of 1000 W/m2 | Visible Light |
| [46]/2019 |
| a-Si:H/a-Si:H/mc-Si:H triple junction cell | NiMo/NiFeOX catalyst | Visible Light |
| [52]/2018 |
| Monolithic perovskite/Si tandem solar cell | NiMo4/MnO3−X catalyst | Visible Light |
| [56]/2021 |
| Perovskite PV | NiFe DLH/Ni foam electrodes | Visible Light |
| [53]/2014 |
| DSSCs | Bimetallic Cu-Ni/TiO2 | Visible Light | H2 production: 694.84 μmol | [54]/2017 |
| DSSCs | RGO@g-C3N4/BiVO4 | Visible Light |
| [57]/2020 |
| DSSCs | Cu-Ni/TiO2 | Visible Light |
| [58]/2020 |
| DSSCs | BiVO4 | Visible Light |
| [23]/2020 |
| DSSCs | SrTiO3 | UV illumination | Short-circuit current (JSC): 3.36 mA·cm−2, efficiency: 1.12% | [55]/2022 |
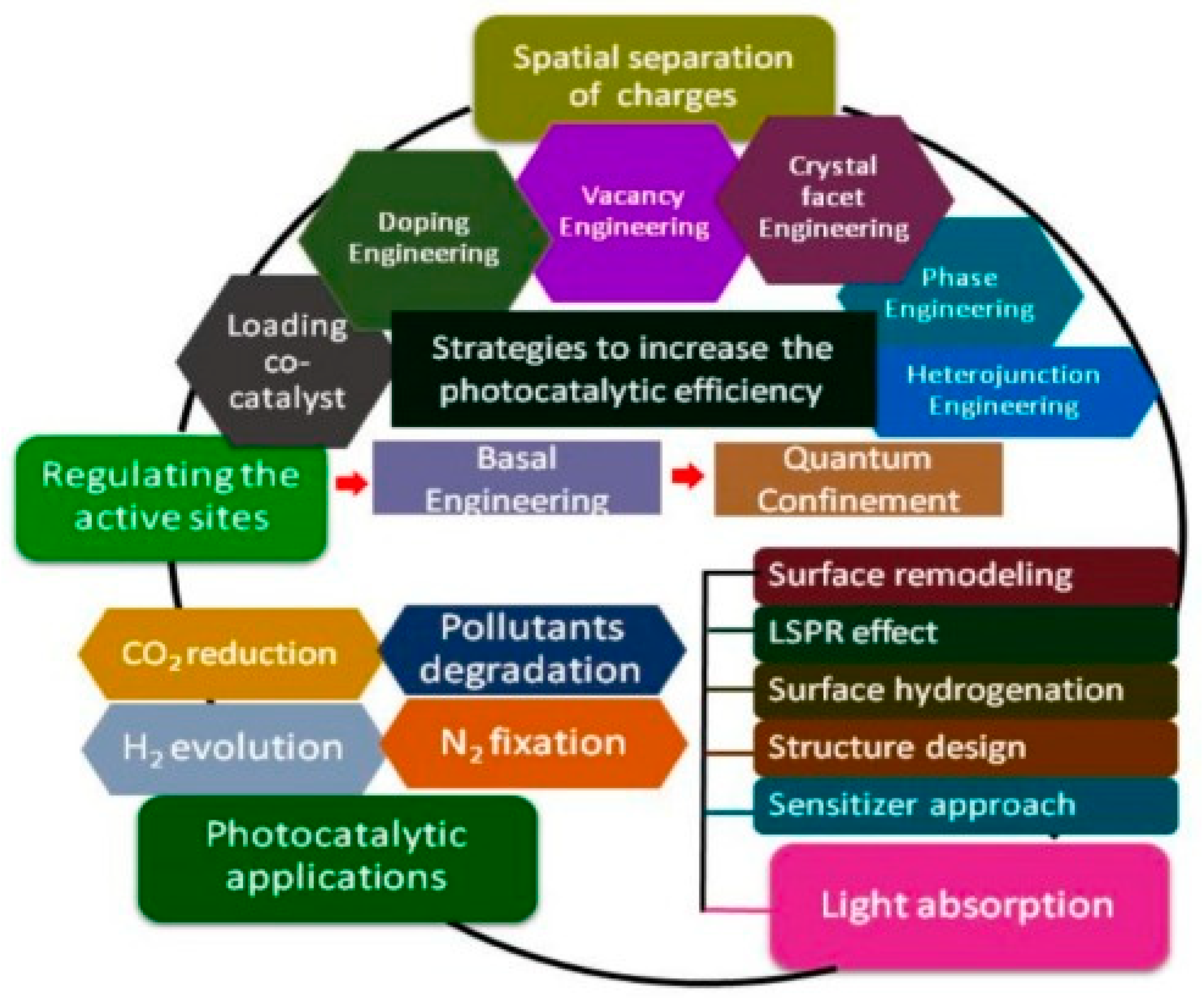
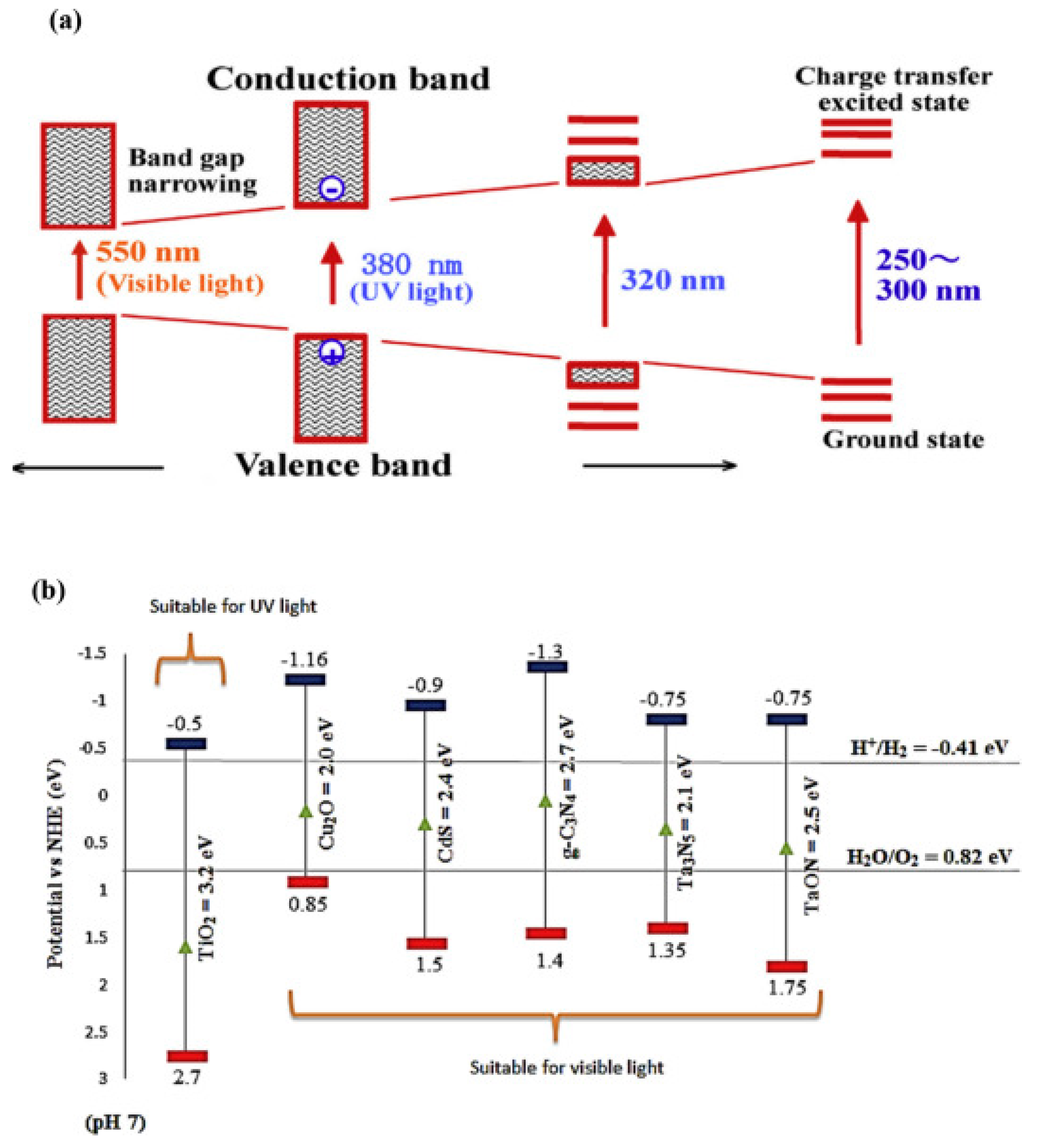
5. Different Types of Semiconductor Materials Used in PEC System
6. Challenges in the PV-PEC System
7. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Huang, X.; Wang, X. Recent Progresses in the Design of BiVO4-Based Photocatalysts for Efficient Solar Water Splitting. Catal. Today 2019, 335, 31–38. [Google Scholar] [CrossRef]
- Allouhi, A.; Rehman, S.; Buker, M.S.; Said, Z. Up-to-Date Literature Review on Solar PV Systems: Technology Progress, Market Status and R&D. J. Clean. Prod. 2022, 362, 132339. [Google Scholar]
- Cherubini, F. The Biorefinery Concept: Using Biomass Instead of Oil for Producing Energy and Chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Mekhilef, S.; Safari, A.; Mustaffa, W.E.S.; Saidur, R.; Omar, R.; Younis, M.A.A. Solar Energy in Malaysia: Current State and Prospects. Renew. Sustain. Energy Rev. 2012, 16, 386–396. [Google Scholar] [CrossRef]
- Younis, S.A.; Kwon, E.E.; Qasim, M.; Kim, K.H.; Kim, T.; Kukkar, D.; Dou, X.; Ali, I. Metal-Organic Framework as a Photocatalyst: Progress in Modulation Strategies and Environmental/Energy Applications. Prog. Energy Combust. Sci. 2020, 81, 100870. [Google Scholar] [CrossRef]
- He, Y.; Hamann, T.; Wang, D. Thin Film Photoelectrodes for Solar Water Splitting. Chem. Soc. Rev. 2019, 48, 2182–2215. [Google Scholar] [CrossRef] [PubMed]
- Licht, S.; Wang, B.; Mukerji, S.; Soga, T.; Umeno, M.; Tributsch, H. Over 18% Solar Energy Conversion to Generation of Hydrogen Fuel; Theory and Experiment for Efficient Solar Water Splitting. Int. J. Hydrogen Energy 2001, 26, 653–659. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Frebilot, C.; Kaddoury, Y.; Sufian, S.; Ong, W.J. Bifunctional Z-Scheme Ag/AgVO3/g-C3N4 photocatalysts for expired ciprofloxacin degradation and hydrogen production from natural rainwater without using scavengers. J. Environ. Manag. 2020, 270, 110803. [Google Scholar] [CrossRef] [PubMed]
- Rekioua, D.; Matagne, E. Optimization of Photovoltaic Power Systems: Modelization, Simulation and Control; Springer: Berlin/Heidelberg, Germany, 2012; Volume 102. [Google Scholar]
- Rekioua, D.; Rekioua, T.; Soufi, Y. Control of a Grid Connected Photovoltaic System. In Proceedings of the 2015 International Conference on Renewable Energy Research and Applications (ICRERA) 2015, Palermo, Italy, 22–25 November 2015; pp. 1382–1387. [Google Scholar]
- Yan, J.; Saunders, B.R. Third-Generation Solar Cells: A Review and Comparison of Polymer:Fullerene, Hybrid Polymer and Perovskite Solar Cells. RSC Adv. 2014, 4, 43286–43314. [Google Scholar] [CrossRef]
- Tao, M.; Azzolini, J.A.; Stechel, E.B.; Ayers, K.E.; Valdez, T.I. Review—Engineering Challenges in Green Hydrogen Production Systems. J. Electrochem. Soc. 2022, 169, 054503. [Google Scholar] [CrossRef]
- Fischer, M. Review of Hydrogen Production with Photovoltaic Electrolysis Systems. Int. J. Hydrogen Energy 1986, 11, 495–501. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Y.; Wang, L. Revisiting Solar Hydrogen Production through Photovoltaic-Electrocatalytic and Photoelectrochemical Water Splitting. Front. Energy 2021, 15, 596–599. [Google Scholar] [CrossRef]
- Khan, M.A.; Al-Shankiti, I.; Ziani, A.; Idriss, H. Demonstration of Green Hydrogen Production Using Solar Energy at 28% Efficiency and Evaluation of Its Economic Viability. Sustain. Energy Fuels 2021, 5, 1085–1094. [Google Scholar] [CrossRef]
- Özçelep, Y.; Bekdaş, G.; Apak, S. Meeting the Electricity Demand for the Heating of Greenhouses with Hydrogen: Solar Photovoltaic-Hydrogen-Heat Pump System Application in Turkey. Int. J. Hydrogen Energy 2023, 48, 2510–2517. [Google Scholar] [CrossRef]
- Pastuszak, J.; Węgierek, P. Photovoltaic Cell Generations and Current Research Directions for Their Development. Materials 2022, 15, 5542. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Ullah, H.; Bashiri, R.; Mohamed, N.M.; Sufian, S.; Ng, Y.H. Experimental and DFT Insights on Microflower g-C3N4/BiVO4 Photocatalyst for Enhanced Photoelectrochemical Hydrogen Generation from Lake Water. ACS Sustain. Chem. Eng. 2020, 8, 9393–9403. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Bacho, N.; Sufian, S. Recent Development of Graphitic Carbon Nitride-Based Photocatalyst for Environmental Pollution Remediation. In Nanocatalysts; IntechOpen: London, UK, 2018; Volume 1, pp. 1–15. [Google Scholar]
- Ahmad, I.; Zou, Y.; Yan, J.; Liu, Y.; Shukrullah, S.; Naz, M.Y.; Hussain, H.; Khan, W.Q.; Khalid, N.R. Semiconductor Photocatalysts: A Critical Review Highlighting the Various Strategies to Boost the Photocatalytic Performances for Diverse Applications. Adv. Colloid Interface Sci. 2023, 311, 102830. [Google Scholar] [CrossRef]
- Zhao, F.; Li, X.; Zuo, M.; Liang, Y.; Qin, P. Preparation of Photocatalysts Decorated by Carbon Quantum Dots (CQDs) and Their Applications: A Review. J. Environ. Chem. Eng. 2023, 11, 109487. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Sufian, S.; Hameed, B.H. Epigrammatic Progress and Perspective on the Photocatalytic Properties of BiVO4-Based Photocatalyst in Photocatalytic Water Treatment Technology: A Review. J. Mol. Liq. 2018, 268, 438–459. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Bashiri, R.; Mohamed, N.M.; Ng, Y.H.; Sufian, S. Tailoring the Morphological Structure of BiVO4 Photocatalyst for Enhanced Photoelectrochemical Solar Hydrogen Production from Natural Lake Water. Appl. Surf. Sci. 2020, 504, 144417. [Google Scholar] [CrossRef]
- Saafie, N.; Zulfiqar, M.; Samsudin, M.F.R.; Sufian, S. Current Scenario of MXene-Based Nanomaterials for Wastewater Remediation: A Review. Chemistry 2022, 4, 1576–1608. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A Review and Recent Developments in Photocatalytic Water-Splitting Using TiO2 for Hydrogen Production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A Review on BiVO4 photocatalyst: Activity Enhancement Methods for Solar Photocatalytic Applications. Appl. Catal. A Gen. 2018, 555, 47–74. [Google Scholar]
- Joy, J.; Mathew, J.; George, S.C. Nanomaterials for Photoelectrochemical Water Splitting—Review. Int. J. Hydrogen Energy 2018, 43, 4804–4817. [Google Scholar] [CrossRef]
- Peerakiatkhajohn, P.; Yun, J.-H.; Wang, S.; Wang, L. Review of Recent Progress in Unassisted Photoelectrochemical Water Splitting: From Material Modification to Configuration Design. J. Photon. Energy 2016, 7, 012006. [Google Scholar] [CrossRef]
- Mahmoud, B.; Rahman, A.; Ahmed, K. Methods and Strategies for Producing Porous Photocatalysts: Review. J. Solid State Chem. 2023, 320, 123834. [Google Scholar]
- Yang, M.; Ma, G.; Yang, H.; Zhan, X.; Yang, W.; Hou, H. Advanced Strategies for Promoting the Photocatalytic Performance of FeVO4 Based Photocatalysts: A Review of Recent Progress. J. Alloys Compd. 2023, 941, 168995. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Dai, K.; Zhang, J. Review on inorganic-organic S-scheme photocatalysts. J. Mater. Sci. Technol. 2023, 165, 187–218. [Google Scholar] [CrossRef]
- Saraswat, S.K.; Rodene, D.D.; Gupta, R.B. Recent Advancements in Semiconductor Materials for Photoelectrochemical Water Splitting for Hydrogen Production Using Visible Light. Renew. Sustain. Energy Rev. 2018, 89, 228–248. [Google Scholar] [CrossRef]
- Shaker, M.; Riahifar, R.; Li, Y. A Review on the Superb Contribution of Carbon and Graphene Quantum Dots to Electrochemical Capacitors’ Performance: Synthesis and Application. FlatChem 2020, 22, 100171. [Google Scholar] [CrossRef]
- Bedia, J.; Muelas-Ramos, V.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodríguez, J.J.; Belver, C. A Review on the Synthesis and Characterization of Metal Organic Frameworks for Photocatalytic Water Purification. Catalysts 2019, 9, 52. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- El Hammoumi, A.; Chtita, S.; Motahhir, S.; El Ghzizal, A. Solar PV Energy: From Material to Use, and the Most Commonly Used Techniques to Maximize the Power Output of PV Systems: A Focus on Solar Trackers and Floating Solar Panels. Energy Rep. 2022, 8, 11992–12010. [Google Scholar] [CrossRef]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical Devices for Solar Water Splitting-Materials and Challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef]
- Wu, H.; Tan, H.L.; Toe, C.Y.; Scott, J.; Wang, L.; Amal, R.; Ng, Y.H. Photocatalytic and Photoelectrochemical Systems: Similarities and Differences. Adv. Mater. 2020, 32, 1904717. [Google Scholar] [CrossRef]
- Chan, C.K.; Tüysüz, H.; Braun, A.; Ranjan, C.; La Mantia, F.; Miller, B.K.; Zhang, L.; Crozier, P.A.; Haber, J.A.; Gregoire, J.M.; et al. Advanced and in Situ Analytical Methods for Solar Fuel Materials; Springer: Berlin/Heidelberg, Germany, 2016; Volume 371, ISBN 9783319230986. [Google Scholar]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar]
- Saga, T. Advances in Crystalline Silicon Solar Cell Technology for Industrial Mass Production. NPG Asia Mater. 2010, 2, 96–102. [Google Scholar] [CrossRef]
- Kenu, E. Sarah A Review of Solar Photovoltaic Technologies. Int. J. Eng. Res. 2020, V9, 741–749. [Google Scholar]
- Huang, C.; Wang, L. Simulation Study on the Degradation Process of Photovoltaic Modules. Energy Convers. Manag. 2018, 165, 236–243. [Google Scholar] [CrossRef]
- Radue, C.; van Dyk, E.E. A Comparison of Degradation in Three Amorphous Silicon PV Module Technologies. Sol. Energy Mater. Sol. Cells 2010, 94, 617–622. [Google Scholar] [CrossRef]
- Mateo, C.; Hernández-Fenollosa, M.A.; Montero; Seguí-Chilet, S. Ageing and Seasonal Effects on Amorphous Silicon Photovoltaic Modules in a Mediterranean Climate. Renew. Energy 2022, 186, 74–88. [Google Scholar] [CrossRef]
- Mikolasek, M.; Kemeny, M.; Chymo, F.; Ondrejka, P.; Huran, J. Amorphous Silicon PEC-PV Hybrid Structure for Photo-Electrochemical Water Splitting. J. Electr. Eng. 2019, 70, 107–111. [Google Scholar] [CrossRef]
- Zeng, Q.; Guo, G.; Meng, Z.; Gao, L.; Meng, H.; Zhou, L. Improvement of Amorphous Silicon/Crystalline Silicon Heterojunction Solar Cells by Light-Thermal Processing. Mater. Sci. Semicond. Process. 2023, 154, 107192. [Google Scholar] [CrossRef]
- Bonnet, D. CdTe Thin-Film PV Modules, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; ISBN 9780123869647. [Google Scholar]
- Nasri, M.S.I.; Samsudin, M.F.R.; Tahir, A.A.; Sufian, S. Effect of MXene Loaded on g-C3N4 Photocatalyst for the Photocatalytic Degradation of Methylene Blue. Energies 2022, 15, 955. [Google Scholar] [CrossRef]
- Ahmad Madzlan, M.K.A.; Samsudin, M.F.R.; Maeght, F.; Goepp, C.; Sufian, S. Enhancement of g-C3N4 via Acid Treatment for the Degradation of Ciprofloxacin Antibiotic. Malaysian J. Microsc. 2020, 16, 105–114. [Google Scholar]
- Landman, A.; Halabi, R.; Dias, P.; Dotan, H.; Mehlmann, A.; Shter, G.E.; Halabi, M.; Naseraldeen, O.; Mendes, A.; Grader, G.S.; et al. Decoupled Photoelectrochemical Water Splitting System for Centralized Hydrogen Production. Joule 2020, 4, 448–471. [Google Scholar] [CrossRef]
- Welter, K.; Hamzelui, N.; Smirnov, V.; Becker, J.P.; Jaegermann, W.; Finger, F. Catalysts from Earth Abundant Materials in a Scalable, Stand-Alone Photovoltaic-Electrochemical Module for Solar Water Splitting. J. Mater. Chem. A 2018, 6, 15968–15976. [Google Scholar] [CrossRef]
- Luo, J.; Im, J.H.; Mayer, M.T.; Schreier, M.; Nazeeruddin, M.K.; Park, N.G.; Tilley, S.D.; Fan, H.J.; Grätzel, M. Water Photolysis at 12.3% Efficiency via Perovskite Photovoltaics and Earth-Abundant Catalysts. Science 2014, 345, 1593–1596. [Google Scholar] [CrossRef]
- Bashiri, R.; Mohamed, N.M.; Fai Kait, C.; Sufian, S.; Khatani, M. Enhanced Hydrogen Production over Incorporated Cu and Ni into Titania Photocatalyst in Glycerol-Based Photoelectrochemical Cell: Effect of Total Metal Loading and Calcination Temperature. Int. J. Hydrogen Energy 2017, 42, 9553–9566. [Google Scholar]
- Aravinthkumar, K.; Praveen, E.; Jacquline Regina Mary, A.; Raja Mohan, C. Investigation on SrTiO3 Nanoparticles as a Photocatalyst for Enhanced Photocatalytic Activity and Photovoltaic Applications. Inorg. Chem. Commun. 2022, 140, 109451. [Google Scholar] [CrossRef]
- Pan, S.; Li, R.; Zhang, Q.; Cui, C.; Wang, M.; Shi, B.; Wang, P.; Zhang, C.; Zhang, B.; Zhao, Y.; et al. An over 20% Solar-to-Hydrogen Efficiency System Comprising a Self-Reconstructed NiCoFe-Based Hydroxide Nanosheet Electrocatalyst and Monolithic Perovskite/Silicon Tandem Solar Cell. J. Mater. Chem. A 2021, 9, 14085–14092. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Sufian, S. Hybrid 2D/3D g-C3N4/BiVO4 Photocatalyst Decorated with RGO for Boosted Photoelectrocatalytic Hydrogen Production from Natural Lake Water and Photocatalytic Degradation of Antibiotics. J. Mol. Liq. 2020, 314, 113530. [Google Scholar] [CrossRef]
- Bashiri, R.; Mohamed, N.M.; Sufian, S.; Kait, C.F. Improved Photoelectrochemical Hydrogen Production over Decorated Titania with Copper and Nickel Oxides by Optimizing the Photoanode and Reaction Characteristics. Mater. Today Chem. 2020, 16, 100241. [Google Scholar] [CrossRef]
- Bora, L.V.; Mewada, R.K. Visible/Solar Light Active Photocatalysts for Organic Effluent Treatment: Fundamentals, Mechanisms and Parametric Review. Renew. Sustain. Energy Rev. 2017, 76, 1393–1421. [Google Scholar]
- Fajrina, N.; Tahir, M. A Critical Review in Strategies to Improve Photocatalytic Water Splitting towards Hydrogen Production. Int. J. Hydrogen Energy 2018, 44, 540–577. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Lee, H.; Park, I.S.; Bang, H.J.; Park, Y.K.; Kim, H.; Ha, H.H.; Kim, B.J.; Jung, S.C. Fabrication of Gd-La Codoped TiO2 Composite via a Liquid Phase Plasma Method and Its Application as Visible-Light Photocatalysts. Appl. Surf. Sci. 2019, 471, 893–899. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, Y.; Meng, X. Fabrication of TiO2 Nanofibers/MXene Ti3C2 Nanocomposites for Photocatalytic H2 Evolution by Electrostatic Self-Assembly. Appl. Surf. Sci. 2019, 496, 143647. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; Zeng, G.; Zhang, C.; Yu, H.; Wan, Q.; Yi, K.; Zhang, W.; Pang, H.; Liu, S.; et al. Efficient Photosynthesis of H2O2 via Two-Electron Oxygen Reduction Reaction by Defective g-C3N4 with Terminal Cyano Groups and Nitrogen Vacancies. Chem. Eng. J. 2023, 463, 142512. [Google Scholar] [CrossRef]
- Wang, J.; Pan, R.; Hao, Q.; Gao, Y.; Ye, J.; Wu, Y.; van Ree, T. Constructing Defect-Mediated CdS/g-C3N4 by an In-Situ Interlocking Strategy for Cocatalyst-Free Photocatalytic H2 Production. Appl. Surf. Sci. 2022, 599, 153875. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Lai, J.; Pan, R.; Fan, Y.; Wu, X.; Ou, M.; Zhu, Y.; Fu, L.; Shi, F.; et al. Two-Dimensional Graphitic Carbon Nitride/N-Doped Carbon with a Direct Z-Scheme Heterojunction for Photocatalytic Generation of Hydrogen. Nanoscale Adv. 2021, 3, 6580–6586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Zhao, J.; Wang, J.; Li, Z. g-C3N4/B Doped g-C3N4 Quantum Dots Heterojunction Photocatalysts for Hydrogen Evolution under Visible Light. Int. J. Hydrogen Energy 2018, 44, 618–628. [Google Scholar] [CrossRef]
- Cui, H.; Li, B.; Li, Z.; Li, X.; Xu, S. Z-Scheme Based CdS/CdWO4 Heterojunction Visible Light Photocatalyst for Dye Degradation and Hydrogen Evolution. Appl. Surf. Sci. 2018, 455, 831–840. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Zhu, W.; Meng, X. Boosted Photocatalytic Degradation of Rhodamine B Pollutants with Z-Scheme CdS/AgBr-RGO Nanocomposite. Appl. Surf. Sci. 2020, 502, 144275. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samsudin, M.F.R. Photovoltaic-Assisted Photo(electro)catalytic Hydrogen Production: A Review. Energies 2023, 16, 5743. https://doi.org/10.3390/en16155743
Samsudin MFR. Photovoltaic-Assisted Photo(electro)catalytic Hydrogen Production: A Review. Energies. 2023; 16(15):5743. https://doi.org/10.3390/en16155743
Chicago/Turabian StyleSamsudin, Mohamad Fakhrul Ridhwan. 2023. "Photovoltaic-Assisted Photo(electro)catalytic Hydrogen Production: A Review" Energies 16, no. 15: 5743. https://doi.org/10.3390/en16155743
APA StyleSamsudin, M. F. R. (2023). Photovoltaic-Assisted Photo(electro)catalytic Hydrogen Production: A Review. Energies, 16(15), 5743. https://doi.org/10.3390/en16155743






