Anaerobic Digestion of Food Waste—A Short Review
Abstract
1. Introduction
2. Key Parameters
2.1. Temperature
2.2. pH
2.3. Volatile Fatty Acids (VFAs) Concentrations
2.4. C/N Ratio
2.5. Organic Loading Rate (OLR)
2.6. Hydraulic Retention Time (HRT)
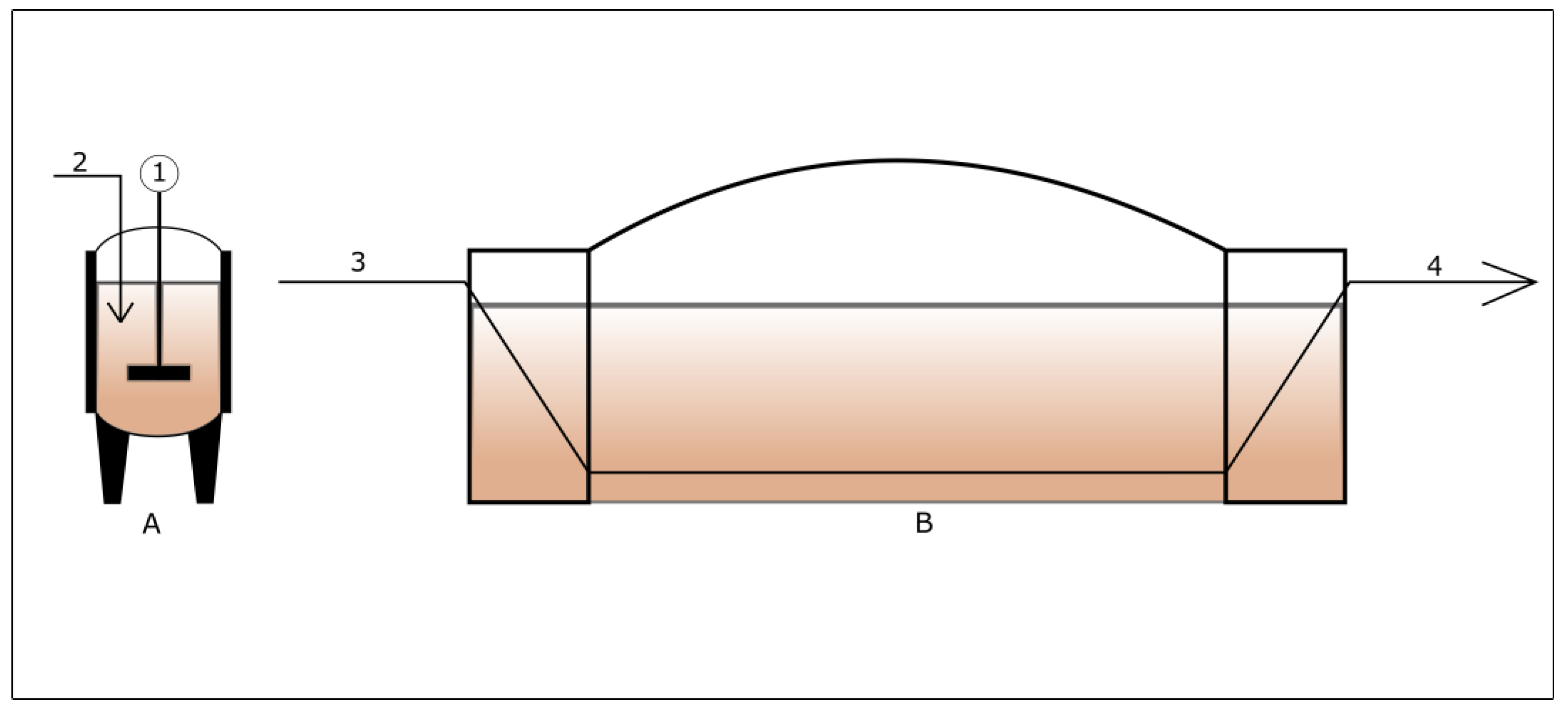
3. Bioreactor Configurations
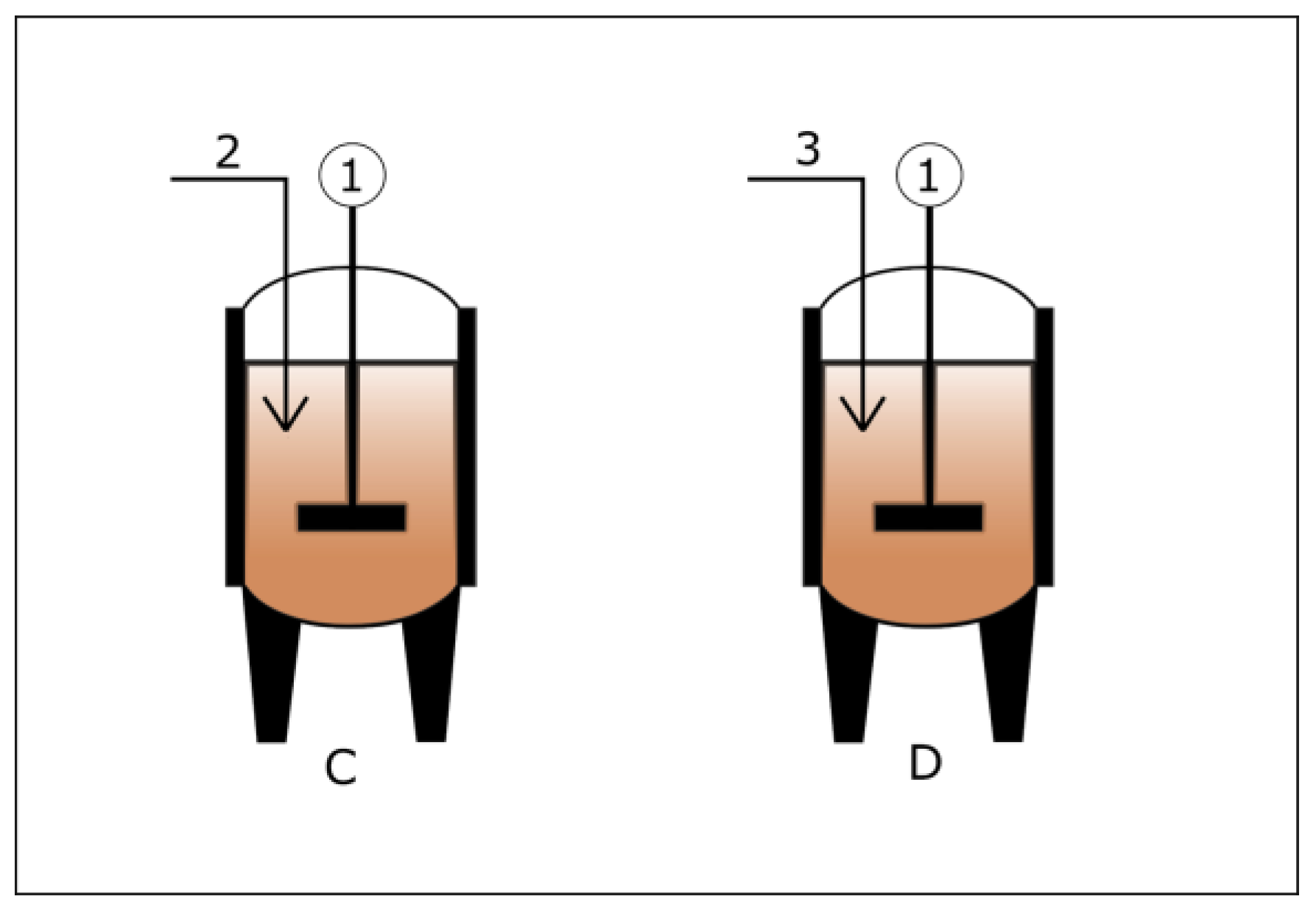
3.1. Wet and Dry Anaerobic Digestion System
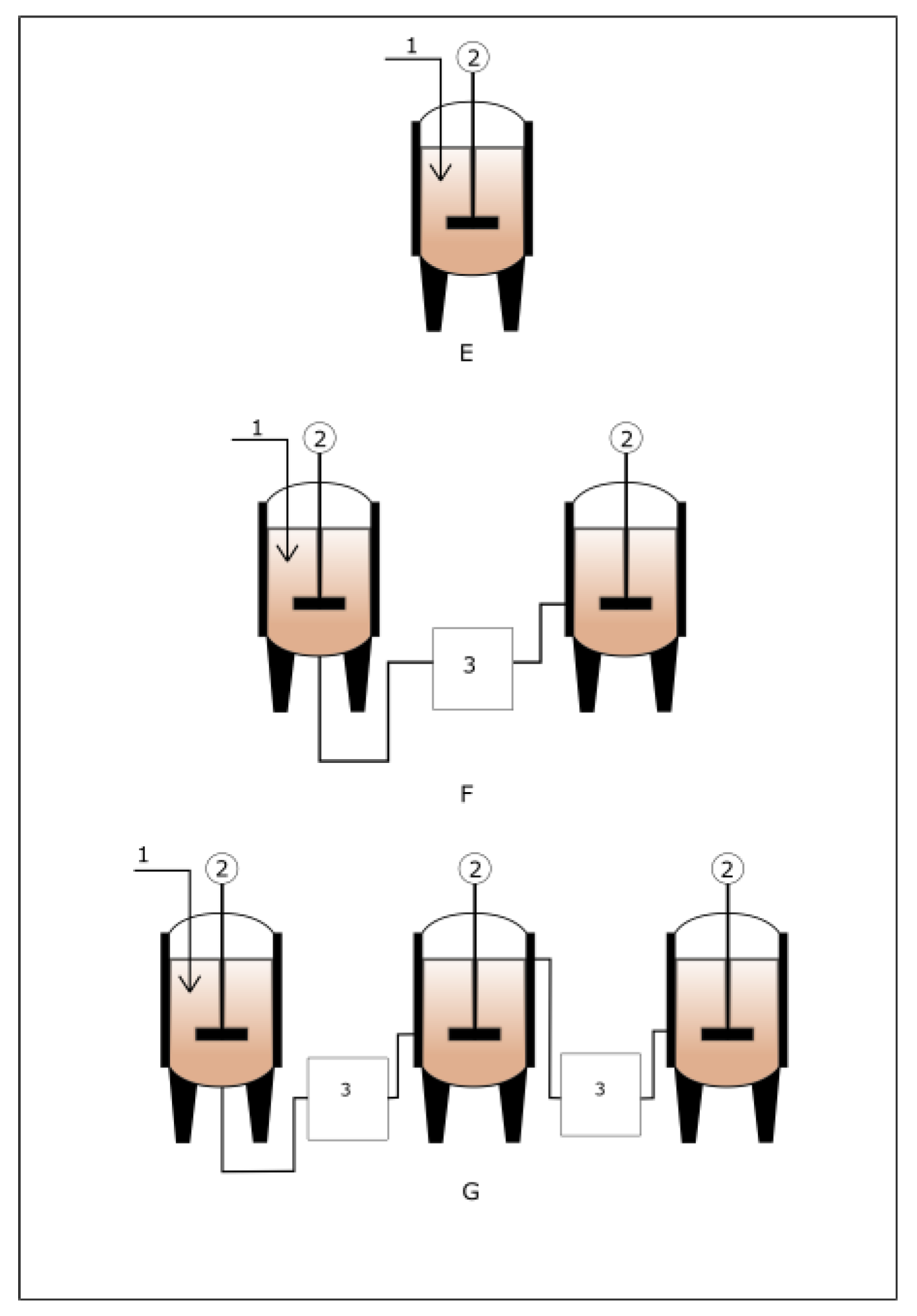
3.2. Operating Modes of an Anaerobic Digester
3.3. Single and Multi-Stage Anaerobic Digester
4. Inhibitors
5. Pretreatments
6. Anaerobic Co-Digestion
6.1. Co-Digestion of Food Waste with Plants, Plant Residues and Algae
6.2. Co-Digestion of Food Waste with Crop Residues
6.3. Co-Digestion of Food Waste with Animal Faeces, Wastewater and Sewage Sludge
7. Efficiency of Anaerobic Digestion of Food Waste
8. Development Perspectives and Recommendations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pilarska, A.A.; Pilarski, K.; Waliszewska, B.; Zborowska, M.; Witaszek, K.; Waliszewska, H.; Kolasiński, M.; Szwarc-Rzepka, K. Evaluation of bio-methane yields for high-energy organic waste and sewage sludge: A pilot-scale study for a wastewater treatment plant. Environ. Eng. Manag. J. 2019, 18, 2019–2030. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sust. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Iglińskin, B.; Buczkowski, R.; Cichosz, M. Biogas production in Poland—Current state, potential and perspectives. Renew. Sust. Energy Rev. 2015, 50, 686–695. [Google Scholar] [CrossRef]
- Kader, F.; Baky, A.H.; Khan, M.N.H.; Chowdhury, H.A. Production of biogas by anaerobic digestion of food waste and process simulation. Am. J. Mech. Eng. 2015, 3, 79–83. [Google Scholar]
- Kavacik, B.; Topaloglu, B. Biogas production from co-digestion of a mixture of cheese whey and dairy manure. Biomass Bioenerg. 2010, 34, 1321–1329. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Pilarski, K.; Wolna-Maruwka, A.; Boniecki, P.; Zaborowicz, M. Use of confectionery waste in biogas production by the anaerobic digestion process. Molecules 2019, 24, 37. [Google Scholar] [CrossRef] [PubMed]
- FAO; FAOSTAT. Food and Agriculture Organization of the United Nations, Rome, Italy. 2020. Available online: http://www.fao.org/faostat/en/#home (accessed on 27 July 2023).
- Kuo, J.; Dow, J. Biogas production from anaerobic digestion of food waste and relevant air quality implications. J. Air. Waste. Manag. 2017, 67, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Papargyropoulou, E.; Lozano, R.; Steinberger, J.K.; Wright, N.; Ujang, Z.B. The food waste hierarchy as a framework for the management of food surplus and food waste. J. Clean. Prod. 2014, 76, 106–115. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste—Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef]
- EU Platform on Food Losses and Food Waste; Activity report—First mandate (2016–2021); Publications Office of the European Union: Luxembourg, 2021.
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Pilarski, K.; Witaszek, K.; Waliszewska, H.; Zborowska, M.; Waliszewska, B.; Kolasiński, M.; Szwarc-Rzepka, K. Treatment of dairy waste by anaerobic digestion with sewage sludge. Ecol. Chem. Eng. 2016, 23, 99–115. [Google Scholar] [CrossRef]
- A/RES/70/1; Transforming our world: The 2030 Agenda for Sustainable Development. United Nations Organization: New York, NY, USA, 2015.
- European Commission. Next steps for a sustainable European future European action for sustainability. In Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Commitee of the Regions; COM(2016); European Commission: Strasbourg, France, 2016. [Google Scholar]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef]
- Paritosh, K.; Kushwaha, S.K.; Yadav, M.; Pareek, N.; Chawade, A.; Vivekanand, V. Food waste to energy: An overview of sustainable approaches for food waste management and nutrient recycling. Biomed Res. Int. 2017, 1, 2370927. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Pilarski, K.; Ryniecki, A.; Tomaszyk, K.; Dach, J.; Wolna-Maruwka, A. Utilization of vegetable dumplings waste from industrial production by anaerobic digestion. Int. Agrophys. 2017, 31, 93–102. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energ. 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Wolna-Maruwka, A.; Pilarski, K.; Janczak, D.; Przybył, K.; Gawrysiak-Witulska, M. The use of lignin as a microbial carrier in the co-digestion of cheese and wafer waste. Polymers 2019, 11, 2073. [Google Scholar] [CrossRef] [PubMed]
- Razaviarani, V.; Buchanan, I.D.; Malik, S.; Katalambula, H. Pilot-scale anaerobic co-digestion of municipal wastewater sludge with restaurant grease trap waste. J. Environ. Manag. 2013, 123, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Bresa, P.; Beily, M.E.; Young, B.J.; Gasulla, J.; Butti, M.; Crespo, D.; Candal, R.; Komilis, D. Performance of semi-continuous anaerobic co-digestion of poultry manure with fruit and vegetable waste and analysis of digestate quality: A bench scale study. Waste Manag. 2018, 82, 276–284. [Google Scholar] [CrossRef]
- De Vrieze, J.; Hennebel, T.; Van den Brande, J.; Bilad, R.M.; Bruton, T.A.; Vankelecom, I.F.J.; Verstraete, W.; Boon, N. Anaerobic digestion of molasses by means of a vibrating and non-vibrating submerged anaerobic membrane bioreactor. Biomass Bioenerg. 2014, 68, 95–105. [Google Scholar] [CrossRef]
- Lunghi, P.; Burzacca, R. Energy recovery from industrial waste of a confectionery plant by means of BIGFC plant. Energy 2004, 29, 2601–2617. [Google Scholar] [CrossRef]
- Rusín, J.; Kašáková, K.; Chamrádová, K. Anaerobic digestion of waste wafer material from the confectionery production. Energy 2015, 85, 194–199. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Pilarski, K.; Adamski, M.; Zaborowicz, M.; Dorota Cais-Sokolińska, D.; Wolna-Maruwka, A.; Niewiadomska, A. Eco-friendly and effective diatomaceous earth/peat (DEP) microbial carriers in the anaerobic biodegradation of food waste products. Energies 2022, 15, 3442. [Google Scholar] [CrossRef]
- Pilarski, K.; Pilarska, A.A.; Boniecki, P.; Niedbała, G.; Durczak, K.; Witaszek, K.; Mioduszewska, N.; Kowalik, I. The efficiency of industrial and laboratory anaerobic digesters of organic substrates: The use of the Biochemical Methane Potential Correction Coefficient. Energies 2020, 13, 1280. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Ye, M.; Liu, J.; Ma, C.; Li, Y.-Y.; Zou, L.; Qian, G.; Xu, Z.P. Improving the stability and efficiency of anaerobic digestion of food waste using additives: A critical review. J. Clean. Prod. 2018, 192, 316–326. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jay, J.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Fdz-Polanco, M. Anaerobic co-digestion of sewage sludge and grease trap: Assessment of enzyme addition. Process Biochem. 2013, 48, 936–940. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Natural carriers in bioremediation: A review. Electron. J. Biotechnol. 2016, 23, 28–36. [Google Scholar]
- Gong, W.; Ran, Z.; Ye, F.; Zhao, G. Lignin from bamboo shoot shells as an activator and novel immobilizing support for α-amylase. Food Chem. 2017, 228, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Ortner, M.; Rameder, M.; Rachbauer, L.; Bochmann, G.; Fuchs, W. Bioavailability of essential trace elements and their impact on anaerobic digestion of slaughterhouse waste. Biochem. Eng. J. 2015, 99, 107–113. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Pilarski, K.; Wolna-Maruwka, A. Cell immobilization on lignin–polyvinylpyrrolidone material used for anaerobic digestion of waste wafers and sewage sludge. Environ. Eng. Sci. 2019, 36, 478–490. [Google Scholar] [CrossRef]
- Chew, K.R.; Leong, H.Y.; Khoo, K.S.; Vo, D.V.N.; Anjum, H.; Chang, C.K.; Show, P.L. Effects of anaerobic digestion of food waste on biogas production and environmental impacts: A review. Environ. Chem. Lett. 2021, 19, 2921–2939. [Google Scholar] [CrossRef]
- Chiu, S.L.H.; Lo, I.M.C. Reviewing the anaerobic digestion and co-digestion process of food waste from the perspectives on biogas production performance and environmental impacts. Environ. Sci. Pollut. Res. 2016, 23, 24435–24450. [Google Scholar] [CrossRef]
- Lou, X.F.; Nair, J.; Ho, G. Field performance of small scale anaerobic digesters treating food waste. Energy Sustain. Dev. 2012, 16, 509–514. [Google Scholar] [CrossRef]
- Muñoz, P.; Cordero, C.; Tapia, X.; Muñoz, L.; Candia, O. Assessment of anaerobic digestion of food waste at psychrophilic conditions and effluent post-treatment by microalgae cultivation. Clean. Techn. Environ. Policy 2020, 22, 725–733. [Google Scholar] [CrossRef]
- Martí-Herrero, J.; Soria-Castellón, G.; Diaz-de-Basurto, A.; Alvarez, R.; Chemisana, D. Biogas from a full scale digester operated in psychrophilic conditions and fed only with fruit and vegetable waste. Renew. Energy 2018, 133, 676–684. [Google Scholar] [CrossRef]
- Park, J.; Lee, B.; Shin, W.; Jo, S.; Jun, H. Psychrophilic methanogenesis of food waste in a bio-electrochemical anaerobic digester with rotating impeller electrode. J. Clean. Prod. 2018, 188, 556–567. [Google Scholar] [CrossRef]
- Guo, X.; Wang, C.; Sun, F.; Zhu, W.; Wu, W. A comparison of microbial characteristics between the thermophilic and mesophilic anaerobic digesters exposed to elevated food waste loadings. Bioresour. Technol. 2014, 152, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Van Lier, J.B.; Rebac, S.; Lettinga, G. High-rate anaerobic wastewater treatment under psychrophilic and thermophilic conditions. Water Sci. Technol. 1997, 35, 199–206. [Google Scholar] [CrossRef]
- Kumar, G.; Sivagurunathan, P.; Park, J.-H.; Kim, S.-H. Anaerobic digestion of food waste to methane at various organic loading rates (OLRs) and hydraulic retention times (HRTs): Thermophilic vs. mesophilic regimes. Environ. Eng. Res. 2016, 21, 69–73. [Google Scholar] [CrossRef]
- Pilarski, K.; Pilarska, A.A.; Boniecki, P.; Niedbała, G.; Witaszek, K.; Piekutowska, M.; Idzior-Haufa, M.; Wawrzyniak, A. Degree of Biomass Conversion in the Integrated Production of Bioethanol and Biogas. Energies 2021, 14, 7763. [Google Scholar] [CrossRef]
- Hwang, M.H.; Jang, N.J.; Hyun, S.H.; Kim, I.S. Anaerobic biohydrogen production from ethanol fermentation: The role of pH. J. Biotechnol. 2004, 111, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Montańés, R.; Pérez, M.; Solera, R. Anaerobic mesophilic co digestion of sewage sludge and sugar beet pulp lixiviation in batch reactors: Effect of pH control. Chem. Eng. J. 2014, 255, 492–499. [Google Scholar] [CrossRef]
- Eastman, J.A.; Ferguson, J.F. Solubilisation of particulate organic carbon during the acid phase of AD. J. Water Pollut. Control Fed. 1981, 53, 352–366. [Google Scholar]
- Ahring, B.; Sandberg, M.; Angelidaki, I. Volatile fatty acids as indicators of process imbalance in anaerobic digestors. Appl. Microbiol. Biotechnol. 1995, 43, 559–565. [Google Scholar] [CrossRef]
- Qamaruz-Zaman, N.; Milke, M.W. VFA and ammonia from residential food waste as indicators of odor potential. Waste Manag. 2012, 32, 2426–2430. [Google Scholar] [CrossRef]
- Annachhatre, A.P. Dry Anaerobic Digestion of Municipal Solid Waste and Digestate Management Strategies. Ph.D. Thesis, Asian Institute of Technology, Pathumthani, Thailand, 2012. [Google Scholar]
- Kondusamy, D.; Kalamdhad, A.S. Pre-Treatment and anaerobic digestion of food waste for high rate methane production—A review. J. Environ. Chem. Eng. 2014, 2, 1821–1830. [Google Scholar] [CrossRef]
- Xue, S.; Wang, Y.; Lyu, X.; Zhao, N.; Song, J.; Wang, X.; Yang, G. Interactive effects of carbohydrate, lipid, protein composition and carbon/nitrogen ratio on biogas production of different food wastes. Bioresour. Technol. 2020, 312, 123566. [Google Scholar] [CrossRef]
- Mattocks, R. Understanding Biogás Generation. Technical Paper, No. 4; Volunteers in Technical Assistance (VITA): Arlington, VA, USA, 1984; p. 13. [Google Scholar]
- Nagao, N.; Tajima, N.; Kawai, M.; Niwa, C.; Kurosawa, N.; Matsuyama, T.; Yusoff, F.M.; Toda, T. Maximum organic loading rate for the single-stage wet anaerobic digestion of food waste. Bioresour. Technol. 2012, 118, 210–218. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Sheng, L.; Liu, X.; Zong, M.; Yao, D. Anaerobic digestion technology for methane production using deer manure under different experimental conditions. Energies 2019, 12, 1819. [Google Scholar] [CrossRef]
- Li, D.; Liu, S.; Mi, L.; Li, Z.; Yuan, Y.; Yan, Z.; Liu, X. Effects of feedstock ratio and organic loading rate on the anaerobic mesophilic co-digestion of rice straw and pig manure. Bioresour Technol. 2015, 187, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2011, 16, 1462–1476. [Google Scholar] [CrossRef]
- Srisowmeya, G.; Chakravarthy, M.; Nandhini Devi, G. Critical considerations in two-stage anaerobic digestion of food waste—A review. Renew. Sustain. Energy Rev. 2020, 119, 109587. [Google Scholar] [CrossRef]
- Alepu, O.E.; Li, Z.; Ikhumhen, H.O.; Kalakodio, L.; Wang, K.; Segun, G.A. Effect of hydraulic retention time on anaerobic digestion of Xiao Jiahe municipal sludge. Int. J. Waste Resour. 2016, 6, 3. [Google Scholar]
- Tchobanoglous, G.; Theisen, H.; Vigil, S.A. Integrated Solid Waste Management: Engineering Principles and Management Issue; McGraw-Hill: New York, NY, USA, 1993. [Google Scholar]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.W.; Fernández, O.; Bajón-Fernández, Y. Dry anaerobic digestion of organic waste: A review of operational parameters and their impact on process performance. Bioresour Technol. 2020, 299, 122681. [Google Scholar] [CrossRef]
- Angelonidi, E.; Smith, S.R. A comparison of wet and dry anaerobic digestion processes for the treatment of municipal solid waste and food waste. Water Environ. J. 2015, 29, 549–557. [Google Scholar] [CrossRef]
- Kothari, R.; Pandey, A.K.; Kumar, S.; Tyagi, V.V.; Tyagi, S.K. Different aspects of dry anaerobic digestion for bio-energy: An overview. Renew. Sust. Energ. Rev. 2014, 39, 174–195. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Suja, F.B.; Zain, S.M.; Pramanik, B.K. The anaerobic digestion process of biogas production from food waste: Prospects and constraints. Bioresour. Technol. Rep. 2019, 8, 100310. [Google Scholar] [CrossRef]
- Zhang, E.; Li, J.; Zhang, K.; Wang, F.; Yang, H.; Zhi, S.; Liu, G. Anaerobic digestion performance of sweet potato vine and animal manure under wet, semi-dry, and dry conditions. AMB Expr 2018, 8, 45. [Google Scholar] [CrossRef]
- Paladino, O. Data Driven Modelling and Control Strategies to Improve Biogas Quality and Production from High Solids Anaerobic Digestion: A Mini Review. Sustainability 2022, 14, 16467. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Biogas generation through anaerobic digestion process-an overview. Res. J. Chem. Environ. 2014, 18, 80–93. [Google Scholar]
- Van, D.P.; Fujiwara, T.; Tho, B.L.; Toan, P.P.S.; Minh, G.H. A review of anaerobic digestion systems for biodegradable waste: Configurations, operating parameters, and current trends. Environ. Eng. Res. 2020, 25, 1–17. [Google Scholar] [CrossRef]
- Salsali, H.; Parker, W.; Sattar, S. Influence of staged operation of mesophilic anaerobic digestion on microbial reduction. Proc. Water Environ. Fed. 2005, 2005, 4571–4586. [Google Scholar] [CrossRef]
- Pilarska, A.; Linda, I.; Wysokowski, M.; Paukszta, D.; Jesionowski, T. Synthesis of Mg(OH)2 from manesium salts and NH4OH by direct functionalisation with poly(ethylene glycols). Physicochem. Probl. Miner. Process. 2012, 48, 631–643. [Google Scholar]
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.J.; Longhurst, P. Ammonia inhibition and toxicity in anaerobic digestion: A critical review. J. Water Proc. Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Haiping, Y.; Nanwen, Z. Progress in inhibition mechanisms and process control of intermediates and by-products in sewage sludge anaerobic digestion. Renew. Sustain. Energy Rev. 2016, 58, 429–438. [Google Scholar]
- Chen, H.; Wang, W.; Xue, L.; Chen, C.; Liu, G.; Zhang, R. Effects of ammonia on anaerobic digestion of food waste: Process performance and microbial community. Energ. Fuel. 2016, 30, 5749–5757. [Google Scholar] [CrossRef]
- Dasa, K.T.; Westman, S.Y.; Millati, R.; Cahyanto, M.N.; Taherzadeh, M.J.; Niklasson, C. Inhibitory Effect of Long-Chain Fatty Acids on Biogas Production and the Protective Effect of Membrane Bioreactor. BioMed Res. Int. 2016, 2016, 7263974. [Google Scholar]
- Tian, G.; Xi, J.; Yeung, M.; Ren, G. Characteristics and mechanisms of H2S production in anaerobic digestion of food waste. Sci. Total Environ. 2020, 724, 137977. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Tabatabaei, M.; Aghbashlo, M. Biogas production from food wastes: A review on recent developments and future perspectives. Bioresour. Technol. Rep. 2019, 7, 100202. [Google Scholar] [CrossRef]
- Izumi, K.; Okishio, Y.K.; Nagao, N.; Niwa, C.; Yamamoto, S.; Toda, T. Effects of particle size on anaerobic digestion of food waste. Int. Biodeterior. Biogdegrad. 2010, 64, 601–608. [Google Scholar] [CrossRef]
- Witaszek, K.; Herkowiak, M.; Pilarska, A.A.; Czekała, W. Methods of handling the cup plant (Silphium perfoliatum L.) for Energy production. Energies 2022, 15, 1897. [Google Scholar] [CrossRef]
- Marañón, E.; Castrillón, L.; Quiroga, C.; Fernández-Nava, Y.; Gómez, L.; García, M.M. Co-digestion of cattle manure with food waste and sludge to increase biogas production. Waste Manag. 2012, 32, 1821–1825. [Google Scholar] [CrossRef]
- Witaszek, K.; Pilarska, A.A.; Pilarski, K. Selected methods of pre-treatment of plant materials used for biogas production. Econ. Environ. 2015, 2, 138–152. [Google Scholar]
- Shahriari, H.; Warith, M.; Hamoda, M.; Kennedy, K. Evaluation of single vs. staged mesophilic anaerobic digestion of kitchen waste with and without microwave pretreatment. J. Environ. Manag. 2013, 125, 74–84. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Zhang, L.; Zhou, Y.L.; Chen, J.C.; Liang, Y.M.; Wei, L. Pilot-scale operation of enhanced anaerobic digestion of nutrient-deficient municipal sludge by ultrasonic pretreatment and co-digestion of kitchen garbage. J. Environ. Chem. Eng. 2013, 1, 73–78. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Ma, J.; Duong, T.H.; Smits, M.; Verstraete, W.; Carballa, M. Enhanced biomethanation of kitchen waste by different pre-treatments. Bioresour. Technol. 2011, 102, 592–599. [Google Scholar] [CrossRef]
- Gonzales, H.B.; Takyu, K.; Sakashita, H.; Nakano, Y.; Nishijima, W.; Okada, M. Biological solubilization and mineralization as novel approach for the pretreatment of food waste. Chemosphere 2005, 58, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Vavouraki, A.I.; Angelis, E.M.; Kornaros, M. Optimization of thermo-chemical hydrolysis of kitchen wastes. Waste Manag. 2013, 33, 740–745. [Google Scholar] [CrossRef]
- Lim, J.W.; Wang, J.Y. Enhanced hydrolysis and methane yield by applying microaeration pretreatment to the anaerobic co-digestion of brown water and food waste. Waste Manag. 2013, 33, 813–819. [Google Scholar] [CrossRef]
- Palmarola-Adrados, B.; Galbe, M.; Zacchi, G. Combined steam pretreatment and enzymatic hydrolysis of starch-free wheat fibers. In Proceedings of the Twenty-Fifth Symposium on Biotechnology for Fuels and Chemicals, Breckenridge, CO, USA, 4–7 May 2003; Humana Press: Totowa, NJ, USA, 2004; Volume 113–116, pp. 989–1002. [Google Scholar]
- Taherzadeh, M.J.; Karimi, K. Acid-based hydrolysis processes for ethanol from lignocellulosic materials: A review. Bioresour. Technol. 2007, 2, 472–499. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Bordeleau, E.L.; Droste, R.L. Comprehensive review and compilation of pretreatments for mesophilic and thermophilic anaerobic digestion. Water Sci. Technol. 2011, 63, 291–296. [Google Scholar] [CrossRef]
- Tongco, J.V.; Kim, S.; Oh, B.R.; Sun-Yeon Heo, S.Y.; Lee, J.; Hwang, S. Enhancement of hydrolysis and biogas production of primary sludge by use of mixtures of protease and lipase. Biotechnol. Bioproc. Eng. 2020, 25, 132–140. [Google Scholar] [CrossRef]
- Moon, H.C.; Song, I.S. Enzymatic hydrolysis of food waste and methane production using UASB bioreactor. Int. J. Green Energy 2011, 8, 361–371. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Qiu, Y.; Ren, L.; Jiang, B. Microbial characteristics in anaerobic digestion process of food waste for methane production—A review. Biores. Technol. 2018, 248, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mehariya, S.; Patel, A.K.; Obulisamy, P.K.; Punniyakotti, E.; Wong, J.W.C. Co-digestion of food waste and sewage sludge for methane production: Current status and perspective. Bioresour. Technol. 2018, 265, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Jiménez, L.M.; Pérez-Vidal, A.; Torres-Lozada, P. Research trends and strategies for the improvement of anaerobic digestion of food waste in psychrophilic temperatures conditions. Heliyon 2022, 8, e11174. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Wicks, M.; Li, Y.; Keener, H. Anaerobic digestion of food waste for bioenergy production. Adv. Food Waste Bioenergy Prod. 2018, 1, 1–8. [Google Scholar]
- Morales-Polo, C.; Cledera-Castro, M.D.M.; Moratilla Soria, B.Y. Reviewing the Anaerobic Digestion of Food Waste: From Waste Generation and Anaerobic Process to Its Perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef]
- Chen, G.; Liu, G.; Yan, B.; Shan, R.; Wang, J.; Li, T.; Xu, W. Experimental study of co-digestion of food waste and tall fescue for biogas production. Renew. Energy 2016, 88, 273–279. [Google Scholar] [CrossRef]
- Oduor, W.W.; Wandera, S.M.; Murunga, S.I.; Raude, J.M. Enhancement of anaerobic digestion by co-digesting food waste and water hyacinth in improving treatment of organic waste and bio-methane recovery. Heliyon 2022, 8, e10580. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.O.; Astals, S.; Passos, F. Anaerobic co-digestion of food waste and microalgae in an integrated treatment plant. J. Chem. Technol. Biotechnol. 2021, 97, 1545–1554. [Google Scholar] [CrossRef]
- Zhao, M.X.; Ruan, W.Q. Biogas performance from co-digestion of Taihu algae and kitchen wastes. Energy Convers. Manag. 2013, 75, 21–24. [Google Scholar] [CrossRef]
- Zhang, L.; Loh, K.-C.; Zhang, J. Food waste enhanced anaerobic digestion of biologically pretreated yard waste: Analysis of cellulose crystallinity and microbial communities. Waste Manag. 2018, 79, 109–119. [Google Scholar] [CrossRef]
- Chen, X.; Yan, W.; Sheng, K.; Sanati, M. Comparison of high-solids to liquid anaerobic co-digestion of food waste and green waste. Bioresour. Technol. 2014, 154, 215–221. [Google Scholar] [CrossRef]
- Yong, Z.; Dong, Y.; Zhang, X.; Tan, T. Anaerobic co-digestion of food waste and straw for biogas production. Renew. Energy 2015, 78, 527–530. [Google Scholar] [CrossRef]
- Pei, Z.; Liu, J.; Shi, F.; Wang, S.; Gao, Y.; Zhang, D. High-solid Anaerobic Co-digestion of Food Waste and Rice Straw for Biogas Production. J. Northeast. Agric. Univ. 2014, 21, 61–66. [Google Scholar]
- Owamah, H.I.; Izinyon, O.C. The Effect of Organic Loading Rates (OLRs) on the Performances of Food Wastes and Maize Husks Anaerobic Co-Digestion in Continuous Mode. Sustain. Energy Technol. Assess. 2015, 11, 71–76. [Google Scholar] [CrossRef][Green Version]
- Haider, M.R.; Zeshan; Yousaf, S.; Malik, R.N.; Visvanathan, C. Effect of mixing ratio of food waste and rice husk co-digestion and substrate to inoculum ratio on biogas production. Bioresour. Technol. 2015, 190, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Shahazi, R.; Nova, S.N.B.; Uddin, M.R.; Hossain, M.S.; Yousuf, A. Biogas production from anaerobic co-digestion using kitchen waste and poultry manure as substrate—Part 1: Substrate ratio and effect of temperature. Biomass Convers. Biorefin. 2021, 13, 6635–6645. [Google Scholar] [CrossRef] [PubMed]
- Chuenchart, W.; Logan, M.; Leelayouthayotin, C.; Visvanathan, C. Enhancement of food waste thermophilic anaerobic digestion through synergistic effect with chicken manure. Biomass Bioenergy 2020, 136, 105541. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, G.; Peng, L.; Su, H.; Tan, T. The anaerobic co-digestion of food waste and cattle manure. Bioresour. Technol. 2013, 129, 170–176. [Google Scholar] [CrossRef]
- Agyeman, F.O.; Tao, W. Anaerobic co-digestion of food waste and dairy manure: Effects of food waste particle size and organic loading rate. J. Environ. Manag. 2014, 133, 268–274. [Google Scholar] [CrossRef]
- Cour Jansen, J.; Gruvberger, C.; Hanner, N.; Aspegren, H.; Svärd, A. Digestion of sludge and organic waste in the sustainability concept for Malmö, Sweden. Water Sci. Technol. 2004, 49, 163–169. [Google Scholar] [CrossRef]
- Kim, H.-W.; Han, S.-K.; Shin, H.-S. The optimisation of food waste addition as a co-substrate in anaerobic digestion of sewage sludge. Waste Manag. Res. 2003, 21, 515–526. [Google Scholar] [CrossRef]
- Heo, N.H.; Park, S.C.; Kang, H. Effects of Mixture Ratio and Hydraulic Retention Time on Single-Stage Anaerobic Co-digestion of Food Waste and Waste Activated Sludge. J. Environ. Sci. Health Part A 2004, 39, 1739–1756. [Google Scholar] [CrossRef]
- Pilarska, A.A. Anaerobic co-Digestion of waste wafers from confectionery production with sewage sludge. Pol. J. Environ. Stud. 2018, 27, 237–245. [Google Scholar] [CrossRef]
- Negri, C.; Ricci, M.; Zilio, M.; D’Imporzano, G.; Qiao, W.; Dong, R.; Adani, F. Anaerobic digestion of food waste for bio-energy production in China and Southeast Asia: A review. Renew. Sustain. Energy Rev. 2020, 133, 110138. [Google Scholar] [CrossRef]
- Uddin, M.N.; Siddiki, S.Y.A.; Mofijur, M.; Djavanroodi, F.; Hazrat, M.A.; Show, P.L.; Ahmed, S.F.; Chu, Y.-M. Prospects of Bioenergy Production from Organic Waste Using Anaerobic Digestion Technology: A Mini Review. Front. Energy Res. 2021, 9, 627093. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Liu, G.; Chen, C.; He, Y.; Liu, X. Comparison of methane production potential, biodegradability, and kinetics of different organic substrates. Bioresour. Technol. 2013, 149, 565–569. [Google Scholar] [CrossRef]
- Zhang, W.; Lang, Q.; Fang, M.; Li, X.; Bah, H.; Dong, H.; Dong, R. Combinedeffect of crude fat content and initial substrate concentration on batch anaerobic digestion characteristics of food waste. Bioresour. Technol. 2017, 232, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Khadka, A.; Parajuli, A.; Dangol, S.; Thapa, B.; Sapkota, L.; Carmona-Martínez, A.A.; Ghimire, A. Effect of the Substrate to Inoculum Ratios on the Kinetics of Biogas Production during the Mesophilic Anaerobic Digestion of Food Waste. Energies 2022, 15, 834. [Google Scholar] [CrossRef]
- Dennehy, C.; Lawlor, P.G.; Croize, T.; Jiang, Y.; Morrison, L.; Gardiner, G.E.; Zhan, X. Synergism and effect of high initial volatile fatty acid concentrations during food waste and pig manure anaerobic co-digestion. Waste Manag. 2016, 56, 173–180. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustacas, K.; Mikulewicz, M. Valorisation of agri-food waste to fertilisers is a challenge in implementing the circular economy concept in practice. Environ. Pollut. 2022, 312, 119906. [Google Scholar] [CrossRef]
- Marks, S.; Dach, J.; Jesus, F.; Morales, F.; Mazurkiewicz, J.; Pochwatka, P.; Gierz, Ł. New trends in substrates and biogas systems in Poland. J. Ecol. Eng. 2020, 21, 19–25. [Google Scholar] [CrossRef]

| Substrates | Conditions | Results | References |
|---|---|---|---|
| kitchen waste |
| specific biogas yield: 12.6 mL/gTSd | [104] |
| kitchen waste |
| methane yield: 541 mL/gVS | [121] |
| student dormitories kitchen waste |
| the rate of cumulative biogas: 312 ± 9 mL | [111] |
| student dormitories kitchen waste |
| the rate of cumulative biogas: 532 ± 17 mL | [111] |
| canteen food waste |
| biogas yield: 357.85 mL/gVS | [102] |
| canteen food waste |
| methane yield: 326.4 mL/gVS | [106] |
| canteen food waste |
| biogas yield: 11.10 L/gVS | [109] |
| canteen food waste |
| biogas production: 621 mL/gVS methane yield: 410 mL/gVS | [113] |
| restaurant food waste |
| average daily biogas: 224 L/d methane production: 120 L/d | [112] |
| restaurant food waste |
| methane yield: 573 mL/gVS | [122] |
| leftovers of cooked foods, meats, rice, breads, noodles and vegetables |
| biogas production: 0.49 m3/kgVS methane production yield: 0.281 m3/kgVS | [107] |
| vegetables, fruits, rice, noodles, meat, fish and eggs |
| biogas yield: 674.40 NmL/gVS | [123] |
| rice, beans and meat |
| methane yield: 241 mL/gVS | [103] |
| lettuce, carrots, and tomato |
| methane yield: 35 mL/gVS | [103] |
| orange peel, banana peel and papaya peel |
| methane yield: 44.8 mL/gVS | [103] |
| fruit and vegetable waste |
| methane yield: 342 mL/gVS | [121] |
| Co-Substrates | Conditions | Results | References |
|---|---|---|---|
| tall fescue |
| biogas yield: 406 mL/gVS methane yield: 296.01 mL/gVS | [101] |
| water hyacinth |
| biogas yield: 616.01 mL/gVS | [102] |
| microalgal biomass |
| methane yield: 514 mL/gVS | [103] |
| Taihu algae |
| specific biogas yield: 14.9 mL/gTSd | [104] |
| yard waste |
| cumulative methane yield: 131 mL/gVS | [105] |
| green waste |
| biogas yield: 390.2 mL/gVS methane yield: 272.1 mL/gVS | [106] |
| straw of maize, sorgos and wheat |
| biogas production: 0.58 m3/kgVS methane production yield: 0.392 m3/kgVS | [107] |
| rice straw |
| methane yield: 60.55 mL/gVSd | [108] |
| maize husk |
| biogas yield: 28.92 L/gVS | [19] |
| rice husk |
| specific biogas yield: 584 L/kgVS | [20] |
| poultry manure |
| the rate of cumulative biogas: 920 ± 11 mL | [109] |
| cattle manure |
| biogas production: 570 mL/gVS methane yield: 388 mL/gVS | [113] |
| pig manure |
| specific methane yield: 521 mL/gVS | [124] |
| sewage sludge |
| methane yield: 326 Nm3/tonVSin | [115] |
| sewage sludge |
| methane yield: 0.215 L/gVS | [116] |
| sewage sludge |
| methane yield: 0.280 L/gVS | [116] |
| waste activated sludge |
| specific methane production: 0.346 m3/kgVS | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilarska, A.A.; Kulupa, T.; Kubiak, A.; Wolna-Maruwka, A.; Pilarski, K.; Niewiadomska, A. Anaerobic Digestion of Food Waste—A Short Review. Energies 2023, 16, 5742. https://doi.org/10.3390/en16155742
Pilarska AA, Kulupa T, Kubiak A, Wolna-Maruwka A, Pilarski K, Niewiadomska A. Anaerobic Digestion of Food Waste—A Short Review. Energies. 2023; 16(15):5742. https://doi.org/10.3390/en16155742
Chicago/Turabian StylePilarska, Agnieszka A., Tomasz Kulupa, Adrianna Kubiak, Agnieszka Wolna-Maruwka, Krzysztof Pilarski, and Alicja Niewiadomska. 2023. "Anaerobic Digestion of Food Waste—A Short Review" Energies 16, no. 15: 5742. https://doi.org/10.3390/en16155742
APA StylePilarska, A. A., Kulupa, T., Kubiak, A., Wolna-Maruwka, A., Pilarski, K., & Niewiadomska, A. (2023). Anaerobic Digestion of Food Waste—A Short Review. Energies, 16(15), 5742. https://doi.org/10.3390/en16155742









