Improved High Temperature Thermoelectric Properties in Misfit Ca3Co4O9 by Thermal Annealing
Abstract
1. Introduction
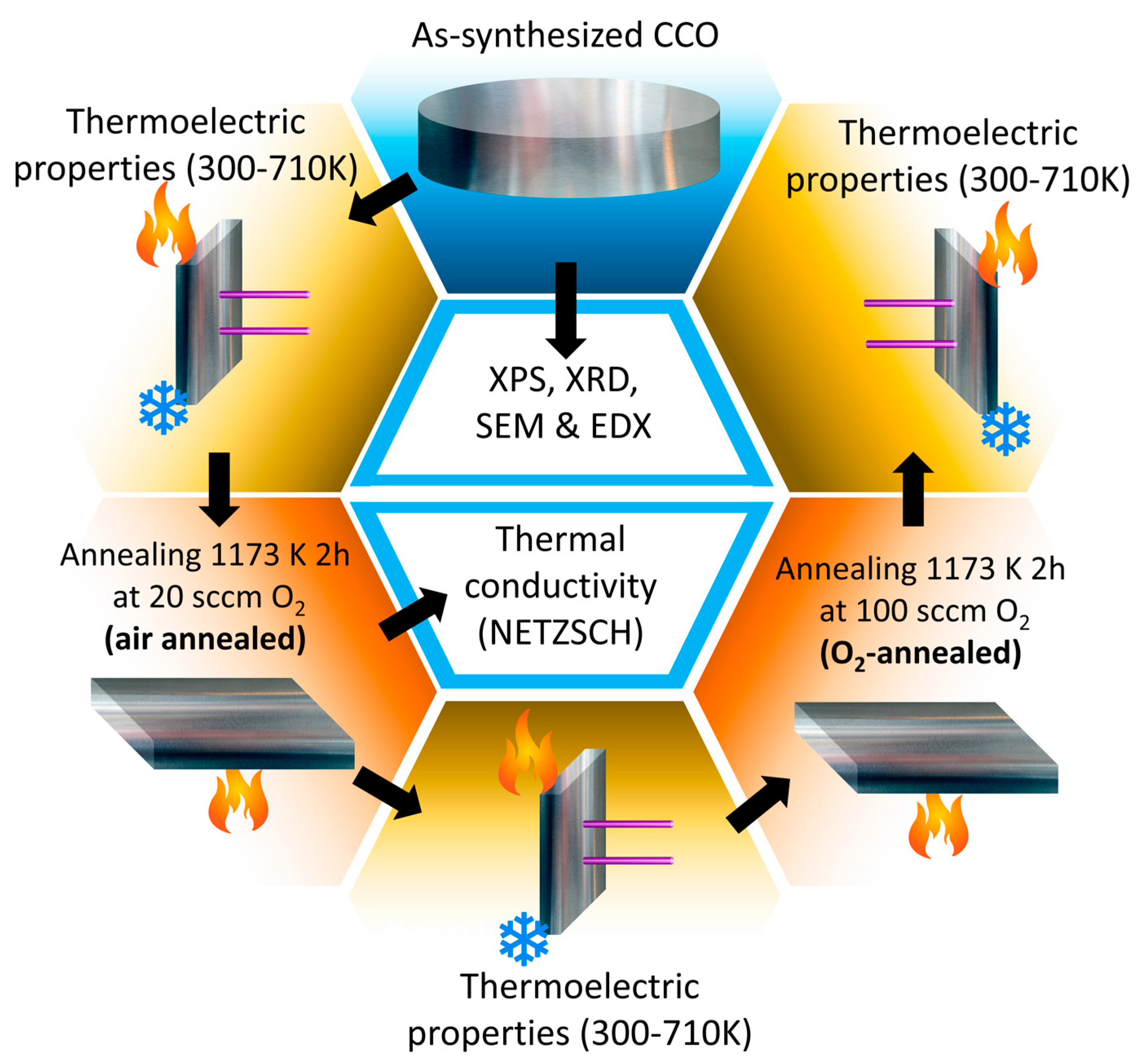
2. Experimental Methods
3. Results
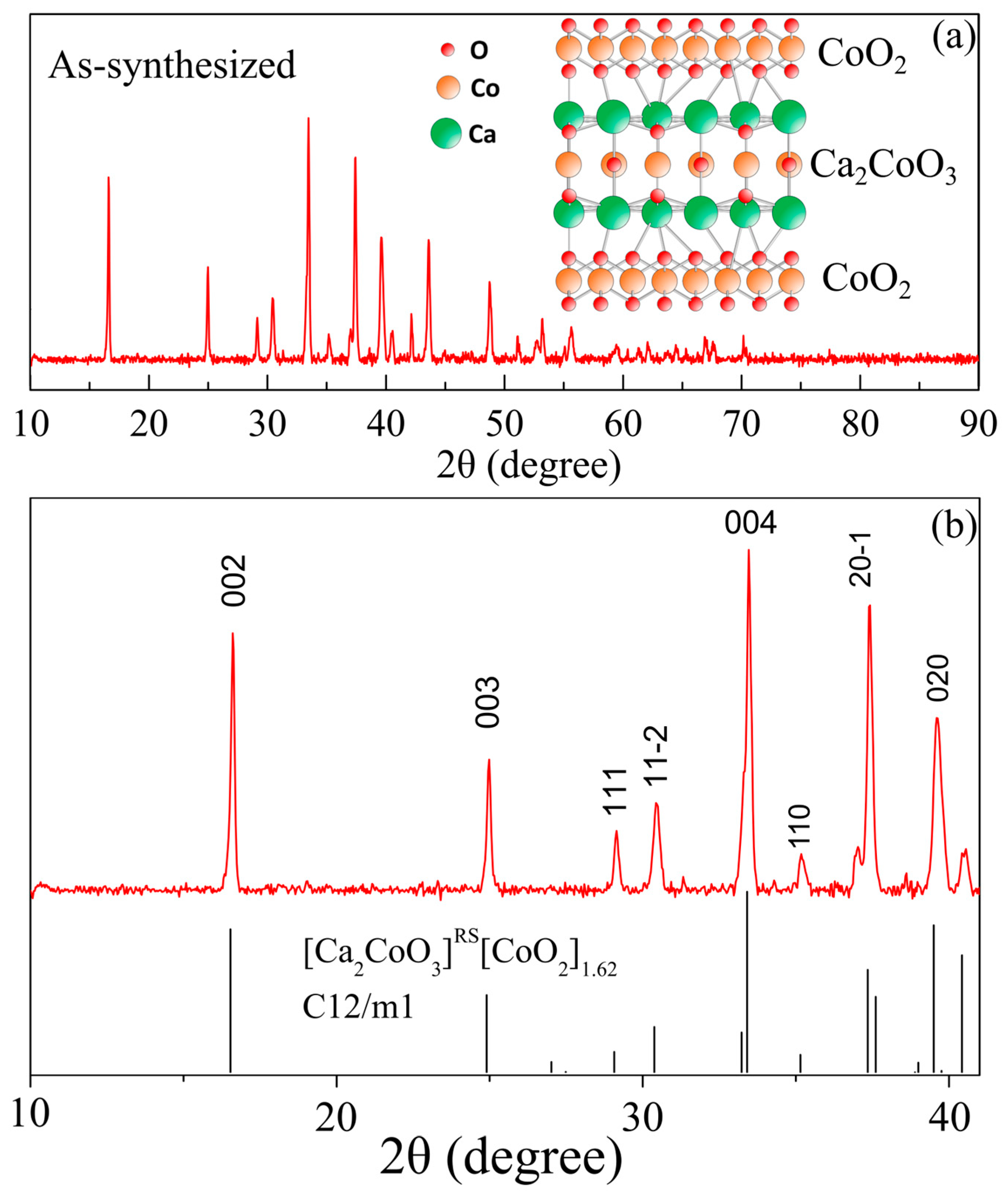
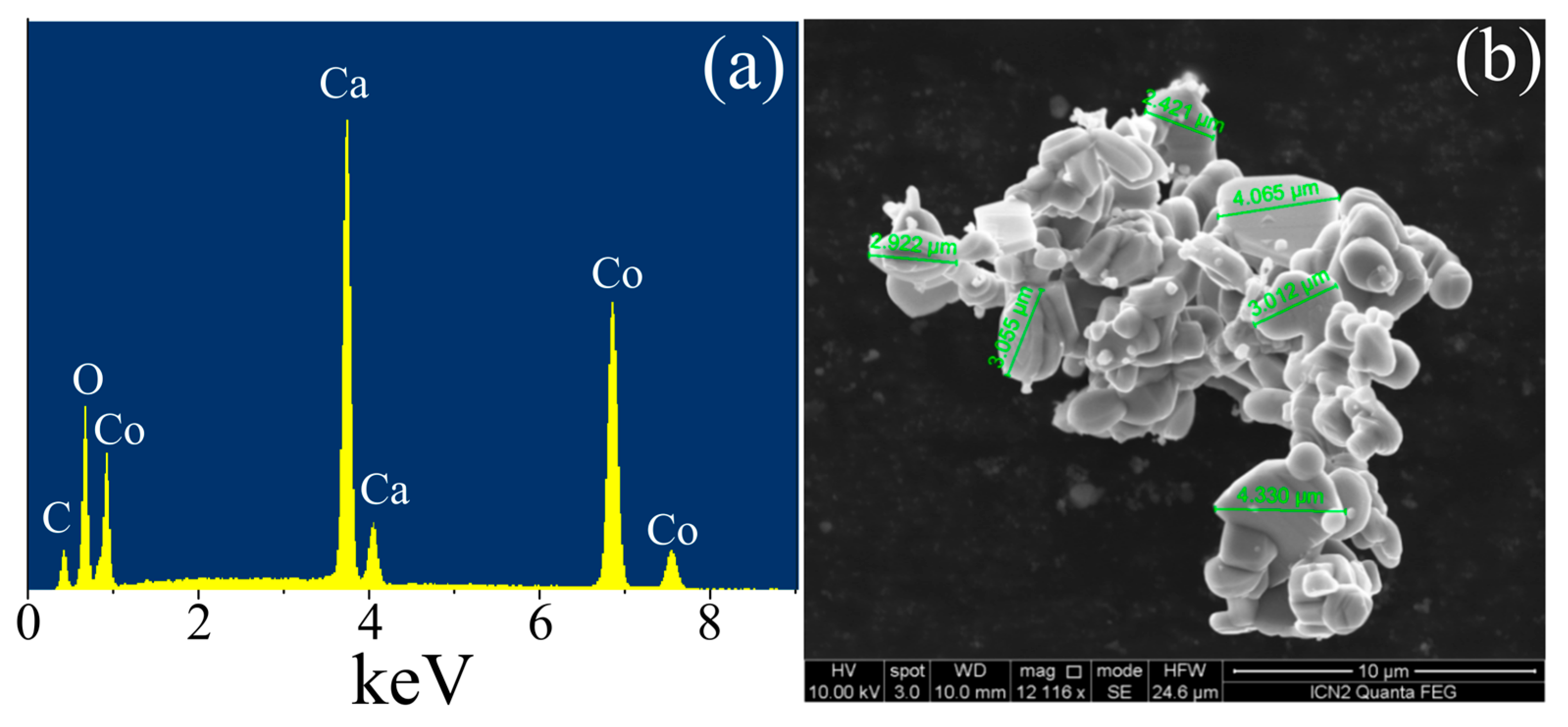
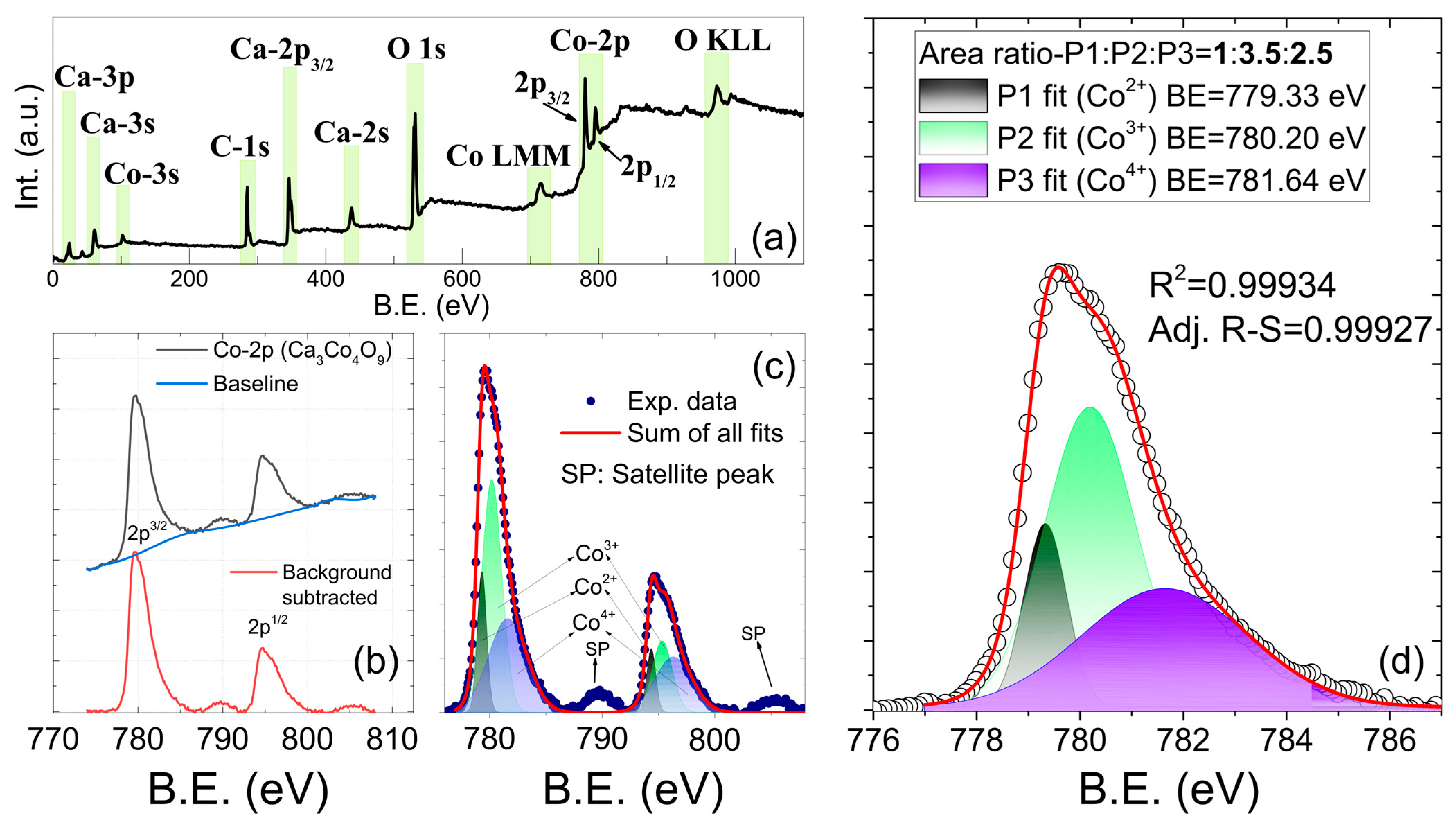
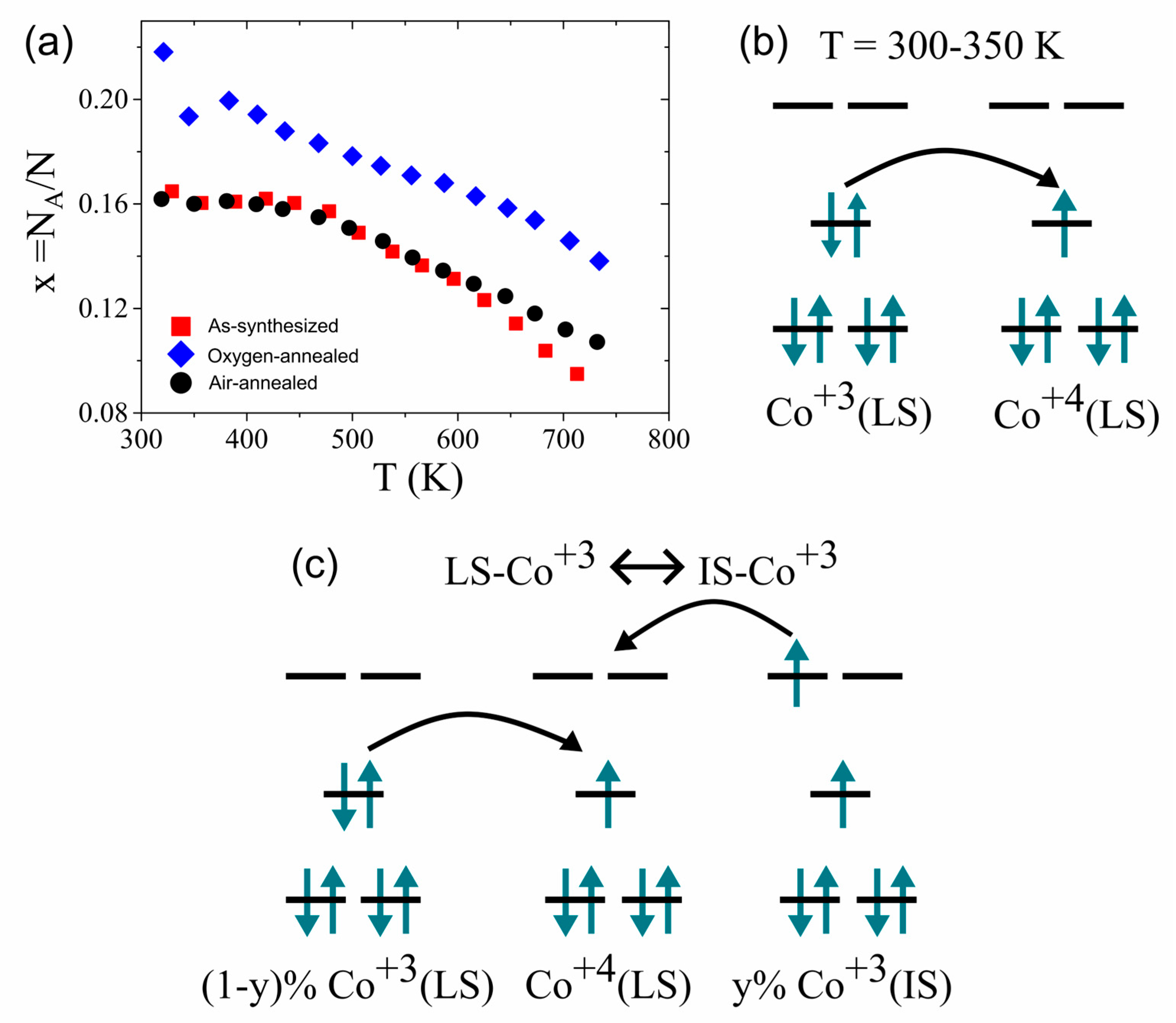
3.1. Structural and Elemental Characterizations
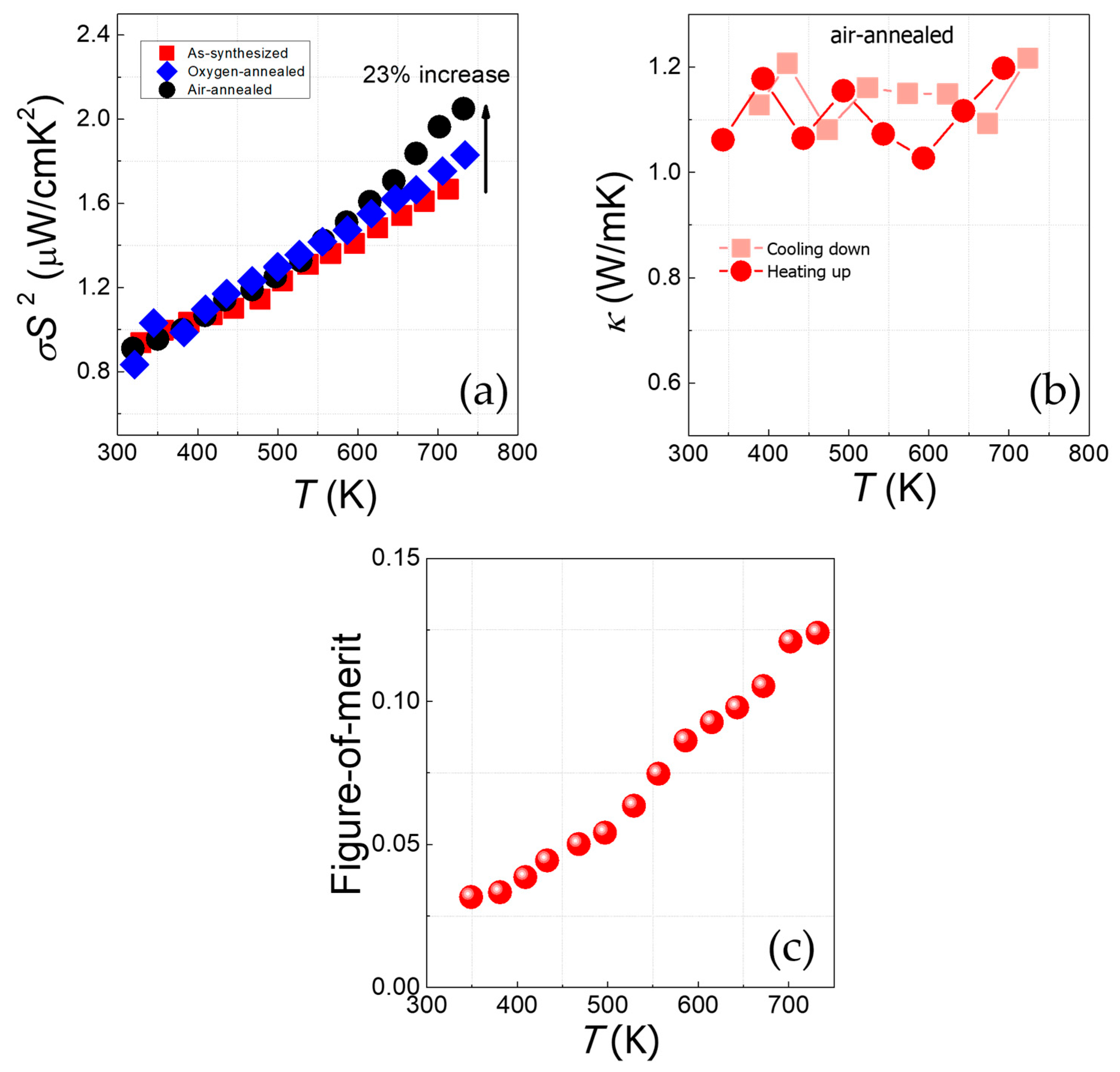
3.2. Electronic Transport Properties and Thermoelectric Power
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brinks, P.; Huijben, M. Thermoelectric Oxides. In Epitaxial Growth of Complex Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2015; pp. 397–441. [Google Scholar]
- Yu, J.; Freer, R. Calcium Cobaltite, a Promising Oxide for Energy Harvesting: Effective Strategies toward Enhanced Thermoelectric Performance. J. Phys. Energy 2022, 4, 022001. [Google Scholar] [CrossRef]
- Terasaki, I. Research Update: Oxide Thermoelectrics: Beyond the Conventional Design Rules. APL Mater. 2016, 4, 104501. [Google Scholar] [CrossRef]
- Satake, A.; Tanaka, H.; Ohkawa, T.; Fujii, T.; Terasaki, I. Thermal Conductivity of the Thermoelectric Layered Cobalt Oxides Measured by the Harman Method. J. Appl. Phys. 2004, 96, 931–933. [Google Scholar] [CrossRef]
- Li, L.; Yan, X.-J.; Dong, S.-T.; Lv, Y.-Y.; Li, X.; Yao, S.-H.; Chen, Y.-B.; Zhang, S.-T.; Zhou, J.; Lu, H.; et al. Ultra-Low Thermal Conductivities along c-Axis of Naturally Misfit Layered Bi2[AE]2Co2Oy (AE = Ca, Ca0.5Sr0.5, Sr, Ba) Single Crystals. Appl. Phys. Lett. 2017, 111, 033902. [Google Scholar] [CrossRef]
- Singh, D.J. Electronic Structure of NaCo2O4. Phys. Rev. B 2000, 61, 13397–13402. [Google Scholar] [CrossRef]
- Kuroki, K.; Arita, R. “Pudding Mold” Band Drives Large Thermopower in NaXCoO2. J. Phys. Soc. Jpn. 2007, 76, 3–6. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kondo, T.; Takami, T.; Takahashi, H.; Ikuta, H.; Mizutani, U.; Soda, K.; Funahashi, R.; Shikano, M.; Mikami, M.; et al. Contribution of Electronic Structure to the Large Thermoelectric Power in Layered Cobalt Oxides. Phys. Rev. B 2004, 69, 125410. [Google Scholar] [CrossRef]
- Chatterjee, A.; Banik, A.; El Sachat, A.; Caicedo Roque, J.M.; Padilla-Pantoja, J.; Sotomayor Torres, C.M.; Biswas, K.; Santiso, J.; Chavez-Angel, E. Enhanced Thermoelectric Properties of Misfit Bi2Sr2-XCaxCo2Oy: Isovalent Substitutions and Selective Phonon Scattering. Materials 2023, 16, 1413. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Leligny, H.; Grebille, D. Three Forms of the Misfit Layered Cobaltite [Ca2CoO3] [CoO2]1.62·A 4D Structural Investigation. J. Solid State Chem. 2001, 160, 322–331. [Google Scholar] [CrossRef]
- Iyazaki, Y.M.; Noda, M.O.; Ku, T.O.; Ikuchi, M.K.; Shii, Y.I. Modulated Structure of the Thermoelectric Compound [Ca2CoO3]0.62CoO2. J. Phys. Soc. Jpn. 2002, 71, 491–497. [Google Scholar] [CrossRef]
- Terasaki, I.; Sasago, Y.; Uchinokura, K. Large Thermoelectric Power in NaCo2O4 Single Crystals. Phys. Rev. B 1997, 56, R12685–R12687. [Google Scholar] [CrossRef]
- Masset, A.; Michel, C.; Maignan, A.; Hervieu, M.; Toulemonde, O.; Studer, F.; Raveau, B.; Hejtmanek, J. Misfit-Layered Cobaltite with an Anisotropic Giant Magnetoresistance. Phys. Rev. B—Condens. Matter Mater. Phys. 2000, 62, 166–175. [Google Scholar] [CrossRef]
- Sakabayashi, H.; Okazaki, R. Crossover from Itinerant to Localized States in the Thermoelectric Oxide [Ca2CoO3]0.62[CoO2]. Phys. Rev. B 2021, 103, 125119. [Google Scholar] [CrossRef]
- Kuno, S.; Takeuchi, T.; Ikuta, H.; Kondo, T.; Kaminski, A.; Saito, Y.; Fujimori, S. Electronic Structure and Thermoelectric Properties of a Layered Cobalt Oxide NaxCoO2 (0.5 << x << 0.8) Investigated by Angle-Resolved Photoemission Spectroscopy. In Proceedings of the 2007 26th International Conference on Thermoelectrics, Jeju, Republic of Korea, 3–7 June 2007; IEEE: Piscataway, NJ, USA, 2007; Volume 2, pp. 99–102. [Google Scholar]
- Chaikin, P.M.; Beni, G. Thermopower in the Correlated Hopping Regime. Phys. Rev. B 1976, 13, 647–651. [Google Scholar] [CrossRef]
- Koshibae, W.; Tsutsui, K.; Maekawa, S. Thermopower in Cobalt Oxides. Phys. Rev. B 2000, 62, 6869–6872. [Google Scholar] [CrossRef]
- Van Nong, N.; Pryds, N.; Linderoth, S.; Ohtaki, M. Enhancement of the Thermoelectric Performance of P-Type Layered Oxide Ca3Co4O9+δ through Heavy Doping and Metallic Nanoinclusions. Adv. Mater. 2011, 23, 2484–2490. [Google Scholar] [CrossRef]
- Amin, B.; Eckern, U.; Schwingenschlögl, U. Thermoelectric Properties of the Misfit Cobaltate Ca3Co4O9. Appl. Phys. Lett. 2017, 110, 233505. [Google Scholar] [CrossRef]
- Paul, B.; Schroeder, J.L.; Kerdsongpanya, S.; Van Nong, N.; Schell, N.; Ostach, D.; Lu, J.; Birch, J.; Eklund, P. Mechanism of Formation of the Thermoelectric Layered Cobaltate Ca3Co4O9 by Annealing of CaO-CoO Thin Films. Adv. Electron. Mater. 2015, 1, 1400022. [Google Scholar] [CrossRef]
- Soret, J.; Lepetit, M.B. Electronic Structure of the Ca3Co4O9 Compound from Ab Initio Local Interactions. Phys. Rev. B—Condens. Matter Mater. Phys. 2012, 85, 165145. [Google Scholar] [CrossRef]
- Muguerra, H.; Grebille, D. Original Disorder-Order Transition Related to Electronic and Magnetic Properties in the Thermoelectric Misfit Phase [Ca2CoO3][CoO3]1.62. Acta Crystallogr. Sect. B Struct. Sci. 2008, 64, 676–683. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Yao, Q.; Li, J. High-Temperature Thermoelectric Properties of Ca3Co4O9+δ with Eu Substitution. Solid State Commun. 2004, 129, 615–618. [Google Scholar] [CrossRef]
- Wu, N.; Holgate, T.C.; Van Nong, N.; Pryds, N.; Linderoth, S. High Temperature Thermoelectric Properties of Ca3Co4O9+δ by Auto-Combustion Synthesis and Spark Plasma Sintering. J. Eur. Ceram. Soc. 2014, 34, 925–931. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.; Shi, Z.; Nan, C.-W.; Shen, Z. Preparation of Ca3Co4O9 and Improvement of Its Thermoelectric Properties by Spark Plasma Sintering. J. Am. Ceram. Soc. 2005, 88, 1337–1340. [Google Scholar] [CrossRef]
- Lee, H.; Vashaee, D.; Wang, D.Z.; Dresselhaus, M.S.; Ren, Z.F.; Chen, G. Effects of Nanoscale Porosity on Thermoelectric Properties of SiGe. J. Appl. Phys. 2010, 107, 094308. [Google Scholar] [CrossRef]
- dos Santos, A.M.; Thomazini, D.; Gelfuso, M.V. Cold Sintering and Thermoelectric Properties of Ca3Co4O9 Ceramics. Ceram. Int. 2020, 46, 14064–14070. [Google Scholar] [CrossRef]
- Wang, X.; Suwardi, A.; Zheng, Y.; Zhou, H.; Chien, S.W.; Xu, J. Enhanced Thermoelectric Performance of Nanocrystalline Indium Tin Oxide Pellets by Modulating the Density and Nanoporosity via Spark Plasma Sintering. ACS Appl. Nano Mater. 2020, 3, 10156–10165. [Google Scholar] [CrossRef]
- Hira, U.; Han, L.; Norrman, K.; Christensen, D.V.; Pryds, N.; Sher, F. High-Temperature Thermoelectric Properties of Na- and W-Doped Ca3Co4O9 System. RSC Adv. 2018, 8, 12211–12221. [Google Scholar] [CrossRef]
- Kahraman, F.; Madre, M.A.; Rasekh, S.; Salvador, C.; Bosque, P.; Torres, M.A.; Diez, J.C.; Sotelo, A. Enhancement of Mechanical and Thermoelectric Properties of Ca3Co4O9 by Ag Addition. J. Eur. Ceram. Soc. 2015, 35, 3835–3841. [Google Scholar] [CrossRef]
- Mele, P.; Kamei, H.; Yasumune, H.; Matsumoto, K.; Miyazaki, K. Development of Thermoelectric Module Based on Dense Ca3Co4O9 and Zn0.98Al0.02O Legs. Met. Mater. Int. 2014, 20, 389–397. [Google Scholar] [CrossRef]
- Hira, U.; Ali, S.S.; Latif, S.; Pryds, N.; Sher, F. Improved High-Temperature Thermoelectric Properties of Dual-Doped Ca3Co4O9. ACS Omega 2022, 7, 6579–6590. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Xu, J.; Zhu, J.; Zhang, Y.; Gao, T.; Qin, M.; Sun, H.; Dong, G.; Gao, F. Effect of Platelet Template Seeds on Microstructure and Thermoelectric Properties of Ca3Co4O9 Ceramics. Ceram. Int. 2019, 45, 1977–1983. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Uday, M.B.; Izman, S. Enhanced Thermoelectric Performance of Ca3Co4O9 Doped with Aluminum. J. Mater. Sci. Mater. Electron. 2020, 31, 16569–16582. [Google Scholar] [CrossRef]
- Büttner, G.; Populoh, S.; Xie, W.; Trottmann, M.; Hertrampf, J.; Döbeli, M.; Karvonen, L.; Yoon, S.; Thiel, P.; Niewa, R.; et al. Thermoelectric Properties of [Ca2CoO3−δ][CoO2]1,62 as a Function of Co/Ca Defects and Co3O4 Inclusions. J. Appl. Phys. 2017, 121, 215101. [Google Scholar] [CrossRef]
- Wu, T.; Tyson, T.A.; Chen, H.; Bai, J.; Wang, H.; Jaye, C. A Structural Change in Ca3Co4O9 Associated with Enhanced Thermoelectric Properties. J. Phys. Condens. Matter 2012, 24, 455602. [Google Scholar] [CrossRef]
- Shimoyama, J.I.; Horii, S.; Otzschi, K.; Sano, M.; Kishio, K. Oxygen Nonstoichiometry in Layered Cobaltite Ca3Co4Oy. Jpn. J. Appl. Phys. 2003, 42, L194. [Google Scholar] [CrossRef]
- Yang, G.; Ramasse, Q.; Klie, R.F. Direct Measurement of Charge Transfer in Thermoelectric Ca3Co4O9. Phys. Rev. B 2008, 78, 153109. [Google Scholar] [CrossRef]
- Ahad, A.; Gautam, K.; Majid, S.S.; Francoual, S.; Rahman, F.; De Groot, F.M.F.; Shukla, D.K. Origin of the High Seebeck Coefficient of the Misfit [Ca2CoO3]0.62[CoO2] Cobaltate from Site-Specific Valency and Spin-State Determinations. Phys. Rev. B 2020, 101, 220202. [Google Scholar] [CrossRef]
- Heikes, R.R.; Ure, R.W. Narrow Band Semiconductors, Ionic Crystals, and Liquids. In Thermoelectricity: Science and Engineering; Interscience Publishers: New York, NY, USA, 1961; pp. 75–90. [Google Scholar]
- Limelette, P.; Hardy, V.; Auban-Senzier, P.; Jérome, D.; Flahaut, D.; Hébert, S.; Frésard, R.; Simon, C.; Noudem, J.; Maignan, A. Strongly Correlated Properties of the Thermoelectric Cobalt Oxide Ca3Co4O9. Phys. Rev. B—Condens. Matter Mater. Phys. 2005, 71, 4–7. [Google Scholar] [CrossRef]
- Limelette, P.; Hébert, S.; Muguerra, H.; Frésard, R.; Simon, C. Dual Electronic States in Thermoelectric Cobalt Oxide [Bi1.7Ca2O4]0.59CoO2. Phys. Rev. B 2008, 77, 235118. [Google Scholar] [CrossRef]
- Cheng, J.; Sui, Y.; Wang, Y.; Wang, X.; Su, W. First-Order Phase Transition Characteristic of the High Temperature Metal-Semiconductor Transition in [Ca2CoO3]0.62[CoO2]. Appl. Phys. A Mater. Sci. Process. 2009, 94, 911–916. [Google Scholar] [CrossRef]
- Wang, Y.; Rogado, N.S.; Cava, R.J.; Ong, N.P. Spin Entropy as the Likely Source of Enhanced Thermopower in NaxCo2O4. Nature 2003, 423, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Koshibae, W.; Maekawa, S. Effects of Spin and Orbital Degeneracy on the Thermopower of Strongly Correlated Systems. Phys. Rev. Lett. 2001, 87, 236603. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.J.; Singh, D.J. Suppression of Thermopower of NaxCoO2 by an External Magnetic Field: Boltzmann Transport Combined with Spin-Polarized Density Functional Theory. Phys. Rev. B 2007, 76, 195111. [Google Scholar] [CrossRef]
- Wakisaka, Y.; Hirata, S.; Mizokawa, T.; Suzuki, Y.; Miyazaki, Y.; Kajitani, T. Electronic Structure of Ca3Co4O9 Photoemission Spectroscopy: Phase Separation and Charge Localization. Phys. Rev. B 2008, 78, 235107. [Google Scholar] [CrossRef]
- Sugiyama, J.; Brewer, J.H.; Ansaldo, E.J.; Itahara, H.; Dohmae, K.; Seno, Y.; Xia, C.; Tani, T. Hidden Magnetic Transitions in Thermoelectric Layered Cobaltite, [Ca2CoO3]0.62[CoO2]. Phys. Rev. B 2003, 68, 134423. [Google Scholar] [CrossRef]
- Terasaki, I.; Tanaka, H.; Satake, A.; Okada, S.; Fujii, T. Out-of-Plane Thermal Conductivity of the Layered Thermoelectric Oxide Bi2−xPbxSr2Co2Oy. Phys. Rev. B 2004, 70, 214106. [Google Scholar] [CrossRef]

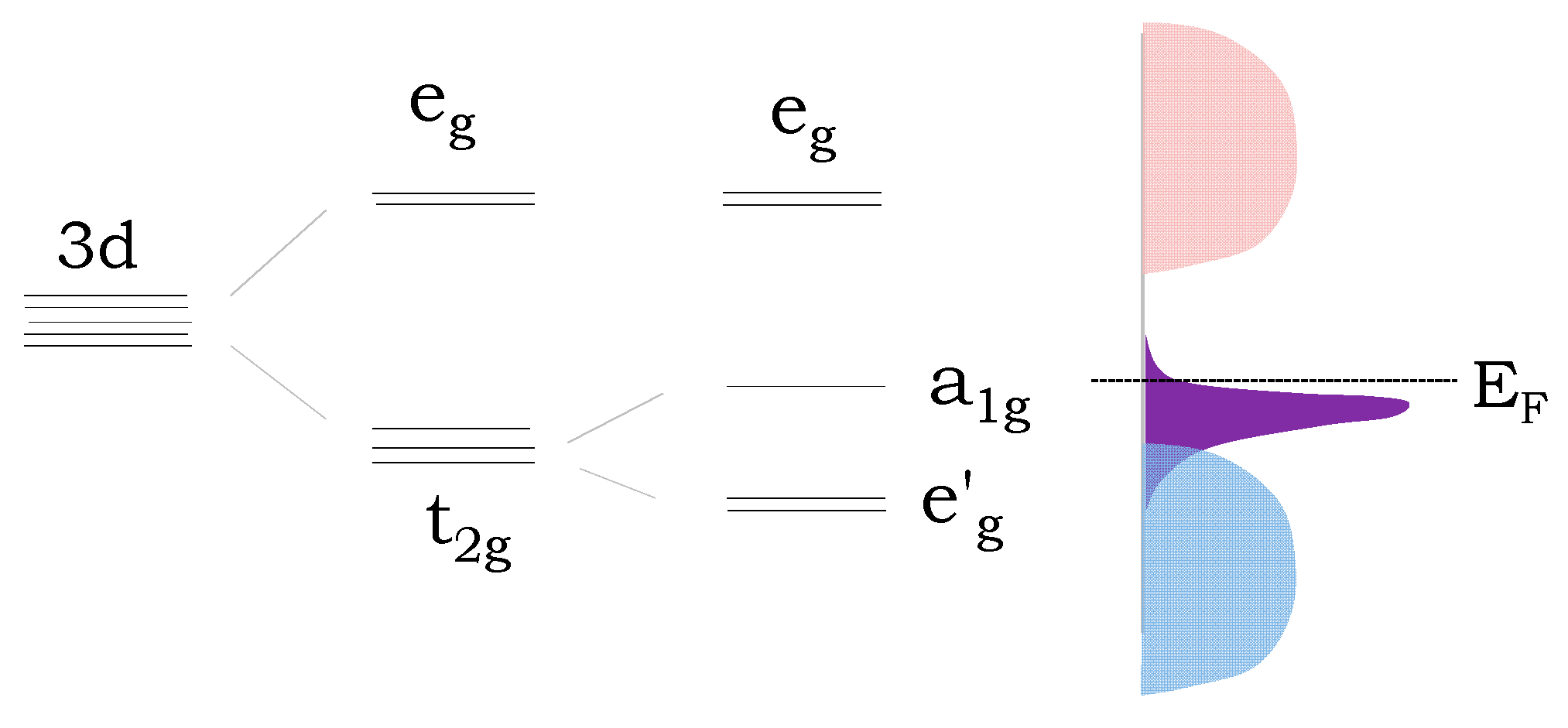
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, A.; El Sachat, A.; Banik, A.; Biswas, K.; Castro-Alvarez, A.; Sotomayor Torres, C.M.; Santiso, J.; Chávez-Ángel, E. Improved High Temperature Thermoelectric Properties in Misfit Ca3Co4O9 by Thermal Annealing. Energies 2023, 16, 5162. https://doi.org/10.3390/en16135162
Chatterjee A, El Sachat A, Banik A, Biswas K, Castro-Alvarez A, Sotomayor Torres CM, Santiso J, Chávez-Ángel E. Improved High Temperature Thermoelectric Properties in Misfit Ca3Co4O9 by Thermal Annealing. Energies. 2023; 16(13):5162. https://doi.org/10.3390/en16135162
Chicago/Turabian StyleChatterjee, Arindom, Alexandros El Sachat, Ananya Banik, Kanishka Biswas, Alejandro Castro-Alvarez, Clivia M. Sotomayor Torres, José Santiso, and Emigdio Chávez-Ángel. 2023. "Improved High Temperature Thermoelectric Properties in Misfit Ca3Co4O9 by Thermal Annealing" Energies 16, no. 13: 5162. https://doi.org/10.3390/en16135162
APA StyleChatterjee, A., El Sachat, A., Banik, A., Biswas, K., Castro-Alvarez, A., Sotomayor Torres, C. M., Santiso, J., & Chávez-Ángel, E. (2023). Improved High Temperature Thermoelectric Properties in Misfit Ca3Co4O9 by Thermal Annealing. Energies, 16(13), 5162. https://doi.org/10.3390/en16135162









