1. Diesel Fuel with Ethyl Tertiary Butyl Ether—Biofuel for Compression Ignition Engines
The twentieth century ushered in significant economic developments around the world, resulting in an increase in energy demand. The main sources of energy have become oil, gas, and coal, which are characterized as cheap resources and are non-renewable raw materials with ending deposits. The increase in demand for transport services results in an increase in the production of mass transport and, as a result, an increase in the demand for fuel increases. The combustion of oil, gas, and coal negatively affects the Earth’s natural environment, and nitrogen oxide, sulfur, and carbon emissions pollute the planet’s atmosphere. In addition, excessive emissions of greenhouse gases in the environment, mainly derived from the combustion in internal combustion engines, promote global warming.
Increasing environmental awareness contributes to taking action to protect the environment. One of the ways to do this is to reduce the impact of emitted pollutants, particularly carbon dioxide. Standards regulating the content of toxins in exhaust gases are tightening, forcing engineers to look for new design solutions for engines that will burn fuels from renewable sources and whose exhaust emissions are less harmful to the environment.
Vehicles powered by electric motors further exemplify the desire of engineers to reduce exhaust emissions. The production of such cars is growing every year. This does not change the fact that the value of these cars are also seemingly increasing, and the cost of replacing an electric car battery is close to the value of the vehicle itself. In the future, electric motors will likely provide a popular means of powering light urban vehicles.
Compression-ignition and spark-ignition engines will be successfully used in transport and trade as the main source of propulsion for vehicles. In order to reduce the harmfulness of exhaust emissions, engineers are aiming to build engines that will successfully burn fuels from renewable sources. In addition, the use of new propulsion systems powered by electricity, as well as various fuels, e.g., hydrogen, alcohol (ethanol, methanol), ethers, natural gas, LPG, vegetable oils, and their esters, will also reduce environmental pollution. Research found in the literature indicates that the idea of using biofuels is correct and that the main impact of using biofuels will likely come in the form of reducing exhaust emissions [
1,
2,
3]. However, the use of biofuels for engines involves the need to carry out technological processes and obtain biofuels with appropriate physicochemical properties. The use of biofuels must also properly affect the engine’s operating processes, and the additives must be properly selected so that the process of consumption of fuel system components is the smallest [
4,
5]. The main purpose of the fuel used is to reduce fuel consumption, reduce exhaust toxicity, and generate high engine efficiency [
6].
Biofuels that can be used for compression-ignition engines [
7,
8,
9]:
Belonging to the group of unconventional liquid fuels, vegetable oils and their esters are mainly produced from plant seeds. The use of vegetable fuels, in particular rapeseed oil, has advantages and disadvantages. The advantages include the following:
- -
There is a large amount of oxygen bound in the fuel, which favorably affects the combustion process;
- -
The high kinematic viscosity seals the fuel injection system;
- -
The use of rapeseed oil has a beneficial effect on the environment;
- -
Low sulfur content (up to 0.05%);
- -
Strengthening the domestic biofuel market and the possibility of developing free land for rapeseed cultivation;
- -
The use of rapeseed oil reduces the NOx content in exhaust gases;
However, the disadvantages are as follows:
- -
High viscosity at low temperatures blocks the fuel filter in the system;
- -
Esters are oxidized, which increases their acidity and contributes to faster wear of the seal;
- -
At high temperatures, glycerin present in the composition turns into acroline, which, in the engine, forms carbon deposits (leading to engine failure);
- -
The use of pure vegetable oil necessitates the modification the engine designs and their supply systems (e.g., two-tank power system equipped with a heated oil tank, additional pumps, filters, etc.);
- -
The use of rapeseed oil reduces the operating parameters of the engine, i.e., power, torque, average pressure indicated, etc.;
Oils of vegetative origin include the following:
- -
corn oil,
- -
cotton oil,
- -
linseed oil,
- -
peanut oil,
- -
soybean oil,
- -
sunflower oil, etc.
Alcohols
Ethanol is the most well-known alcohol used for compression-ignition engines, but it has adverse physicochemical properties. Its mixing with diesel reduces lubricity, and under the influence of water contained in the fuel and low temperature, it tends to delaminate. Additionally, a mixture of ethanol and ON reduces its viscosity, which may result in leaks in the fuel system [
10].
Methyl alcohol, also known as methanol, has only one carbon atom and OH group in its structure. It is considered to be a very dangerous substance that poses a threat to human health and is characterized by a low calorific value in relation to diesel oil and high auto-ignition temperature.
Ethanol has two carbon atoms in its structure and can be mixed with diesel to a limited extent. The problems associated with this alcohol become visible with changes in temperature and include the presence of water in the fuel, which promotes the delamination of the mixture. Used as an oxygen additive by the Gdańsk Refinery for motor gasoline and thus increasing their octane number, 95% ethanol is biodegradable [
11,
12].
Propanol is a chemical compound from the group of alcohols found in two molecular structures: 1-propanol and isopropanol. The use of propanol to power compression-ignition engines is possible after mixing it with oils of vegetable or mineral origin. The increasing share of alcohols in the mixture of propanol and butanol isomers with rapeseed oil increases the emissions of unburned carbohydrates and reduces the cetane number and viscosity of the fuel.
Butyl alcohol—An alcohol with a higher molecular weight that can be used as a fuel is biobutanol. It is obtained through a two-stage biomass fermentation process using appropriate anaerobic bacteria. Butanol has physicochemical properties similar to that of gasoline, particularly in terms of calorific value and density. Unfortunately, butanol is more viscous than gasoline, which worsens the quality of fuel spraying. Ethers are also ecological fuels used for compression-ignition engines. Ethers are chemical substances with different physicochemical properties and complex molecular structures. These include dimethyl ether (DME), diethyl ether (DEE) [
13], methyl-tetra-butyl ether (EMTB), and ethyl-tertiary-butyl ether (EETB), among others. Depending on the structure of the ether, they differ in terms of physicochemical properties. EETB is produced by reacting isobutylene with ethanol, which is obtained from renewable raw materials [
14].
Table 1 shows the physicochemical properties of ethers.
The use of ethers for fuel purposes is very popular in Poland—PKN Orlen S.A. produces EETB. The use of 10% EETB for diesel fuel reduces particulate emissions, as well as cetane number, which extends the delay period of self-ignition of fuel. The effect of extending this period may come in the form of a greater increase in pressure in the combustion chamber, which in turn results in hard engine operation [
15]. EETB is produced from ethanol and isobutylene. It can be obtained from bioethanol, making it a renewable fuel. Bioethanol also produces diethyl ether, which definitely exceeds EETB in terms of cetane number. The use of EETB and DEE to power compression-ignition engines accelerates the wear of friction nodes in the injection apparatus. The use of the above fuels requires an appropriate power supply system and the use of a dual-fuel engine power system. In the literature, data on the physicochemical properties of mixtures of diesel oil and ethyl-tertiary-butyl ether are available [
9,
15].
In order to check the operating parameters of the engine, based on the parameters of the fuel (a mixture of diesel oil with ethyl-tertiary butyl ether), thermal calculations were carried out for a compression-ignition engine powered by the said mixture. Their correctness was checked in matlab/Simulink, where a control system was built and processes were simulated. The results of these studies are presented in this article.
2. Thermal Calculations for a Mixture of EETB and ON
The calculations were carried out for a four-stroke compression-ignition engine intended for use as a power generator. The engine is an eight-cylinder engine with undivided combustion chambers, a volumetric method of formation of the fuel-air mixture, crankshaft speed up to 2600 rpm, and compression ratio of Ɛ = 17. The engine is naturally aspirated with an effective power of Ne = 170 kW and supercharged with an air pressure of pk = 0.17 MPa (centrifugal compressor with cooled body, vane diffuser, and radial turbine with constant pressure in front of the turbine). A calculation algorithm was applied to each mixture.
The calculations were carried out for a mixture of diesel fuel with ethyl-tertiary butyl ether in the share of EETB 5%, 10%, 20%, 30%, 40%. EETB from ON was chosen because it is commercially available domestically and is produced in Poland on the basis of it being a domestic raw material. EETB is obtained from bioethanol, which can be produced from food waste (a renewable energy source). The use EETB for diesel fuels reduces particulate emissions. For the purposes of thermal calculations, calculations were made, and
Table 2 presents the obtained elementary composition of the mixture of chemical composition ON with the participation of 5%, 10%, 20%, 30%, 40% EETB.
For the above average elementary fuel composition for each mixture, the calorific value of
Hu fuel is calculated according to the following formula (according to Mendelejewa) [
16]:
Based on the above formula for the calorific value of fuel in Matlab, Simulink was used to build a model to check the correctness of the equations (
Figure 1). The results obtained in the model agree with the results obtained via our calculations.
Already knowing the calorific value of the fuel, further calculations were carried out to determine the parameters of the working medium. One of these parameters is the theoretical air demand to burn 1 kg of Lo fuel (
Figure 2).
The next stage of calculation is the calculation of the amount of fresh load
Mt and the total amount of combustion products
Mz. In order to calculate the amount of fresh cargo, the coefficient of excess air λ is necessary. It determines the ratio of the actual amount of air in which fuel is burned to the amount of air needed to completely burn the fuel. In order to achieve complete combustion, it is usually necessary to supply more air than the results from the chemical reaction equations indicate. Knowing the λ parameter makes it possible to regulate the combustion process to obtain the appropriate purity of the exhaust gases. The coefficient of excess air depends directly on the type of fuel used. If insufficient oxygen (λ < 1—rich mixture) is supplied for combustion, toxic gases such as carbon monoxide (CO), nitrogen oxide (NO), or sulfur oxide (SO) will appear in the exhaust gases. In order for all the fuel and exhaust gases to contain less harmful compounds, more oxygen must be supplied (λ > 1—lean mixture) [
17]. For naturally aspirated engines λ = 1.4 ÷ 1.5, λ = 1.4 was used for the calculation. Knowing the previously calculated theoretical air demand, we used the following formula to calculate the amount of fresh cargo:
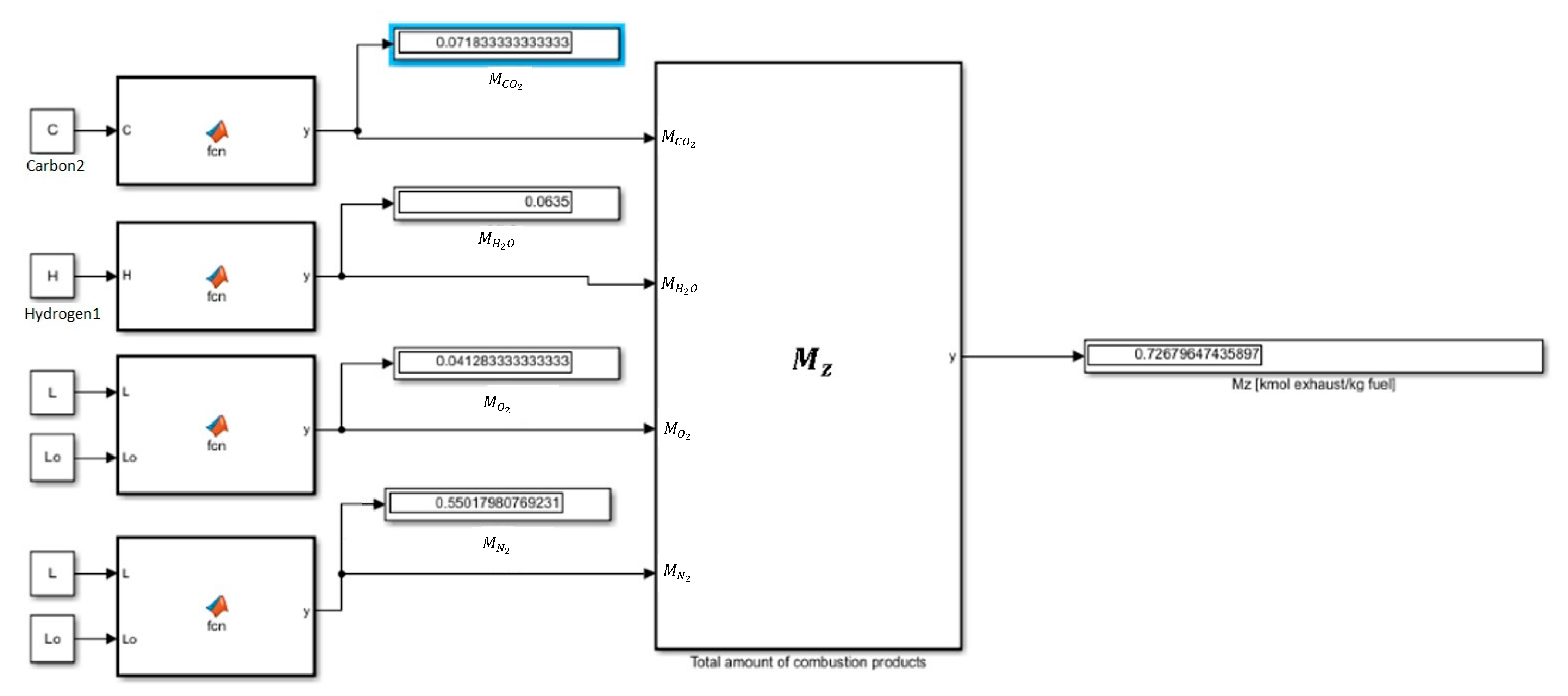
Before proceeding to the calculation of the total amount of combustion products
Mz, it is necessary to calculate the number of individual components of combustion products,
For each mixture of ON and EETB, the following formulae were used to determine the individual combustion components. Knowing the number of combustion components, we calculated the total number of combustion products
Mz, which can be facilitated by using the following formula:
The results of the above calculations coincide with the results of the model (
Figure 3).
The table (
Table 3) below summarizes the results of the working medium parameters depending on the share of EETB in the fuel.
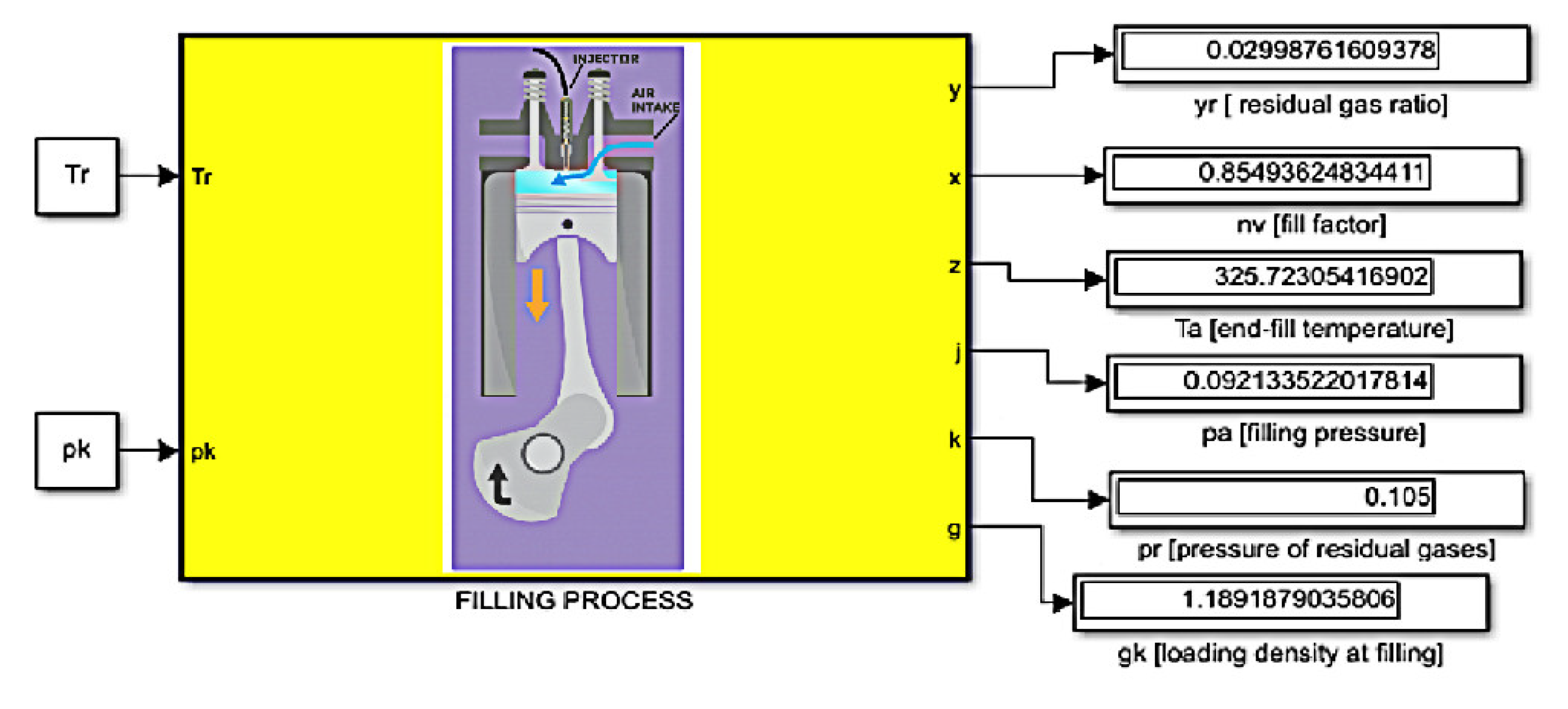
2.1. Filling Process
In the next stage, calculations will be made. The algorithm is the same for each type of EETB share in the fuel, while the results presented are for the compositions of 95% ON and 5% EETB. The calculations take into account the ambient parameters and residual gases, the filling process, the compression process, the combustion process, and the expansion process. The ambient conditions were assumed to be as follows:
Ambient pressure for motor:
Ambient temperature for engine
Both the temperature and pressure of residual gases depend on the compression value, rotational speed, fuel quality, temperature and intake pressure, and fuel temperature and pressure, among other things. Fairly high compression ratio values (e.g., values of approx. Ɛ = 17) lower the temperature of residual gases, while a high frequency of rotational speed increases the temperature and pressure of residual gases. Accordingly, the pressure and temperature of the residual gases are as follows:
By adopting the above-listed parameters, we can proceed to the calculations regarding the filling process. The first parameter is the temperature of the fresh cargo. In the calculations, it was assumed that the engine does not have a special device for heating fresh cargo. The actual heating of the load in naturally aspirated engines can reach ~15 ÷ 20 K. For the calculation, the following values were taken: ΔT = 20 [K]. Other parameters that should be taken into account include the following:
- -
charge density when filling:
- -
pressure trats at filling:
where
.
These parameters were selected in accordance with the speed regime of the engine, taking into account the low hydraulic resistance in the engine intake system.
- -
in the residual gas coefficient:
- -
fill end temperature:
- -
into the filling coefficient:
For the above-mentioned and performed calculations, a model was built (
Figure 4).
2.2. Compression Process
At this stage of the calculation, it was assumed that the average exponent of adiabate and compression polytropics can be determined by the respective nomograms. The approximate value of the polytropic exponent, which is equal to the exponent of the adiabate, was read from the nomogram.
Knowing the compression ratio, adiabate exponents, and polytropics, the pressure and temperature at the end of compression were determined to be as follows:
Next, the average heat and proper at the end of the compression was calculated:
where:
Residual gases (interpolation method)
The pre-compression process model is shown below (
Figure 5).
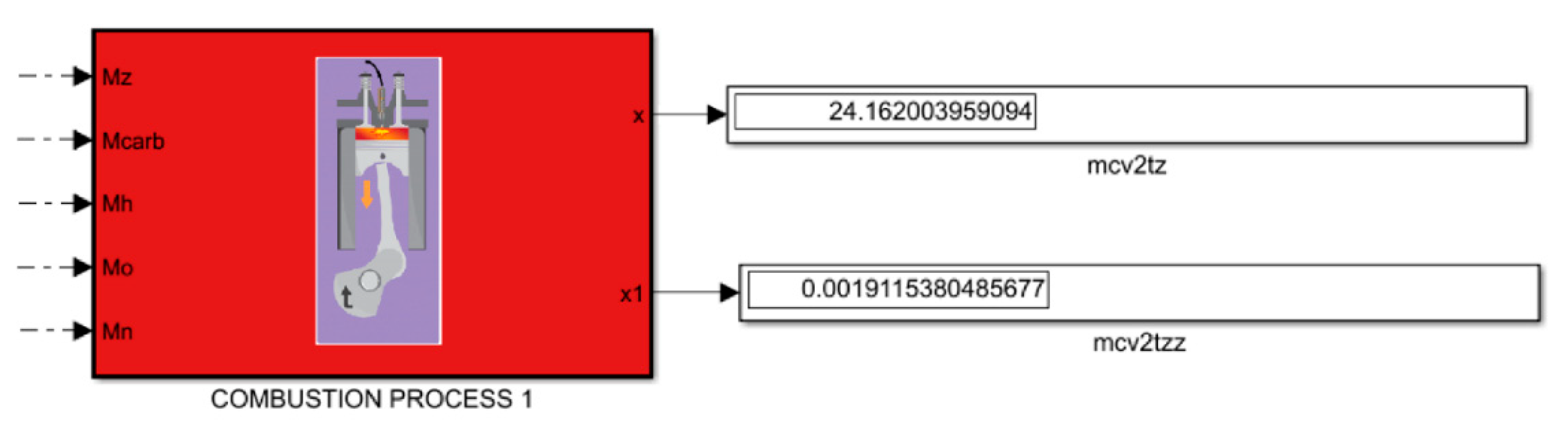
2.3. Combustion Process
The combustion process is a process by which it is possible to analyze the selection of parameters for the operation of the engine, the impact of which significantly affects the effective parameters of the engine. For this purpose, it is necessary for us to perform calculations and analyze the achieved parameters. The first stage of the combustion process is to determine the molecular value of the diesel fuel–air mixture
μo and the molecular value of the mixture change in diesel
μ:
Then, thanks to the previously calculated calorific value of the fuel and the amount of fresh load, the heat of combustion of the working mixture in Hmiesz rob diesels and the average specific heat of the combustion products are calculated
using the following formula:
Average specific heat of combustion products (
Figure 6):
Knowing the above value of the heat of combustion of the mixture in diesel and the average specific heat of the combustion products, we can calculate the temperature at the end of the combustion process
. In order to reduce the bearing load, the engine should have a combustion pressure of no more than 11–12 MPa. Therefore, it is necessary to calculate for diesel without supercharging λ = 2.0.
Maximum pressure for diesel: .
Degree of expansion preparation for diesel (
Figure 7):
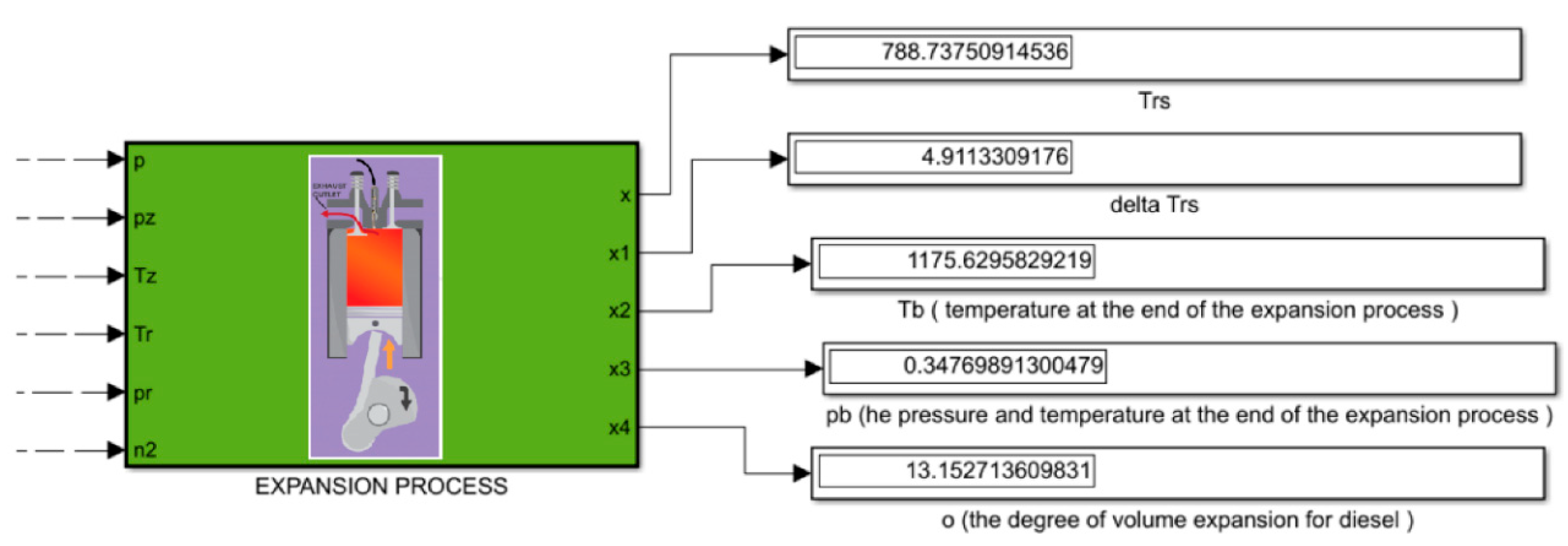
2.4. The Process of Expansion
The last process for which the calculations were made is the process of expansion. As was the case before, on the basis of our previous calculations, the degree of volume increase for diesel
δ was calculated, and on the basis of the aforementioned tables, adiabate and expansion polytropic for diesel were selected.
The average values of adiabate and expansion polytropic for diesel are selected as follows: On the nominal (small) extension you have to take adiabate, and on large cylinders, we take polytope. To determine the average values, we used arrays.
Pressure and temperature finally expand for diesels.
Checking the previously accepted temperature of the final (exhaust) gases (
Figure 8).
3. Engine Efficiency Parameters
Analyzing all the processes of engine operation, you can proceed to the calculation of the effective parameters of the engine. First of all,
= was determined. Mean pressure is indicated by the formula below:
The following parameters are determined in turn:
where
where
= 10
2 [m/c].
The effective and mechanical parameters of the engine operation were also calculated:
The main indicators of the engine are as follows: effective power, effective torque, fuel consumption, and power. Below are the dependencies according to which these parameters were calculated.
The above calculation algorithm has been performed for all EETB and ON mixtures, and the results are summarized in
Table 4.
By analyzing the table above, it can be seen that, with the increase in the share of EETB, the calorific value decreases, which is caused by the decreasing share of coal in the fuel. The effective performance of an engine powered by a mixture of ON and EETB slightly differs from the parameters of a diesel engine. The pressure and efficiency indicated decrease as the proportion of ether in the fuel increases. The highest of these values are characterized by a mixture of ON and 5% EETB, which are and , respectively. It is also worth noting that, with the decrease in the above parameters, the indicated fuel consumption increases by 6.3% compared to ON.
The mechanical efficiency for mixtures of ON and EETB is almost at one level (on average, varying by 0.51% compared to the efficiency for diesel). The increasing amount of EETB added to diesel increases hourly fuel consumption. It is worth noting that, for a mixture of 5%, EETB+95%ON is the lowest fuel consumption, amounting to 41.741 g/h, 1.9% less than 100% diesel. No mixture of ON and EETB achieved such high effective power and effective torque as diesel. The obtained results coincide with the results reported by other authors in the literature [
8,
15].