An Experimental Study of High-Pressure Microscopy and Enhanced Oil Recovery with Nanoparticle-Stabilised Foams in Carbonate Oil Reservoir
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.1.1. Liquid Phase Components
2.1.2. Gas Phase Components
2.1.3. Porous Media
2.1.4. Crude Oil
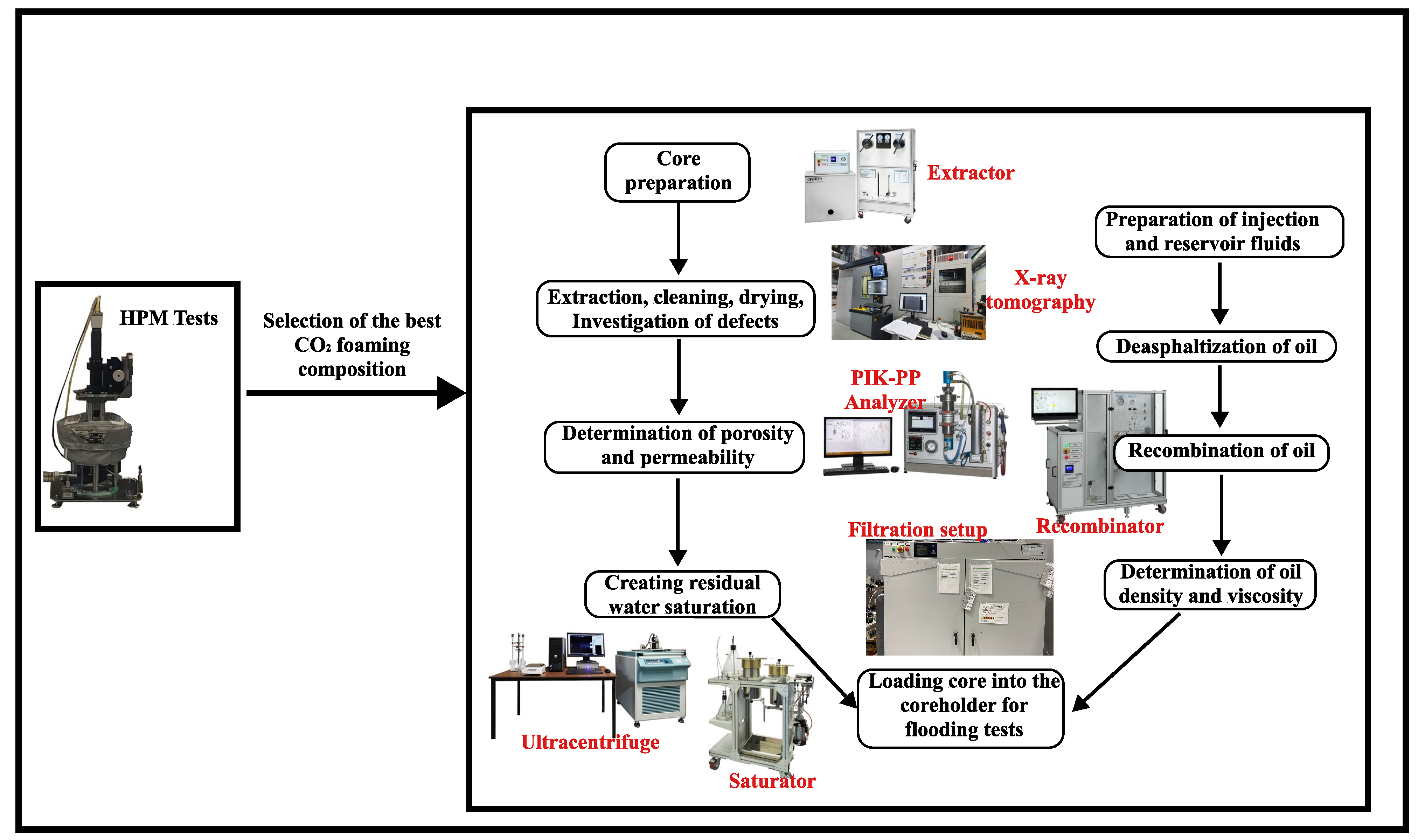
2.2. Methodology
2.2.1. Preparation of Nanofluids
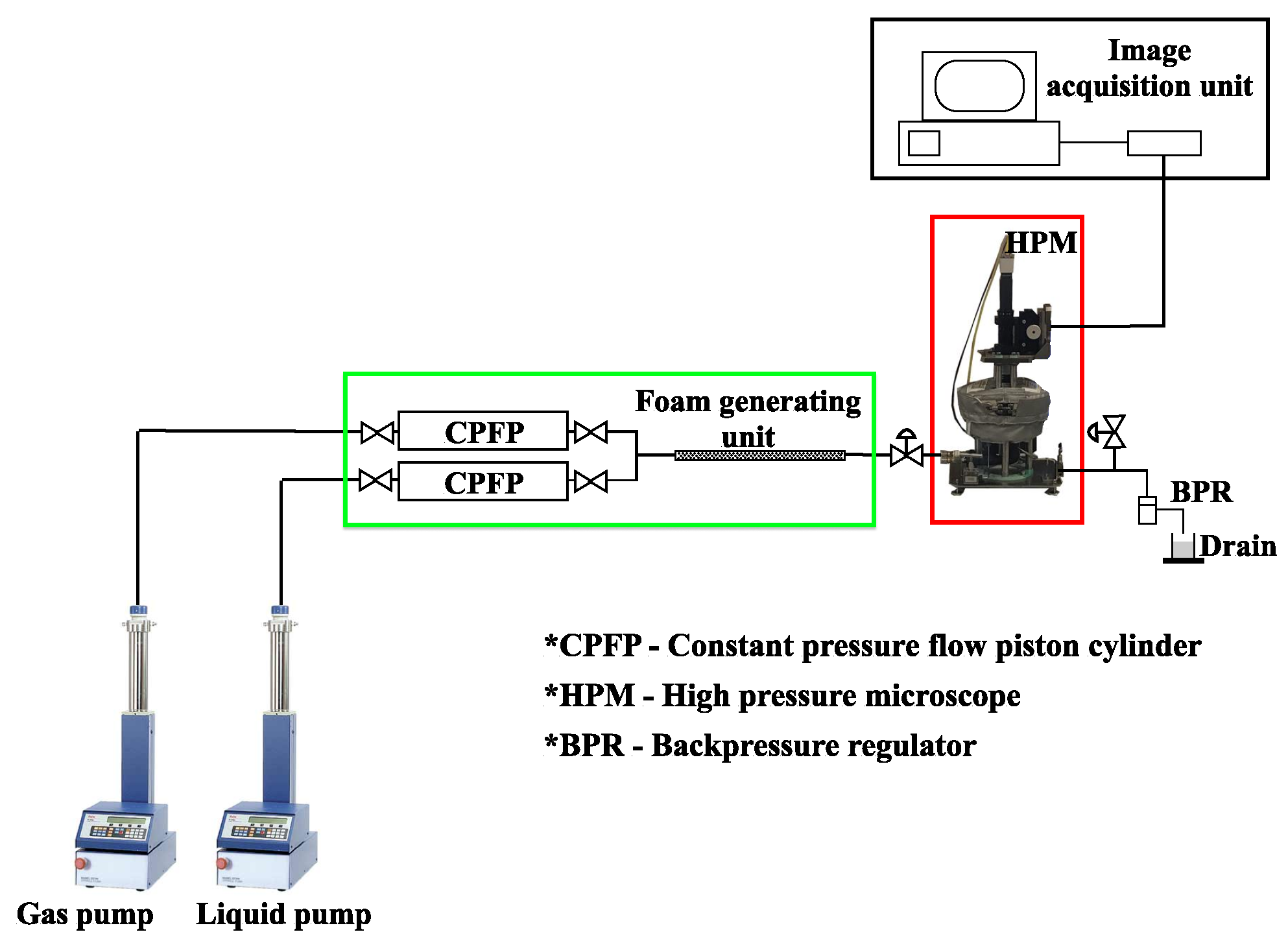
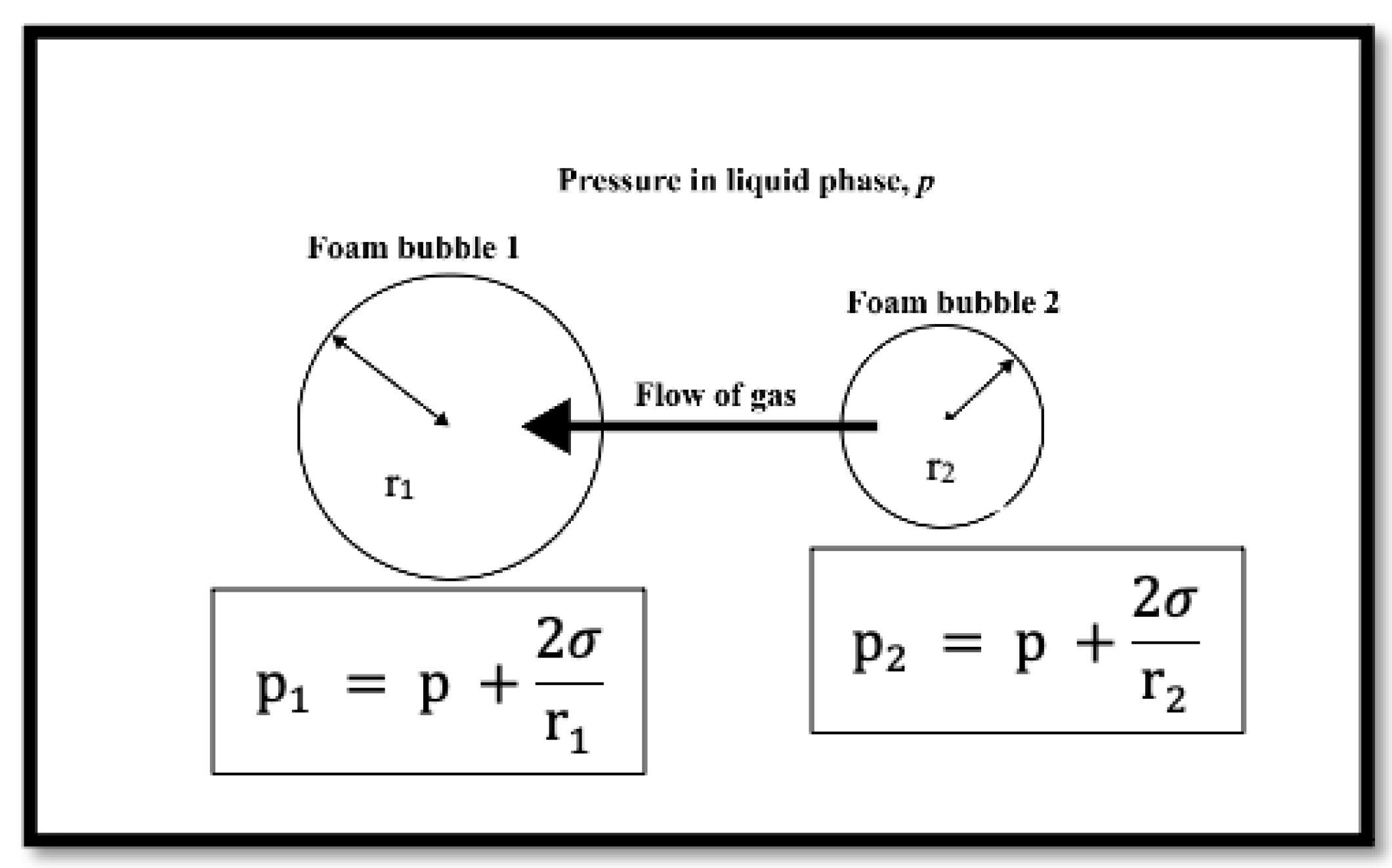
2.2.2. High-Pressure Microscopy Foam Stability
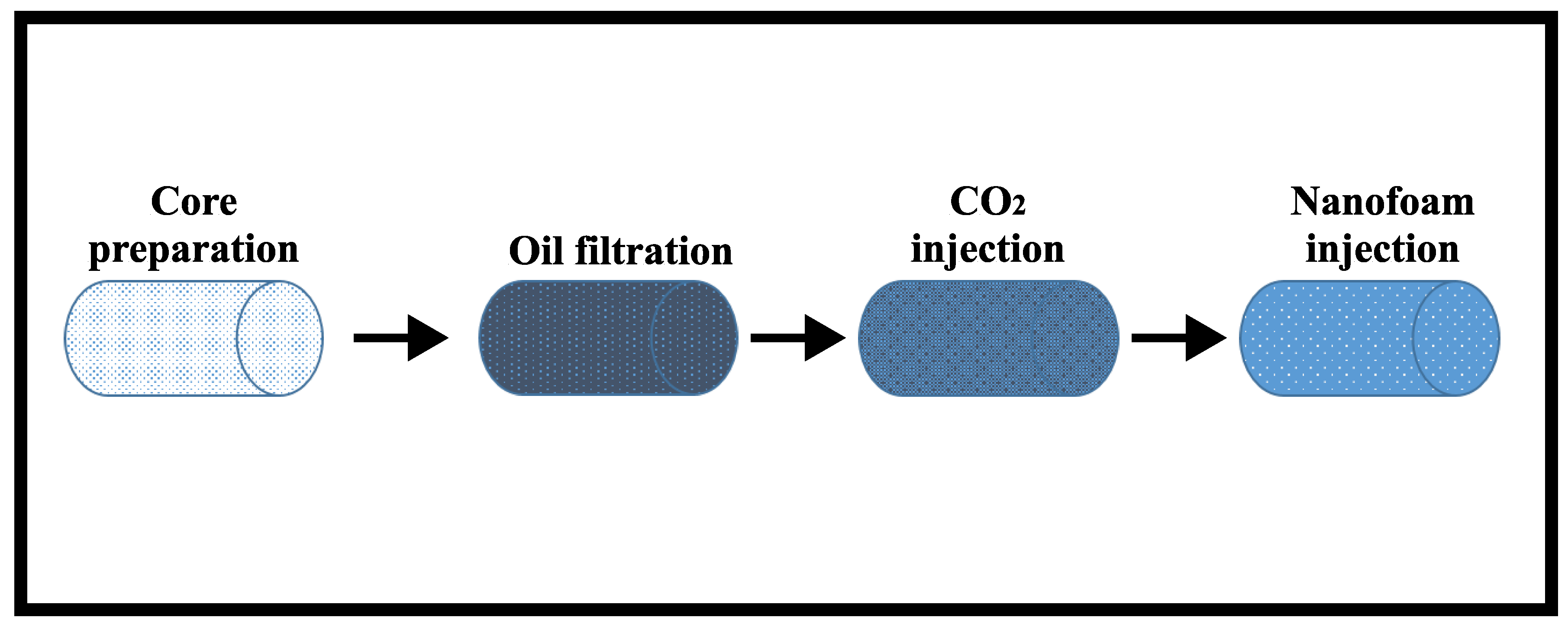
2.2.3. Preparation of Core Samples
2.2.4. Preparation of Oil for Filtration Experiment
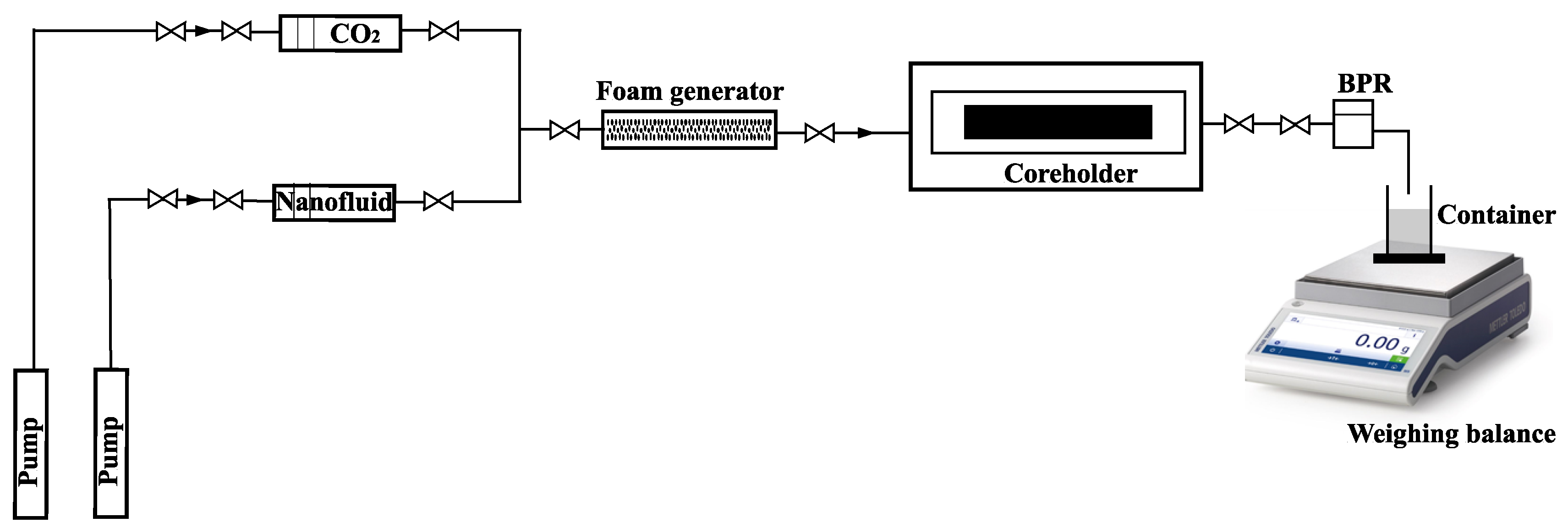
2.2.5. Filtration Experiments
- Stage I—During core preparation, the core samples were dried to a constant weight, and then, their filtration–capacitive properties were determined, which are summarised in Table 1. The cores were then saturated in water and centrifuged to create residual water saturation. Afterwards, the cores were filtered with decane in order to convert the system from atmospheric conditions to thermobaric conditions, since a recombined oil is used to represent the reservoir oil. Filtration with decane also serves as a test run experiment to determine if there are any cracks in the core sample or leakages within the core flooding unit.
- Stage II—To achieve irreducible water saturation, the filtration experiment began with the injection of oil. The injection process was repeated until the differential pressure stabilised. During oil filtration, thermobaric conditions (temperature of 38 °C and pressure of 20.8 MPa) were created by replacing the decane in the core sample with a recombined model of the reservoir oil.
- Stage III— was injected until a stable recovery factor with a constant flow rate was obtained. This was determined until a stable pressure gradient was obtained.
- Stage IV—Finally, nanofluid (low salinity brine + 0.3 wt.% AOS surfactant + 0.1 wt.% silica nanoparticles) and were injected as foam until a stable recovery factor was obtained, and then, the injection rate was increased.
3. Results and Discussion
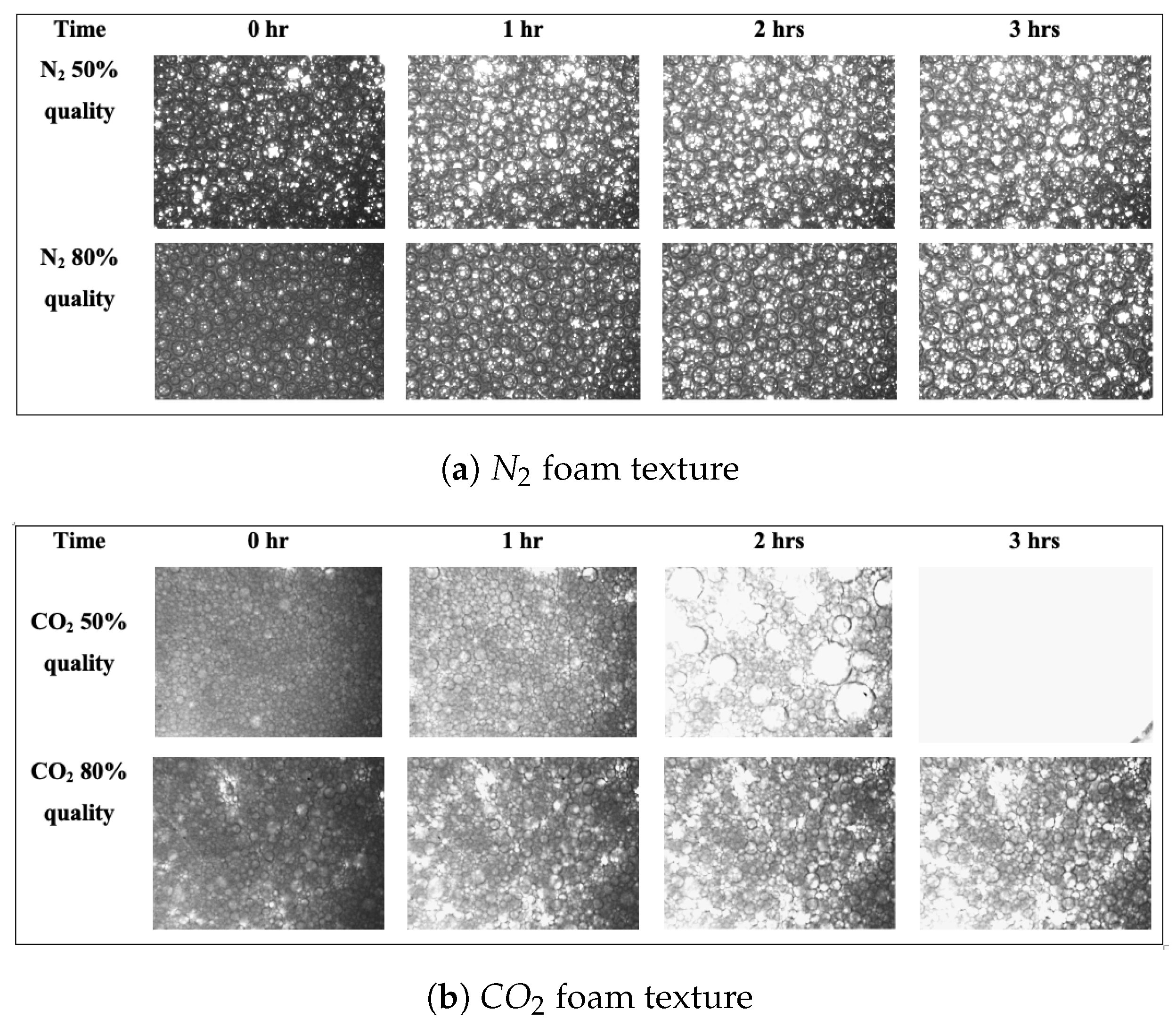
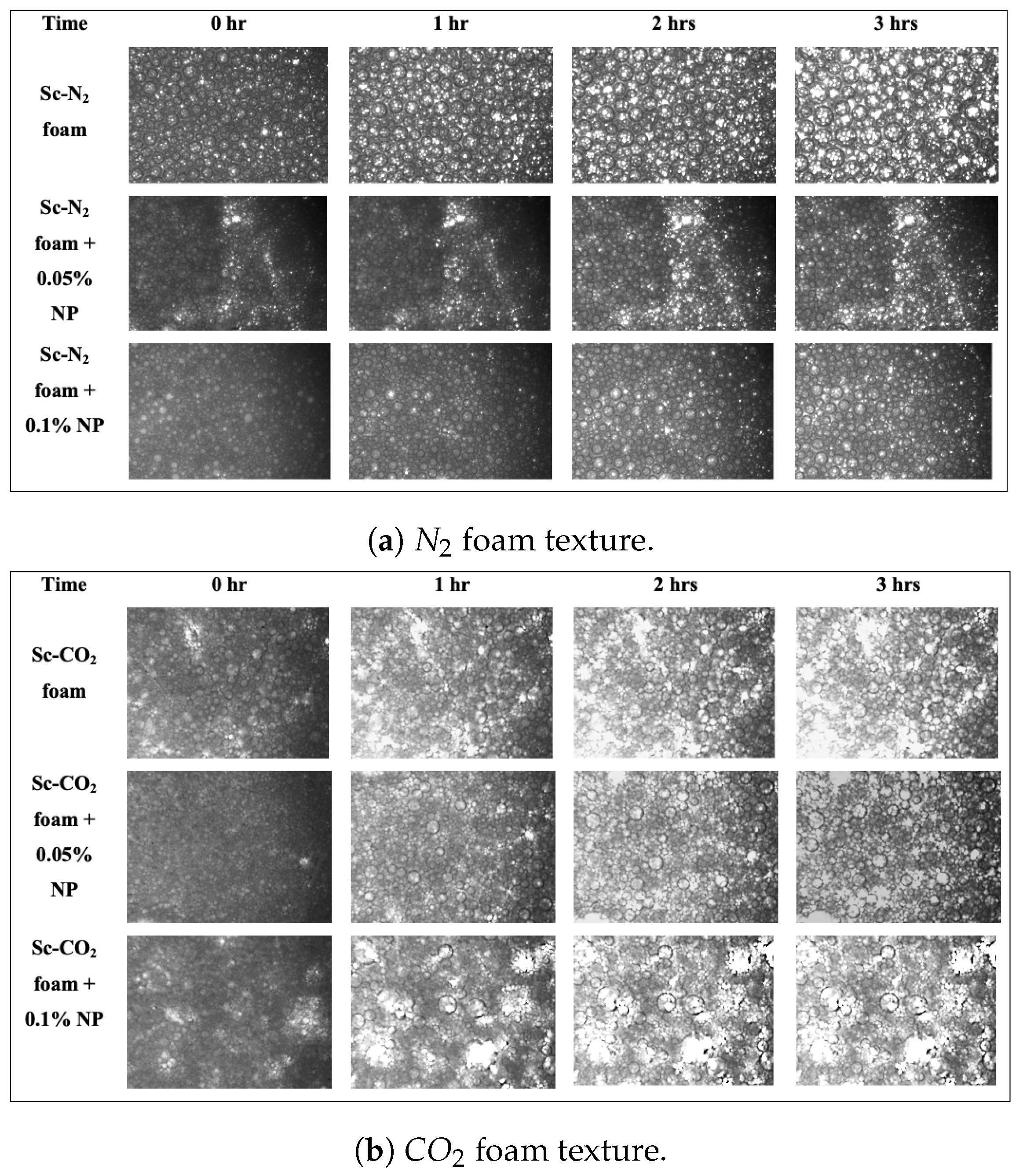
3.1. Effect of Foam Quality on the Stability of Foams without Nanoparticles
3.2. Effect of Nanoparticles on the Stability of Foams at High Pressure
3.3. Role of the Gas Phase in Foam Generation and Stability
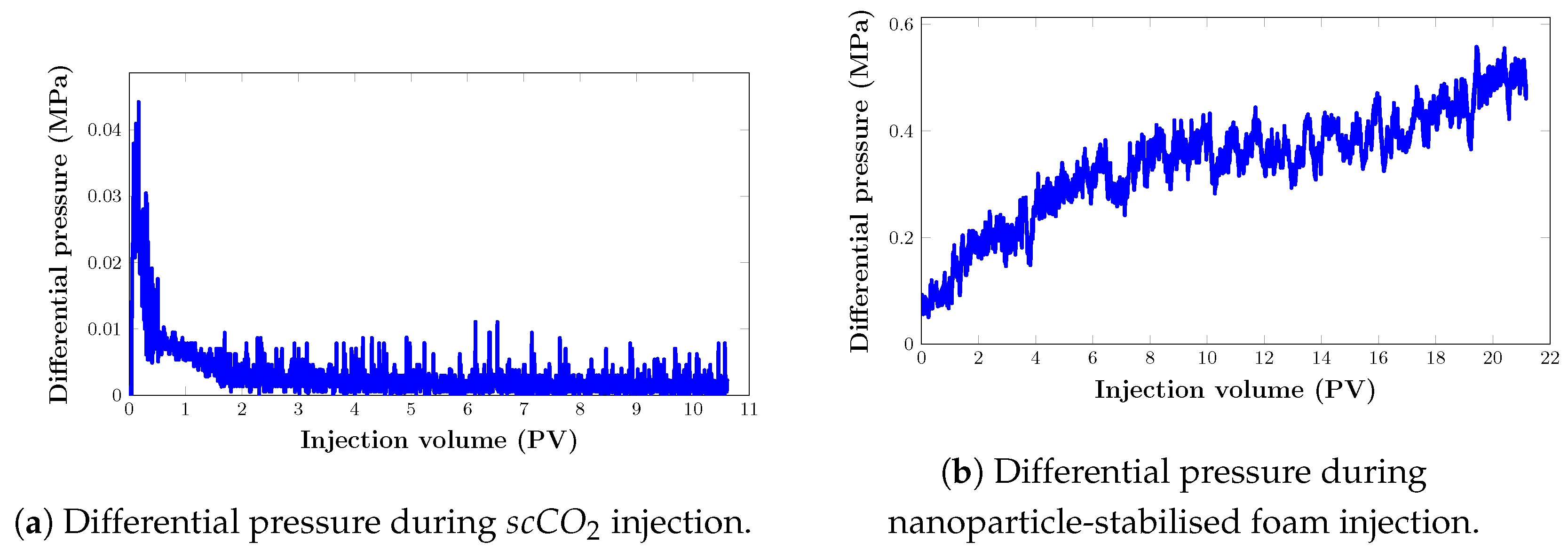
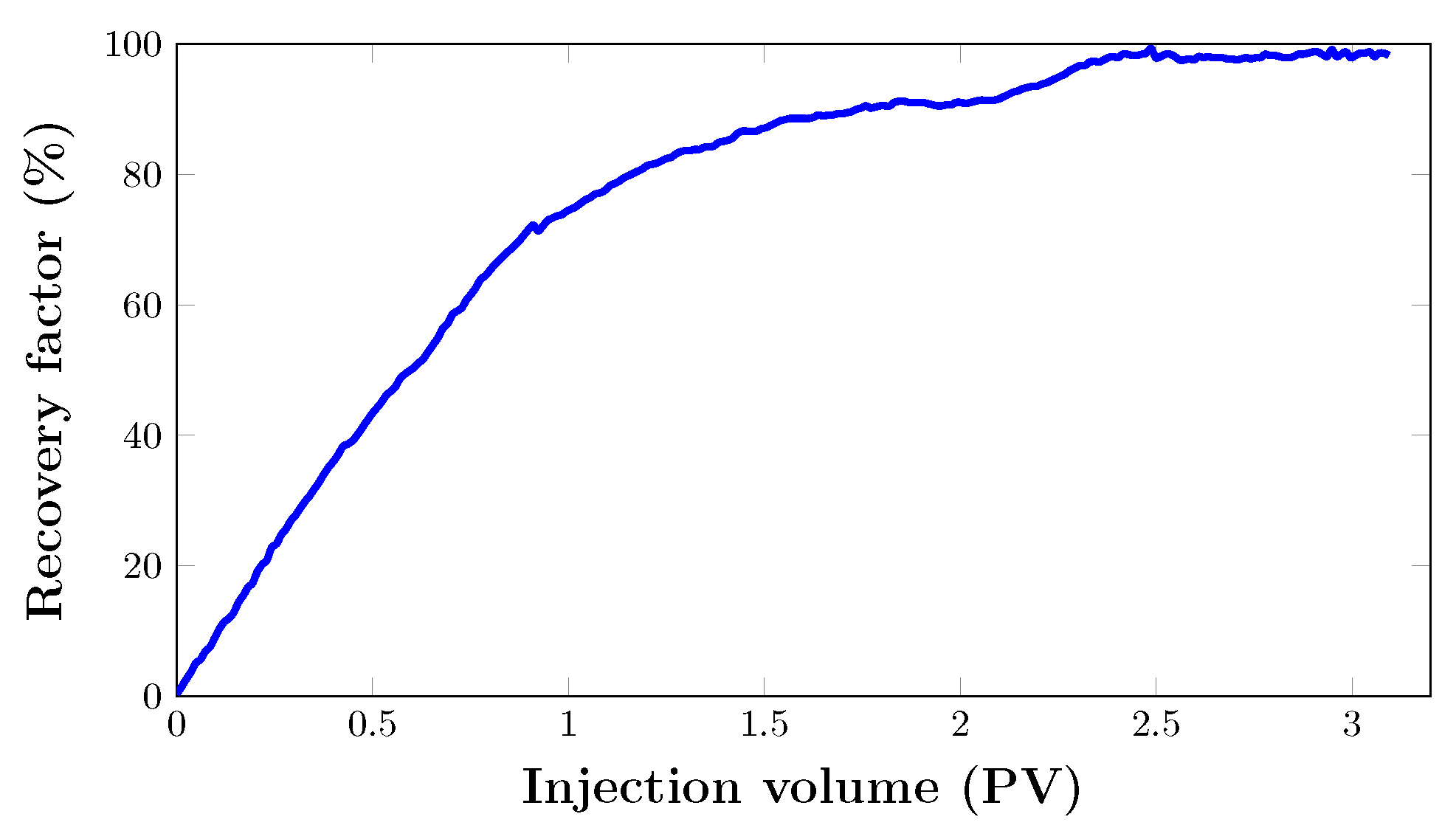
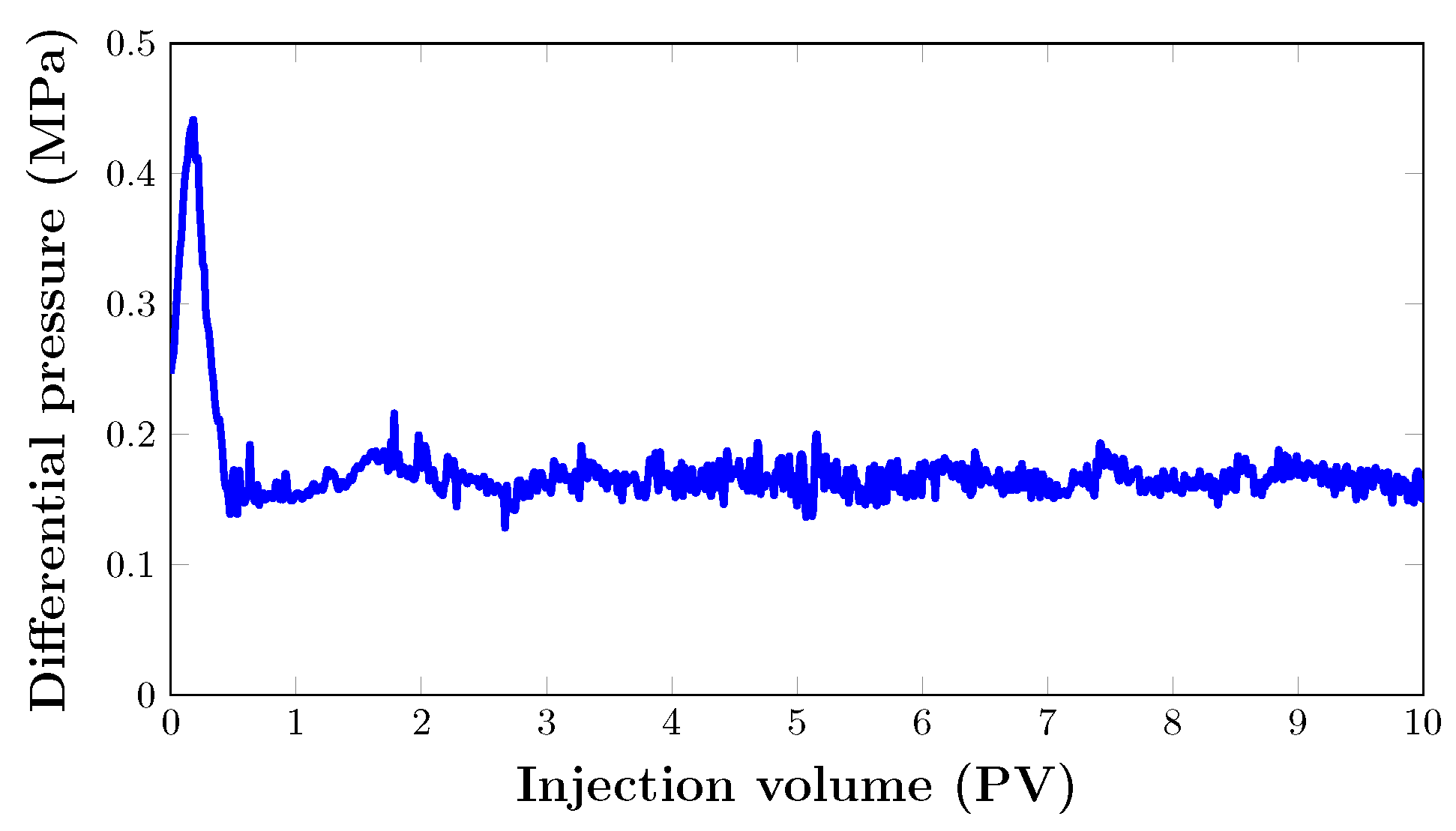
3.4. Oil Recovery Performance of Core Flooding Experiments
3.4.1. Core Flooding in an Artificial Core Sample (Set 1)
Oil Recovery Factor
Mobility Control
3.4.2. Core Flooding in a Real Core Sample with an Injection Pressure of 20.8 MPa (Set 2)
3.4.3. Core Flooding in a Real Core Sample with an Injection Pressure of 8.5 MPa (Set 3)
4. Conclusions
- Since easily dissolves in liquid, the stability of and foams significantly differs. Gas penetration between foam bubbles is significantly increased, which has a detrimental effect on foam stability.
- As pressure increases, the foam becomes more stable. This is more pronounced in foams. Foams generated with are significantly less stable than foams made with in both the vapour and supercritical states. However, generating foams with supercritical is more stable than with vapour because of the increased pressure.
- Core flooding tests showed that nanoparticle-stabilised foam helped to improve oil displacement efficiency from the porous media. At some point in both the artificial and real core samples, injection could no longer recover oil. However, implementing foam as a tertiary recovery technique resulted in recovery factors of 40.8% and 28.91%, respectively. This can be attributed to the enhanced viscosity of the propagating phase during the injection of foam, which helped to eliminate the gas breakthrough that was observed during the injection of .
- The mobility reduction factor in artificial core flooding was determined as 12.6, which suggests that the injected nanofoam was able to lower the mobility of in the porous medium by a factor of more than ten.
- Finally, the difference in EOR results could be seen in the implementation of foam injection in artificial and real core samples. Our study has demonstrated that these differences might be attributable to rock permeability, the length of the core sample, minimum miscibility pressure, and the mode of foam injection. Therefore, before performing laboratory core flooding studies, it is crucial to precisely identify, characterise, and validate these parameters.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johns, R.T.; Dindoruk, B. Chapter 18—In situ combustion. In Enhanced Oil Recovery Field Case Studies; Gulf Professional Publishing: Oxford, UK, 2013; pp. 1–22. [Google Scholar]
- Zhang, P.Y.; Huang, S.; Sayegh, S.; Zhou, X.L. Effect of CO2 impurities on gas-injection EOR processes. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 17–21 April 2004. [Google Scholar] [CrossRef]
- Song, Z.J.; Li, M.; Zhao, C.; Yang, Y.L.; Hou, J.R. Gas injection for enhanced oil recovery in two-dimensional geology-based physical model of Tahe fractured-vuggy carbonate reservoirs: Karst fault system. Pet. Sci. 2020, 17, 419–433. [Google Scholar] [CrossRef]
- Teletzke, G.F.; Patel, P.D.; Chen, A.L. Methodology for miscible gas injection EOR screening. In Proceedings of the SPE International Improved Oil Recovery Conference in Asia Pacific, Kuala Lumpur, Malaysia, 5–6 December 2005; pp. 315–325. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Z.; Lu, X.; Cheng, S.; He, Y.; Shi, L.; Xian, B.; Shi, T. Experimental Study on EOR Performance of Natural Gas Injection in Tight Oil Reservoirs. IOP Conf. Ser. Earth Environ. Sci. 2019, 252, 052021. [Google Scholar] [CrossRef]
- Hudgins, D.A.; Llave, F.M.; Chung, F.T. Nitrogen miscible displacement of light crude oil: A laboratory study. SPE Reserv. Eng. 1990, 5, 100–106. [Google Scholar] [CrossRef]
- Asgarpour, S. An Overview of Miscible Flooding. J. Can. Pet. Technol. 1994, 33, 5–12. [Google Scholar] [CrossRef]
- Awan, A.R.; Teigland, R.; Kleppe, J. A survey of North Sea enhanced-oil-recovery projects initiated during the years 1975 to 2005. SPE Reserv. Eval. Eng. 2008, 11, 497–512. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Andrianov, A.; Krastev, R.; Hirasaki, G.J.; Rossen, W.R. Foam-oil interaction in porous media: Implications for foam assisted enhanced oil recovery. Adv. Colloid Interface Sci. 2012, 183–184, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Afzali, S.; Rezaei, N.; Zendehboudi, S. A comprehensive review on Enhanced Oil Recovery by Water Alternating Gas (WAG) injection. Fuel 2018, 227, 218–246. [Google Scholar] [CrossRef]
- Chen, Q.; Gerritsen, M.G.; Kovscek, A.R. Modeling Foam Displacement with the Local-Equilibrium Approximation: Theory and Experimental Verification. SPE J. 2010, 15, 171–183. [Google Scholar] [CrossRef]
- Li, S.; Zhang, K.; Jia, N.; Liu, L. Evaluation of four CO2 injection schemes for unlocking oils from low-permeability formations under immiscible conditions. Fuel 2018, 234, 814–823. [Google Scholar] [CrossRef]
- Xu, Z.X.; Li, S.Y.; Li, B.F.; Chen, D.Q.; Liu, Z.Y.; Li, Z.M. A review of development methods and EOR technologies for carbonate reservoirs. Pet. Sci. 2020, 17, 990–1013. [Google Scholar] [CrossRef]
- Spirov, P.; Rudyk, S.N.; Khan, A.A. Foam assisted WAG, snorre revisit with new foam screening model. In Proceedings of the North Africa Technical Conference and Exhibition, Cairo, Egypt, 20–22 February 2012; Volume 1, pp. 624–641. [Google Scholar] [CrossRef]
- Bello, A.; Ivanova, A.; Cheremisin, A. Foam EOR as an Optimisation Technique for Gas EOR: A Comprehensive Review of Laboratory and Field Implementations. Energies 2023, 16, 972. [Google Scholar] [CrossRef]
- Yang, W.; Wang, T.; Fan, Z.; Miao, Q.; Deng, Z.; Zhu, Y. Foams Stabilized by in Situ-Modified Nanoparticles and Anionic Surfactants for Enhanced Oil Recovery. Energy Fuels 2017, 31, 4721–4730. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Wang, P. Experimental Study of the Stabilisation of CO2 Foam by Sodium Dodecyl Sulfate and Hydrophobic Nanoparticles. Ind. Eng. Chem. Res. 2016, 55, 1243–1253. [Google Scholar] [CrossRef]
- Karakashev, S.I.; Ozdemir, O.; Hampton, M.A.; Nguyen, A.V. Formation and stability of foams stabilized by fine particles with similar size, contact angle and different shapes. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 382, 132–138. [Google Scholar] [CrossRef]
- Yekeen, N.; Manan, M.A.; Idris, A.K.; Padmanabhan, E.; Junin, R.; Samin, A.M.; Gbadamosi, A.O.; Oguamah, I. A comprehensive review of experimental studies of nanoparticles-stabilized foam for enhanced oil recovery. J. Pet. Sci. Eng. 2018, 164, 43–74. [Google Scholar] [CrossRef]
- Singh, R.; Mohanty, K.K. Foam flow in a layered, heterogeneous porous medium: A visualisation study. Fuel 2017, 197, 58–69. [Google Scholar] [CrossRef]
- Bello, A.; Ozoani, J.; Adebayo, A.; Kuriashov, D. Rheological study of nanoparticle-based cationic surfactant solutions. Petroleum 2022, 8, 522–528. [Google Scholar] [CrossRef]
- Son, H.A.; Lee, T. Enhanced Oil Recovery with Size-Dependent Interactions of Nanoparticles Surface-Modified by Zwitterionic Surfactants. Appl. Sci. 2021, 11, 7184. [Google Scholar] [CrossRef]
- Maestro, A.; Rio, E.; Drenckhan, W.; Langevin, D.; Salonen, A. Foams stabilised by mixtures of nanoparticles and oppositely charged surfactants: Relationship between bubble shrinkage and foam coarsening. Soft Matter 2014, 10, 6975–6983. [Google Scholar] [CrossRef]
- Bello, A.; Ivanova, A.; Cheremisin, A. Application of Nanoparticles in Foam Flooding for Enhanced Oil Recovery and Foam Stability in Carbonate Reservoirs. In Proceedings of the 83rd EAGE Annual Conference & Exhibition, Madrid, Spain, 6–9 June 2022. [Google Scholar] [CrossRef]
- Zargartalebi, M.; Kharrat, R.; Barati, N. Enhancement of surfactant flooding performance by the use of silica nanoparticles. Fuel 2015, 143, 21–27. [Google Scholar] [CrossRef]
- Arain, Z.U.A.; Al-Anssari, S.; Ali, M.; Memon, S.; Bhatti, M.A.; Lagat, C.; Sarmadivaleh, M. Reversible and irreversible adsorption of bare and hybrid silica nanoparticles onto carbonate surface at reservoir condition. Petroleum 2020, 6, 277–285. [Google Scholar] [CrossRef]
- Horozov, T. Foams and foam films stabilised by solid particles. Curr. Opin. Colloid Interface Sci. 2008, 13, 134–140. [Google Scholar] [CrossRef]
- Kim, I.; Worthen, A.J.; Johnston, K.P.; DiCarlo, D.A.; Huh, C. Size-dependent properties of silica nanoparticles for Pickering stabilisation of emulsions and foams. J. Nanoparticle Res. 2016, 18, 82. [Google Scholar] [CrossRef]
- Kristiansen, T.; Holt, T. Properties of Flowing Foam in Porous Media Containing Oil. In Proceedings of the SPE/DOE Enhanced Oil Recovery Symposium, Tulsa, OK, USA, 22–24 April 1992. [Google Scholar] [CrossRef]
- Zitha, P.L.J.; Nguyen, Q.P.; Currie, P.K.; Buijse, M.A. Coupling of Foam Drainage and Viscous Fingering in Porous Media Revealed by X-ray Computed Tomography. Transp. Porous Media 2006, 64, 301–313. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Andrianov, A.; Bruining, H.; Zitha, P.L.J. Comparative Study of CO2 and N2 Foams in Porous Media at Low and High Pressure Temperatures. Ind. Eng. Chem. Res. 2009, 48, 6423–6431. [Google Scholar] [CrossRef]
- Singh, R.; Mohanty, K.K. Synergistic stabilisation of foams by a mixture of nanoparticles and surfactants. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014; Volume 3, pp. 1379–1391. [Google Scholar]
- Sun, Q.; Li, Z.; Li, S.; Jiang, L.; Wang, J.; Wang, P. Utilisation of surfactant-stabilized foam for enhanced oil recovery by adding nanoparticles. Energy Fuels 2014, 28, 2384–2394. [Google Scholar] [CrossRef]
- Bello, A.; Ivanova, A.; Cheremisin, A. Enhancing N2 and CO2 foam stability by surfactants and nanoparticles at high temperature and various salinities. J. Pet. Sci. Eng. 2022, 215, 110720. [Google Scholar] [CrossRef]
- Zhu, T.; Ogbe, D.O.; Khataniar, S. Improving the foam performance for mobility control and improved sweep efficiency in gas flooding. Ind. Eng. Chem. Res. 2004, 43, 4413–4421. [Google Scholar] [CrossRef]
- Farhadi, H.; Riahi, S.; Ayatollahi, S.; Ahmadi, H. Experimental study of nanoparticle-surfactant-stabilized CO2 foam: Stability and mobility control. Chem. Eng. Res. Des. 2016, 111, 449–460. [Google Scholar] [CrossRef]
- Yekeen, N.; Idris, A.K.; Manan, M.A.; Samin, A.M.; Risal, A.R.; Kun, T.X. Bulk and bubble-scale experimental studies of influence of nanoparticles on foam stability. Chin. J. Chem. Eng. 2017, 25, 347–357. [Google Scholar] [CrossRef]
- Caps, H.; Vandewalle, N.; Broze, G. Foaming dynamics in hele-shaw cells. Phys. Rev. E—Stat. Nonlinear Soft Matter Phys. 2006, 73, 5–8. [Google Scholar] [CrossRef]
- Yu, J.; Khalil, M.; Liu, N.; Lee, R. Effect of particle hydrophobicity on CO2 foam generation and foam flow behaviour in porous media. Fuel 2014, 126, 104–108. [Google Scholar] [CrossRef]
- Osei-Bonsu, K.; Shokri, N.; Grassia, P. Fundamental investigation of foam flow in a liquid-filled Hele-Shaw cell. J. Colloid Interface Sci. 2016, 462, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Tyrode, E.; Pizzino, A.; Rojas, O.J. Foamability and foam stability at high pressures and temperatures. I. Instrument validation. Rev. Sci. Instruments 2003, 74, 2925–2932. [Google Scholar] [CrossRef]
- Fu, C.; Liu, N. Rheology and stability of nanoparticle-stabilized CO2 foam under reservoir conditions. J. Pet. Sci. Eng. 2021, 196, 107671. [Google Scholar] [CrossRef]
- Stevenson, P. Inter-bubble gas diffusion in liquid foam. Curr. Opin. Colloid Interface Sci. 2010, 15, 374–381. [Google Scholar] [CrossRef]
- Koelsch, P.; Motschmann, H. Relating foam lamella stability and surface dilational rheology. Langmuir 2005, 21, 6265–6269. [Google Scholar] [CrossRef]
- Ivanova, A.; Cheremisin, A.; Spasennykh, M. Application of Nanoparticles in Chemical EOR. In Proceedings of the 19th European Symposium on Improved Oil Recovery, Stavanger, Norway, 24–27 April 2017. [Google Scholar] [CrossRef]
- Georgieva, D.; Cagna, A.; Langevin, D. Link between surface elasticity and foam stability. Soft Matter 2009, 5, 2063–2071. [Google Scholar] [CrossRef]
- Salameh, S.; Schneider, J.; Laube, J.; Alessandrini, A.; Facci, P.; Seo, J.W.; Ciacchi, L.C.; Mädler, L. Adhesion mechanisms of the contact interface of TiO 2 nanoparticles in films and aggregates. Langmuir 2012, 28, 11457–11464. [Google Scholar] [CrossRef]
- Dehdari, B.; Parsaei, R.; Riazi, M.; Rezaei, N.; Zendehboudi, S. New insight into foam stability enhancement mechanism, using polyvinyl alcohol (PVA) and nanoparticles. J. Mol. Liq. 2020, 307, 112755. [Google Scholar] [CrossRef]
- Binks, B.P.; Horozov, T.S. Aqueous foams stabilized solely by silica nanoparticles. Angew. Chem.—Int. Ed. 2005, 44, 3722–3725. [Google Scholar] [CrossRef]
- Johnston, K.P.; Shah, P.S. Making Nanoscale Materials with Supercritical Fluids. Science 2004, 303, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Cleaves, H.J. Supercritical Fluid. In Encyclopedia of Astrobiology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1636–1637. [Google Scholar] [CrossRef]
- Montalbán, M.G.; Víllora, G. Supercritical Fluids: Properties and Applications. In Phase Equilibria With Supercritical Carbon Dioxide—Application to the Components of a Biocatalytic Process; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Zeng, Y.; Farajzadeh, R.; Eftekhari, A.A.; Vincent-Bonnieu, S.; Muthuswamy, A.; Rossen, W.R.; Hirasaki, G.J.; Biswal, S.L. Role of gas type on foam transport in porous media. Langmuir 2016, 32, 6239–6245. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Orekhov, A.; Markovic, S.; Iglauer, S.; Grishin, P.; Cheremisin, A. Live imaging of micro and macro wettability variations of carbonate oil reservoirs for enhanced oil recovery and CO2 trapping/storage. Sci. Rep. 2022, 12, 1262. [Google Scholar] [CrossRef] [PubMed]
- Szabries, M.; Jaeger, P.; Amro, M.M. Foam Analysis at Elevated Pressures for Enhanced Oil Recovery Applications. Energy Fuels 2018, 33, 3743–3752. [Google Scholar] [CrossRef]
- Ghoodjani, E.; Bolouri, S. Experimental Study of CO2-EOR and N2-EOR with Focus on Relative Permeability Effect. J. Pet. Environ. Biotechnol. 2011, 2, 1–5. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Sheng, J.J. Effect of the Injection Pressure on Enhancing Oil Recovery in Shale Cores during the CO2 Huff-n-Puff Process When It Is above and below the Minimum Miscibility Pressure. Energy Fuels 2017, 31, 3856–3867. [Google Scholar] [CrossRef]
- Shyeh-Yung, J.G. Mechanisms of Miscible Oil Recovery: Effects of Pressure on Miscible and Near-Miscible Displacements of Oil by Carbon Dioxide. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 6–9 October 1991. [Google Scholar] [CrossRef]
- Song, C.; Yang, D. Performance Evaluation of CO2 Huff-n-puff Processes in Tight Oil Formations. In Proceedings of the SPE Unconventional Resources Conference Canada, Calgary, AB, Canada, 5–7 November 2013. [Google Scholar] [CrossRef]






| Experimental Set | Core Sample | Length (cm) | Diameter (cm) | Porosity (%) | Absolute Permeability (mD) |
|---|---|---|---|---|---|
| Set 1 | Artificial core | 7.58 | 2.91 | 11.68 | 102.81 |
| Set 2 | Real core 1 | 3.03 | 2.96 | 19.20 | 0.767 |
| Real core 2 | 3.11 | 2.97 | 15.67 | 0.743 | |
| Real core 3 | 3.09 | 2.96 | 15.53 | 0.576 | |
| Set 3 | Real core 4 | 2.84 | 2.96 | 10.91 | 0.359 |
| Real core 5 | 3.07 | 2.96 | 8.25 | 0.145 | |
| Real core 6 | 3.05 | 2.96 | 8.65 | 0.236 |
| Oil Model | Density (g/cm) | Viscosity (mPas) | Gas Factor |
|---|---|---|---|
| Oil model A | 0.915 | 3.13 | 72 |
| Oil model B | 0.785 | 1.71 | 60 |
| Average Bubble Size, m | t = 0 | t = 1 h | t = 2 h | t = 3 h |
|---|---|---|---|---|
| foam | 25.841 | 45.383 | 68.335 | 94.09 |
| foam with 0.05% NP | 15.279 | 37.131 | 59.413 | 73.382 |
| foam with 0.1% NP | 23.962 | 43.64 | 58.827 | 75.169 |
| Average Bubble Size, m | t = 0 | t = 1 h | t = 2 h | t = 3 h |
|---|---|---|---|---|
| foam | 7.682 | 35.502 | 53.607 | 82.374 |
| foam with 0.05% NP | 4.222 | 13.128 | 28.42 | 50.068 |
| foam with 0.1% NP | 5.881 | 21.822 | 44.746 | 72.180 |
| Bubble Count | t = 0 | t = 1 h | t = 2 h | t = 3 h |
|---|---|---|---|---|
| foam | 6825 | 4960 | 3493 | 3299 |
| foam with 0.05% NP | 9182 | 8070 | 6392 | 5460 |
| foam with 0.1% NP | 11,629 | 8299 | 6736 | 5959 |
| Bubble Count | t = 0 | t = 1 h | t = 2 h | t = 3 h |
|---|---|---|---|---|
| foam | 11,817 | 8705 | 5999 | 3932 |
| foam with 0.05% NP | 14,740 | 11,604 | 8293 | 4828 |
| foam with 0.1% NP | 14,636 | 13,848 | 12,751 | 12,078 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bello, A.; Ivanova, A.; Rodionov, A.; Aminev, T.; Mishin, A.; Bakulin, D.; Grishin, P.; Belovus, P.; Penigin, A.; Kyzyma, K.; et al. An Experimental Study of High-Pressure Microscopy and Enhanced Oil Recovery with Nanoparticle-Stabilised Foams in Carbonate Oil Reservoir. Energies 2023, 16, 5120. https://doi.org/10.3390/en16135120
Bello A, Ivanova A, Rodionov A, Aminev T, Mishin A, Bakulin D, Grishin P, Belovus P, Penigin A, Kyzyma K, et al. An Experimental Study of High-Pressure Microscopy and Enhanced Oil Recovery with Nanoparticle-Stabilised Foams in Carbonate Oil Reservoir. Energies. 2023; 16(13):5120. https://doi.org/10.3390/en16135120
Chicago/Turabian StyleBello, Ayomikun, Anastasia Ivanova, Alexander Rodionov, Timur Aminev, Alexander Mishin, Denis Bakulin, Pavel Grishin, Pavel Belovus, Artem Penigin, Konstantin Kyzyma, and et al. 2023. "An Experimental Study of High-Pressure Microscopy and Enhanced Oil Recovery with Nanoparticle-Stabilised Foams in Carbonate Oil Reservoir" Energies 16, no. 13: 5120. https://doi.org/10.3390/en16135120
APA StyleBello, A., Ivanova, A., Rodionov, A., Aminev, T., Mishin, A., Bakulin, D., Grishin, P., Belovus, P., Penigin, A., Kyzyma, K., & Cheremisin, A. (2023). An Experimental Study of High-Pressure Microscopy and Enhanced Oil Recovery with Nanoparticle-Stabilised Foams in Carbonate Oil Reservoir. Energies, 16(13), 5120. https://doi.org/10.3390/en16135120







