Crude Oil Pyrolysis Studies: Application to In Situ Superheat Steam Enhanced Oil Recovery
Abstract
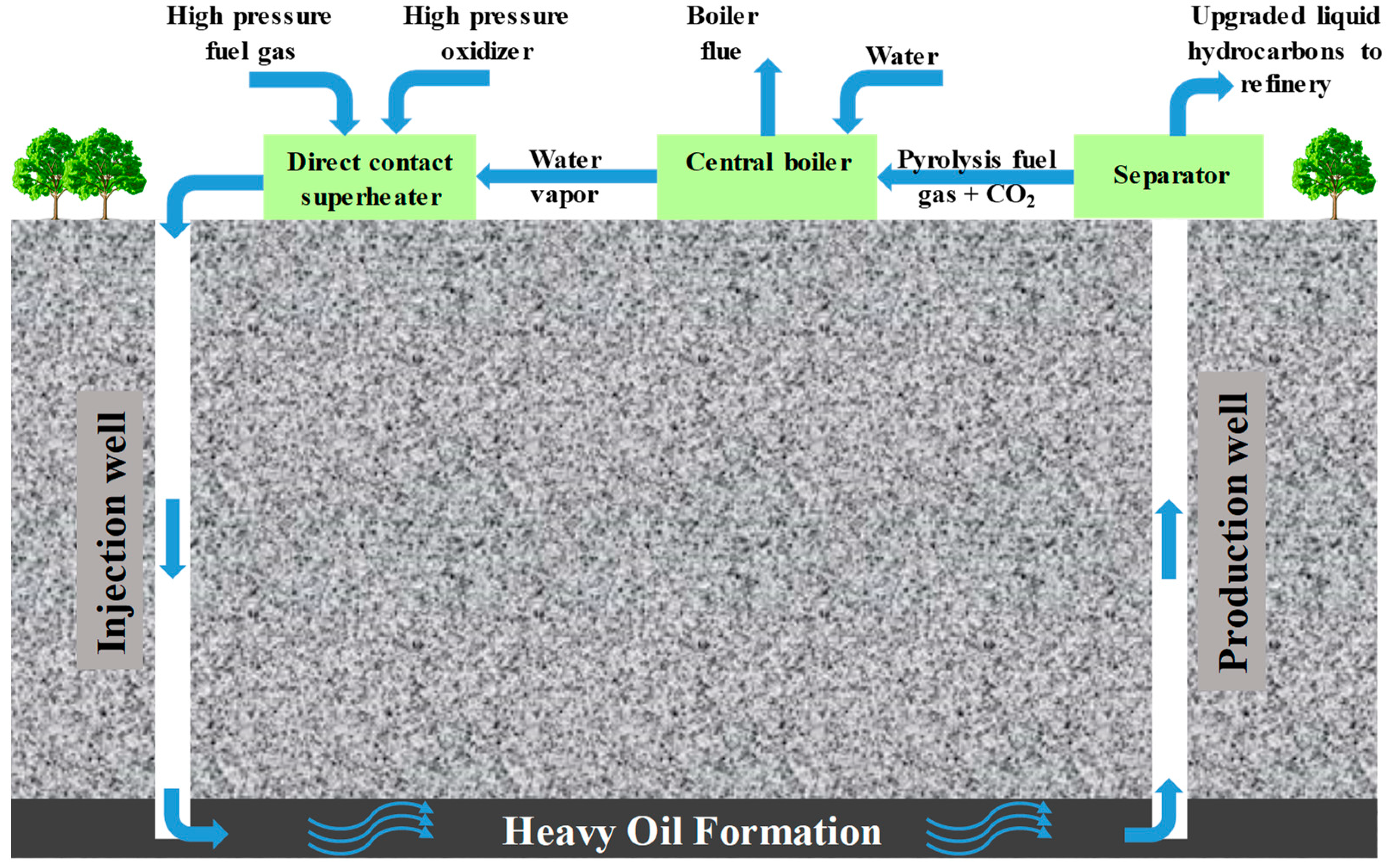
:1. Introduction
2. Experimental Details
2.1. Thermogravimetric Analysis and Differential Scanning Calorimetry
2.2. Mass Spectrometry
3. Results
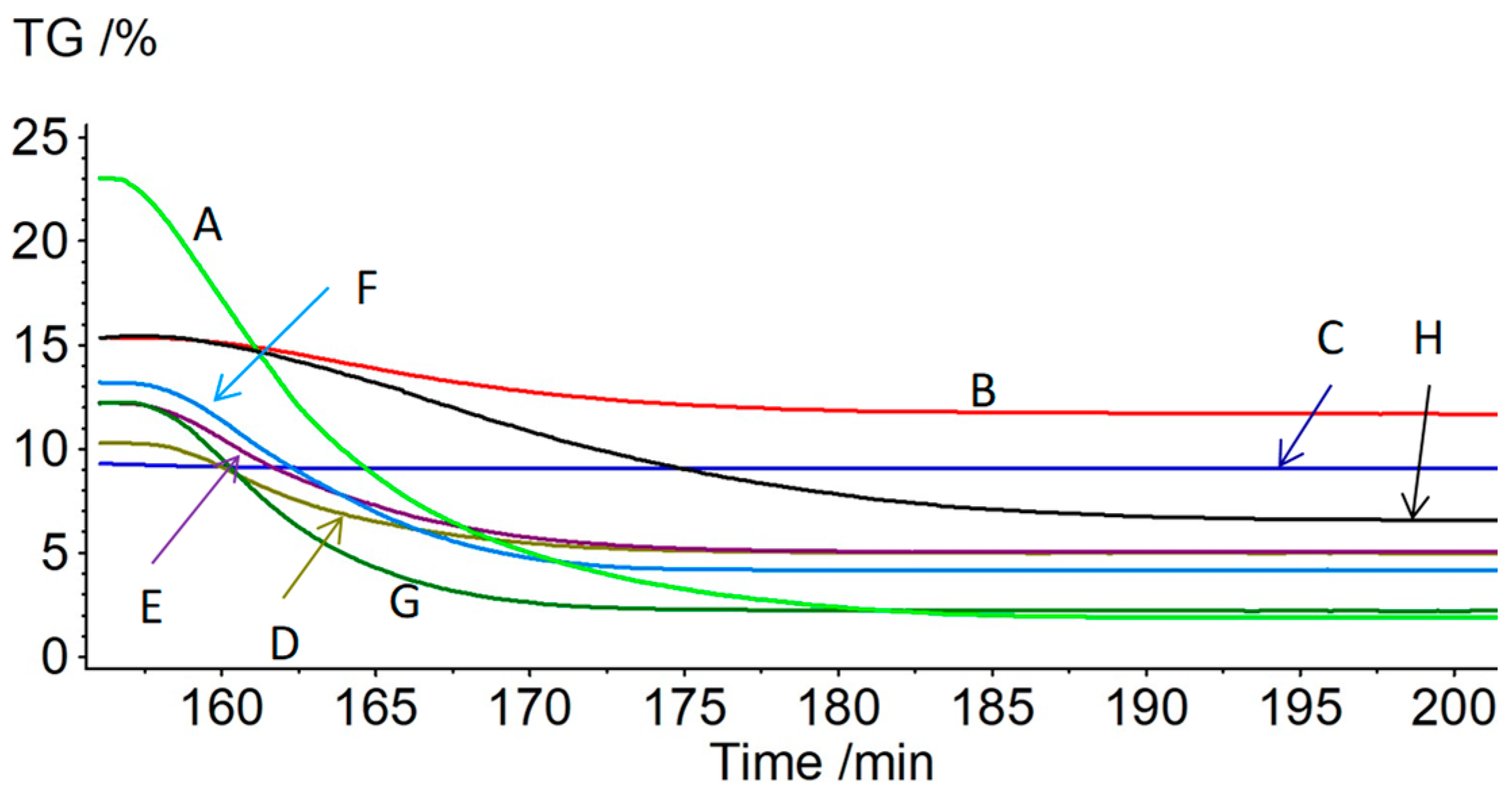
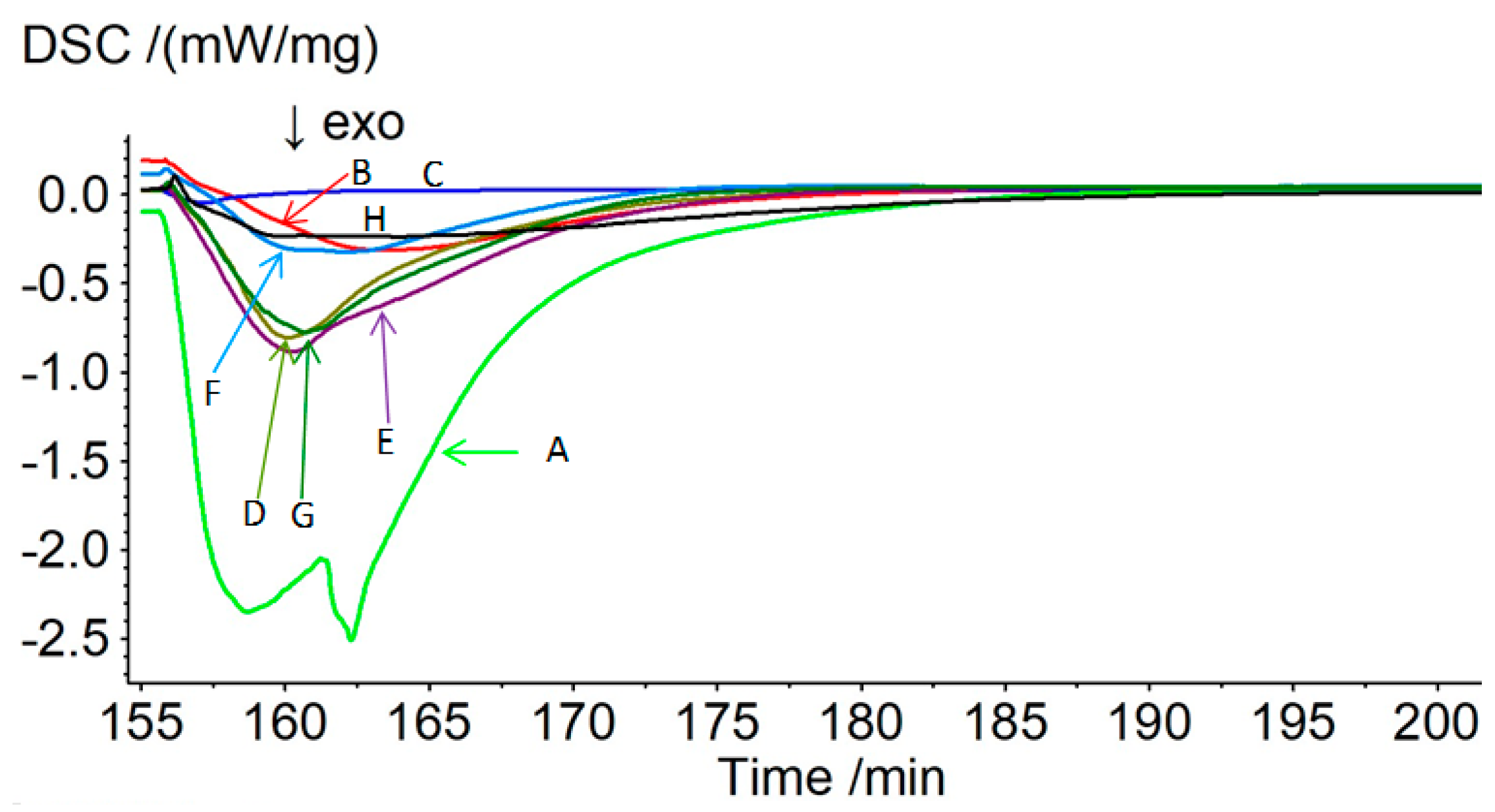
3.1. Pyrolysis under Moist Carbon Dioxide
3.2. Oxidation of Residual Char Post-Pyrolysis
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, A.Z.; Mu, L.X.; Wu, X.H.; Fan, Z.F.; Zhao, L. Superiority of Superheated Steam Flooding in Development of High Water-cut Heavy Oil Reservoir. Adv. Mater. Res. 2013, 616–618, 992–995. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, H.Q.; Pang, Z.X.; Li, F. A Study on Effect Evaluating and Parameters Sensitivity Analyzing of Superheated Steam Soak in Heavy Oil Reservoirs. Adv. Mater. Res. 2011, 239–242, 3069–3073. [Google Scholar] [CrossRef]
- Xu, A.; Mu, L.; Fan, Z.; Wu, X.; Zhao, L.; Bo, B.; Xu, T. Mechanism of Heavy Oil Recovery by Cyclic Superheated Steam Stimulation. J. Pet. Sci. Eng. 2013, 111, 197–207. [Google Scholar] [CrossRef]
- Xu, A.; Fan, Z.; Zhao, L.; Xue, X.; Wang, C. Significant Superiorities of Superheated Steam in Heavy Oil Thermal Recovery. Adv. Pet. Explor. Dev. 2012, 4, 46–51. [Google Scholar]
- Wu, Y.; Ma, D.; Liu, S. EOR of Superheated Steam Injection in Shallow Heavy Oil Reservoir: A Case Study. In Proceedings of the International Oil and Gas Conference, Beijing, China, 8–10 June 2010. [Google Scholar]
- Zhao, X.; Gao, P.; Wu, Y. Superheated Steam Injection in North Кенкияк Shallow Heavy Oil Reservoir. J. Geol. Min. Res. 2013, 5, 136–146. [Google Scholar] [CrossRef]
- Zhou, T.Y. Improvement of Porous Medium Permeability by Injecting Overheated Steam. Adv. Mater. Res. 2013, 668, 279–282. [Google Scholar] [CrossRef]
- John, S.; Sperry, F.S.; Robert, Y., Jr.; Poston, S. Field Testing of the Vapor Therm™ Process in the Eastburn (Cherokee) Field Vernon Co., Missouri. In Proceedings of the Fifth Annual Department of Energy Symposium on Enhanced Oil and Gas Recovery and Improved Drilling Technology, Tulsa, OK, USA, 22–24 August 1979; Linville, B., Ed.; Bartlesville Energy Technology Center, Carmel Energy, Inc.: Bartlesville, OK, USA, 1979; Volume 2, pp. H1/1–H1/16. [Google Scholar]
- NASA CEA. Available online: https://cearun.grc.nasa.gov/ (accessed on 20 December 2022).
- Listiohadi, Y.; Hourigan, J.; Sleigh, R.; Steele, R. Thermal Analysis of Amorphous Lactose and α-Lactose Monohydrate. Dairy Sci. Technol. 2009, 89, 43–67. [Google Scholar] [CrossRef]
- Manapov, E.; Ishkil’din, A.; Akhmetov, A. Hydrovisbreaking Petroleum Resids. Chem. Technol. Fuels Oils 1997, 33, 251–253. [Google Scholar] [CrossRef]
- Chiesa, P.; Romano, M.C.; Kreutz, T.G. Use of Membranes in Systems for Electric Energy and Hydrogen Production from Fossil Fuels. In Handbook of Membrane Reactors; Woodhead Publishing: Sawston, UK, 2013; Volume 2, pp. 416–455. [Google Scholar]


| Compound; Atomic Weight | Major Fragmentation Products/m/z | Analyzed Signal/m/z |
|---|---|---|
| Hydrogen; 2 | 2, 1 | 2 |
| Methane; 16 | 16, 15, 14, (13, 2, 12, 17) | 15 |
| Water; 18 | 18, 17, (16) | 18 |
| Carbon monoxide; 28 | 28, (12, 29) | - |
| Nitrogen; 28 | 28, (14) | - |
| Ethylene; 28 | 28, 27, 26, 25, (14, 24, 13, 29, 12) | - |
| Ethane; 30 | 28, 27, 30, 26, (12, 25, 14) | 27 |
| Oxygen; 32 | 32, 16 | - |
| Propylene; 42 | 41, 39, 42, 27, 40, 38, 37, 26, (15, 14) | - |
| Propane; 44 | 29, 26, 28, 15, 27, 44, 14, 43, 39, 41, (42, 38, 37, 40, 30) | 29 |
| Carbon dioxide; 44 | 44, 28, (16, 12, 45, 22) | 44 |
| Benzene; 78 | 78, 52, 51, 50, 77, 39, (38) | 78 |
| Oil | API. Deg. | Peak H2 % | T, C @ Peak H2 | Peak CH4% | T, C @ Peak CH4 | Peak C2H6 % | T, C@ Peak C2H6 |
|---|---|---|---|---|---|---|---|
| Bakkens | 35 | NA | NA | NA | NA | NA | NA |
| SPR | ~35 | 2.6 | 490 | 2.3 | 490 | 3.6 | 485 |
| Monterrey 16-31 | 14 | 2.4 | 480 | 1.3 | 480 | 1.3 | 460 |
| Diatomite 1312 | 14 | 2.2 | 460 | 0.8 | 450 | 1.1 | 440 |
| Orcutt S2–S6 | 14 | 3.9 | 470 | 1.5 | 460 | 1.7 | 450 |
| Orcutt S!B | 14 | 2.5 | 480 | 1.1 | 465 | 1.3 | 450 |
| Grassy Creek | 16 | 5.9 | 500 | 4.3 | 505 | 4.8 | 490 |
| Specimen; (API Gravity/°) | Mass Loss during Pyrolysis/% | Mass Loss during Oxidation/% | Residue after Oxidation /% | Enthalpy of Oxidation a/J g−1 | Enthalpy of Oxidation b/J g−1 |
|---|---|---|---|---|---|
| Lactose; (N/A) | 76.5 | 21.7 | 1.82 | −1411 | −6497 |
| SPR; (35) | 84.2 | 4.21 | 11.58 | −364.7 | −8668 |
| Bakken; (35) | 90.7 | 0.23 | 9.07 | −11.61 | −5046 |
| Monterey Well 16-31; (14) | 89.7 | 5.33 | 4.97 | −384.9 | −7221 |
| Diatomite Well 1312; (14) | 87.8 | 7.18 | 5.04 | −498.3 | −6929 |
| Orcutt zones S2–S6; (14) | 86.8 | 9.00 | 4.2 | −243.4 | −2704 |
| Orcutt zone S1B; (14) | 87.7 | 10.13 | 2.19 | −396.4 | −3907 |
| Grassy Creek #1; (16) | 84.3 | 9.23 | 6.51 | −293.7 | −3174 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coker, E.N.; Donaldson, B.; Hughes, B.; Yilmaz, N. Crude Oil Pyrolysis Studies: Application to In Situ Superheat Steam Enhanced Oil Recovery. Energies 2023, 16, 1544. https://doi.org/10.3390/en16031544
Coker EN, Donaldson B, Hughes B, Yilmaz N. Crude Oil Pyrolysis Studies: Application to In Situ Superheat Steam Enhanced Oil Recovery. Energies. 2023; 16(3):1544. https://doi.org/10.3390/en16031544
Chicago/Turabian StyleCoker, Eric N., Burl Donaldson, Brian Hughes, and Nadir Yilmaz. 2023. "Crude Oil Pyrolysis Studies: Application to In Situ Superheat Steam Enhanced Oil Recovery" Energies 16, no. 3: 1544. https://doi.org/10.3390/en16031544
APA StyleCoker, E. N., Donaldson, B., Hughes, B., & Yilmaz, N. (2023). Crude Oil Pyrolysis Studies: Application to In Situ Superheat Steam Enhanced Oil Recovery. Energies, 16(3), 1544. https://doi.org/10.3390/en16031544








