Sewage Sludge Gasification Process Optimization for Combined Heat and Power Generation
Abstract
1. Introduction
- It reduces the pressure on finite fossil fuel reserves to generate electricity to run WWTPs, as the electrical energy can support a fraction of the demand to complete the wastewater treatment;
- -
- Energy recovery potential of SS as CHP;
- -
- CO2 emission reduction over a gas turbine system;
- -
- Emission profile to assess the pollutant concentration.
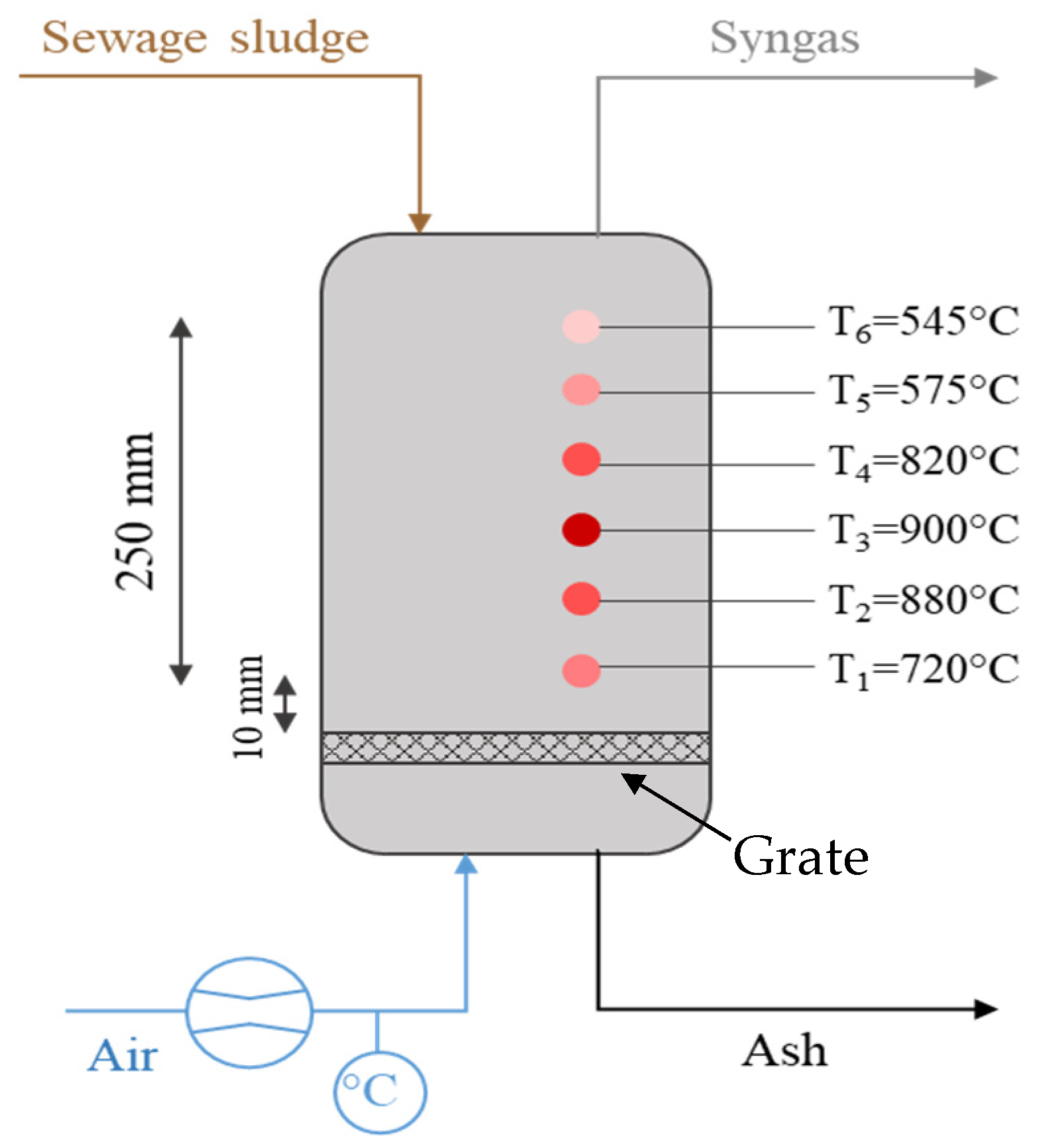
2. Experimental Analysis: Sewage Sludge to Syngas
2.1. Sewage Sludge Collection and Characterization
2.2. Energy Recovery from Sewage Sludge
3. Aspen plus Simulation on the Transformation of Sewage Sludge Energy Content to Combined Heat and Power
3.1. Gasification Modeling
- The model was zero-dimensional;
- The stream fed to the pyrolysis zone was instantaneously disintegrated;
- There was no temperature gradient inside the gasifier in the radial direction, which ensured isothermal conditions;
- The hydrodynamic features of the gasifier were neglected;
- Equilibrium conditions were reached by all the considered gasification reactions;
- Intermediate product formation pathways during the gasification process were not considered;
- Char was full of carbon and combusted to generate thermal energy;
- Ideal behavior was shown by all the gaseous components;
- Gasification was completed under atmospheric pressure;
- Tar formation was not assessed as assumed by other researchers during SS gasification model development [9,28,29,44,49]. The LHV of syngas generated from biomass, including SS deposition, changed with the tar content deposition due to the alteration in the operating conditions. The tar content was deposited at higher temperatures and lower ER. However, the effect on syngas LHV alteration was less than 5% [37,62]. Therefore, neglecting tar formation did not significantly affect the potentiality of electrical and thermal energy cogeneration, which was the main aim of the current research.
Calibration and Validation of Gasification Model
3.2. Cogeneration Modeling
- Steady-state condition;
- Potential and kinetic energy remains unchanged, and pressure drops in the different blocks connected to complete the ICE system are neglected
3.3. Process Performance Evaluation
3.3.1. Gasification System
3.3.2. Cogeneration System
3.4. Cogeneration Process Simulation: Operating Parameters
4. Results and Discussion
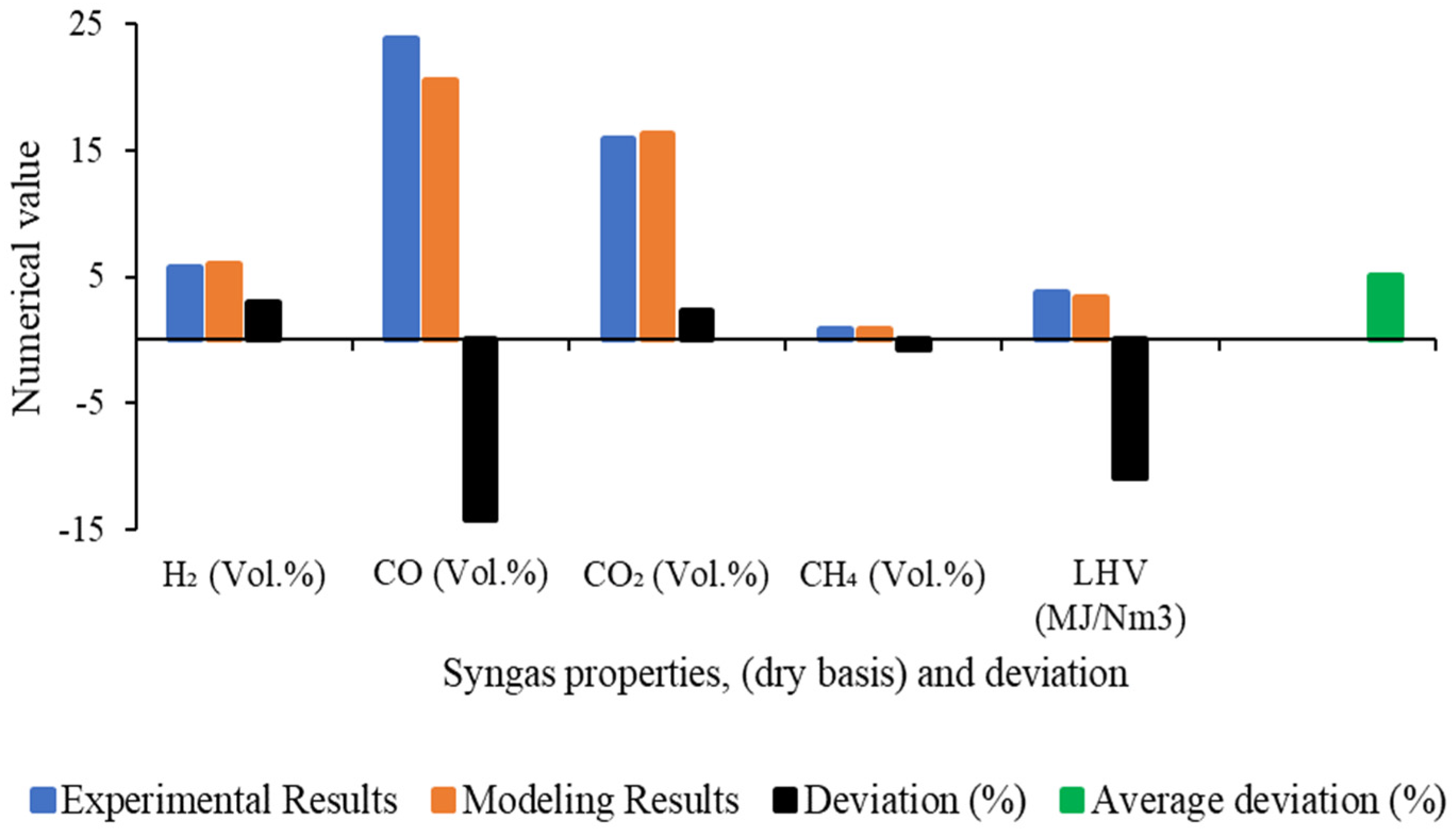
4.1. Development of Gasification Model
4.2. Sensitivity Analysis
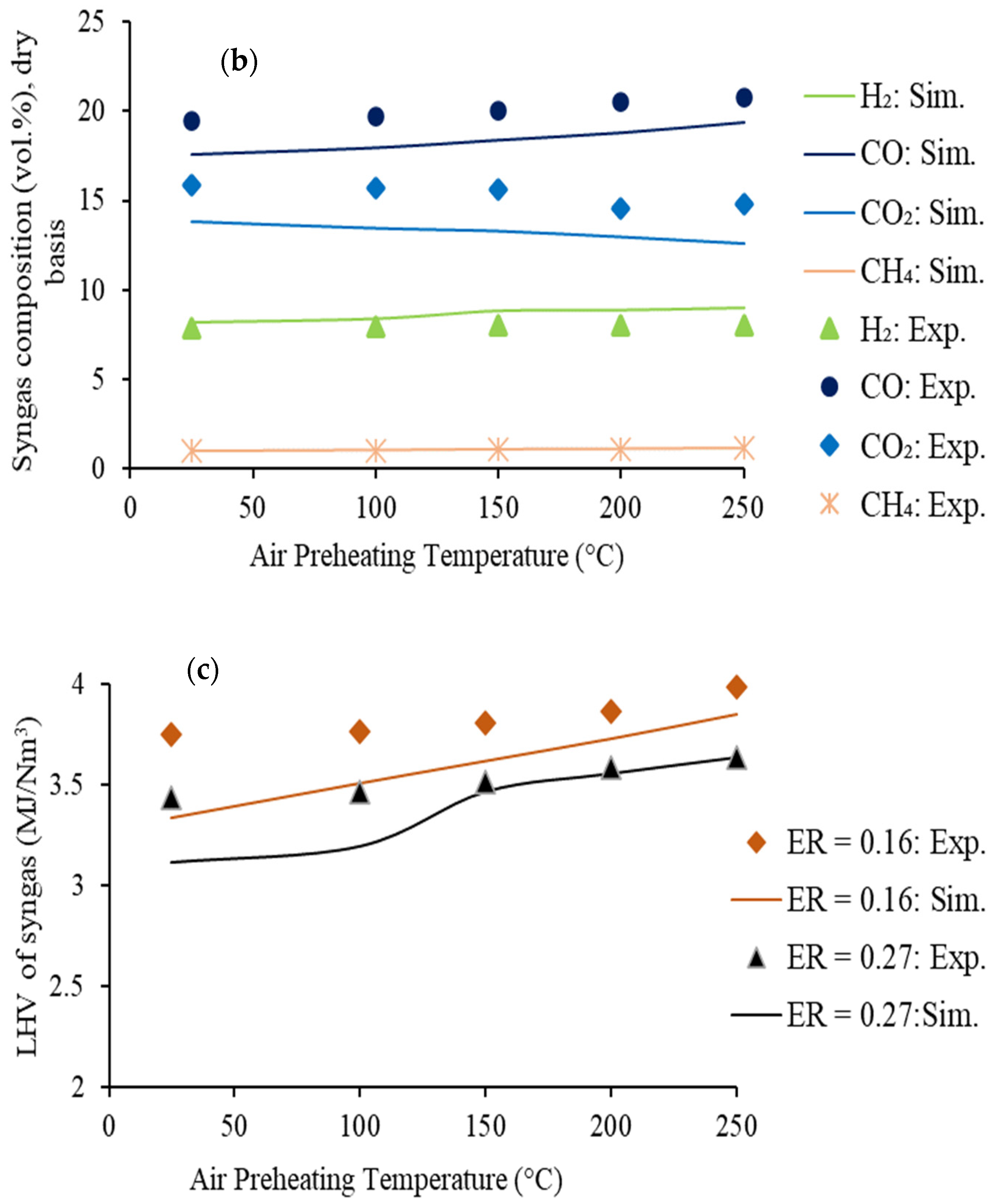
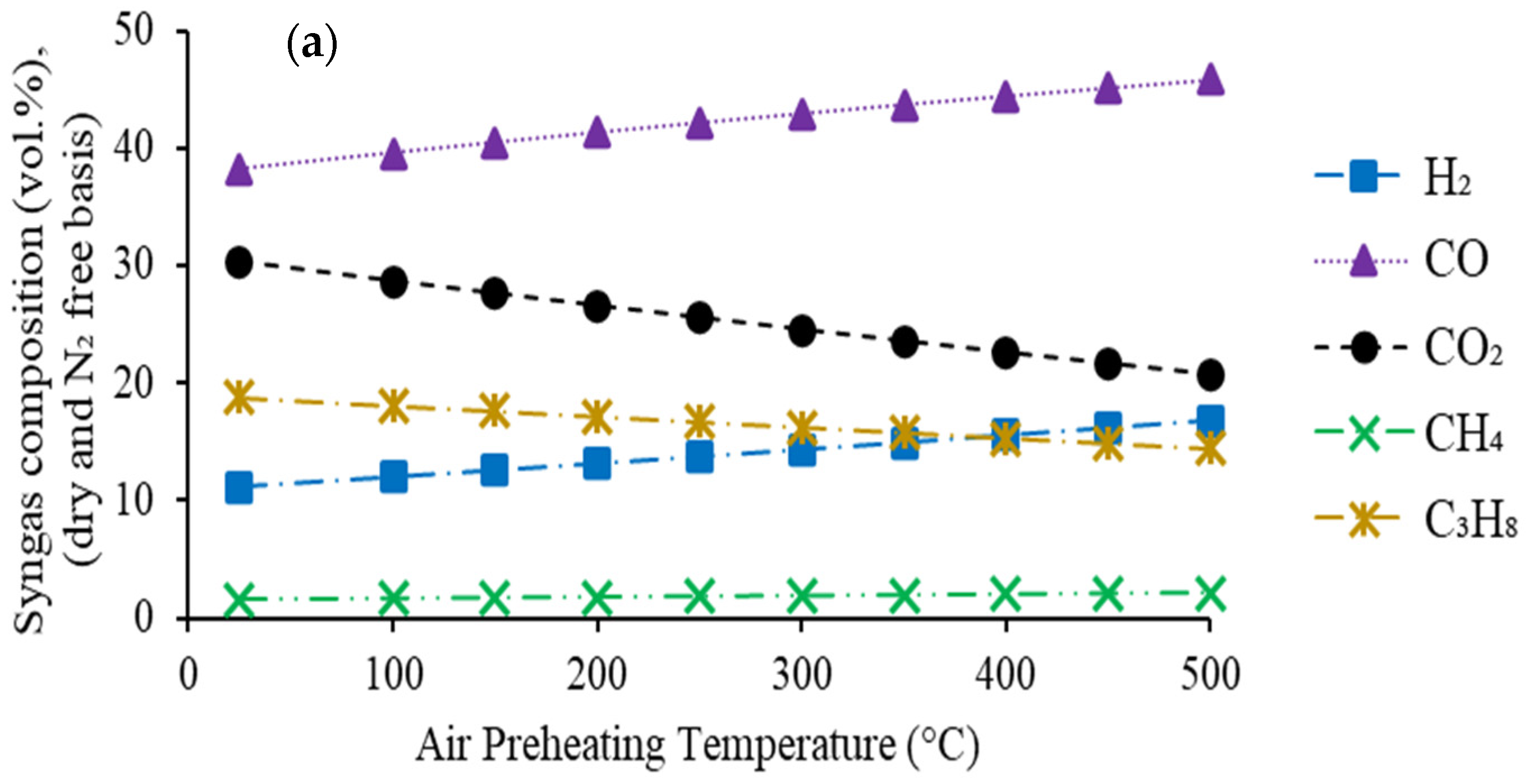
4.2.1. Effect of Air Preheating Temperature
- -
- the concentration of two syngas components (CO and H2) in syngas increases continuously
- -
- the concentration of other two components (CO2 and C3H8) shows a decreasing trend
- -
- the concentration of CH4 shows a slowly increasing trend.
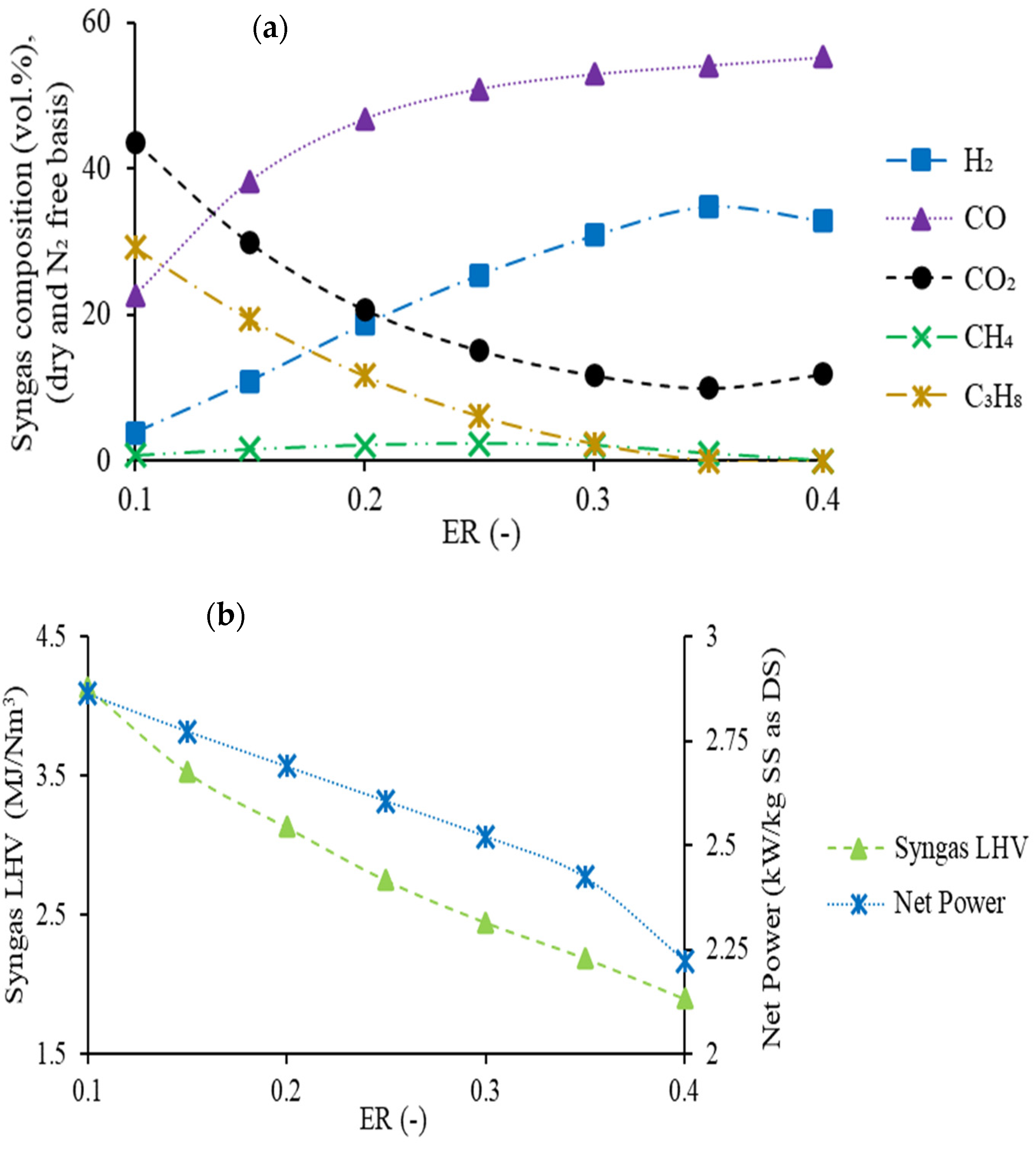
4.2.2. Influence of the Equivalence Ratio
4.3. Cogeneration Process Performances
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IEA Key World Energy Statistics 2021, IEA, Paris. Available online: https://www.iea.org/reports/key-world-energy-statistics-2021 (accessed on 12 December 2022).
- Ritchie, H.; Roser, M. Energy. Available online: https://ourworldindata.org/energy (accessed on 10 January 2022).
- Roser, M. Future Population Growth. Available online: https://ourworldindata.org/future-population-growth (accessed on 13 December 2022).
- Londoño-Pulgarin, D.; Cardona-Montoya, G.; Restrepo, J.C.; Muñoz-Leiva, F. Fossil or bioenergy? Global fuel market trends. Renew. Sustain. Energy Rev. 2021, 143, 110905. [Google Scholar] [CrossRef]
- Sharma, P.; Sivaramakrishnaiah, M.; Deepanraj, B.; Saravanan, R.; Reddy, M.V. A novel optimization approach for biohydrogen production using algal biomass. Int. J. Hydrogen Energy 2022, in press. [CrossRef]
- Hossain, M.S.; ul Karim, T.; Onik, M.H.; Kumar, D.; Rahman, M.A.; Yousuf, A.; Uddin, M.R. Impact of temperature, inoculum flow pattern, inoculum type, and their ratio on dry anaerobic digestion for biogas production. Sci. Rep. 2022, 12, 6162. [Google Scholar] [CrossRef]
- Uddin, M.N.; Salam, M.A.; Uddin, M.R.; Khan, M.R. Biodiesel from Nahor Seed Oil: Synthesis, Evaluation, and Compatibility with Petrodiesel. Pet. Chem. 2022, 62, 1211–1222. [Google Scholar] [CrossRef]
- IEA Global Energy Review: CO2 Emissions in 2020, IEA, Paris. Available online: https://www.iea.org/articles/global-energy-review-co2-emissions-in-2020 (accessed on 12 December 2022).
- Di Fraia, S.; Massarotti, N.; Uddin, M.R.; Vanoli, L. Conversion of Sewage Sludge to combined heat and power: Modeling and optimization. Smart Energy 2021, 5, 100061. [Google Scholar] [CrossRef]
- European Commission Environmental, Economic and Social Impacts of the Use of Sewage Sludge on Land. Consult. Rep. Options Impacts, Rep. by RPA. Milieu Ltd. WRc Eur. Comm. DG Environ. Under Study Contract DG ENV.G.4/ ETU/2008/0076r. 2009, pp. 1–20. Available online: https://www.efar.be/wp-content/uploads/Draft-Impact-Assessment-Sludge-Directive.pdf (accessed on 10 December 2022).
- Mininni, G.; Mauro, E.; Piccioli, B.; Colarullo, G.; Brandolini, F.; Giacomelli, P. Production and characteristics of sewage sludge in Italy. Water Sci. Technol. 2019, 79, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Monte, M.C.; Fuente, E.; Blanco, A.; Negro, C. Waste management from pulp and paper production in the European Union. Waste Manag. 2009, 29, 293–308. [Google Scholar] [CrossRef]
- Lenssen, N.J.L.; Schmidt, G.A.; Hansen, J.E.; Menne, M.J.; Persin, A.; Ruedy, R.; Zyss, D. Improvements in the GISTEMP Uncertainty Model. J. Geophys. Res. Atmos. 2019, 124, 6307–6326. [Google Scholar] [CrossRef]
- European Commission. European Green Deal : Commission Proposes Transformation of EU Economy and Society to Meet Climate Ambitions. 2021. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal/delivering-european-green-deal_en (accessed on 15 December 2022).
- Eurostat Sewage Sludge Generation and Management. Available online: https://appsso.eurostat.ec.europa.eu/nui/submitViewTableAction.do (accessed on 30 January 2022).
- Eurostat Population Projection. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?oldid=497115#Population_projections (accessed on 27 July 2022).
- Liu, X.; Chang, F.; Wang, C.; Jin, Z.; Wu, J.; Zuo, J.; Wang, K. Pyrolysis and subsequent direct combustion of pyrolytic gases for sewage sludge treatment in China. Appl. Therm. Eng. 2018, 128, 464–470. [Google Scholar] [CrossRef]
- Zaker, A.; Chen, Z.; Zaheer-Uddin, M. Catalytic pyrolysis of sewage sludge with HZSM5 and sludge-derived activated char: A comparative study using TGA-MS and artificial neural networks. J. Environ. Chem. Eng. 2021, 9, 105891. [Google Scholar] [CrossRef]
- Miricioiu, M.G.; Zaharioiu, A.; Oancea, S.; Bucura, F.; Raboaca, M.S.; Filote, C.; Ionete, R.E.; Niculescu, V.C.; Constantinescu, M. Sewage sludge derived materials for CO2 adsorption. Appl. Sci. 2021, 11, 7139. [Google Scholar] [CrossRef]
- Carotenuto, A.; Di Fraia, S.; Massarotti, N.; Sobek, S.; Uddin, M.R.; Vanoli, L.; Werle, S. Predictive modeling for energy recovery from sewage sludge gasification. Energy 2023, 263, 125838. [Google Scholar] [CrossRef]
- Vishwajeet; Pawlak-Kruczek, H.; Baranowski, M.; Czerep, M.; Chorążyczewski, A.; Krochmalny, K.; Ostrycharczyk, M.; Ziółkowski, P.; Madejski, P.; Mączka, T.; et al. Entrained Flow Plasma Gasification of Sewage Sludge–Proof-of-Concept and Fate of Inorganics. Energies 2022, 15, 1948. [Google Scholar] [CrossRef]
- Seggiani, M.; Vitolo, S.; Puccini, M.; Bellini, A. Cogasification of sewage sludge in an updraft gasifier. Fuel 2012, 93, 486–491. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Choi, Y.K.; Park, K.B.; Kim, J.S. Air co-gasification of coal and dried sewage sludge in a two-stage gasifier: Effect of blending ratio on the producer gas composition and tar removal. Energy 2019, 185, 708–716. [Google Scholar] [CrossRef]
- Martínez, E.J.; Sotres, A.; Arenas, C.B.; Blanco, D.; Martínez, O.; Gómez, X. Improving Anaerobic Digestion of Sewage Sludge by Hydrogen Addition: Analysis of Microbial Populations and Process Performance. Energies 2019, 12, 1228. [Google Scholar] [CrossRef]
- Costa, M.; Buono, A.; Caputo, C.; Carotenuto, A.; Cirillo, D.; Costagliola, M.A.; Di Blasio, G.; La Villetta, M.; Macaluso, A.; Martoriello, G.; et al. The “INNOVARE” project: Innovative plants for distributed poly-generation by residual biomass. Energies 2020, 13, 4020. [Google Scholar] [CrossRef]
- Werle, S.; Wilk, R.K. A review of methods for the thermal utilization of sewage sludge: The Polish perspective. Renew. Energy 2010, 35, 1914–1919. [Google Scholar] [CrossRef]
- Di Fraia, S.; Uddin, M.R. Energy Recovery from Waste Paper and Deinking Sludge to Support the Demand of the Paper Industry: A Numerical Analysis. Sustainability 2022, 14, 4669. [Google Scholar] [CrossRef]
- Abdelrahim, A.; Brachi, P.; Ruoppolo, G.; Di Fraia, S.; Vanoli, L. Experimental and Numerical Investigation of Biosolid Gasification: Equilibrium-Based Modeling with Emphasis on the Effects of Different Pretreatment Methods. Ind. Eng. Chem. Res. 2020, 59, 299–307. [Google Scholar] [CrossRef]
- de Andrés, J.M.; Vedrenne, M.; Brambilla, M.; Rodríguez, E. Modeling and model performance evaluation of sewage sludge gasification in fluidized-bed gasifiers using Aspen Plus. J. Air Waste Manag. Assoc. 2019, 69, 23–33. [Google Scholar] [CrossRef]
- De Andrés, J.M.; Narros, A.; Rodríguez, M.E. Air-steam gasification of sewage sludge in a bubbling bed reactor: Effect of alumina as a primary catalyst. Fuel Process. Technol. 2011, 92, 433–440. [Google Scholar] [CrossRef]
- Freda, C.; Cornacchia, G.; Romanelli, A.; Valerio, V.; Grieco, M. Sewage sludge gasification in a bench scale rotary kiln. Fuel 2018, 212, 88–94. [Google Scholar] [CrossRef]
- Mun, T.Y.; Kim, J.W.; Kim, J.S. Air gasification of dried sewage sludge in a two-stage gasifier: Part 1. the effects and reusability of additives on the removal of tar and hydrogen production. Int. J. Hydrogen Energy 2013, 38, 5226–5234. [Google Scholar] [CrossRef]
- Mun, T.Y.; Kim, J.S. Air gasification of dried sewage sludge in a two-stage gasifier. Part 2: Calcined dolomite as a bed material and effect of moisture content of dried sewage sludge for the hydrogen production and tar removal. Int. J. Hydrogen Energy 2013, 38, 5235–5242. [Google Scholar] [CrossRef]
- Mun, T.Y.; Cho, M.H.; Kim, J.S. Air gasification of dried sewage sludge in a two-stage gasifier. Part 3: Application of olivine as a bed material and nickel coated distributor for the production of a clean hydrogen-rich producer gas. Int. J. Hydrogen Energy 2014, 39, 5634–5643. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lan Thao Ngo, T.N.; Chiang, K.Y. Enhanced hydrogen production in co-gasification of sewage sludge and industrial wastewater sludge by a pilot-scale fluidized bed gasifier. Int. J. Hydrogen Energy 2021, 46, 14083–14095. [Google Scholar] [CrossRef]
- Cirillo, D.; Di Palma, M.; La Villetta, M.; Macaluso, A.; Mauro, A.; Vanoli, L. A novel biomass gasification micro-cogeneration plant: Experimental and numerical analysis. Energy Convers. Manag. 2021, 243, 114349. [Google Scholar] [CrossRef]
- Tian, Y.; He, D.; Zeng, Y.; Hu, L.; Du, J.; Luo, Z.; Ma, W.; Zhang, Z. Experimental research on hydrogen-rich syngas yield by catalytic biomass air-gasification over Ni/olivine as in-situ tar destruction catalyst. J. Energy Inst. 2023, 108, 101263. [Google Scholar] [CrossRef]
- Chae, H.J.; Kim, J.; Lee, S.C.; Kim, H.; Jo, S.B.; Ryu, J.; Kim, T.Y.; Lee, C.H.; Kim, S.J.; Kang, S. Catalytic Technologies for CO Hydrogenation for the Production of Light Hydrocarbons and Middle Distillates. Catalysts 2020, 10, 99. [Google Scholar] [CrossRef]
- Wibowo, H.; Susanto, H.; Grisdanurak, N.; Hantoko, D.; Yoshikawa, K.; Qun, H.; Yan, M. Recent developments of deep eutectic solvent as absorbent for CO2 removal from syngas produced from gasification: Current status, challenges, and further research. J. Environ. Chem. Eng. 2021, 9, 105439. [Google Scholar] [CrossRef]
- Di Fraia, S.; Massarotti, N.; Vanoli, L.; Costa, M. Thermo-economic analysis of a novel cogeneration system for sewage sludge treatment. Energy 2016, 115, 1560–1571. [Google Scholar] [CrossRef]
- Singh, V.; Phuleria, H.C.; Chandel, M.K. Estimation of energy recovery potential of sewage sludge in India : Waste to watt approach. J. Clean. Prod. 2020, 276, 122538. [Google Scholar] [CrossRef]
- Francois, J.; Abdelouahed, L.; Mauviel, G.; Patisson, F.; Mirgaux, O.; Rogaume, C.; Rogaume, Y.; Feidt, M.; Dufour, A. Detailed process modeling of a wood gasification combined heat and power plant. Biomass Bioenergy 2013, 51, 68–82. [Google Scholar] [CrossRef]
- Damartzis, T.; Michailos, S.; Zabaniotou, A. Energetic assessment of a combined heat and power integrated biomass gasification-internal combustion engine system by using Aspen Plus®. Fuel Process. Technol. 2012, 95, 37–44. [Google Scholar] [CrossRef]
- Brachi, P.; Di Fraia, S.; Massarotti, N.; Vanoli, L. Combined heat and power production based on sewage sludge gasification: An energy-efficient solution for wastewater treatment plants. Energy Convers. Manag. X 2022, 13, 100171. [Google Scholar] [CrossRef]
- Fatema, J.; Ahmed, T.; Islam, M.M.; Sakib, M.N.; Chowdhury, A.M.S.; Haque, P. Gasification of kitchen wastes in an updraft fluidized bed gasifier and simulation of the process with Aspen Plus. J. Clean. Prod. 2022, 371, 133670. [Google Scholar] [CrossRef]
- Singh, M.; Salaudeen, S.A.; Gilroyed, B.H.; Dutta, A. Simulation of biomass-plastic co-gasification in a fluidized bed reactor using Aspen plus. Fuel 2022, 319, 123708. [Google Scholar] [CrossRef]
- Hoo, K.K.; Md Said, M.S. Simulation of air gasification of Napier grass using Aspen Plus. Sustain. Energy Technol. Assess. 2022, 50, 101837. [Google Scholar] [CrossRef]
- Carotenuto, A.; Di Fraia, S.; Massarotti, N.; Uddin, M.R.; Vanoli, L. Combined Heat and Power Generation from Mechanically Dewatered Sewage Sludge: Numerical Modelling. Chem. Eng. Trans. 2022, 92, 283–288. [Google Scholar] [CrossRef]
- Migliaccio, R.; Brachi, P.; Montagnaro, F.; Papa, S.; Tavano, A.; Montesarchio, P.; Ruoppolo, G.; Urciuolo, M. Sewage Sludge Gasification in a Fluidized Bed: Experimental Investigation and Modeling. Ind. Eng. Chem. Res. 2021, 60, 5034–5047. [Google Scholar] [CrossRef]
- Werle, S.; Dudziak, M. Gaseous fuels production from dried sewage sludge via air gasification. Waste Manag. Res. 2015, 32, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Werle, S. Impact of feedstock properties and operating conditions on sewage sludge gasification in a fixed bed gasifier. Waste Manag. Res. 2014, 32, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Werle, S. Gasification of a dried sewage sludge in a laboratory scale fixed bed reactor. Energies 2015, 8, 8562–8572. [Google Scholar] [CrossRef]
- Giltrap, D.L.; McKibbin, R.; Barnes, G.R.G. A steady state model of gas-char reactions in a downdraft biomass gasifier. Sol. Energy 2003, 74, 85–91. [Google Scholar] [CrossRef]
- Zainal, Z.A.; Ali, R.; Lean, C.H.; Seetharamu, K.N. Prediction of performance of a downdraft gasifier using equilibrium modeling for different biomass materials. Energy Convers. Manag. 2001, 42, 1499–1515. [Google Scholar] [CrossRef]
- Acar, M.C.; Böke, Y.E. Simulation of biomass gasification in a BFBG using chemical equilibrium model and restricted chemical equilibrium method. Biomass Bioenergy 2019, 125, 131–138. [Google Scholar] [CrossRef]
- Villarini, M.; Marcantonio, V.; Colantoni, A. Sensitivity Analysis of Different Parameters on the Performance of a CHP Internal Combustion Engine System Fed by a Biomass Waste Gasifier. Energies 2019, 12, 688. [Google Scholar] [CrossRef]
- Lan, W.; Chen, G.; Zhu, X.; Wang, X.; Liu, C.; Xu, B. Biomass gasification-gas turbine combustion for power generation system model based on ASPEN PLUS. Sci. Total Environ. 2018, 628–629, 1278–1286. [Google Scholar] [CrossRef]
- François, J.; Abdelouahed, L.; Mauviel, G.; Feidt, M.; Rogaume, C.; Mirgaux, O.; Patisson, F.; Dufour, A. Estimation of the energy efficiency of a wood gasification CHP plant using Aspen Plus. Chem. Eng. Trans. 2012, 29, 769–774. [Google Scholar] [CrossRef]
- Násner, A.M.L.; Lora, E.E.S.; Palacio, J.C.E.; Rocha, M.H.; Restrepo, J.C.; Venturini, O.J.; Ratner, A. Refuse Derived Fuel (RDF) production and gasification in a pilot plant integrated with an Otto cycle ICE through Aspen plusTM modelling: Thermodynamic and economic viability. Waste Manag. 2017, 69, 187–201. [Google Scholar] [CrossRef]
- Niu, M.; Huang, Y.; Jin, B.; Wang, X. Simulation of syngas production from municipal solid waste gasification in a bubbling fluidized bed using aspen plus. Ind. Eng. Chem. Res. 2013, 52, 14768–14775. [Google Scholar] [CrossRef]
- Chen, C.; Jin, Y.Q.; Yan, J.H.; Chi, Y. Simulation of municipal solid waste gasification in two different types of fixed bed reactors. Fuel 2013, 103, 58–63. [Google Scholar] [CrossRef]
- Roche, E.; De Andrés, J.M.; Narros, A.; Rodríguez, M.E. Air and air-steam gasification of sewage sludge. The influence of dolomite and throughput in tar production and composition. Fuel 2014, 115, 54–61. [Google Scholar] [CrossRef]
- Sreejith, C.C.; Muraleedharan, C.; Arun, P. Performance prediction of steam gasification of wood using an ASPEN PLUS thermodynamic equilibrium model. Int. J. Sustain. Energy 2014, 33, 416–434. [Google Scholar] [CrossRef]
- Pala, L.P.R.; Wang, Q.; Kolb, G.; Hessel, V. Steam gasification of biomass with subsequent syngas adjustment using shift reaction for syngas production: An Aspen Plus model. Renew. Energy 2017, 101, 484–492. [Google Scholar] [CrossRef]
- Franco, C.; Pinto, F.; Gulyurtlu, I.; Cabrita, I. The study of reactions influencing the biomass steam gasification process. Fuel 2003, 82, 835–842. [Google Scholar] [CrossRef]
- Barman, N.S.; Ghosh, S.; De, S. Gasification of biomass in a fixed bed downdraft gasifier—A realistic model including tar. Bioresour. Technol. 2012, 107, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A. Carbonization ranking of selected biomass for charcoal, liquid and gaseous products. Energy Convers. Manag. 2001, 42, 1229–1238. [Google Scholar] [CrossRef]
- Morris, R.D. Chapter Four. In Composition with Pitch-Classes; Yale University Press: London, UK, 2018; pp. 123–183. [Google Scholar]
- Ruya, P.M.; Purwadi, R.; Lim, S.S. Supercritical water gasification of sewage sludge for power generation– thermodynamic study on auto-thermal operation using Aspen Plus. Energy Convers. Manag. 2020, 206, 112458. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, W.; Ying, Z.; Huang, J.; Ji, S.; Wang, B. Thermodynamic investigation on gasification performance of sewage sludge-derived hydrochar: Effect of hydrothermal carbonization. Int. J. Hydrogen Energy 2019, 44, 10374–10383. [Google Scholar] [CrossRef]
- Hossain, M.S.; Kumar, D.; Paul, N.; Rahman, S.J.; Rahman, M.A.; Uddin, M.R.; Pirozzi, D.; Uddin, M.N.; Yousuf, A. Design and Development of Bubbling Fluidized Bed Gasifier for Non-Woody Biomass Gasification. Chem. Eng. Trans. 2022, 92, 289–294. [Google Scholar] [CrossRef]
- Turkcan, A. The effects of different types of biodiesels and biodiesel-bioethanol-diesel blends on the cyclic variations and correlation coefficient. Fuel 2020, 261, 116453. [Google Scholar] [CrossRef]
- Carotenuto, A.; Di Fraia, S.; Uddin, M.R.; Vanoli, L. Comparison of Combustion and Gasification for Energy Recovery from Residual Woody Biomass. Int. J. Heat Technol. 2022, 40, 888–894. [Google Scholar] [CrossRef]
- Moretti, L.; Arpino, F.; Cortellessa, G.; Di Fraia, S.; Di Palma, M.; Vanoli, L. Reliability of equilibrium gasification models for selected biomass types and compositions: An overview. Energies 2022, 15, 61. [Google Scholar] [CrossRef]
- Martínez González, A.; Silva Lora, E.E.; Escobar Palacio, J.C. Syngas production from oil sludge gasification and its potential use in power generation systems: An energy and exergy analysis. Energy 2019, 169, 1175–1190. [Google Scholar] [CrossRef]
- Castillo Santiago, Y.; Martínez González, A.; Venturini, O.J.; Yepes Maya, D.M. Assessment of the energy recovery potential of oil sludge through gasification aiming electricity generation. Energy 2021, 215, 119210. [Google Scholar] [CrossRef]
- Cavalaglio, G.; Coccia, V.; Cotana, F.; Gelosia, M.; Nicolini, A.; Petrozzi, A. Energy from poultry waste: An Aspen Plus-based approach to the thermo-chemical processes. Waste Manag. 2018, 73, 496–503. [Google Scholar] [CrossRef]
- Sheth, P.N.; Babu, B.V. Production of hydrogen energy through biomass (waste wood) gasification. Int. J. Hydrogen Energy 2010, 35, 10803–10810. [Google Scholar] [CrossRef]
- Kaewluan, S.; Pipatmanomai, S. Potential of synthesis gas production from rubber wood chip gasification in a bubbling fluidised bed gasifier. Energy Convers. Manag. 2011, 52, 75–84. [Google Scholar] [CrossRef]
- Skoulou, V.; Koufodimos, G.; Samaras, Z.; Zabaniotou, A. Low temperature gasification of olive kernels in a 5-kW fluidized bed reactor for H2-rich producer gas. Int. J. Hydrogen Energy 2008, 33, 6515–6524. [Google Scholar] [CrossRef]
- De Andrés, J.M.; Narros, A.; Rodríguez, M.E. Behaviour of dolomite, olivine and alumina as primary catalysts in air-steam gasification of sewage sludge. Fuel 2011, 90, 521–527. [Google Scholar] [CrossRef]
- Ahmed, R.; Chandra, M.S. Equilibrium modeling in updraft gasifier for refinery sludge gasification using ASPEN PLUS simulator. Appl. Mech. Mater. 2013, 393, 729–734. [Google Scholar] [CrossRef]
- Zhang, J.; Huo, X.; Dong, C.; Yang, Y. High-temperature oxygen gasification of sludge. In Proceedings of the 1st International Conference on Sustainable Power Generation and Supply, SUPERGEN ’09, Nanjing, China, 6–7 April 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 1–5. [Google Scholar]
- Groß, B.; Eder, C.; Grziwa, P.; Horst, J.; Kimmerle, K. Energy recovery from sewage sludge by means of fluidised bed gasification. Waste Manag. 2008, 28, 1819–1826. [Google Scholar] [CrossRef]
- Elsner, W.; Wysocki, M.; Niegodajew, P.; Borecki, R. Experimental and economic study of small-scale CHP installation equipped with downdraft gasifier and internal combustion engine. Appl. Energy 2017, 202, 213–227. [Google Scholar] [CrossRef]
- Longo, S.; d’Antoni, B.M.; Bongards, M.; Chaparro, A.; Cronrath, A.; Fatone, F.; Lema, J.M.; Mauricio-Iglesias, M.; Soares, A.; Hospido, A. Monitoring and diagnosis of energy consumption in wastewater treatment plants. A state of the art and proposals for improvement. Appl. Energy 2016, 179, 1251–1268. [Google Scholar] [CrossRef]
- Faizan, M.; Song, H. Critical review on catalytic biomass gasification: State-of-Art progress, technical challenges, and perspectives in future development. J. Clean. Prod. 2023, 408, 137224. [Google Scholar] [CrossRef]
- Bocci, E.; Marcantonio, V.; Di Carlo, A. Tar and inorganic contaminant removal from syngas: Modeling and simulation. In Advances in Synthesis Gas: Methods, Technologies and Applications; Elsevier: San Diego, CA, USA, 2023; pp. 241–273. [Google Scholar]
- Peck, D.; Holmes, W.; Zappi, M.; Hernandez, R.; Gang, D.; Buchireddy, P. Novel nickel ceramic filter for hot gas cleanup of tars from syngas. Fuel Process. Technol. 2023, 244, 107708. [Google Scholar] [CrossRef]





| Proximate Analysis (wt.%, db) | Ultimate Analysis (wt.%, db) | LHV (MJ/kg, db) | LHV (kWh/kg, db) | ||
|---|---|---|---|---|---|
| Moisture | 5.30 ± 0.06 | C | 31.79 ± 0.2 | 12.96 ± 0.4 | 3.60 ± 0.1 |
| H2 | 4.36 ± 0.05 | ||||
| Volatile matter | 51.00 ± 0.96 | N2 | 4.88 ± 0.07 | ||
| S | 1.67 ± 0.01 | ||||
| Fixed carbon | 7.20 ± 0.06 | F₂ | 0.013 ± 0.001 | ||
| Cl₂ | 0.22 ± 0.001 | ||||
| Ash | 36.50 ± 0.1 | O2 | 20.57 | ||
| Test Condition | ER (-) | SS Flow Rate (g/s) | Air Flow Rate (g/s) | * APT (°C) | Dry Syngas Composition (vol.%) | LHV (MJ/Nm3) | |||
|---|---|---|---|---|---|---|---|---|---|
| H₂ | CO | CO₂ | CH₄ | ||||||
| I | 0.16 | 1.0 | 0.78 | 25 | 5.80 ± 0.1 | 23.90 ± 0.6 | 15.90 ± 0.4 | 0.85 ± 0.04 | 3.75 ± 0.6 |
| II | 100 | 5.90 ± 0.1 | 24.00 ± 0.7 | 15.40 ± 0.5 | 0.85 ± 0.05 | 3.77 ± 0.7 | |||
| III | 150 | 6.00 ± 0.2 | 24.20 ± 0.7 | 15.35 ± 0.4 | 0.87 ± 0.04 | 3.81 ± 0.6 | |||
| IV | 200 | 6.20 ± 0.1 | 24.50 ± 0.6 | 15.00 ± 0.5 | 0.89 ± 0.03 | 3.87 ± 0.5 | |||
| V | 250 | 6.80 ± 0.1 | 24.90 ± 0.7 | 14.80 ± 0.6 | 0.96 ± 0.04 | 3.99 ± 0.4 | |||
| VI | 0.27 | 1.32 | 25 | 7.90 ± 0.2 | 19.50 ± 0.8 | 15.90 ± 0.5 | 1.00 ± 0.05 | 3.44 ± 0.5 | |
| VII | 100 | 7.92 ± 0.1 | 19.70 ± 0.6 | 15.70 ± 0.4 | 1.05 ± 0.06 | 3.47 ± 0.4 | |||
| VIII | 150 | 8.00 ± 0.2 | 20.00 ± 0.8 | 15.65 ± 0.6 | 1.06 ± 0.05 | 3.52 ± 0.5 | |||
| IX | 200 | 8.02 ± 0.1 | 20.50 ± 0.7 | 14.60 ± 0.7 | 1.10 ± 0.07 | 3.59 ± 0.6 | |||
| X | 250 | 8.05 ± 0.2 | 20.80 ± 0.6 | 14.80 ± 0.7 | 1.21 ± 0.06 | 3.64 ± 0.5 | |||
| Reaction No. | Reaction Scheme | Reaction Name | ΔH, (kJ/mol) |
|---|---|---|---|
| R1 | C + H2O → H2 + CO | Water gas | +131.0 |
| R2 | C + O2 → CO2 | Carbon combustion | −393.0 |
| R3 | C + 2H2 → CH4 | Methanation | −74.0 |
| R4 | CO + H2O → H2 + CO2 | Water gas shift | −41.0 |
| R5 | 2C2H6 + 7O2 → 6H2O + 4CO2 | Ethane combustion | −1559.8 |
| R6 | C3H8 + 5O2 → 4H2O + 3CO2 | Propane combustion | −2220.0 |
| R7 | 2H2 + O2 → 2H2O | Hydrogen combustion | −242.0 |
| Operating Parameters, Unit | Value | |
|---|---|---|
| Incoming syngas temperature to the ICE combustion chamber (°C) | 30.0 | |
| Incoming air temperature to the ICE (°C) | 20.0 | |
| Equivalence ratio used for syngas combustion (-) | 1.11 | |
| Pressure of ICE system for compression and combustion (bar) | 9.45 | |
| Energy losses during syngas combustion (% of syngas energy) | 10.0 | |
| Isentropic efficiency (%) | Compression process | 85.0 |
| Expansion process | 87.0 | |
| Mechanical efficiency (%) | Compression process | 99.0 |
| Expansion process | ||
| Exhaust stream pressure from the ICE (bar) | 1.0 | |
| Exhaust stream Temperature from the ICE (°C) | 338.0 | |
| Exhaust fume utilization temperature (°C) | 80.0 | |
| Cooling water temperature to the ICE radiator | Inlet (°C) | 80.0 |
| Exit (°C) | 90.0 | |
| Reaction No. | |
|---|---|
| R1 | −292.7 |
| R2 | 51.1 |
| R3 | −493.2 |
| R4 | 500 |
| R5 | −320.8 |
| R6 | 252.2 |
| R7 | −469.7 |
| Fraction of carbon moving to char formation | 0.0879 |
| ER = 0.16 | |||||
| Syngas component | H₂ | CO | CO₂ | CH₄ | LHV |
| Experimental | 0.93 | 0.99 | −0.97 | 0.95 | 0.93 |
| Simulation | 0.999 | 0.999 | −0.999 | 0.998 | 0.999 |
| ER = 0.27 | |||||
| Experimental | 0.97 | 0.98 | −0.88 | 0.93 | 0.98 |
| Simulation | 0.96 | 0.93 | −0.96 | 0.997 | 0.97 |
| Biomass | CGE (%) | Reference | |||
|---|---|---|---|---|---|
| SS | 74.1 | 25.7 | 51.5 | 74.9 | Present study |
| 74.6 | 25.6 | 47.8 | 76.7 | [20] | |
| 71.9 | 24.6 | 45.9 | 76.8 | ||
| 72.3 | 24.8 | 46.3 | 67.4 | [48] | |
| n.r. | 32.0 | n.r. | n.r. | [84] | |
| 69.0 | 20.0 | n.r. | n.r. | [85] | |
| Wood | n.r. | 27.0 | 40.0 | 67.0 | [58] |
| MSW | 59.0 | 19.1 | 20.0 | 40.1 | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carotenuto, A.; Di Fraia, S.; Massarotti, N.; Sobek, S.; Uddin, M.R.; Vanoli, L.; Werle, S. Sewage Sludge Gasification Process Optimization for Combined Heat and Power Generation. Energies 2023, 16, 4742. https://doi.org/10.3390/en16124742
Carotenuto A, Di Fraia S, Massarotti N, Sobek S, Uddin MR, Vanoli L, Werle S. Sewage Sludge Gasification Process Optimization for Combined Heat and Power Generation. Energies. 2023; 16(12):4742. https://doi.org/10.3390/en16124742
Chicago/Turabian StyleCarotenuto, Alberto, Simona Di Fraia, Nicola Massarotti, Szymon Sobek, M. Rakib Uddin, Laura Vanoli, and Sebastian Werle. 2023. "Sewage Sludge Gasification Process Optimization for Combined Heat and Power Generation" Energies 16, no. 12: 4742. https://doi.org/10.3390/en16124742
APA StyleCarotenuto, A., Di Fraia, S., Massarotti, N., Sobek, S., Uddin, M. R., Vanoli, L., & Werle, S. (2023). Sewage Sludge Gasification Process Optimization for Combined Heat and Power Generation. Energies, 16(12), 4742. https://doi.org/10.3390/en16124742









