Investigation of the Impact of Biochar Application on Foaming Slags with Varied Compositions in Electric Arc Furnace-Based Steel Production
Abstract
:1. Introduction
2. Materials and Methods
- (1)
- In the first scenario, the slags had a constant FeO content of 25 wt.%, and the basicity (B2) was varied within the range of 1.0 to 3.4. Basicity was defined as the ratio of CaO to SiO2. Additionally, another basicity (B3) was defined as the ratio of CaO to the sum of SiO2 and Al2O3, and it varied between 0.8 and 2.0. The MgO content ranged from 15.0 to 6.0 wt.%, as shown in Table 1;
- (2)
- In the second scenario, the slags had a constant basicity B2 of 1.4 and basicity B3 of 1.0, as well as the content of Al2O3 8 wt.%. Nevertheless, the FeO content varied between 24.0 and 30.7 wt.%, and the MgO content varied between 9.34 and 16.03 wt.%, as shown in Table 2;
- (3)
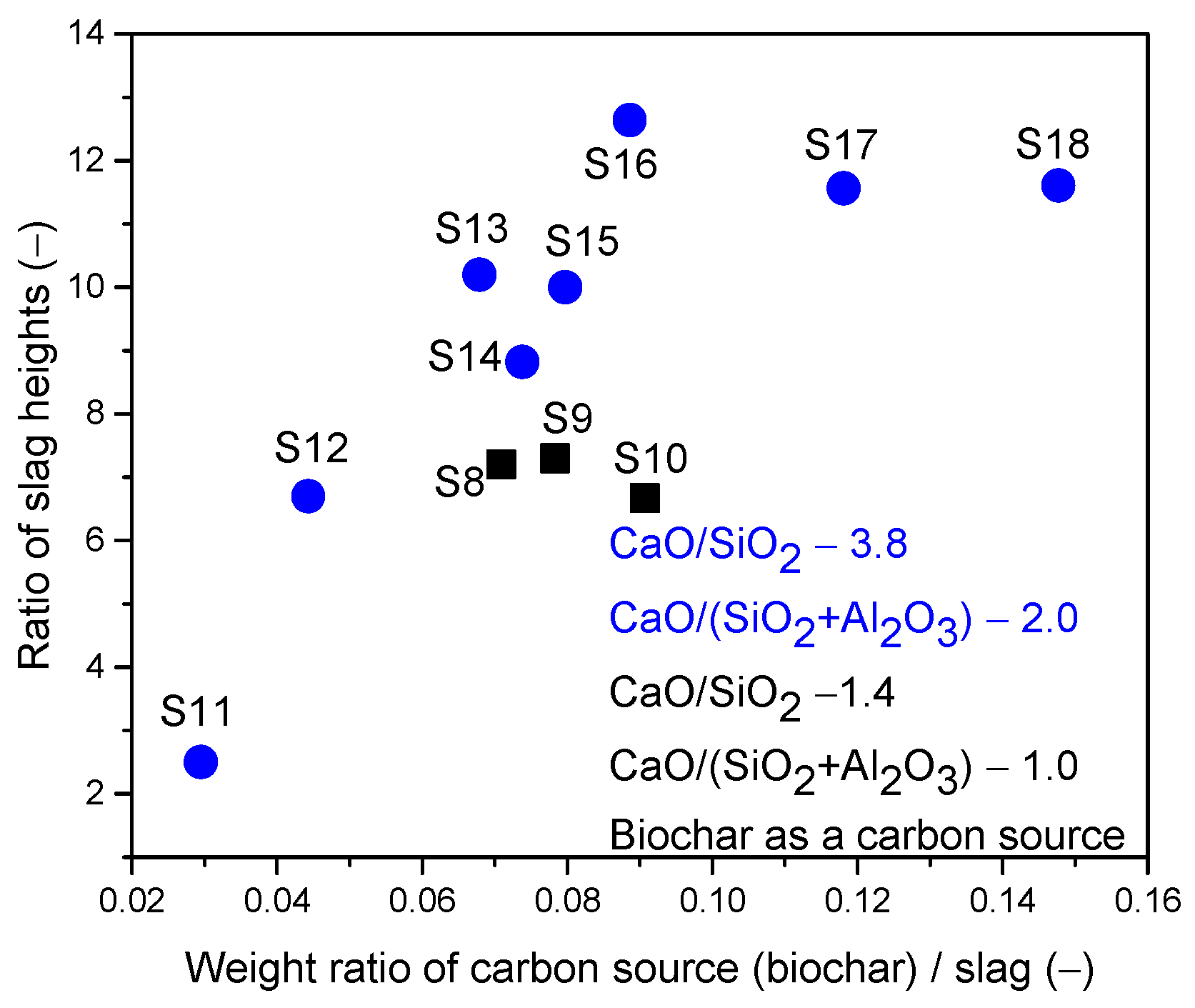
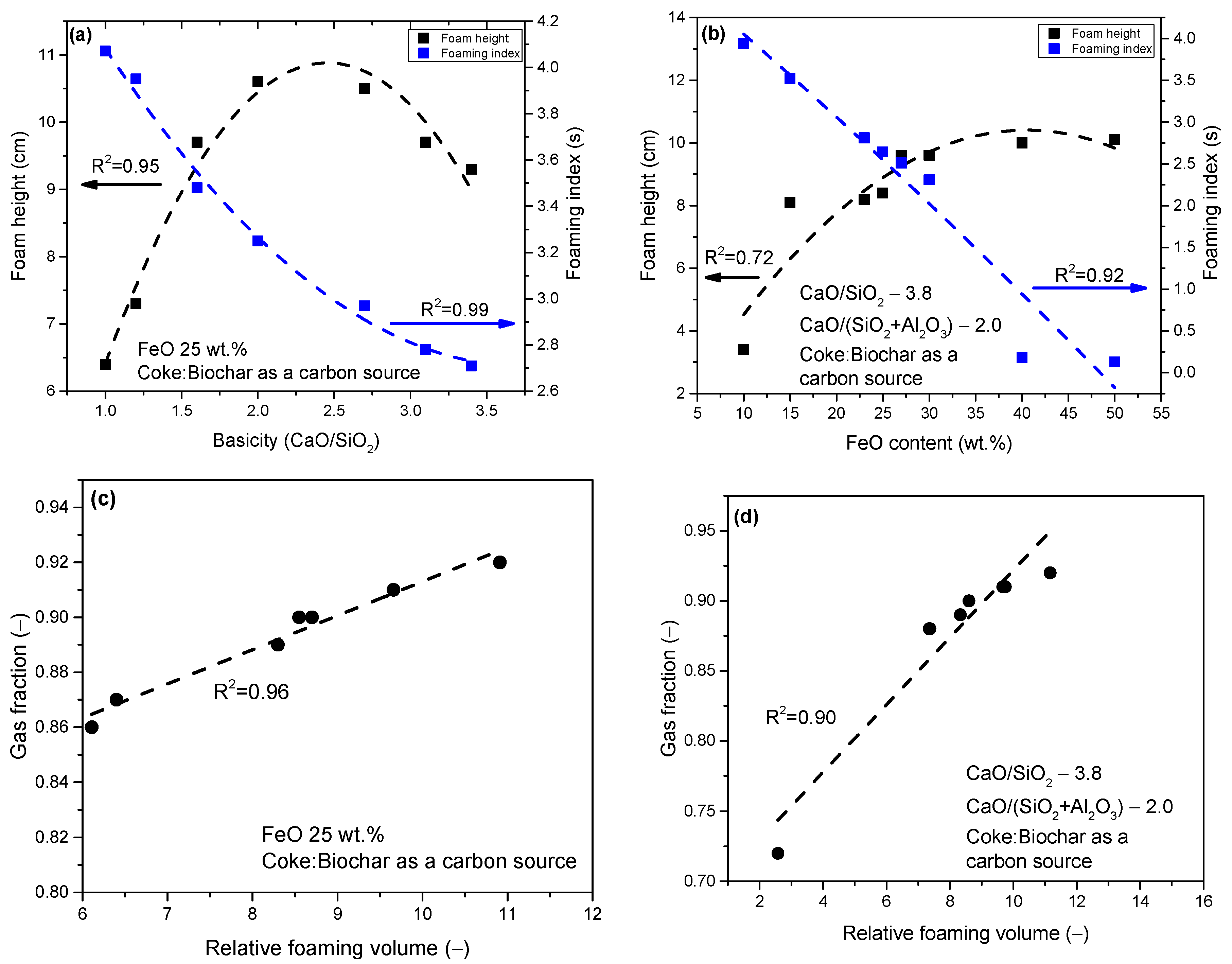
- In the third scenario, the slags have a constant basicity B2 of 3.8 and a basicity B3 of 2.0. However, the FeO content varied between 10 and 50 wt.%, and the MgO content varied between 5.72 and 11.16 wt.%, as shown in Table 3.
3. Results and Discussion
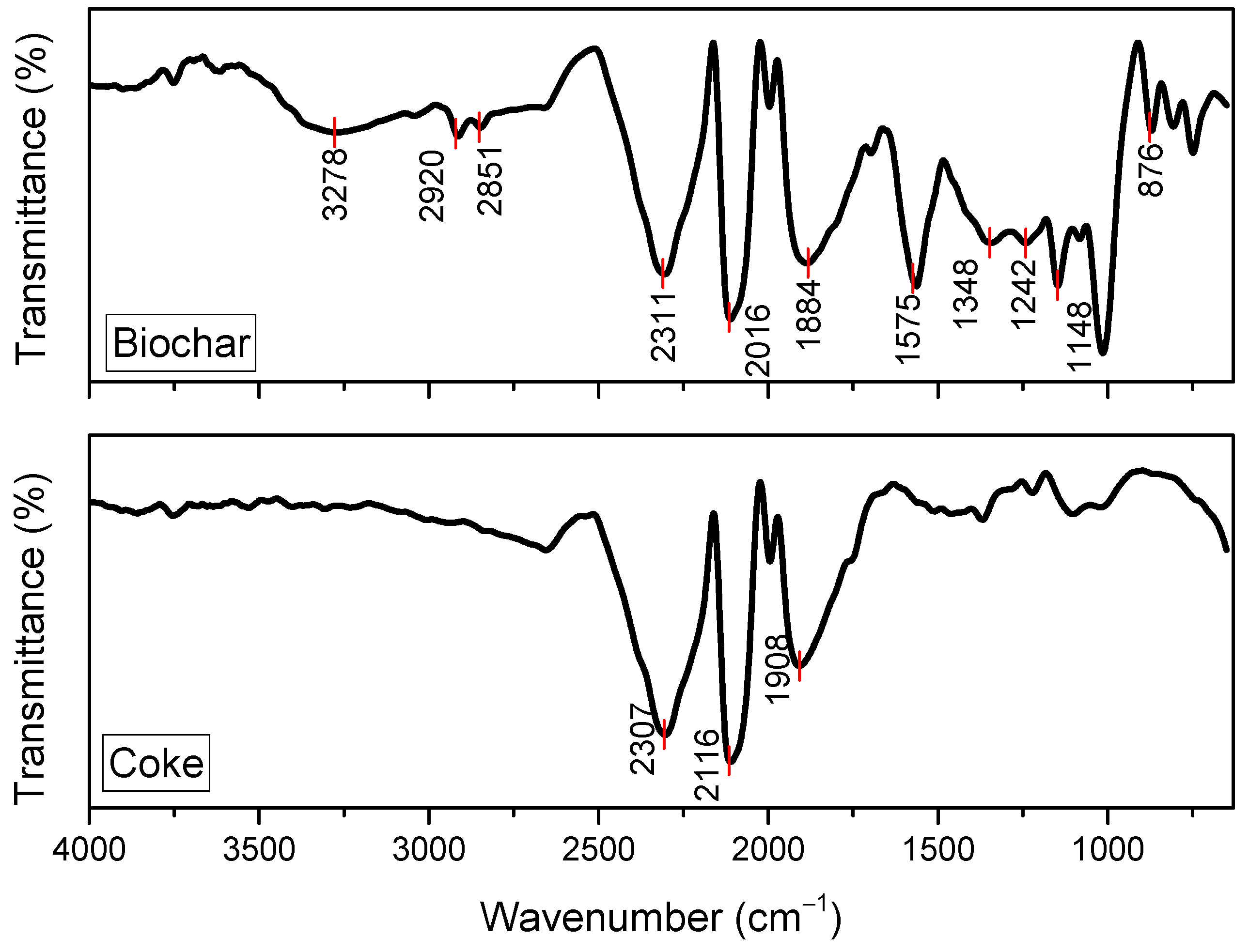
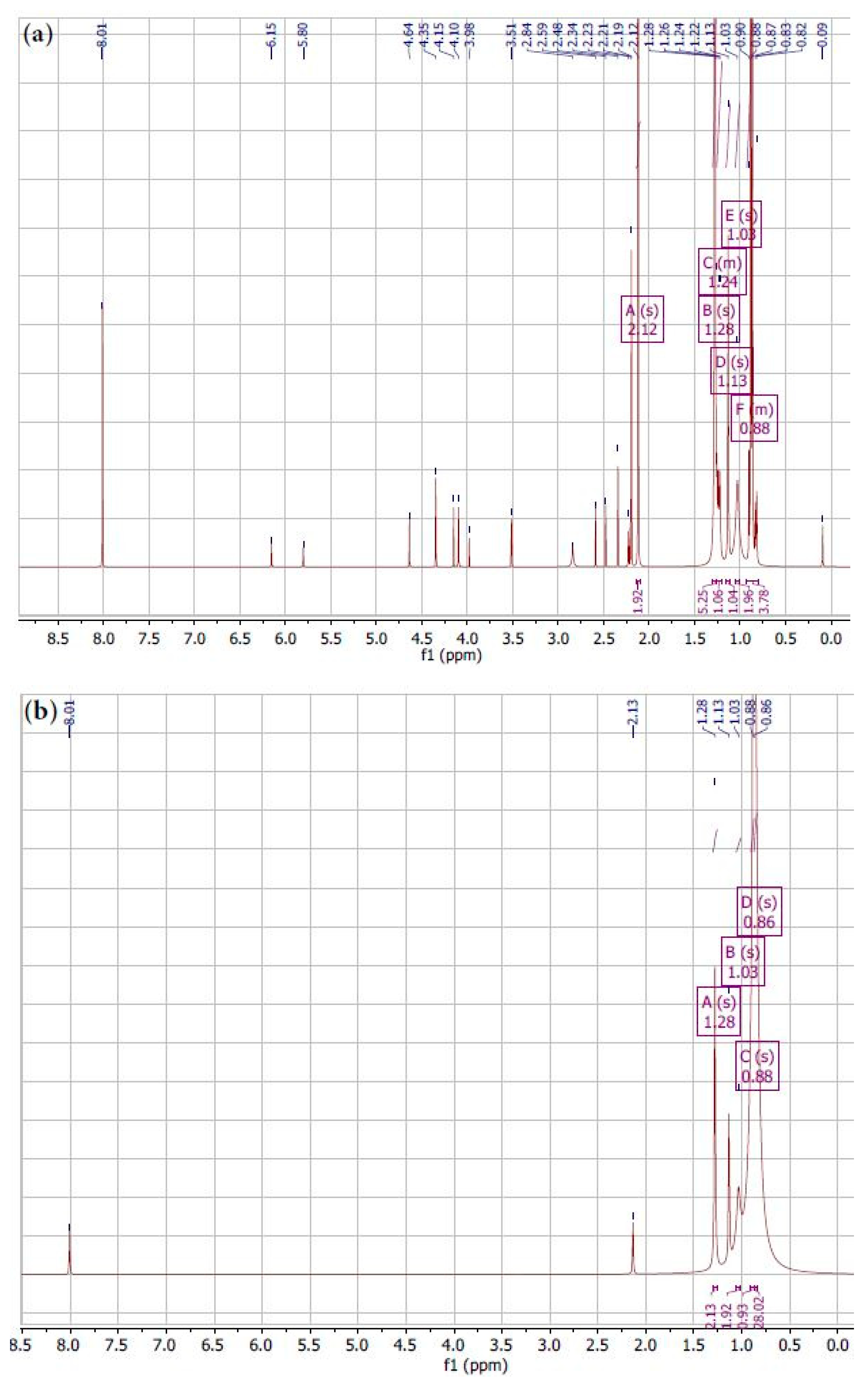
3.1. Evaluation of Carbon Sources via Fourier Transform Infrared Spectroscopy and Proton Nuclear Magnetic Resonance Spectroscopy
3.2. Evaluation of Slag Foaming Behavior: The Case of 100 wt.% Coke as a Carbon Source
3.3. Evaluation of Slag Foaming Behavior: Case of 100 wt.% Biochar as a Carbon Source
3.4. Evaluation of Slag Foaming Behavior: The Case of Coke and Biochar Mixture with a Ratio of 1:1 as a Carbon Source
4. Conclusions
- (1)
- FTIR analysis indicates clear chemical distinctions between biochar and coke, with different functional groups in each carbon source. The reduction in certain functional groups of coke can be attributed to the higher carbonization temperature, leading to the loss of volatile and oxygen-containing functional groups. These results were confirmed judging by the 1H NMR spectra;
- (2)
- Employing 100 wt.% coke as the carbon source resulted in optimal foaming characters for slags with different basicity values ranging from 1.2 to 2.7 and FeO content from 15 to 30 wt.%. For stable basicity (B2) at 1.4 and consistent Al2O3 content, the highest foam height and volume were achieved with 26.5 wt.% FeO and 13.52 wt.% MgO;
- (3)
- When using 100 wt.% biochar as a carbon source, the most optimal foaming characters were obtained for a group of slags with different basicities from 1.2 to 3.4. Notably, the influence on foam height was not observed with the increase in basicity B2 from 1.2 to 3.1. For group slags with different FeO content, stable foaming was obtained for samples containing this oxide from 15 to 27 wt.%. In the slag group, where stable basicity of 1.4 and Al2O3 content were used, foaming characters were lower than coke, there were no significant changes, and the characters were within experimental error;
- (4)
- A mixture of coke and biochar led to stable foaming with a minimum basicity value of 1.0 and 25 wt.% FeO content. Unlike the other carbon sources, this mixture provided stable foaming across all slag compositions with varying basicity values. Stable foaming was observed in the presence of 15 wt.% to 40 wt.% FeO, presenting a broader range compared to individual applications of coke or biochar. For slags with stable basicity and Al2O3 content, similar foaming tendencies were observed compared to using coke.
- (5)
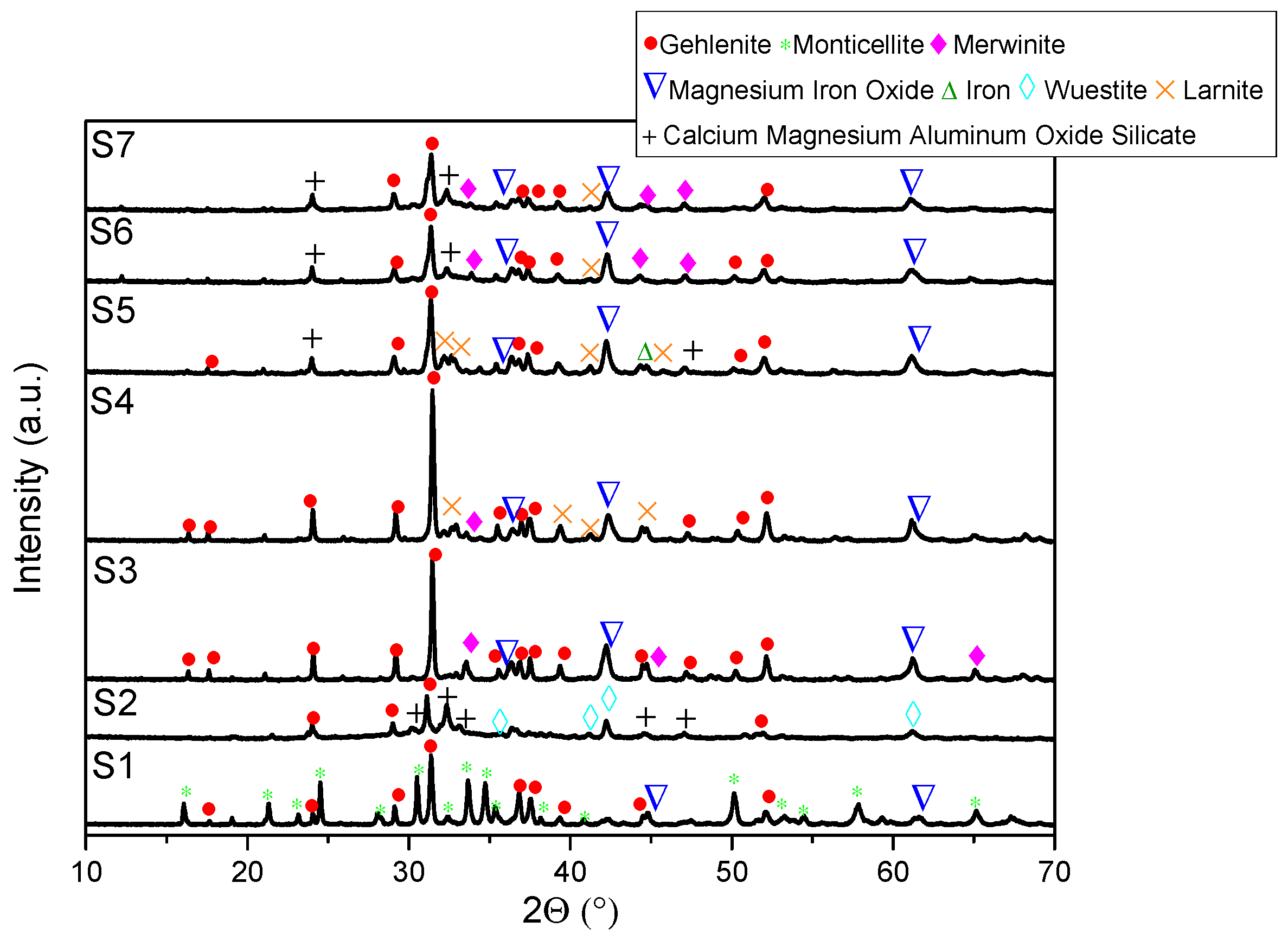
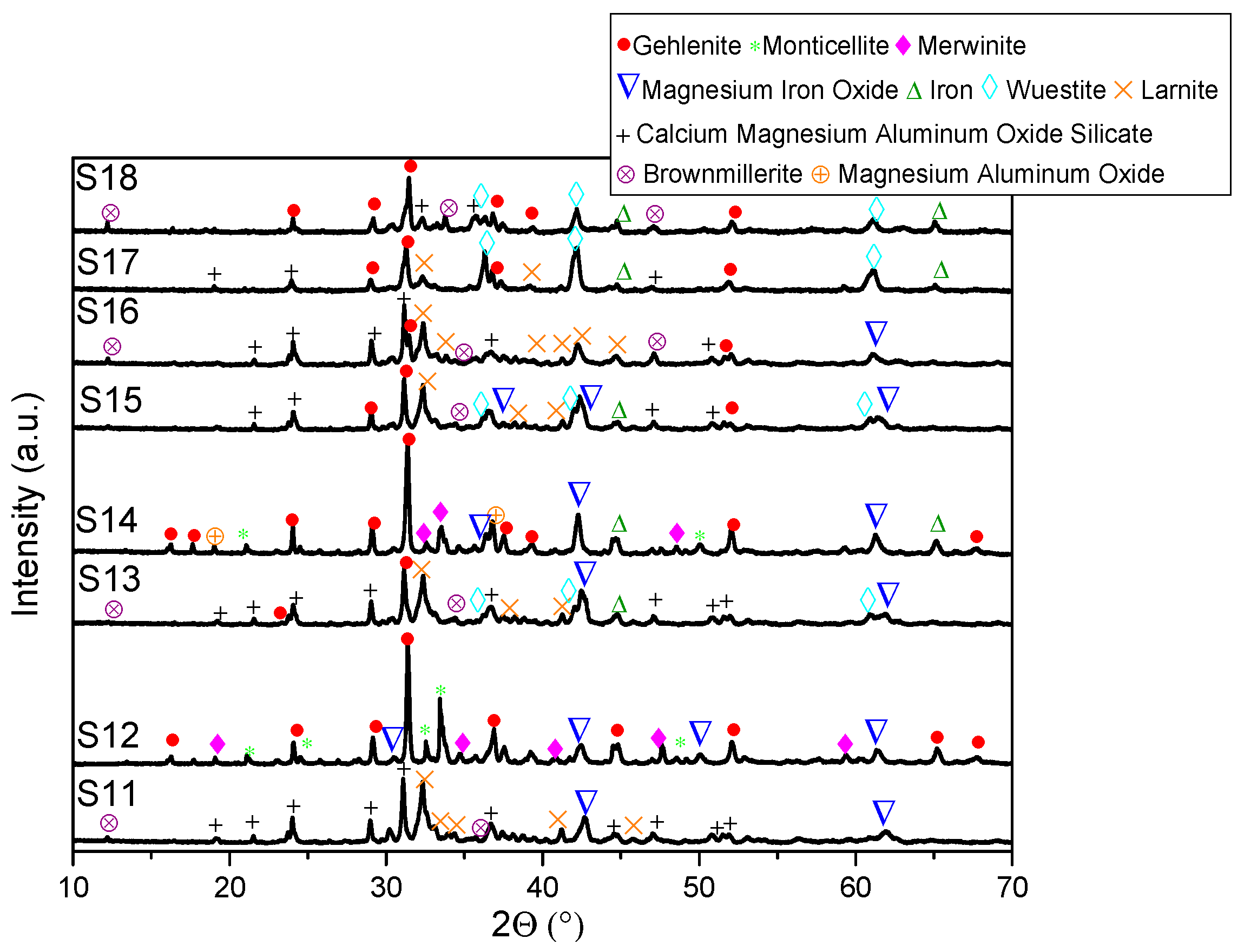
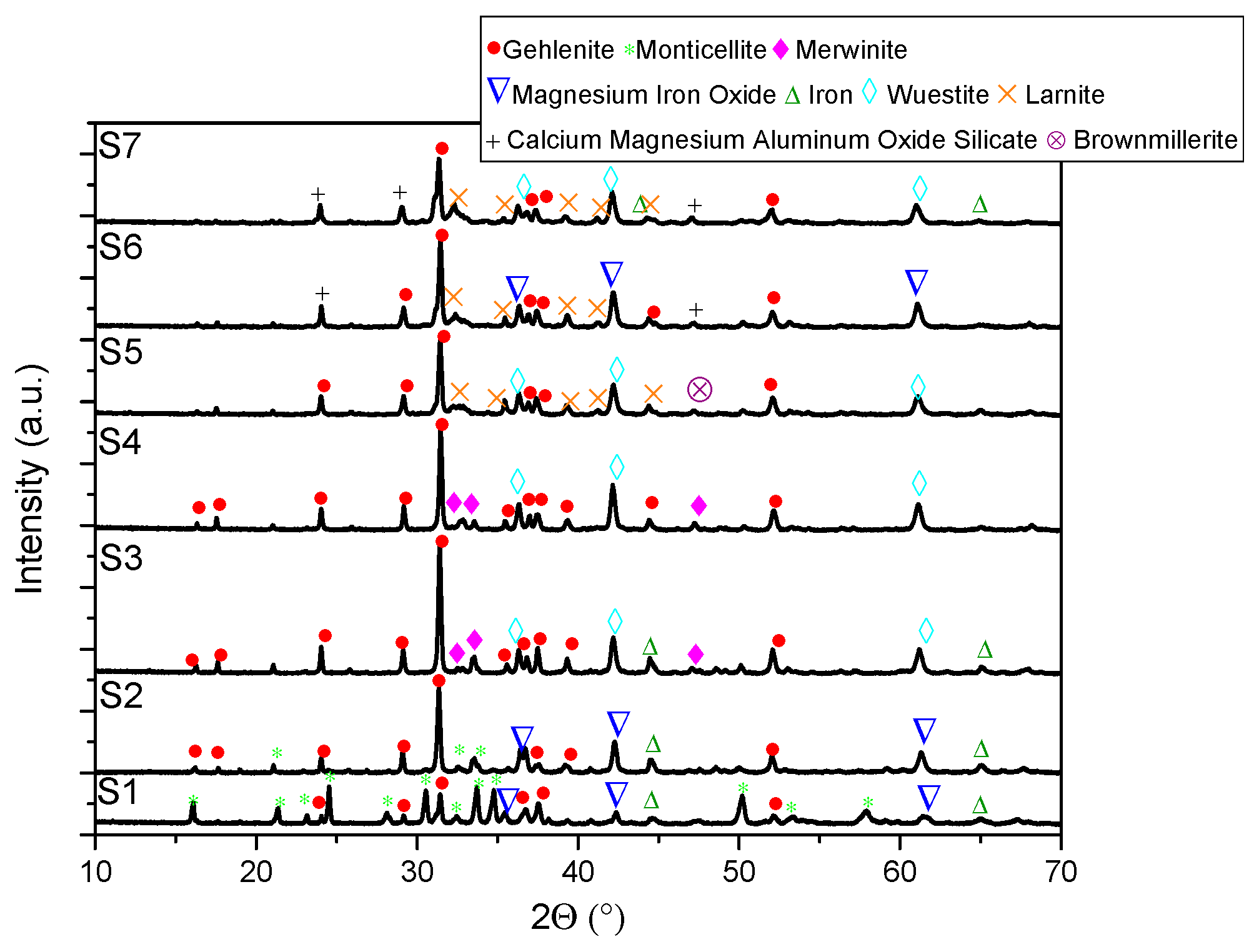
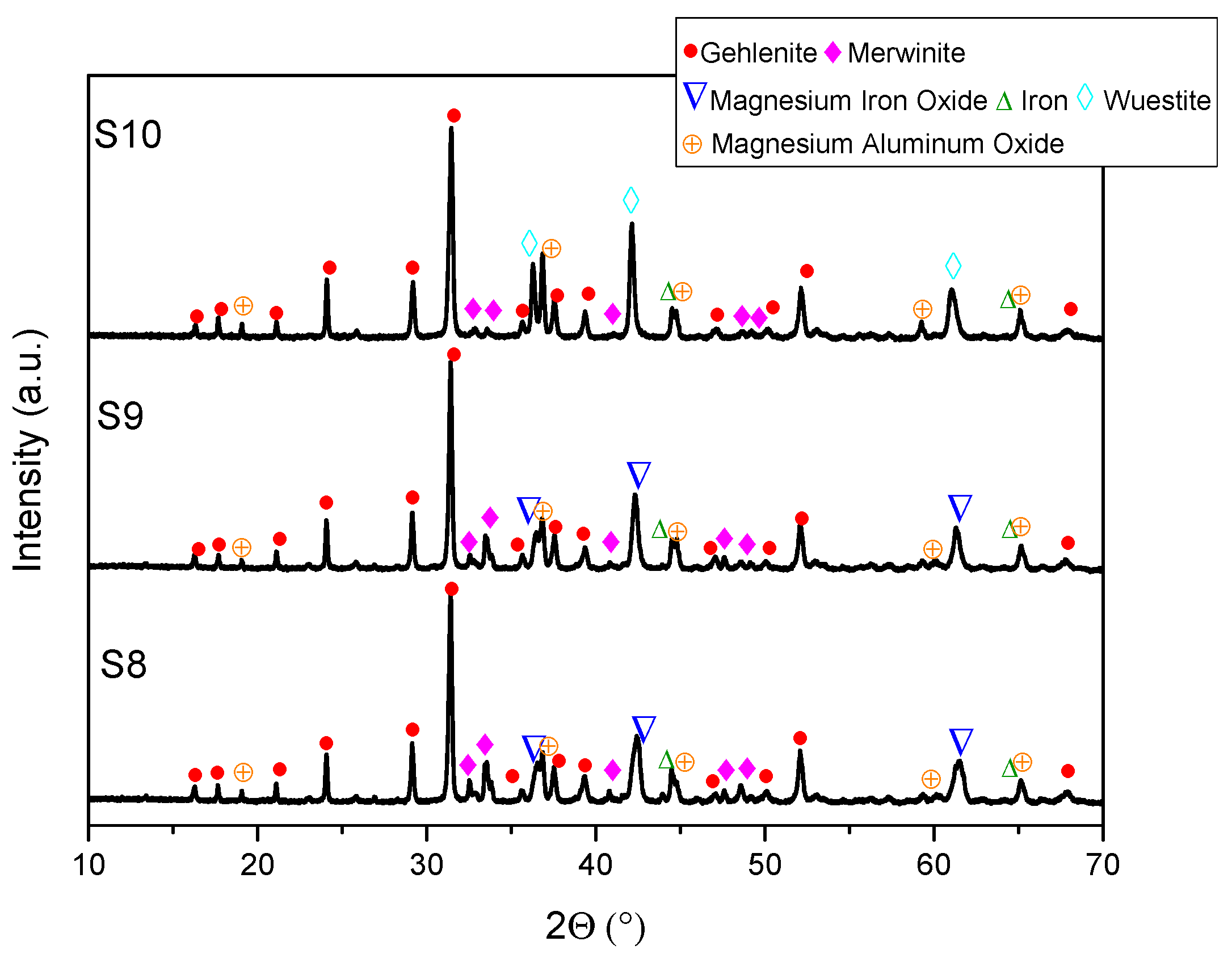
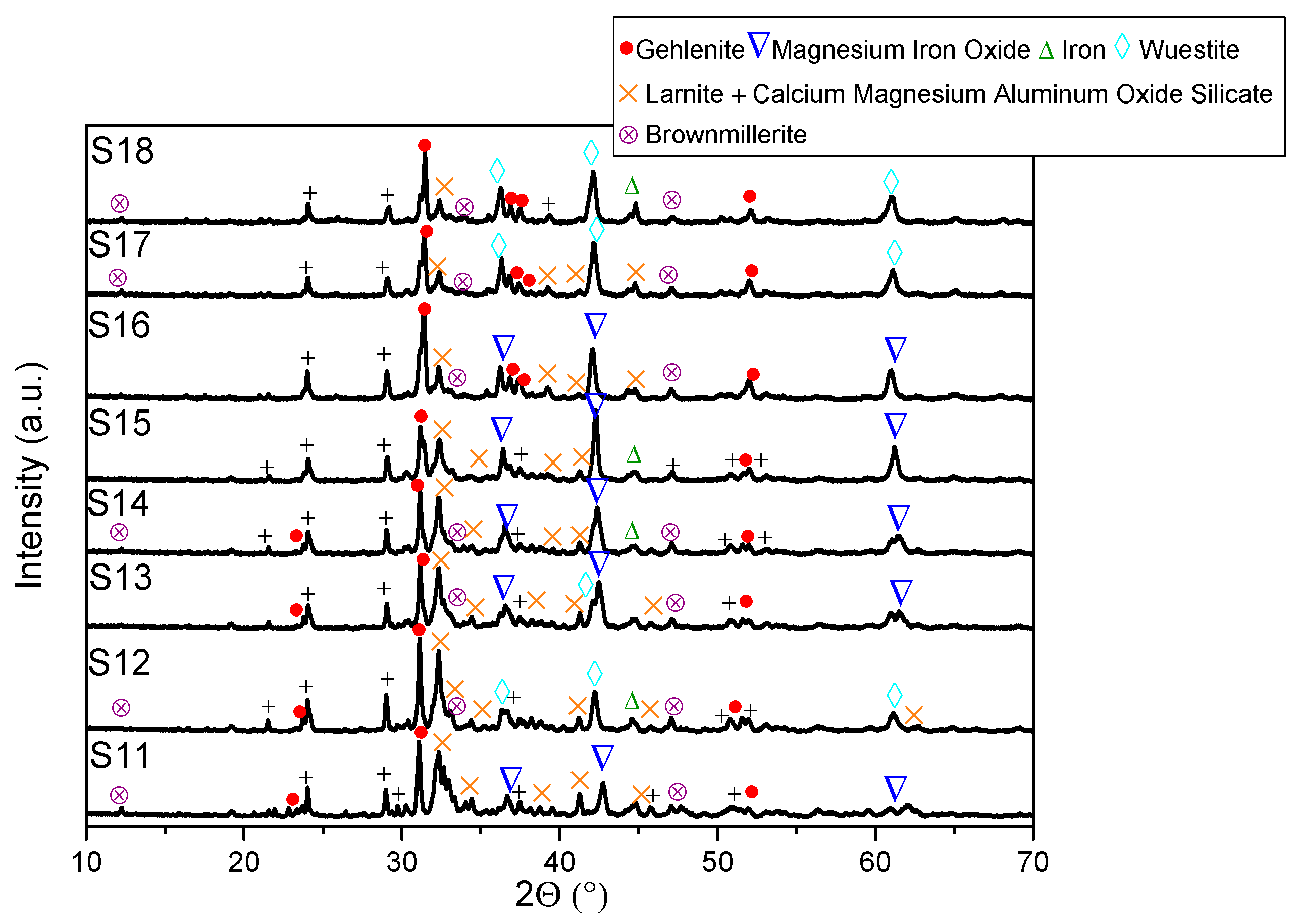
- XRD analysis revealed consistent trends in the composition of slag foam samples unrelated to the carbon source used. Higher MgO content was associated with predominant phases such as monticellite and magnesium iron oxide. Slag foam samples with the lowest basicity showed the presence of monticellite and merwinite, while those with the highest basicity contained calcium magnesium aluminum oxide silicate, larnite, or dicalcium silicate;
- (6)
- When a mixture of coke and biochar was used, it produced different foaming behavior than coke or biochar. From experimental observation, biochar reacts fast enough at the initial stages of foaming, and, probably, coke has a major effect throughout the entire foaming process. It could be assumed that this mixture might generate more CO/CO2 than using 100 wt.% coke or 100 wt.% biochar individually. Consequently, this increased gas generation could lead to a prolonged reaction time compared to using 100 wt.% biochar, thereby enhancing the overall foaming process.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, T.X.; Coley, K.S.; Irons, G.A. Progress in Slag Foaming in Metallurgical Processes. Metall. Mater. Trans. B 2012, 43, 751–757. [Google Scholar] [CrossRef]
- Matsuura, H.; Fruehan, R.J. Slag Foaming in an Electric Arc Furnace. ISIJ Int. 2009, 49, 1530–1535. [Google Scholar] [CrossRef]
- Chychko, A.; Seetharaman, S. Foaming in Electric Arc Furnace Part I: Laboratory Studies of Enthalpy Changes of Carbonate Additions to Slag Melts. Metall. Mater. Trans. B 2011, 42, 20–29. [Google Scholar] [CrossRef]
- Vieira, D.; Almeida, R.A.M.D.; Bielefeldt, W.V.; Vilela, A.C.F. Slag Evaluation to Reduce Energy Consumption and EAF Electrical Instability. Mater. Res. 2016, 19, 1127–1131. [Google Scholar] [CrossRef]
- Ji, F.-Z.; Barati, M.; Coley, K.; Irons, G.A. Kinetics of Coal Injection into Iron Oxide Containing Slags. Can. Metall. Q. 2005, 44, 85–94. [Google Scholar] [CrossRef]
- Agnihotri, A.; Singh, P.K.; Singh, D.; Gupta, M. Foamy Slag Practice to Enhance the Energy Efficiency of Electric Arc Furnace: An Industrial Scale Validation. Mater. Today Proc. 2021, 46, 1537–1542. [Google Scholar] [CrossRef]
- de Almeida, R.A.M.; Vieira, D.; Bielefeldt, W.V.; Vilela, A.C.F. Slag Foaming Fundamentals—A Critical Assessment. Mater. Res. 2017, 20, 474–480. [Google Scholar] [CrossRef]
- Kieush, L.; Koveria, A.; Schenk, J.; Rysbekov, K.; Lozynskyi, V.; Zheng, H.; Matayev, A. Investigation into the Effect of Multi-Component Coal Blends on Properties of Metallurgical Coke via Petrographic Analysis under Industrial Conditions. Sustainability 2022, 14, 9947. [Google Scholar] [CrossRef]
- Ogawa, Y.; Katayama, H.; Hirata, H.; Tokumitsu, N.; Yamauchi, M. Slag Foaming in Smelting Reduction and Its Control with Carbonaceous Materials. ISIJ Int. 1992, 32, 87–94. [Google Scholar] [CrossRef]
- Wu, K.; Qian, W.; Chu, S.; Niu, Q.; Luo, H. Behavior of Slag Foaming Caused by Blowing Gas in Molten Slags. ISIJ Int. 2000, 40, 954–957. [Google Scholar] [CrossRef]
- Zhang, Y.; Fruehan, R.J. Effect of Carbonaceous Particles on Slag Foaming. Metall. Mater. Trans. B 1995, 26, 813–819. [Google Scholar] [CrossRef]
- Bhoi, B.; Jouhari, A.K.; Ray, H.S.; Misra, V.N. Smelting Reduction Reactions by Solid Carbon Using Induction Furnace: Foaming Behaviour and Kinetics of FeO Reduction in CaO–SiO2–FeO Slag. Ironmak. Steelmak. 2006, 33, 245–252. [Google Scholar] [CrossRef]
- Zhang, Y.; Fruehan, R.J. Effect of Gas Type and Pressure on Slag Foaming. Metall. Mater. Trans. B 1995, 26, 1088–1091. [Google Scholar] [CrossRef]
- Kieush, L.; Schenk, J.; Koveria, A.; Hrubiak, A.; Hopfinger, H.; Zheng, H. Evaluation of Slag Foaming Behavior Using Renewable Carbon Sources in Electric Arc Furnace-Based Steel Production. Energies 2023, 16, 4673. [Google Scholar] [CrossRef]
- European Commission. Directorate General for Research and Innovation. In Sustainable EAF Steel Production (GREENEAF); Publications Office: Luxembourg, 2013. [Google Scholar]
- Cirilli, F.; Baracchini, G.; Bianco, L. EAF Long Term Industrial Trials of Utilization of Char from Biomass as Fossil Coal Substitute. La Metall. Ital. 2017, 109, 13–17. [Google Scholar]
- Nwachukwu, C.M.; Wang, C.; Wetterlund, E. Exploring the Role of Forest Biomass in Abating Fossil CO2 Emissions in the Iron and Steel Industry—The Case of Sweden. Appl. Energy 2021, 288, 116558. [Google Scholar] [CrossRef]
- Funke, A.; Demus, T.; Willms, T.; Schenke, L.; Echterhof, T.; Niebel, A.; Pfeifer, H.; Dahmen, N. Application of Fast Pyrolysis Char in an Electric Arc Furnace. Fuel Process. Technol. 2018, 174, 61–68. [Google Scholar] [CrossRef]
- Kalde, A.; Demus, T.; Echterhof, T.; Pfeifer, H. Determining the Reactivity of Biochar-Agglomerates to Replace Fossil Coal in Electric Arc Furnace Steelmaking. In Proceedings of the EUBCE 2015 Online Conference Proceedings, Online, 1–4 June 2015; pp. 497–507. [Google Scholar]
- Chen, W.-H.; Peng, J.; Bi, X.T. A State-of-the-Art Review of Biomass Torrefaction, Densification and Applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Ru, B.; Wang, S.; Dai, G.; Zhang, L. Effect of Torrefaction on Biomass Physicochemical Characteristics and the Resulting Pyrolysis Behavior. Energy Fuels 2015, 29, 5865–5874. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. In-Depth Investigation of Biomass Pyrolysis Based on Three Major Components: Hemicellulose, Cellulose and Lignin. Energy Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Kieush, L.; Koveria, A.; Boyko, M.; Yaholnyk, M.; Hrubiak, A.; Molchanov, L.; Moklyak, V. Influence of Biocoke on Iron Ore Sintering Performance and Strength Properties of Sinter. Min. Miner. Depos. 2022, 16, 55–63. [Google Scholar] [CrossRef]
- Shukla, I. Potential of Renewable Agricultural Wastes in the Smart and Sustainable Steelmaking Process. J. Clean. Prod. 2022, 370, 133422. [Google Scholar] [CrossRef]
- Fidalgo, B.; Berrueco, C.; Millan, M. Chars from Agricultural Wastes as Greener Fuels for Electric Arc Furnaces. J. Anal. Appl. Pyrolysis 2015, 113, 274–280. [Google Scholar] [CrossRef]
- Echterhof, T. Review on the Use of Alternative Carbon Sources in EAF Steelmaking. Metals 2021, 11, 222. [Google Scholar] [CrossRef]
- Huang, X.; Ng, K.W.; Giroux, L.; Duchesne, M.; Li, D.; Todoschuk, T. Interaction Behavior of Biogenic Material with Electric Arc Furnace Slag. Fuels 2021, 2, 420–436. [Google Scholar] [CrossRef]
- Robinson, R.; Brabie, L.; Pettersson, M.; Amovic, M.; Ljunggren, R. An Empirical Comparative Study of Renewable Biochar and Fossil Carbon as Carburizer in Steelmaking. ISIJ Int. 2022, 62, 2522–2528. [Google Scholar] [CrossRef]
- Demus, T.; Reichel, T.; Schulten, M.; Echterhof, T.; Pfeifer, H. Increasing the Sustainability of Steel Production in the Electric Arc Furnace by Substituting Fossil Coal with Biochar Agglomerates. Ironmak. Steelmak. 2016, 43, 564–570. [Google Scholar] [CrossRef]
- Yunos, N.F.M.; Zaharia, M.; Idris, M.A.; Nath, D.; Khanna, R.; Sahajwalla, V. Recycling Agricultural Waste from Palm Shells during Electric Arc Furnace Steelmaking. Energy Fuels 2012, 26, 278–286. [Google Scholar] [CrossRef]
- Hoikkaniemi, E.; Sulasalmi, P.; Visuri, V.-V.; Fabritius, T. Biochar as a Slag Foaming Agent in EAF—A Novel Experimental Setup. In Proceedings of the 5th European Academic Symposium on EAF Steelmaking, Oulu, Finland, 5 June 2023; pp. 1–5. [Google Scholar]
- Di Giovanni, C.; Li, D.; Ng, K.W.; Huang, X. Ranking of Injection Biochar for Slag Foaming Applications in Steelmaking. Metals 2023, 13, 1003. [Google Scholar] [CrossRef]
- Kieush, L.; Rieger, J.; Schenk, J.; Brondi, C.; Rovelli, D.; Echterhof, T.; Cirilli, F.; Thaler, C.; Jaeger, N.; Snaet, D.; et al. A Comprehensive Review of Secondary Carbon Bio-Carriers for Application in Metallurgical Processes: Utilization of Torrefied Biomass in Steel Production. Metals 2022, 12, 2005. [Google Scholar] [CrossRef]
- Kieush, L.; Schenk, J.; Koveria, A.; Rantitsch, G.; Hrubiak, A.; Hopfinger, H. Utilization of Renewable Carbon in Electric Arc Furnace-Based Steel Production: Comparative Evaluation of Properties of Conventional and Non-Conventional Carbon-Bearing Sources. Metals 2023, 13, 722. [Google Scholar] [CrossRef]
- Menad, N.-E.; Kana, N.; Seron, A.; Kanari, N. New EAF Slag Characterization Methodology for Strategic Metal Recovery. Materials 2021, 14, 1513. [Google Scholar] [CrossRef]
- Heo, J.H.; Park, J.H. Assessment of Physicochemical Properties of Electrical Arc Furnace Slag and Their Effects on Foamability. Metall. Mater. Trans. B 2019, 50, 2959–2968. [Google Scholar] [CrossRef]
- Jiang, R.; Fruehan, R.J. Slag Foaming in Bath Smelting. Metall. Mater. Trans. B 1991, 22, 481–489. [Google Scholar] [CrossRef]
- Lahiri, A.K.; Seetharaman, S. Foaming Behavior of Slags. Metall. Mater. Trans. B 2002, 33, 499–502. [Google Scholar] [CrossRef]
- Hong, L.; Hirasawa, M.; Sano, M. Behavior of Slag Foaming with Reduction of Iron Oxide in Molten Slags by Graphite. ISIJ Int. 1998, 38, 1339–1345. [Google Scholar] [CrossRef]
- Xiang, J.; Wang, X.; Yang, M.; Wang, J.; Shan, C.; Fan, G.; Qiu, G.; Lv, X. Slag-Foaming Phenomenon Originating from Reaction of Titanium-Bearing Blast Furnace Slag: Continuous Monitoring of Foaming Height and Calibration. J. Mater. Res. Technol. 2021, 11, 1184–1192. [Google Scholar] [CrossRef]
- Ren, X.; Cai, H.; Chang, J.-M.; Fan, Y.-M. TG-FTIR Study on the Pyrolysis Properties of Lignin from Different Kinds of Woody Biomass. Mater. Sci. 2018, 3, 1–7. [Google Scholar] [CrossRef]
- Ben, H.; Hao, N.; Liu, Q.; Ragauskas, A.J. Solid-State NMR Investigation of Bio-Chars Produced from Biomass Components and Whole Biomasses. Bioenerg. Res. 2017, 10, 1036–1044. [Google Scholar] [CrossRef]
- Jennings, K.R. Spectrometric Identification of Organic Compounds (Fifth Edition). Wiley, New York. Org. Mass Spectrom. 1991, 26, 813. [Google Scholar] [CrossRef]
- Vidacak, B.; Arvanitidis, I.; Jönsson, P.G.; Sjöberg, P. Observation on Foaming of EAF Slags in the Production of Stainless Steel: Observation on Foaming of EAF Slags. Scand. J. Metall. 2002, 31, 321–327. [Google Scholar] [CrossRef]
- Zhang, Y.; Fruehan, R.J. Effect of the Bubble Size and Chemical Reactions on Slag Foaming. MMTB 1995, 26, 803–812. [Google Scholar] [CrossRef]
- Herbelin, M.; Bascou, J.; Lavastre, V.; Guillaume, D.; Benbakkar, M.; Peuble, S.; Baron, J.-P. Steel Slag Characterisation—Benefit of Coupling Chemical, Mineralogical and Magnetic Techniques. Minerals 2020, 10, 705. [Google Scholar] [CrossRef]
- Mombelli, D.; Mapelli, C.; Barella, S.; Gruttadauria, A.; Le Saout, G.; Garcia-Diaz, E. The Efficiency of Quartz Addition on Electric Arc Furnace (EAF) Carbon Steel Slag Stability. J. Hazard. Mater. 2014, 279, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Riboldi, A.; Borgese, L.; Vassalini, I.; Cornacchia, G.; Gelfi, M.; Boniardi, M.V.; Casaroli, A.; Depero, L.E. Micro-Raman Spectroscopy Investigation of Crystalline Phases in EAF Slag. Appl. Sci. 2020, 10, 4115. [Google Scholar] [CrossRef]
- Gaye, H.; Lehmann, J. Steelmaking Slags. In Slag Atlas; Verlag Stahleisen GmbH: Dusseldorf, Germany, 1995. [Google Scholar]
- Corbari, R.; Matsuura, H.; Halder, S.; Walker, M.; Fruehan, R.J. Foaming and the Rate of the Carbon-Iron Oxide Reaction in Slag. Metall. Mater. Trans. B 2009, 40, 940–948. [Google Scholar] [CrossRef]
- Luz, A.P.; Avila, T.A.; Bonadia, P.; Pandolfelli, V.C. Slag Foaming: Fundamentals, Experimental Evaluation and Application in the Steelmaking Industry. Refract. Worldforum 2011, 3, 91–98. [Google Scholar]
- Ito, K.; Fruehan, R.J. Study on the Foaming of CaO-SiO2-FeO Slags: Part I. Foaming Parameters and Experimental Results. Metall. Trans. B 1989, 20, 509–514. [Google Scholar] [CrossRef]
- Luz, A.P.; Tomba Martinez, A.G.; López, F.; Bonadia, P.; Pandolfelli, V.C. Slag Foaming Practice in the Steelmaking Process. Ceram. Int. 2018, 44, 8727–8741. [Google Scholar] [CrossRef]
- Teasdale, S.L.; Hayes, P.C. Observations of the Reduction of FeO from Slag by Graphite, Coke and Coal Char. ISIJ Int. 2005, 45, 634–641. [Google Scholar] [CrossRef]
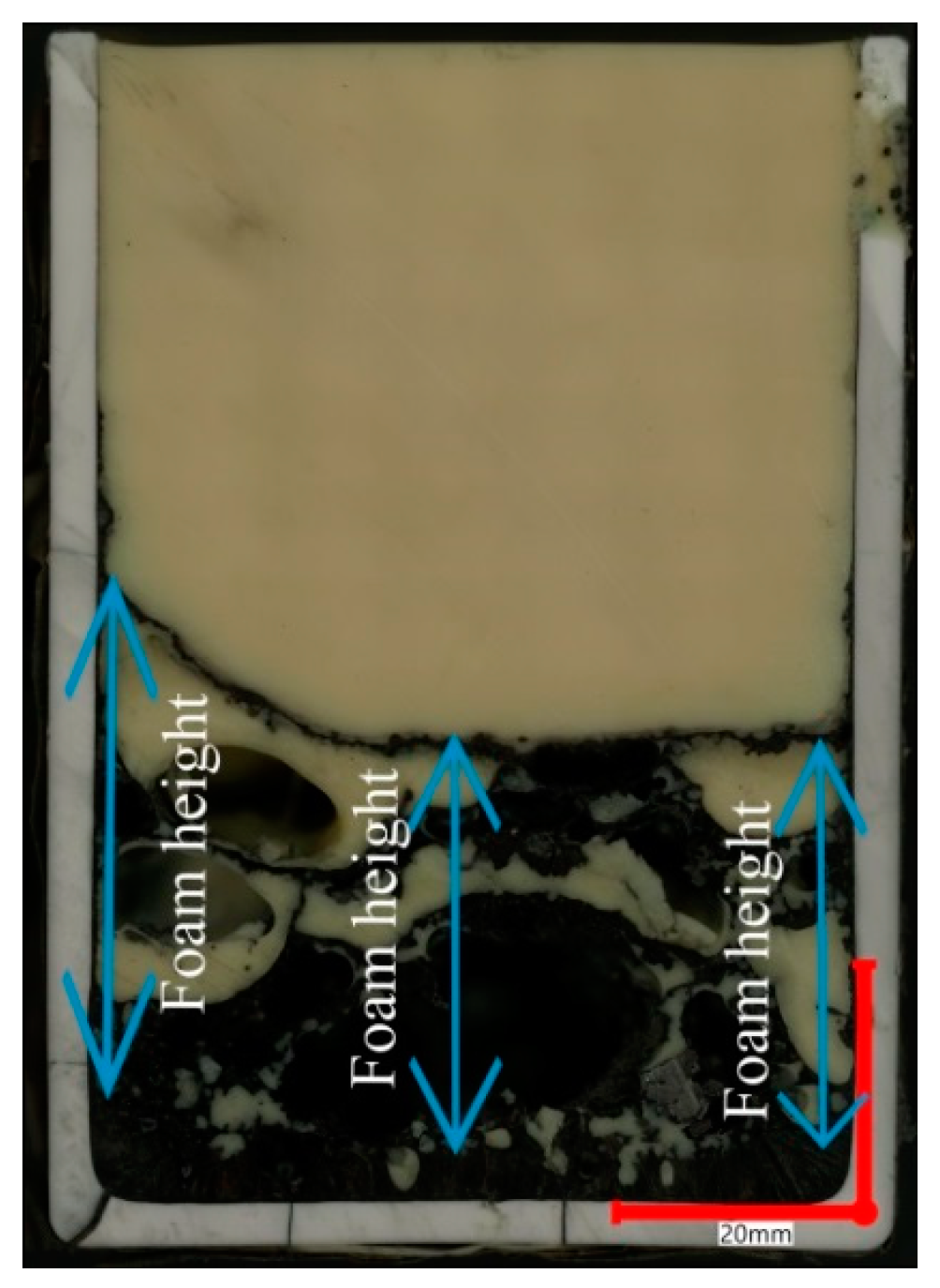
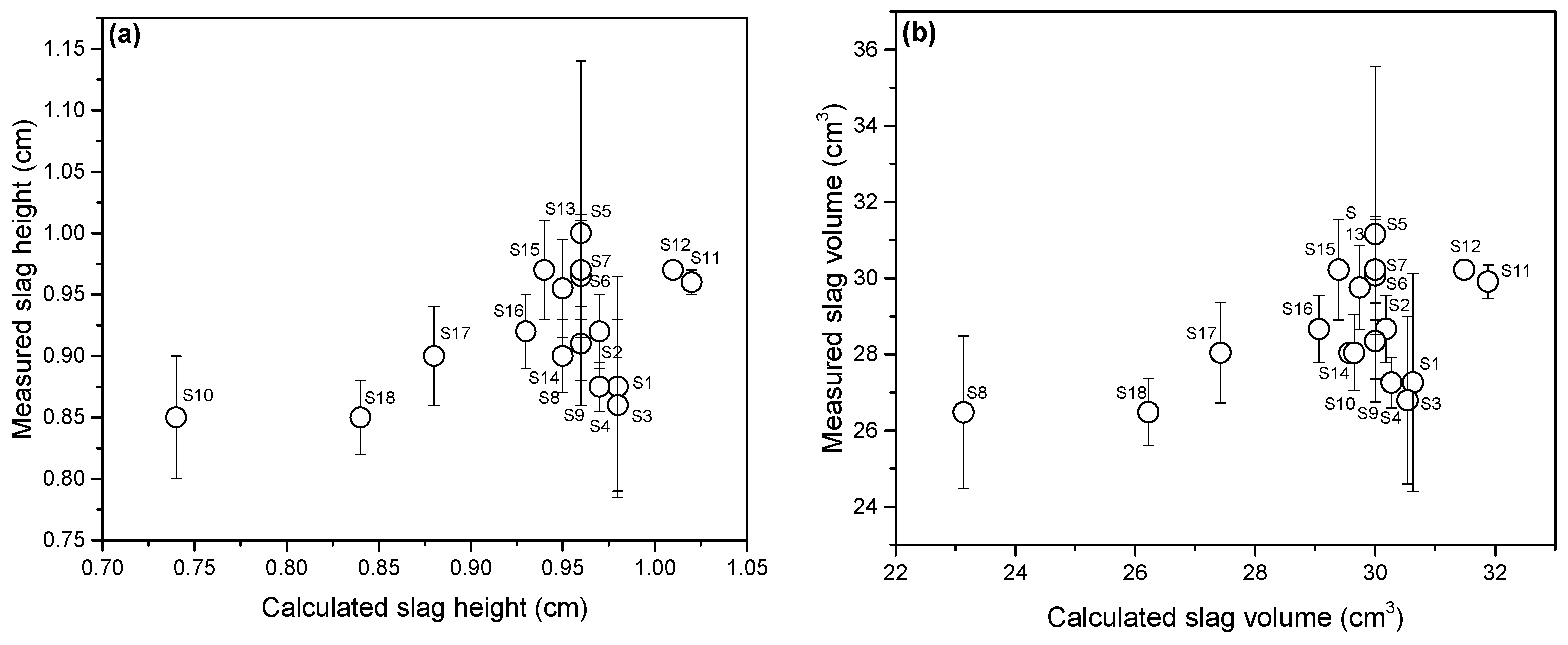
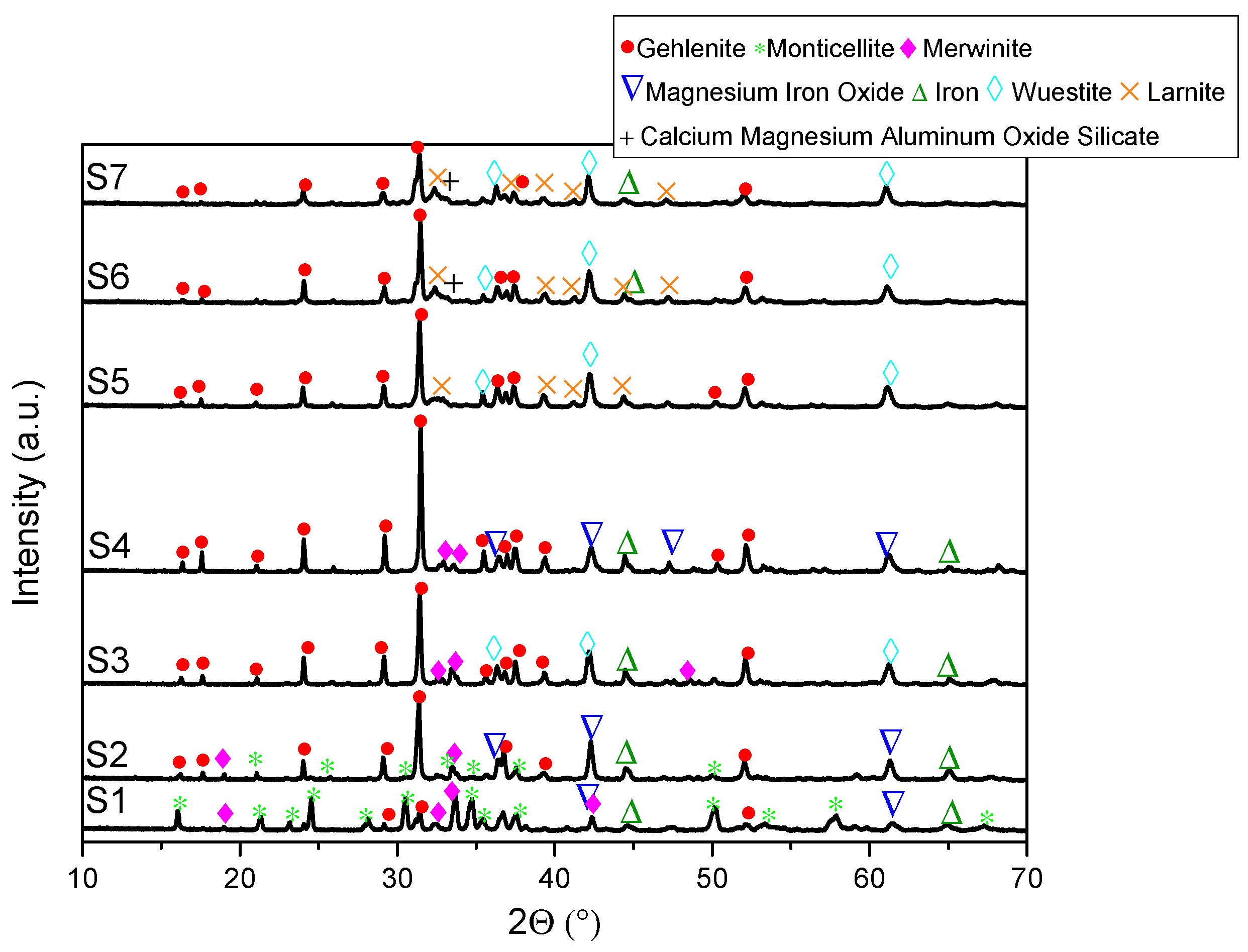
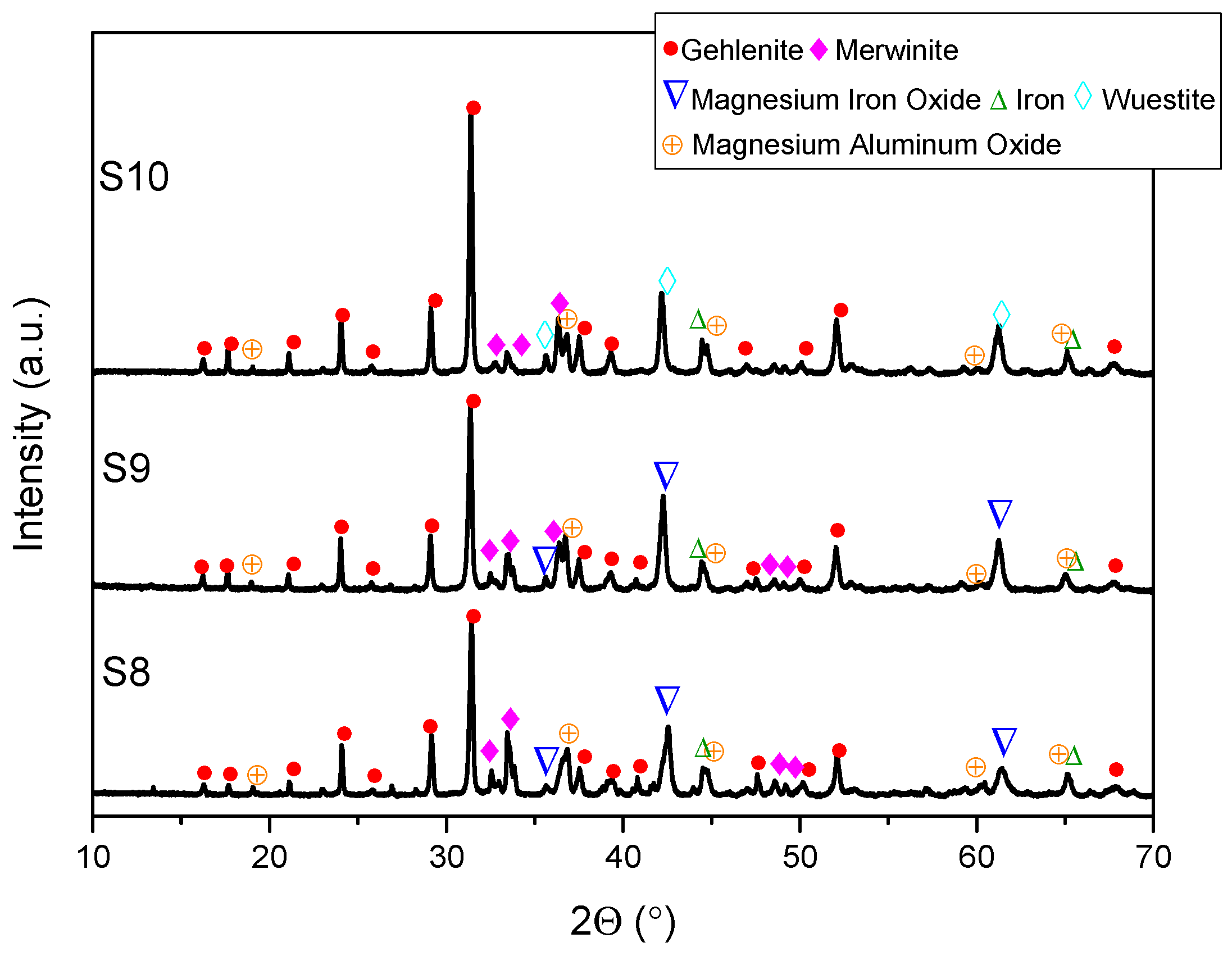
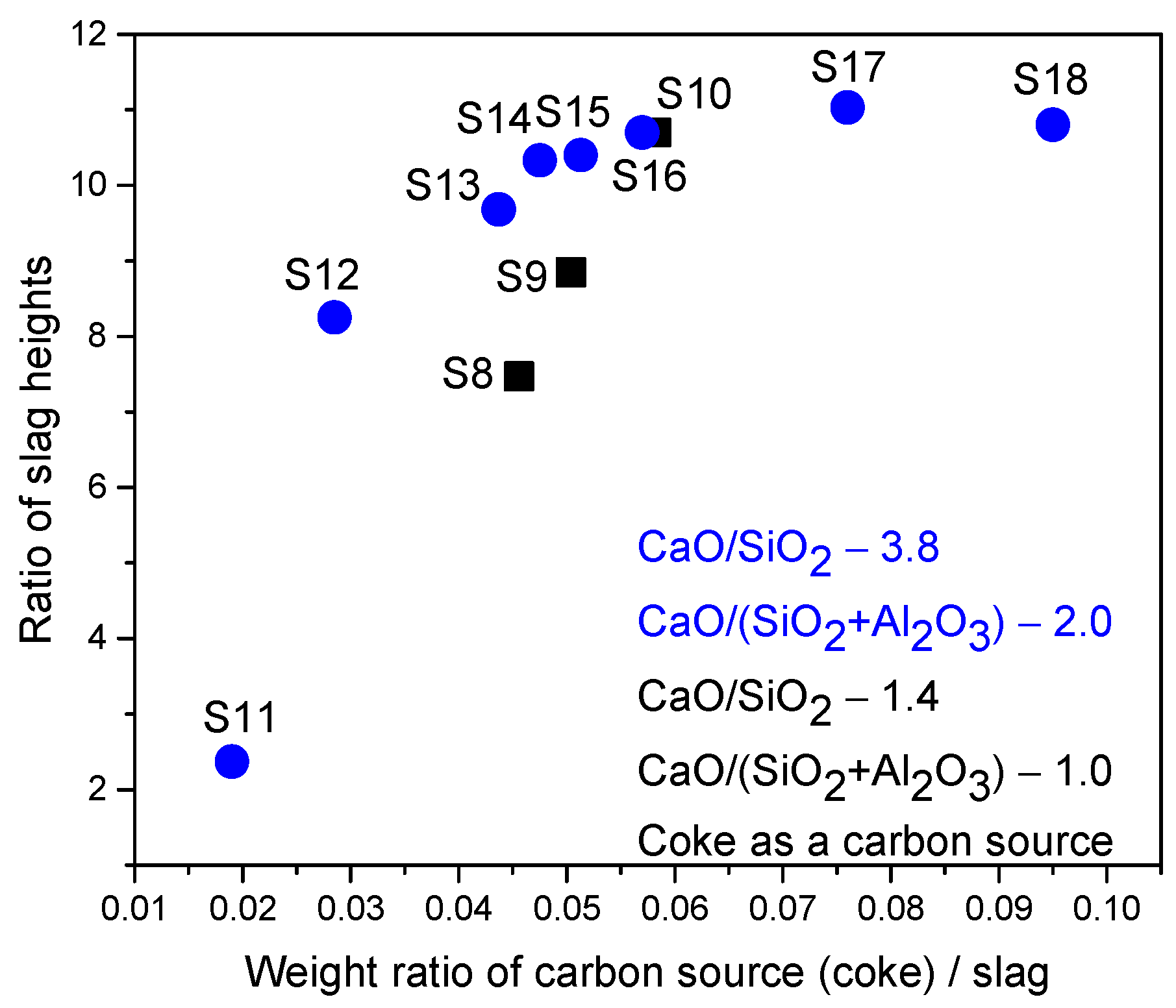
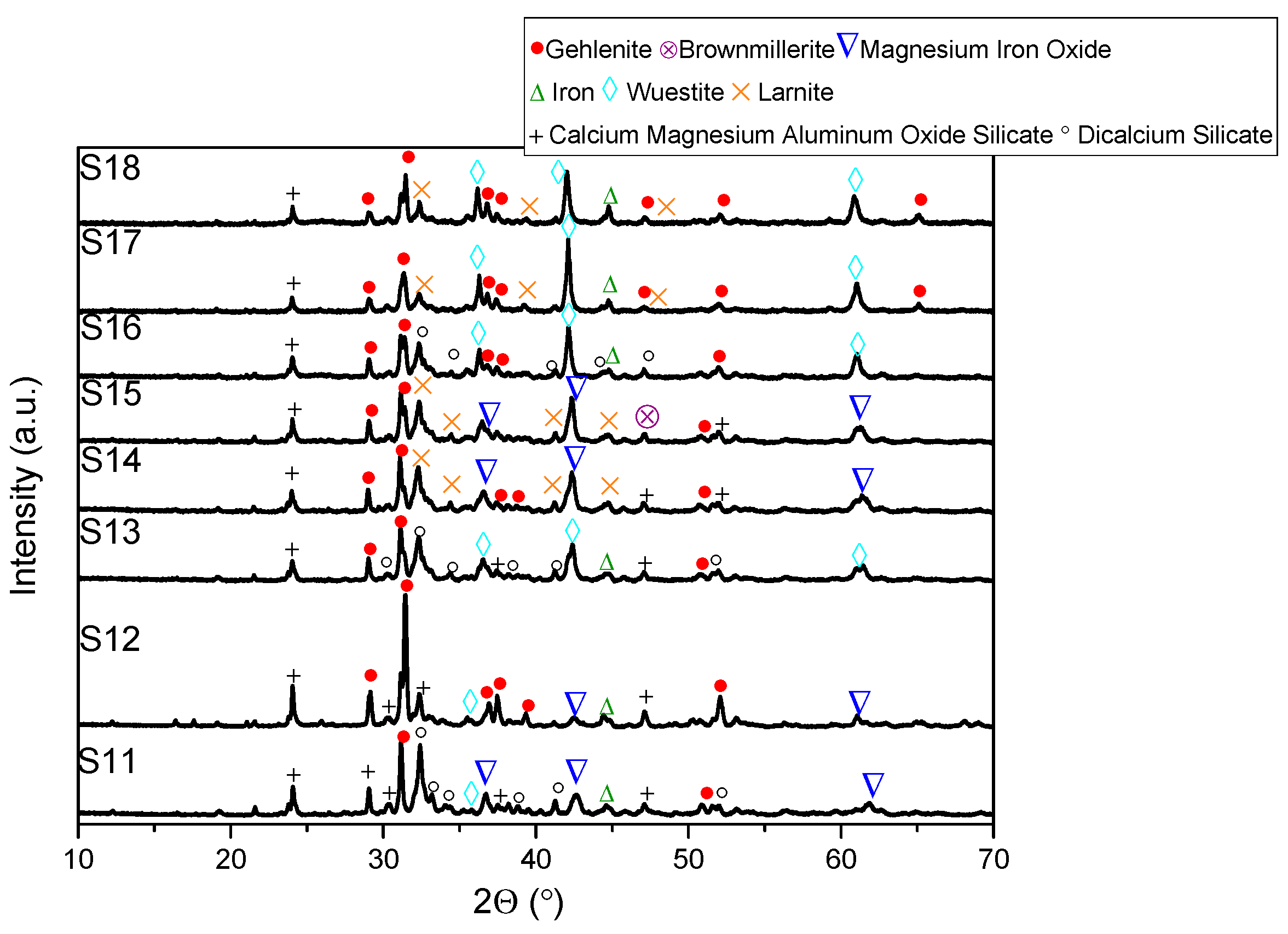



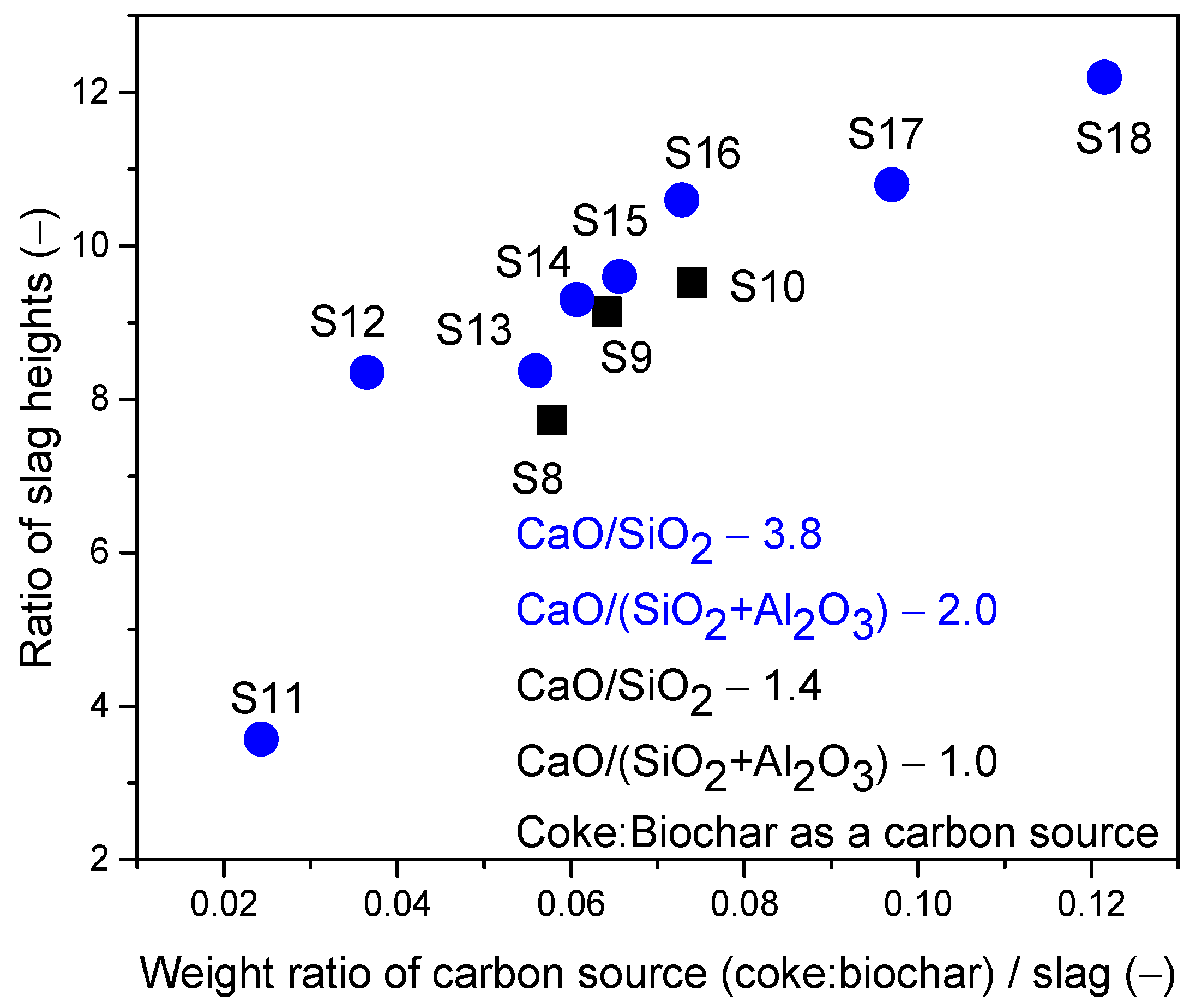
| Sample | FeO, wt.% | CaO, wt.% | SiO2, wt.% | MgO, wt.% | Al2O3, wt.% | Total | B2 | B3 | μ, Pa·s | ρ, g/cm3 | σ, N/m | Ʃ, s |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 25.0 | 27.2 | 27.2 | 15.0 | 5.5 | 100.0 | 1.0 | 0.8 | 0.0475 | 3.33 | 0.54 | 4.07 |
| S2 | 25.0 | 28.3 | 23.2 | 14.2 | 9.3 | 100.0 | 1.2 | 0.9 | 0.0465 | 3.38 | 0.54 | 3.95 |
| S3 | 25.0 | 35.6 | 22.9 | 9.7 | 6.8 | 100.0 | 1.6 | 1.2 | 0.0412 | 3.34 | 0.56 | 3.48 |
| S4 | 25.0 | 38.5 | 19.0 | 8.0 | 9.4 | 100.0 | 2.0 | 1.4 | 0.0391 | 3.37 | 0.57 | 3.25 |
| S5 | 25.0 | 41.5 | 15.5 | 7.5 | 10.5 | 100.0 | 2.7 | 1.6 | 0.0363 | 3.40 | 0.58 | 2.97 |
| S6 | 25.0 | 44.5 | 14.3 | 6.4 | 9.8 | 100.0 | 3.1 | 1.8 | 0.0341 | 3.40 | 0.59 | 2.78 |
| S7 | 25.0 | 45.7 | 13.5 | 6.0 | 9.8 | 100.0 | 3.4 | 2.0 | 0.0333 | 3.40 | 0.59 | 2.71 |
| Sample | FeO, wt.% | CaO, wt.% | SiO2, wt.% | MgO, wt.% | Al2O3, wt.% | Total | B2 | B3 | μ, Pa·s | ρ, g/cm3 | σ, N/m | Ʃ, s |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S8 | 24.0 | 30.0 | 22.0 | 16.0 | 8.0 | 100.0 | 1.4 | 1.0 | 0.044 | 4.41 | 0.73 | 2.83 |
| S9 | 26.5 | 30.0 | 22.0 | 13.5 | 8.0 | 100.0 | 1.4 | 1.0 | 0.041 | 3.40 | 0.56 | 3.42 |
| S10 | 30.7 | 30.0 | 22.0 | 9.3 | 8.0 | 100.0 | 1.4 | 1.0 | 0.038 | 3.44 | 0.56 | 3.15 |
| Sample | FeO, wt.% | CaO, wt.% | SiO2, wt.% | MgO, wt.% | Al2O3, wt.% | Total | B2 | B3 | μ, Pa·s | ρ, g/cm3 | σ, N/m | Ʃ, s |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S11 | 10.0 | 53.1 | 14.0 | 10.9 | 12.0 | 100.0 | 3.8 | 2.0 | 0.0466 | 3.20 | 0.58 | 3.94 |
| S12 | 15.0 | 53.1 | 14.0 | 6.1 | 12.0 | 100.0 | 3.8 | 2.0 | 0.0420 | 3.24 | 0.58 | 3.52 |
| S13 | 23.0 | 43.6 | 11.6 | 11.2 | 10.6 | 100.0 | 3.8 | 2.0 | 0.0348 | 3.43 | 0.59 | 2.81 |
| S14 | 25.0 | 43.3 | 11.4 | 10.3 | 10.0 | 100.0 | 3.8 | 2.0 | 0.0328 | 3.45 | 0.59 | 2.64 |
| S15 | 27.0 | 43.2 | 11.4 | 8.4 | 10.0 | 100.0 | 3.8 | 2.0 | 0.0314 | 3.47 | 0.60 | 2.51 |
| S16 | 30.0 | 43.1 | 11.2 | 5.7 | 10.0 | 100.0 | 3.8 | 2.0 | 0.0291 | 3.51 | 0.60 | 2.31 |
| S17 | 40.0 | 33.1 | 8.8 | 10.1 | 8.0 | 100.0 | 3.8 | 2.0 | 0.0023 | 3.72 | 0.61 | 0.18 |
| S18 | 50.0 | 28.1 | 7.4 | 7.5 | 7.0 | 100.0 | 3.8 | 2.0 | 0.0018 | 3.89 | 0.62 | 0.13 |
| Characters | Coke | Bch |
|---|---|---|
| Proximate analysis | ||
| M | 1.04 | 2.67 |
| VM (daf) | 1.57 | 41.04 |
| Ash (db) | 10.88 | 4.29 |
| Elemental analysis | ||
| S (db) | 0.77 | n.d. |
| C (db) | 86.25 | 72.74 |
| H (db) | 0.29 | 4.62 |
| N (db) | 1.21 | 0.24 |
| * Others, mainly O (db) | 0.60 | 18.11 |
| Cfix (db) | 87.70 | 56.43 |
| Sample | Coke | Biochar | Coke + Biochar |
|---|---|---|---|
| S1–S7 | 4.75 | 7.38 | 2.38 + 3.69 |
| S8 | 4.56 | 7.09 | 2.28 + 3.55 |
| S9 | 5.04 | 7.83 | 2.52 + 3.92 |
| S10 | 5.83 | 9.07 | 2.92 + 4.54 |
| S11 | 1.90 | 2.95 | 0.95 + 1.48 |
| S12 | 2.85 | 4.43 | 1.43 + 2.22 |
| S13 | 4.37 | 6.79 | 2.19 + 3.40 |
| S14 | 4.75 | 7.38 | 2.38 + 3.69 |
| S15 | 5.13 | 7.97 | 2.57 + 3.99 |
| S16 | 5.70 | 8.86 | 2.85 + 4.43 |
| S17 | 7.60 | 11.81 | 3.80 + 5.90 |
| S18 | 9.50 | 14.77 | 4.75 + 7.40 |
| Sample | Foam Height hfoam, cm | Foam Volume Vfoam, cm3 | Relative Foaming Volume, ΔV/V0 | Volumetric Gas Fraction, Xgas |
|---|---|---|---|---|
| S1 | 3.5 * | 109.05 | 3.32 | 0.77 |
| S2 | 7.0 | 218.10 | 6.45 | 0.87 |
| S3 | 9.6 | 299.10 | 10.85 | 0.92 |
| S4 | 10.6 | 330.26 | 11.33 | 0.92 |
| S5 | 11.0 | 342.72 | 11.22 | 0.92 |
| S6 | 8.5 | 264.83 | 8.04 | 0.89 |
| S7 | 8.0 | 249.25 | 7.60 | 0.88 |
| Sample | O | Mg | Al | Si | Ca | Fe |
|---|---|---|---|---|---|---|
| S1 | 39.62 | 7.38 | 6.57 | 10.32 | 15.82 | 20.30 |
| S2 | 37.98 | 7.48 | 13.11 | 8.25 | 15.32 | 17.86 |
| S3 | 38.26 | 4.50 | 8.56 | 9.92 | 24.35 | 14.41 |
| S4 | 35.76 | 3.17 | 10.60 | 6.46 | 30.62 | 13.38 |
| S5 | 36.31 | 2.71 | 10.68 | 5.41 | 27.37 | 17.52 |
| S6 | 36.41 | 2.27 | 14.04 | 4.93 | 29.23 | 13.11 |
| S7 | 38.16 | 2.27 | 13.72 | 4.92 | 26.08 | 14.85 |
| Sample | Foam Height hfoam, cm | Foam Volume Vfoam, cm3 | Relative Foaming Volume, ΔV/V0 | Volumetric Gas Fraction, Xgas |
|---|---|---|---|---|
| S8 | 7.1 | 221.21 | 6.89 | 0.87 |
| S9 | 8.5 | 264.83 | 8.44 | 0.89 |
| S10 | 7.9 | 246.14 | 8.29 | 0.89 |
| Sample | O | Mg | Al | Si | Ca | Fe |
|---|---|---|---|---|---|---|
| S8 | 38.82 | 7.06 | 5.20 | 9.87 | 21.40 | 17.65 |
| S9 | 35.80 | 7.80 | 4.79 | 10.69 | 20.71 | 20.21 |
| S10 | 36.07 | 5.43 | 4.48 | 10.52 | 21.18 | 22.32 |
| Sample | Foam Height hfoam, cm | Foam Volume Vfoam, cm3 | Relative Foaming Volume, ΔV/V0 | Volumetric Gas Fraction, Xgas |
|---|---|---|---|---|
| S11 | 2.3 * | 71.66 | 1.37 | 0.58 |
| S12 | 8.0 | 249.25 | 7.25 | 0.88 |
| S13 | 9.0 | 280.41 | 8.68 | 0.90 |
| S14 | 9.3 | 289.76 | 9.33 | 0.90 |
| S15 | 9.8 | 305.34 | 9.43 | 0.90 |
| S16 | 10.1 | 314.68 | 9.74 | 0.91 |
| S17 | 9.6 | 299.10 | 10.03 | 0.91 |
| S18 | 9.4 | 292.87 | 9.80 | 0.91 |
| Sample | O | Mg | Al | Si | Ca | Fe |
|---|---|---|---|---|---|---|
| S11 | 39.74 | 3.49 | 13.01 | 4.57 | 31.15 | 8.03 |
| S12 | 38.34 | 1.36 | 12.54 | 3.61 | 37.56 | 6.59 |
| S13 | 34.50 | 1.80 | 12.55 | 5.18 | 35.13 | 10.83 |
| S14 | 33.94 | 3.61 | 9.43 | 3.29 | 30.44 | 19.28 |
| S15 | 32.26 | 2.49 | 13.72 | 4.95 | 30.09 | 16.49 |
| S16 | 37.48 | 6.18 | 5.75 | 8.25 | 22.39 | 19.95 |
| S17 | 31.72 | 2.03 | 9.86 | 2.53 | 27.23 | 26.63 |
| S18 | 31.98 | 4.81 | 13.24 | 2.87 | 15.30 | 31.81 |
| Sample | Foam Height hfoam, cm | Foam Volume Vfoam, cm3 | Relative Foaming Volume ΔV/V0 | Volumetric Gas Fraction Xgas |
|---|---|---|---|---|
| S1 | 3.1 * | 96.59 | 2.30 | 0.70 |
| S2 | 8.7 | 271.06 | 8.70 | 0.89 |
| S3 | 8.6 | 267.94 | 7.60 | 0.88 |
| S4 | 8.7 | 271.06 | 8.00 | 0.89 |
| S5 | 8.9 | 277.29 | 7.90 | 0.89 |
| S6 | 8.6 | 267.94 | 7.60 | 0.88 |
| S7 | 10.0 | 311.56 | 9.10 | 0.90 |
| Sample | O | Mg | Al | Si | Ca | Fe |
|---|---|---|---|---|---|---|
| S1 | 37.53 | 9.08 | 5.65 | 12.23 | 20.82 | 14.69 |
| S2 | 35.23 | 2.09 | 10.98 | 3.72 | 27.40 | 20.59 |
| S3 | 38.50 | 2.71 | 11.43 | 7.03 | 23.07 | 17.25 |
| S4 | 36.15 | 4.69 | 10.68 | 7.62 | 26.72 | 14.15 |
| S5 | 37.73 | 2.35 | 16.15 | 5.49 | 24.95 | 13.33 |
| S6 | 38.25 | 2.53 | 13.33 | 4.35 | 23.92 | 17.61 |
| S7 | 37.25 | 2.29 | 12.22 | 3.97 | 29.89 | 14.38 |
| Sample | Foam Height hfoam, cm | Foam Volume Vfoam, cm3 | Relative Foaming Volume, ΔV/V0 | Volumetric Gas Fraction, Xgas |
|---|---|---|---|---|
| S8 | 6.7 | 208.75 | 6.20 | 0.86 |
| S9 | 6.9 | 214.98 | 6.34 | 0.86 |
| S10 | 6.8 | 186.94 | 5.67 | 0.85 |
| Sample | O | Mg | Al | Si | Ca | Fe |
|---|---|---|---|---|---|---|
| S8 | 36.24 | 9.08 | 4.39 | 10.14 | 21.28 | 18.87 |
| S9 | 37.65 | 6.34 | 4.76 | 10.37 | 20.01 | 20.87 |
| S10 | 37.12 | 5.22 | 5.23 | 10.09 | 19.29 | 23.05 |
| Sample | Foam Height hfoam, cm | Foam Volume Vfoam, cm3 | Relative Foaming Volume ΔV/V0 | Volumetric Gas Fraction, Xgas |
|---|---|---|---|---|
| S11 | 2.5 * | 77.89 | 2.60 | 0.72 |
| S12 | 6.7 | 208.75 | 5.80 | 0.85 |
| S13 | 10.1 | 314.68 | 9.20 | 0.90 |
| S14 | 8.0 | 249.25 | 7.90 | 0.89 |
| S15 | 9.5 | 295.99 | 9.00 | 0.90 |
| S16 | 11.0 | 343.66 | 11.70 | 0.92 |
| S17 | 10.4 | 324.03 | 10.60 | 0.91 |
| S18 | 10.8 | 336.49 | 10.60 | 0.91 |
| Sample | O | Mg | Al | Si | Ca | Fe |
|---|---|---|---|---|---|---|
| S11 | 39.74 | 3.49 | 13.01 | 4.57 | 31.15 | 8.03 |
| S12 | 38.34 | 1.36 | 12.54 | 3.61 | 37.56 | 6.59 |
| S13 | 34.50 | 1.80 | 12.55 | 5.18 | 35.13 | 10.83 |
| S14 | 33.94 | 3.61 | 9.43 | 3.29 | 30.44 | 19.28 |
| S15 | 32.26 | 2.49 | 13.72 | 4.95 | 30.09 | 16.49 |
| S16 | 37.48 | 6.18 | 5.75 | 8.25 | 22.39 | 19.95 |
| S17 | 31.72 | 2.03 | 9.86 | 2.53 | 27.23 | 26.63 |
| S18 | 31.98 | 4.71 | 13.24 | 2.87 | 15.30 | 31.81 |
| Sample | Foam Height hfoam, cm | Foam Volume Vfoam, cm3 | Relative Foaming Volume, ΔV/V0 | Volumetric Gas Fraction, Xgas |
|---|---|---|---|---|
| S1 | 6.4 | 199.40 | 6.11 | 0.86 |
| S2 | 7.3 | 227.44 | 6.40 | 0.87 |
| S3 | 9.7 | 302.22 | 9.66 | 0.91 |
| S4 | 10.6 | 330.26 | 10.91 | 0.92 |
| S5 | 10.5 | 327.14 | 8.55 | 0.90 |
| S6 | 9.7 | 302.22 | 8.70 | 0.90 |
| S7 | 9.3 | 289.76 | 8.30 | 0.89 |
| Sample | O | Mg | Al | Si | Ca | Fe |
|---|---|---|---|---|---|---|
| S1 | 37.80 | 3.75 | 15.42 | 4.62 | 22.99 | 15.42 |
| S2 | 38.24 | 6.16 | 10.35 | 9.25 | 21.39 | 14.61 |
| S3 | 34.72 | 3.48 | 7.05 | 8.81 | 27.89 | 18.05 |
| S4 | 40.70 | 2.16 | 12.69 | 5.32 | 25.08 | 14.06 |
| S5 | 34.06 | 2.55 | 11.21 | 7.35 | 26.98 | 17.84 |
| S6 | 31.90 | 2.68 | 13.24 | 5.52 | 30.71 | 15.95 |
| S7 | 34.66 | 2.32 | 12.13 | 4.55 | 29.66 | 16.68 |
| Sample | Foam Height hfoam, cm | Foam Volume Vfoam, cm3 | Relative Foaming Volume, ΔV/V0 | Volumetric Gas Fraction, Xgas |
|---|---|---|---|---|
| S8 | 7.5 | 233.67 | 7.06 | 0.88 |
| S9 | 8.6 | 267.95 | 8.15 | 0.89 |
| S10 | 8.0 | 249.25 | 8.52 | 0.90 |
| Sample | O | Mg | Al | Si | Ca | Fe |
|---|---|---|---|---|---|---|
| S8 | 38.86 | 9.88 | 5.08 | 9.16 | 19.15 | 17.87 |
| S9 | 39.79 | 7.83 | 4.60 | 10.84 | 18.78 | 18.16 |
| S10 | 40.76 | 5.05 | 4.82 | 9.09 | 20.22 | 20.06 |
| Sample | Foam Height hfoam, cm | Foam Volume Vfoam, cm3 | Relative Foaming Volume, ΔV/V0 | Volumetric Gas Fraction, Xgas |
|---|---|---|---|---|
| S11 | 3.4 * | 105.93 | 2.58 | 0.72 |
| S12 | 8.1 | 252.37 | 7.35 | 0.88 |
| S13 | 8.2 | 255.48 | 7.37 | 0.88 |
| S14 | 8.4 | 261.72 | 8.33 | 0.89 |
| S15 | 9.6 | 299.10 | 8.60 | 0.90 |
| S16 | 9.6 | 299.10 | 9.67 | 0.91 |
| S17 | 10.0 | 311.57 | 9.75 | 0.91 |
| S18 | 10.1 | 314.68 | 11.16 | 0.92 |
| Sample | O | Mg | Al | Si | Ca | Fe |
|---|---|---|---|---|---|---|
| S11 | 41.58 | 3.35 | 11.47 | 3.88 | 32.39 | 7.31 |
| S12 | 31.85 | 1.42 | 8.86 | 2.78 | 41.59 | 13.49 |
| S13 | 37.24 | 4.35 | 10.16 | 4.27 | 26.14 | 17.83 |
| S14 | 47.17 | 3.03 | 13.64 | 3.39 | 14.83 | 17.93 |
| S15 | 28.49 | 1.43 | 9.58 | 2.43 | 35.43 | 22.63 |
| S16 | 26.16 | 1.16 | 13.04 | 2.81 | 30.05 | 26.77 |
| S17 | 33.61 | 2.67 | 10.94 | 2.65 | 24.81 | 25.33 |
| S18 | 27.11 | 1.69 | 7.86 | 1.77 | 29.44 | 32.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kieush, L.; Schenk, J. Investigation of the Impact of Biochar Application on Foaming Slags with Varied Compositions in Electric Arc Furnace-Based Steel Production. Energies 2023, 16, 6325. https://doi.org/10.3390/en16176325
Kieush L, Schenk J. Investigation of the Impact of Biochar Application on Foaming Slags with Varied Compositions in Electric Arc Furnace-Based Steel Production. Energies. 2023; 16(17):6325. https://doi.org/10.3390/en16176325
Chicago/Turabian StyleKieush, Lina, and Johannes Schenk. 2023. "Investigation of the Impact of Biochar Application on Foaming Slags with Varied Compositions in Electric Arc Furnace-Based Steel Production" Energies 16, no. 17: 6325. https://doi.org/10.3390/en16176325
APA StyleKieush, L., & Schenk, J. (2023). Investigation of the Impact of Biochar Application on Foaming Slags with Varied Compositions in Electric Arc Furnace-Based Steel Production. Energies, 16(17), 6325. https://doi.org/10.3390/en16176325









