Current Status and Future Trends of In Situ Catalytic Upgrading of Extra Heavy Oil
Abstract
1. Introduction
1.1. Problems in Thermal Recovery for Heavy Oil
1.2. In-Situ Upgrading of Heavy Oil
1.3. Overview of Catalysts in In-Situ Upgrading
2. Catalysts for Upgrading and Viscosity Reduction
2.1. Water-Soluble Catalysts
2.2. Oil-Soluble Catalysts
2.3. Amphiphilic Catalysts
2.4. Solid Catalysts
2.5. Dispersed Catalysts
2.6. Ionic Liquid Catalysts
2.7. Bio-Based Catalysts
3. Catalytic Upgrading and Viscosity Reduction Methods
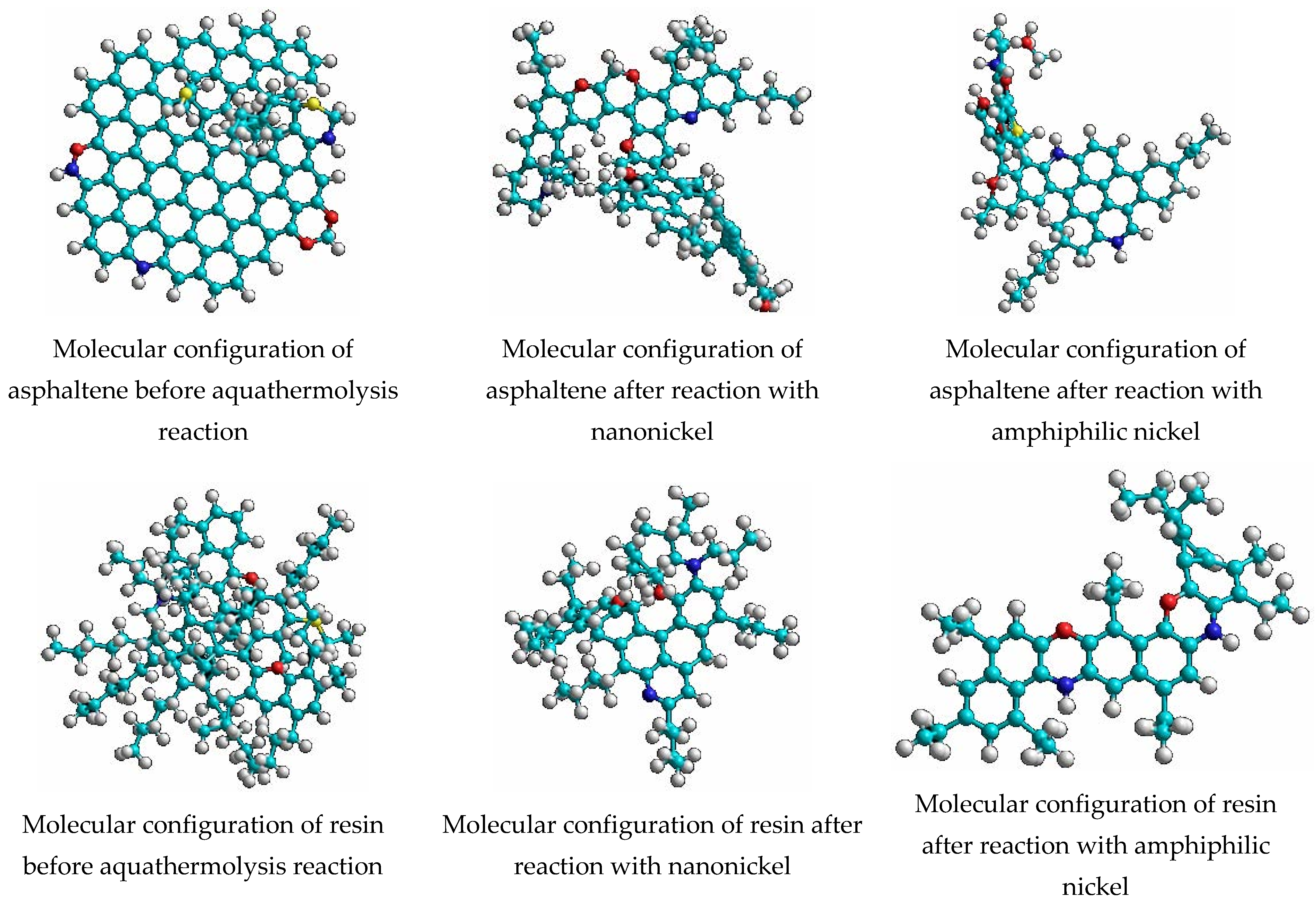
3.1. Catalytic Aquathermolysis for Heavy Oil
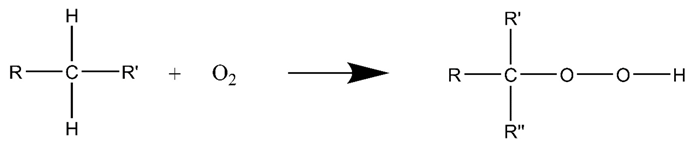
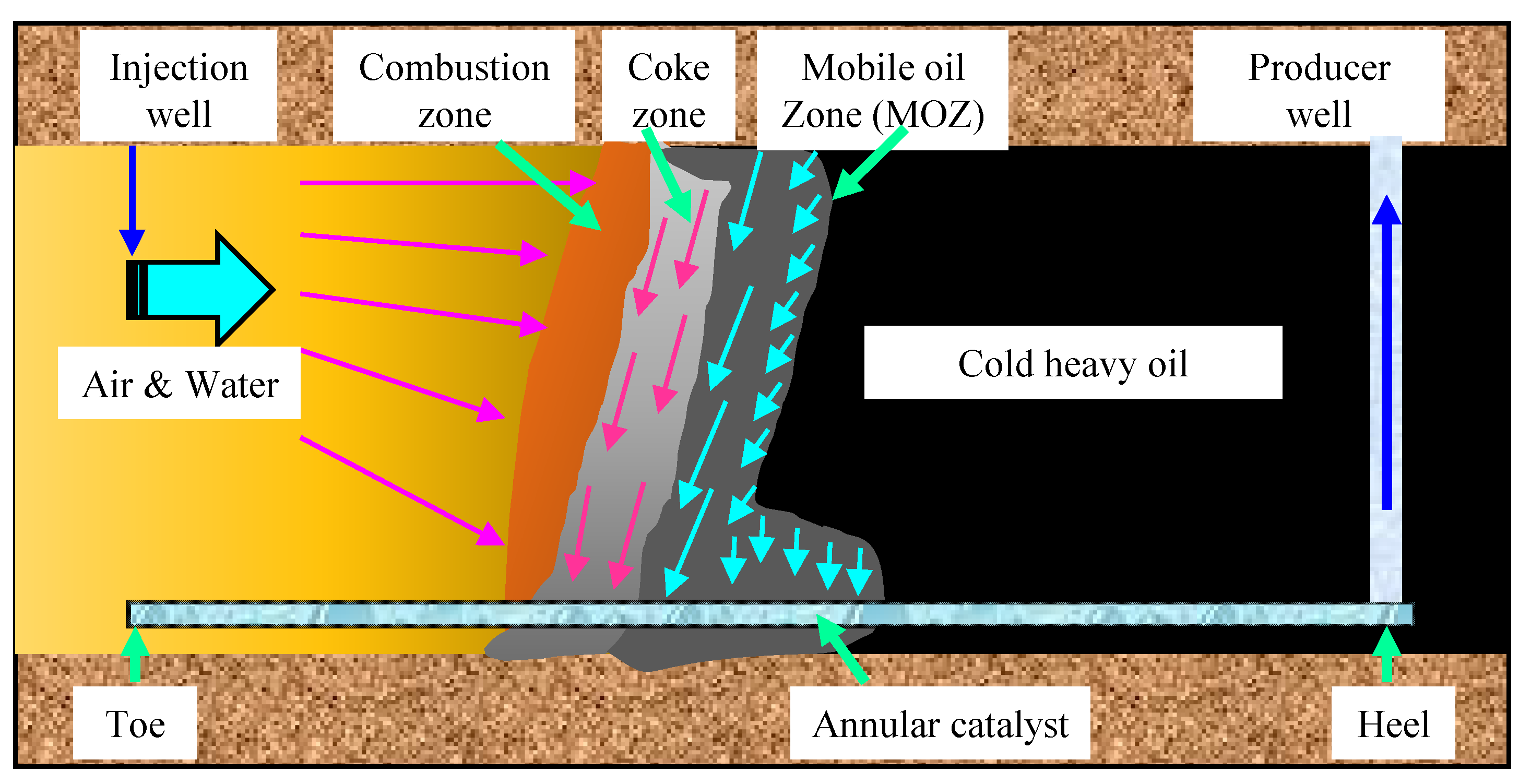
3.2. Air Injection Catalytic Oxidation for Heavy Oil

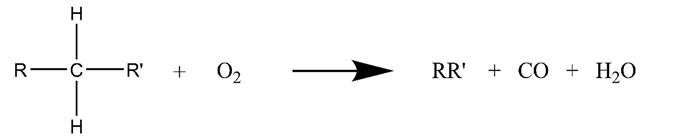
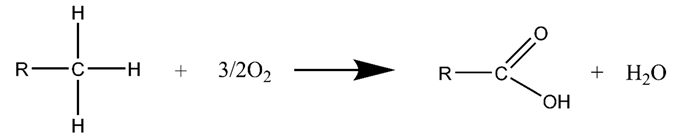
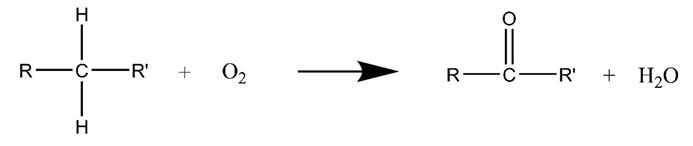
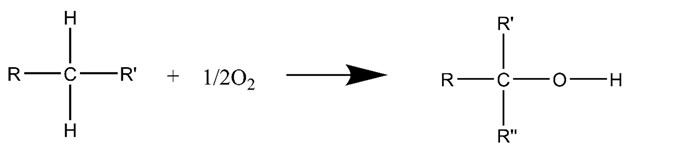
3.3. Microwave-Assisted Catalytic Upgrading
3.4. Ultrasonic-Assisted Catalytic Upgrading
4. Current Research Challenges and Future Directions
4.1. Costs
4.2. Catalytic Upgrading Scale
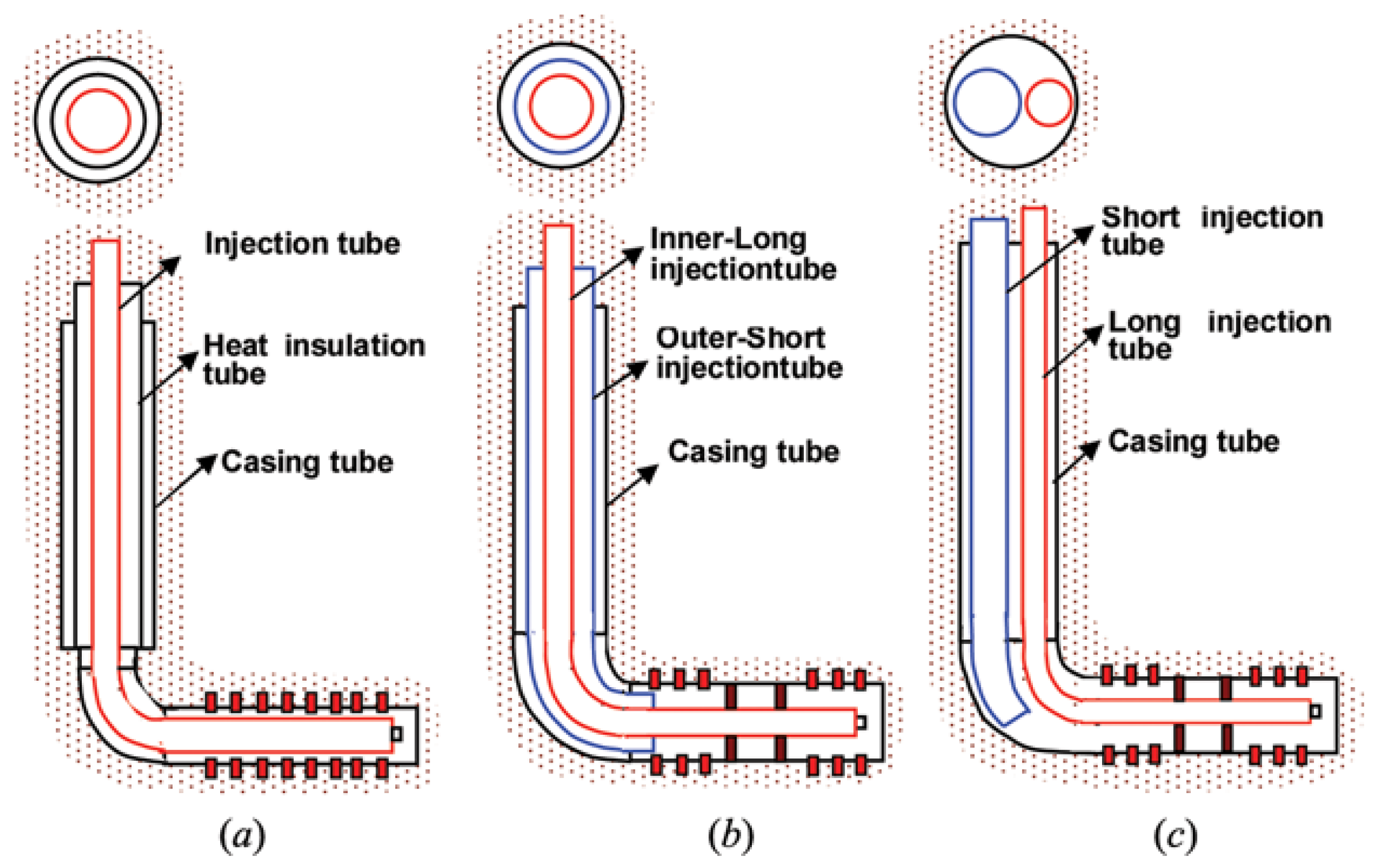
4.3. Novel Wellbore Configurations
4.3.1. Inflow Control Devices
4.3.2. Concentric/Parallel Dual Tubing Wellbore Configurations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, X.; Liu, H.; Chen, Z.; Wu, K.; Lu, N.; Zhang, Q. Enhanced oil recovery techniques for heavy oil and oilsands reservoirs after steam injection. Appl. Energy 2019, 239, 1190–1211. [Google Scholar] [CrossRef]
- Gou, Q.; Xu, S.; Hao, F.; Lu, Y.; Shu, Z.; Liu, R. Evaluation of the exploration prospect and risk of marine gas shale, southern China: A case study of Wufeng-Longmaxi shales in the Jiaoshiba area and Niutitang shales in the Cen’gong area. GSA Bull. 2022, 134, 1585–1602. [Google Scholar] [CrossRef]
- Gou, Q.; Xu, S.; Hao, F.; Shu, Z.; Zhang, Z. Making sense of micro-fractures to the Longmaxi shale reservoir quality in the Jiaoshiba area, Sichuan Basin, China: Implications for the accumulation of shale gas. J. Nat. Gas Sci. Eng. 2021, 94, 104107. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, S.; Xie, C.; Chen, K.; Zhang, Z. Production Performance of Multiple-Fractured Horizontal Well Based on Potential Theory. J. Energy Resour. Technol. 2022, 144, 103005. [Google Scholar] [CrossRef]
- Wu, Z.; Huiqing, L.; Cao, P.; Yang, R. Experimental investigation on improved vertical sweep efficiency by steam-air injection for heavy oil reservoirs. Fuel 2021, 285, 119138. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, S.; Wang, L.; Zhang, Y. Thermophysical Properties of Steam–Air under High Temperature and High Pressure. J. Energy Resour. Technol. 2020, 142, 042001. [Google Scholar] [CrossRef]
- Cui, F.; Jin, X.; Liu, H.; Wu, H.; Wang, F. Molecular modeling on Gulong shale oil and wettability of reservoir matrix. Capillarity 2022, 5, 65–74. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Sun, S. Phase equilibrium calculations in shale gas reservoirs. Capillarity 2019, 2, 8–16. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Blackbourn, G.; Ma, F.; He, Z.; Wen, Z.; Wang, Z.; Yang, Z.; Luan, T.; Wu, Z. Heavy Oils and Oil Sands: Global Distribution and Resource Assessment. Acta Geol. Sin. Engl. Ed. 2019, 93, 199–212. [Google Scholar] [CrossRef]
- Wu, Z.; Huiqing, L.; Wang, X.; Zhang, Z. Emulsification and improved oil recovery with viscosity reducer during steam injection process for heavy oil. J. Ind. Eng. Chem. 2018, 61, 348–355. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, H.; Pang, Z.; Wu, C.; Gao, M. Pore-Scale Experiment on Blocking Characteristics and EOR Mechanisms of Nitrogen Foam for Heavy Oil: A 2D Visualized Study. Energy Fuels 2016, 30, 9106–9113. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, H.; Pang, Z.; Wu, Y.; Wang, X.; Liu, D.; Gao, M. A visual investigation of enhanced heavy oil recovery by foam flooding after hot water injection. J. Pet. Sci. Eng. 2016, 147, 361–370. [Google Scholar] [CrossRef]
- Ahmadi, M.; Chen, Z. Challenges and future of chemical assisted heavy oil recovery processes. Adv. Colloid Interface Sci. 2020, 275, 102081. [Google Scholar] [CrossRef]
- Rui, Z.; Wang, X.; Zhang, Z.; Lu, J.; Chen, G.; Zhou, X.; Patil, S. A realistic and integrated model for evaluating oil sands development with Steam Assisted Gravity Drainage technology in Canada. Appl. Energy 2018, 213, 76–91. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vardhan, K.H.; Jeevanantham, S.; Karishma, S.B.; Yaashikaa, P.R.; Vellaichamy, P. A review on systematic approach for microbial enhanced oil recovery technologies: Opportunities and challenges. J. Clean. Prod. 2020, 258, 120777. [Google Scholar] [CrossRef]
- Muraza, O.; Galadima, A. Aquathermolysis of heavy oil: A review and perspective on catalyst development. Fuel 2015, 157, 219–231. [Google Scholar] [CrossRef]
- Hamedi Shokrlu, Y.; Babadagli, T. In-Situ Upgrading of Heavy Oil/Bitumen during Steam Injection by Use of Metal Nanoparticles: A Study on In-Situ Catalysis and Catalyst Transportation. SPE Reserv. Eval. Eng. 2013, 16, 333–344. [Google Scholar] [CrossRef]
- Elahi, S.M.; Scott, C.E.; Chen, Z.; Pereira-Almao, P. In-situ upgrading and enhanced recovery of heavy oil from carbonate reservoirs using nano-catalysts: Upgrading reactions analysis. Fuel 2019, 252, 262–271. [Google Scholar] [CrossRef]
- Nares, H.R.; Schacht-Hernández, P.; Ramirez-Garnica, M.A.; Cabrera-Reyes, M.C.; Noe-Valencia, L. Heavy-Crude-Oil Upgrading with Transition Metals. J. Pet. Technol. 2007, 59, 49–50. [Google Scholar] [CrossRef]
- Akhmadiyarov, A.; Rakipov, I.; Salikhov, R.; Petrov, A.; Varfolomeev, M. Oxidation of heavy crude oils under reservoir conditions: Influence of catalysts and the gas phase. J. Pet. Sci. Eng. 2022, 214, 110507. [Google Scholar] [CrossRef]
- Xiong, P.; Yang, H.; Wu, P.; Liao, Y.; Tan, D.; Ma, Z.; Yan, X. Study on catalytic aquathermolysis of heavy oil by simple synthesis of highly dispersed nickel-loaded nitrogen-doped carbon catalysts. Mol. Catal. 2022, 529, 112528. [Google Scholar] [CrossRef]
- Mehrabi-Kalajahi, S.; Varfolomeev, M.A.; Yuan, C.; Zinnatullin, A.L.; Rodionov, N.O.; Vagizov, F.G.; Osin, Y.N.; Yakimova, L.S. Improving heavy oil oxidation performance by oil-dispersed CoFe2O4 nanoparticles in In-Situ combustion process for enhanced oil recovery. Fuel 2021, 285, 119216. [Google Scholar] [CrossRef]
- Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Ziganshina, M.R.; Onishchenko, Y.V.; Sharifullin, A.V.; Vakhin, A.V. In-Situ Heavy Oil Aquathermolysis in the Presence of Nanodispersed Catalysts Based on Transition Metals. Processes 2021, 9, 127. [Google Scholar] [CrossRef]
- Mehrabi-Kalajahi, S.; Varfolomeev, M.A.; Yuan, C.; Rodionov, N.O.; Zinnatullin, A.L.; Vagizov, F.G.; Osin, Y.N. Oil-Dispersed α-Fe2O3 Nanoparticles as a Catalyst for Improving Heavy Oil Oxidation. Energy Fuels 2021, 35, 10498–10511. [Google Scholar]
- Golafshani, M.B.; Varfolomeev, M.A.; Mehrabi-Kalajahi, S.; Rodionov, N.O.; Tahay, P.; Zinnatullin, A.L.; Emelianov, D.A.; Vagizov, F.G.; Sadikov, K.G.; Osin, Y.N. Oxidation of Heavy Oil Using Oil-Dispersed Transition Metal Acetylacetonate Catalysts for Enhanced Oil Recovery. Energy Fuels 2021, 35, 20284–20299. [Google Scholar] [CrossRef]
- Sviridenko, N.N.; Akimov, A.S. Effect of pre-oxidation of dispersed catalysts on heavy oil cracking. Pet. Sci. Technol. 2023, 41, 1147–1161. [Google Scholar] [CrossRef]
- Ibrahim, M.; Al-Zahrani, F.A.; Diaz, F.J.; Al-Attas, T.; Zahir, H.; Ali, S.A.; Siddiqui, M.A.B.; Hossain, M.M. Experimental Investigation of Metal-Based Calixarenes as Dispersed Catalyst Precursors for Heavy Oil Hydrocracking. Catalysts 2022, 12, 1255. [Google Scholar] [CrossRef]
- Yeletsky, P.M.; Zaikina, O.O.; Sosnin, G.A.; Kukushkin, R.G.; Yakovlev, V.A. Heavy oil cracking in the presence of steam and nanodispersed catalysts based on different metals. Fuel Process. Technol. 2020, 199, 106239. [Google Scholar] [CrossRef]
- Djimasbe, R.; Varfolomeev, M.A.; Al-Muntaser, A.A.; Yuan, C.; Feoktistov, D.A.; Suwaid, M.A.; Kirgizov, A.J.; Davletshin, R.R.; Zinnatullin, A.L.; Fatou, S.D.; et al. Oil dispersed nickel-based catalyst for catalytic upgrading of heavy oil using supercritical water. Fuel 2022, 313, 122702. [Google Scholar] [CrossRef]
- Shokrlu, Y.H.; Babadagli, T. Viscosity reduction of heavy oil/bitumen using micro- and nano-metal particles during aqueous and non-aqueous thermal applications. J. Pet. Sci. Eng. 2014, 119, 210–220. [Google Scholar] [CrossRef]
- Farooqui, J.; Babadagli, T.; Li, H.A. Improvement of the recovery factor using nano-metal particles at the late stages of cyclic steam stimulation. In Proceedings of the SPE Canada Heavy Oil Technical Conference, Calgary, AB, Canada, 9–11 June 2015. [Google Scholar]
- Wu, C.; Su, J.; Zhang, R.; Lei, G.; Cao, Y. The Use of a Nano-nickel Catalyst for Upgrading Extra-heavy Oil by an Aquathermolysis Treatment under Steam Injection Conditions. Pet. Sci. Technol. 2013, 31, 2211–2218. [Google Scholar] [CrossRef]
- Ovalles, C.; Rivero, V.; Salazar, A. Downhole Upgrading of Orinoco Basin Extra-Heavy Crude Oil Using Hydrogen Donors under Steam Injection Conditions. Eff. Presence Iron Nanocatalysts. Catalysts 2015, 5, 286–297. [Google Scholar] [CrossRef]
- Galarraga, C.E.; Pereira-Almao, P. Hydrocracking of Athabasca Bitumen Using Submicronic Multimetallic Catalysts at Near In-Reservoir Conditions. Energy Fuels 2010, 24, 2383–2389. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Almao, P.P. Enhanced Heavy Oil Recovery by in Situ Prepared Ultradispersed Multimetallic Nanoparticles: A Study of Hot Fluid Flooding for Athabasca Bitumen Recovery. Energy Fuels 2013, 27, 2194–2201. [Google Scholar] [CrossRef]
- Yakasai, F.; Jaafar, M.Z.; Bandyopadhyay, S.; Agi, A.; Sidek, M.A. Application of iron oxide nanoparticles in oil recovery—A critical review of the properties, formulation, recent advances, and prospects. J. Pet. Sci. Eng. 2022, 208, 109438. [Google Scholar] [CrossRef]
- Yuan, C.; Pu, W.-F.; Emelianov, D.A.; Mehrabi-Kalajahi, S.; Varfolomeev, M.A. Catalytic Oxidation of Alkanes by Organometallics in an In Situ Combustion Process. Energy Fuels 2022, 36, 10167–10176. [Google Scholar] [CrossRef]
- Yuan, C.; Pu, W.-F.; Ifticene, M.A.; Zhao, S.; Varfolomeev, M.A. Crude Oil Oxidation in an Air Injection Based Enhanced Oil Recovery Process: Chemical Reaction Mechanism and Catalysis. Energy Fuels 2022, 36, 5209–5227. [Google Scholar] [CrossRef]
- Yuan, C.; Emelianov, D.A.; Varfolomeev, M.A.; Rodionov, N.O.; Suwaid, M.A.; Vakhitov, I.R. Mechanistic and kinetic insight into catalytic oxidation process of heavy oil in in-situ combustion process using copper (II) stearate as oil soluble catalyst. Fuel 2021, 284, 118981. [Google Scholar] [CrossRef]
- Yuan, C.; Varfolomeev, M.A.; Emelianov, D.A.; Suwaid, M.A.; Khachatrian, A.A.; Starshinova, V.L.; Vakhitov, I.R.; Al-Muntaser, A.A. Copper stearate as a catalyst for improving the oxidation performance of heavy oil in in-situ combustion process. Appl. Catal. A Gen. 2018, 564, 79–89. [Google Scholar] [CrossRef]
- Yuan, C.; Mehrabi-Kalajahi, S.S.; Sadikov, K.; Varfolomeev, M.A.; Emelianov, D.A.; Rodionov, N.O.; Amerkhanov, M.I. Potential of Copper-Based Oil Soluble Catalyst for Improving Efficiency of In-Situ Combustion Process: Catalytic Combustion, Catalytic In-Situ Oil Upgrading, and Increased Oil Recovery. In Proceedings of the the SPE Kuwait Oil & Gas Conference and Show, Mishref, Kuwait, 13–16 October 2019. [Google Scholar]
- Varfolomeev, M.A.; Yuan, C.; Bolotov, A.V.; Minkhanov, I.F.; Mehrabi-Kalajahi, S.; Saifullin, E.R.; Marvanov, M.M.; Baygildin, E.R.; Sabiryanov, R.M.; Rojas, A.; et al. Effect of copper stearate as catalysts on the performance of in-situ combustion process for heavy oil recovery and upgrading. J. Pet. Sci. Eng. 2021, 207, 109125. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Eskin, A.A.; Vakhin, A.V. Kinetic Study on Heavy Oil Oxidation by Copper Tallates. Energy Fuels 2019, 33, 12690–12695. [Google Scholar] [CrossRef]
- Zhao, R.; Heng, M.; Chen, C.; Li, T.; Shi, Y.; Wang, J. Catalytic effects of Al2O3 nano-particles on thermal cracking of heavy oil during in-situ combustion process. J. Pet. Sci. Eng. 2021, 205, 108978. [Google Scholar] [CrossRef]
- Bera, A.; Babadagli, T. Effect of native and injected nano-particles on the efficiency of heavy oil recovery by radio frequency electromagnetic heating. J. Pet. Sci. Eng. 2017, 153, 244–256. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Z.; Liu, X.; Liu, L.; Peng, J. Analysis of the viscosity reduction of crude oil with nano-Ni catalyst by acoustic cavitation. Fuel 2020, 275, 117976. [Google Scholar] [CrossRef]
- Gopinath, R.; Dalai, A.K.; Adjaye, J. Effects of Ultrasound Treatment on the Upgradation of Heavy Gas Oil. Energy Fuels 2006, 20, 271–277. [Google Scholar] [CrossRef]
- Ershov, M.A.; Baranov, D.A.; Mullakaev, M.S.; Abramov, V.O. Reducing viscosity of paraffinic oils in ultrasonic field. Chem. Pet. Eng. 2011, 47, 457–461. [Google Scholar] [CrossRef]
- Hamidi, H.; Mohammadian, E.; Junin, R.; Rafati, R.; Manan, M.; Azdarpour, A.; Junid, M. A technique for evaluating the oil/heavy-oil viscosity changes under ultrasound in a simulated porous medium. Ultrasonics 2014, 54, 655–662. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. In-situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Clark, P.D.; Hyne, J.B. Steam-oil chemical reactions: Mechanisms for the aquathermolysis of heavy oil. AOSTRA J. Res. 1984, 1, 15–20. [Google Scholar]
- Clark, P.D.; Kirk, M.J. Studies on the Upgrading of Bituminous Oils with Water and Transition Metal Catalysts. Energy Fuels 1994, 8, 380–387. [Google Scholar] [CrossRef]
- Clark, P.D.; Hyne, J.B.; Tyrer, J. Chemistry of organosulphur compound types occurring in heavy oil sands1: High temperature hydrolysis and thermolysis of tetrahydrothiophene in relation to steam stimulation processes. Fuel 1983, 62, 959–962. [Google Scholar] [CrossRef]
- Clark, P.D.; Hyne, J.B.; Tyrer, J.D. Chemistry of organosulfur compound type occurring in heavy oil sands2: Influence of pH on the high temperature hydrolysis of tetraothiophene and thiophene. Fuel 1984, 63, 125–128. [Google Scholar] [CrossRef]
- Clark, P.D.; Hyne, J.B.; Tyrer, J.D. Chemistry of organosulfur compound type occurring in heavy oil sands3: Reaction of thiophene and tetrahydro-thiophene with vanadyl and nickel salts. Fuel 1984, 63, 1645–1649. [Google Scholar]
- Clark, P.D.; Hyne, J.B.; Tyrer, J.D. Chemistry of organosulfur compound type occurring in heavy oil sands4: The high-temperature reaction of hiophene and tetrahydro—Thiophene with aqueous solution of aluminium and first row transition—Metal cations. Fuels 1987, 66, 1353–1357. [Google Scholar] [CrossRef]
- Clark, P.D.; Hyne, J.B.; Tyrer, J.D. Chemistry of organosulfur compound type occurring in heavy oil sands5: Reaction of thiophene and tetrahydro-thiophene with aqueous group: Metal species at high temperature. Fuels 1987, 66, 1699–1702. [Google Scholar] [CrossRef]
- Chen, E.; Liu, Y.; Wen, S. A study on the degradation of the resin in Liaohe heavy oil during catalytic aquathermolysis reaction. Chem. Eng. Oil Gas 2006, 35, 49–50+87. [Google Scholar]
- Wang, Y.; Chen, Y.; He, J.; Li, P.; Yang, C. Mechanism of Catalytic Aquathermolysis: Influences on Heavy Oil by Two Types of Efficient Catalytic Ions: Fe3+ and Mo6+. Energy Fuels 2010, 24, 1502–1510. [Google Scholar] [CrossRef]
- Fan, Z.; Zhao, F.; Wang, J.; Gong, Y. Upgrading and viscosity reduction of super heavy oil by aquathermolysis with hydrogen donor. J. Fuel Chem. Technol. 2006, 34, 315–318. [Google Scholar]
- Wang, J.; Fan, Z.; Ren, S. An Experimental study on catalytic aquathermolysis of Shanjiasi heavy oil. Oilfield Chem. 2006, 23, 205–208. [Google Scholar]
- Feng, X.; Wang, Q.; Lyu, W. Application of sulfonated organometallic catalyst in viscosity reducing of heavy oil. Spec. Petrochem. 2018, 35, 16–20. [Google Scholar]
- Tang, X.-D.; Zhu, H.; Li, J.-J.; Wang, F.; Qing, D.-Y. Catalytic Aquathermolysis of Heavy Oil with Oil-soluble Multicomponent Acrylic Copolymers Combined with Cu2+. Pet. Sci. Technol. 2015, 33, 1721–1727. [Google Scholar] [CrossRef]
- Wu, C.; Lei, G.-L.; Yao, C.-J.; Sun, K.-J.; Gai, P.-Y.; Cao, Y.-B. Mechanism for reducing the viscosity of extra-heavy oil by aquathermolysis with an amphiphilic catalyst. J. Fuel Chem. Technol. 2010, 38, 684–690. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, H.; Zhao, C. Effects and mechanism of combined nano-catalysts on viscosity reduction of heavy oil. China Powder Sci. Technol. 2020, 26, 68–74. [Google Scholar]
- Ma, Y.; Zhang, J.; Wu, W.; Cai, Z.; Cao, Y.; Huang, K.; Jiang, L. Trialkylmethylammonium molybdate ionic liquids as novel oil-soluble precursors of dispersed metal catalysts for slurry-phase hydrocracking of heavy oils. Chem. Eng. Sci. 2022, 253, 117516. [Google Scholar] [CrossRef]
- Tajik, A.; Farhadian, A.; Khelkhal, M.A.; Rezaeisadat, M.; Petrov, S.M.; Eskin, A.A.; Vakhin, A.V.; Golafshani, M.B.; Lapuk, S.E.; Buzurov, A.E.; et al. Sunflower oil as renewable biomass source to develop highly effective oil-soluble catalysts for in-situ combustion of heavy oil. Chem. Eng. J. 2023, 453, 139813. [Google Scholar] [CrossRef]
- Suwaid, M.A.; Varfolomeev, M.A.; Al-Muntaser, A.A.; Yuan, C.; Starshinova, V.L.; Zinnatullin, A.; Vagizov, F.G.; Rakhmatullin, I.Z.; Emelianov, D.A.; Chemodanov, A.E. In-situ catalytic upgrading of heavy oil using oil-soluble transition metal-based catalysts. Fuel 2020, 281, 118753. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Tang, X.; Deng, L.; Wei, Y. Upgrading heavy and extra-heavy crude oil by iron oil-soluble catalyst for transportation. Pet. Sci. Technol. 2017, 35, 1160–1165. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Nguyen, N.T.; Cho, J.; Park, C.; Park, S.; Jung, J.; Lee, C.W. A review on the oil-soluble dispersed catalyst for slurry-phase hydrocracking of heavy oil. J. Ind. Eng. Chem. 2016, 43, 1–12. [Google Scholar] [CrossRef]
- Wu, C.; Su, J.; Zhang, R.; Zhang, Z. Study on the molecular dynamics mechanism of extra-heavy oil by catalytic aquathermolysis. Energy Sources Part A Recover. Util. Environ. Eff. 2016, 38, 763–768. [Google Scholar] [CrossRef]
- Wu, C.; Su, J.; Zhang, R.; Lei, G.; Cao, Y. The Use of Amphiphilic Nickel Chelate for Catalytic Aquathermolysis of Extra-heavy Oil under Steam Injection Conditions. Energy Sources Part A Recover. Util. Environ. Eff. 2014, 36, 1437–1444. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Wu, C.; Xia, F. Laboratory Experiments and Field Tests of an Amphiphilic Metallic Chelate for Catalytic Aquathermolysis of Heavy Oil. Energy Fuels 2008, 22, 1502–1508. [Google Scholar] [CrossRef]
- Yusuf, A.; Al-Hajri, R.S.; Al-Waheibi, Y.M.; Jibril, B.Y. Upgrading of Omani heavy oil with bimetallic amphiphilic catalysts. J. Taiwan Inst. Chem. Eng. 2016, 67, 45–53. [Google Scholar] [CrossRef]
- Aliev, F.; Akhunov, A.A.; Mirzaev, O.; Vakhin, A. Development of New Amphiphilic Catalytic Steam Additives for Hydrothermal Enhanced Oil Recovery Techniques. Catalysts 2022, 12, 921. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Zhang, L.; Li, Z. Aquathermolysis of Heavy Crude Oil with Amphiphilic Nickel and Iron Catalysts. Energy Fuels 2014, 28, 7440–7447. [Google Scholar] [CrossRef]
- Cao, Y.-B.; Zhang, L.-L.; Xia, D.-H. Catalytic aquathermolysis of Shengli heavy crude oil with an amphiphilic cobalt catalyst. Pet. Sci. 2016, 13, 463–475. [Google Scholar] [CrossRef]
- Li, C.; Su, L.; Li, Q.; Wang, X.; Li, X.; Yang, J.; Zhang, Z. Enhanced Heavy Oil Recovery in Mild Conditions by SO42−/TiO2-ZrO2 Solid Superacid Prepared by Different Methods. J. Nanomater. 2016, 2016, 7436057. [Google Scholar] [CrossRef]
- Mironenko, O.O.; Sosnin, G.A.; Eletskii, P.M.; Gulyaeva, Y.K.; Bulavchenko, O.A.; Stonkus, O.A.; Rodina, V.O.; Yakovlev, V.A. A Study of the Catalytic Steam Cracking of Heavy Crude Oil in the Presence of a Dispersed Molybdenum-Containing Catalyst. Nanogeterogennyi Katal. 2017, 2, 74–87. [Google Scholar] [CrossRef]
- Mironenko, O.O.; Sosnin, G.A.; Eletskii, P.M.; Gulyaeva, Y.K.; Bulavchenko, O.A.; Stonkus, O.A.; Rodina, V.O.; Yakovlev, V.A. Catalytic steam cracking of heavy crude oil with molybdenum and nickel nanodispersed catalysts. Catal. Ind. 2017, 9, 221–229. [Google Scholar] [CrossRef]
- Ortiz-Moreno, H.; Ramírez, J.; Cuevas, R.; Marroquín, G.; Ancheyta, J. Heavy oil upgrading at moderate pressure using dispersed catalysts: Effects of temperature, pressure and catalytic precursor. Fuel 2012, 100, 186–192. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, M.; Zhao, S.; Xu, C. Upgrading Heavy Oil Using Syngas as the Hydrogen Source with Dispersed Catalysts. Pet. Sci. Technol. 2009, 27, 712–732. [Google Scholar] [CrossRef]
- Al-Marshed, A.; Hart, A.; Leeke, G.; Greaves, M.; Wood, J. Effectiveness of Different Transition Metal Dispersed Catalysts for In Situ Heavy Oil Upgrading. Ind. Eng. Chem. Res. 2015, 54, 10645–10655. [Google Scholar] [CrossRef]
- Al-Marshed, A.; Hart, A.; Leeke, G.; Greaves, M.; Wood, J. Optimization of Heavy Oil Upgrading Using Dispersed Nanoparticulate Iron Oxide as a Catalyst. Energy Fuels 2015, 29, 6306–6316. [Google Scholar] [CrossRef]
- Brown, A.R.; Hart, A.; Coker, V.S.; Lloyd, J.R.; Wood, J. Upgrading of heavy oil by dispersed biogenic magnetite catalysts. Fuel 2016, 185, 442–448. [Google Scholar] [CrossRef]
- Omajali, J.B.; Hart, A.; Walker, M.; Wood, J.; Macaskie, L.E. In-situ catalytic upgrading of heavy oil using dispersed bionanoparticles supported on gram-positive and gram-negative bacteria. Appl. Catal. B Environ. 2017, 203, 807–819. [Google Scholar] [CrossRef]
- Al-Attas, T.A.; Zahir, M.H.; Ali, S.A.; Al-Bogami, S.A.; Malaibari, Z.; Razzak, S.A.; Hossain, M.M. Kinetics of the synergy effects in heavy oil upgrading using novel Ni-p-tertbutylcalix[4]arene as a dispersed catalyst with a supported catalyst. Fuel Process. Technol. 2019, 185, 158–168. [Google Scholar] [CrossRef]
- Al-Attas, T.A.; Ali, S.A.; Zahir, M.H.; Xiong, Q.; Al-Bogami, S.A.; Malaibari, Z.O.; Razzak, S.A.; Hossain, M.M. Recent Advances in Heavy Oil Upgrading Using Dispersed Catalysts. Energy Fuels 2019, 33, 7917–7949. [Google Scholar] [CrossRef]
- Pereira, J.F.; Costa, R.; Foios, N.; Coutinho, J.A. Ionic liquid enhanced oil recovery in sand-pack columns. Fuel 2014, 134, 196–200. [Google Scholar] [CrossRef]
- Perez-Sanchez, J.F.; Diaz-Zavala, N.P.; Gonzalez-Santana, S.; Izquierdo-Kulich, E.F.; Suarez-Dominguez, E.J. Water-In-Oil Emulsions through Porous Media and the Effect of Surfactants: Theoretical Approaches. Processes 2019, 7, 620. [Google Scholar] [CrossRef]
- Fan, H.-F.; Li, Z.-B.; Liang, T. Experimental study on using ionic liquids to upgrade heavy oil. J. Fuel Chem. Technol. 2007, 35, 32–35. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, T.; He, Y. Upgrading and viscosity reducing of heavy oils by [BMIM][AlCl4] ionic liquid. J. Fuel Chem. Technol. 2009, 37, 690–693. [Google Scholar] [CrossRef]
- Tang, X.-D.; Zhou, T.-D.; Li, J.-J.; Deng, C.-L.; Qin, G.-F. Experimental study on a biomass-based catalyst for catalytic upgrading and viscosity reduction of heavy oil. J. Anal. Appl. Pyrolysis 2019, 143, 104684. [Google Scholar] [CrossRef]
- Wu, G.; Huang, H.; Chen, X.; Cai, Z.; Jiang, Y.; Chen, X. Facile synthesis of clean Pt nanoparticles supported on reduced graphene oxide composites: Their growth mechanism and tuning of their methanol electro-catalytic oxidation property. Electrochim. Acta 2013, 111, 779–783. [Google Scholar] [CrossRef]
- Omri, A.; Benzina, M.; Bennour, F. Industrial application of photocatalysts prepared by hydrothermal and sol–gel methods. J. Ind. Eng. Chem. 2015, 21, 356–362. [Google Scholar] [CrossRef]
- Yadav, T.P.; Yadav, R.M.; Singh, D. Mechanical Milling: A Top down Approach for the Synthesis of Nanomaterials and Nanocomposites. Nanosci. Nanotechnol. 2012, 2, 22–48. [Google Scholar] [CrossRef]
- Quester, K.; Avalos-Borja, M.; Castro-Longoria, E. Biosynthesis and microscopic study of metallic nanoparticles. Micron 2013, 54–55, 1–27. [Google Scholar] [CrossRef]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Luo, K.; Jung, S.; Park, K.-H.; Kim, Y.-R. Microbial Biosynthesis of Silver Nanoparticles in Different Culture Media. J. Agric. Food Chem. 2018, 66, 957–962. [Google Scholar] [CrossRef]
- Crookes-Goodson, W.J.; Slocik, J.M.; Naik, R.R. Bio-directed synthesis and assembly of nanomaterials. Chem. Soc. Rev. 2008, 37, 2403–2412. [Google Scholar] [CrossRef]
- Mirhendi, M.; Emtiazi, G.; Roghanian, R. Production of nano zinc, zinc sulphide and nanocomplex of magnetite zinc oxide by Brevundimonas diminuta and Pseudomonas stutzeri. IET Nanobiotechnol. 2013, 7, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Kitching, M.; Ramani, M.; Marsili, E. Fungal biosynthesis of gold nanoparticles: Mechanism and scale up. Microb. Biotechnol. 2015, 8, 904–917. [Google Scholar] [CrossRef]
- Shantkriti, S.; Rani, P. Biological synthesis of copper nanoparticles using Pseudomonas fluorescens. Int. J. Med. Microbiol. 2014, 3, 374–383. [Google Scholar]
- Ha, C.; Zhu, N.; Shang, R.; Shi, C.; Cui, J.; Sohoo, I.; Wu, P.; Cao, Y. Biorecovery of palladium as nanoparticles by Enterococcus faecalis and its catalysis for chromate reduction. Chem. Eng. J. 2016, 288, 246–254. [Google Scholar] [CrossRef]
- De Corte, S.; Hennebel, T.; De Gusseme, B.; Verstraete, W.; Boon, N. Bio-palladium: From metal recovery to catalytic applications. Microb. Biotechnol. 2012, 5, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.D.; Hyne, J.B. Studies on the chemical reactions of heavy oils under steam stimulation condition. AOSTRA J. Res. 1990, 29, 29–39. [Google Scholar]
- Kapadia, P.R.; Kallos, M.S.; Gates, I.D. A review of pyrolysis, aquathermolysis, and oxidation of Athabasca bitumen. Fuel Process. Technol. 2015, 131, 270–289. [Google Scholar] [CrossRef]
- Yi, S.; Babadagli, T.; Li, H.A. Use of Nickel Nanoparticles for Promoting Aquathermolysis Reaction during Cyclic Steam Stimulation. SPE J. 2018, 23, 145–156. [Google Scholar] [CrossRef]
- Hou, J.; Li, C.; Gao, H.; Chen, M.; Huang, W.; Chen, Y.; Zhou, C. Recyclable oleic acid modified magnetic NiFe2O4 nanoparticles for catalytic aquathermolysis of Liaohe heavy oil. Fuel 2017, 200, 193–198. [Google Scholar] [CrossRef]
- Li, C.; Huang, W.; Zhou, C.; Chen, Y. Advances on the transition-metal based catalysts for aquathermolysis upgrading of heavy crude oil. Fuel 2019, 257, 115779. [Google Scholar] [CrossRef]
- Li, W.; Zhu, J.-H.; Qi, J.-H. Application of nano-nickel catalyst in the viscosity reduction of Liaohe extra-heavy oil by aqua-thermolysis. J. Fuel Chem. Technol. 2007, 35, 176–180. [Google Scholar] [CrossRef]
- Sitnov, S.A.; Khelkhal, M.A.; Mukhamatdinov, I.I.; Feoktistov, D.A.; Vakhin, A.V. Iron oxide nanoparticles impact on improving reservoir rock minerals catalytic effect on heavy oil aquathermolysis. Fuel 2022, 327, 124956. [Google Scholar] [CrossRef]
- Al-Ghefeili, H.M.A.; Devi, M.G.; Al-Abri, M.Z. Magnetite nanoparticle mediated catalytic aquathermolysis of Omani heavy crude oil. J. Indian Chem. Soc. 2022, 99, 100314. [Google Scholar] [CrossRef]
- Fan, H.; Liu, Y.; Zhao, X. First field experimental of recover heavy oil using down-hole catalytic method in China. Oil Drill. Prod. Technol. 2001, 23, 42–44. [Google Scholar]
- Fan, H.; Liu, Y.; Yang, F. A Study on Heavy Oil Recovery by in-Situ Catalytic Aquathermal Cracking. Oilfield Chem. 2002, 18, 13–16. [Google Scholar]
- Biyouki, A.A.; Hosseinpour, N.; Bahramian, A.; Vatani, A. In-situ upgrading of reservoir oils by in-situ preparation of NiO nanoparticles in thermal enhanced oil recovery processes. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 520, 289–300. [Google Scholar] [CrossRef]
- Ayasse, C.; Bloomer, C.; Lyngberg, E.; Boddy, W.; Donnelly, J.; Greaves, M. First field pilot of the THAI™ Process. In Proceedings of the Petroleum Society’s 6th Canadian International Petroleum Conference (56th Annual Technical Meeting), Calgary, AB, Canada, 7–9 June 2005. [Google Scholar]
- He, B.; Chen, Q.; Castanier, L.M.; Kovscek, A.R. Improved in-situ combustion performance with metallic salt additives. In Proceedings of the 2005 SPE Western Regional Meeting, Irvine, CA, USA, 30 March–1 April 2005. [Google Scholar]
- Javadli, R.; de Klerk, A. Desulfurization of heavy oil-oxidative desulfurization (ODS) as potential upgrading pathway for oil sands derived bitumen. Energy Fuels 2012, 26, 594–602. [Google Scholar] [CrossRef]
- Pu, W.-F.; Liu, P.-G.; Li, Y.-B.; Jin, F.-Y.; Liu, Z.-Z. Thermal Characteristics and Combustion Kinetics Analysis of Heavy Crude Oil Catalyzed by Metallic Additives. Ind. Eng. Chem. Res. 2015, 54, 11525–11533. [Google Scholar] [CrossRef]
- Jia, H.; Liu, P.-G.; Pu, W.-F.; Ma, X.-P.; Zhang, J.; Gan, L. In situ catalytic upgrading of heavy crude oil through low-temperature oxidation. Pet. Sci. 2016, 13, 476–488. [Google Scholar] [CrossRef]
- Galukhin, A.; Khelkhal, M.A.; Gerasimov, A.; Biktagirov, T.; Gafurov, M.; Rodionov, A.; Orlinskii, S. Mn-Catalyzed Oxidation of Heavy Oil in Porous Media: Kinetics and Some Aspects of the Mechanism. Energy Fuels 2016, 30, 7731–7737. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Pu, J.; Pu, C.; Li, Q.; Gu, X. Accelerated oxidation during air flooding in a low temperature reservoir. Pet. Sci. Technol. 2016, 35, 86–91. [Google Scholar] [CrossRef]
- Castanier, L.M.; Baena, C.J.; Holt, R.J.; Brigham, W.E.; Tavares, C. In situ combustion with metallic additives. In Proceedings of the SPE Latin America Petroleum Engineering Conference, Caracas, Venezuela, 8–12 March 1992. [Google Scholar]
- Wang, T.; Yang, W.; Wang, J.; Kalitaani, S.; Deng, Z. Low temperature oxidation of crude oil: Reaction progress and catalytic mechanism of metallic salts. Fuel 2018, 225, 336–342. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Fan, Z.; Liu, Y. Enhanced heavy oil recovery and performance by application of catalytic in-situ combustion. Pet. Sci. Technol. 2019, 37, 493–499. [Google Scholar] [CrossRef]
- Mazloom, M.S.; Hemmati-Sarapardeh, A.; Husein, M.M.; Behbahani, H.S.; Zendehboudi, S. Application of nanoparticles for asphaltenes adsorption and oxidation: A critical review of challenges and recent progress. Fuel 2020, 279, 117763. [Google Scholar] [CrossRef]
- Tang, X.; Chen, T.; Guo, E.; Guan, W.; Jiang, Y.; Li, J. Experiment on Enhanced Oil Recovery by In-situ Catalytic Reforming of Super heavy Oil. Spec. Oil Gas Reserv. 2022, 29, 114–120. [Google Scholar]
- Tang, X.; Wei, Y.; Li, J.; Qing, D.; Deng, L. Advances in oxidation reaction of air injection and its kinetics. Chem. Ind. Eng. Process 2016, 35, 83–90. [Google Scholar]
- Jiang, S.; Tang, X.; Zhang, Y.; Li, J.; Cui, Y. Adaptability of Air—Injection Low—Temperature Catalytic Oxidation Technology in Heavy Oil Production. Spec. Oil Gas Reserv. 2014, 21, 130–133+157–158; [Google Scholar]
- Tang, X.; Su, X.; Cui, Y.; Yang, K.; Zheng, C. Effect of low temperature air oxidation on the composition of heavy oil. J. Southwest Pet. Univ. (Sci. Technol. Ed.) 2011, 33, 149–152. [Google Scholar]
- Tang, X.; Cui, Y.; He, B.; Liu, Q.; Meng, K. Experimental Study on the Viscosity Reduction of Liaohe Heavy Oil by Catalytic Air Oxidation. Oilfield Chem. 2008, 25, 316–319. [Google Scholar]
- Li, J.; Wei, Y.; Tang, X.; Liu, X.; Deng, L. Catalytic effect of zinc naphthenate on the heavy oil low-temperature oxidation in an air injection process. Pet. Sci. Technol. 2016, 34, 813–818. [Google Scholar] [CrossRef]
- Xia, T.; Greaves, M. 3-D physical model studies of downhole catalytic upgrading of Wolf Lake heavy oil using THAI. In Proceedings of the Petroleum Society’s Canadian International Petroleum Conference 2001, Calgary, AB, Canada, 12–14 June 2001. [Google Scholar]
- Xia, T.X.; Greaves, M. Upgrading Athabasca tar sand using toe-to-heel air injection. In Proceedings of the 2000 SPE/Petroleum Society of CIM International Conference on Horizontal Well Technology, Calgary, AB, Canada, 6–8 November 2000. [Google Scholar]
- Xia, T.X.; Greaves, M.; Werfilli, W.S.; Rathbone, R.R. Downhole conversion of Lloydminster heavy oil using THAI-CAPRI process. In Proceedings of the SPE International Thermal Operations and Heavy Oil Symposium and International Horizontal Well Technology Conference, Calgary, AB, Canada, 4–7 November 2002. [Google Scholar]
- Xia, T.X.; Greaves, M. Downhole Upgrading of Athabasca Tar Sand Bitumen Using THAI—SARA Analysis. In Proceedings of the 2001 SPE International Thermal Operations and Heavy Oil Symposium, Porlamar, Margarita Island, Venezuela, 12 March 2001. [Google Scholar]
- Greaves, M.; Xia, T.X. CAPRI-Downhole catalytic process for upgrading heavy oil: Produced oil properties and composition. In Proceedings of the Petroleum Society’s Canadian International Petroleum Conference 2001, Calgary, AB, Canada, 12–14 June 2001. [Google Scholar]
- Greaves, M.; Xia, T.X.; Turta, A.T.; Ayasse, C. Recent Laboratory Results of THAI and Its Comparison with Other IOR Processes. In Proceedings of the 2000 SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 3–5 April 2000. [Google Scholar]
- Hart, A.; Greaves, M.; Wood, J. A comparative study of fixed-bed and dispersed catalytic upgrading of heavy crude oil using-CAPRI. Chem. Eng. J. 2015, 282, 213–223. [Google Scholar] [CrossRef]
- Hart, A.; Wood, J.; Greaves, M. In situ catalytic upgrading of heavy oil using a pelletized Ni-Mo/Al2O3 catalyst in the THAI process. J. Pet. Sci. Eng. 2017, 156, 958–965. [Google Scholar] [CrossRef]
- Hart, A.; Leeke, G.; Greaves, M.; Wood, J. Down-hole heavy crude oil upgrading by CAPRI: Effect of hydrogen and methane gases upon upgrading and coke formation. Fuel 2014, 119, 226–235. [Google Scholar] [CrossRef]
- Hart, A.; Leeke, G.; Greaves, M. Downhole heavy crude oil upgrading using CAPRI: Effect of steam upon ipgrading and coke formation. Energy Fuels 2014, 28, 1811–1819. [Google Scholar] [CrossRef]
- Hart, A.; Shah, A.; Leeke, G.; Greaves, M.; Wood, J. Optimization of the CAPRI Process for Heavy Oil Upgrading: Effect of Hydrogen and Guard Bed. Ind. Eng. Chem. Res. 2013, 52, 15394–15406. [Google Scholar] [CrossRef]
- Hart, A.; Wood, J. In Situ Catalytic Upgrading of Heavy Crude with CAPRI: Influence of Hydrogen on Catalyst Pore Plugging and Deactivation due to Coke. Energies 2018, 11, 636. [Google Scholar] [CrossRef]
- Hart, A.; Adam, M.; Robinson, J.P.; Rigby, S.P.; Wood, J. Inductive Heating Assisted-Catalytic Dehydrogenation of Tetralin as a Hydrogen Source for Downhole Catalytic Upgrading of Heavy Oil. Top. Catal. 2020, 63, 268–280. [Google Scholar] [CrossRef]
- Hart, A.; Adam, M.; Robinson, J.P.; Rigby, S.P.; Wood, J. Tetralin and Decalin H-Donor Effect on Catalytic Upgrading of Heavy Oil Inductively Heated with Steel Balls. Catalysts 2020, 10, 393. [Google Scholar] [CrossRef]
- Vossoughi, S.; Willhite, G.; El Shoubary, Y.; Bartlett, G. Study of the clay effect on crude oil combustion by thermogravimetry and differential scanning calorimetry. J. Therm. Anal. Calorim. 1983, 27, 17–36. [Google Scholar] [CrossRef]
- Kozlowski, M.L.; Punase, A.; Nasr-El-Din, H.A.; Hascakir, B. The catalytic effect of clay on in-situ combustion performance. In Proceedings of the SPE Latin American, and Caribbean Petroleum Engineering Conference, Quito, Ecuador, 18–20 November 2015. [Google Scholar]
- Yu, X.; Qu, Z.; Kan, C.; Zhao, X. Effect of Different Clay Minerals on Heavy Oil Oxidation during Ignition Process. Energy Fuels 2017, 31, 12839–12847. [Google Scholar] [CrossRef]
- Jiang, S. Experimental study and application of air assisted steam flooding in Qi40 block. Spec. Oil Gas Reserv. 2012, 19, 117Ġ, 157. [Google Scholar]
- Yu, H.; Yang, B.; Xu, G.; Wang, J.; Ren, S.; Lin, W.; Xiao, L.; Gao, H. Air Foam Injection for IOR: From Laboratory to Field Implementation in ZhongYuan Oilfield China. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20–23 April 2008. [Google Scholar]
- Dong, X.; Liu, H.; Sun, P.; Zheng, J.; Sun, R. Air-Foam-Injection Process: An Improved-Oil-Recovery Technique for Waterflooded Light-Oil Reservoirs. SPE Reserv. Eval. Eng. 2012, 15, 436–444. [Google Scholar] [CrossRef]
- Liu, X. Study on Low Temperature Oxidation Process of Heavy Oil with Air Injection and Its Influence on Enhanced Oil Recovery; Southwest Petroleum University: Chengdu, China, 2016. [Google Scholar]
- Abdulrahman, M.M.; Meribout, M. Antenna array design for enhanced oil recovery under oil reservoir constraints with experimental validation. Energy 2014, 66, 868–880. [Google Scholar] [CrossRef]
- Rehman, M.M.; Meribout, M. Conventional versus electrical enhanced oil recovery: A review. J. Pet. Explor. Prod. Technol. 2012, 2, 157–167. [Google Scholar] [CrossRef]
- Mozafari, M.; Nasri, Z. Operational conditions effects on Iranian heavy oil upgrading using microwave irradiation. J. Pet. Sci. Eng. 2017, 151, 40–48. [Google Scholar] [CrossRef]
- Taheri-Shakib, J.; Shekarifard, A.; Naderi, H. Heavy crude oil upgrading using nanoparticles by applying electromagnetic technique. Fuel 2018, 232, 704–711. [Google Scholar] [CrossRef]
- Taheri-Shakib, J.; Shekarifard, A.; Naderi, H. Experimental investigation of the asphaltene deposition in porous media: Accounting for the microwave and ultrasonic effects. J. Pet. Sci. Eng. 2018, 163, 453–462. [Google Scholar] [CrossRef]
- Taheri-Shakib, J.; Shekarifard, A.; Naderi, H. The experimental investigation of effect of microwave and ultrasonic waves on the key characteristics of heavy crude oil. J. Anal. Appl. Pyrolysis 2017, 128, 92–101. [Google Scholar] [CrossRef]
- Li, H.; Gao, H.; Zhao, X.; Xia, Z.; Yu, B.; Sun, D. Experimental study on viscosity reduction of heavy oil with water content by synergistic effect of microwave and nano-catalyst. J. Pet. Sci. Eng. 2022, 208, 109271. [Google Scholar] [CrossRef]
- Li, H.; Cui, K.; Jin, L.; Wang, L.; Yu, B. Experimental study on the viscosity reduction of heavy oil with nanocatalyst by microwave heating under low reaction temperature. J. Pet. Sci. Eng. 2018, 170, 374–382. [Google Scholar]
- Jiang, H.; Huang, L.; Wei, A. Experimental study on the dehydration of heavy oil by microwave. J. Xi’an Shiyou Univ. (Nat. Sci. Ed.) 2005, 20, 61–63+10. [Google Scholar]
- Wan, J.K.S.; Wolf, K.; Heyding, R.D. Some chemical aspects of the microwave assisted catalytic hydrocracking processes. Stud. Surf. Sci. Catal. 1984, 19, 561–568. [Google Scholar]
- Iskandar, F.; Fitriani, P.; Merissa, S.; Mukti, R.R.; Khairurrijal; Abdullah, M. Fe3O4/Zeolite nanocomposites synthesized by microwave assisted coprecipitation and its performance in reducing viscosity of heavy oil. AIP Conf. Proc. 2014, 1586, 132–135. [Google Scholar]
- Greff, J.; Babadagli, T. Use of nano-metal particles as catalyst under electromagnetic heating for in-situ heavy oil recovery. J. Pet. Sci. Eng. 2013, 112, 258–265. [Google Scholar] [CrossRef]
- Li, K.; Hou, B.; Wang, L.; Cui, Y. Application of Carbon Nanocatalysts in Upgrading Heavy Crude Oil Assisted with Microwave Heating. Nano Lett. 2014, 14, 3002–3008. [Google Scholar] [CrossRef]
- Adeyemi, I.; Meribout, M.; Khezzar, L. Recent developments, challenges, and prospects of ultrasound-assisted oil technologies. Ultrason. Sonochem. 2022, 82, 105902. [Google Scholar] [CrossRef]
- Agi, A.; Junin, R.; Jaafar, M.Z.; Amin, N.A.S.; Sidek, M.A.; Nyakuma, B.B.; Yakasai, F.; Gbadamosi, A.; Oseh, J.; Azli, N.B. Ultrasound-assisted nanofluid flooding to enhance heavy oil recovery in a simulated porous media. Arab. J. Chem. 2022, 15, 103784. [Google Scholar] [CrossRef]
- Agi, A.; Junin, R.; Jaafar, M.Z.; Sidek, M.A.; Yakasai, F.; Gbadamosi, A.; Oseh, J. Laboratory evaluation to field application of ultrasound: A state-of-the art review on the effect of ultrasonication on enhanced oil recovery mechanisms. Journal of Industrial and Engineering Chemistry. J. Ind. Eng. Chem. 2022, 110, 100–119. [Google Scholar] [CrossRef]
- Ghahremani, H.; Nasri, Z.; Eikani, M.H. Ultrasound-assisted oxidative desulfurization (UAOD) of Iranian heavy crude oil: Investigation of process variables. J. Pet. Sci. Eng. 2021, 204, 108709. [Google Scholar] [CrossRef]
- Gao, J.; Li, C.; Xu, D.; Wu, P.; Lin, W.; Wang, X. The mechanism of ultrasonic irradiation effect on viscosity variations of heavy crude oil. Ultrason. Sonochem. 2021, 81, 105842. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, H.; Haddad, A.S.; Otumudia, E.W.; Rafati, R.; Mohammadian, E.; Azdarpour, A.; Pilcher, W.G.; Fuehrmann, P.W.; Sosa, L.R.; Cota, N.; et al. Recent applications of ultrasonic waves in improved oil recovery: A review of techniques and results. Ultrasonics 2021, 110, 106288. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-X.; Pu, C.-S. Experimental study of heavy oil underground aquathermolysis using catalyst and ultrasonic. J. Fuel Chem. Technol. 2011, 39, 606–610. [Google Scholar] [CrossRef]
- Xu, L.; Tao, F.; Yu, T. Anovel practical method for generation of dichlorocarbene by use of ultrasonic irradiation and phase transfer catalyst. Acta Chmica Sin. 1988, 46, 340–344. [Google Scholar]
- Bianchi, C.; Gotti, E.; Toscano, L.; Ragaini, V. Preparation of Pd/C catalysts via ultrasound: A study of the metal distribution. Ultrason. Sonochem. 1997, 4, 317–320. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, C.; Liu, X. Ultrasound enhanced catalytic oxidative deep desulfurization of diesel with H2O2-trichloroacetic acid. J. Fuel Chem. Technol. 2009, 37, 324–328. [Google Scholar]
- Liu, X.F.; Zhang, L.; Shi, Y.H. Preparation of NiW/Al2O3 hydrodesulfurization catalyst by ultrasound-microwave treatment. Chin. J. Catal. 2004, 25, 748–752. [Google Scholar]
- Mei, H.; Mei, B.; Yen, T.F. A new method for obtaining ultra-low sulfur diesel fuel via ultrasound assisted oxidative desulfurization. Fuel 2003, 82, 405–414. [Google Scholar] [CrossRef]
- Ilyushin, Y.V. Development of a Process Control System for the Production of High-Paraffin Oil. Energies 2022, 15, 6462. [Google Scholar] [CrossRef]
- Kukharova, T.V.; Ilyushin, Y.V.; Asadulagi, M.-A.M. Investigation of the OA-300M Electrolysis Cell Temperature Field of Metallurgical Production. Energies 2022, 15, 9001. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Hou, J.; Wang, Q.; Dong, K. A Mathematical Model and Inflow Control Effect Analysis of Inflow Control Devices Completed Horizontal Well. J. Energy Resour. Technol. 2017, 139, 034501. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Liu, Y.; Jiao, Y.; Wu, J.; Kang, A. Mechanism and sensitivity analysis of an inflow control devices (ICDs) for reducing water production in heterogeneous oil reservoir with bottom water. J. Pet. Sci. Eng. 2016, 146, 971–982. [Google Scholar] [CrossRef]
- Banerjee, S.; Hascakir, B. Flow control devices in SAGD completion design: Enhanced heavy oil/bitumen recovery through improved thermal efficiency. J. Pet. Sci. Eng. 2018, 169, 197–308. [Google Scholar] [CrossRef]
- Olsen, J.J.; Hemmingsen, C.S.; Bergmann, L.; Nielsen, K.K.; Glimberg, S.L.; Walther, J.H. Characterization and Erosion Modeling of a Nozzle-Based Inflow-Control Device. SPE Drill. Complet. 2017, 27, 44–51. [Google Scholar] [CrossRef]
- Bashtani, F.; Irani, M.; Kantzas, A. Scale Up of Pore-Network Models into Reservoir Scale: Optimization of Inflow Control Devices Placement. SPE J. 2022, 27, 3100–3118. [Google Scholar] [CrossRef]
- Sabet, N.; Irani, M.; Hassanzadeh, H. Inflow Control Devices Placement: A Computational Fluid Dynamics Approach. SPE J. 2022, 27, 1562–1576. [Google Scholar] [CrossRef]
- Konopczynski, M.; Moradi, M.; Krishnan, T.; Sandhu, H.; Lai, C.-L. Case Study: Oil Production Optimized with Autonomous Inflow Control Devices Offshore Malaysia. J. Pet. Technol. 2022, 74, 44–51. [Google Scholar] [CrossRef]
- Zaripov, A.T.; Shaikhutdinov, D.K.; Bisenova, A.A. Assessment of feasibility of inflow control devices to produce extra-heavy oil reserves of Tatneft PJSC. Oil Ind. 2019, 7, 44–46. [Google Scholar]
- Zaikin, I.; Kempf, K.V.; Voll, B.A.; Budlov, S.S.; Laidlaw, D. Well Productivity and Oil Recovery Enhancement in East and West Siberian Fields as a Result of Inflow Control Technology and Application. In Proceedings of the IADC/SPE Asia Pacific Drilling Technology Conference and Exhibition, Jakarta, Indonesia, 25–27 August 2008. [Google Scholar]
- Adam, W. Inflow-Control Device, Inflow-Control Valves Aid Kuwait’s First Smart Multilateral Well. J. Pet. Technol. 2013, 65, 113–117. [Google Scholar]
- Zhang, S.; Sun, X.; Gou, Y.; Zhang, Z.; Wang, H.; Zhou, X.; Xie, Y.; Lv, B. Application status and thinking of flow control devices in SAGD. Acta Pet. Sin. 2021, 42, 1395–1404. [Google Scholar]
- Sun, F.; Yao, Y.; Li, G.; Li, X.; Li, Q.; Yang, J.; Wu, J. A coupled model for CO2 & superheated steam flow in full-length concentric dual-tube horizontal wells to predict the thermophysical properties of CO2 & superheated steam mixture considering condensation. J. Pet. Sci. Eng. 2018, 170, 151–165. [Google Scholar] [CrossRef]
- Sun, F.; Yao, Y.; Li, X.; Tian, J.; Zhu, G.; Chen, Z. The flow and heat transfer characteristics of superheated steam in concentric dual-tubing wells. Int. J. Heat Mass Transf. 2017, 115, 1099–1108. [Google Scholar] [CrossRef]
- Sun, F.; Yao, Y.; Li, G.; Li, X.; Chen, M.; Chen, G.; Zhang, T. Analysis of superheated steam performance in offshore concentric dual-tubing wells. J. Pet. Sci. Eng. 2018, 166, 984–999. [Google Scholar] [CrossRef]
- Gu, H.; Cheng, L.; Huang, S.; Du, B.; Hu, C. Prediction of thermophysical properties of saturated steam and wellbore heat losses in concentric dual-tubing steam injection wells. Energy 2014, 75, 419–429. [Google Scholar] [CrossRef]
- Dong, X.; Liu, H.; Pang, Z.; Wang, C.; Lu, C. Flow and Heat Transfer Characteristics of Multi-Thermal Fluid in a Dual-String Horizontal Well. Numer. Heat Transf. Part A Appl. Int. J. Comput. Methodol. 2014, 66, 185–204. [Google Scholar] [CrossRef]



| Well Group | Before Measurement | After Measurement | Increment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Liquid Production/(t/d) | Oil Production/(t/d) | Water Cut/% | Liquid Production/(t/d) | Oil Production/(t/d) | Water Cut/% | Production Time/d | Liquid /(t) | Oil/(t) | |
| S1-42-039 | 0 | 0 | / | 4 | 1.4 | 65 | 317 | 1268 | 444 |
| S1-42-041 | 3 | 0.4 | 86.7 | 4.7 | 1.3 | 72.3 | 284 | 483 | 256 |
| S1-38-38C | 1.6 | 0.5 | 68.8 | 6.3 | 2.8 | 55.6 | 315 | 1481 | 725 |
| S1-45-23 | 3.5 | 1.6 | 54.3 | 19.9 | 6.9 | 65.3 | 165 | 2706 | 875 |
| D80-26-68 | 8.4 | 2.5 | 70.2 | 24.5 | 8.5 | 65.3 | 174 | 2801 | 1044 |
| D48-DH1 | 3.1 | 0.6 | 80.6 | 13.4 | 3.2 | 76.1 | 187 | 1926 | 486 |
| D212-DH13 | 0 | 0 | / | 19 | 8.1 | 57.4 | 89 | 1691 | 721 |
| D212-DH16 | 1.3 | 0.6 | 53.8 | 8.4 | 3.9 | 53.6 | 104 | 738 | 343 |
| Researchers | Catalyst | Remarks |
|---|---|---|
| Xiong et al. [21] | Dispersed nickel-loaded nitrogen-doped carbon catalysts | Through a series of reactions, the catalyst can decrease 82.21% of viscosity and 13.04% of heavy component. |
| Mehrabi-Kalajahi et al. [22] | CoFe2O4 nanoparticles | The CoFe2O4 nanoparticles were used in ISC process, which slightly promoted LTO and significantly facilitated HTO. |
| Mehrabi-Kalajahi et al. [24] | Oil-dispersed α-Fe2O3 nanoparticles | The α-Fe2O3 nanoparticles were used in ISC process, which decreased the activation energy significantly from 537 kJ/mol to 246 kJ/mol. |
| Babapour Golafshani et al. [25] | Oil-dispersed transition metal acetylacetonate (Ni, Cu, and Fe) catalysts | The catalysts slightly promoted LTO and significantly facilitated HTO. |
| Sviridenko and Akimov [26] | Dispersed NiCrWC catalyst | The pre-oxidation of the dispersed catalyst showed an optimal performance at a temperature of 450 °C and a duration of 2 h in the air, at which condition the macromolecular components, sulfur, by-products were decreased. |
| Yeletsky et al. [28] | Nanodispersed catalysts based on K, Fe, Ni, Mo | The use of Mo-based catalysts achieved highest upgrading efficiency, which got lowest sulphur content and H:C ratio. |
| Djimasbe et al. [29] | Oil dispersed nickel-based catalyst | The Ni-based catalyst decreases the production of gases and coke from 14.92% and 3.09% to 8% and 2.27%, while increases oil production from 81.99% to 89.73%. |
| Yuan et al. [39] | Oil-soluble catalysts based on copper (II) stearate | The catalysts largely reduced the activation energy of LTO, FD, and HTO. |
| Khelkhal et al. [43] | Copper tallates | The copper tallates promoted the combustion front, thus facilitated the LTO and HTO processes. |
| Cui et al. [46] | Nano-Ni catalyst and ultrasonic wave | The asphaltene molecules were destroyed and decomposed into small molecular hydrocarbons. |
| Tajik et al. [67] | Oil soluble catalysts | Sunflower oil is an ideal resource to synthesize and prepare Fe, Ni, Co and Cu-based oil soluble catalysts used for ISC. The synthesized catalysts showed excellent activity on decreasing the activation energy of high-temperature oxidation. |
| Aliev et al. [75] | Amphiphilic catalytic | A novel amphiphilic catalyst was synthesized based on nickel and aluminum. The use of the amphiphilic catalyst can improve the oil mobility. |
| Al-Attas et al. [88] | Ni-TBC[4] as dispersed catalyst | It decreases the activation energy from 65.39 to 57.32 kcal/mol. |
| Tang et al. [94] | Biomass-based catalyst | The oil viscosity was reduced significantly from 508,800 to 22,230 mPa·s at 50 °C with a 0.2 wt% addition of the catalyst. |
| Sitnov et al. [112] | Fe3O4 nanoparticles and hydrogen donor | The resins and asphaltenes decrease from 19.6 wt% and 5.1 wt% to 8.9 wt% and 1.5 wt%. |
| Al-Ghefeili et al. [113] | Magnetite nanoparticles | A more than 50% reduction in viscosity and API gravity increase to 21° was observed. |
| Tang et al. [131] | Oil solubility organozinc | Physical experiments were performed to select high-efficiency in-situ reforming catalysts. Oil viscosity was reduced from 145,000 mPa·s to 54,260 mPa·s. The density and acid value of the heavy oil reformed by physical model experiment were decreased, the content of heavy components (colloid and asphaltene) was decreased by 10.85%, and the fractions before 300 °C and 500 °C were increased by 6.75% and 17.29%, respectively. |
| Hart et al. [147] | Tetralin and decalin as H-donor | The induction heat in CAPRI increases the activity of catalyst, thus improving the produced oil quality. |
| Taheri-Shakib [160] | Electromagnetic and nanoparticles (Fe, TO, CA) | Nanoparticles together with microwave decreased the content of heavy components, such as OH, S-H, alkyl groups, carbonyl, carboxylic acid, etc. |
| Li et al. [161] | Microwave and nano-catalyst | Under the synergistic effect of nano-catalyst and microwave, significant increase in tricyclic aromatic Hydrocarbons and hopanidanes, and decrease in bicyclic aromatic hydrocarbons was observed. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Chen, H.; Cai, X.; Gou, Q.; Jiang, L.; Chen, K.; Chen, Z.; Jiang, S. Current Status and Future Trends of In Situ Catalytic Upgrading of Extra Heavy Oil. Energies 2023, 16, 4610. https://doi.org/10.3390/en16124610
Wu Z, Chen H, Cai X, Gou Q, Jiang L, Chen K, Chen Z, Jiang S. Current Status and Future Trends of In Situ Catalytic Upgrading of Extra Heavy Oil. Energies. 2023; 16(12):4610. https://doi.org/10.3390/en16124610
Chicago/Turabian StyleWu, Zhengbin, Hanzhao Chen, Xidong Cai, Qiyang Gou, Liangliang Jiang, Kai Chen, Zhangxin Chen, and Shu Jiang. 2023. "Current Status and Future Trends of In Situ Catalytic Upgrading of Extra Heavy Oil" Energies 16, no. 12: 4610. https://doi.org/10.3390/en16124610
APA StyleWu, Z., Chen, H., Cai, X., Gou, Q., Jiang, L., Chen, K., Chen, Z., & Jiang, S. (2023). Current Status and Future Trends of In Situ Catalytic Upgrading of Extra Heavy Oil. Energies, 16(12), 4610. https://doi.org/10.3390/en16124610







