Investigation of the Effect of Injected CO2 on the Morrow B Sandstone through Laboratory Batch Reaction Experiments: Implications for CO2 Sequestration in the Farnsworth Unit, Northern Texas, USA
Abstract
1. Introduction
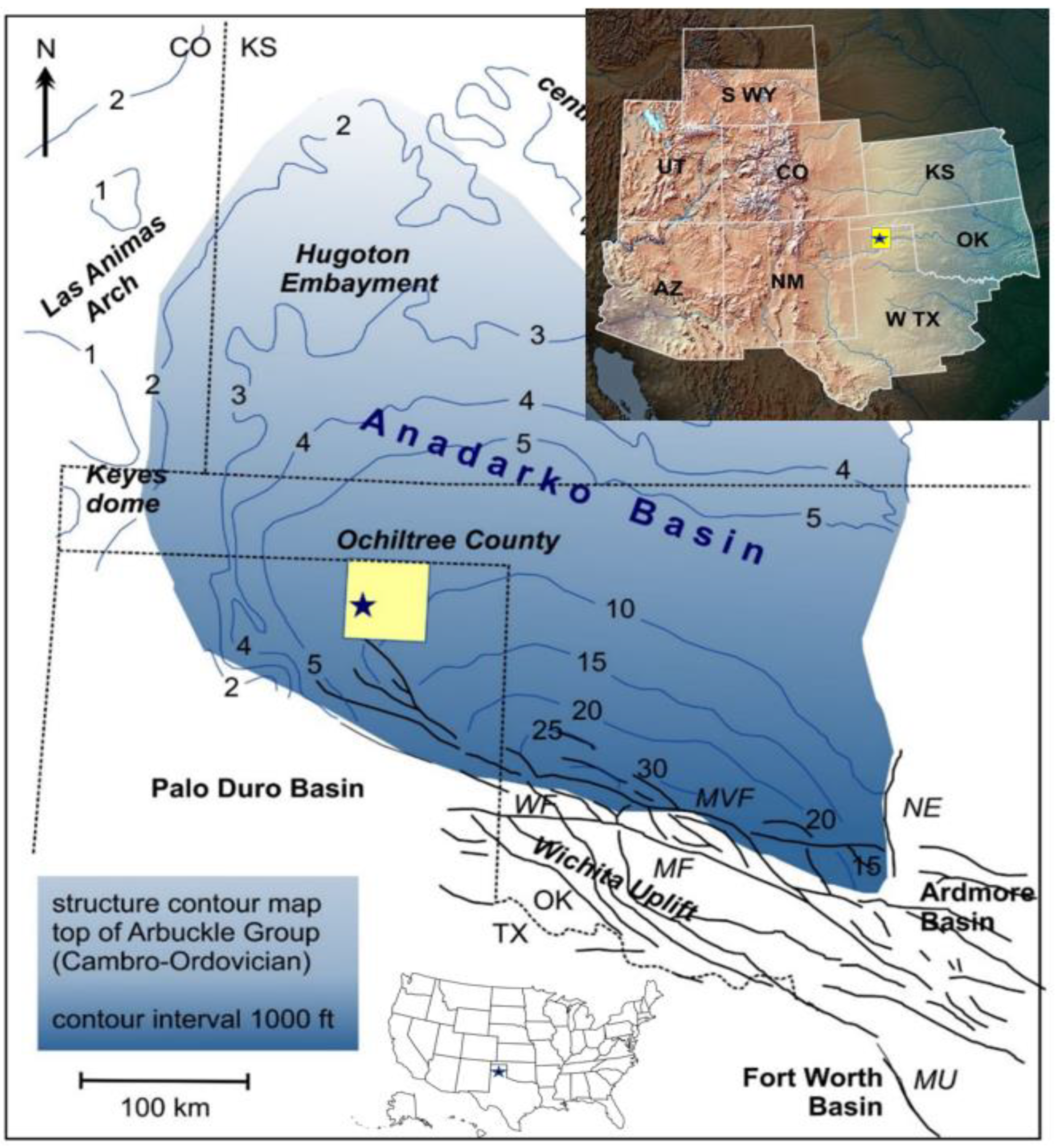
2. Geological Background
3. Experimental Methods
3.1. Sample Preparation and Initial Mineral Characterization
3.2. Water Sample Analysis
3.3. Batch Reaction Experiments
4. Results
4.1. Aqueous Chemistry
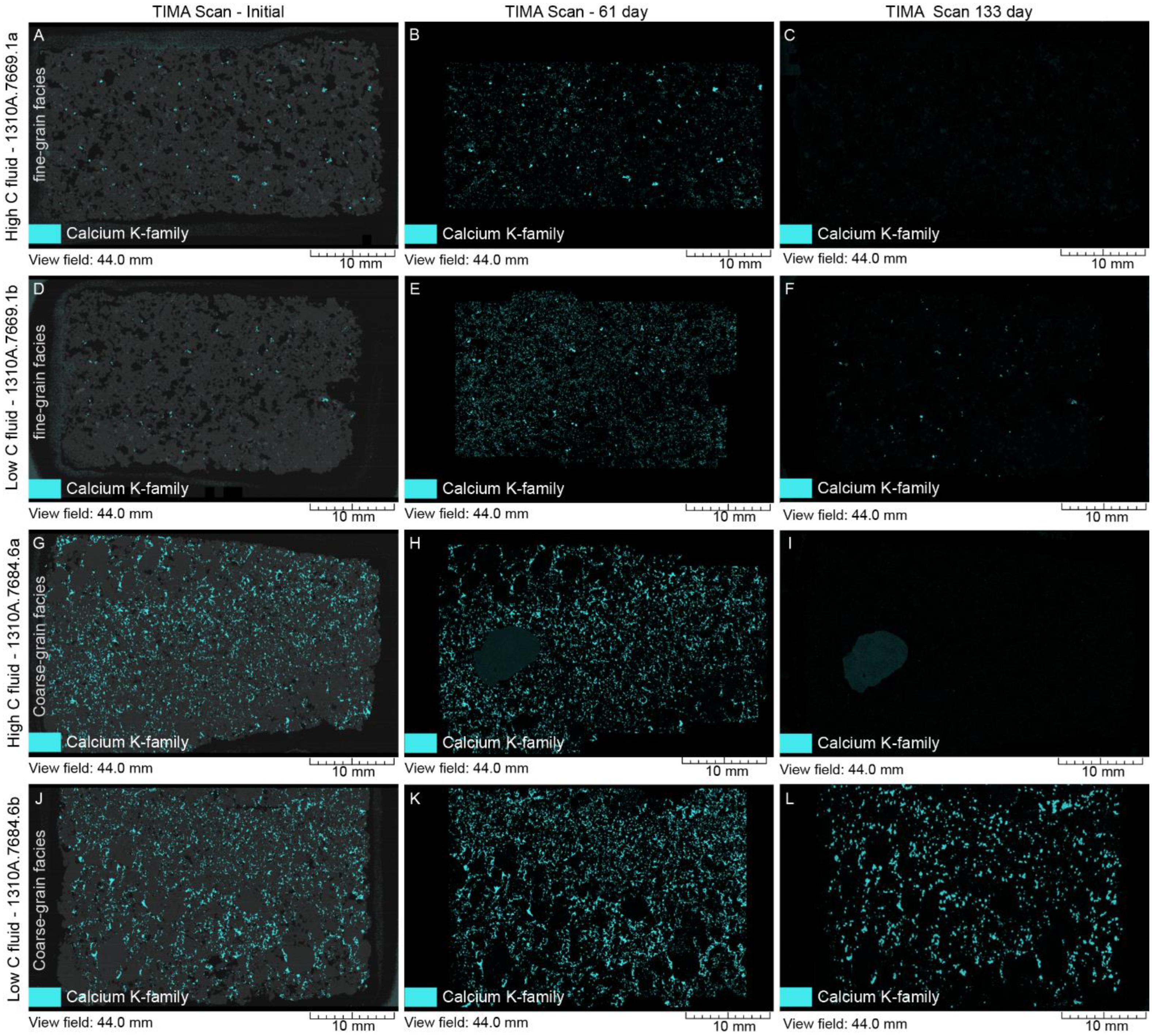
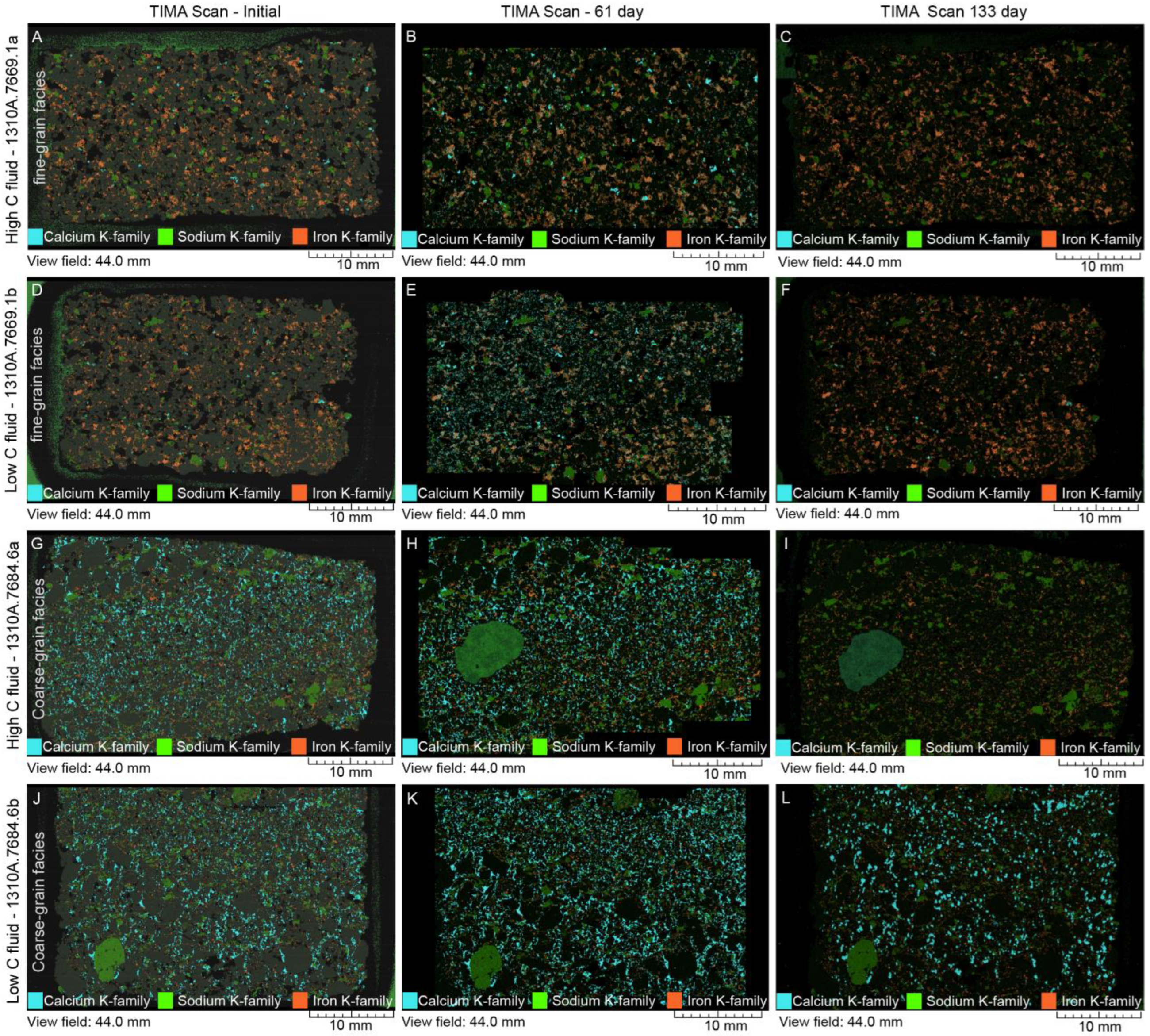
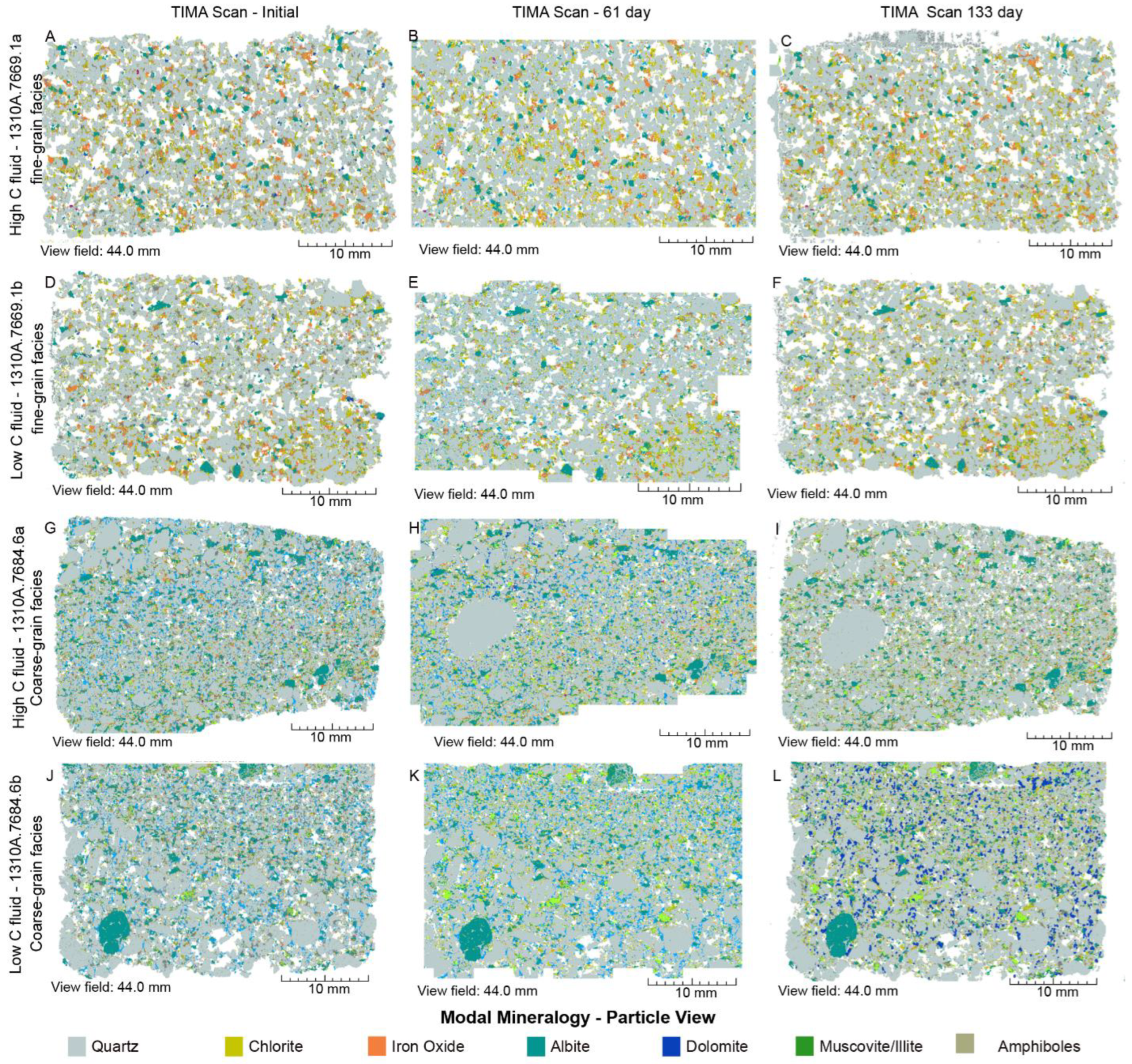
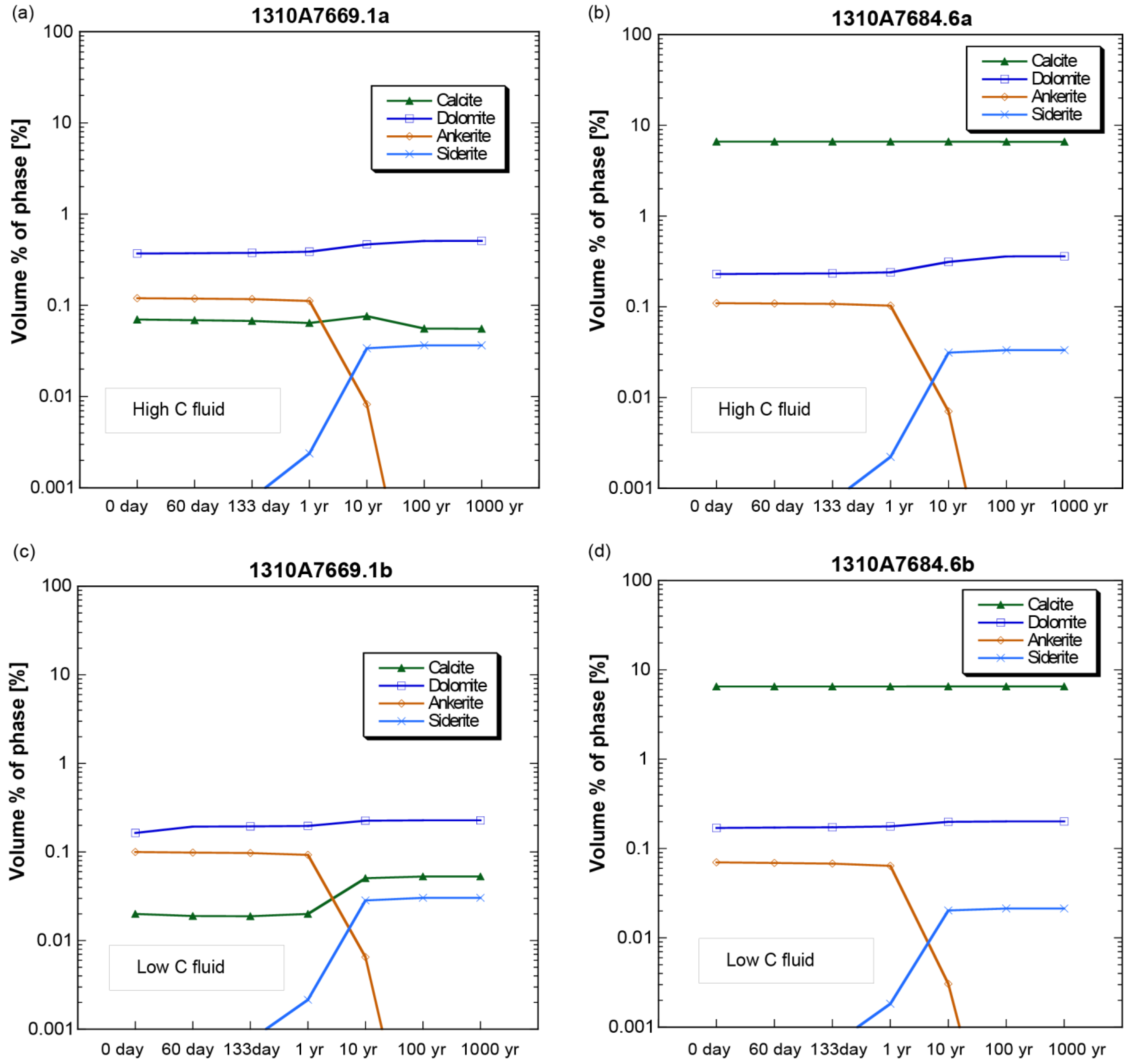
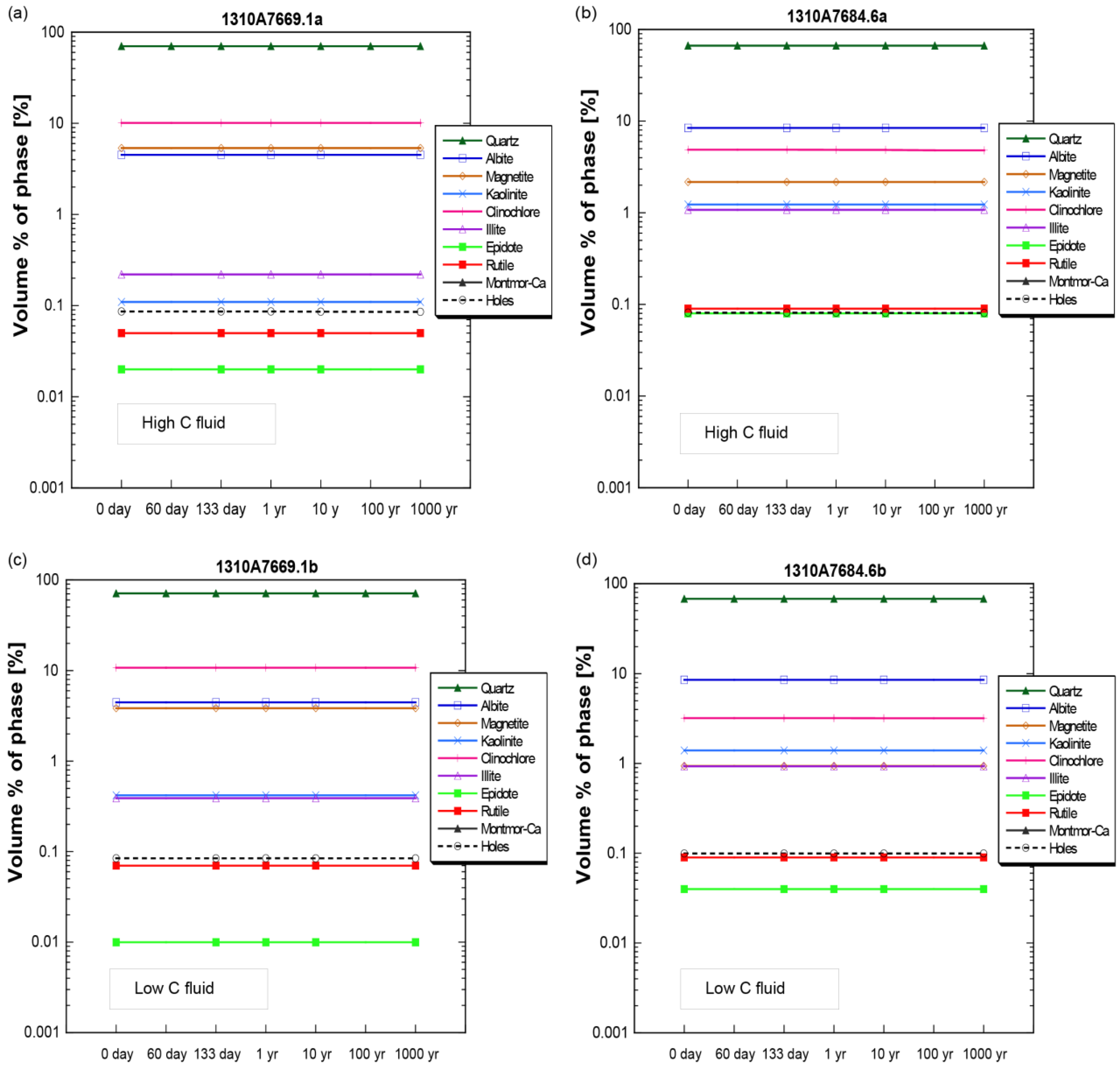
4.2. Mineral Chemistry
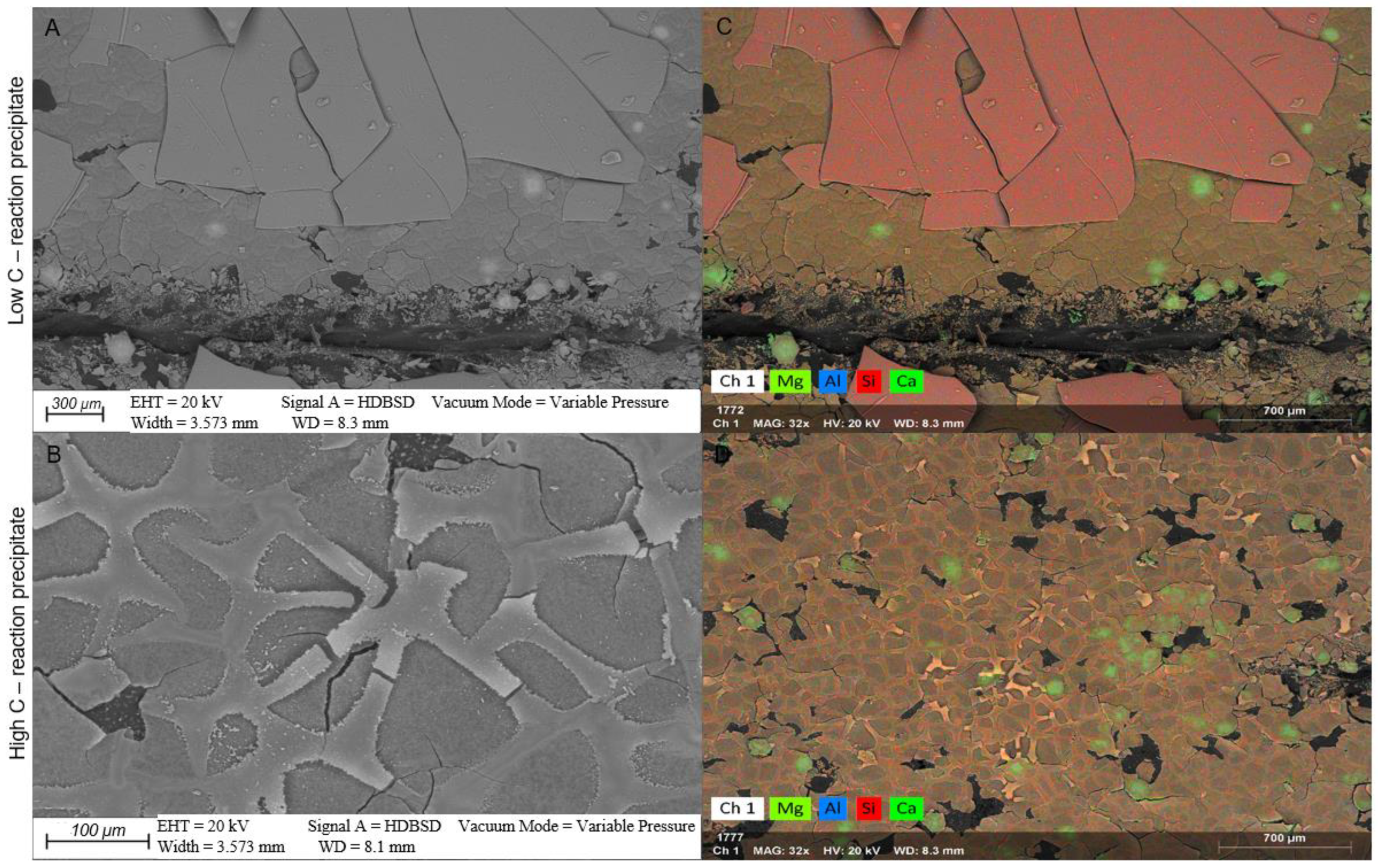
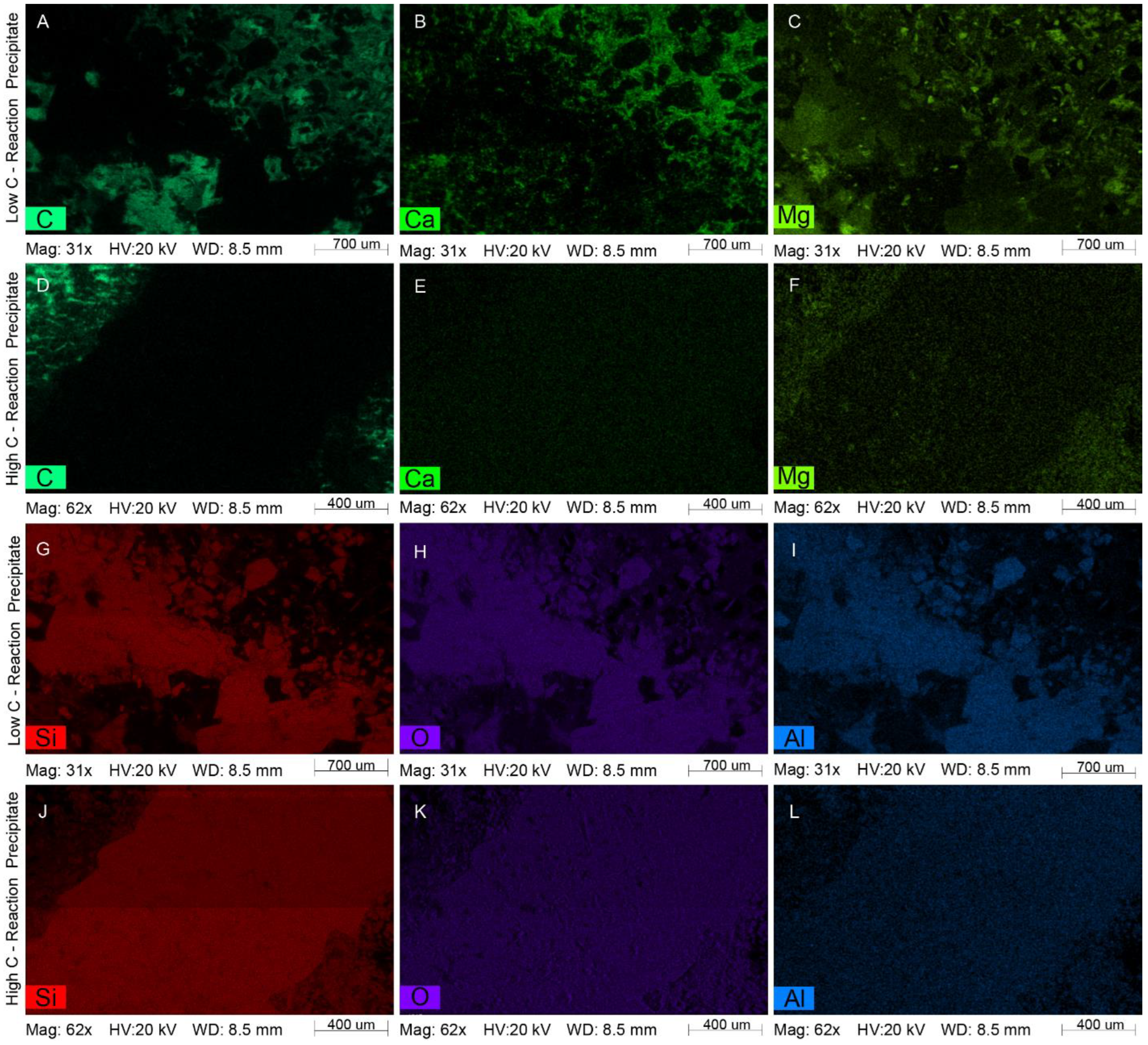
4.2.1. White Fine-Grained Precipitate
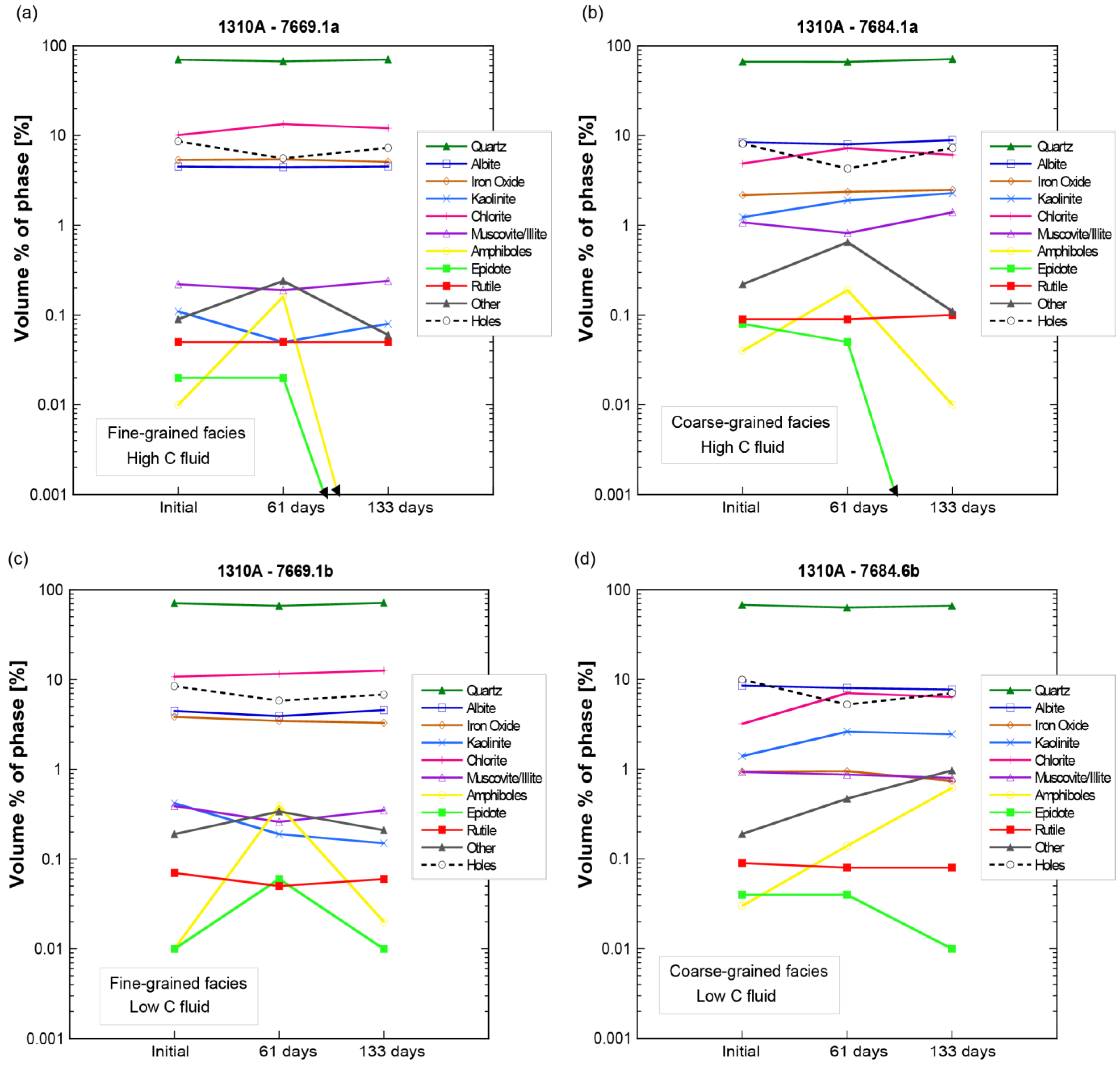
4.2.2. SGS Modal Minerals Analysis
4.3. Numerical Modeling and Predictions
5. Discussion
6. Conclusions
- The batch reaction experiments consistently formed a precipitate that coated the thin sections and consisted of a mixture of silica and dolomite, similar to theoretical model predictions.
- In the thin sections themselves, carbonate minerals (ankerite, dolomite, and calcite) were found mostly to precipitate during the stage 1 experiments from 0 to 61 days, but largely dissolved during the second stage of the experiments by 133 days.
- Mineral abundance changes over time in the laboratory experiments were much more variable and non-monotonic than in the theoretical reaction path models, perhaps reflecting heterogeneities in the mineral grain size surface area to volume ratios, and mineral distributions.
- The laboratory experiments affirm the results of theoretical models that predict dolomite to be the principal carbonate mineral sink for injected CO2 in the FWU and the Morrow B Sandstone formation water’s supersaturation with respect to silica.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bachu, S.; Adams, J.J. Sequestration of CO2 in geological media in response to climate change: Capacity of deep saline aquifers to sequester CO2 in solution. Energy Convers. Manag. 2003, 44, 3151–3175. [Google Scholar] [CrossRef]
- Litynski, J.; Plasynski, S.; Spangler, L.; Finley, R.; Steadman, E.; Ball, D.; Nemeth, K.J.; McPherson, B.; Myer, L.U.S. Department of Energy’s Regional Carbon Sequestration Partnership Program: Overview. Energy Procedia 2009, 1, 3959–3967. [Google Scholar] [CrossRef]
- DOE. Issue Brief Carbon Capture, Utilization, and Storage: Climate Change, Economic Competitiveness, and Energy Security; DOE Issue Brief. 2016; pp. 1–12. Available online: https://www.energy.gov/epsa/downloads/carbon-capture-utilization-and-storage-climate-change-economic-competitiveness-and (accessed on 8 May 2023).
- Ahmmed, B.; Appold, M.S.; Fan, T.; McPherson, B.J.O.L.; Grigg, R.B.; White, M.D. Chemical Effects of Carbon Dioxide Sequestration in the Upper Morrow Sandstone in the Farnsworth, Texas, hydrocarbon unit. Environ. Geosci. 2016, 23, 81–93. [Google Scholar] [CrossRef]
- Ampomah, W.; Balch, R.; Cather, M.; Rose-Coss, D.; Dai, Z.; Heath, J.; Dewers, T.; Mozley, P. Evaluation of CO2 Storage Mechanisms in CO2 Enhanced Oil Recovery Sites: Application to Morrow Sandstone Reservoir. Energy Fuels 2016, 30, 8545–8555. [Google Scholar] [CrossRef]
- Kutsienyo, E.J.; Appold, M.S.; White, M.D.; Ampomah, W. Field-Scale reactive transport assessment of CO2 storage in the farnsworth unit through enhanced oil recovery practices. Greenh. Gases Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Jia, W.; Xiao, T.; Wu, Z.; Dai, Z.; McPherson, B. Impact of mineral reactive surface area on forecasting geological carbon sequestration in a co2-eor field. Energies 2021, 14, 1608. [Google Scholar] [CrossRef]
- Gyamfi, B.A.; Ampomah, W.; Tu, J.; Sun, Q.; Erzuah, S.; Acheampong, S. Assessment of chemo-mechanical impacts of CO2 sequestration on the caprock formation in Farnsworth oil field, Texas. Sci. Rep. 2022, 12, 13023. [Google Scholar] [CrossRef]
- Ampomah, W.; Balch, R.S.; Grigg, R.B.; McPherson, B.; Will, R.A.; Lee, S.-Y.; Dai, Z.; Pan, F. Co-optimization of CO2-EOR and Storage Processes in Mature Oil Reservoirs. Greenh. Gases Sci. Technol. 2016, 7, 128–142. [Google Scholar] [CrossRef]
- Pan, F.; McPherson, B.J.; Esser, R.; Xiao, T.; Appold, M.S.; Jia, W.; Moodie, N. Forecasting evolution of formation water chemistry and long-term mineral alteration for GCS in a typical clastic reservoir of the Southwestern United States. Int. J. Greenh. Gas Control 2016, 54, 524–537. [Google Scholar] [CrossRef]
- Khan, R. Evaluation of the Geologic CO2 Sequestration Potential of the Morrow B Sandstone in the Farnsworth, Texas Hydrocarbon Field Using Reactive Transport Modeling. Master’s Thesis, University of Missouri-Columbia, Columbia, MO, USA, 2017. [Google Scholar]
- Kutsienyo, E.J.; Ampomah, W.; Sun, Q.; Balch, R.S.; You, J.; Aggrey, W.N.; Cather, M. Evaluation of CO2-EOR Performance and Storage Mechanisms in an Active Partially Depleted Oil Reservoir. In Proceedings of the SPE Europec Featured at 81st EAGE Conference and Exhibition, London, UK, 3–6 June 2019; pp. 1–19. [Google Scholar] [CrossRef]
- Sun, Q.; Ampomah, W.; Kutsienyo, E.J.; Appold, M.; Adu-Gyamfi, B.; Dai, Z.; Soltanian, M.R. Assessment of CO2 trapping mechanisms in partially depleted oil-bearing sands. Fuel 2020, 278, 118356. [Google Scholar] [CrossRef]
- You, J.; Ampomah, W.; Sun, Q.; Kutsienyo, E.J.; Balch, R.S.; Dai, Z.; Cather, M.; Zhang, X. Machine learning based co-optimization of carbon dioxide sequestration and oil recovery in CO2-EOR project. J. Clean. Prod. 2020, 260, 120866. [Google Scholar] [CrossRef]
- Kutsienyo, E.J.; Appold, M.S.; White, M.D.; Ampomah, W. Numerical Modeling of CO2 Sequestration within a Five-Spot Well Pattern in the Morrow B Sandstone of the Farnsworth Hydrocarbon Field: Comparison of the Toughreact, Stomp-Eor, and GEM Simulators. Energies 2021, 14, 5337. [Google Scholar] [CrossRef]
- Kutsienyo, E.J.; Ampomah, W.; Balch, R.; Cather, M.; Luhmann, A. Three-Phase Compositional Simulation Modeling Coupled with Reactive Transport: Application to Farnsworth Field CO2-EOR and Storage Project. Earth Sp. Sci. Open Arch. 2019. [Google Scholar] [CrossRef]
- Gunter, W.D.; Perkins, E.H.; McCann, T.J. Aquifer disposal of CO2-rich gases: Reaction design for added capacity. Energy Convers. Manag. 1993, 34, 941–948. [Google Scholar] [CrossRef]
- Weir, G.J.; White, S.P.; Kissling, W.M. Reservoir storage and containment of greenhouse gases. Transp. Porous Media 1996, 23, 37–60. [Google Scholar] [CrossRef]
- Bickle, M.; Chadwick, A.; Huppert, H.E.; Hallworth, M.; Lyle, S. Modelling carbon dioxide accumulation at Sleipner: Implications for underground carbon storage. Earth Planet. Sci. Lett. 2007, 255, 164–176. [Google Scholar] [CrossRef]
- Gherardi, F.; Xu, T.; Pruess, K. Numerical modeling of self-limiting and self-enhancing caprock alteration induced by CO2 storage in a depleted gas reservoir. Chem. Geol. 2007, 244, 103–129. [Google Scholar] [CrossRef]
- Xu, T.; Apps, J.A.; Pruess, K.; Yamamoto, H. Numerical modeling of injection and mineral trapping of CO2 with H2S and SO2 in a sandstone formation. Chem. Geol. 2007, 242, 319–346. [Google Scholar] [CrossRef]
- Akai, T.; Kuriyama, T.; Kato, S.; Okabe, H. Numerical modelling of long-term CO2 storage mechanisms in saline aquifers using the Sleipner benchmark dataset. Int. J. Greenh. Gas Control 2021, 110, 103405. [Google Scholar] [CrossRef]
- Zhan, J.; Niu, Z.; Li, M.; Zhang, Y.; Ma, X.; Fan, C.; Wang, R. Numerical Simulation and Modeling on CO2 Sequestration Coupled with Enhanced Gas Recovery in Shale Gas Reservoirs. Geofluids 2021, 2021, 9975296. [Google Scholar] [CrossRef]
- Gunter, W.D.; Wiwehar, B.; Perkins, E.H. Aquifer disposal of CO2-rich greenhouse gases: Extension of the time scale of experiment for CO2-sequestering reactions by geochemical modelling. Mineral. Petrol. 1997, 59, 121–140. [Google Scholar] [CrossRef]
- Gunter, W.D.; Perkins, E.H.; Hutcheon, I. Aquifer disposal of acid gases: Modelling of water-rock reactions for trapping of acid wastes. Appl. Geochem. 2000, 15, 1085–1095. [Google Scholar] [CrossRef]
- White, S.P.; Weir, G.J.; Kissling, W.M. Numerical simulation of CO2 sequestration in natural CO2 reservoirs on the Colorado Plateau. In Proceedings of the First National Conference on Carbon Sequestration, Washington, DC, USA, 14–17 May 2001. [Google Scholar]
- Johnson, J.W.; Nitao, J.J.; Knauss, K.G. Reactive transport modelling of CO2 storage in saline aquifers to elucidate fundamental processes, trapping mechanisms and sequestration partitioning. Geol. Soc. Spec. Publ. 2004, 233, 107–128. [Google Scholar] [CrossRef]
- Zwingmann, N.; Mito, S.; Sorai, M.; Ohsumi, T. Preinjection characterisation and evaluation of CO2 sequestration potential in the Haizume Formation, Niigata Basin, Japan. Geochemical modelling of water-minerals-CO2 interaction. Oil Gas Sci. Technol. 2005, 60, 249–258. [Google Scholar] [CrossRef]
- White, S.P.; Allis, R.G.; Moore, J.; Chidsey, T.; Morgan, C.; Gwynn, W.; Adams, M. Simulation of reactive transport of injected CO2 on the Colorado Plateau, Utah, USA. Chem. Geol. 2005, 217, 387–405. [Google Scholar] [CrossRef]
- Gaus, I.; Azaroual, M.; Czernichowski-Lauriol, I. Reactive transport modelling of the impact of CO2 injection on the clayey cap rock at Sleipner (North Sea). Chem. Geol. 2005, 217, 319–337. [Google Scholar] [CrossRef]
- André, L.; Audigane, P.; Azaroual, M.; Menjoz, A. Numerical modeling of fluid–rock chemical interactions at the supercritical CO2–liquid interface during CO2 injection into a carbonate reservoir, the Dogger aquifer (Paris Basin, France). Energy Convers. Manag. 2007, 48, 1782–1797. [Google Scholar] [CrossRef]
- Wu, Z.; Luhmann, A.J.; Rinehart, A.J.; Mozley, P.S.; Dewers, T.A.; Heath, J.E.; Majumdar, B.S. Chemo-mechanical Alterations Induced From CO2 Injection in Carbonate-Cemented Sandstone: An Experimental Study at 71 °C and 29 MPa. J. Geophys. Res. Solid Earth 2020, 125, e2019JB019096. [Google Scholar] [CrossRef]
- Munson, T.W. Depositional, Diagenetic, and Production History of the upper Morrowan Buckhaults Sandstone, Farnsworth Field Ochiltree County Texas. OCGS—Shale Shak. Dig. XIII 1989, XXXX–XXXXIV, 1–20. [Google Scholar]
- Gallagher, S.R. Depositional and Diagenetic Controls on Reservoir Heterogeneity: Upper Morrow Sandstone, Farnsworth Unit, Ochiltree County, Texas. Master’s Thesis, New Mexico Institute of Mining and Technology, Socorro, NM, USA, 2014; pp. 1–233. [Google Scholar]
- Cather, M.; Rose-Coss, D.; Gallagher, S.; Trujillo, N.; Cather, S.; Hollingworth, R.S.; Mozley, P.; Leary, R.J. Deposition, Diagenesis, and Sequence Stratigraphy of the Pennsylvanian Morrowan and Atokan Intervals at Farnsworth. Energies 2021, 14, 1057. [Google Scholar] [CrossRef]
- Perdure Petroleum, LLC. Farnsworth Unit, Texas (FWU) Monitoring, Reporting, and Verification Plan. 2021. Available online: https://www.epa.gov/system/files/documents/2021-07/fwu_decision.pdf (accessed on 8 May 2023).
- Van Wijk, J.; Hobbs, N.; Rose, P.; Mella, M.; Axen, G.; Gragg, E. Analysis of geologic CO2 migration pathways in farnsworth field, nw anadarko basin. Energies 2021, 14, 7818. [Google Scholar] [CrossRef]
- SWP. The Southwest Regional Partnership on Carbon Sequestration, 2022. Available online: https://www.netl.doe.gov/coal/carbon-storage/atlas/swp/phase-III/farnsworth (accessed on 9 December 2022).




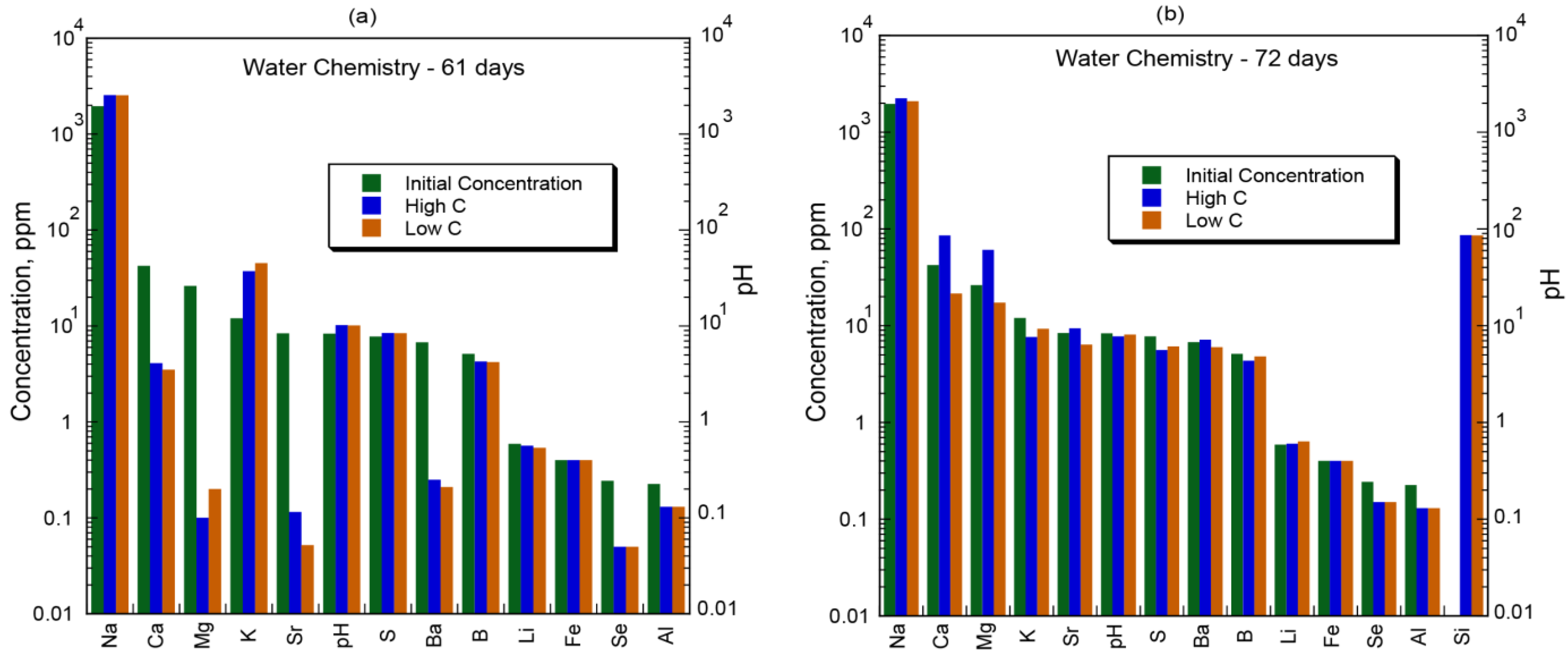





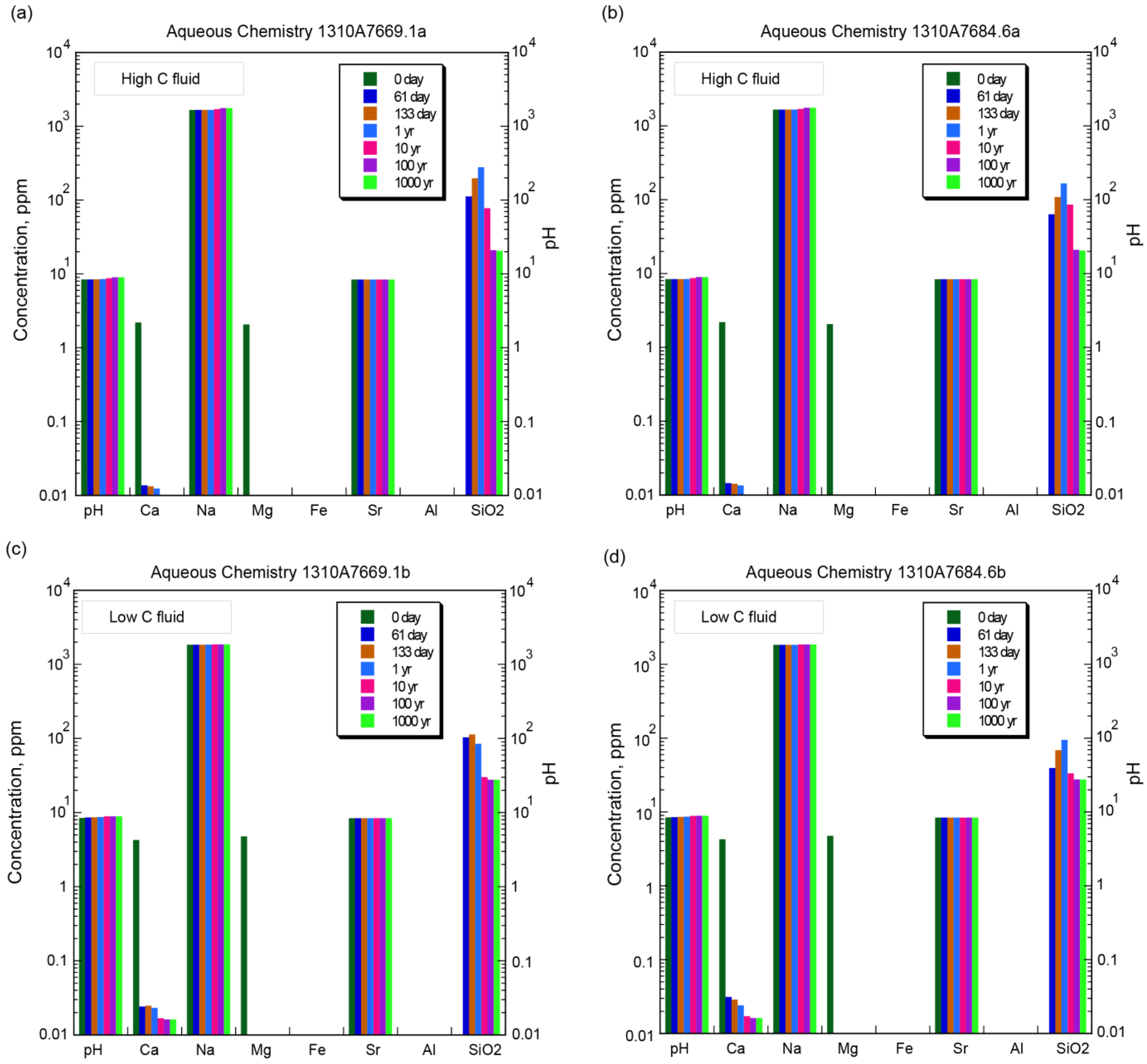
| Elements ** | Concentration *** (ppm *) | ||||
|---|---|---|---|---|---|
| Initial | 61 Days | 72 Days | |||
| High C | Low C | High C | Low C | ||
| S | 7.76 | 7.76 | 8.45 | 8.4 | 5.59 |
| Ca | 42.3 | 42.3 | 4.1 | 3.5 | 85.8 |
| K | 12 | 12 | 37.3 | 45.2 | 7.65 |
| Na | 1955 | 1955 | 2550 | 2540 | 2240 |
| Mg | 26.2 | 26.2 | <0.1 | 0.2 | 60.9 |
| Fe | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 |
| Li | 0.591 | 0.591 | 0.565 | 0.537 | 0.604 |
| B | 5.13 | 5.13 | 4.27 | 4.21 | 4.35 |
| Sr | 8.38 | 8.38 | 0.115 | 0.052 | 9.41 |
| Ba | 6.75 | 6.75 | 0.25 | 0.21 | 7.17 |
| Al | 0.226 | 0.226 | <0.13 | <0.13 | <0.13 |
| Se | 0.243 | 0.243 | <0.05 | <0.05 | 0.15 |
| pH | 8.33 | 10.19 | 10.15 | 7.76 | 8.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutsienyo, E.J.; Appold, M.S.; Cather, M.E. Investigation of the Effect of Injected CO2 on the Morrow B Sandstone through Laboratory Batch Reaction Experiments: Implications for CO2 Sequestration in the Farnsworth Unit, Northern Texas, USA. Energies 2023, 16, 4611. https://doi.org/10.3390/en16124611
Kutsienyo EJ, Appold MS, Cather ME. Investigation of the Effect of Injected CO2 on the Morrow B Sandstone through Laboratory Batch Reaction Experiments: Implications for CO2 Sequestration in the Farnsworth Unit, Northern Texas, USA. Energies. 2023; 16(12):4611. https://doi.org/10.3390/en16124611
Chicago/Turabian StyleKutsienyo, Eusebius J., Martin S. Appold, and Martha E. Cather. 2023. "Investigation of the Effect of Injected CO2 on the Morrow B Sandstone through Laboratory Batch Reaction Experiments: Implications for CO2 Sequestration in the Farnsworth Unit, Northern Texas, USA" Energies 16, no. 12: 4611. https://doi.org/10.3390/en16124611
APA StyleKutsienyo, E. J., Appold, M. S., & Cather, M. E. (2023). Investigation of the Effect of Injected CO2 on the Morrow B Sandstone through Laboratory Batch Reaction Experiments: Implications for CO2 Sequestration in the Farnsworth Unit, Northern Texas, USA. Energies, 16(12), 4611. https://doi.org/10.3390/en16124611





