Aluminosilicate Clay Minerals: Kaolin, Bentonite, and Halloysite as Fuel Additives for Thermal Conversion of Biomass and Waste
Abstract
1. Introduction
- Aluminosilicates-based additives;
- Calcium-based additives;
- Sulphur-based additives;
- Phosphorous additives.
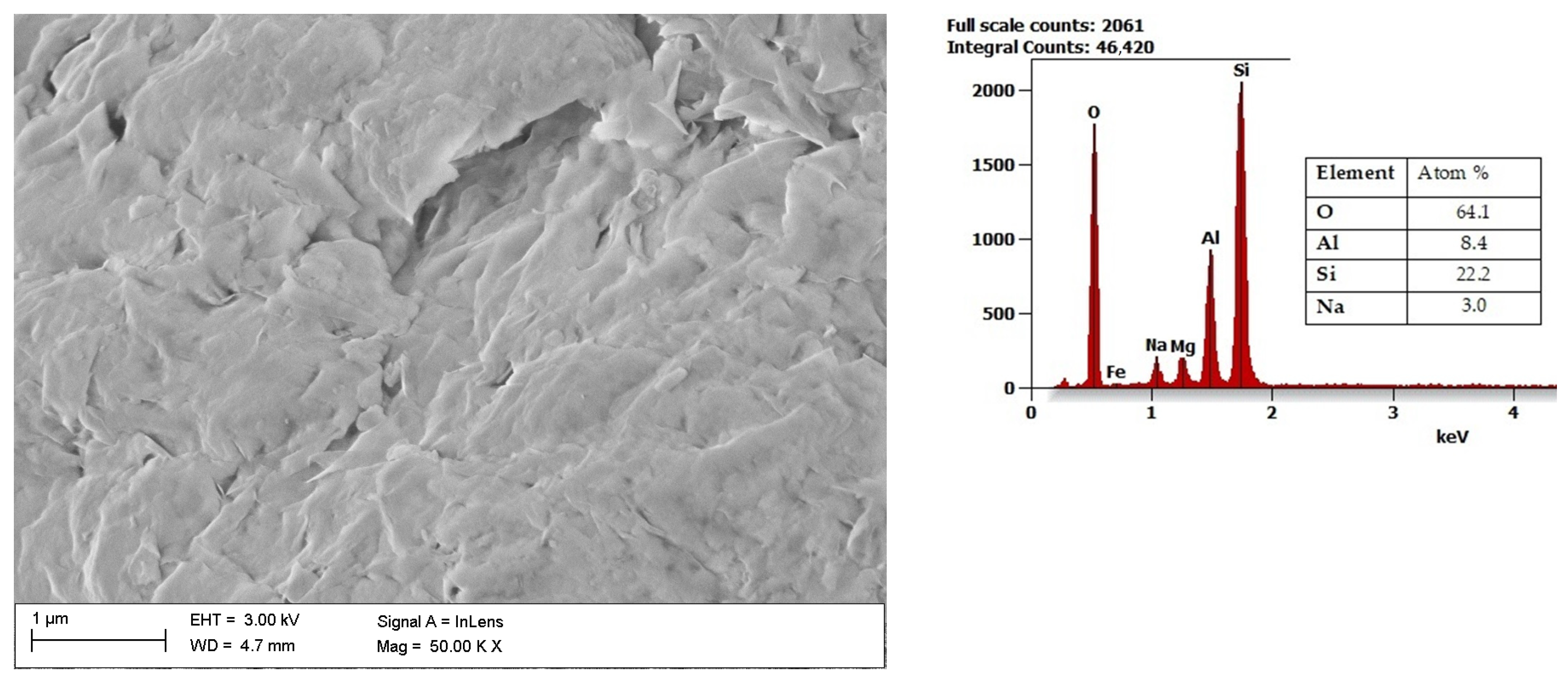
2. Characteristics of Aluminosilicate Clay Minerals
2.1. Halloysite
2.2. Kaolin
2.3. Bentonite
3. Mitigation of Combustion-Related Problems
3.1. The Influence of Aluminosilicate Clay Minerals on Potassium Retention
3.2. The Influence of Aluminosilicate Clay Minerals on Ash Fusion Temperatures and Deposition Tendency
3.3. Aluminosilicate Clay Minerals in Fluidized Bed Combustion
3.4. The Influence of Aluminosilicate Clay Minerals on Particulate Matter Emission
3.5. Other Fuel-Related Applications of Aluminosilicate Clay Minerals
4. Mitigation of the Heavy Metals Emission
5. Ash Disposal
6. Application and Optimal Dosage of Aluminosilicate Clay Minerals
6.1. Determination of the Optimal Additive Dose
6.2. Application
7. Limitations
8. Conclusions
- (1)
- The ash fusion temperatures are elevated as a result of bonding potassium into the compounds with melting points of above 1500 °C. This leads directly to the reduction of slagging, fouling, ash deposition, and bed agglomeration in CFB furnaces.
- (2)
- The risk of chlorine-induced corrosion is reduced, as aluminosilicates favor the release of chlorine in the form of hydrogen chloride and thus reduce its presence in ash deposits.
- (3)
- The formation and emission of the particulate matter are minimized, as aluminosilicates reduce the release of ash-forming components into the gas phase.
- (4)
- Heavy metals are expected to get fixed in ash; consequently, their emission into the gas phase is expected to be reduced.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Askarova, A.; Zamorano, M.; Martín-Pascual, J.; Nugymanova, A.; Bolegenova, S. A Review of the Energy Potential of Residual Biomass for Coincineration in Kazakhstan. Energies 2022, 15, 6482. [Google Scholar] [CrossRef]
- Li, H.; Min, X.; Dai, M.; Dong, X. The Biomass Potential and GHG (Greenhouse Gas) Emissions Mitigation of Straw-Based Biomass Power Plant: A Case Study in Anhui Province, China. Processes 2019, 7, 608. [Google Scholar] [CrossRef]
- Chen, Y.-C. Effects of Urbanization on Municipal Solid Waste Composition. Waste Manag. 2018, 79, 828–836. [Google Scholar] [CrossRef]
- Antczak, E. Regionally Divergent Patterns in Factors Affecting Municipal Waste Production: The Polish Perspective. Sustainability 2020, 12, 6885. [Google Scholar] [CrossRef]
- Wilson, D.C.; Velis, C.A. Waste Management—Still a Global Challenge in the 21st Century: An Evidence-Based Call for Action. Waste Manag. Res. J. A Sustain. Circ. Econ. 2015, 33, 1049–1051. [Google Scholar] [CrossRef]
- The World Bank—Trends in Solid Waste Management. Available online: https://Datatopics.Worldbank.Org/What-a-Waste/Trends_in_solid_waste_management (accessed on 16 May 2023).
- Sarquah, K.; Narra, S.; Beck, G.; Bassey, U.; Antwi, E.; Hartmann, M.; Derkyi, N.S.A.; Awafo, E.A.; Nelles, M. Characterization of Municipal Solid Waste and Assessment of Its Potential for Refuse-Derived Fuel (RDF) Valorization. Energies 2022, 16, 200. [Google Scholar] [CrossRef]
- Rajca, P.; Skibiński, A.; Biniek-Poskart, A.; Zajemska, M. Review of Selected Determinants Affecting Use of Municipal Waste for Energy Purposes. Energies 2022, 15, 9057. [Google Scholar] [CrossRef]
- Gałko, G.; Mazur, I.; Rejdak, M.; Jagustyn, B.; Hrabak, J.; Ouadi, M.; Jahangiri, H.; Sajdak, M. Evaluation of Alternative Refuse-Derived Fuel Use as a Valuable Resource in Various Valorised Applications. Energy 2023, 263, 125920. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Vaskalis, I. Economic Assessment of Polypropylene Waste (PP) Pyrolysis in Circular Economy and Industrial Symbiosis. Energies 2023, 16, 593. [Google Scholar] [CrossRef]
- Bourtsalas, A.C.; Shen, T.; Tian, Y. A Comprehensive Assessment of Products Management and Energy Recovery from Waste Products in the United States. Energies 2022, 15, 6581. [Google Scholar] [CrossRef]
- Muzyka, R.; Gałko, G.; Ouadi, M.; Sajdak, M. Impact of Plastic Blends on the Gaseous Product Composition from the Co-Pyrolysis Process. Energies 2023, 16, 947. [Google Scholar] [CrossRef]
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R. A Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Vassilev, S.v.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Composition and Application of Biomass Ash. Fuel 2013, 105, 19–39. [Google Scholar] [CrossRef]
- Díaz-Ramírez, M.; Frandsen, F.J.; Glarborg, P.; Sebastián, F.; Royo, J. Partitioning of K, Cl, S and P during Combustion of Poplar and Brassica Energy Crops. Fuel 2014, 134, 209–219. [Google Scholar] [CrossRef]
- Maj, I. Significance and Challenges of Poultry Litter and Cattle Manure as Sustainable Fuels: A Review. Energies 2022, 15, 8981. [Google Scholar] [CrossRef]
- Maj, I.; Kalisz, S.; Ciukaj, S. Properties of Animal-Origin Ash—A Valuable Material for Circular Economy. Energies 2022, 15, 1274. [Google Scholar] [CrossRef]
- Tran, Q.T.; Maeda, M.; Oshita, K.; Takaoka, M. Phosphorus Release from Cattle Manure Ash as Soil Amendment in Laboratory-Scale Tests. Soil. Sci. Plant Nutr. 2017, 63, 369–376. [Google Scholar] [CrossRef]
- Liikanen, M.; Sahimaa, O.; Hupponen, M.; Havukainen, J.; Sorvari, J.; Horttanainen, M. Updating and Testing of a Finnish Method for Mixed Municipal Solid Waste Composition Studies. Waste Manag. 2016, 52, 25–33. [Google Scholar] [CrossRef]
- Kofstad, P. High Temperature Corrosion; Elsevier: London, UK, 1988. [Google Scholar]
- Vassilev, S.v.; Baxter, D.; Vassileva, C.G. An Overview of the Behaviour of Biomass during Combustion: Part II. Ash Fusion and Ash Formation Mechanisms of Biomass Types. Fuel 2014, 117, 152–183. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Preto, F.; Zhu, J.; Xu, C. Ash Deposition in Biomass Combustion or Co-Firing for Power/Heat Generation. Energies 2012, 5, 5171–5189. [Google Scholar] [CrossRef]
- Niu, Y.; Tan, H.; Hui, S. Ash-Related Issues during Biomass Combustion: Alkali-Induced Slagging, Silicate Melt-Induced Slagging (Ash Fusion), Agglomeration, Corrosion, Ash Utilization, and Related Countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. [Google Scholar] [CrossRef]
- Persson, K.; Broström, M.; Carlsson, J.; Nordin, A.; Backman, R. High Temperature Corrosion in a 65 MW Waste to Energy Plant. Fuel Process. Technol. 2007, 88, 1178–1182. [Google Scholar] [CrossRef]
- Król, D.; Motyl, P.; Poskrobko, S. Chlorine Corrosion in a Low-Power Boiler Fired with Agricultural Biomass. Energies 2022, 15, 382. [Google Scholar] [CrossRef]
- Maj, I.; Kalisz, S.; Szymajda, A.; Łaska, G.; Gołombek, K. The Influence of Cow Dung and Mixed Straw Ashes on Steel Corrosion. Renew. Energy 2021, 177, 1198–1211. [Google Scholar] [CrossRef]
- Ma, W.; Wenga, T.; Frandsen, F.J.; Yan, B.; Chen, G. The Fate of Chlorine during MSW Incineration: Vaporization, Transformation, Deposition, Corrosion and Remedies. Prog. Energy Combust. Sci. 2020, 76, 100789. [Google Scholar] [CrossRef]
- Pronobis, M. Evaluation of the Influence of Biomass Co-Combustion on Boiler Furnace Slagging by Means of Fusibility Correlations. Biomass Bioenergy 2005, 28, 375–383. [Google Scholar] [CrossRef]
- Lachman, J.; Baláš, M.; Lisý, M.; Lisá, H.; Milčák, P.; Elbl, P. An Overview of Slagging and Fouling Indicators and Their Applicability to Biomass Fuels. Fuel Process. Technol. 2021, 217, 106804. [Google Scholar] [CrossRef]
- Jiang, D.; Song, W.; Wang, X.; Zhu, Z. Physicochemical Properties of Bottom Ash Obtained from an Industrial CFB Gasifier. J. Energy Inst. 2021, 95, 375–383. [Google Scholar] [CrossRef]
- Yao, X.; Mao, J.; Li, L.; Sun, L.; Xu, K.; Ma, X.; Hu, Y.; Zhao, Z.; Chen, S.; Xu, K. Characterization Comparison of Bottom Ash and Fly Ash during Gasification of Agricultural Residues at an Industrial-Scale Gasification Plant–Experiments and Analysis. Fuel 2021, 285, 119122. [Google Scholar] [CrossRef]
- Kelleher, B.P.; Leahy, J.J.; Henihan, A.M.; O’Dwyer, T.F.; Sutton, D.; Leahy, M.J. Advances in Poultry Litter Disposal Technology—A Review. Bioresour. Technol. 2002, 83, 27–36. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, C.; Luo, Z.; Li, Y. Investigation of Ash Deposition Dynamic Process in an Industrial Biomass CFB Boiler Burning High-Alkali and Low-Chlorine Fuel. Energies 2020, 13, 1092. [Google Scholar] [CrossRef]
- Singhal, A.; Konttinen, J.; Joronen, T. Effect of Different Washing Parameters on the Fuel Properties and Elemental Composition of Wheat Straw in Water-Washing Pre-Treatment. Part 2: Effect of Washing Temperature and Solid-to-Liquid Ratio. Fuel 2021, 292, 120209. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, T.; An, Y.; Zhang, W.; Dong, Y. Influence of Water Leaching on Alkali-Induced Slagging Properties of Biomass Straw. J. Fuel Chem. Technol. 2021, 49, 1839–1849. [Google Scholar] [CrossRef]
- Suleimenova, B.; Aimbetov, B.; Zhakupov, D.; Shah, D.; Sarbassov, Y. Co-Firing of Refuse-Derived Fuel with Ekibastuz Coal in a Bubbling Fluidized Bed Reactor: Analysis of Emissions and Ash Characteristics. Energies 2022, 15, 5785. [Google Scholar] [CrossRef]
- Wu, S.; Chen, J.; Peng, D.; Wu, Z.; Li, Q.; Huang, T. Effects of Water Leaching on the Ash Sintering Problems of Wheat Straw. Energies 2019, 12, 387. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Shen, Y.-H.; Tsai, W.-T. Effect of Alkaline Pretreatment on the Fuel Properties of Torrefied Biomass from Rice Husk. Energies 2023, 16, 679. [Google Scholar] [CrossRef]
- Míguez, J.L.; Porteiro, J.; Behrendt, F.; Blanco, D.; Patiño, D.; Dieguez-Alonso, A. Review of the Use of Additives to Mitigate Operational Problems Associated with the Combustion of Biomass with High Content in Ash-Forming Species. Renew. Sustain. Energy Rev. 2021, 141, 110502. [Google Scholar] [CrossRef]
- Wang, L.; Hustad, J.E.; Skreiberg, Ø.; Skjevrak, G.; Grønli, M. A Critical Review on Additives to Reduce Ash Related Operation Problems in Biomass Combustion Applications. Energy Procedia 2012, 20, 20–29. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, L.; Pant, R.; Yu, Y.; Wei, Z.; Zhang, G. Effects of Interlayer Interactions on the Nanoindentation Behavior and Hardness of 2:1 Phyllosilicates. Appl. Clay Sci. 2013, 80, 267–280. [Google Scholar] [CrossRef]
- Nickovic, S.; Vukovic, A.; Vujadinovic, M.; Djurdjevic, V.; Pejanovic, G. Technical Note: High-Resolution Mineralogical Database of Dust-Productive Soils for Atmospheric Dust Modeling. Atmos. Chem. Phys. 2012, 12, 845–855. [Google Scholar] [CrossRef]
- Bilans Zasobów Złóż Kopalin W Polsce Wg Stanu Na 31 XII 2021 r; Państwowy Instytut Geologiczny—Państwowy Instytut Badawczy: Warszawa, Poland, 2022; pp. 89–90, 458–459.
- Kaolin Market Size, Share & Trends Analysis Report by Application (Paper, Ceramics, Paint & Coatings, Fiber Glass, Plastic, Rubber, Cosmetics), by Region, and Segment Forecasts, 2022–2030. Available online: https://Www.Grandviewresearch.Com/Industry-Analysis/Kaolin-Market (accessed on 15 March 2023).
- Singh, N.B. Clays and Clay Minerals in the Construction Industry. Minerals 2022, 12, 301. [Google Scholar] [CrossRef]
- Singh, B.K.; Um, W. Application of Clay Materials for Sorption of Radionuclides from Waste Solutions. Minerals 2023, 13, 239. [Google Scholar] [CrossRef]
- Gaaz, T.; Sulong, A.; Kadhum, A.; Al-Amiery, A.; Nassir, M.; Jaaz, A. The Impact of Halloysite on the Thermo-Mechanical Properties of Polymer Composites. Molecules 2017, 22, 838. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R. A Comprehensive Study on the Applications of Clays into Advanced Technologies, with a Particular Attention on Biomedicine and Environmental Remediation. Inorganics 2022, 10, 40. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Wu, C.; Xiong, S.; Zhou, C. Chitosan/Halloysite Nanotubes Bionanocomposites: Structure, Mechanical Properties and Biocompatibility. Int. J. Biol. Macromol. 2012, 51, 566–575. [Google Scholar] [CrossRef]
- Murray, H.H. Applied Clay Mineralogy Today and Tomorrow. Clay Miner. 1999, 34, 39–49. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. Chapter 1 General Introduction: Clays, Clay Minerals, and Clay Science. Dev. Clay Sci. 2006, 1, 1–18. [Google Scholar]
- Wejkowski, R.; Kalisz, S.; Tymoszuk, M.; Ciukaj, S.; Maj, I. Full-Scale Investigation of Dry Sorbent Injection for NOx Emission Control and Mercury Retention. Energies 2021, 14, 7787. [Google Scholar] [CrossRef]
- Kalisz, S.; Ciukaj, S.; Mroczek, K.; Tymoszuk, M.; Wejkowski, R.; Pronobis, M.; Kubiczek, H. Full-Scale Study on Halloysite Fireside Additive in 230 t/h Pulverized Coal Utility Boiler. Energy 2015, 92, 33–39. [Google Scholar] [CrossRef]
- Lutyński, M.; Sakiewicz, P.; Lutyńska, S. Characterization of Diatomaceous Earth and Halloysite Resources of Poland. Minerals 2019, 9, 670. [Google Scholar] [CrossRef]
- Rooj, S.; Das, A.; Thakur, V.; Mahaling, R.N.; Bhowmick, A.K.; Heinrich, G. Preparation and Properties of Natural Nanocomposites Based on Natural Rubber and Naturally Occurring Halloysite Nanotubes. Mater. Des. 2010, 31, 2151–2156. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Stetsyshyn, Y. Stability of Halloysite, Imogolite, and Boron Nitride Nanotubes in Solvent Media. Appl. Sci. 2018, 8, 1068. [Google Scholar] [CrossRef]
- Prabhu, R.; Shetty, K.; Jeevananda, T.; Ananda Murthy, H.C.; Sillanpaa, M.; Nhat, T. Novel Polyaniline–Halloysite Nanoclay Hybrid Composites: Synthesis, Physico-Chemical, Thermal and Electrical Properties. Inorg. Chem. Commun. 2023, 148, 110328. [Google Scholar] [CrossRef]
- Shahabi, N.; Soleimani, S.; Ghorbani, M. Investigating Functional Properties of Halloysite Nanotubes and Propolis Used in Reinforced Composite Film Based on Soy Protein/Basil Seed Gum for Food Packaging Application. Int. J. Biol. Macromol. 2023, 231, 123350. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, O.V.; Smirnova, D.N.; Noskov, A.V.; Kuznetsov, O.Y.; Kirilenko, M.A.; Agafonov, A.V. Mesoporous Halloysite/Magnetite Composite: Synthesis, Characterization and in Vitro Evaluation of the Effect on the Bacteria Viability. Mater. Today Commun. 2022, 32, 103877. [Google Scholar] [CrossRef]
- Sakiewicz, P.; Lutyński, M.; Sołtys, J.; Pytliński, A. Purification of Halloysite by Magnetic Separation. Physicochem. Probl. Miner. Process. 2016, 52, 991–1001. [Google Scholar]
- Ciftbudak, S.; Kalkan, B.; Bozbay, R.; Er, M.; Orakdogen, N. Structure-Property Relationships of Kaolin-Nanocomposite Beads Decorated with Tertiary Amines: Influence of Shape on Network Elasticity and Multi-Responsivity. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130471. [Google Scholar] [CrossRef]
- Gasparini, E.; Tarantino, S.C.; Ghigna, P.; Riccardi, M.P.; Cedillo-González, E.I.; Siligardi, C.; Zema, M. Thermal Dehydroxylation of Kaolinite under Isothermal Conditions. Appl. Clay Sci. 2013, 80, 417–425. [Google Scholar] [CrossRef]
- Murray, H.H. Chapter 5 Kaolin Applications. Dev. Clay Sci. 2006, 2, 85–109. [Google Scholar]
- Kwaśniewska, A.; Chocyk, D.; Gładyszewski, G.; Borc, J.; Świetlicki, M.; Gładyszewska, B. The Influence of Kaolin Clay on the Mechanical Properties and Structure of Thermoplastic Starch Films. Polymers 2020, 12, 73. [Google Scholar] [CrossRef]
- Shakeel, A.; Ali, W.; Chassagne, C.; Kirichek, A. Tuning the Rheological Properties of Kaolin Suspensions Using Biopolymers. Colloids. Surf. A Physicochem. Eng. Asp. 2022, 654, 130120. [Google Scholar] [CrossRef]
- Cui, Q.; Chen, B. Review of Polymer-Amended Bentonite: Categories, Mechanism, Modification Processes and Application in Barriers for Isolating Contaminants. Appl. Clay Sci. 2023, 235, 106869. [Google Scholar] [CrossRef]
- Alzamel, M.; Fall, M.; Haruna, S. Swelling Ability and Behaviour of Bentonite-Based Materials for Deep Repository Engineered Barrier Systems: Influence of Physical, Chemical and Thermal Factors. J. Rock Mech. Geotech. Eng. 2022, 14, 689–702. [Google Scholar] [CrossRef]
- Zhong, H.; Guan, Y.; Qiu, Z.; Grady, B.P.; Su, J.; Huang, W. Application of Carbon Coated Bentonite Composite as an Ultra-High Temperature Filtration Reducer in Water-Based Drilling Fluid. J. Mol. Liq. 2023, 375, 121360. [Google Scholar] [CrossRef]
- Punjak, W.A.; Shadman, F. Aluminosilicate Sorbents for Control of Alkali Vapors during Coal Combustion and Gasification. Energy Fuels 1988, 2, 702–708. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Yang, Y.; Shen, F.; Zhang, Z. Theoretical Investigation of Sodium Capture Mechanism on Kaolinite Surfaces. Fuel 2018, 234, 318–325. [Google Scholar] [CrossRef]
- Liao, Y.; Wu, S.; Chen, T.; Cao, Y.; Ma, X. The Alkali Metal Characteristic During Biomass Combustion with Additives. Energy Procedia 2015, 75, 124–129. [Google Scholar] [CrossRef]
- Hardy, T.; Kordylweski, W.; Mościcki, K. Use of Aluminosilicate Sorbents for Control of KCl Vapors in Biomass Combustion Gases. J. Power Technol. 2013, 93, 37–43. [Google Scholar]
- Clery, D.S.; Mason, P.E.; Rayner, C.M.; Jones, J.M. The Effects of an Additive on the Release of Potassium in Biomass Combustion. Fuel 2018, 214, 647–655. [Google Scholar] [CrossRef]
- Dragutinovic, N.; Höfer, I.; Kaltschmitt, M. Effect of Additives on Thermochemical Conversion of Solid Biofuel Blends from Wheat Straw, Corn Stover, and Corn Cob. Biomass Convers. Biorefin. 2019, 9, 35–54. [Google Scholar] [CrossRef]
- Mroczek, K.; Kalisz, S.; Pronobis, M.; Sołtys, J. The Effect of Halloysite Additive on Operation of Boilers Firing Agricultural Biomass. Fuel Process. Technol. 2011, 92, 845–855. [Google Scholar] [CrossRef]
- Roberts, L.J.; Mason, P.E.; Jones, J.M.; Gale, W.F.; Williams, A.; Hunt, A.; Ashman, J. The Impact of Aluminosilicate-Based Additives upon the Sintering and Melting Behaviour of Biomass Ash. Biomass Bioenergy 2019, 127, 105284. [Google Scholar] [CrossRef]
- Sobieraj, J.; Gądek, W.; Jagodzińska, K.; Kalisz, S. Investigations of Optimal Additive Dose for Cl-Rich Biomasses. Renew. Energy 2021, 163, 2008–2017. [Google Scholar] [CrossRef]
- Hui, S.; Lv, Y.; Niu, Y.; Li, S.; Lei, Y.; Li, P. Effects of Leaching and Additives on the Formation of Deposits on the Heating Surface during High-Na/Ca Zhundong Coal Combustion. J. Energy Inst. 2021, 94, 319–328. [Google Scholar] [CrossRef]
- Maj, I.; Kalisz, S.; Wejkowski, R.; Pronobis, M.; Gołombek, K. High-Temperature Corrosion in a Multifuel Circulating Fluidized Bed (CFB) Boiler Co-Firing Refuse Derived Fuel (RDF) and Hard Coal. Fuel 2022, 324, 124749. [Google Scholar] [CrossRef]
- Zuwała, J.; Lasek, J.; Głód, K. Study on Bed Agglomeration, Fouling and Slagging Remedies in Biomass Fired BFB Combustors Based on Laboratory Tests and Long Term Operational Experiences. Eur. Biomass Conf. Exhib. Proc. 2018, 458–464. [Google Scholar] [CrossRef]
- Morris, J.D.; Daood, S.S.; Nimmo, W. The Use of Kaolin and Dolomite Bed Additives as an Agglomeration Mitigation Method for Wheat Straw and Miscanthus Biomass Fuels in a Pilot-Scale Fluidized Bed Combustor. Renew. Energy 2022, 196, 749–762. [Google Scholar] [CrossRef]
- Nguyen, H.K.; Moon, J.H.; Jo, S.H.; Park, S.J.; Bae, D.H.; Seo, M.W.; Ra, H.W.; Yoon, S.-J.; Yoon, S.-M.; Lee, J.G.; et al. Ash Characteristics of Oxy-Biomass Combustion in a Circulating Fluidized Bed with Kaolin Addition. Energy 2021, 230, 120871. [Google Scholar] [CrossRef]
- Bäfver, L.S.; Rönnbäck, M.; Leckner, B.; Claesson, F.; Tullin, C. Particle Emission from Combustion of Oat Grain and Its Potential Reduction by Addition of Limestone or Kaolin. Fuel Process. Technol. 2009, 90, 353–359. [Google Scholar] [CrossRef]
- Dragutinović, N.; Höfer, I.; Kaltschmitt, M. Fuel Improvement Measures for Particulate Matter Emission Reduction during Corn Cob Combustion. Energies 2021, 14, 4548. [Google Scholar] [CrossRef]
- Höfer, I.; Gollmer, C.; Kaltschmitt, M. Inorganic PM and K Emissions during Ashing of Solid Biofuels and Kaolinite–Data Measurement in Laboratory Scale. Fuel 2021, 296, 120704. [Google Scholar] [CrossRef]
- Chen, J.; Yao, H.; Zhang, P.; Xiao, L.; Luo, G.; Xu, M. Control of PM1 by Kaolin or Limestone during O2/CO2 Pulverized Coal Combustion. Proc. Combust. Inst. 2011, 33, 2837–2843. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Qi, J.; Wang, H.; Zhang, T.; Xu, M. Effects of Kaolin-Limestone Blended Additive on the Formation and Emission of Particulate Matter: Field Study on a 1000 MW Coal-Firing Power Station. J. Hazard. Mater. 2020, 399, 123091. [Google Scholar] [CrossRef]
- Seliverstov, E.S.; Furda, L.V.; Lebedeva, O.E. Thermocatalytic Conversion of Plastics into Liquid Fuels over Clays. Polymers 2022, 14, 2115. [Google Scholar] [CrossRef] [PubMed]
- Fadillah, G.; Fatimah, I.; Sahroni, I.; Musawwa, M.M.; Mahlia, T.M.I.; Muraza, O. Recent Progress in Low-Cost Catalysts for Pyrolysis of Plastic Waste to Fuels. Catalysts 2021, 11, 837. [Google Scholar] [CrossRef]
- Auxilio, A.R.; Choo, W.-L.; Kohli, I.; Chakravartula Srivatsa, S.; Bhattacharya, S. An Experimental Study on Thermo-Catalytic Pyrolysis of Plastic Waste Using a Continuous Pyrolyser. Waste Manag. 2017, 67, 143–154. [Google Scholar] [CrossRef]
- Abnisa, F. Enhanced Liquid Fuel Production from Pyrolysis of Plastic Waste Mixtures Using a Natural Mineral Catalyst. Energies 2023, 16, 1224. [Google Scholar] [CrossRef]
- Elordi, G.; Olazar, M.; Castaño, P.; Artetxe, M.; Bilbao, J. Polyethylene Cracking on a Spent FCC Catalyst in a Conical Spouted Bed. Ind. Eng. Chem. Res. 2012, 51, 14008–14017. [Google Scholar] [CrossRef]
- Erawati, E.; Hamid; Martenda, D. Kinetic Study on the Pyrolysis of Low-Density Polyethylene (LDPE) Waste Using Kaolin as Catalyst. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012071. [Google Scholar] [CrossRef]
- Kulok, M.; Kolacz, R.; Dobrzański, Z.; Wolska, I. The Influence of Halloysite on the Content of Bacteria, Fungi and Mycotoxins in Feed Mixtures. In Proceedings of the XIIth International Congress on Animal Hygiene ISAH, Warsaw, Poland, 4–8 September 2005. [Google Scholar]
- Szul, M.; Iluk, T.; Sobolewski, A. High-Temperature, Dry Scrubbing of Syngas with Use of Mineral Sorbents and Ceramic Rigid Filters. Energies 2020, 13, 1528. [Google Scholar] [CrossRef]
- Du, H.; Zhong, Z.; Zhang, B.; Zhao, D.; Lai, X.; Wang, N.; Li, J. Comparative Study on Intercalation-Exfoliation and Thermal Activation Modified Kaolin for Heavy Metals Immobilization during High-Organic Solid Waste Pyrolysis. Chemosphere 2021, 280, 130714. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, Y.; Zhong, Z.; Yan, Y.; Niu, M.; Wang, Y. Control of Inhalable Particulate Lead Emission from Incinerator Using Kaolin in Two Addition Modes. Fuel Process. Technol. 2014, 119, 228–235. [Google Scholar] [CrossRef]
- Bury, M.; Dziok, T.; Borovec, K.; Burmistrz, P. Influence of RDF Composition on Mercury Release during Thermal Pretreatment. Energies 2023, 16, 772. [Google Scholar] [CrossRef]
- Yang, J.; Xu, H.; Zhao, Y.; Li, H.; Zhang, J. Mercury Removal from Flue Gas by Noncarbon Sorbents. Energy Fuels 2021, 35, 3581–3610. [Google Scholar] [CrossRef]
- Ishag, A.; Yue, Y.; Xiao, J.; Huang, X.; Sun, Y. Recent Advances on the Adsorption and Oxidation of Mercury from Coal-Fired Flue Gas: A Review. J. Clean. Prod. 2022, 367, 133111. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, G.; Zhang, Q.; Li, Z.; Zhang, S.; Cui, W. Cost-Effective Sulfurized Sorbents Derived from One-Step Pyrolysis of Wood and Scrap Tire for Elemental Mercury Removal from Flue Gas. Fuel 2021, 285, 119221. [Google Scholar] [CrossRef]
- Trobajo, J.R.; Antuña-Nieto, C.; Rodríguez, E.; García, R.; López-Antón, M.A.; Martínez-Tarazona, M.R. Carbon-Based Sorbents Impregnated with Iron Oxides for Removing Mercury in Energy Generation Processes. Energy 2018, 159, 648–655. [Google Scholar] [CrossRef]
- Marczak-Grzesik, M.; Piersa, P.; Karczewski, M.; Szufa, S.; Ünyay, H.; Kędzierska-Sar, A.; Bochenek, P. Modified Fly Ash-Based Adsorbents (MFA) for Mercury and Carbon Dioxide Removal from Coal-Fired Flue Gases. Energies 2021, 14, 7101. [Google Scholar] [CrossRef]
- Xing, L.; Xu, Y.; Zhong, Q. Mn and Fe Modified Fly Ash As a Superior Catalyst for Elemental Mercury Capture under Air Conditions. Energy Fuels 2012, 26, 4903–4909. [Google Scholar] [CrossRef]
- Duan, X.-L.; Yuan, C.-G.; Guo, Q.; Niu, S.-L.; He, K.-Q.; Xia, G.-W. Preparation of Halloysite Nanotubes-Encapsulated Magnetic Microspheres for Elemental Mercury Removal from Coal-Fired Flue Gas. J. Hazard. Mater. 2021, 406, 124683. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, 170, 1–114. [Google Scholar]
- Jarosz-Krzemińska, E.; Poluszyńska, J. Repurposing Fly Ash Derived from Biomass Combustion in Fluidized Bed Boilers in Large Energy Power Plants as a Mineral Soil Amendment. Energies 2020, 13, 4805. [Google Scholar] [CrossRef]
- Saletnik, B.; Zagula, G.; Bajcar, M.; Czernicka, M.; Puchalski, C. Biochar and Biomass Ash as a Soil Ameliorant: The Effect on Selected Soil Properties and Yield of Giant Miscanthus (Miscanthus × Giganteus). Energies 2018, 11, 2535. [Google Scholar] [CrossRef]
- Wei, H.; Wang, H.; Chu, H.; Li, J. Preparation and Characterization of Slow-Release and Water-Retention Fertilizer Based on Starch and Halloysite. Int. J. Biol. Macromol. 2019, 133, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Gilkes, R.J.; Prakongkep, N. How the Unique Properties of Soil Kaolin Affect the Fertility of Tropical Soils. Appl. Clay Sci. 2016, 131, 100–106. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Greinert, A.; Mrówczyńska, M.; Szefner, W. Study on the Possibilities of Natural Use of Ash Granulate Obtained from the Combustion of Pellets from Plant Biomass. Energies 2019, 12, 2569. [Google Scholar] [CrossRef]
- Rożek, P.; Florek, P.; Król, M.; Mozgawa, W. Immobilization of Heavy Metals in Boroaluminosilicate Geopolymers. Materials 2021, 14, 214. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E. Glassy Wasteforms for Nuclear Waste Immobilization. Metall. Mater. Trans. A 2011, 42, 837–851. [Google Scholar] [CrossRef]
- Wang, G.; Poulsen, J.N.F.; Poulsen, S.N.F.; Jensen, P.A.; Frandsen, F.J. Influence of Kaolin and Coal Fly Ash Addition on Biomass Ash Deposition in an Entrained Flow Reactor. Fuel 2022, 313, 123041. [Google Scholar] [CrossRef]
- Čepauskienė, D.; Vaškevičienė, I.; Praspaliauskas, M.; Pedišius, N. Comparison of the Influence of Additives on the Melting Behaviour of Wheat Straw and Fibre Hemp Ash. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Kuptz, D.; Kuchler, C.; Rist, E.; Eickenscheidt, T.; Mack, R.; Schön, C.; Drösler, M.; Hartmann, H. Combustion Behaviour and Slagging Tendencies of Pure, Blended and Kaolin Additivated Biomass Pellets from Fen Paludicultures in Two Small-Scale Boilers <30 KW. Biomass Bioenergy 2022, 164, 106532. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Zhao, C.; Li, Y.; Guo, M.; Fan, H.; Guo, Q.; Fang, Y. Influence of Additives on Potassium Retention Behaviors during Straw Combustion: A Mechanism Study. Bioresour Technol. 2020, 299, 122515. [Google Scholar] [CrossRef]
- Dal Pozzo, A.; Moricone, R.; Tugnoli, A.; Cozzani, V. Experimental Investigation of the Reactivity of Sodium Bicarbonate toward Hydrogen Chloride and Sulfur Dioxide at Low Temperatures. Ind. Eng. Chem. Res. 2019, 58, 6316–6324. [Google Scholar] [CrossRef]
- Walawska, B.; Szymanek, A.; Pajdak, A.; Nowak, M. Flue Gas Desulfurization by Mechanically and Thermally Activated Sodium Bicarbonate. Pol. J. Chem. Technol. 2014, 16, 56–62. [Google Scholar] [CrossRef]
- Krause, H.H. Corrosion by Chlorine in Wastefueled Boilers. In Proceedings of the International Conference on Fireside Problems While Incinerating Municipal and Industrial Waste, Palm Coast, FL, USA, 8–12 October 1989. [Google Scholar]
- Ma, W.; Hoffmann, G.; Schirmer, M.; Chen, G.; Rotter, V.S. Chlorine Characterization and Thermal Behavior in MSW and RDF. J. Hazard. Mater. 2010, 178, 489–498. [Google Scholar] [CrossRef]
- Becidan, M.; Sørum, L.; Frandsen, F.; Pedersen, A.J. Corrosion in Waste-Fired Boilers: A Thermodynamic Study. Fuel 2009, 88, 595–604. [Google Scholar] [CrossRef]
- Wang, L.; Skreiberg, Ø.; Becidan, M. Investigation of Additives for Preventing Ash Fouling and Sintering during Barley Straw Combustion. Appl. Therm. Eng. 2014, 70, 1262–1269. [Google Scholar] [CrossRef]
- Nowak Delgado, R.; Bieli, P.; de Riese, T.; Fendt, S.; Spliethoff, H. Influence of Additive Surface Area Degradation on Fine Particle Formation during Biomass Pulverised-Fuel Combustion. Fuel 2023, 338, 127247. [Google Scholar] [CrossRef]
- Sakiewicz, P.; Piotrowski, K.; Rajca, M.; Maj, I.; Kalisz, S.; Ober, J.; Karwot, J.; Pagilla, K. Innovative Technological Approach for the Cyclic Nutrients Adsorption by Post-Digestion Sewage Sludge-Based Ash Co-Formed with Some Nanostructural Additives under a Circular Economy Framework. Int. J. Environ. Res. Public. Health 2022, 19, 11119. [Google Scholar] [CrossRef]
- Sakiewicz, P.; Lutyński, M.; Sobieraj, J.; Piotrowski, K.; Miccio, F.; Kalisz, S. Adsorption of CO2 on In Situ Functionalized Straw Burning Ashes—An Innovative, Circular Economy-Based Concept for Limitation of Industrial-Scale Greenhouse Gas Emission. Energies 2022, 15, 1352. [Google Scholar] [CrossRef]
- Andrejkovičová, S.; Alves, C.; Velosa, A.; Rocha, F. Bentonite as a Natural Additive for Lime and Lime–Metakaolin Mortars Used for Restoration of Adobe Buildings. Cem. Concr. Compos. 2015, 60, 99–110. [Google Scholar] [CrossRef]
- Statista Statistics. Available online: www.Statista.com (accessed on 4 February 2023).
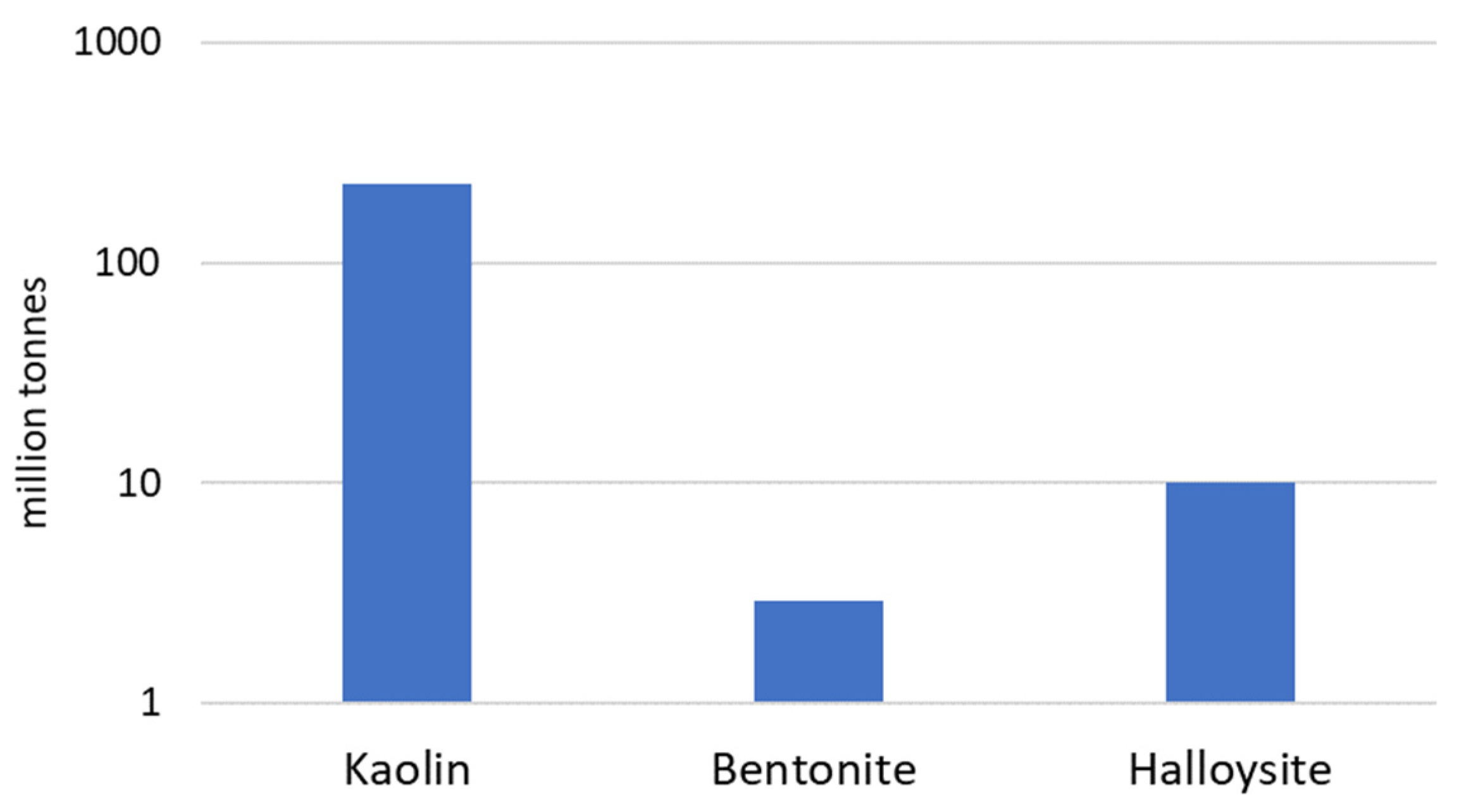
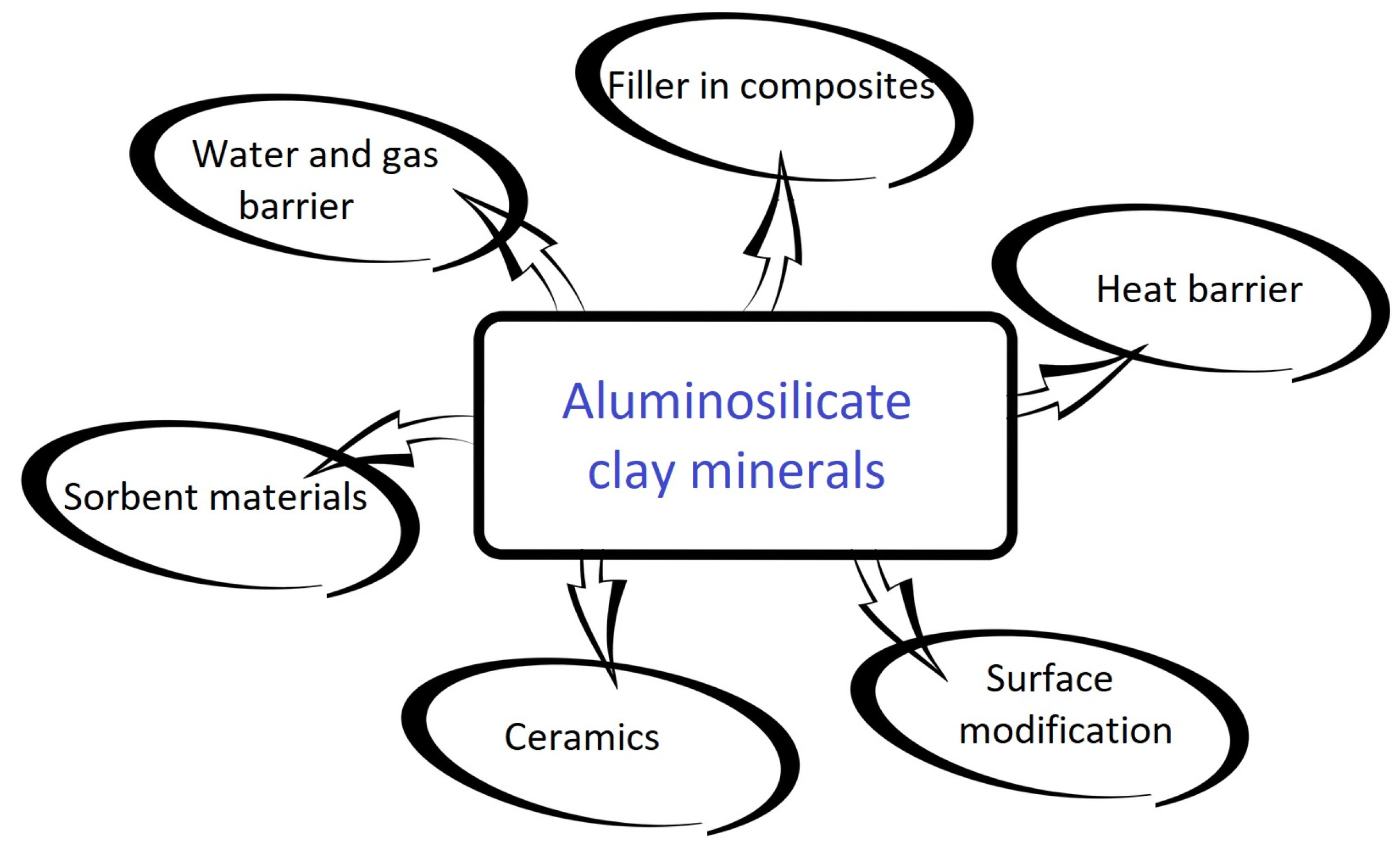
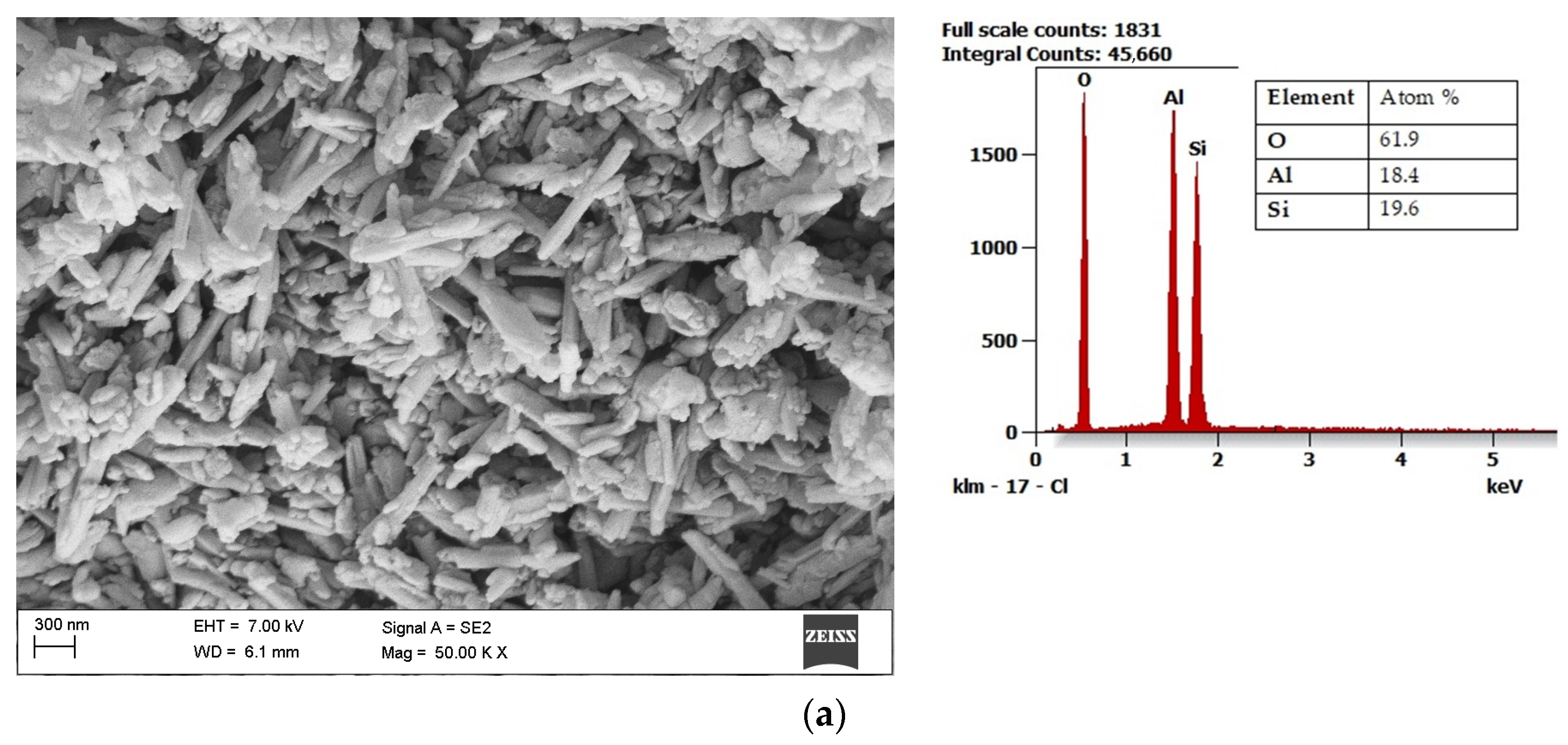

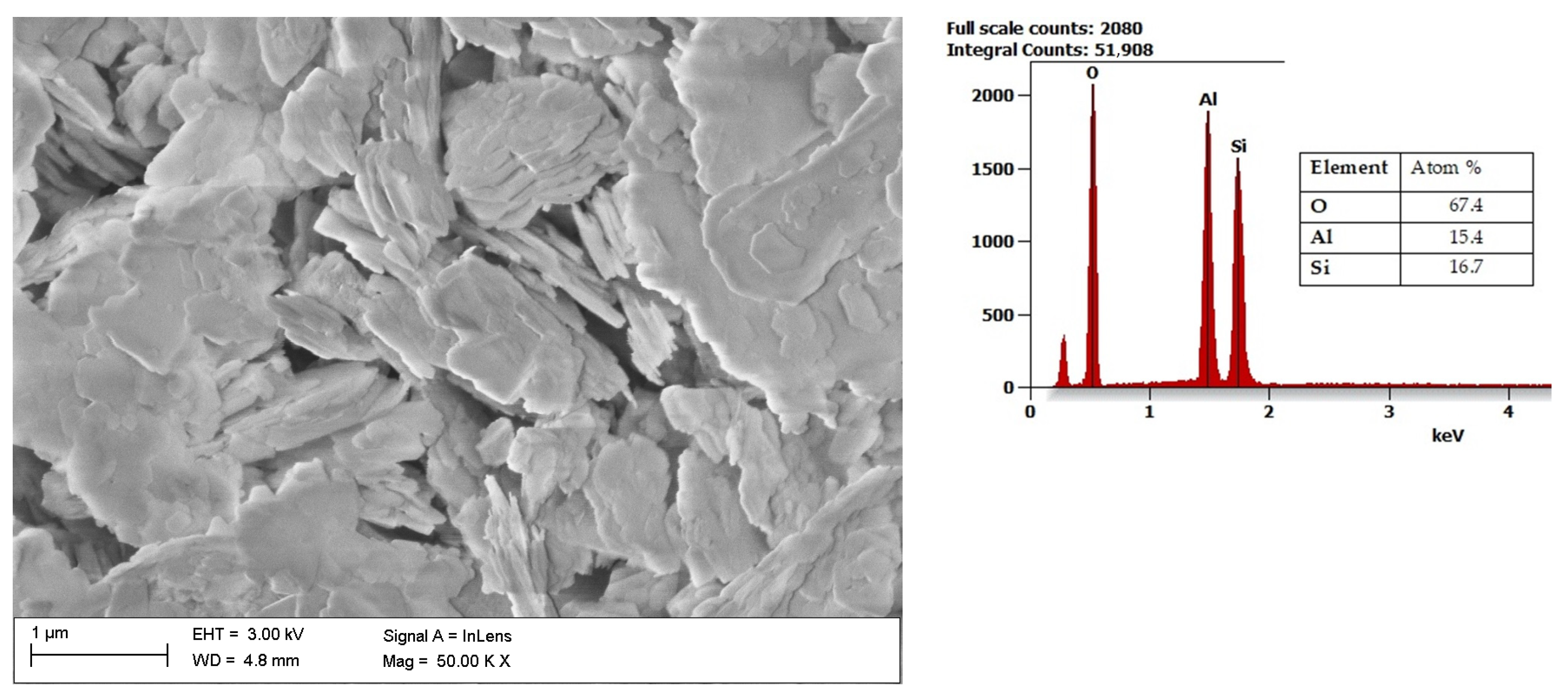
| Energy Source | Primary Energy Consumption in the World | |||
|---|---|---|---|---|
| 2015 | 2020 | |||
| EJ | % | EJ | % | |
| Fossil fuels | ||||
| Oil | 183.63 | 33.7 | 173.73 | 31.2 |
| Natural Gas | 125.22 | 23 | 137.62 | 24.7 |
| Coal | 158.64 | 29.1 | 151.42 | 27.2 |
| Total fossil | 467.49 | 85.9 | 462.77 | 83.1 |
| Non-Fossil fuels | ||||
| Nuclear | 23.46 | 4.3 | 23.98 | 4.3 |
| Hydro | 35.38 | 6.5 | 38.16 | 6.9 |
| Renewable | 18.1 | 3.3 | 31.71 | 5.7 |
| Total non-fossil | 76.94 | 14.1 | 93.85 | 16.9 |
| Total all sources | 544.43 | 100.0 | 556.62 | 100.0 |
| Fuel | Additive | AFT Change | Standard Used | Reference |
|---|---|---|---|---|
| Wheat straw | Halloysite 1 wt.% | Sintering temperature from 840 to 850 °C Softening temperature from 960 to 1080 °C Melting temperature from 1180 to 1240 °C | PN-G-04535:1982 | [75] |
| Sunflower husk | Halloysite 1 wt.% | Sintering temperature from 810 to 900 °C Softening temperature from 900 to 1070 °C Melting temperature from 1110 to 1210 °C | PN-G-04535:1982 | [75] |
| Wheat-rye straw | Halloysite 2 wt.% | Shrinkage starting temperature from 750 to 860 °C Initial deformation temperature from 960 to 1030 °C Hemispherical temperature from 1070 to 1220 °C Fluid temperature from 1170 to 1260 °C | CEN/TS 15370-1:2007 | [77] |
| Miscanthus | Halloysite 4 wt.% | Shrinkage starting temperature from 780 to 930 °C Initial deformation temperature from 940 to 1210 °C Hemispherical temperature from 1170 to 1260 °C Flow temperature from 1260 to 1270 °C | CEN/TS 15370-1:2007 | [77] |
| Olive cake | Kaolin 5 wt.% | Initial deformation temperature from 935 to 1135 °C Softening temperature from 1290 to 1445 °C Fluid temperature from 1325 to 1500 °C | ASTM D1857 | [76] |
| White wood | Kaolin 5 wt.% | Initial deformation temperature from 1075 to 927.5 °C Softening temperature from 1275 to above 1585 °C Fluid temperature from 1290 to above 1585 °C | ASTM D1857 | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maj, I.; Matus, K. Aluminosilicate Clay Minerals: Kaolin, Bentonite, and Halloysite as Fuel Additives for Thermal Conversion of Biomass and Waste. Energies 2023, 16, 4359. https://doi.org/10.3390/en16114359
Maj I, Matus K. Aluminosilicate Clay Minerals: Kaolin, Bentonite, and Halloysite as Fuel Additives for Thermal Conversion of Biomass and Waste. Energies. 2023; 16(11):4359. https://doi.org/10.3390/en16114359
Chicago/Turabian StyleMaj, Izabella, and Krzysztof Matus. 2023. "Aluminosilicate Clay Minerals: Kaolin, Bentonite, and Halloysite as Fuel Additives for Thermal Conversion of Biomass and Waste" Energies 16, no. 11: 4359. https://doi.org/10.3390/en16114359
APA StyleMaj, I., & Matus, K. (2023). Aluminosilicate Clay Minerals: Kaolin, Bentonite, and Halloysite as Fuel Additives for Thermal Conversion of Biomass and Waste. Energies, 16(11), 4359. https://doi.org/10.3390/en16114359







