Prospective of Response Surface Methodology as an Optimization Tool for Biomass Gasification Process
Abstract
1. Introduction
2. Parameters that Affect Biomass Gasification
2.1. Types of Feedstock
2.2. Biomass Size
2.3. Biomass Feeding Rate
2.4. Type of Reactor
2.5. Temperature
2.6. Pressure
2.7. Air Equivalence Ratio (ER)
2.8. Steam/Biomass Ratio
2.9. Gasification Agent
2.10. Catalyst
2.11. Residence Time
3. Parametric Studies on Biomass Gasification
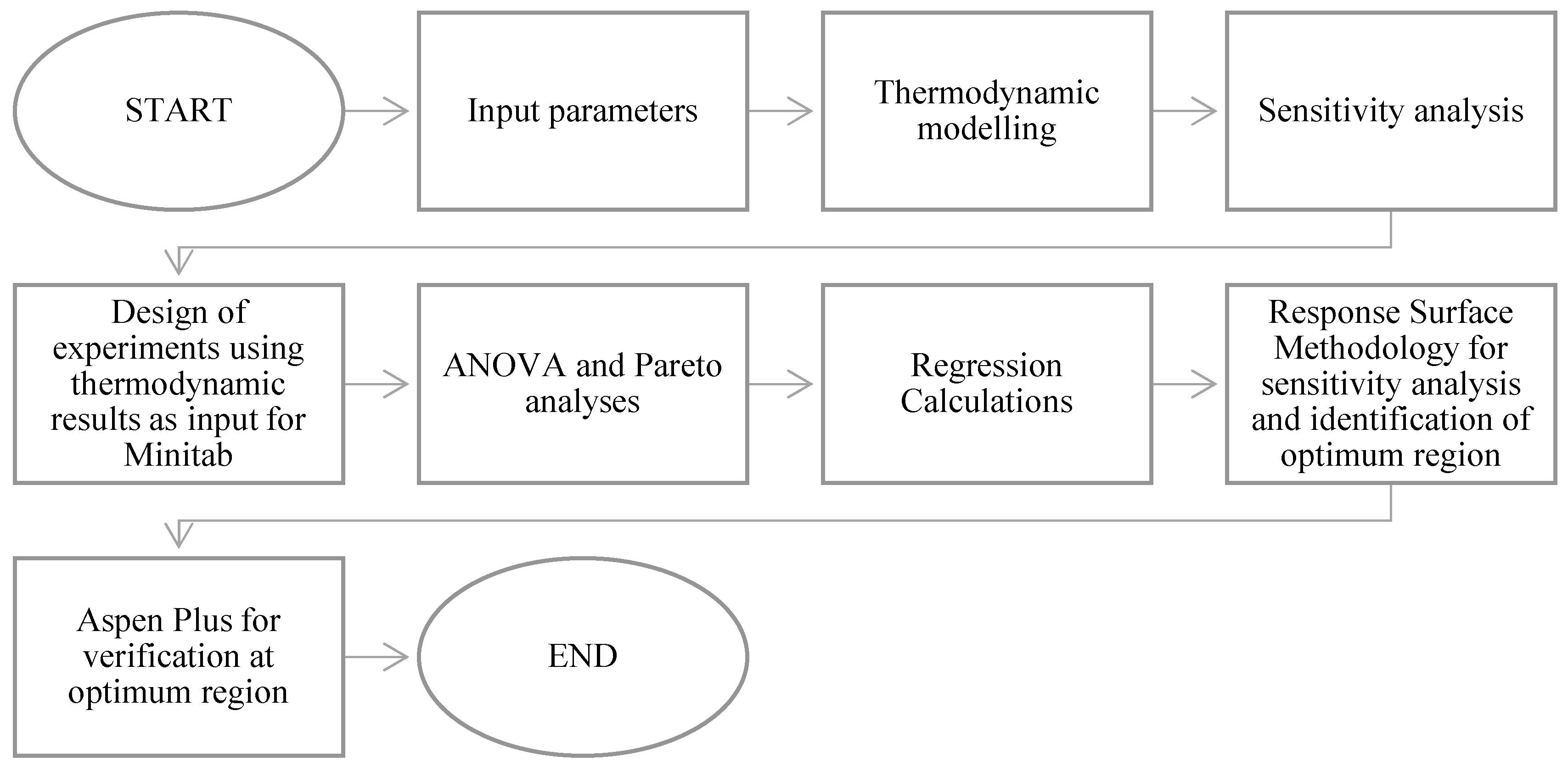
4. Optimization Using Response Surface Methodology (RSM)
5. Gaps, Challenges, and Possibilities
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- AlMallahi, M.N.; Faroukh, A.M.; Alketbi, H.H.; Inayat, A.; Rocha-Meneses, L.; Said, Z. Fast Pyrolysis Process for Bio-oil Production from Coffee Waste in the UAE. In Proceedings of the 2022 Advances in Science and Engineering Technology International Conferences (ASET), Dubai, United Arab Emirates, 21–24 February 2022; pp. 1–4. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Ferreira, J.A.; Mushtaq, M.; Karimi, S.; Orupõld, K.; Kikas, T. Genetic modification of cereal plants: A strategy to enhance bioethanol yields from agricultural waste. Ind. Crop. Prod. 2020, 150, 112408. [Google Scholar] [CrossRef]
- Calabria, U.; Tursi, A. A review on biomass: Importance, chemistry, classification, and conversion A review on biomass: Importance, chemistry, classification, and conversion. Biofuel Res. J. 2019, 6, 962. [Google Scholar] [CrossRef]
- World Bionenergy Association. Global Biomass Potential Towards 2035. World Bioenergy Assoc. Factsheet, No. March P. 6. 2016. Available online: https://www.worldbioenergy.org/uploads/Factsheet_Biomass potential.pdf (accessed on 30 June 2022).
- Ashraf, M.T.; Fang, C.; Bochenski, T.; Cybulska, I.; Alassali, A.; Sowunmi, A.; Farzanah, R.; Brudecki, G.P.; Chaturvedi, T.; Haris, S.; et al. Estimation of Bioenergy Potential for Local Biomass in the United Arab Emirates. Emir. J. Food Agric. 2020, 28, 99–106. [Google Scholar] [CrossRef]
- Bajpai, P. Biomass to Energy Conversion Technologies: The Road to Commercialization; Elsevier Science: Amsterdam, The Netherlands, 2019; Available online: https://books.google.ae/books?id=tGq4DwAAQBAJ (accessed on 30 June 2022).
- SMishra, S.; Upadhyay, R.K. Review on biomass gasification: Gasifiers, gasifying mediums, and operational parameters. Mater. Sci. Energy Technol. 2021, 4, 329–340. [Google Scholar] [CrossRef]
- Pradana, Y.S.; Budiman, A. Bio-syngas derived from Indonesian oil palm empty fruit bunch (EFB) using middle-scale gasification. J. Eng. Sci. Technol. 2015, 10, 1–8. [Google Scholar]
- Speight, J.G. Types and properties of fuels from nonfossil fuel sources. In The Refinery of the Future; Elsevier: Amsterdam, The Netherlands, 2020; pp. 469–513. [Google Scholar] [CrossRef]
- Luo, X.; Wu, T.; Shi, K.; Song, M.; Rao, Y. Biomass Gasification: An Overview of Technological Barriers and Socio-Environmental Impact. In Gasification for Low-Grade Feedstock; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Kiang, Y.-H. Other and emerging alternative energy technology. In Fuel Property Estimation and Combustion Process Characterization; Elsevier: Amsterdam, The Netherlands, 2018; pp. 363–401. [Google Scholar] [CrossRef]
- Pereira, E.G.; Martins, M.A. Gasification Technologies. In Encyclopedia of Sustainable Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 315–325. [Google Scholar] [CrossRef]
- Xu, C.; Liao, B.; Pang, S.; Nazari, L.; Mahmood, N.; Tushar, M.; Dutta, A.; Ray, M. 1.19 Biomass Energy. In Comprehensive Energy Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 770–794. [Google Scholar] [CrossRef]
- Heidenreich, S.; Müller, M.; Foscolo, P.U. Advanced Biomass Gasification: New Concepts for Efficiency Increase and Product Flexibility; Elsevier Science: Amsterdam, The Netherlands, 2016; Available online: https://books.google.ae/books?id=XwNKCgAAQBAJ (accessed on 2 July 2022).
- Basu, P. Design of Biomass Gasifiers, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2010. [Google Scholar] [CrossRef]
- AlNouss, A.; McKay, G.; Al-Ansari, T. Superstructure Optimization for the Production of Fuels, Fertilizers and Power Using Biomass Gasification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 301–306. [Google Scholar] [CrossRef]
- De, S.; Agarwal, A.K.; Moholkar, V.S.; Thallada, B. Coal and Biomass Gasification: Recent Advances and Future Challenges; Springer: Singapore, 2017; Available online: https://books.google.ae/books?id=vQlDDwAAQBAJ (accessed on 3 July 2022).
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Wang, L. Sustainable Bioenergy Production; Taylor & Francis: Abingdon, UK, 2014; Available online: https://books.google.ae/books?id=izoyAwAAQBAJ (accessed on 27 July 2022).
- Chuayboon, S.; Abanades, S.; Rodat, S. Analysis of process parameters influence on syngas yields and biomass gasification rates in a continuous particle-fed solar-irradiated gasifier. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2020; Volume 2303. [Google Scholar] [CrossRef]
- Bressanin, J.M.; Klein, B.C.; Chagas, M.F.; Watanabe, M.D.B.; Sampaio, I.L.D.M.; Bonomi, A.; De Morais, E.R.; Cavalett, O. Techno-Economic and Environmental Assessment of Biomass Gasification and Fischer–Tropsch Synthesis Integrated to Sugarcane Biorefineries. Energies 2020, 13, 4576. [Google Scholar] [CrossRef]
- Asadullah, M. Biomass gasification gas cleaning for downstream applications: A comparative critical review. Renew. Sustain. Energy Rev. 2014, 40, 118–132. [Google Scholar] [CrossRef]
- Breeze, P. Fluidized Bed Combustion and Coal Gasification. In Coal-Fired Generation; Academic Press: Cambridge, MA, USA, 2015; pp. 41–52. [Google Scholar] [CrossRef]
- Dutta, A.; Acharya, B. Production of Bio-Syngas and Biohydrogen via Gasification; Woodhead Publishing Limited: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Bermudez, J.M.; Fidalgo, B. Production of bio-syngas and bio-hydrogen via gasification. In Handbook of Biofuels Production; Elsevier: Amsterdam, The Netherlands, 2016; pp. 431–494. [Google Scholar] [CrossRef]
- Jamin, N.A.; Saleh, S.; Samad, N.A.F.A. Influences of gasification temperature and equivalence ratio on fluidized bed gasification of raw and torrefied wood wastes. Chem. Eng. Trans. 2020, 80, 127–132. [Google Scholar] [CrossRef]
- Wongsiriamnuay, T.; Kannang, N.; Tippayawong, N. Effect of Operating Conditions on Catalytic Gasification of Bamboo in a Fluidized Bed. Int. J. Chem. Eng. 2013, 2013. [Google Scholar] [CrossRef]
- Higman, C.; van der Burgt, M. Gasification; Elsevier Science: Amsterdam, The Netherlands, 2011; Available online: https://books.google.ae/books?id=IjlMBi%5C_Q6kIC (accessed on 15 November 2022).
- Valin, S.; Ravel, S.; Guillaudeau, J.; Thiery, S. Comprehensive study of the influence of total pressure on products yields in fluidized bed gasification of wood sawdust. Fuel Process. Technol. 2010, 91, 1222–1228. [Google Scholar] [CrossRef]
- Timofeeva, S.; Ermolaev, D. Study of the Effect of Gasification Pressure on the Composition of the Producer Gas From Coal. IOP Conf. Ser. Earth Environ. Sci. 2022, 988, 032043. [Google Scholar] [CrossRef]
- Motta, I.L.; Miranda, N.T.; Filho, R.M.; Maciel, M.R.W. Biomass gasification in fluidized beds: A review of biomass moisture content and operating pressure effects. Renew. Sustain. Energy Rev. 2018, 94, 998–1023. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M. Biomass Gasification. In Encyclopedia of Sustainable Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 205–216. [Google Scholar] [CrossRef]
- Mai, T.P.; Nguyen, D.Q. Gasification of Biomass. In Biotechnological Applications of Biomass; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Ciuta, S.; Tsiamis, D.; Castaldi, M.J. Gasification of Waste Materials: Technologies for Generating Energy, Gas, and Chemicals from Municipal Solid Waste, Biomass, Nonrecycled Plastics, Sludges, and Wet Solid Wastes; Elsevier Science: Amsterdam, The Netherlands, 2017; Available online: https://books.google.ae/books?id=mKnRDgAAQBAJ (accessed on 8 July 2022).
- Narnaware, S.L.; Panwar, N.L. Catalysts and Their Role in Biomass Gasification and Tar Abetment: A Review; no. October; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Agu, C.E.; Pfeifer, C.; Eikeland, M.; Tokheim, L.-A.; Moldestad, B.M. Measurement and characterization of biomass mean residence time in an air-blown bubbling fluidized bed gasification reactor. Fuel 2019, 253, 1414–1423. [Google Scholar] [CrossRef]
- Inayat, A.; Ahmad, M.M.; Yusup, S.; Mutalib, M.I.A. Biomass Steam Gasification with In-Situ CO2 Capture for Enriched Hydrogen Gas Production: A Reaction Kinetics Modelling Approach. Energies 2010, 3, 1472–1484. [Google Scholar] [CrossRef]
- Ghassemi, H.; Shahsavan-Markadeh, R. Effects of various operational parameters on biomass gasification process; a modified equilibrium model. Energy Convers. Manag. 2014, 79, 18–24. [Google Scholar] [CrossRef]
- Ibrahim, S.M. Syngas Compositions, Cold Gas and Carbon Conversion Efficiencies for Different Coal Gasification Processes and all Coal Ranks. J. Min. Mech. Eng. 2020, 1, 59–72. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Patil, K.N.; Bellmer, D.D.; Wang, D.; Yuan, W.; Huhnke, R.L. Effects of Biomass Feedstocks and Gasification Conditions on the Physiochemical Properties of Char. Energies 2013, 6, 3972–3986. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S.; Rodat, S.; Boujjat, H. Experimental assessment of biomass feedstock gasification in a high-temperature continuous solar gasifier. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2126. [Google Scholar] [CrossRef]
- Hernández, J.J.; Aranda-Almansa, G.; Bula, A. Gasification of biomass wastes in an entrained flow gasifier: Effect of the particle size and the residence time. Fuel Process. Technol. 2010, 91, 681–692. [Google Scholar] [CrossRef]
- Szul, M.; Głód, K.; Iluk, T. Influence of pressure and CO2 in fluidized bed gasification of waste biomasses. Biomass Convers. Biorefin. 2021, 11, 69–81. [Google Scholar] [CrossRef]
- Huang, F.-X.; Jin, S.-P.; Lin, Y.-X.; Shu, Z.-H.; Li, S. The Effect of Different Operational Parameters on Biomass(The Pine Wood) Gasification. E3S Web Conf. 2018, 53, 03005. [Google Scholar] [CrossRef]
- Rupesh, S.; Muraleedharan, C.; Arun, P. Influence of Residence Time on Syngas Composition in CaO Enhanced Air–Steam Gasification of Biomass. Environ. Dev. Sustain. 2022, 24, 8363–8377. [Google Scholar] [CrossRef]
- James, A.M.; Yuan, W.; Boyette, M.D.; Wang, D. The Effect of Air Flow Rate and Biomass Type on the Performance of an Updraft Biomass Gasifier. Bioresources 2015, 10, 3615–3624. [Google Scholar] [CrossRef]
- Mahallati, M.N. Advances in modeling saffron growth and development at different scales. In Saffron; Elsevier: Amsterdam, The Netherlands, 2020; pp. 139–167. [Google Scholar] [CrossRef]
- Khedmati, M.; Khodaii, A.; Haghshenas, H. A study on moisture susceptibility of stone matrix warm mix asphalt. Constr. Build. Mater. 2017, 144, 42–49. [Google Scholar] [CrossRef]
- Karimifard, S.; Moghaddam, M.R.A. Application of response surface methodology in physicochemical removal of dyes from wastewater: A critical review. Sci. Total Environ. 2018, 640–641, 772–797. [Google Scholar] [CrossRef]
- Zaman, S.A.; Roy, D.; Ghosh, S. Process modeling and optimization for biomass steam-gasification employing response surface methodology. Biomass-Bioenergy 2020, 143, 105847. [Google Scholar] [CrossRef]
- Kumar, M.S. Analysis of Process Parameters on the Biomass Gasification Using Response Surface Methodology. Int. J. Eng. Trends Technol. 2018, 65, 155–162. [Google Scholar] [CrossRef]
- Halim, N.H.A.; Saleh, S.; Samad, N.A.F.A. Optimization of oil palm empty fruit bunch gasification temperature and steam to biomass ratio using response surface methodology. IOP Conf. Ser. Mater. Sci. Eng. 2019, 702, 012006. [Google Scholar] [CrossRef]
- Kombe, E.Y.; Lang’At, N.; Njogu, P.; Malessa, R.; Weber, C.-T.; Njoka, F.; Krause, U. Numerical investigation of sugarcane bagasse gasification using Aspen Plus and response surface methodology. Energy Convers. Manag. 2022, 254, 115198. [Google Scholar] [CrossRef]
- Singh, D.K.; Tirkey, J.V. Modeling and multi-objective optimization of variable air gasification performance parameters using Syzygium cumini biomass by integrating ASPEN Plus with Response surface methodology (RSM). Int. J. Hydrogen Energy 2021, 46, 18816–18831. [Google Scholar] [CrossRef]
- Silva, V.; Rouboa, A. Optimizing the gasification operating conditions of forest residues by coupling a two-stage equilibrium model with a response surface methodology. Fuel Process. Technol. 2014, 122, 163–169. [Google Scholar] [CrossRef]
- Seçer, A.; Hasanoğlu, A. Evaluation of the effects of process parameters on co–gasification of Çan lignite and sorghum biomass with response surface methodology: An optimization study for high yield hydrogen production. Fuel 2020, 259, 116230. [Google Scholar] [CrossRef]
- Yahaya, A.Z.; Somalu, M.R.; Muchtar, A.; Sulaiman, S.A.; Daud, W.R.W. Effect of particle size and temperature on gasification performance of coconut and palm kernel shells in downdraft fixed-bed reactor. Energy 2019, 175, 931–940. [Google Scholar] [CrossRef]
- Umar, H.A.; Sulaiman, S.A.; Said, M.A.M.; Gungor, A. Use of Response Surface Methodology to Measure the Impact of Operating Variables on the Co-gasification of Oil Palm Biomass. J. Hunan 2021. Available online: http://jonuns.com/index.php/journal/article/view/561%0Ahttp://jonuns.com/index.php/journal/article/download/561/558 (accessed on 18 August 2022).
- Inayat, M.; Sulaiman, S.A.; Kurnia, J.C. Catalytic co-gasification of coconut shells and oil palm fronds blends in the presence of cement, dolomite, and limestone: Parametric optimization via Box Behnken Design. J. Energy Inst. 2019, 92, 871–882. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Khan, Z.; Yusup, S.; Aslam, M.; Inayat, A.; Shahbaz, M.; Raza Naqvi, S.; Farooq, R.; Watson, I. NO and SO2 emissions in palm kernel shell catalytic steam gasification with in-situ CO2 adsorption for hydrogen production in a pilot-scale fluidized bed gasification system. J. Clean. Prod. 2019, 236, 117636. [Google Scholar] [CrossRef]
- Inayat, A.; Ahmad, M.M.; Mutalib, M.I.A.; Yusup, S. Flowsheet development and modeling of hydrogen production from Empty Fruit Bunch via steam gasification. Chem. Eng. Trans. 2010, 21, 427–432. [Google Scholar]
- Jamil, F.; Aslam, M.; Al-Muhtaseb, A.A.H.; Bokhari, A.; Rafiq, S.; Khan, Z.; Inayat, A.; Ahmed, A.; Hossain, S.; Khurram, M.S.; et al. Greener and sustainable production of bioethylene from bioethanol: Current status, opportunities and perspectives. Rev. Chem. Eng. 2022, 38, 185–207. [Google Scholar] [CrossRef]
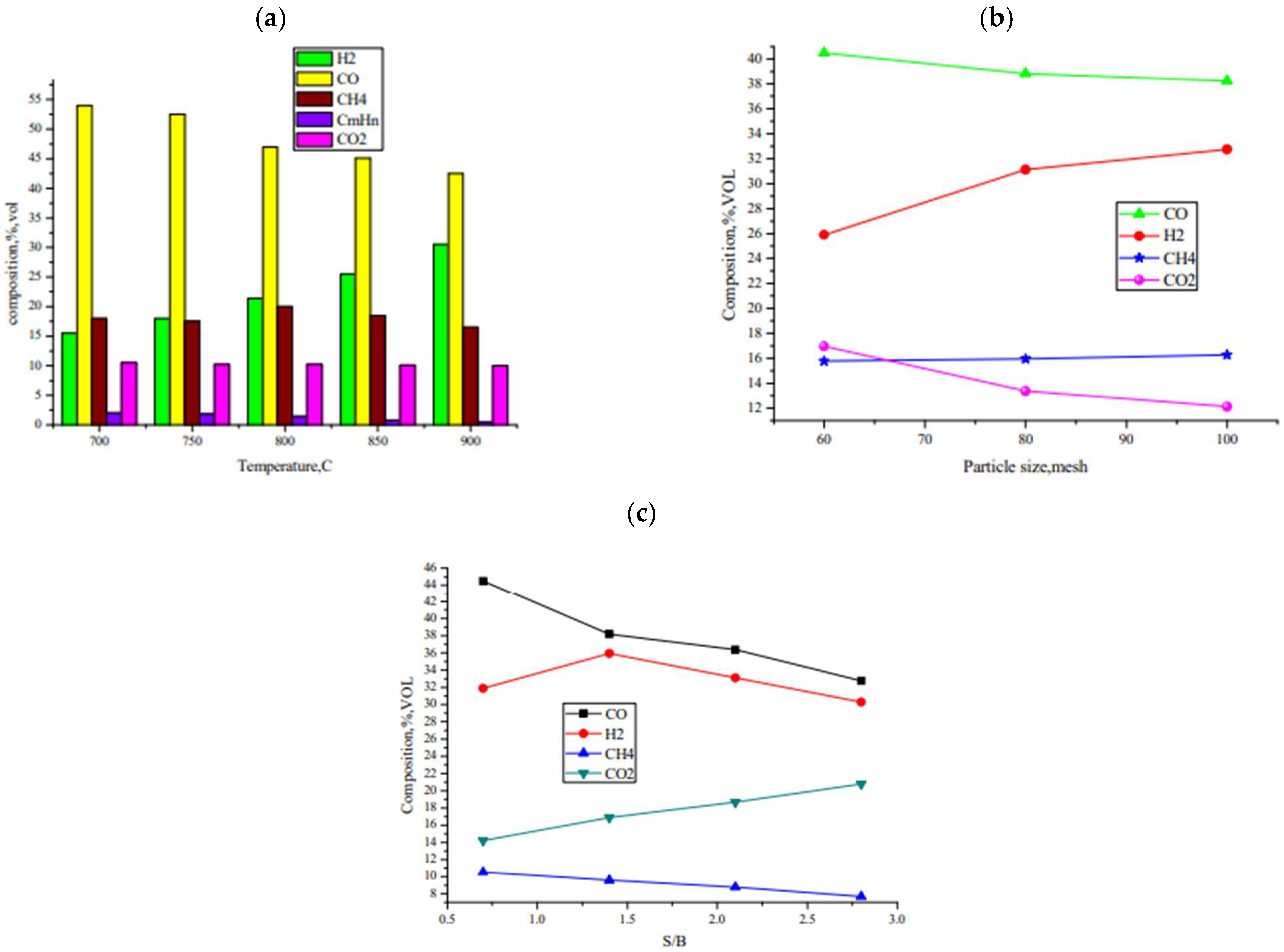



| Type of Feedstock | Reactor | Parameters Studied | Affected Parameters Studied | Reference |
|---|---|---|---|---|
| Wood | - |
|
| [37] |
| Pine saw dust | - |
|
| [38] |
| Bamboo | Fluidized bed reactor |
|
| [27] |
| Switchgrass/sorghum straw/red cedar | Fluidized bed reactor |
|
| [40] |
| Beech wood/mix of pine and spruce wood | Solar biomass gasifier |
|
| [41] |
| Grapevine pruning/sawdust wastes/marc of grape/blend of coal–coke | Entrained flow gasifier |
|
| [42] |
| Bark/lignin/softwood pellet (for reference) | Autothermal fluidized bed reactor |
|
| [43] |
| Pine wood chips | Downdraft gasifier |
|
| [44] |
| - | - |
|
| [45] |
| Prairie hay, sorghum biomass, wood chips | Updraft gasifier |
|
| [46] |
| Type of Biomass | Reactor | Type of Study | Parameters Studied | Range | Optimum Parameters | Optimum Production | References |
|---|---|---|---|---|---|---|---|
| Rice husk | RGIBBS | Modelling |
|
|
|
| [50] |
| Wood powder | Updraft fluidized bed reactor | Experimental and Modelling |
|
|
|
| [51] |
| Empty fruit bunch | Bubbling fluidized bed | Experimental and Modelling |
|
|
|
| [52] |
| Sugarcane bagasse | Fixed bed downdraft gasifier | Modelling |
|
|
|
| [53] |
| Syzygium cumini | Downdraft gasifier | Modelling |
|
|
|
| [54] |
| Forest residues | Up-flow fluidized bed gasifier | Experimental and Modelling |
|
|
|
| [55] |
| Çan lignite and sorghum biomass with coal | Fixed bed | Experimental and Modelling |
|
|
|
| [56] |
| Coconut and palm kernel shells | Downdraft fixed bed reactor | Experimental and Modelling |
|
|
|
| [57] |
| Oil palm trunks and fronds | Downdraft gasifier | Experimental and Modelling |
|
|
|
| [58] |
| Coconut shell and oil palm frond blends | Downdraft gasifier | Experimental and Modelling |
|
|
|
| [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asaad, S.M.; Inayat, A.; Rocha-Meneses, L.; Jamil, F.; Ghenai, C.; Shanableh, A. Prospective of Response Surface Methodology as an Optimization Tool for Biomass Gasification Process. Energies 2023, 16, 40. https://doi.org/10.3390/en16010040
Asaad SM, Inayat A, Rocha-Meneses L, Jamil F, Ghenai C, Shanableh A. Prospective of Response Surface Methodology as an Optimization Tool for Biomass Gasification Process. Energies. 2023; 16(1):40. https://doi.org/10.3390/en16010040
Chicago/Turabian StyleAsaad, Sara Maen, Abrar Inayat, Lisandra Rocha-Meneses, Farrukh Jamil, Chaouki Ghenai, and Abdallah Shanableh. 2023. "Prospective of Response Surface Methodology as an Optimization Tool for Biomass Gasification Process" Energies 16, no. 1: 40. https://doi.org/10.3390/en16010040
APA StyleAsaad, S. M., Inayat, A., Rocha-Meneses, L., Jamil, F., Ghenai, C., & Shanableh, A. (2023). Prospective of Response Surface Methodology as an Optimization Tool for Biomass Gasification Process. Energies, 16(1), 40. https://doi.org/10.3390/en16010040









