FTIR Analysis of Changes in Chipboard Properties after Pretreatment with Pleurotus ostreatus (Jacq.) P. Kumm
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Pretreatment
2.2. Research Procedure
2.3. Indexes for Evaluation of Pretreatment
2.4. Selection of Indexes
2.5. Statistical Analysis
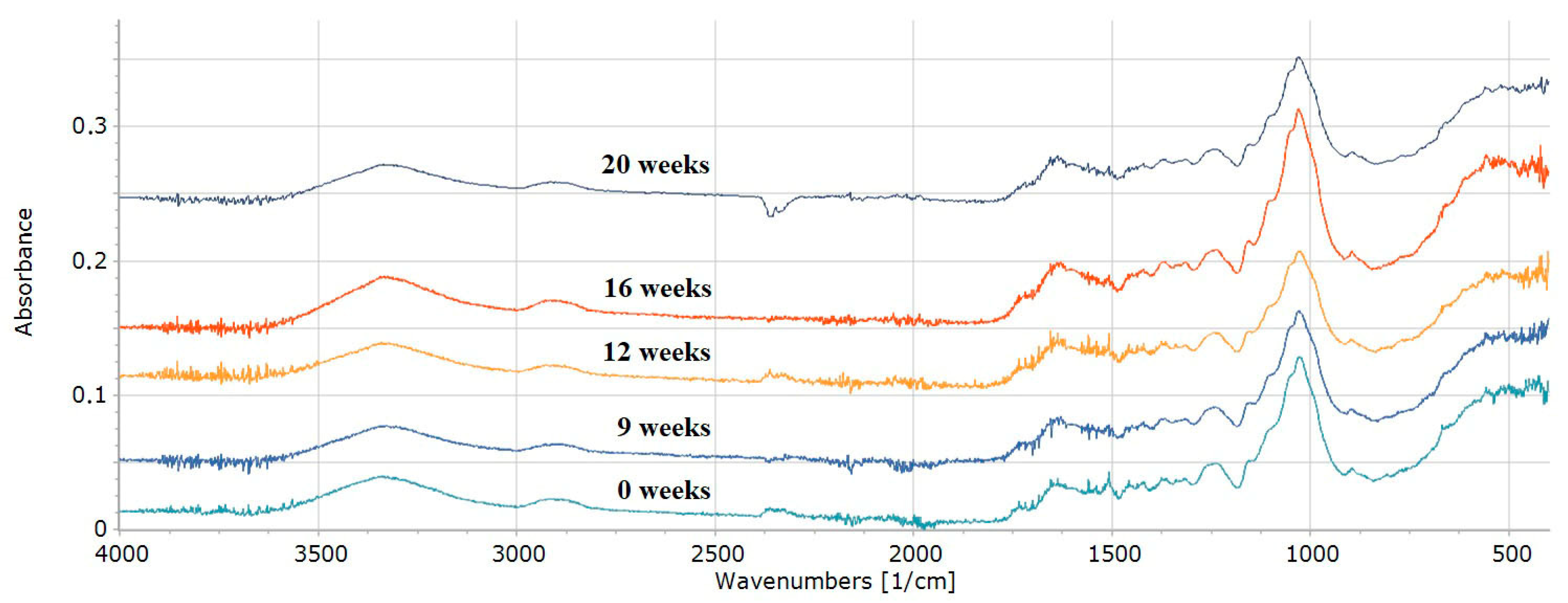
3. Results and Discussion
3.1. Delignification
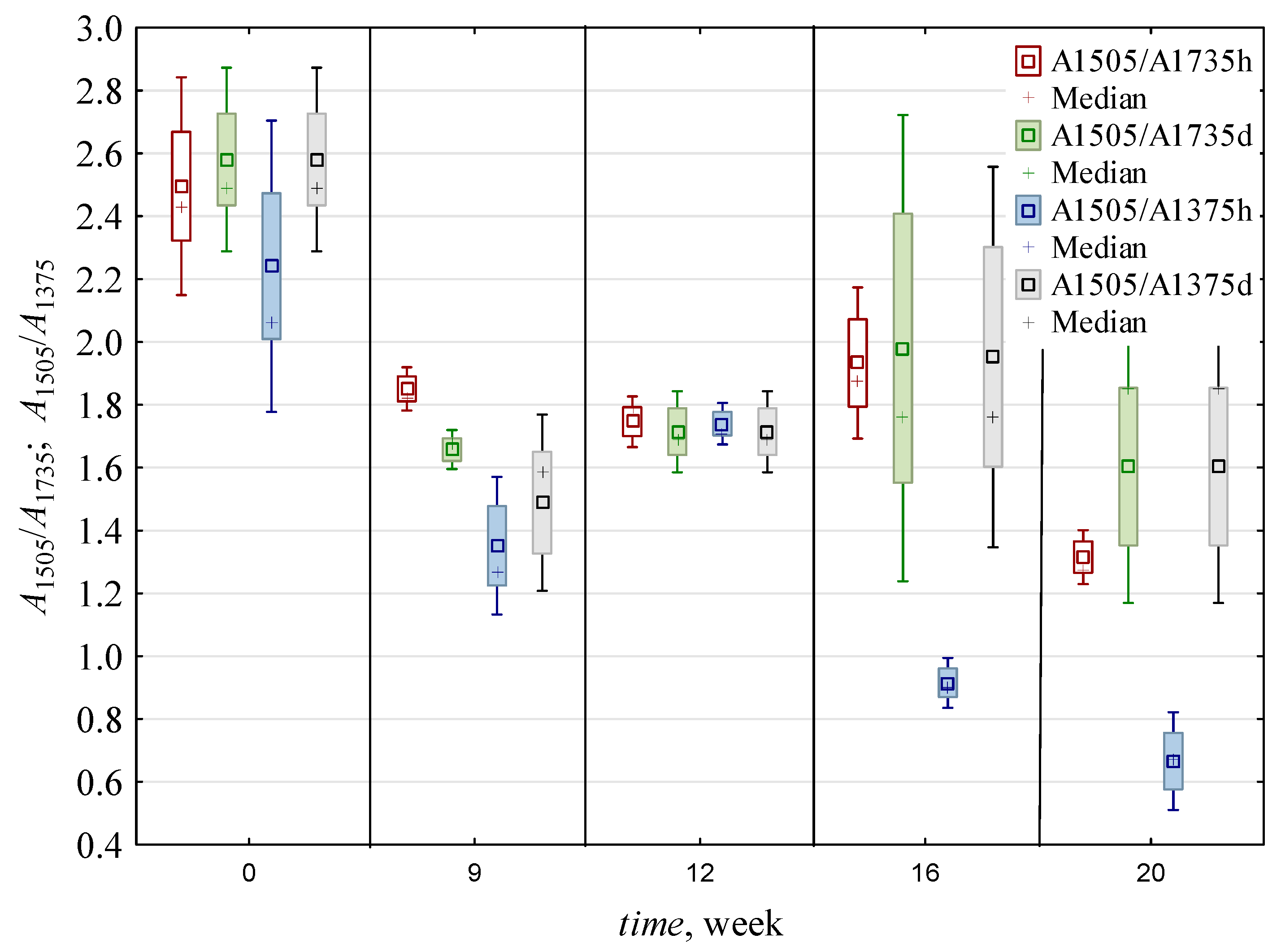
3.2. Delignification Based on Comparison with Polysaccharides
3.2.1. Delignification Based on Comparison with Hemicelluloses
3.2.2. Delignification Based on Comparison with Holocellulose
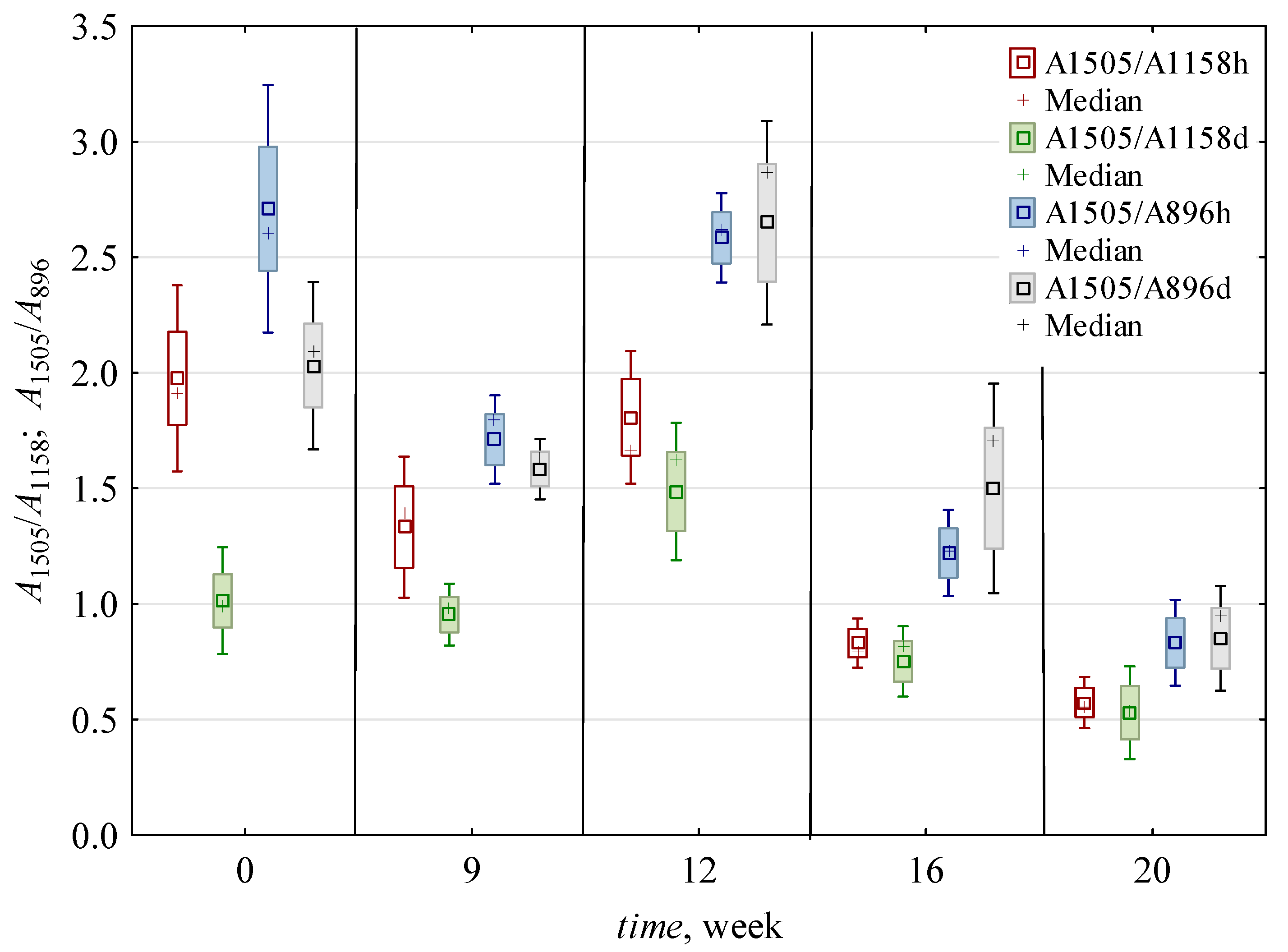
3.2.3. Delignification Based on Comparison with Cellulose
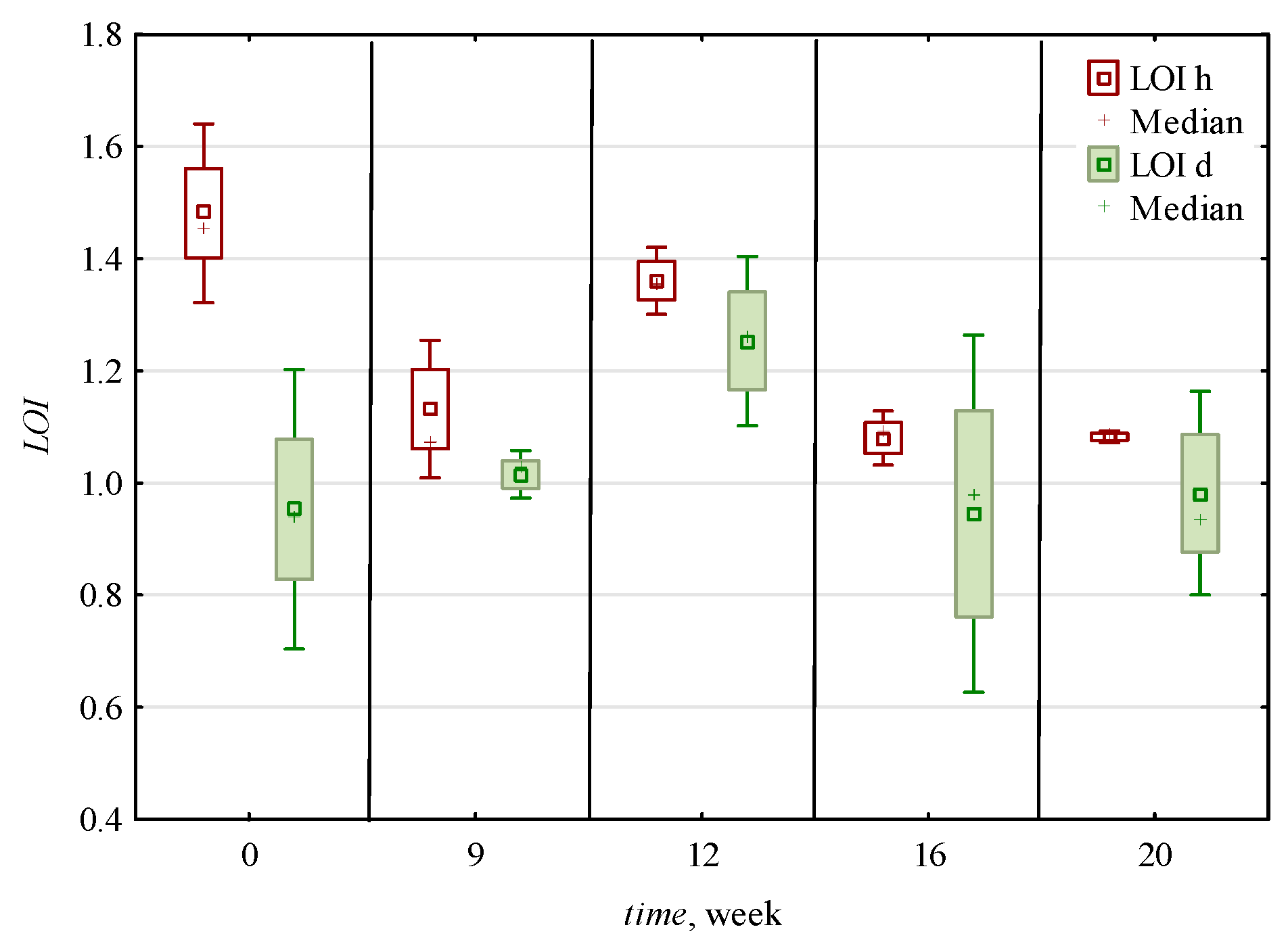
3.3. Changes in Indexes of Crystallinity
Indexes of Crystallinity
3.4. Results of Feature Correlation Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- CSO. Statistical Yearbook; CSO: Cork, Ireland, 2019. [Google Scholar]
- Nemli, G.; Öztürk, I. Influences of some factors on the formaldehyde content of particleboard. Build. Environ. 2006, 41, 770–774. [Google Scholar] [CrossRef]
- Szadkowska, D. Suitability of biomass from waste wood composites for liquid biofuel production Możliwość wykorzystania biomasy poużytkowych tworzyw drzewnych w technologii ciekłych biopaliw. Przem. Chem. 2015, 1, 74–76. [Google Scholar] [CrossRef]
- Althuri, A.; Gujjala, L.K.S.; Banerjee, R. Partially consolidated bioprocessing of mixed lignocellulosic feedstocks for ethanol production. Bioresour. Technol. 2017, 245, 530–539. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Zhu, J.Y.; Ralph, S.A. Enzymatic hydrolysis of biomass: Effects of crystallinity, particle size, and lignin removal. In Proceedings of the 16th International Symposium on Wood, Fiber, and Pulping Chemistry—Proceedings, ISWFPC, Tianjin, China, 8–10 June 2011; Volume 2, pp. 908–912. [Google Scholar]
- Yoshida, M.; Liu, Y.; Uchida, S.; Kawarada, K.; Ukagami, Y.; Ichinose, H.; Kaneko, S.; Fukuda, K. Effects of cellulose crystallinity, hemicellulose, and lignin on the enzymatic hydrolysis of Miscanthus sinensis to monosaccharides. Biosci. Biotechnol. Biochem. 2008, 72, 805–810. [Google Scholar] [CrossRef]
- Ghasemzadeh, R.; Mosavian, M.T.H.; Karimi, A. Analysis of biological pretreatment of rapeseed straw with white rot fungi for enzymatic hydrolysis. Maderas Cienc. Tecnol. 2018, 20, 725–736. [Google Scholar] [CrossRef]
- Phongpreecha, T.; Christy, K.F.; Singh, S.K.; Hao, P.; Hodge, D.B. Effect of catalyst and reaction conditions on aromatic monomer yields, product distribution, and sugar yields during lignin hydrogenolysis of silver birch wood. Bioresour. Technol. 2020, 316, 123907. [Google Scholar] [CrossRef] [PubMed]
- Torr, K.M.; Love, K.T.; Simmons, B.A.; Hill, S.J. Structural features affecting the enzymatic digestibility of pine wood pretreated with ionic liquids. Biotechnol. Bioeng. 2016, 113, 540–549. [Google Scholar] [CrossRef]
- Studer, M.H.; DeMartini, J.D.; Davis, M.F.; Sykes, R.W.; Davison, B.; Keller, M.; Tuskan, G.A.; Wyman, C.E. Lignin content in natural populus variants affects sugar release. Proc. Natl. Acad. Sci. USA 2011, 108, 6300–6305. [Google Scholar] [CrossRef]
- Ohlsson, J.A.; Hallingbäck, H.R.; Jebrane, M.; Harman-Ware, A.E.; Shollenberger, T.; Decker, S.R.; Sandgren, M.; Rönnberg-Wästljung, A.C. Genetic variation of biomass recalcitrance in a natural Salix viminalis (L.) population. Biotechnol. Biofuels 2019, 12, 135. [Google Scholar] [CrossRef]
- Healey, A.L.; Lupoi, J.S.; Lee, D.J.; Sykes, R.W.; Guenther, J.M.; Tran, K.; Decker, S.R.; Singh, S.; Simmons, B.A.; Henry, R.J. Effect of aging on lignin content, composition and enzymatic saccharification in Corymbia hybrids and parental taxa between years 9 and 12. Biomass Bioenergy 2016, 93, 50–59. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Sun, W.; Li, X.; Wang, F.; Zhao, J.; Qu, Y. Differences in the adsorption of enzymes onto lignins from diverse types of lignocellulosic biomass and the underlying mechanism. Biotechnol. Biofuels 2014, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Pimienta, J.A.P.; Papa, G.; Rodriguez, A.; Barcelos, C.A.; Liang, L.; Stavila, V.; Sanchez, A.; Gladden, J.M.; Simmons, B.A. Pilot-scale hydrothermal pretreatment and optimized saccharification enables bisabolene production from multiple feedstocks. Green Chem. 2019, 21, 3152–3164. [Google Scholar] [CrossRef]
- Xu, J.K.; Sun, Y.C.; Sun, R.C. Synergistic effects of ionic liquid plus alkaline pretreatments on eucalyptus: Lignin structure and cellulose hydrolysis. Process Biochem. 2015, 50, 955–965. [Google Scholar] [CrossRef]
- Govender, M.; Bush, T.; Spark, A.; Bose, S.K.; Francis, R.C. An accurate and non-labor intensive method for the determination of syringyl to guaiacyl ratio in lignin. Bioresour. Technol. 2009, 100, 5834–5839. [Google Scholar] [CrossRef]
- Sequeiros, A.; Labidi, J. Characterization and determination of the S/G ratio via Py-GC/MS of agricultural and industrial residues. Ind. Crops Prod. 2017, 97, 469–476. [Google Scholar] [CrossRef]
- Ding, D.; Zhou, X.; Ji, Z.; You, T.; Xu, F. How Does Hemicelluloses Removal Alter Plant Cell Wall Nanoscale Architecture and Correlate with Enzymatic Digestibility? Bioenergy Res. 2016, 9, 601–609. [Google Scholar] [CrossRef]
- Ding, C.; Wang, X.; Li, M. Evaluation of six white-rot fungal pretreatments on corn stover for the production of cellulolytic and ligninolytic enzymes, reducing sugars, and ethanol. Appl. Microbiol. Biotechnol. 2019, 103, 5641–5652. [Google Scholar] [CrossRef] [PubMed]
- Sahuand, S.; Pramanik, K. Evaluating fungal mixed culture for pretreatment of cotton gin waste to bioethanol by enzymatic hydrolysis and fermentation using co-culture. Polish J. Environ. Stud. 2017, 26, 1215–1223. [Google Scholar] [CrossRef]
- Singh, T.; Vaidya, A.A.; Donaldson, L.A.; Singh, A.P. Improvement in the enzymatic hydrolysis of biofuel substrate by a combined thermochemical and fungal pretreatment. Wood Sci. Technol. 2016, 50, 1003–1014. [Google Scholar] [CrossRef]
- Pandey, K.K.; Pitman, A.J. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Li, M.; Marek, S.M.; Peng, J.; Liu, Z.; Wilkins, M.R. Effect of moisture content and inoculum size on cell wall composition and ethanol yield from switchgrass after solid-state Pleurotus ostreatus treatment. Trans. ASABE 2018, 61, 1997–2006. [Google Scholar] [CrossRef]
- Schmidt, O. Fundamentals of Mold Growth in Indoor Environments and Strategies for Healthy Living; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 9789086867226. [Google Scholar]
- Vaverková, M.D.; Adamcová, D.; Radziemska, M.; Voběrková, S.; Mazur, Z.; Zloch, J. Assessment and Evaluation of Heavy Metals Removal from Landfill Leachate by Pleurotus ostreatus. Waste Biomass Valorization 2018, 9, 503–511. [Google Scholar] [CrossRef]
- Pozdniakova, N.N.; Nikitina, V.E.; Turkovskaia, O.V. Bioremediation of oil-polluted soil with an association including the fungus Pleurotus ostreatus and soil microflora. Prikl. Biokhim. Mikrobiol. 2008, 44, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.N. Microbial Degradation of Synthetic Dyes in Wastewaters; Environmental Science and Engineering; Sub Series Environmental Science; Springer: Berlin/Heidelberg, Germany, 2015; pp. iii–iv. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Elkenawy, N.M.; Yassin, A.S.; Amin, M.A. Laccase production by Pleurotus ostreatus and its application in synthesis of gold nanoparticles. Biotechnol. Rep. 2015, 5, 31–39. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, H.; Meng, F. Pleurotus ostreatus decreases cornstalk lignin content, potentially improving its suitability for animal feed. J. Sci. Food Agric. 2017, 97, 1592–1598. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar] [CrossRef]
- Fabiyi, J.S.; McDonald, A.G.; Morrell, J.J.; Freitag, C. Effects of wood species on durability and chemical changes of fungal decayed wood plastic composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 501–510. [Google Scholar] [CrossRef]
- Harrington, K.J.; Higgins, H.G.; Michell, A.J. Infrared Spectra of Eucalyptus regnans F. Muell. and Pinus radiata D. Don. Holzforschung 1964, 18, 108–113. [Google Scholar] [CrossRef]
- Zhong, R.; Gu, J.; Gao, Z.; Tu, D.; Hu, C. Impacts of urea-formaldehyde resin residue on recycling and reconstitution of wood-based panels. Int. J. Adhes. Adhes. 2017, 78, 60–66. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, Y.; Ma, F.; Zhang, X.; Yu, H. Effect of biopretreatment on thermogravimetric and chemical characteristics of corn stover by different white-rot fungi. Bioresour. Technol. 2010, 101, 5475–5479. [Google Scholar] [CrossRef]
- O’Connor, R.T.; Dupré, E.F.; Mitcham, D. Applications of Infrared Absorption Spectroscopy to Investigations of Cotton and Modified Cottons:Part I: Physical and Crystalline Modifications and Oxidation. Text. Res. J. 1958, 28, 382–392. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A critical review of analytical methods in pretreatment of lignocelluloses: Composition, imaging, and crystallinity. Bioresour. Technol. 2016, 200, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Q.; Yang, J.S.; Zhu, N.; Wang, E.T.; Yuan, H.L. Sugarcane bagasse degradation and characterization of three white-rot fungi. Bioresour. Technol. 2013, 131, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Miller, G. The magical number seven, plus or minus two: Some limits on our capacity for processing information. Psychol. Rev. 1994, 101, 343–352. [Google Scholar] [CrossRef]
- Hopkins, W.G. A New View of Statistics. Sportscience 2000, 1–7. Available online: https://www.sportsci.org/resource/stats/newview.html (accessed on 29 October 2022).
- Huang, X.; Korányi, T.I.; Boot, M.D.; Hensen, E.J.M. Ethanol as capping agent and formaldehyde scavenger for efficient depolymerization of lignin to aromatics. Green Chem. 2015, 17, 4941–4950. [Google Scholar] [CrossRef]
- Pan, H. Wood Liquefaction in the Presence of Phenol With a Weak Acid Catalyst and Its Potential for Novolac Type Wood Adhesives. Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA, 2007; pp. 1–109. [Google Scholar]
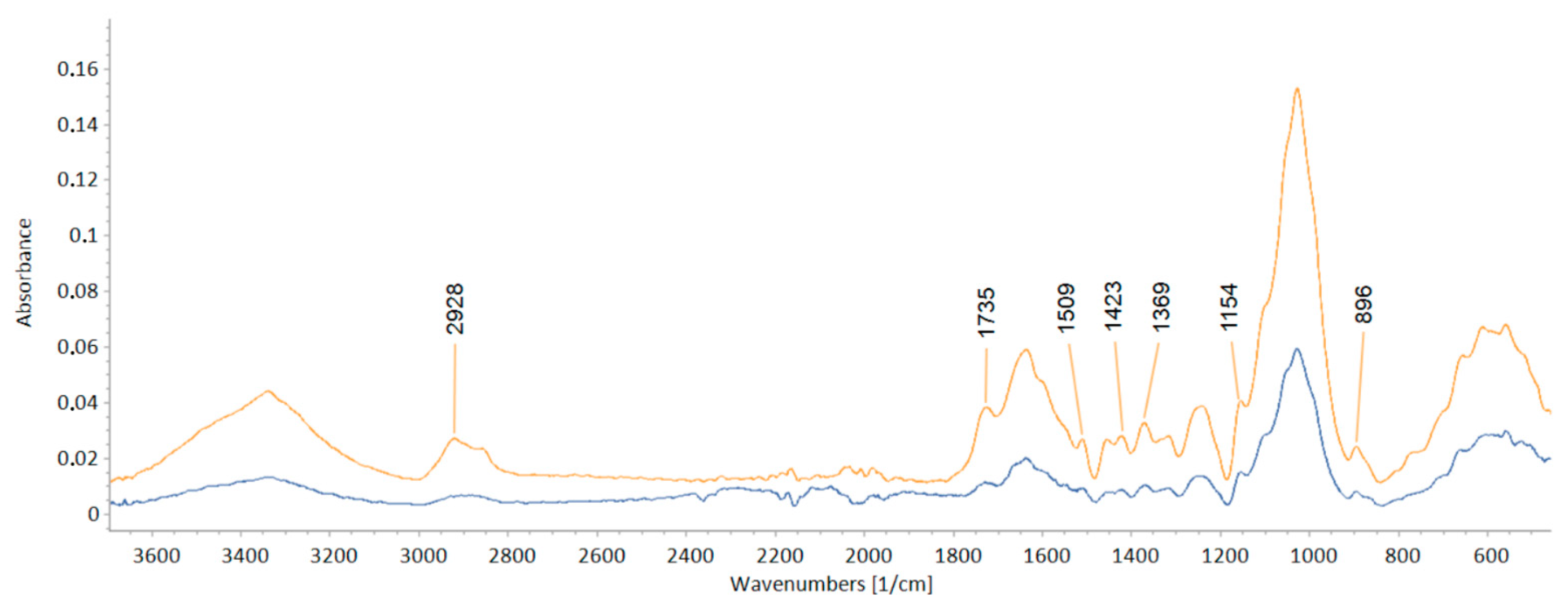


| Wavenumber cm−1 | Assignment | Reference |
|---|---|---|
| 2900 | C–H and CH2 stretching | [30] |
| 1735 | unconjugated C=O in xylans (hemicellulose) [22]; unconjugated C–O in acetyl group (xylan in hardwoods, glucomannan in softwoods) [31] | [22,31] |
| 1505 | aromatic skeletal in lignin [22]; aromatic skeletal vibration C=C [31]; stretching modes of benzene ring in lignin [32] | [22,31,32] |
| 1420 | CH2 scissoring motion [30]; CH2 scissor vibration in cellulose and CH bonds in methoxyl groups in lignin [32]; C–H deformation in lignin and carbohydrates [22]; C–H in plane deformation with aromatic ring stretching, in lignin [31] | [22,31,32] |
| 1375 | CH bending modes in cellulose and hemicellulose [32]; C–H bending mode [30]; C–H deformation in cellulose and hemicellulose [31]; CH stretching in CH2 in urea–formaldehyde resin [33] | [30,31,32,33] |
| 1158 | C–O–C vibration in cellulose and hemicellulose | [31] |
| 1120 | aromatic skeletal and C–O stretching | [34] |
| 1078 | C–O stretch in cellulose and hemicellulose | [34] |
| 896 | C–H deformation in cellulose [22]; vibrational mode involving C1 and the four atoms attached to it [32] | [22,32] |
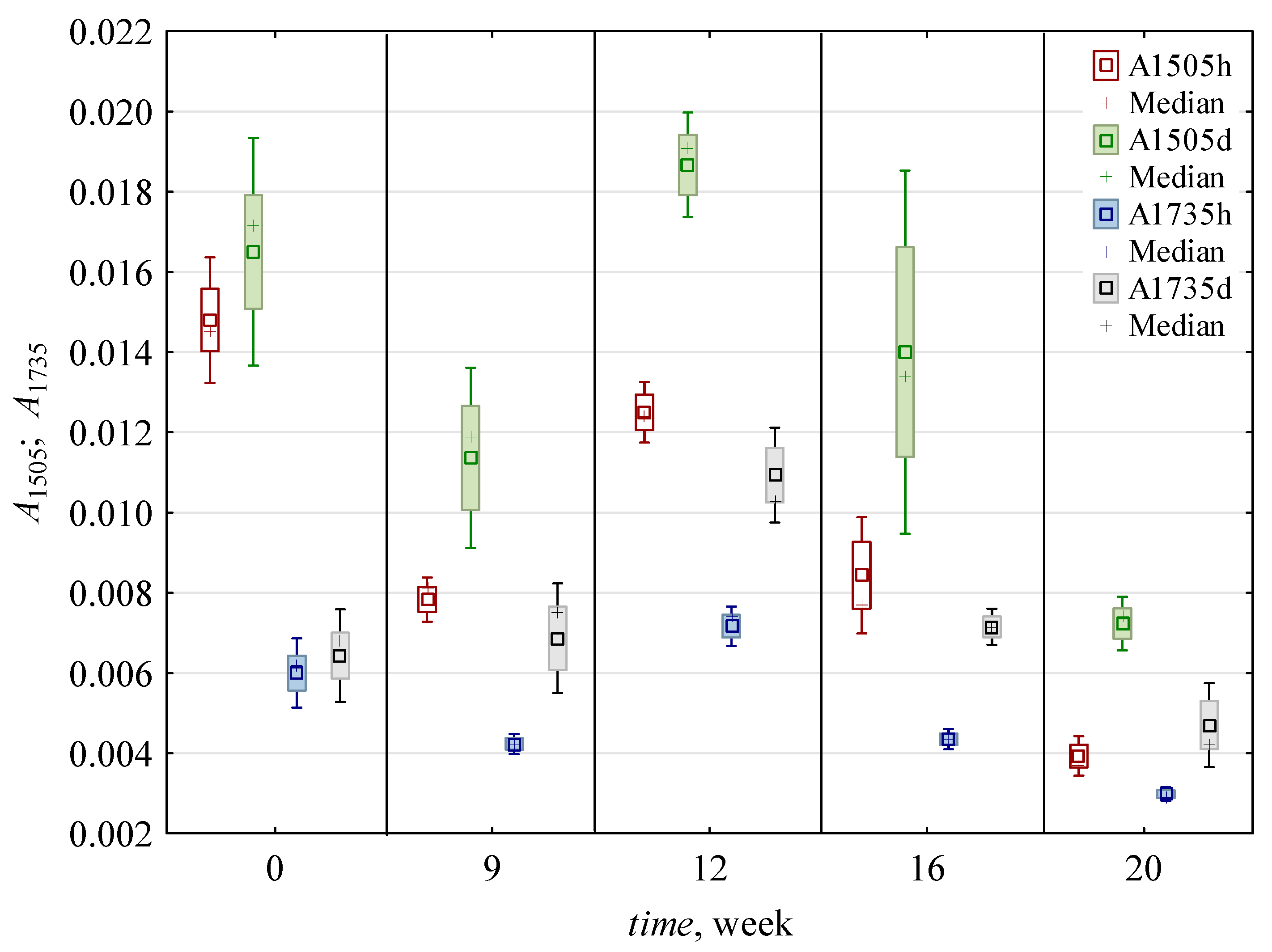
| F ** | A1505 | A1735 | A1505/A1735 | A1505/A1375 | A1505/A1158 | A1505/A896 | A1375b | A1375n | A1158 | A896 | LOI | A1420 | A2900 | TCIb | TCIn | A1120/A1078 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | ||||||||||||||||
| t | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.083 | 0.028 | 0.015 | 0.021 | 0.118 | 0.006 | 0.003 | 0.307 | 0.005 |
| M | <0.001 | <0.001 | 0.738 | <0.001 | <0.001 | 0.468 | 0.425 | ND | <0.001 | <0.001 | 0.003 | 0.003 | 0.004 | <0.001 | ND | ND |
| t × M | <0.001 | <0.001 | 0.774 | 0.032 | 0.015 | 0.115 | <0.001 | ND | 0.792 | 0.714 | 0.094 | 0.183 | 0.594 | 0.013 | ND | ND |
| A ± SD | A ± SD | A ± SD | A ± SD | A ± SD | A ± SD | A ± SD | A ± SD | A ± SD | A ± SD | A ± SD | A ± SD | A ± SD | A ± SD | A ± SD | A ± SD | |
| t, w | ||||||||||||||||
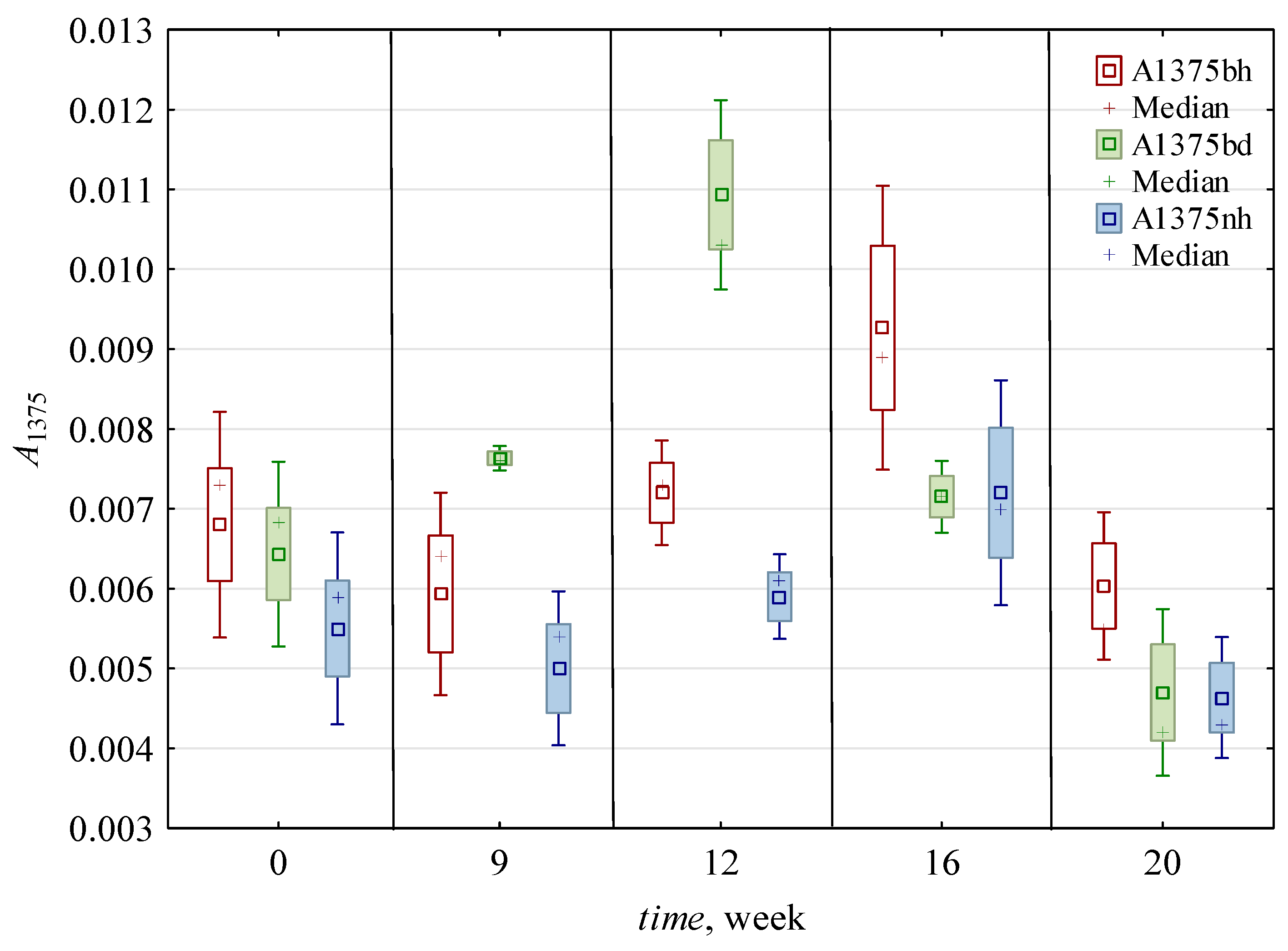
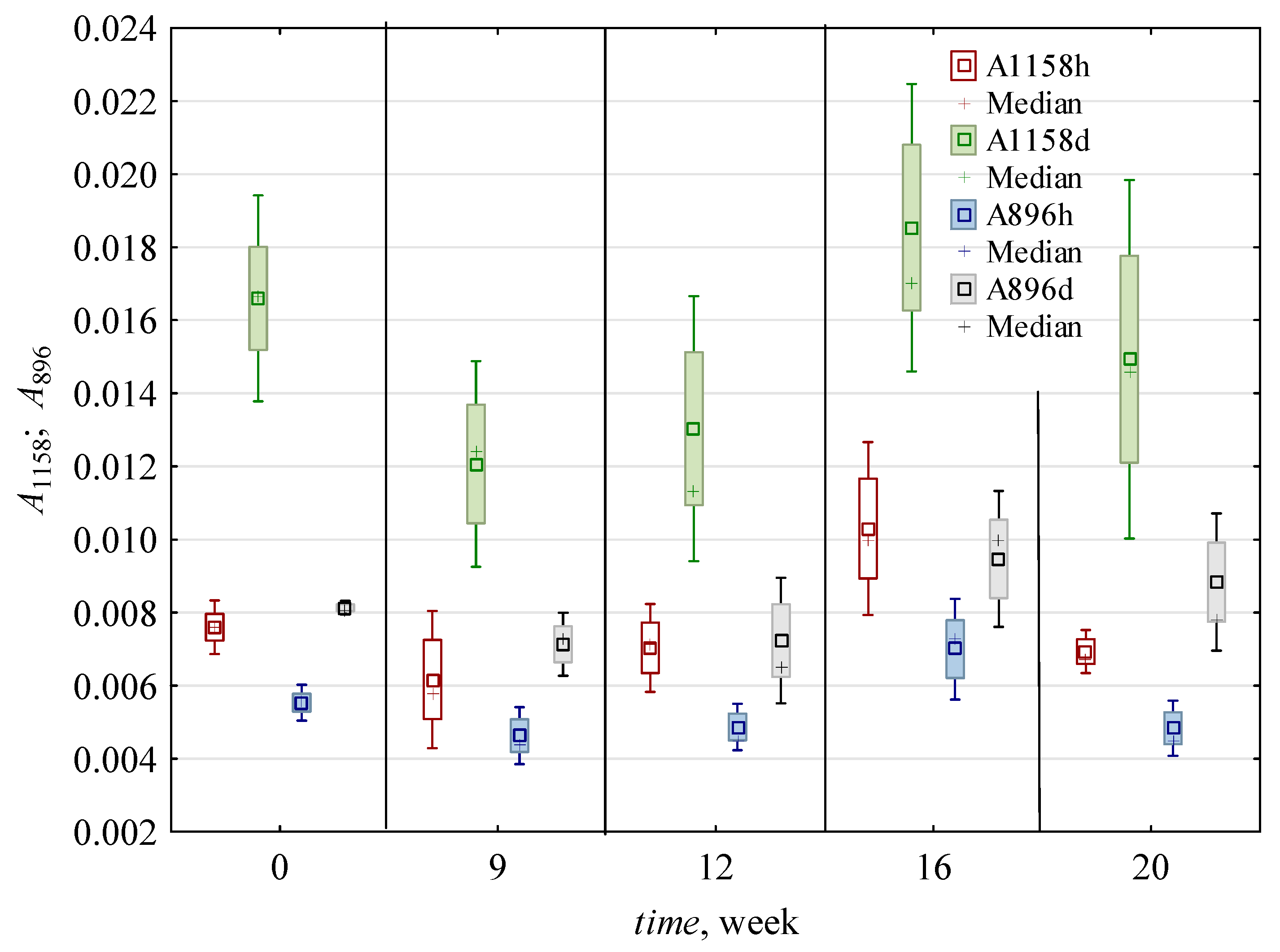
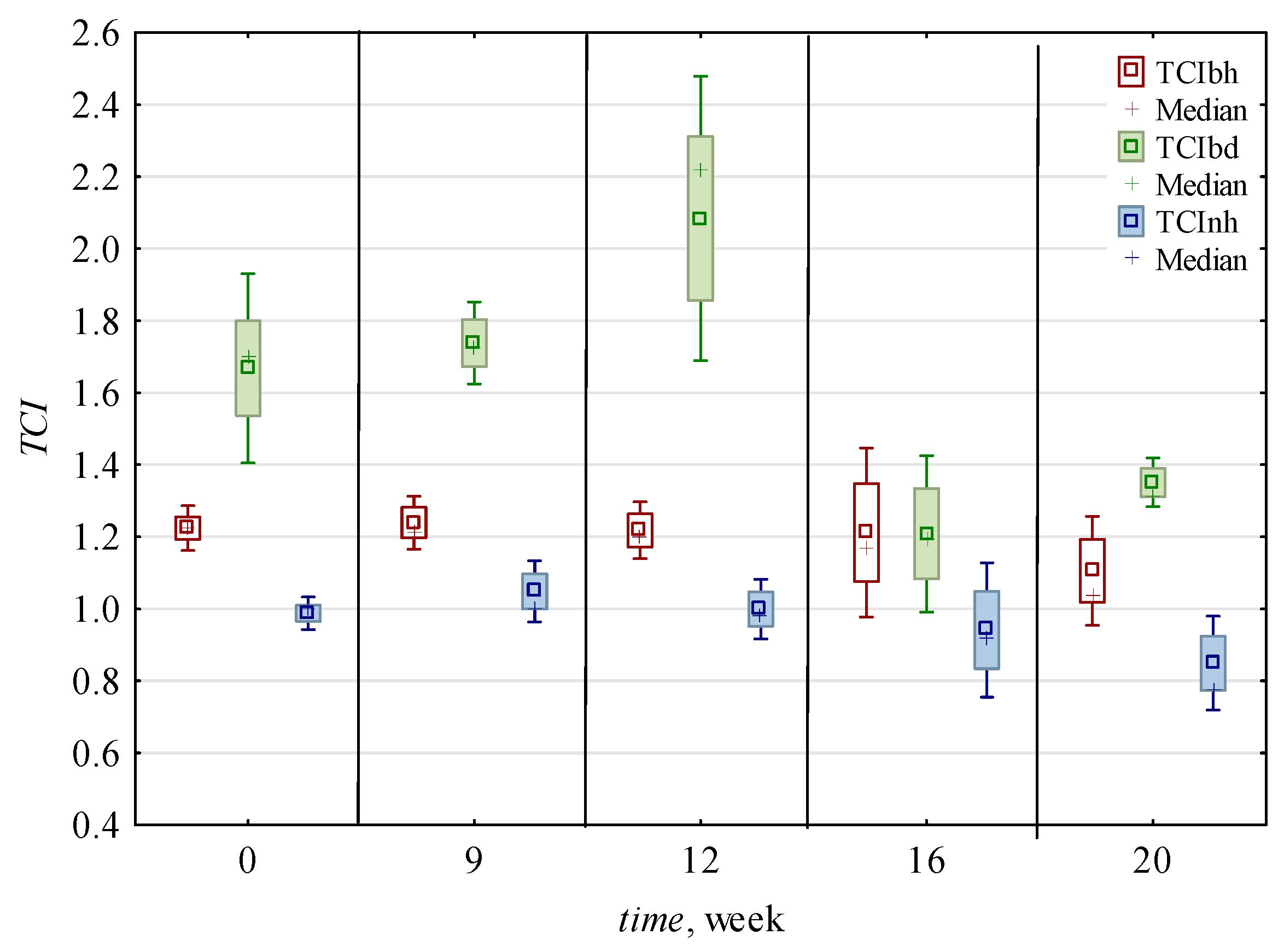
| 0 | 0.016 c,* ± 0.002 | 0.006 b ± 0.001 | 2.54 b ± 0.3 | 2.41 c ± 0.4 | 1.49 c,d ± 0.6 | 2.37 c ± 0.56 | 0.007 a,b ± 0.001 | 0.006 a ± 0.001 | 0.012 a,b ± 0.005 | 0.007 a,b ± 0.001 | 1.22 a,b ± 0.34 | 0.008 a ± 0.001 | 0.005 a ± 0.001 | 1.45 a,b ± 0.3 | 0.99 a ± 0.05 | 0.75 b ± 0.01 |
| 9 | 0.010 c ± 0.002 | 0.006 b ± 0.002 | 1.75 a ± 0.12 | 1.42 a,b ± 0.24 | 1.14 b,c ± 0.3 | 1.65 b ± 0.16 | 0.007 a,b ± 0.001 | 0.005 a ± 0.001 | 0.009 a ± 0.004 | 0.006 a ± 0.002 | 1.07 a,b ± 0.1 | 0.006 a ± 0.001 | 0.005 a ± 0.001 | 1.49 a,b ± 0.29 | 1.05 a ± 0.08 | 0.61 a,b ± 0.14 |
| 12 | 0.016 b ± 0.004 | 0.009 c ± 0.002 | 1.73 a ± 0.1 | 1.73 b ± 0.09 | 1.65 d ± 0.31 | 2.62 c ± 0.31 | 0.009 c ± 0.002 | 0.006 a ± 0.001 | 0.010 a,b ± 0.004 | 0.006 a ± 0.002 | 1.31 b ± 0.12 | 0.008 a ± 0.001 | 0.006 a,b ± 0.001 | 1.65 b ± 0.54 | 1.00 a ± 0.08 | 0.51 a ± 0.03 |
| 16 | 0.011 b ± 0.004 | 0.006 b ± 0.002 | 1.96 a ± 0.49 | 1.43 a,b ± 0.69 | 0.79 a,b ± 0.13 | 1.36 a,b ± 0.35 | 0.008 b,c ± 0.002 | 0.007 a ± 0.001 | 0.014 b ± 0.005 | 0.008 b ± 0.002 | 1.01 a ± 0.22 | 0.008 a ± 0.002 | 0.007 b ± 0.001 | 1.21 a ± 0.2 | 0.94 a ± 0.19 | 0.50 a ± 0.04 |
| 20 | 0.006 a ± 0.002 | 0.004 a ± 0.001 | 1.46 a ± 0.32 | 1.13 a ± 0.59 | 0.55 a ± 0.15 | 0.84 a ± 0.19 | 0.005 a ± 0.001 | 0.005 a ± 0.001 | 0.011 a,b ± 0.005 | 0.007 a,b ± 0.003 | 1.03 a,b ± 0.13 | 0.007 a ± 0.002 | 0.005 a ± 0.002 | 1.23 a ± 0.17 | 0.85 a ± 0.13 | 0.53 a ± 0.11 |
| M | ||||||||||||||||
| h | 0.010 a ± 0.004 | 0.005 a ± 0.002 | 1.91 a ± 0.45 | 1.44 a ± 0.65 | 1.35 b ± 0.62 | 1.87 a ± 0.82 | 0.007 a ± 0.002 | 0.006 ± 0.001 | 0.008 a ± 0.002 | 0.005 a ± 0.001 | 1.24 b ± 0.2 | 0.007 a ± 0.001 | 0.006 b ± 0.001 | 1.20 a ± 0.12 | 0.97 ± 0.12 | ND |
| d | 0.014 b ± 0.005 | 0.007 b ± 0.002 | 1.95 a ± 0.53 | 1.91 b ± 0.53 | 0.95 a ± 0.37 | 1.74 a ± 0.67 | 0.007 a ± 0.002 | ND | 0.015 b ± 0.004 | 0.008 b ± 0.001 | 1.02 a ± 0.22 | 0.008 b ± 0.002 | 0.005 a ± 0.001 | 1.61 b ± 0.37 | ND | 0.59 ± 0.12 |
| Parameter | A1505 | A1735 | A1505/A1735 | A1505/A1375 | A1375b | A1505/A1158 | A1158 | A1505/A896 | A896 | LOI | A1420 | TCIb | A2900 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1505 | 1.000 | ||||||||||||
| A1735 | 0.803 a | 1.000 | |||||||||||
| A1505/A1735 | 0.615 a | 0.042 | 1.000 | ||||||||||
| A1505/A1375 | 0.755 a | 0.356 a | 0.825 a | 1.000 | |||||||||
| A1375b | 0.473 a | 0.664 a | −0.085 | −0.188 | 1.000 | ||||||||
| A1505/A1158 | 0.502 a | 0.365 a | 0.378 a | 0.390 a | 0.195 | 1.000 | |||||||
| A1158 | 0.425 a | 0.373 a | 0.221 | 0.393 a | 0.132 | −0.498 a | 1.000 | ||||||
| A1505/A896 | 0.769 a | 0.597 a | 0.506 a | 0.586 a | 0.326 | 0.912 a | −0.190 | 1.000 | |||||
| A896 | 0.307 | 0.322 | 0.108 | 0.257 | 0.163 | −0.528 a | 0.931 a | −0.315 | 1.000 | ||||
| LOI | 0.288 | 0.186 | 0.227 | 0.148 | 0.206 | 0.772 a | −0.524 a | 0.699 a | −0.579 a | 1.000 | |||
| A1420 | 0.618 a | 0.537 a | 0.330 | 0.403 a | 0.425 a | 0.011 | 0.624 a | 0.217 | 0.641 a | 0.225 | 1.000 | ||
| TCIb | 0.522 a | 0.584 a | 0.116 | 0.303 | 0.377 a | 0.103 | 0.288 | 0.342 | 0.193 | 0.033 | 0.333 | 1.000 | |
| A2900 | −0.002 | 0.112 | −0.169 | −0.436 a | 0.618 a | 0.083 | −0.120 | 0.010 | −0.010 | 0.133 | 0.085 | −0.476 a | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tryjarski, P.; Gawron, J.; Andres, B.; Obiedzińska, A.; Lisowski, A. FTIR Analysis of Changes in Chipboard Properties after Pretreatment with Pleurotus ostreatus (Jacq.) P. Kumm. Energies 2022, 15, 9101. https://doi.org/10.3390/en15239101
Tryjarski P, Gawron J, Andres B, Obiedzińska A, Lisowski A. FTIR Analysis of Changes in Chipboard Properties after Pretreatment with Pleurotus ostreatus (Jacq.) P. Kumm. Energies. 2022; 15(23):9101. https://doi.org/10.3390/en15239101
Chicago/Turabian StyleTryjarski, Paweł, Jakub Gawron, Bogusław Andres, Agnieszka Obiedzińska, and Aleksander Lisowski. 2022. "FTIR Analysis of Changes in Chipboard Properties after Pretreatment with Pleurotus ostreatus (Jacq.) P. Kumm" Energies 15, no. 23: 9101. https://doi.org/10.3390/en15239101
APA StyleTryjarski, P., Gawron, J., Andres, B., Obiedzińska, A., & Lisowski, A. (2022). FTIR Analysis of Changes in Chipboard Properties after Pretreatment with Pleurotus ostreatus (Jacq.) P. Kumm. Energies, 15(23), 9101. https://doi.org/10.3390/en15239101