Enzymatic Conversion of Hydrolysis Lignin—A Potential Biorefinery Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Acid Precipitated Lignin (APL) and Britton & Robinson (B&R) Buffer
2.3. Enzymatic Treatment of APL
2.4. Lignin Concentration Determination by UV-Vis Spectroscopy
2.5. Size Exclusion Chromatography (SEC)
2.6. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.7. Two-Dimensional NMR Spectroscopy
3. Results and Discussion
3.1. The Molecular Weight Distribution of Acid Precipitated Lignin (APL)
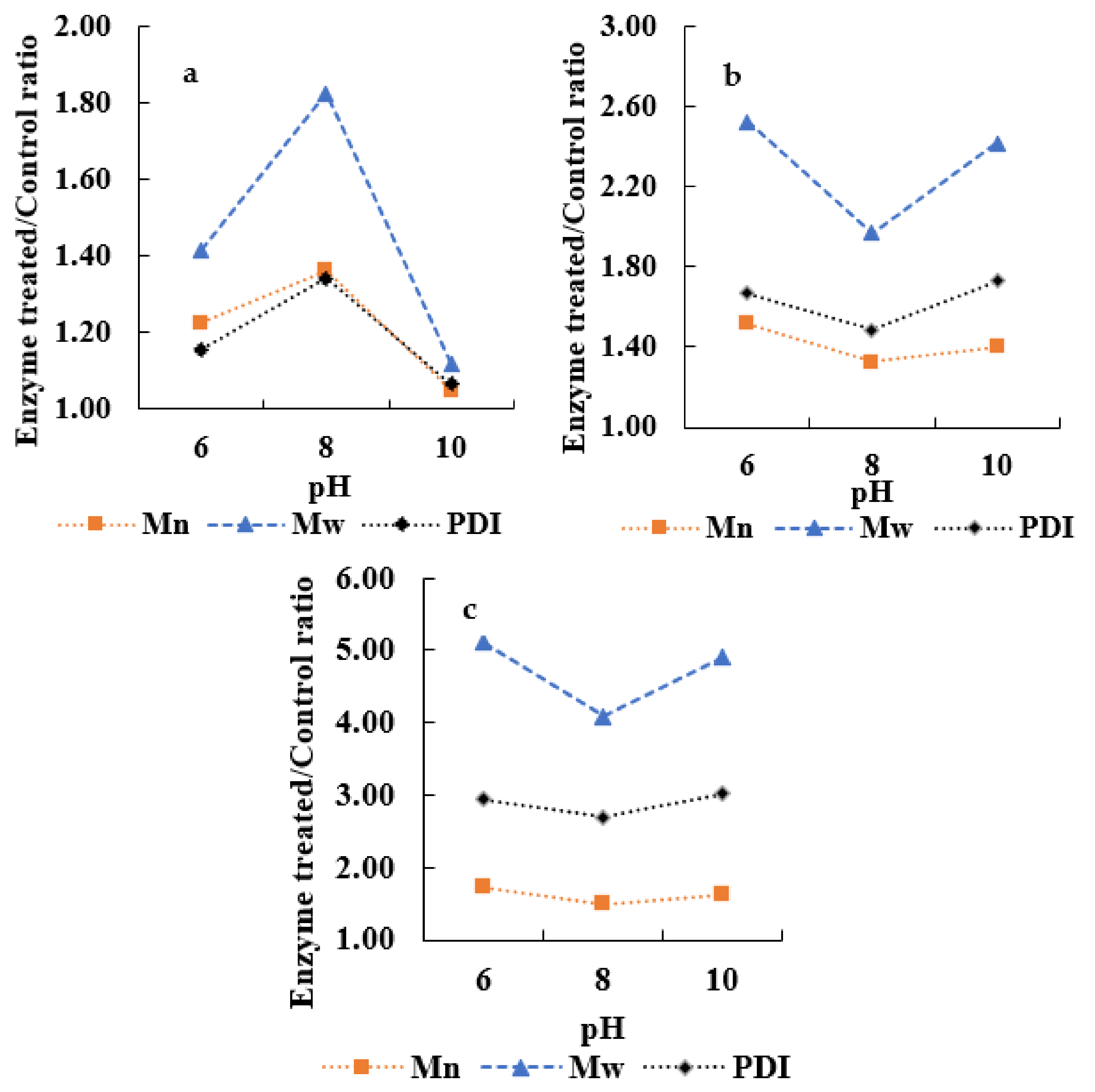
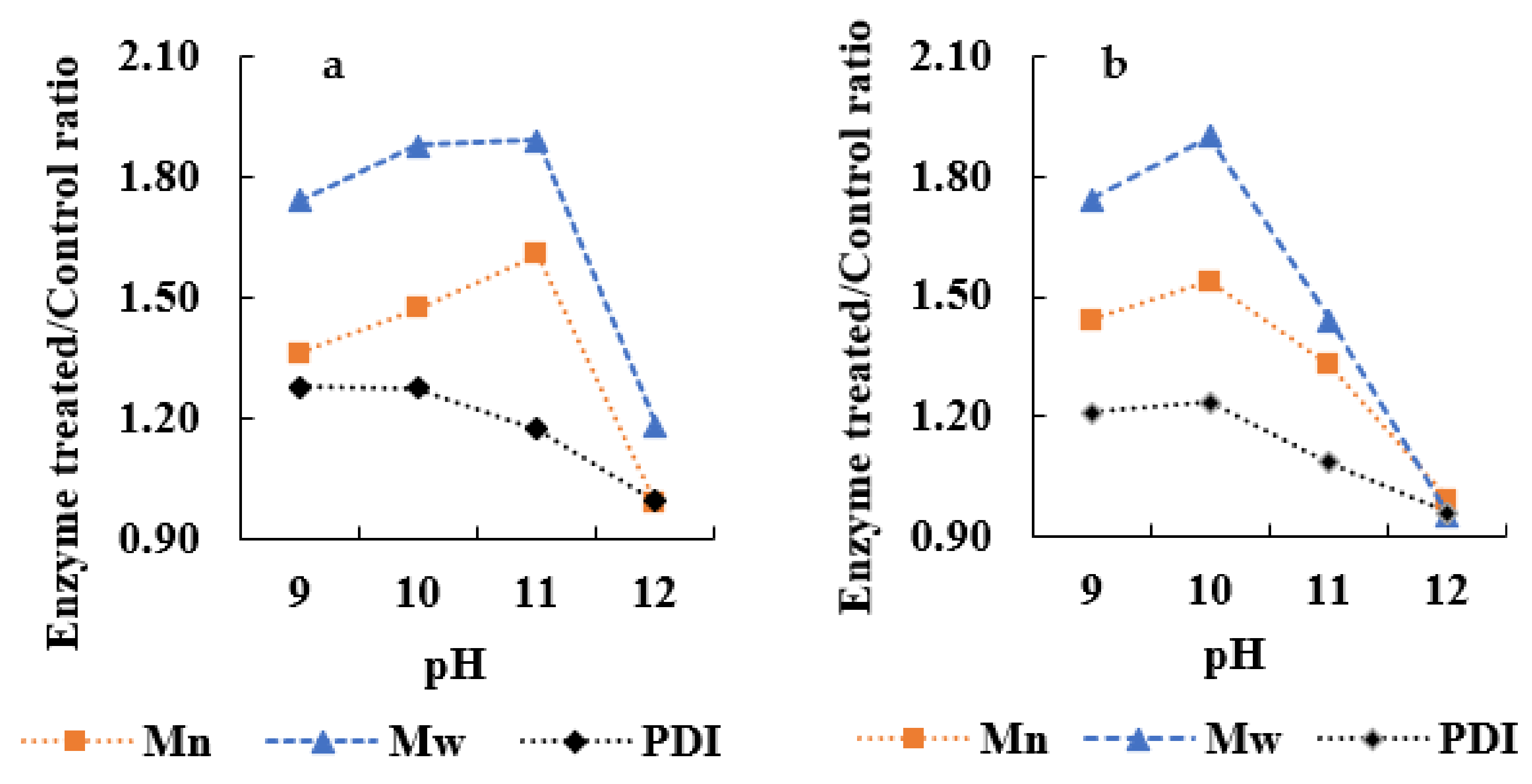
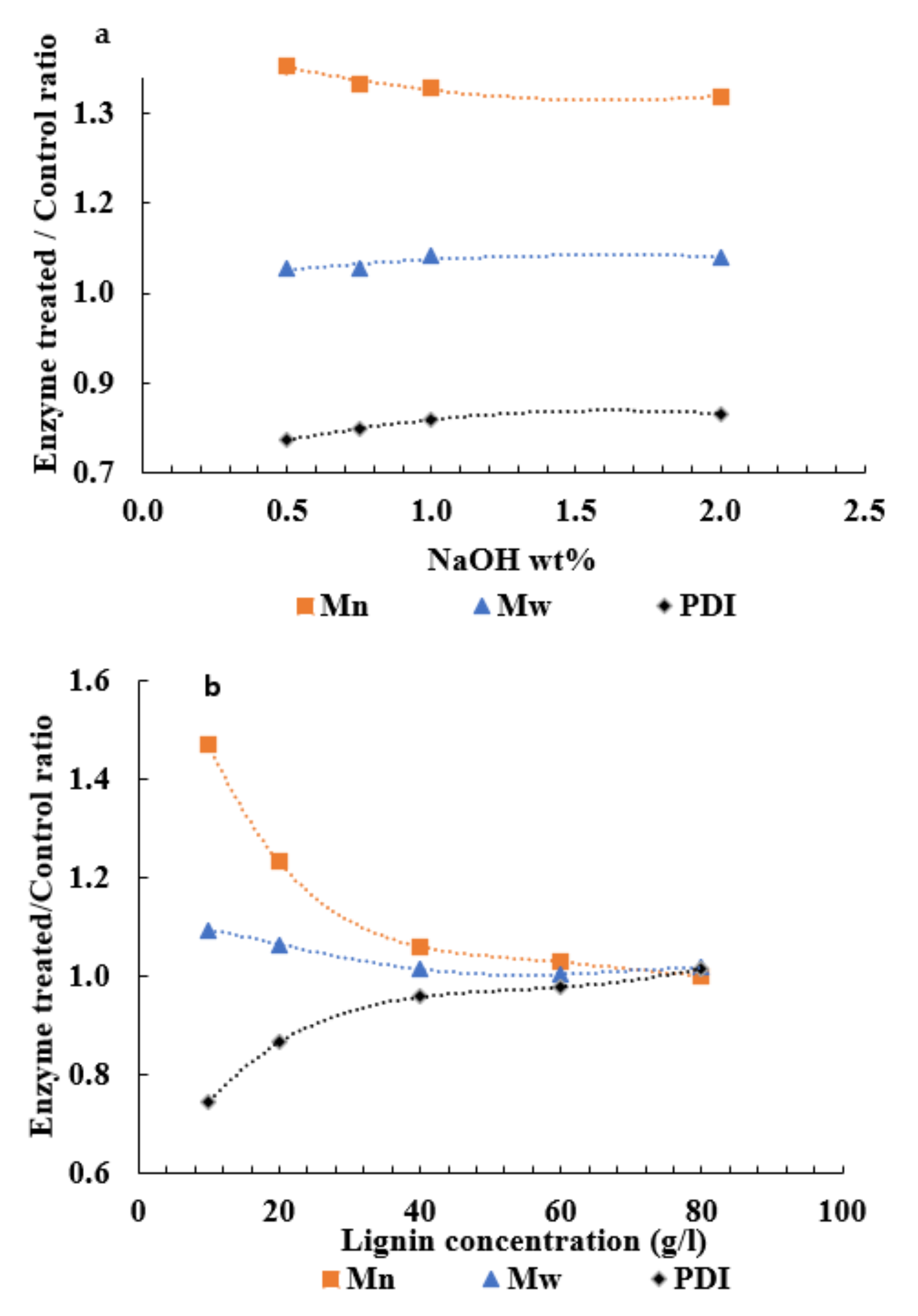
3.2. Enzymatic Oxidation of APL
3.3. Determination of Enzymatic Inhibition
3.4. FT-IR Specstra of ScLac-Treated and Nontreated (Control) APL
3.5. Semiquantitative HSQC Spectra of Enzymatically Treated APL
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, O.; Kim, K.H. Lignin to materials: A focused review on recent novel lignin applications. Appl. Sci. 2020, 10, 4626. [Google Scholar] [CrossRef]
- Tofani, G.; Cornet, I.; Tavernier, S. Separation and recovery of lignin and hydrocarbon derivatives from cardboard. Biomass Convers. Biorefinery 2022, 12, 3409–3424. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Weng, C.; Peng, X.; Han, Y. Depolymerization and conversion of lignin to value-added bioproducts by microbial and enzymatic catalysis. Biotechnol. Biofuels 2021, 14, 84. [Google Scholar] [CrossRef]
- Tofani, G.; Cornet, I.; Tavernier, S. Estimation of hydrogen peroxide effectivity during bleaching using the Kappa number. Chem. Pap. 2021, 75, 5749–5758. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C.C. Hydrolytic depolymerization of hydrolysis lignin: Effects of catalysts and solvents. Bioresour. Technol. 2015, 190, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Svensson, I.; Roncal, T.; De Winter, K.; Van Canneyt, A.; Tamminen, T.; Mikkelson, A.; Barrio, A. Valorisation of Hydrolysis Lignin Rest from Bioethanol Pilot Plant: Process Development and Upscaling. Ind. Crops Prod. 2020, 156, 112869. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; De Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Christopher, L.P.; Yao, B.; Ji, Y. Lignin biodegradation with laccase-mediator systems. Front. Energy Res. 2014, 2, 12. [Google Scholar] [CrossRef]
- Agustin, M.B.; de Carvalho, D.M.; Lahtinen, M.H.; Hilden, K.; Lundell, T.; Mikkonen, K.S. Laccase as a Tool in Building Advanced Lignin-Based Materials. ChemSusChem 2021, 14, 4615–4635. [Google Scholar] [CrossRef]
- Patel, A.; Shah, A.R. Integrated lignocellulosic biorefinery: Gateway for production of second generation ethanol and value added products. J. Bioresour. Bioprod. 2021, 6, 108–128. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Eugenio, M.E.; Tomás-Pejó, E. Laccases as versatile enzymes: From industrial uses to novel applications. J. Chem. Technol. Biotechnol. 2020, 95, 481–494. [Google Scholar] [CrossRef]
- Berlin, A.; Balakshin, M. Industrial Lignins: Analysis, Properties, and Applications. In Bioenergy Research: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Osma, J.F.; Toca-Herrera, J.L.; Rodríguez-Couto, S. Cost analysis in laccase production. J. Environ. Manag. 2011, 92, 2907–2912. [Google Scholar] [CrossRef]
- Lee, S.; Kang, M.; Bae, J.H.; Sohn, J.H.; Sung, B.H. Bacterial Valorization of Lignin: Strains, Enzymes, Conversion Pathways, Biosensors, and Perspectives. Front. Bioeng. Biotechnol. 2019, 7, 209. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Paice, M.G. Oxidation of non-phenolic substrates. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef]
- Zhu, D.; Liang, N.; Zhang, R.; Ahmad, F.; Zhang, W.; Yang, B.; Wu, J.; Geng, A.; Gabriel, M.; Sun, J. Insight into Depolymerization Mechanism of Bacterial Laccase for Lignin. ACS Sustain. Chem. Eng. 2020, 8, 12920–12933. [Google Scholar] [CrossRef]
- Balakshin, M.Y.; Capanema, E.A.; Sulaeva, I.; Schlee, P.; Huang, Z.; Feng, M.; Borghei, M.; Rojas, O.J.; Potthast, A.; Rosenau, T. New Opportunities in the Valorization of Technical Lignins. ChemSusChem 2021, 14, 1016–1036. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef]
- Xue, Y.; Li, Y.; Liu, Z.; Hou, Y. Structural changes of lignin in soda delignification process and associations with pollution load. BioResources 2019, 14, 7869–7885. [Google Scholar] [CrossRef]
- Mattinen, M.L.; Suortti, T.; Gosselink, R.; Argyropoulos, D.S.; Evtuguin, D.; Suurnäkki, A.; De Jong, E.; Tamminen, T. Polymerization of different lignins by laccase. BioResources 2008, 3, 549–565. [Google Scholar] [CrossRef]
- Majumdar, S.; Lukk, T.; Solbiati, J.O.; Bauer, S.; Nair, S.K.; Cronan, J.E.; Gerlt, J.A. Roles of small laccases from streptomyces in lignin degradation. Biochemistry 2014, 53, 4047–4058. [Google Scholar] [CrossRef] [PubMed]
- Zovo, K.; Pupart, H.; Van Wieren, A.; Gillilan, R.E.; Huang, Q.; Majumdar, S.; Lukk, T. Substitution of the Methionine Axial Ligand of the T1 Copper for the Fungal-like Phenylalanine Ligand (M298F) Causes Local Structural Perturbations that Lead to Thermal Instability and Reduced Catalytic Efficiency of the Small Laccase from Streptomyces coelicolor A3 (2). ACS Omega 2022, 7, 6184–6194. [Google Scholar] [CrossRef]
- Ebihara, A.; Kawamoto, S.; Shibata, N.; Yamaguchi, T.; Suzuki, F.; Nakagawa, T. Development of a modified Britton-Robinson buffer with improved linearity in the alkaline pH region. Bioj. Sci. Technol. 2016, 3, 2016. Available online: http://www.bjst.bio-journal.com/2016/m15006/ (accessed on 20 February 2016).
- Hämäläinen, V.; Grönroos, T.; Suonpää, A.; Heikkilä, M.W.; Romein, B.; Ihalainen, P.; Malandra, S.; Birikh, K.R. Enzymatic processes to unlock the lignin value. Front. Bioeng. Biotechnol. 2018, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Rauber, D.; Shanmugam, S.; Kay, C.W.M.; Konist, A.; Kikas, T. Efficient Lignin Fractionation from Scots Pine (Pinus sylvestris) Using Ammonium-Based Protic Ionic Liquid: Process Optimization and Characterization of Recovered Lignin. Polymers 2022, 14, 4637. [Google Scholar] [CrossRef] [PubMed]
- Dillies, J.; Vivien, C.; Chevalier, M.; Rulence, A.; Châtaigné, G.; Flahaut, C.; Senez, V.; Froidevaux, R. Enzymatic depolymerization of industrial lignins by laccase-mediator systems in 1,4-dioxane/water. Biotechnol. Appl. Biochem. 2020, 67, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xiao, J.; Wang, G.; Chen, G. Enzymatic hydrolysis of lignin by ligninolytic enzymes and analysis of the hydrolyzed lignin products. Bioresour. Technol. 2020, 304, 122975. [Google Scholar] [CrossRef]
- Chan, J.C.; Paice, M.; Zhang, X. Enzymatic Oxidation of Lignin: Challenges and Barriers Toward Practical Applications. ChemCatChem 2020, 12, 401–425. [Google Scholar] [CrossRef]
- Sewring, T.; Theliander, H. Acid precipitation of kraft lignin from aqueous solutions: The influence of anionic specificity and concentration level of the salt. Holzforschung 2019, 73, 937–945. [Google Scholar] [CrossRef]
- Li, M.; Pu, Y.; Ragauskas, A.J. Current understanding of the correlation of lignin structure with biomass recalcitrance. Front. Chem. 2016, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.C.; Ximenes, E.; Kim, Y.; Ladisch, M.R. Lignin–Enzyme Interactions in the Hydrolysis of Lignocellulosic Biomass. Trends Biotechnol. 2019, 37, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, Y. Lignin-enzyme interaction: Mechanism, mitigation approach, modeling, and research prospects. Biotechnol. Adv. 2017, 35, 466–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tan, L.; Hu, L.; Wang, X.; Koppolu, R.; Tirri, T.; van Bochove, B.; Ihalainen, P.; Sobhanadhas, L.S.S.; Xu, C.; et al. On Laccase-Catalyzed Polymerization of Biorefinery Lignin Fractions and Alignment of Lignin Nanoparticles on the Nanocellulose SurfaceviaOne-Pot Water-Phase Synthesis. ACS Sustain. Chem. Eng. 2021, 9, 8770–8782. [Google Scholar] [CrossRef]
- Curran, L.M.L.K.; Pham, L.T.M.; Sale, K.L.; Simmons, B.A. Review of advances in the development of laccases for the valorization of lignin to enable the production of lignocellulosic biofuels and bioproducts. Biotechnol. Adv. 2021, 54, 107809. [Google Scholar] [CrossRef]
- Lange, H.; Decina, S.; Crestini, C. Oxidative upgrade of lignin—Recent routes reviewed. Eur. Polym. J. 2013, 49, 1151–1173. [Google Scholar] [CrossRef]
- Subbotina, E.; Rukkijakan, T.; Marquez-Medina, M.D.; Yu, X.; Johnsson, M.; Samec, J.S.M. Oxidative cleavage of C–C bonds in lignin. Nat. Chem. 2021, 13, 1118–1125. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, R.; Gao, W.; Yao, X. Investigation of [Emim][OAc] as a mild pretreatment solvent for enhancing the sulfonation efficiency of alkali lignin. RSC Adv. 2017, 7, 31009–31017. [Google Scholar] [CrossRef]
- Ibrahim, M.N.M.; Iqbal, A.; Shen, C.C.; Bhawani, S.A.; Adam, F. Synthesis of lignin based composites of TiO2 for potential application as radical scavengers in sunscreen formulation. BMC Chem. 2019, 13, 17. [Google Scholar] [CrossRef]
- Sette, M.; Wechselberger, R.; Crestini, C. Elucidation of lignin structure by quantitative 2D NMR. Chem. Eur. J. 2011, 17, 9529–9535. [Google Scholar] [CrossRef]
- Zikeli, F.; Vinciguerra, V.; D’Annibale, A.; Capitani, D.; Romagnoli, M.; Mugnozza, G.S. Preparation of lignin nanoparticles from wood waste for wood surface treatment. Nanomaterials 2019, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Lee, J.H.; Raja, A.A.; Choi, J.W. Effects of gamma-valerolactone assisted fractionation of ball-milled pine wood on lignin extraction and its characterization aswell as its corresponding cellulose digestion. Appl. Sci. 2020, 10, 1599. [Google Scholar] [CrossRef]
- Arefmanesh, M.; Vuong, T.V.; Nikafshar, S.; Wallmo, H.; Nejad, M.; Master, E.R. Enzymatic synthesis of kraft lignin-acrylate copolymers using an alkaline tolerant laccase. Appl. Microbiol. Biotechnol. 2022, 106, 2969–2979. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Bai, R.; Zhang, Y.; Wang, Q.; Fan, X.; Yuan, J.; Cui, L.; Wang, P. Laccase-catalyzed oxidative polymerization of phenolic compounds. Appl. Biochem. Biotechnol. 2013, 171, 1673–1680. [Google Scholar] [CrossRef]
- Hilgers, R.; Vincken, J.P.; Gruppen, H.; Kabel, M.A. Laccase/Mediator Systems: Their Reactivity toward Phenolic Lignin Structures. ACS Sustain. Chem. Eng. 2018, 6, 2037–2046. [Google Scholar] [CrossRef]
- Domínguez, G.; Blánquez, A.; Borrero-López, A.M.; Valencia, C.; Eugenio, M.E.; Arias, M.E.; Rodríguez, J.; Hernández, M. Eco-Friendly Oleogels from Functionalized Kraft Lignin with Laccase SilA from Streptomyces ipomoeae: An Opportunity to Replace Commercial Lubricants. ACS Sustain. Chem. Eng. 2021, 9, 4611–4616. [Google Scholar] [CrossRef]

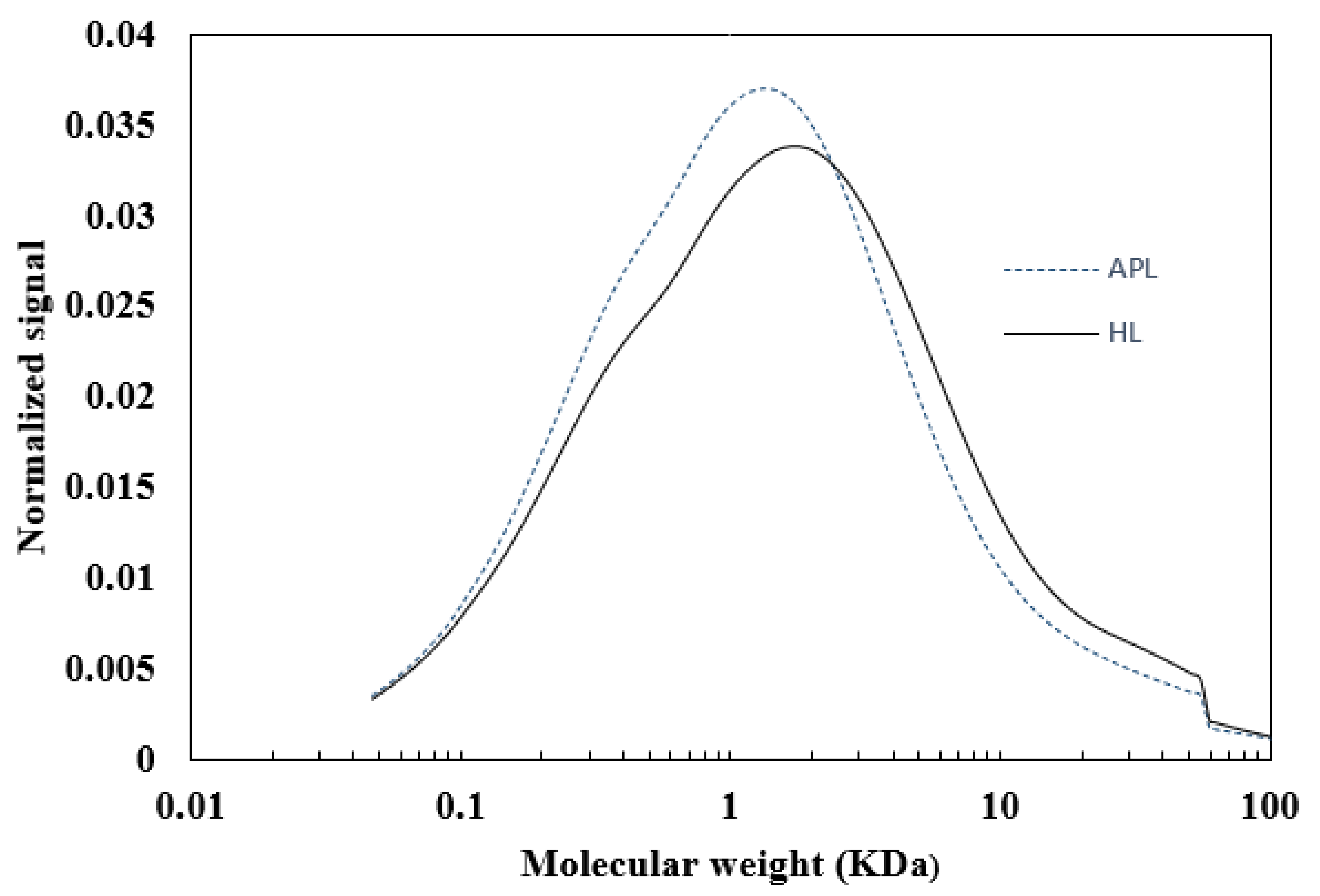

| Lignin | Mn | Mw | PDI |
|---|---|---|---|
| HL | 0.66 | 8.89 | 7.2 |
| APL | 0.53 | 6.86 | 7.6 |
| pH | B&R Buffer | 0.1 M NaOH Solution | 0.25 M NaOH Solution |
|---|---|---|---|
| 6 | 12 ± 3 | 20 ± 3 | 25 ± 1 |
| 8 | 13 ± 4 | 22 ± 2 | 35 ± 2 |
| 10 | 15 ± 4 | 28 ± 1.2 | 45 ± 5 |
| Enzyme | Solution | pH | Mn | Mw | PDI |
|---|---|---|---|---|---|
| 0.25 M NaOH Solution | 9 | 0.56 | 6.57 | 11.74 | |
| 0.80 | 11.46 | 14.19 | |||
| 10 | 0.56 | 6.83 | 12.16 | ||
| 0.86 | 12.97 | 15.01 | |||
| 11 | 0.57 | 6.67 | 11.67 | ||
| 0.76 | 9.63 | 12.65 | |||
| 12 | 0.65 | 8.70 | 13.27 | ||
| 0.65 | 8.29 | 12.75 | |||
| ScLac | 0.1 M NaOH Solution | ||||
| 9 | 0.53 | 6.37 | 11.92 | ||
| 0.72 | 11.10 | 15.26 | |||
| 10 | 0.56 | 6.63 | 11.78 | ||
| 0.83 | 12.47 | 15.02 | |||
| 11 | 0.52 | 6.37 | 12.23 | ||
| 0.84 | 12.07 | 14.37 | |||
| 12 | 0.53 | 6.23 | 11.83 | ||
| 0.52 | 6.18 | 11.75 |
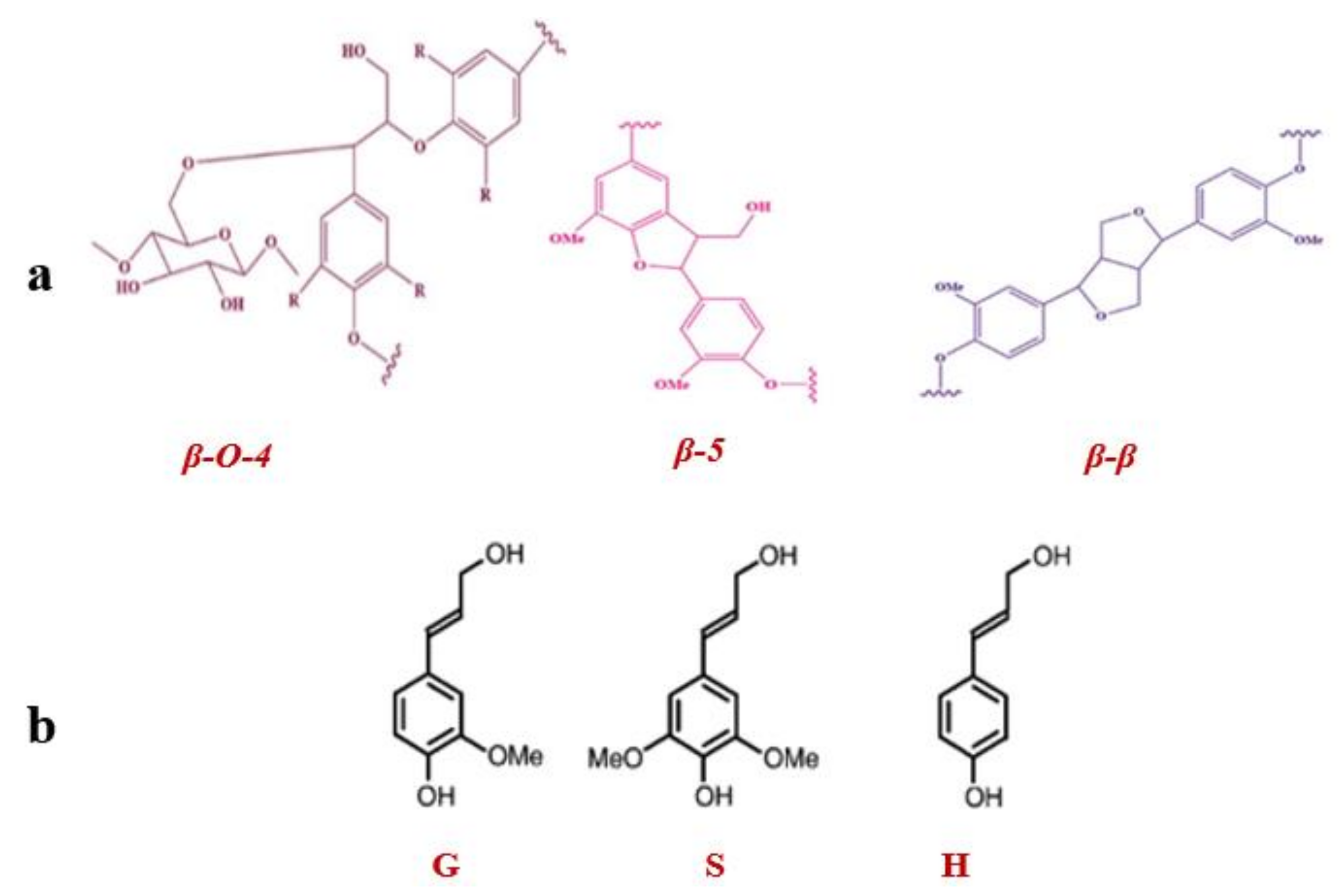
| Monomeric Ratio % | S/G Ratio | Inter-Unit Linkages % | |||||
|---|---|---|---|---|---|---|---|
| G | S | H | β-O-4 | β-5 | β-β | ||
| ScLac treated | 22 | 75 | 3 | 3 | 71 | 3 | 26 |
| Control sample | 20 | 77 | 3 | 4 | 52 | 5 | 43 |
| δC/δH (ppm) | Assignment (Label) |
|---|---|
| 104.1/6.64 | C2,6-H2,6 in syringyl units (S2,6) |
| 107.0/7.05 | C2,6-H2,6 in sinapaldehyde units (SA2,6) |
| 107.0/7.25 | C2,6-H2,6 in α-oxidized (Cα=O) syringyl units (S’2,6) |
| 111.3/7.01 | C2-H2 in guaiacyl units (G2) |
| 112.5/6.67 | C3,5-H3,5 in p-hydroxyphenyl units (H3,5) |
| 112.6/7.49 | C2-H2 in oxidized guaiacyl units (G’2) |
| 115.9/6.96 + 6.63 | C5-H5 and C6-H6 in guaiacyl units (G5, G6) |
| 119.1/6.84 | C6-H6 in guaiacyl units (G6) |
| 119.5/6.84 | C6-H6 in coniferaldehyde (CA6) |
| 128.9/7.14 | C2,6-H2,6 in p-hydroxyphenyl units (H2,6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Puss, K.K.; Lukk, T.; Loog, M.; Kikas, T.; Salmar, S. Enzymatic Conversion of Hydrolysis Lignin—A Potential Biorefinery Approach. Energies 2023, 16, 370. https://doi.org/10.3390/en16010370
Khan S, Puss KK, Lukk T, Loog M, Kikas T, Salmar S. Enzymatic Conversion of Hydrolysis Lignin—A Potential Biorefinery Approach. Energies. 2023; 16(1):370. https://doi.org/10.3390/en16010370
Chicago/Turabian StyleKhan, Sharib, Kait Kaarel Puss, Tiit Lukk, Mart Loog, Timo Kikas, and Siim Salmar. 2023. "Enzymatic Conversion of Hydrolysis Lignin—A Potential Biorefinery Approach" Energies 16, no. 1: 370. https://doi.org/10.3390/en16010370
APA StyleKhan, S., Puss, K. K., Lukk, T., Loog, M., Kikas, T., & Salmar, S. (2023). Enzymatic Conversion of Hydrolysis Lignin—A Potential Biorefinery Approach. Energies, 16(1), 370. https://doi.org/10.3390/en16010370










