Actual Trends in the Usability of Biochar as a High-Value Product of Biomass Obtained through Pyrolysis
Abstract
1. Introduction
2. Application of Biochar
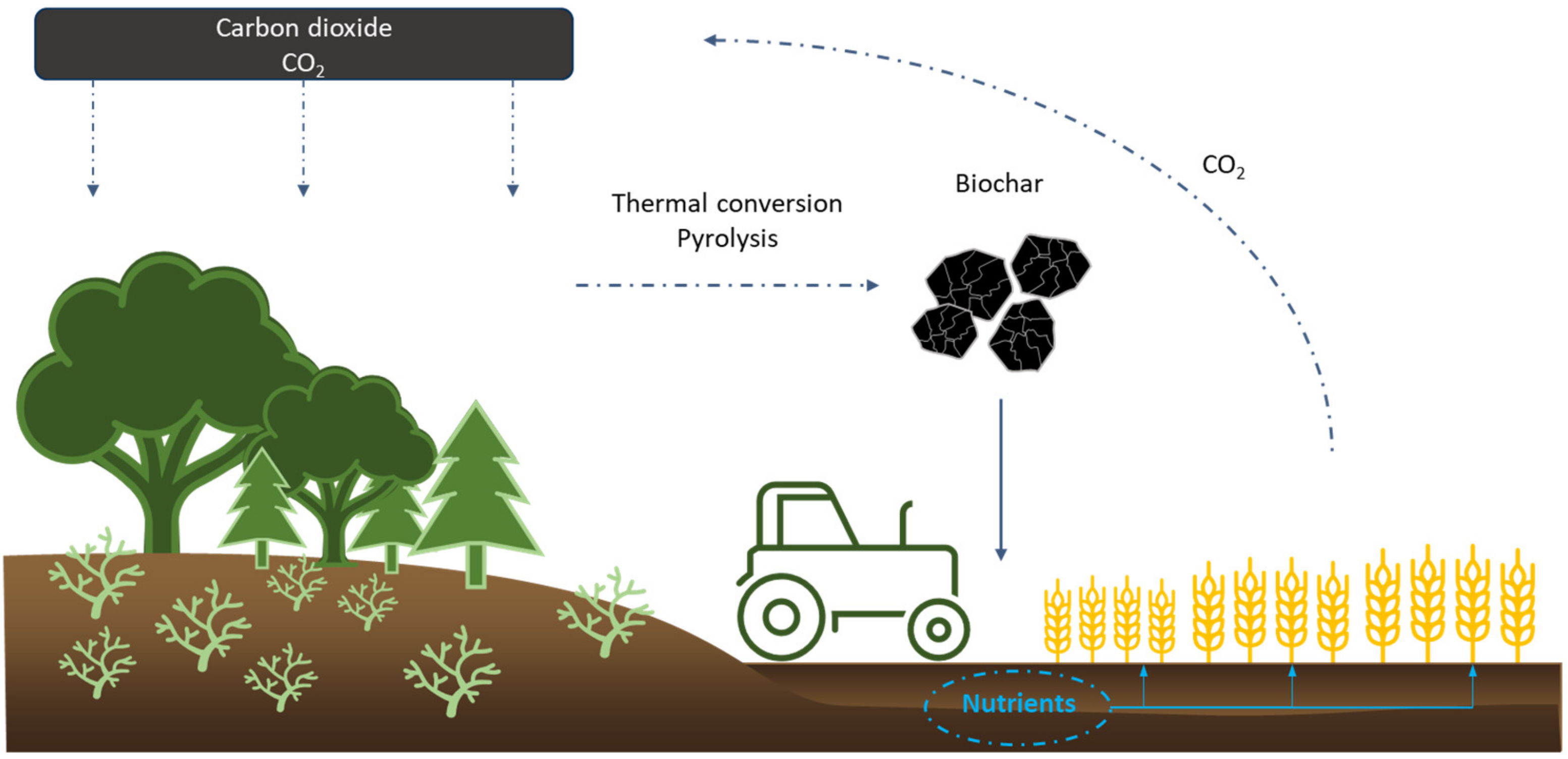
2.1. Biochar in CO2 Sequestration and Soil Improvement
- Guarantees a reasonable and equitable transition;
- Ensures a level playing field concerning third-world countries while preserving and enhancing the creativity and competitiveness of EU industry;
- Economic operators;
- Supports EU’s role as a worldwide leader in the battle against climate change.
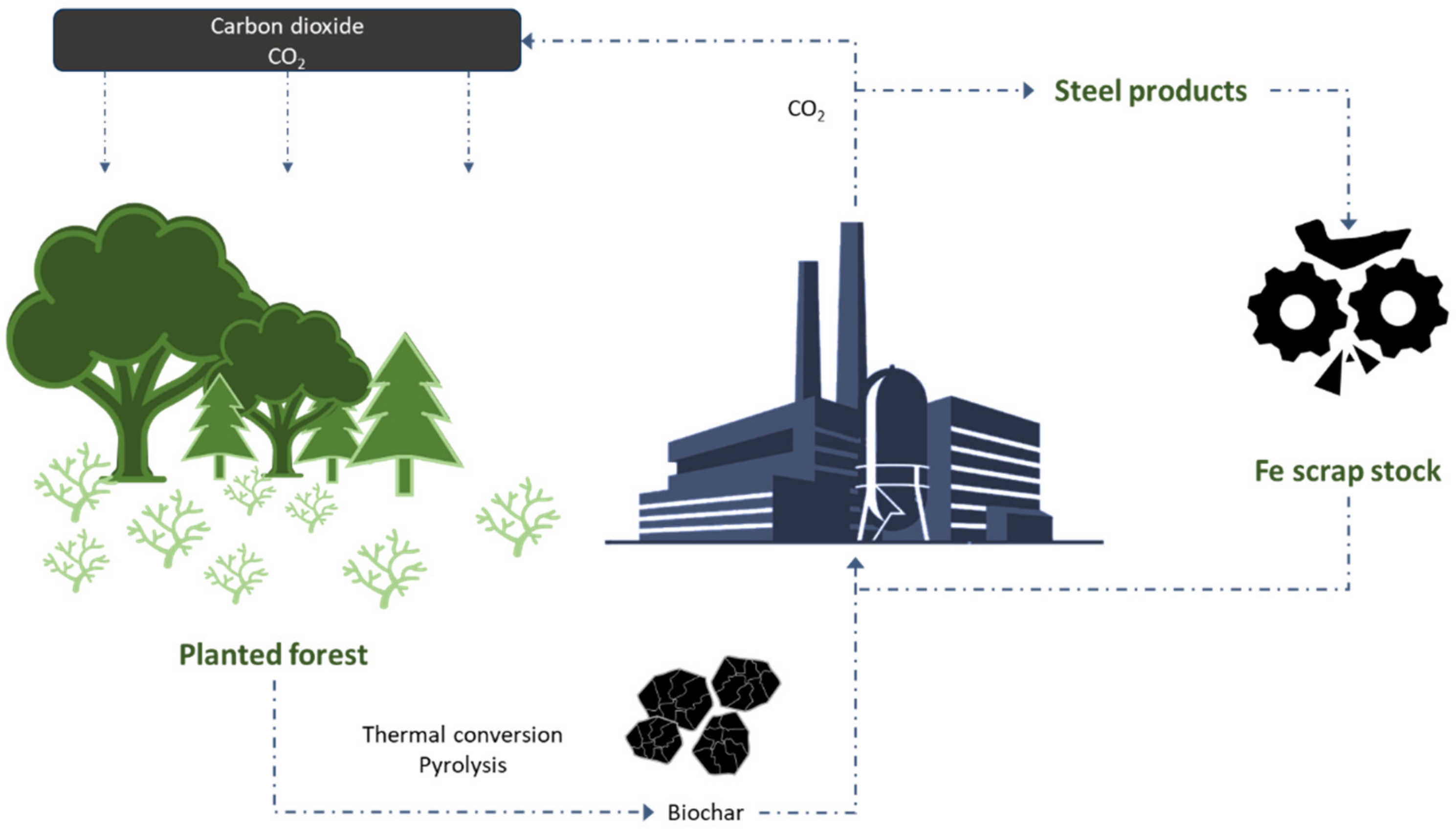
2.2. Biochars in Iron and Steel Industries
- (i)
- Cokemaking for the production of bio-coke;
- (ii)
- Sintering process for the production of bio-sinter;
- (iii)
- Pelletizing/briquetting for the production of bio-composites and/or bio-briquettes;
- (iv)
- Partial replacement of nut coke, coke or PC in the blast furnace;
- (v)
- Bio-carburization of steel in ladle furnace.
- -
- Coking coal (charcoal substitution rate: 2–10%);
- -
- Blast Furnace tuyere injection (charcoal substitution rate: 0–100%);
- -
- Blast Furnace nut coke (charcoal substitution rate: 50–100%);
- -
- Blast furnace briquettes (charcoal substitution rate: 0–100%);
- -
- Sintering solid fuel (charcoal substitution rate: 50–100%).
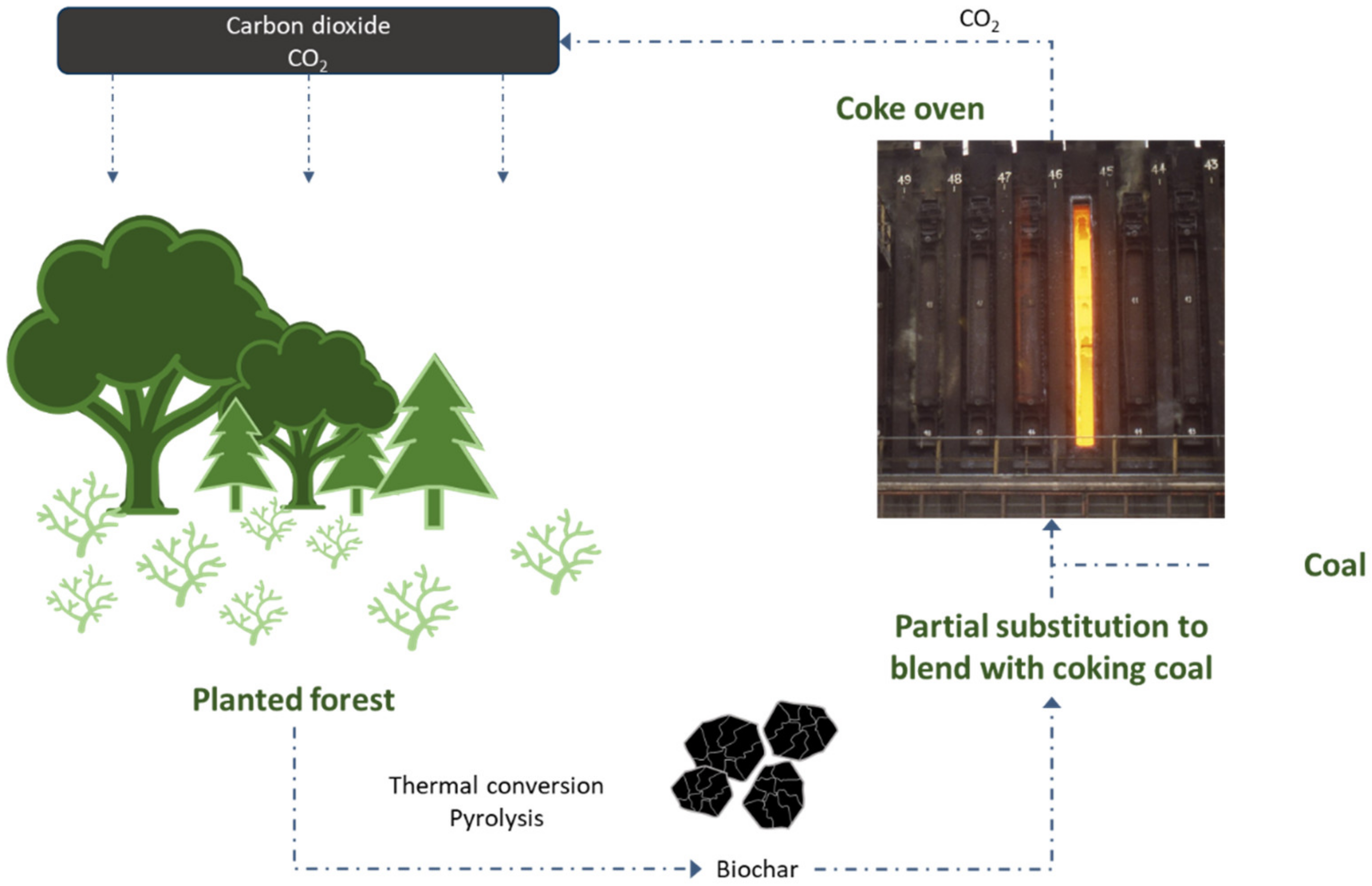
2.3. Usability of Carbonite Materials in Cokemaking
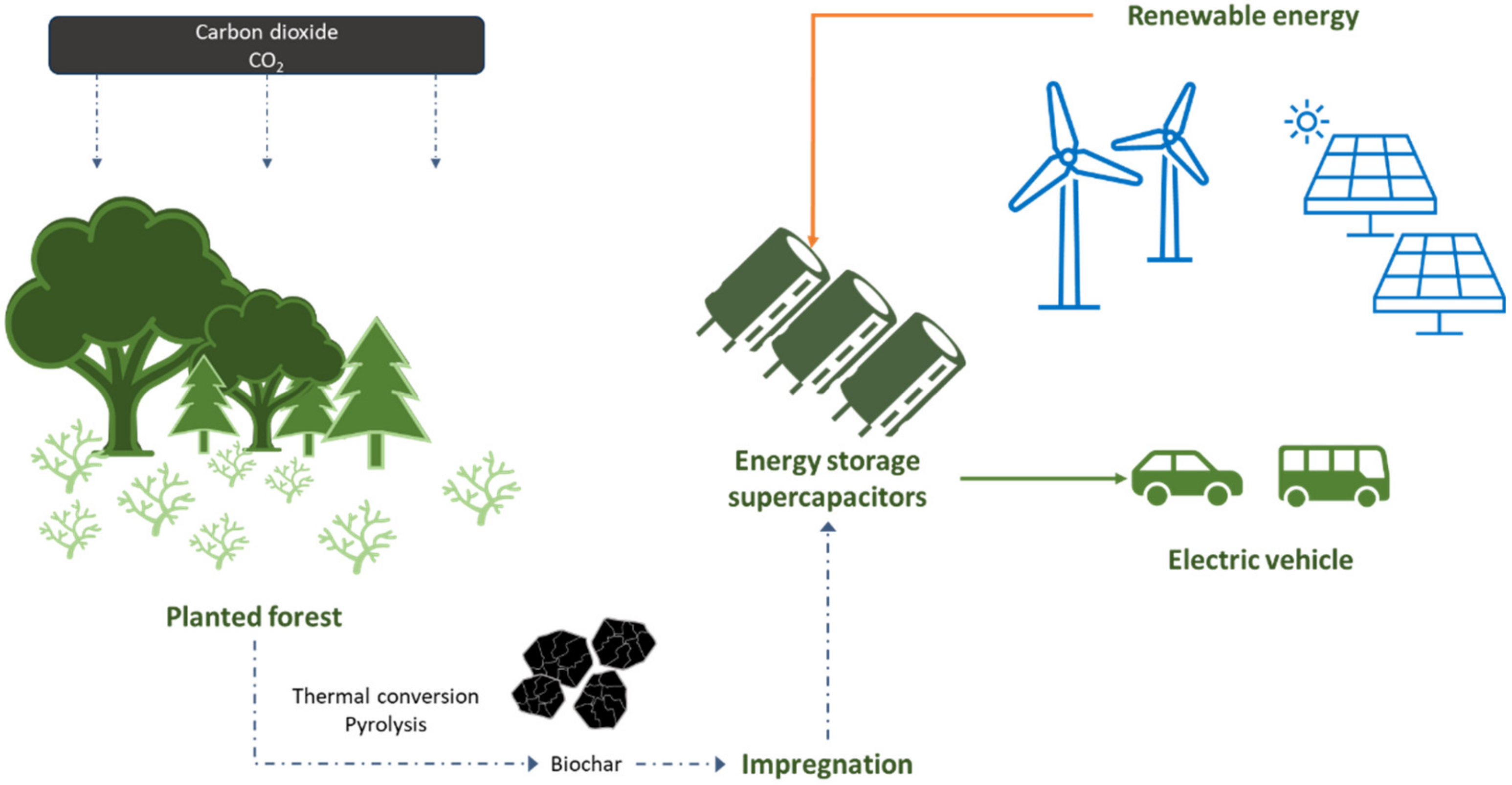
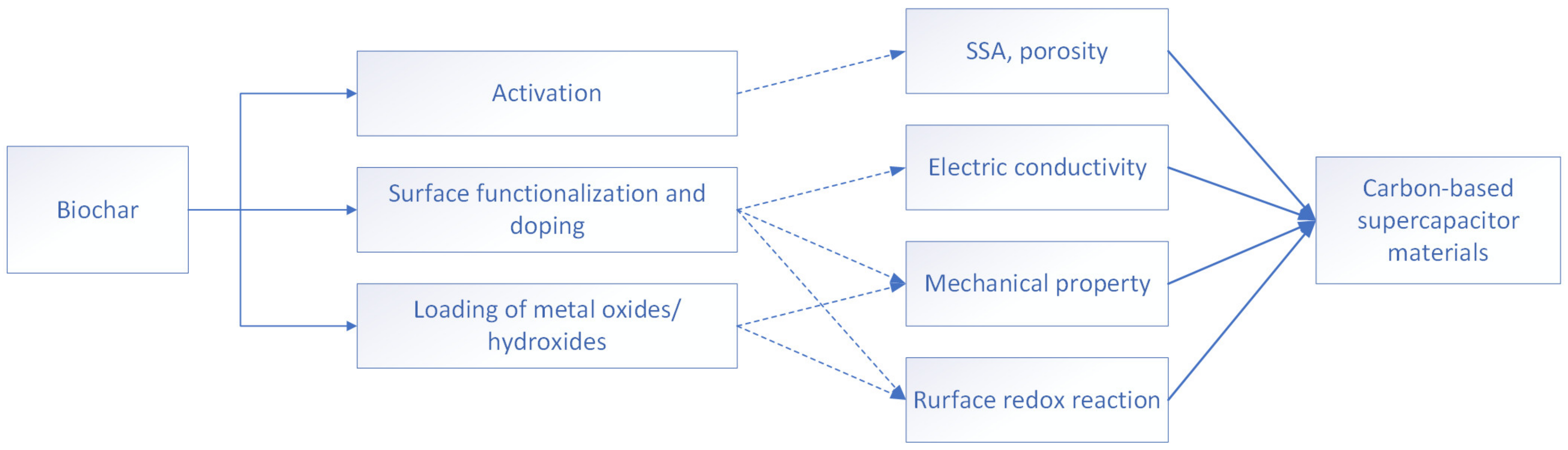
2.4. The Use of Biochar in Energy Storage Technologies
2.5. The Use of Carbonisate in Pickering Emulsions and Energy Storage
2.6. Hydrothermal Carbonization (HTC) as a Biomass Upgrade Method
3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Laird, D.A.; Brown, R.C.; Amonette, J.E.; Lehmann, J. Review of the Pyrolysis Platform for Coproducing Bio-Oil and Biochar. Biofuels Bioprod. Biorefining 2009, 3, 547–562. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar Properties and Eco-Friendly Applications for Climate Change Mitigation, Waste Management, and Wastewater Treatment: A Review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Routledge: London, UK, 2012; pp. 1–416. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A Synthesis of Its Agronomic Impact beyond Carbon Sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of Pyrolysis Temperature and Manure Source on Physicochemical Characteristics of Biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Mašek, O.; Brownsort, P.; Cross, A.; Sohi, S. Influence of Production Conditions on the Yield and Environmental Stability of Biochar. Fuel 2013, 103, 151–155. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of Low-Temperature Pyrolysis Conditions on Biochar for Agricultural Use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The Forms of Alkalis in the Biochar Produced from Crop Residues at Different Temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Strezov Vladimir, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of Pyrolysis Temperature on Production and Nutrient Properties of Wastewater Sludge Biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Kearns, J.P.; Wellborn, L.S.; Summers, R.S.; Knappe, D.R.U. 2,4-D Adsorption to Biochars: Effect of Preparation Conditions on Equilibrium Adsorption Capacity and Comparison with Commercial Activated Carbon Literature Data. Water Res. 2014, 62, 20–28. [Google Scholar] [CrossRef]
- Han Weng, Z.; van Zwieten, L.; Singh, B.P.; Tavakkoli, E.; Joseph, S.; Macdonald, L.M.; Rose, T.J.; Rose, M.T.; Kimber, S.W.L.; Morris, S.; et al. Biochar Built Soil Carbon over a Decade by Stabilizing Rhizodeposits. Nat. Clim. Change 2017, 7, 371–376. [Google Scholar] [CrossRef]
- The Emissions Gap Report 2017 A UN Environment Synthesis Report; United Nations Environment Programme (UNEP): Nairobi, Kenya, 2017.
- Roy, P.; Dias, G. Prospects for Pyrolysis Technologies in the Bioenergy Sector: A Review. Renew. Sustain. Energy Rev. 2017, 77, 59–69. [Google Scholar] [CrossRef]
- Joseph, S.; Graber, E.R.; Chia, C.; Munroe, P.; Donne, S.; Thomas, T.; Nielsen, S.; Marjo, C.; Rutlidge, H.; Pan, G.X.; et al. Shifting Paradigms: Development of High-Efficiency Biochar Fertilizers Based on Nano-Structures and Soluble Components. Carbon Manag. 2013, 4, 323–343. [Google Scholar] [CrossRef]
- Smith, P. Soil Carbon Sequestration and Biochar as Negative Emission Technologies. Glob. Change Biol. 2016, 22, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Fuss, S.; Lamb, W.F.; Callaghan, M.W.; Hilaire, J.; Creutzig, F.; Amann, T.; Beringer, T.; de Oliveira Garcia, W.; Hartmann, J.; Khanna, T.; et al. Negative Emissions—Part 2: Costs, Potentials and Side Effects. Environ. Res. Lett. 2018, 13, 063002. [Google Scholar] [CrossRef]
- Roberts, D.A.; Paul, N.A.; Dworjanyn, S.A.; Bird, M.I.; de Nys, R. Biochar from Commercially Cultivated Seaweed for Soil Amelioration. Sci. Rep. 2015, 5, 9665. [Google Scholar] [CrossRef] [PubMed]
- Shackley, S.; Hammond, J.; Gaunt, J.; Ibarrola, R. The Feasibility and Costs of Biochar Deployment in the UK. Carbon Manag. 2014, 2, 335–356. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Song, L.; Song, X.; Hänninen, H.; Wu, J. Biochar Enhances Nut Quality of Torreya Grandis and Soil Fertility under Simulated Nitrogen Deposition. Ecol. Manag. 2017, 391, 321–329. [Google Scholar] [CrossRef]
- Garciá, A.C.; de Souza, L.G.A.; Pereira, M.G.; Castro, R.N.; Garciá-Mina, J.M.; Zonta, E.; Lisboa, F.J.G.; Berbara, R.L.L. Structure-Property-Function Relationship in Humic Substances to Explain the Biological Activity in Plants. Sci. Rep. 2016, 6, 20798. [Google Scholar] [CrossRef]
- Bonanomi, G.; Ippolito, F.; Cesarano, G.; Nanni, B.; Lombardi, N.; Rita, A.; Saracino, A.; Scala, F. Biochar as Plant Growth Promoter: Better off Alone or Mixed with Organic Amendments? Front. Plant Sci. 2017, 8, 1570. [Google Scholar] [CrossRef]
- Dume, B.; Mosissa, T.; Nebiyu, A. Effect of Biochar on Soil Properties and Lead (Pb) Availability in a Military Camp in South West Ethiopia. Afr. J. Environ. Sci. Technol. 2016, 10, 77–85. [Google Scholar] [CrossRef]
- Verheijen, F.; Jeffery, S.; Bastos, A.C.; Van der Velde, M.; Diafas, I. Biochar Application to Soils—A Critical Scientific Review of Effects on Soil Properties, Processes and Functions. Environment 2010, 8, 144. [Google Scholar] [CrossRef]
- Nigussie, A.; Kissi, E.; Misganaw, M.; Ambaw, G. Effect of Biochar Application on Soil Properties and Nutrient Uptake of Lettuces (Lactuca Sativa) Grown in Chromium Polluted Soils. J. Agric. Environ. Sci. 2012, 12, 369–376. [Google Scholar]
- Placek, A.; Grobelak, A.; Kacprzak, M. Improving the Phytoremediation of Heavy Metals Contaminated Soil by Use of Sewage Sludge. Int. J. Phytoremediation 2016, 18, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Mensah, A.K.; Frimpong, K.A. Biochar and/or Compost Applications Improve Soil Properties, Growth, and Yield of Maize Grown in Acidic Rainforest and Coastal Savannah Soils in Ghana. Int. J. Agron. 2018, 2018, 6837404. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating Physical and Chemical Properties of Highly Weathered Soils in the Tropics with Charcoal—A Review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient Availability and Leaching in an Archaeological Anthrosol and a Ferralsol of the Central Amazon Basin: Fertilizer, Manure and Charcoal Amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Zhang, A.; Cui, L.; Pan, G.; Li, L.; Hussain, Q.; Zhang, X.; Zheng, J.; Crowley, D. Effect of Biochar Amendment on Yield and Methane and Nitrous Oxide Emissions from a Rice Paddy from Tai Lake Plain, China. Agric. Ecosyst. Environ. 2010, 139, 469–475. [Google Scholar] [CrossRef]
- Spokas, K.A.; Koskinen, W.C.; Baker, J.M.; Reicosky, D.C. Impacts of Woodchip Biochar Additions on Greenhouse Gas Production and Sorption/Degradation of Two Herbicides in a Minnesota Soil. Chemosphere 2009, 77, 574–581. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Downie, A.; Morris, S.; Petty, S.; Rust, J.; Chan, K.Y. A Glasshouse Study on the Interaction of Low Mineral Ash Biochar with Nitrogen in a Sandy Soil. Soil Res. 2010, 48, 569–576. [Google Scholar] [CrossRef]
- Chan, K.Y.; van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic Values of Greenwaste Biochar as a Soil Amendment. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Major, J.; Lehmann, J.; Rondon, M.; Goodale, C. Fate of Soil-Applied Black Carbon: Downward Migration, Leaching and Soil Respiration. Glob. Change Biol. 2010, 16, 1366–1379. [Google Scholar] [CrossRef]
- Singh, B.P.; Hatton, B.J.; Singh, B.; Cowie, A.L.; Kathuria, A. Influence of Biochars on Nitrous Oxide Emission and Nitrogen Leaching from Two Contrasting Soils. J. Environ. Qual. 2010, 39, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Xu, Y.; Liu, G.; Liu, Q.; Zhu, J.; Tu, C.; Amonette, J.E.; Cadisch, G.; Yong, J.W.H.; Hu, S. Impact of Biochar Application on Nitrogen Nutrition of Rice, Greenhouse-Gas Emissions and Soil Organic Carbon Dynamics in Two Paddy Soils of China. Plant Soil 2013, 370, 527–540. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Speir, R.A.; Harris, K.; Das, K.C.; Lee, R.D.; Morris, L.A.; Fisher, D.S. Effect of Peanut Hull and Pine Chip Biochar on Soil Nutrients, Corn Nutrient Status, and Yield. Agron. J. 2010, 102, 623–633. [Google Scholar] [CrossRef]
- Liu, H.; Idem, R.; Tontiwachwuthikul, P. Post-Combustion CO2 Capture Technology; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Kopczyński, M.; Lasek, J.A.; Iluk, A.; Zuwała, J. The Co-Combustion of Hard Coal with Raw and Torrefied Biomasses (Willow (Salix Viminalis), Olive Oil Residue and Waste Wood from Furniture Manufacturing). Energy 2017, 140, 1316–1325. [Google Scholar] [CrossRef]
- Ksepko, E.; Lysowski, R. Reactivity Study of Bimetallic Fe-Mn Oxides with Addition of TiO2 for Chemical Looping Combustion Purposes. Catalysts 2021, 11, 1437. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Modestra, J.A.; Amulya, K.; Butti, S.K.; Velvizhi, G. A Circular Bioeconomy with Biobased Products from CO2 Sequestration. Trends Biotechnol. 2016, 34, 506–519. [Google Scholar] [CrossRef]
- Zahed, M.A.; Movahed, E.; Khodayari, A.; Zanganeh, S.; Badamaki, M. Biotechnology for Carbon Capture and Fixation: Critical Review and Future Directions. J. Environ. Manag. 2021, 293, 112830. [Google Scholar] [CrossRef] [PubMed]
- Suali, E.; Sarbatly, R. Conversion of Microalgae to Biofuel. Renew. Sustain. Energy Rev. 2012, 16, 4316–4342. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-Value Products from Microalgae—Their Development and Commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Hanson, D.T. Breaking the Rules of Rubisco Catalysis. J. Exp. Bot. 2016, 67, 3180–3182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klinthong, W.; Yang, Y.H.; Huang, C.H.; Tan, C.S. A Review: Microalgae and Their Applications in CO2 Capture and Renewable Energy. Aerosol. Air Qual. Res. 2015, 15, 712–742. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Tazikeh, S.; Zendehboudi, S.; Ghafoori, S.; Lohi, A.; Mahinpey, N. Algal Bioenergy Production and Utilization: Technologies, Challenges, and Prospects. J. Environ. Chem. Eng. 2022, 10, 107863. [Google Scholar] [CrossRef]
- Kim, J.; Sovacool, B.K.; Bazilian, M.; Griffiths, S.; Lee, J.; Yang, M.; Lee, J. Decarbonizing the Iron and Steel Industry: A Systematic Review of Sociotechnical Systems, Technological Innovations, and Policy Options. Energy Res. Soc. Sci. 2022, 89, 102565. [Google Scholar] [CrossRef]
- Wang, R.Q.; Jiang, L.; Wang, Y.D.; Roskilly, A.P. Energy Saving Technologies and Mass-Thermal Network Optimization for Decarbonized Iron and Steel Industry: A Review. J. Clean. Prod. 2020, 274, 122997. [Google Scholar] [CrossRef]
- Lin, B.; Wu, R. Designing Energy Policy Based on Dynamic Change in Energy and Carbon Dioxide Emission Performance of China’s Iron and Steel Industry. J. Clean. Prod. 2020, 256, 120412. [Google Scholar] [CrossRef]
- Li, Z.; Hanaoka, T. Plant-Level Mitigation Strategies Could Enable Carbon Neutrality by 2060 and Reduce Non-CO2 Emissions in China’s Iron and Steel Sector. One Earth 2022, 5, 932–943. [Google Scholar] [CrossRef]
- Zhang, S.; Yi, B.W.; Worrell, E.; Wagner, F.; Crijns-Graus, W.; Purohit, P.; Wada, Y.; Varis, O. Integrated Assessment of Resource-Energy-Environment Nexus in China’s Iron and Steel Industry. J. Clean. Prod. 2019, 232, 235–249. [Google Scholar] [CrossRef]
- Khanna, R.; Li, K.; Wang, Z.; Sun, M.; Zhang, J.; Mukherjee, P.S. Biochars in Iron and Steel Industries; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 2017, ISBN 9780128148945. [Google Scholar]
- Huang, D.; Dinga, C.D.; Wen, Z.; Razmadze, D. Industrial-Environmental Management in China’s Iron and Steel Industry under Multiple Objectives and Uncertainties. J. Environ. Manag. 2022, 310, 114785. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Wang, S.; Lu, C.; Jiang, N.; Long, W.; Zhang, M.; Zhang, R. Environmental Impact Evaluation of an Iron and Steel Plant in China: Normalized Data and Direct/Indirect Contribution. J. Clean. Prod. 2020, 264, 121697. [Google Scholar] [CrossRef]
- Zhang, S.; Yi, B.; Guo, F.; Zhu, P. Exploring Selected Pathways to Low and Zero CO2 Emissions in China’s Iron and Steel Industry and Their Impacts on Resources and Energy. J. Clean. Prod. 2022, 340, 130813. [Google Scholar] [CrossRef]
- Ren, M.; Lu, P.; Liu, X.; Hossain, M.S.; Fang, Y.; Hanaoka, T.; O’Gallachoir, B.; Glynn, J.; Dai, H. Decarbonizing China’s Iron and Steel Industry from the Supply and Demand Sides for Carbon Neutrality. Appl. Energy 2021, 298, 117209. [Google Scholar] [CrossRef]
- Huang, D.; Dinga, C.D.; Tao, Y.; Wen, Z.; Wang, Y. Multi-Objective Optimization of Energy Conservation and Emission Reduction in China’s Iron and Steel Industry Based on Dimensionality Reduction. J. Clean. Prod. 2022, 368, 133131. [Google Scholar] [CrossRef]
- Yu, X.; Tan, C. China’s Pathway to Carbon Neutrality for the Iron and Steel Industry. Glob. Environ. Change 2022, 76, 102574. [Google Scholar] [CrossRef]
- Fan, Z.; Friedmann, S.J. Low-Carbon Production of Iron and Steel: Technology Options, Economic Assessment, and Policy. Joule 2021, 5, 829–862. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, Q.; Xu, L.; Tian, S.; Wang, P. Future CO2 Emission Trends and Radical Decarbonization Path of Iron and Steel Industry in China. J. Clean. Prod. 2021, 326, 129354. [Google Scholar] [CrossRef]
- Wu, R.; Lin, B. Environmental Regulation and Its Influence on Energy-Environmental Performance: Evidence on the Porter Hypothesis from China’s Iron and Steel Industry. Resour. Conserv. Recycl. 2022, 176, 105954. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Lu, C. Research on the Driving Factors and Carbon Emission Reduction Pathways of China’s Iron and Steel Industry under the Vision of Carbon Neutrality. J. Clean. Prod. 2022, 357, 131990, Erratum in 2022, 361, 132237.. [Google Scholar] [CrossRef]
- Na, H.; Sun, J.; Qiu, Z.; He, J.; Yuan, Y.; Yan, T.; Du, T. A Novel Evaluation Method for Energy Efficiency of Process Industry—A Case Study of Typical Iron and Steel Manufacturing Process. Energy 2021, 233, 121081. [Google Scholar] [CrossRef]
- Shukla, I. Potential of Renewable Agricultural Wastes in the Smart and Sustainable Steelmaking Process. J. Clean. Prod. 2022, 370, 133422. [Google Scholar] [CrossRef]
- Karakaya, E.; Nuur, C.; Assbring, L. Potential Transitions in the Iron and Steel Industry in Sweden: Towards a Hydrogen-Based Future? J. Clean. Prod. 2018, 195, 651–663. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona, P.; Peña, B.; Romeo, L.M. A Review on CO2 mitigation in the Iron and Steel Industry through Power to X Processes. J. CO2 Util. 2021, 46, 101456. [Google Scholar] [CrossRef]
- Conejo, A.N.; Birat, J.; Dutta, A. A Review of the Current Environmental Challenges of the Steel Industry and Its Value Chain. J. Environ. Manag. 2020, 259, 109782. [Google Scholar] [CrossRef]
- Ren, L.; Zhou, S.; Peng, T.; Ou, X. A Review of CO2 Emissions Reduction Technologies and Low-Carbon Development in the Iron and Steel Industry Focusing on China. Renew. Sustain. Energy Rev. 2021, 143, 110846. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, Z.; Yao, J.; Doh Dinga, C. Multi-Objective Optimization of Synergic Energy Conservation and CO2 Emission Reduction in China’s Iron and Steel Industry under Uncertainty. Renew. Sustain. Energy Rev. 2020, 134, 110128. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, Y.; Li, H.; Tao, Y.; De Clercq, D. Quantitative Analysis of the Precise Energy Conservation and Emission Reduction Path in China’s Iron and Steel Industry. J. Environ. Manag. 2019, 246, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.; Adams, T.A. Process Design and Techno-Economic Analysis of Biomass Pyrolysis By-Product Utilization in the Ontario and Aichi Steel Industries; Elsevier Masson SAS: Amsterdam, The Netherlands, 2022; Volume 49, ISBN 9780323851596. [Google Scholar]
- Sakhiya, A.K.; Anand, A.; Kaushal, P. Production, Activation, and Applications of Biochar in Recent Times; Springer: Singapore, 2020; Volume 2, ISBN 0123456789. [Google Scholar]
- Xie, Y.; Wang, L.; Li, H.; Westholm, L.J.; Carvalho, L.; Thorin, E.; Yu, Z.; Yu, X.; Skreiberg, Ø. A Critical Review on Production, Modification and Utilization of Biochar. J. Anal. Appl. Pyrolysis 2022, 161, 105405. [Google Scholar] [CrossRef]
- Praes, G.E.; De Arruda, J.D.; Lemos, L.R.; Tavares, R.P. Assessment of Iron Ore Pellets Production Using Two Charcoals with Different Content of Materials Volatile Replacing Partially Anthracite Fines. J. Mater. Res. Technol. 2019, 8, 1150–1160. [Google Scholar] [CrossRef]
- Jahanshahi, S.; Mathieson, J.G.; Somerville, M.A.; Haque, N.; Norgate, T.E.; Deev, A.; Pan, Y.; Xie, D.; Ridgeway, P.; Zulli, P. Development of Low-Emission Integrated Steelmaking Process. J. Sustain. Metall. 2015, 1, 94–114. [Google Scholar] [CrossRef]
- Rodrigues, T.; Braghini Junior, A. Technological Prospecting in the Production of Charcoal: A Patent Study. Renew. Sustain. Energy Rev. 2019, 111, 170–183. [Google Scholar] [CrossRef]
- Zajemska, M.; Zawada, A.; Poskart, A. Vitri Fi Cation of Environmentally Harmful By-Products from Biomass Torrefaction Process. J. Clean. Prod. 2020, 249, 119427. [Google Scholar] [CrossRef]
- Szwaja, S.; Poskart, A.; Zajemska, M. A New Approach for Evaluating Biochar Quality from Virginia Mallow Biomass Thermal Processing. J. Clean. Prod. 2019, 214, 356–364. [Google Scholar] [CrossRef]
- Coleti, J.L.; Manfredi, G.V.P.; Vinhal, J.T.; Junca, E.; Espinosa, D.C.R.; Tenório, J.A.S. Kinetic Investigation of Self-Reduction Basic Oxygen Furnace Dust Briquettes Using Charcoals from Different Biomass. J. Mater. Res. Technol. 2020, 9, 13282–13293. [Google Scholar] [CrossRef]
- Pinto, R.G.D.; Szklo, A.S.; Rathmann, R. CO2 Emissions Mitigation Strategy in the Brazilian Iron and Steel Sector–From Structural to Intensity Effects. Energy Policy 2018, 114, 380–393. [Google Scholar] [CrossRef]
- Rousset, P.; Figueiredo, C.; De Souza, M.; Quirino, W. Pressure Effect on the Quality of Eucalyptus Wood Charcoal for the Steel Industry: A Statistical Analysis Approach. Fuel Process. Technol. 2011, 92, 1890–1897. [Google Scholar] [CrossRef]
- De Castro, J.A.; da Silva, L.M.; de Medeiros, G.A.; de Oliveira, E.M.; Nogami, H. Analysis of a Compact Iron Ore Sintering Process Based on Agglomerated Biochar and Gaseous Fuels Using a 3D Multiphase Multicomponent Mathematical Model. J. Mater. Res. Technol. 2020, 9, 6001–6013. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R.; Sinha, O.P. Characterization of Charcoals Produced from Acacia, Albizia and Leucaena for Application in Ironmaking. Fuel 2022, 320, 123991. [Google Scholar] [CrossRef]
- De Paula Protásio, T.; Roque Lima, M.D.; Scatolino, M.V.; Silva, A.B.; Rodrigues de Figueiredo, I.C.; Gherardi Hein, P.R.; Trugilho, P.F. Charcoal Productivity and Quality Parameters for Reliable Classification of Eucalyptus Clones from Brazilian Energy Forests. Renew. Energy 2021, 164, 34–45. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Q.; Zheng, Z.; Cai, J. Material–Energy–Emission Nexus in the Integrated Iron and Steel Industry. Energy Convers. Manag. 2020, 213, 112828. [Google Scholar] [CrossRef]
- Lu, L.; Scientific, T.C.; Adam, M.; Scientific, T.C.; Sarath, H.; Scientific, T.C.; Jahanshahi, S. Iron Ore Sintering with Charcoal. In Proceedings of the 6th International Congress on the Science and Technology of Ironmaking, Rio de Janeiro, Brazil, 14–18 October 2012. [Google Scholar]
- Purwanto, H.; Zakiyuddin, A.M.; Rozhan, A.N.; Mohamad, A.S.; Salleh, H.M. Effect of Charcoal Derived from Oil Palm Empty Fruit Bunch on the Sinter Characteristics of Low Grade Iron Ore. J. Clean. Prod. 2018, 200, 954–959. [Google Scholar] [CrossRef]
- Venkataraman, M.; Csereklyei, Z.; Aisbett, E.; Rahbari, A.; Jotzo, F.; Lord, M.; Pye, J. Zero-Carbon Steel Production: The Opportunities and Role for Australia. Energy Policy 2022, 163, 112811. [Google Scholar] [CrossRef]
- Mousa, E.; Wang, C.; Riesbeck, J.; Larsson, M. Biomass Applications in Iron and Steel Industry: An Overview of Challenges and Opportunities. Renew. Sustain. Energy Rev. 2016, 65, 1247–1266. [Google Scholar] [CrossRef]
- Han, H.; Duan, D.; Yuan, P. Exploration of Straw Fiber as Reducing Agent Utilization in Rotary Hearth Furnace Process for Direct Reduced Iron Production. Steel Res. Int. 2015, 86, 1361–1369. [Google Scholar] [CrossRef]
- Cheng, Z.; Tan, Z.; Guo, Z.; Yang, J.; Wang, Q. Recent Progress in Sustainable and Energy-Efficient Technologies for Sinter Production in the Iron and Steel Industry. Renew. Sustain. Energy Rev. 2020, 131, 110034. [Google Scholar] [CrossRef]
- Ahlström, J.M.; Zetterholm, J.; Pettersson, K.; Harvey, S.; Wetterlund, E. Economic Potential for Substitution of Fossil Fuels with Liquefied Biomethane in Swedish Iron and Steel Industry—Synergy and Competition with Other Sectors. Energy Convers. Manag. 2020, 209, 112641. [Google Scholar] [CrossRef]
- He, K.; Wang, L. A Review of Energy Use and Energy—Efficient Technologies for the Iron and Steel Industry. Renew. Sustain. Energy Rev. 2017, 70, 1022–1039. [Google Scholar] [CrossRef]
- ArcelorMittal. Climate Action Report 2 July 2021 Forward-Looking Statements; ArcelorMittal: Luxembourg, 2021. [Google Scholar]
- Gul, E.; Riva, L.; Nielsen, H.K.; Yang, H.; Zhou, H.; Yang, Q.; Skreiberg, Ø.; Wang, L.; Barbanera, M.; Zampilli, M.; et al. Substitution of Coke with Pelletized Biocarbon in the European and Chinese Steel Industries: An LCA Analysis. Appl. Energy 2021, 304, 117644. [Google Scholar] [CrossRef]
- Bianco, L.; Baracchini, G.; Cirilli, F.; Di Sante, L.; Moriconi, A.; Moriconi, E.; Agorio, M.M.; Pfeifer, H.; Echterhof, T.; Demus, T.; et al. Sustainable Electric Arc Furnace Steel Production: GREENEAF. Berg-Und Hüttenmännische Mon. 2013, 158, 17–23. [Google Scholar] [CrossRef]
- Mousa, E.; Ahmed, H. Utilization of Biomass as an Alternative Fuel in Iron and Steel Making. In Iron Ore; Woodhead Publishing: Sawston, UK, 2021; pp. 665–690. [Google Scholar] [CrossRef]
- Echterhof, T.; Pfeifer, H. Study on Biochar Usage in the Electric Arc Furnace. In Proceedings of the 2nd International Conference Clean Technologies in the Steel Industry, Budapest, Hungary, 26–28 September 2011; pp. 1–10. [Google Scholar]
- Ooi, T.C.; Thompson, D.; Anderson, D.R.; Fisher, R.; Fray, T.; Zandi, M. The Effect of Charcoal Combustion on Iron-Ore Sintering Performance and Emission of Persistent Organic Pollutants. Combust Flame 2011, 158, 979–987. [Google Scholar] [CrossRef]
- Variny, M.; Varga, A.; Rimár, M.; Janošovský, J.; Kizek, J.; Lukáč, L.; Jablonský, G.; Mierka, O. Advances in Biomass Co-Combustion with Fossil Fuels in the European Context: A Review. Processes 2021, 9, 100. [Google Scholar] [CrossRef]
- Nwachukwu, C.M.; Wang, C.; Wetterlund, E. Exploring the Role of Forest Biomass in Abating Fossil CO2 Emissions in the Iron and Steel Industry—The Case of Sweden. Appl. Energy 2021, 288, 116558. [Google Scholar] [CrossRef]
- Schettini, B.L.S.; Jacovine, L.A.G.; Torres, C.M.M.E.; Carneiro, A.C.O.; Villanova, P.H.; da Rocha, S.J.S.S.; Rufino, M.P.M.X.; Silva, L.B.; Castro, R.V.O. Furnace-Kiln System: How Does the Use of New Technologies in Charcoal Production Affect the Carbon Balance? Ind. Crops Prod. 2022, 187, 115330. [Google Scholar] [CrossRef]
- De Souza, J.F.T.; Pacca, S.A. Carbon Reduction Potential and Costs through Circular Bioeconomy in the Brazilian Steel Industry. Resour. Conserv. Recycl. 2021, 169, 105517. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, J.; Zhou, L.; Liu, Y.; Guo, Z.; Wang, Q. Experimental Study of Commercial Charcoal as Alternative Fuel for Coke Breeze in Iron Ore Sintering Process. Energy Convers. Manag. 2016, 125, 254–263. [Google Scholar] [CrossRef]
- Cardarelli, A.; De Santis, M.; Cirilli, F.; Barbanera, M. Computational Fluid Dynamics Analysis of Biochar Combustion in a Simulated Ironmaking Electric Arc Furnace. Fuel 2022, 328, 125267. [Google Scholar] [CrossRef]
- Feliciano-Bruzual, C. Charcoal Injection in Blast Furnaces (Bio-PCI): CO2 Reduction Potential and Economic Prospects. J. Mater. Res. Technol. 2014, 3, 233–243. [Google Scholar] [CrossRef]
- Bazaluk, O.; Kieush, L.; Koveria, A.; Schenk, J.; Pfeiffer, A.; Zheng, H.; Lozynskyi, V. Metallurgical Coke Production with Biomass Additives: Study of Biocoke Properties for Blast Furnace and Submerged Arc Furnace Purposes. Materials 2022, 15, 1147. [Google Scholar] [CrossRef]
- Ahmed, H. New Trends in the Application of Carbon-Bearing Materials in Blast Furnace Iron-Making. Minerals 2018, 8, 561. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Dahl, E.; Kemppainen, A.; Gornostayev, S.; Koskela, A.; Fabritius, T. Effect of Charcoal and Kraft-Lignin Addition on Coke Compression Strength and Reactivity. Energies 2017, 10, 1850. [Google Scholar] [CrossRef]
- El-tawil, A.A.; Björkman, B.; Lundgren, M.; Bäck, F.; Ökvist, L.S. Influence of Modified Bio-Coals on Carbonization and Bio-Coke Reactivity. Metals 2021, 12, 61. [Google Scholar] [CrossRef]
- Yustanti, E.; Wardhono, E.Y.; Mursito, A.T.; Alhamidi, A. Types and Composition of Biomass in Biocoke Synthesis with the Coal Blending Method. Energies 2021, 14, 6570. [Google Scholar] [CrossRef]
- Somerville, M.; Jahanshahi, S. The Effect of Temperature and Compression during Pyrolysis on the Density of Charcoal Made from Australian Eucalypt Wood. Renew. Energy 2015, 80, 471–478. [Google Scholar] [CrossRef]
- Abdullah, I.; Ahmad, N.; Hussain, M.; Ahmed, A.; Ahmed, U.; Park, Y.K. Conversion of Biomass Blends (Walnut Shell and Pearl Millet) for the Production of Solid Biofuel via Torrefaction under Different Conditions. Chemosphere 2022, 295, 133894. [Google Scholar] [CrossRef] [PubMed]
- Kieush, L.; Schenk, J.; Pfeiffer, A.; Koveria, A.; Rantitsch, G.; Hopfinger, H. Investigation on the Influence of Wood Pellets on the Reactivity of Coke with CO2 and Its Microstructure Properties. Fuel 2022, 309, 122151. [Google Scholar] [CrossRef]
- MacPhee, J.A.; Gransden, J.F.; Giroux, L.; Price, J.T. Possible CO2 Mitigation via Addition of Charcoal to Coking Coal Blends. Fuel Process. Technol. 2009, 90, 16–20. [Google Scholar] [CrossRef]
- Latocha, W.; Kaczmarek, W.; Strugała, A.; Żarczyński, P. Rozszerzenie Bazy Węglowej Polskiego Koksownictwa Przez Wdrożenie Wstępnego Podsuszania Wsadu Oraz Zastosowanie Węgli Importowanych. Polityka Energetyczna 2011, 14, 215–229. [Google Scholar]
- Montiano, M.G.; Díaz-Faes, E.; Barriocanal, C. Partial Briquetting vs Direct Addition of Biomass in Coking Blends. Fuel 2014, 137, 313–320. [Google Scholar] [CrossRef]
- Flores, B.D.; Flores, I.V.; Guerrero, A.; Orellana, D.R.; Pohlmann, J.G.; Diez, M.A.; Borrego, A.G.; Osório, E.; Vilela, A.C.F. Effect of Charcoal Blending with a Vitrinite Rich Coking Coal on Coke Reactivity. Fuel Process. Technol. 2017, 155, 97–105. [Google Scholar] [CrossRef]
- Joseph, K.M.; Kasparian, H.J.; Shanov, V. Carbon Nanotube Fiber-Based Wearable Supercapacitors—A Review on Recent Advances. Energies 2022, 15, 6506. [Google Scholar] [CrossRef]
- Frackowiak, E.; Béguin, F. Electrochemical Storage of Energy in Carbon Nanotubes and Nanostructured Carbons. Carbon N. Y. 2002, 40, 1775–1787. [Google Scholar] [CrossRef]
- De, S.; Acharya, S.; Sahoo, S.; Chandra Nayak, G. Present Status of Biomass-Derived Carbon-Based Composites for Supercapacitor Application. In Nanostructured, Functional, and Flexible Materials for Energy Conversion and Storage Systems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 373–415. [Google Scholar] [CrossRef]
- Kouchachvili, L.; Maffei, N.; Entchev, E. Infested Ash Trees as a Carbon Source for Supercapacitor Electrodes. J. Porous Mater. 2015, 22, 979–988. [Google Scholar] [CrossRef]
- Kouchachvili, L.; Entchev, E. Ag/Biochar Composite for Supercapacitor Electrodes. Mater. Today Energy 2017, 6, 136–145. [Google Scholar] [CrossRef]
- Soffian, M.S.; Abdul Halim, F.Z.; Aziz, F.; Rahman, M.A.; Mohamed Amin, M.A.; Awang Chee, D.N. Carbon-Based Material Derived from Biomass Waste for Wastewater Treatment. Environ. Adv. 2022, 9, 100259. [Google Scholar] [CrossRef]
- Treviño-Cordero, H.; Juárez-Aguilar, L.G.; Mendoza-Castillo, D.I.; Hernández-Montoya, V.; Bonilla-Petriciolet, A.; Montes-Morán, M.A. Synthesis and Adsorption Properties of Activated Carbons from Biomass of Prunus Domestica and Jacaranda Mimosifolia for the Removal of Heavy Metals and Dyes from Water. Ind. Crops Prod. 2013, 42, 315–323. [Google Scholar] [CrossRef]
- Kalyani, P.; Anitha, A. Biomass Carbon & Its Prospects in Electrochemical Energy Systems. Int. J. Hydrogen Energy 2013, 38, 4034–4045. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, L.; Wang, X.; Holm, N.; Rajagopalan, K.; Chen, F.; Ma, S. Highly Ordered Macroporous Woody Biochar with Ultra-High Carbon Content as Supercapacitor Electrodes. Electrochim. Acta 2013, 113, 481–489. [Google Scholar] [CrossRef]
- Wang, L.; Ok, Y.S.; Tsang, D.C.W.; Alessi, D.S.; Rinklebe, J.; Wang, H.; Mašek, O.; Hou, R.; O’Connor, D.; Hou, D. New Trends in Biochar Pyrolysis and Modification Strategies: Feedstock, Pyrolysis Conditions, Sustainability Concerns and Implications for Soil Amendment. Soil Use Manag. 2020, 36, 358–386. [Google Scholar] [CrossRef]
- Monticelli, D.; Binda, G.; Spanu, D.; Cancelliere, R.; Cianciaruso, M.; Carbone, K.; Micheli, L. Biochar: A Sustainable Alternative in the Development of Electrochemical Printed Platforms. Chemosensors 2022, 10, 344. [Google Scholar] [CrossRef]
- Patwardhan, S.B.; Pandit, S.; Kumar Gupta, P.; Kumar Jha, N.; Rawat, J.; Joshi, H.C.; Priya, K.; Gupta, M.; Lahiri, D.; Nag, M.; et al. Recent Advances in the Application of Biochar in Microbial Electrochemical Cells. Fuel 2022, 311, 122501. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dubey, M.; Kharel, P.; Gu, Z.; Fan, Q.H. Biochar Activated by Oxygen Plasma for Supercapacitors. J. Power Sources 2015, 274, 1300–1305. [Google Scholar] [CrossRef]
- Cheng, B.H.; Zeng, R.J.; Jiang, H. Recent Developments of Post-Modification of Biochar for Electrochemical Energy Storage. Bioresour. Technol. 2017, 246, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yan, J.; Hao, L.; Xue, R.; Sun, G.; Yi, B. High Rate Performance Activated Carbons Prepared from Ginkgo Shells for Electrochemical Supercapacitors. Carbon N. Y. 2013, 56, 146–154. [Google Scholar] [CrossRef]
- Oluwafikayo Adegoke, S.; Akanni Adeleke, A.; Pelumi Ikubanni, P.; Timothy Nnodim, C.; Olubusayo Balogun, A.; Adebanjo Falode, O.; Olawumi Adetona, S.; Kumar Shukla, S. Energy from Biomass and Plastics Recycling: A Review Energy from Biomass and Plastics Recycling: A Review Public Interest Statement. Cogent Eng. 2021, 8, 1994106. [Google Scholar] [CrossRef]
- Li, H.; Aguirre-Villegas, H.A.; Allen, R.D.; Bai, X.; Benson, C.H.; Beckham, G.T.; Bradshaw, S.L.; Brown, J.L.; Brown, R.C.; Cecon, V.S.; et al. Expanding Plastics Recycling Technologies: Chemical Aspects, Technology Status and Challenges. Green Chem. 2022, 24, 8899–9002. [Google Scholar] [CrossRef]
- Sieradzka, M.; Kirczuk, C.; Kalemba-rec, I.; Mlonka-mędrala, A.; Magdziarz, A. Pyrolysis of Biomass Wastes into Carbon Materials. Energies 2022, 15, 1941. [Google Scholar] [CrossRef]
- Wan, Z.; Chen, C.; Meng, T.; Mojtaba, M.; Teng, Y.; Feng, Q.; Li, D. Multifunctional Wet-Spun Filaments through Robust Nanocellulose Networks Wrapping to Single-Walled Carbon Nanotubes. ACS Appl. Mater. Interfaces 2019, 11, 42808–42817. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Ding, X.; Li, Y.; Zhang, P.; Shu, M.; Zhang, Q.; Gong, Y.; Zheng, K.; Wu, B.; et al. Enhanced Thermal Conductivity of Carbon Nitride-Doped Graphene/Polyimide Composite Film via a “Deciduous-like” Strategy. Compos. Sci. Technol. 2021, 205, 108693. [Google Scholar] [CrossRef]
- Cao, Q.; Cui, Q.; Yang, Y.; Xu, J.; Han, C.; Li, L. Graphitic Carbon Nitride as a Distinct Solid Stabilizer for Emulsion Polymerization. Chem. Eur. J. 2018, 24, 2286–2291. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as Surfactants—Similarities and Differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Leunissen, M.E.; van Blaaderen, A.; Hollingsworth, A.D.; Sullivan, M.T.; Chaikin, P.M. Electrostatics at the Oil-Water Interface, Stability, and Order in Emulsions and Colloids. Proc. Natl. Acad. Sci. USA 2007, 104, 2585–2590. [Google Scholar] [CrossRef]
- Yuan, Q.; Cayre, O.J.; Manga, M.; Williams, R.A.; Biggs, S. Preparation of Particle-Stabilized Emulsions Using Membrane Emulsification. Soft Matter 2010, 6, 1580–1588. [Google Scholar] [CrossRef]
- Jin, Y.; Lee, W.; Musina, Z.; Ding, Y. A One-Step Method for Producing Microencapsulated Phase Change Materials. Particuology 2010, 8, 588–590. [Google Scholar] [CrossRef]
- Xiao, B.; Yuan, Q.; Williams, R.A. Exceptional Function of Nanoporous Metal Organic Framework Particles in Emulsion Stabilisation. Chem. Commun. 2013, 49, 8208–8210. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Cairns, A.J.; Liu, Y.; Belmabkhout, Y.; Zeng, H.C.; Eddaoudi, M. Synthesis and Integration of Fe-Soc-MOF Cubes into Colloidosomes via a Single-Step Emulsion-Based Approach. J. Am. Chem. Soc. 2013, 135, 10234–10237. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Resasco, D.E. Emulsions Stabilized by Carbon Nanotube-Silica Nanohybrids. Langmuir 2009, 25, 10843–10851. [Google Scholar] [CrossRef]
- Yuan, Q.; Williams, R.A. CO-Stabilisation Mechanisms of Nanoparticles and Surfactants in Pickering Emulsions Produced by Membrane Emulsification. J. Membr. Sci. 2016, 497, 221–228. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A Review of Microencapsulation Methods of Phase Change Materials (PCMs) as a Thermal Energy Storage (TES) Medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Ghani, S.A.A.; Jamari, S.S.; Abidin, S.Z. Waste Materials as the Potential Phase Change Material Substitute in Thermal Energy Storage System: A Review. Chem. Eng. Commun. 2020, 208, 687–707. [Google Scholar] [CrossRef]
- Wan, Y.C.; Chen, Y.; Cui, Z.X.; Ding, H.; Gao, S.F.; Han, Z.; Gao, J.K. A Promising Form-Stable Phase Change Material Prepared Using Cost Effective Pinecone Biochar as the Matrix of Palmitic Acid for Thermal Energy Storage. Sci. Rep. 2019, 9, 11535. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Wi, S.; Yun, B.Y.; Kim, S. Engineering Biochar with Multiwalled Carbon Nanotube for Efficient Phase Change Material Encapsulation and Thermal Energy Storage. Energy 2021, 216, 119294. [Google Scholar] [CrossRef]
- Gondora, W.; Doudin, K.; Nowakowski, D.J.; Xiao, B.; Ding, Y.; Bridgwater, T.; Yuan, Q. Encapsulation of Phase Change Materials Using Rice-Husk-Char. Appl. Energy 2016, 182, 274–281. [Google Scholar] [CrossRef]
- Advincula, P.A.; de Leon, A.C.; Rodier, B.J.; Kwon, J.; Advincula, R.C.; Pentzer, E.B. Accommodating Volume Change and Imparting Thermal Conductivity by Encapsulation of Phase Change Materials in Carbon Nanoparticles. J. Mater. Chem. A Mater. 2018, 6, 2461–2467. [Google Scholar] [CrossRef]
- ALOthman, Z.A.; Naushad, M.; Ali, R. Kinetic, Equilibrium Isotherm and Thermodynamic Studies of Cr(VI) Adsorption onto Low-Cost Adsorbent Developed from Peanut Shell Activated with Phosphoric Acid. Environ. Sci. Pollut. Res. 2013, 20, 3351–3365. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, M.K.; Thakur, M.; Gurung, R.B.; Srivastava, R. Graphene Quantum Dots for Cell Proliferation, Nucleus Imaging, and Photoluminescent Sensing Applications. Sci. Rep. 2017, 7, 15858. [Google Scholar] [CrossRef]
- Nirala, N.R.; Khandelwal, G.; Kumar, B.; Vinita; Prakash, R.; Kumar, V. One Step Electro-Oxidative Preparation of Graphene Quantum Dots from Wood Charcoal as a Peroxidase Mimetic. Talanta 2017, 173, 36–43. [Google Scholar] [CrossRef]
- Bach, Q.V.; Skreiberg, O. Upgrading Biomass Fuels via Wet Torrefaction: A Review and Comparison with Dry Torrefaction. Renew. Sustain. Energy Rev. 2016, 54, 665–677. [Google Scholar] [CrossRef]
- Marcus, Y. Extraction by Subcritical and Supercriticalwater, Methanol, Ethanol and Their Mixtures. Separations 2018, 5, 4. [Google Scholar] [CrossRef]
- Kubátová, A.; Miller, D.J.; Hawthorne, S.B. Comparison of Subcritical Water and Organic Solvents for Extracting Kava Lactones from Kava Root. J. Chromatogr. A 2001, 923, 187–194. [Google Scholar] [CrossRef]
- Tran, K.Q. Fast Hydrothermal Liquefaction for Production of Chemicals and Biofuels from Wet Biomass—The Need to Develop a Plug-Flow Reactor. Bioresour. Technol. 2016, 213, 327–332. [Google Scholar] [CrossRef]
- Sharma, R.; Jasrotia, K.; Singh, N.; Ghosh, P.; Srivastava, S.; Sharma, N.R.; Singh, J.; Kanwar, R.; Kumar, A. A Comprehensive Review on Hydrothermal Carbonization of Biomass and Its Applications. Chem. Afr. 2020, 3, 1–19. [Google Scholar] [CrossRef]
- Sobek, S.; Tran, Q.-K.; Junga, R.; Werle, S. Hydrothermal Carbonization of the Waste Straw: A Study of the Biomass Transient Heating Behavior and Solid Products Combustion Kinetics. Fuel 2021, 314, 122725. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Jayaraman, K.; Szymańska-Chargot, M.; Gökalp, I. Hydrothermal Carbonization Characteristics of Sewage Sludge and Lignocellulosic Biomass. A Comparative Study. Biomass Bioenergy 2019, 120, 166–175. [Google Scholar] [CrossRef]
- Huang, H.; Su, Q.; Li, J.; Niu, Z.; Wang, D.; Wei, C.; Long, S.; Ren, J.; Wang, J.; Shan, B.; et al. Effects of Process Water Obtained from Hydrothermal Carbonization of Poultry Litter on Soil Microbial Community, Nitrogen Transformation, and Plant Nitrogen Uptake. J. Environ. Manag. 2022, 323, 116307. [Google Scholar] [CrossRef]
- Ma, Q.; Han, L.; Huang, G. Effect of Water-Washing of Wheat Straw and Hydrothermal Temperature on Its Hydrochar Evolution and Combustion Properties. Bioresour. Technol. 2018, 269, 96–103. [Google Scholar] [CrossRef]
- Reza, M.T.; Rottler, E.; Herklotz, L.; Wirth, B. Hydrothermal Carbonization (HTC) of Wheat Straw: Influence of Feedwater PH Prepared by Acetic Acid and Potassium Hydroxide. Bioresour. Technol. 2015, 182, 336–344. [Google Scholar] [CrossRef]
- Vega, M.F.; Florentino-Madiedo, L.; Díaz-Faes, E.; Barriocanal, C. Influence of Feedwater PH on the CO2 Reactivity of Hydrochars. Co-Carbonisation with a Bituminous Coal. Renew. Energy 2021, 170, 824–831. [Google Scholar] [CrossRef]
- Hu, Y.; Gallant, R.; Salaudeen, S.; Farooque, A.A.; He, S. Hydrothermal Carbonization of Spent Coffee Grounds for Producing Solid Fuel. Sustainability 2022, 14, 8818. [Google Scholar] [CrossRef]
- Arauzo, P.J.; Lucian, M.; Du, L.; Olszewski, M.P.; Fiori, L.; Kruse, A. Improving the Recovery of Phenolic Compounds from Spent Coffee Grounds by Using Hydrothermal Delignification Coupled with Ultrasound Assisted Extraction. Biomass Bioenergy 2020, 139, 105616. [Google Scholar] [CrossRef]
- Junga, R.; Werle, S.; Chabiński, M.; Ziółkowski, Ł. Experimental Analysis of the Fixed Bed Gasification Process of the Mixtures of the Chicken Manure with Biomass. Renew. Energy 2019, 136, 1055–1063. [Google Scholar] [CrossRef]
- Ghanim, B.M.; Pandey, D.S.; Kwapinski, W.; Leahy, J.J. Hydrothermal Carbonisation of Poultry Litter: Effects of Treatment Temperature and Residence Time on Yields and Chemical Properties of Hydrochars. Bioresour. Technol. 2016, 216, 373–380. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.I.; Grasham, O.; Ross, A.B.; Dupont, V.; Camargo-Valero, M.A. Hydrothermal Carbonization of Sewage Digestate at Wastewater Treatment Works: Influence of Solid Loading on Characteristics of Hydrochar, Process Water and Plant Energetics. Renew. Energy 2020, 157, 959–973. [Google Scholar] [CrossRef]
- Smith, A.M.; Singh, S.; Ross, A.B. Fate of Inorganic Material during Hydrothermal Carbonisation of Biomass: Influence of Feedstock on Combustion Behaviour of Hydrochar. Fuel 2016, 169, 135–145. [Google Scholar] [CrossRef]
- Werle, S.; Dudziak, M. Analysis of Organic and Inorganic Contaminants in Dried Sewage Sludge and By-Products of Dried Sewage Sludge Gasification. Energies 2014, 7, 462–476. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Shama, G.; Wheatley, A.D.; Martin, S.J.; Holdich, R.G. Hydrothermal Carbonisation of Sewage Sludge: Effect of Process Conditions on Product Characteristics and Methane Production. Bioresour. Technol. 2015, 177, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, A.; Kokko, M.; Kinnunen, V.; Hilli, T.; Rintala, J. Hydrothermal Carbonisation of Mechanically Dewatered Digested Sewage Sludge—Energy and Nutrient Recovery in Centralised Biogas Plant. Water Res. 2021, 201, 117284. [Google Scholar] [CrossRef]
- Afolabi, O.O.D.; Sohail, M.; Cheng, Y.L. Optimisation and Characterisation of Hydrochar Production from Spent Coffee Grounds by Hydrothermal Carbonisation. Renew. Energy 2020, 147, 1380–1391. [Google Scholar] [CrossRef]
- Weldekidan, H.; Strezov, V.; Li, R.; Kan, T.; Town, G.; Kumar, R.; He, J.; Flamant, G. Distribution of Solar Pyrolysis Products and Product Gas Composition Produced from Agricultural Residues and Animal Wastes at Different Operating Parameters. Renew. Energy 2020, 151, 1102–1109. [Google Scholar] [CrossRef]
- Sobek, S.; Werle, S. Solar Pyrolysis of Waste Biomass: A Comparative Study of Products Distribution, in Situ Heating Behavior, and Application of Model-Free Kinetic Predictions. Fuel 2021, 292, 120365. [Google Scholar] [CrossRef]
- Han, S.; Bai, L.; Chi, M.; Xu, X.; Chen, Z.; Yu, K. Conversion of Waste Corn Straw to Value-Added Fuel via Hydrothermal Carbonization after Acid Washing. Energies 2022, 15, 1828. [Google Scholar] [CrossRef]
- Yu, Y.; Lau, A.; Sokhansanj, S. Hydrothermal Carbonization and Pelletization of Moistened Wheat Straw. Renew. Energy 2022, 190, 1018–1028. [Google Scholar] [CrossRef]
- Hejna, M.; Świechowski, K.; Rasaq, W.A.; Białowiec, A. Study on the Effect of Hydrothermal Carbonization Parameters on Fuel Properties of Chicken Manure Hydrochar. Materials 2022, 15, 5564. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Gholizadeh, M.; Hu, X.; Yuan, X.; Sarkar, B.; Vithanage, M.; Mašek, O.; Ok, Y.S. Co-Hydrothermal Carbonization of Swine and Chicken Manure: Influence of Cross-Interaction on Hydrochar and Liquid Characteristics. Sci. Total Environ. 2021, 786, 147381. [Google Scholar] [CrossRef] [PubMed]
- Danso-Boateng, E.; Holdich, R.G.; Shama, G.; Wheatley, A.D.; Sohail, M.; Martin, S.J. Kinetics of Faecal Biomass Hydrothermal Carbonisation for Hydrochar Production. Appl. Energy 2013, 111, 351–357. [Google Scholar] [CrossRef]

| Biomass Type | Ultimate Analysis [wt%] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Carbon | Hydrogen | Oxygen | Nitrogen | Sulphur | ||||||
| Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | |
| Agro-industrial resides | 68.37 | 91.61 | 0.75 | 3.44 | 6.22 | 21.68 | 0.85 | 9.25 | 0.07 | 0.14 |
| Grass | 54.28 | 70.26 | 2.77 | 3.84 | 27.47 | 41.12 | 1.71 | 1.8 | 0.03 | 0.19 |
| Olive husk and pit | 69.95 | 94.39 | 0.53 | 5.62 | 4.31 | 18.77 | 0.39 | 2.59 | 0.04 | 0.04 |
| Peanut shells | 70.6 | 85.1 | 0.8 | 4.7 | 11.5 | 23.5 | 1 | 1.2 | n/d | n/d |
| Straw | 47.92 | 84.33 | 2.78 | 6.54 | 10 | 44.8 | 0.53 | 1.93 | 0.09 | 0.97 |
| Wheat straw | 73.05 | 88.11 | 2.28 | 3.85 | 8.05 | 22.03 | 0.72 | 1.45 | 0.11 | 0.13 |
| Wood | 64.55 | 92.99 | 0.7 | 6.13 | 2.99 | 23.89 | 0.01 | 3.1 | 0.02 | 1.01 |
| Parameters | Coke | Blast Furnace Coke | Charcoal | Steel Quality Charcoal |
|---|---|---|---|---|
| Fixed carbon (%) | 88–92 | 88 | 65–75 | 74–77 |
| Volatile matter (%) | 7.8–11 | 1 | 25–35 | 22–25 |
| Ash (%) | 0.1–0.5 | 10–12 | 2–5 | 1–1.5 |
| Alkalis | low | Low | High | <20% |
| Resistance to compression (kg/cm2) | - | 130–160 | 10–80 | 50–100 |
| Feedstock Type and Raw Biomass HHV | T, °C | Time | MY, % | HHV, MJ/kg | EY, % | Ref. |
|---|---|---|---|---|---|---|
| Wheat straw (HHV = 20.0 MJ/kg) | 200 | 6 h | 53.1 ± 2.24 | 20.95 ± 0.37 | 55.61 ± 2.27 | Reza et al. [167] |
| 260 | 34.3 ± 2.54 | 26.4 ± 0.31 | 45.28 ± 2.26 | |||
| Waste straw (HHV = 17.04 MJ/kg) | 225 | 10–40 min | 64.18 ± 3.19 | 19.45 ± 0.59 | 73.19 ± 1.65 | Sobek et al. [163] |
| 250 | 60.74 ± 3.19 | 20.71 ± 1.05 | 73.79 ± 3.19 | |||
| 275 | 42.9 ± 3.19 | 25.00 ± 1.34 | 62.69 ± 3.65 | |||
| Waste corn straw, no acid washing (HHV = 16.3 MJ/kg) | 180 | 60 min | 74.5 ± 0.2 | 17.30 | 77.3 | Han et al. [181] |
| 210 | 67.9 ± 0.4 | 18.60 | 77.1 | |||
| 240 | 42.6 ± 1.1 | 24.20 | 63.1 | |||
| 270 | 38.5 ± 1.3 | 25.90 | 60.8 | |||
| Wheat straw (HHV = 16.1 ± 0.1 MJ/kg) * | 100 | 15–30 min | 99 ± 1.0 | 16.90 | 105 ± 2.5 | Yu et al. [182] |
| 140 | 90 ± 2.1 | 18.25 | 106 ± 2.0 | |||
| 180 | 70 ± 2.0 | 20.55 | 90 ± 2.0 | |||
| 220 | 63 ± 2.3 | 22.05 | 82 ± 2.0 | |||
| Spent coffee grains (HHV = 22.83 MJ/kg) | 150 | 30 min | 80.5 ± 2.0 | 23.54 ± 0.08 | 83.93 | Hu et al. [169] |
| 170 | 68 ± 5.0 | 26.55 ± 2.46 | 79.95 | |||
| 190 | 63.0 ± 0.5 | 26.44 ± 0.40 | 73.88 | |||
| 210 | 62.0 ± 0.5 | 27.65 ± 0.11 | 77.05 | |||
| Spent coffee grounds (HHV n.a.) * | 180 | 1–5 h | n.a. | 26.00 | 81.16 ± 1.75 | Afolabi et al. [178] |
| 200 | n.a. | 28.00 | 85.14 ± 0.64 | |||
| 220 | n.a. | 32.00 | 83.69 ± 4.09 | |||
| Poultry litter (HHV = 17.18 ± 0.02 MJ/kg) | 150 | 30–480 min | 79.67 ± 7.93 | 17.75 ± 0.43 | 82.27 ± 8.06 | Ghanim et al. [172] |
| 200 | 42.48 ± 2.99 | 21.98 ± 0.65 | 54.42 ± 5.17 | |||
| 250 | 32.35 ± 0.01 | 24.39 ± 0.94 | 46.50 ± 1.81 | |||
| 300 | 26.13 ± 3.20 | 23.64 ± 1.23 | 36.10 ± 6.33 | |||
| Poultry litter (HHV = 15.55 ± 0.052 MJ/kg) | 180 | 30–180 min | 66.08 ± 5.43 | 17.40 ± 0.78 | 74.05 ± 8.46 | Hejna et al. [183] |
| 240 | 62.00 ± 5.56 | 21.43 ± 0.40 | 85.36 ± 6.03 | |||
| 300 | 42.12 ± 2.51 | 23.17 ± 0.95 | 62.66 ± 1.50 | |||
| Chicken manure (HHV = 11.95 MJ/kg) | 240 | 10 h | 46.6 | 11.4 ± 0.25 | 42.55 ± 1.56 | Li et al. [184] |
| Sewage sludge (HHV = n.a.) | 140 | 15–240 min | 74.64 | 16.66 | 76.14 | Danso-Boateng et al. [185] |
| 160 | 73.11 ± 7.23 | 17.97 ± 0.25 | 81.35 ± 9.60 | |||
| 180 | 66.03 ± 3.82 | 17.95 ± 0.44 | 72.80 ± 5.11 | |||
| 200 | 64.40 ± 3.71 | 18.085 ± 0.80 | 70.74 ± 5.13 | |||
| Sewage sludge, digestate (HHV = 11.49 MJ/kg) | 210 | 30–120 min | 87.02 ± 0.79 | 11.35 ± 0.04 | 86.00 ± 1.05 | Hämäläinen et al. [177] |
| 230 | 81.80 ± 0.25 | 11.76 ± 0.18 | 83.72 ± 1.06 | |||
| 250 | 73.74 ± 2.12 | 11.98 ± 0.19 | 76.93 ± 3.44 | |||
| Sewage sludge, dilluted digestate (HHV = 11.9 MJ/kg) | 210 | 30–120 min | 83.47 ± 6.82 | 11.37 ± 0.02 | 79.72 ± 6.66 | |
| 230 | 77.53 ± 0.18 | 11.65 ± 0.24 | 75.89 ± 1.39 | |||
| 250 | 74.79 ± 3.95 | 12.03 ± 0.23 | 75.54 ± 2.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajdak, M.; Muzyka, R.; Gałko, G.; Ksepko, E.; Zajemska, M.; Sobek, S.; Tercki, D. Actual Trends in the Usability of Biochar as a High-Value Product of Biomass Obtained through Pyrolysis. Energies 2023, 16, 355. https://doi.org/10.3390/en16010355
Sajdak M, Muzyka R, Gałko G, Ksepko E, Zajemska M, Sobek S, Tercki D. Actual Trends in the Usability of Biochar as a High-Value Product of Biomass Obtained through Pyrolysis. Energies. 2023; 16(1):355. https://doi.org/10.3390/en16010355
Chicago/Turabian StyleSajdak, Marcin, Roksana Muzyka, Grzegorz Gałko, Ewelina Ksepko, Monika Zajemska, Szymon Sobek, and Dariusz Tercki. 2023. "Actual Trends in the Usability of Biochar as a High-Value Product of Biomass Obtained through Pyrolysis" Energies 16, no. 1: 355. https://doi.org/10.3390/en16010355
APA StyleSajdak, M., Muzyka, R., Gałko, G., Ksepko, E., Zajemska, M., Sobek, S., & Tercki, D. (2023). Actual Trends in the Usability of Biochar as a High-Value Product of Biomass Obtained through Pyrolysis. Energies, 16(1), 355. https://doi.org/10.3390/en16010355










