Eco-Friendly and Effective Diatomaceous Earth/Peat (DEP) Microbial Carriers in the Anaerobic Biodegradation of Food Waste Products
Abstract
:1. Introduction
2. Materials and Methods
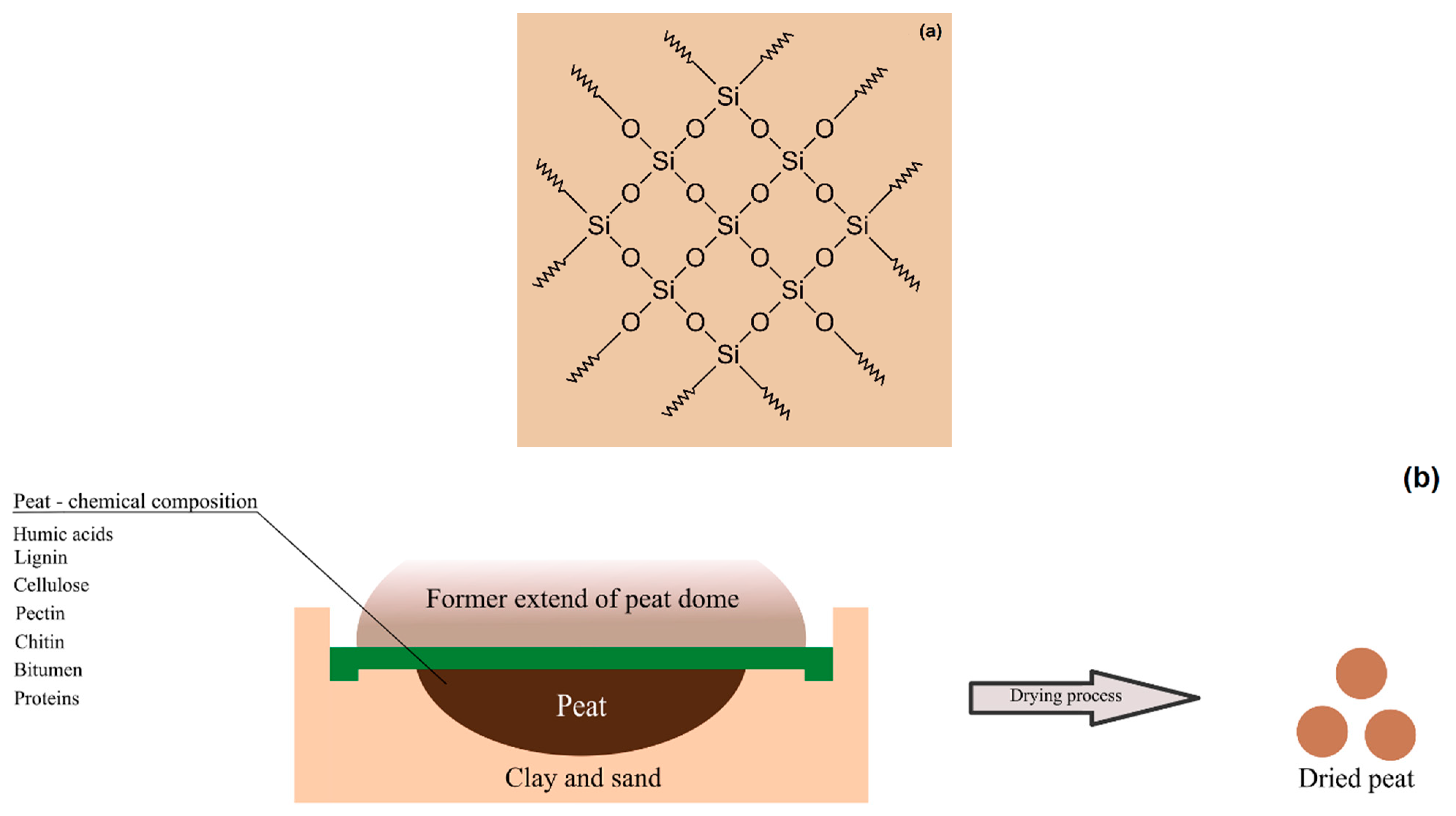
2.1. Substrate and Carriers
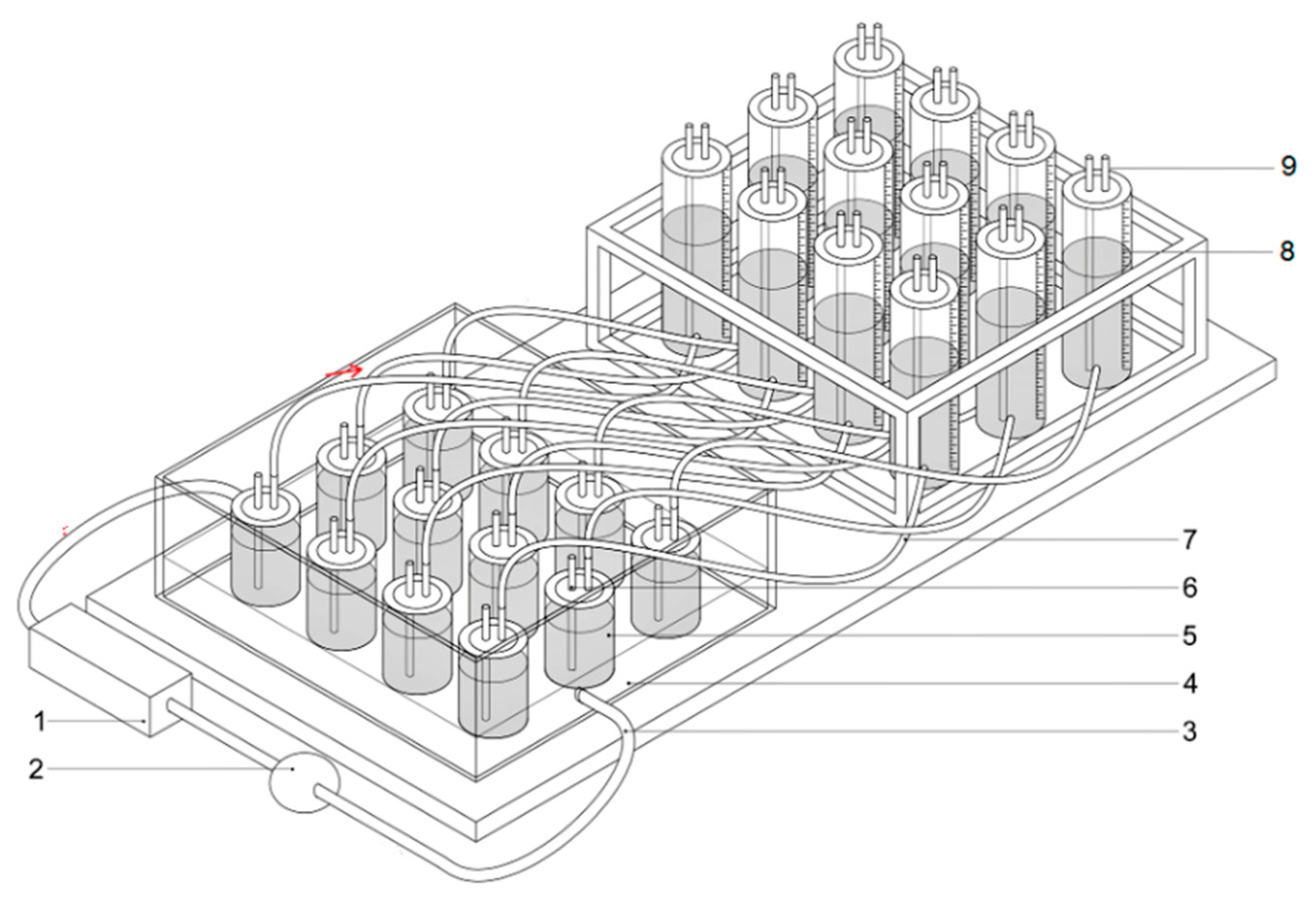
2.2. Biogas Production Set-Up
2.2.1. Batch Preparation
2.2.2. Anaerobic Digestion
2.3. Analytical Methods
2.3.1. Physicochemical Research of the Carrier
2.3.2. Physicochemical Analysis of Substrate and Samples
2.3.3. Biochemical Analysis of Digestate
2.3.4. Bacillus Amyloliquefaciens Biomass Determination
3. Results and Discussion
3.1. Feedstock and Inoculum Characterisation
3.2. Diatomaceous Earth/Peat Properties and Productivity
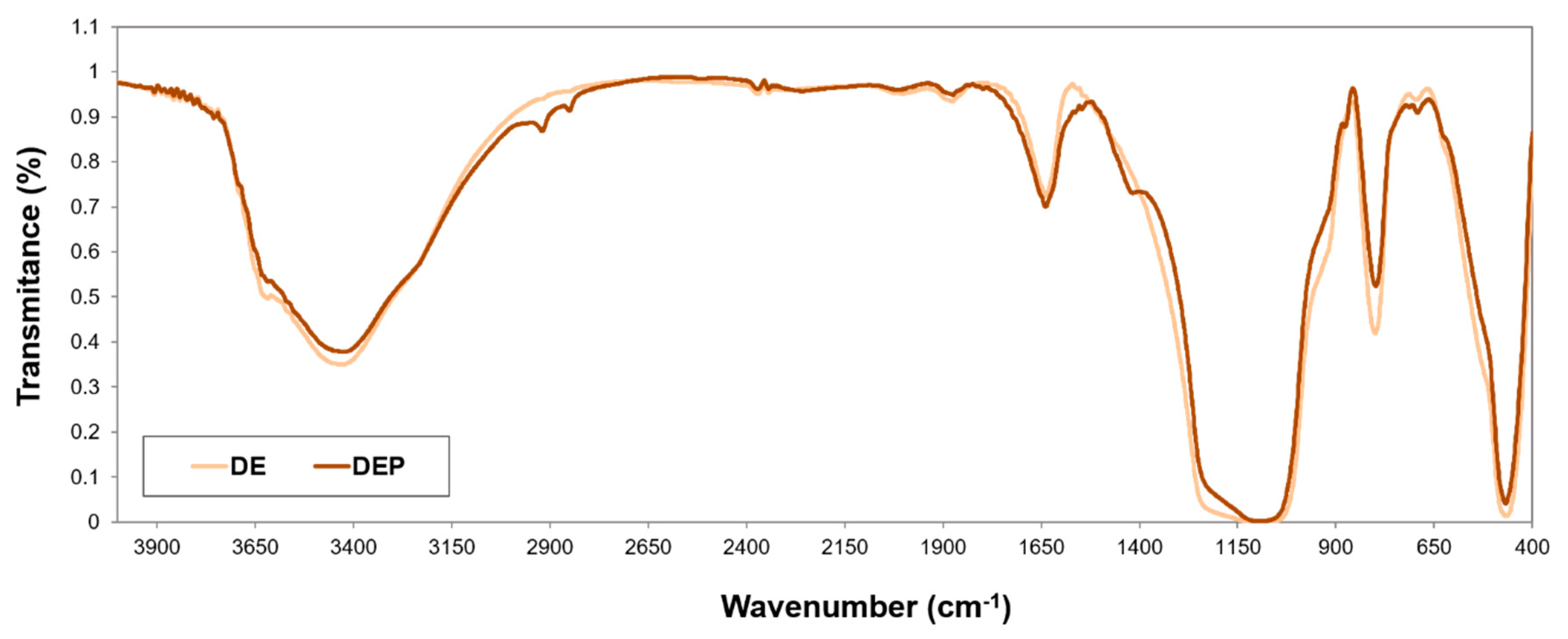
3.2.1. FT-IR Spectra and Elemental Analysis
3.2.2. Dispersive and Morphological Properties
3.2.3. Adsorptive Properties
3.2.4. Thermal Properties
3.2.5. Determining the Microbial Biomass
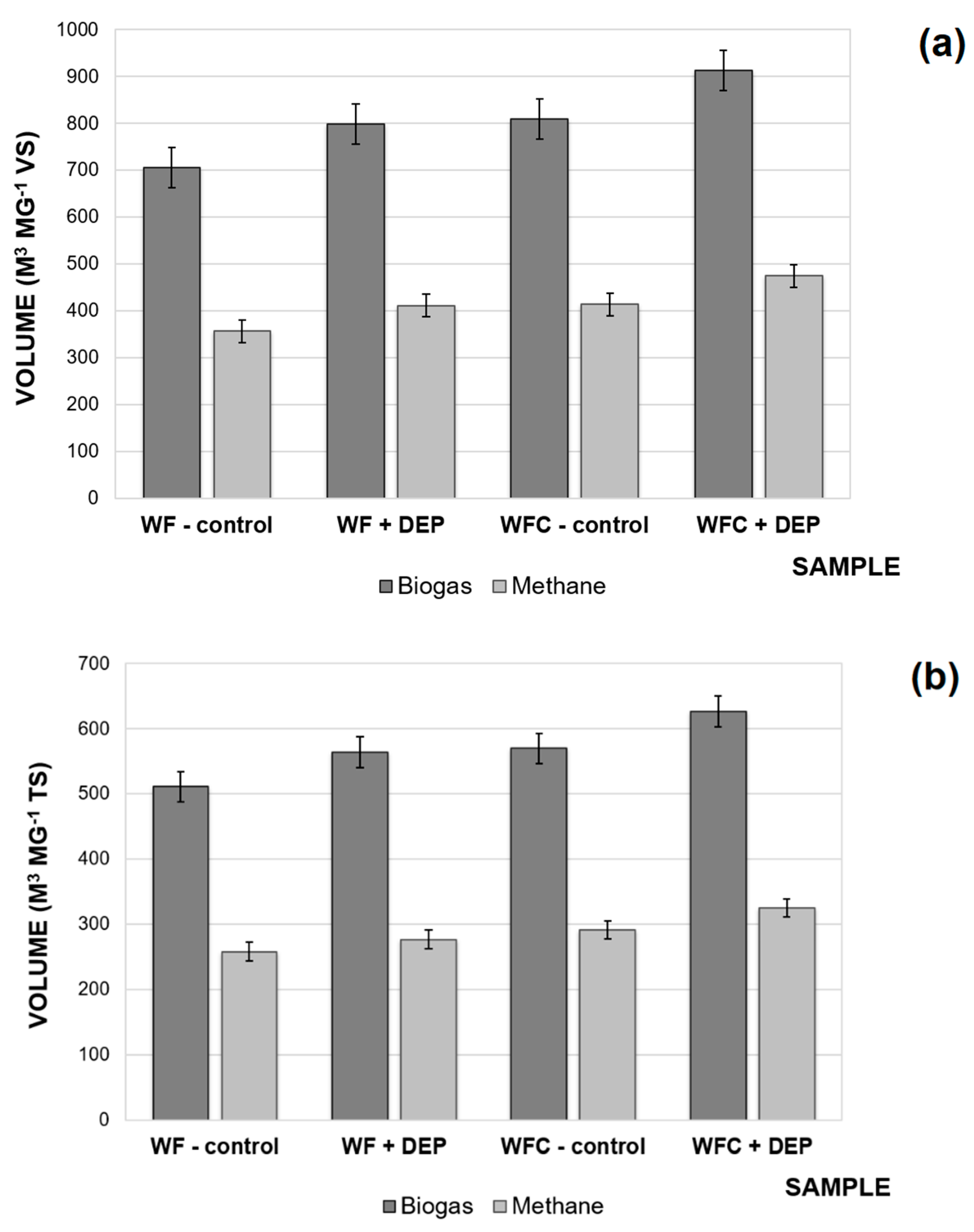
3.3. Process Stability and Efficiency
3.4. Dehydrogenase Activity
- Biogas emission (WFC-control) = 818.95 pH2—5459.1, R2 = 0.53
- Biogas emission (WFC + DEP) = 595.59 pH2—3830.2, R2 = 0.79
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kuo, J.; Dow, J. Biogas production from anaerobic digestion of food waste and relevant air quality implications. J. Air Waste Manag. Assoc. 2017, 67, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste—Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Pilarska, A.A.; Pilarski, K.; Witaszek, K.; Waliszewska, H.; Zborowska, M.; Waliszewska, B.; Kolasiński, M.; Szwarc-Rzepka, K. Treatment of dairy waste by anaerobic digestion with sewage sludge. Ecol. Chem. Eng. S 2016, 23, 99–115. [Google Scholar] [CrossRef] [Green Version]
- Pilarska, A.A.; Pilarski, K.; Ryniecki, A.; Tomaszyk, K.; Dach, J.; Wolna-Maruwka, A. Utilization of vegetable dumplings waste from industrial production by anaerobic digestion. Int. Agrophys. 2017, 31, 93–102. [Google Scholar] [CrossRef]
- Mehariya, S.; Patel, A.K.; Obulisamy, P.K.; Punniyakotti, E.; Jonathan, W.C.; Wong, J.W.C. Co-digestion of food waste and sewage sludge for methane production: Current status and perspective. Bioresour. Technol. 2018, 265, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Pilarska, A.A. Anaerobic co-digestion of waste wafers from the confectionery production with sewage sludge. Pol. J. Environ. Stud. 2018, 27, 237–245. [Google Scholar] [CrossRef]
- Xie, S.; Wickham, R.; Nghiem, L.D. Synergistic effect from anaerobic co-digestion of sewage sludge and organic wastes. Int. Biodeter. Biodegrad. 2017, 116, 191–197. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Wolna-Maruwka, A.; Pilarski, K.; Janczak, D.; Przybył, K.; Gawrysiak-Witulska, M. The use of lignin as a microbial carrier in the co-digestion of cheese and wafer waste. Polymers 2019, 11, 2073. [Google Scholar] [CrossRef] [Green Version]
- Pilarska, A.A.; Wolna-Maruwka, A.; Niewiadomska; Pilarski, K.; Adamski, M.; Grzyb, A.; Grządziel, J.; Gałązka, A. Silica/lignin carrier as a factor increasing the process performance and genetic diversity of microbial communities in laboratory-scale anaerobic digesters. Energies 2021, 14, 4429. [Google Scholar] [CrossRef]
- Lafitte-Trouqué, S.; Forster, C.F. Dual anaerobic co-digestion of sewage sludge and confectionery waste. Bioresour. Technol. 2000, 71, 77–82. [Google Scholar] [CrossRef]
- Rusín, J.; Kašáková, K.; Chamrádová, K. Anaerobic digestion of waste wafer material from the confectionery production. Energy 2015, 85, 194–199. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Pilarski, K.; Wolna-Maruwka, A.; Boniecki, P.; Zaborowicz, M. Use of confectionery waste in biogas production by the anaerobic digestion process. Molecules 2019, 24, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guisan, J.M. Methods in Biotechnology: Immobilization of Enzymes and Cells; Humana Press: Totowa, NJ, USA, 2006. [Google Scholar]
- Lalov, I.G.; Krysteva, M.A.; Phelouzat, J.L. Improvement of biogas production from vinasse via covalently immobilized methanogens. Bioresour. Technol. 2001, 79, 83–85. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Wolna-Maruwka, A.; Pilarski, K. Kraft lignin grafted with polyvinylpyrrolidone as a novel microbial carrier in biogas production. Energies 2018, 11, 3246. [Google Scholar] [CrossRef] [Green Version]
- Pilarska, A.A.; Pilarski, K.; Wolna-Maruwka, A. Cell immobilization on lignin–polyvinylpyrrolidone material used for anaerobic digestion of waste wafers and sewage sludge. Environ. Eng. Sci. 2019, 36, 478–490. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Natural carriers in bioremediation: A review. Electron. J. Biotechnol. 2016, 23, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Weiß, S.; Zankel, A.; Lebuhn, M.; Petrak, S.; Somitsch, W.; Guebitz, G.M. Investigation of microorganisms colonising activated zeolites during anaerobic biogas production from grass silage. Bioresour. Technol. 2011, 102, 4353–4359. [Google Scholar] [CrossRef]
- Chauhan, A.; Ogram, A. Evaluation of support matrices for immobilization of anaerobic consortia for efficient carbon cycling in waste regeneration. Biochem. Biophys. Res. Commun. 2005, 327, 884–893. [Google Scholar] [CrossRef]
- Seelert, T.; Ghosh, D.; Yargeau, V. Improving biohydrogen production using Clostridium beijerinckii immobilized with magnetite nanoparticles. Appl. Microbiol. Biotechnol. 2015, 99, 4107. [Google Scholar] [CrossRef] [Green Version]
- Pilarska, A.A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarski, K.; Olesienkiewicz, A. A Comparison of the influence of kraft lignin and the kraft lignin/silica system as cell carriers on the stability and efficiency of the anaerobic digestion proces. Energies 2020, 13, 5803. [Google Scholar] [CrossRef]
- Lutyński, M.; Sakiewicz, P.; Lutyńska, S. Characterization of diatomaceous earth and halloysite resources of Poland. Minerals 2019, 9, 670. [Google Scholar] [CrossRef] [Green Version]
- Tramontano, C.; Chianese, G.; Terracciano, M.; de Stefano, L.; Rea, I. Nanostructured biosilica of diatoms: From water world to biomedical applications. Appl. Sci. 2020, 10, 6811. [Google Scholar] [CrossRef]
- Sardo, A.; Orefice, I.; Balzano, S.; Barra, S.; Romano, G. Mini-review: Potential of diatom-derived silica for biomedical applications. Appl. Sci. 2021, 11, 4533. [Google Scholar] [CrossRef]
- Thangaraj, S.; Sun, J. The biotechnological potential of the marine diatom skeletonema dohrnii to the elevated temperature and pCO2. Mar. Drugs 2020, 18, 259. [Google Scholar] [CrossRef] [PubMed]
- Delasoie, J.; Zobi, F. Natural diatom biosilica as microshuttles in drug delivery systems. Pharmaceutics 2019, 11, 537. [Google Scholar] [CrossRef] [Green Version]
- Benkacem, T.; Hamdi, B.; Chamayou, A.; Balard, H.; Calvet, R. Physicochemical characterization of a diatomaceous upon an acid treatment: A focus on surface properties by inverse gas chromatography. Powder Technol. 2016, 294, 498–507. [Google Scholar] [CrossRef] [Green Version]
- Hussein, H.A.; Abdullah, M.A. Anticancer compounds derived from marine diatoms. Mar. Drugs 2020, 18, 356. [Google Scholar] [CrossRef]
- Janićijević, J.; Krajišnik, D.; Čalija, B.; Vasiljević, B.N.; Dobričić, V.; Daković, A.; Antonijević, M.D.; Milić, J. Modified local diatomite as potential functional drug carrier—A model study for diclofenac sodium. Int. J. Pharm. 2015, 496, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Qian, T.; Li, J.; Min, X.; Deng, Y.; Guan, W.; Ning, L. Diatomite: A promising natural candidate as carrier material for low, middle and high temperature phase change material. Energy Convers. Manag. 2015, 98, 34–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Z.; Hu, Y.; Song, C.; Li, F.; He, W.; Wang, X.; Li, Z.; Lin, H. Immobilization of nitrifying bacteria in magnetic PVA–SA-diatomite carrier for efficient removal of NH-N from effluents. Environ. Technol. Innov. 2021, 22, 101407. [Google Scholar] [CrossRef]
- Saidi, T.; Hasan, M. The effect of partial replacement of cement with diatomaceous earth (DE) on the compressive strength and absorption of mortar. J. King Saud Univ. Eng. Sci. 2020, in press. [Google Scholar] [CrossRef]
- Korunić, Z. Diatomaceous Earths—Natural Insecticides. Pestic. Phytomed. 2013, 28, 77–95. [Google Scholar] [CrossRef]
- Ðặng, T.H.; Nguyễn, X.H.; Chou, C.L.; Chen, B.H. Preparation of cancrinite-type zeolite from diatomaceous earth as transesterification catalysts for biodiesel production. Renew. Energy 2021, 174, 347–358. [Google Scholar]
- Basri, H.F.; Anuar, A.N.; Yuzir, A.; Ab Halim, M.H. Diatomite carrier for rapid formation of aerobic granular sludge. IOP Conf. Ser. Earth Environ. Sci. 2020, 479, 012028. [Google Scholar] [CrossRef]
- Slebi-Acevedo, C.J.; Zuluaga-Astudillo, D.A.; Ruge, J.C.; Castro-Fresno, D. Influence of the diatomite specie on the peak and residual shear strength of the fine-grained soil. Appl. Sci. 2021, 11, 1352. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wei, W.; Wang, Y.; Ni, B.J. Enhancing methane production from algae anaerobic digestion using diatomite. J. Clean. Prod. 2021, 315, 128138. [Google Scholar] [CrossRef]
- Albareda, M.; Rodríguez-Navarro, D.N.; Camacho, M.; Temprano, F.J. Alternatives to peat as a carrier for rhizobia inoculants: Solid and liquid formulations. Soil Biol. Biochem. 2008, 40, 2771–2779. [Google Scholar] [CrossRef]
- Paleckiene, R.; Navikaite, R.; Slinksiene, R. Peat as a raw material for plant nutrients and humic substances. Sustainability 2021, 13, 6354. [Google Scholar] [CrossRef]
- Bartczak, B.; Norman, M.; Klapiszewski, Ł.; Karwańska, N.; Kawalec, M.; Baczyńska, M.; Wysokowski, M.; Zdarta, J.; Ciesielczyk, F.; Jesionowski, T. Removal of nickel(II) and lead(II) ions from aqueous solution using peat as a low-cost adsorbent: A kinetic and equilibrium study. Arab. J. Chem. 2018, 11, 1209–1222. [Google Scholar] [CrossRef] [Green Version]
- Michel, J.C. The physical properties of peat: A key factor for modern growing media. Mires Peat 2010, 6, 1–6. [Google Scholar]
- Lee, S.Y.; Kim, E.G.; Park, J.R.; Ryu, Y.H.; Moon, W.; Park, G.H.; Ubaidillah, M.; Ryu, N.S.; Kim, K.M. Effect on chemical and physical properties of soil each peat moss, elemental sulfur, and sulfur-oxidizing bacteria. Plants 2021, 10, 1901. [Google Scholar] [CrossRef] [PubMed]
- Norm VDI 4630; Fermentation of Organic Materials Characterization of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. German Engineers Club: Düsseldorf, Germany, 2006.
- DIN Guideline 38 414-S8; Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. German Institute for Standardization: Berlin, Germany, 1985.
- Pilarska, A.A.; Pilarski, K.; Waliszewska, B.; Zborowska, M.; Witaszek, K.; Waliszewska, H.; Kolasiński, M.; Szwarc-Rzepka, K. Evaluation of bio-methane yields for high-energy organic waste and sewage sludge: A pilot-scale study for a wastewater treatment plant. Environ. Eng. Manag. J. 2019, 18, 2019–2030. [Google Scholar] [CrossRef]
- Camiña, F.; Trasar-Cepeda, C.; Gil-Sotres, F.; Leirós, C. Measurement of dehydrogenase activity in acid soilsrich in organic matter. Soil Biol. Biochem. 1998, 30, 1005–1011. [Google Scholar] [CrossRef]
- Szaja, A.; Montusiewicz, A. Enhancing the co-digestion efficiency of sewage sludge and cheese whey using brewery spent grain as an additional substrate. Bioresour. Technol. 2019, 291, 121863. [Google Scholar] [CrossRef]
- Chen, J.L.; Ortiz, R.; Steele, T.W.J.; Stuckey, D.C. Toxicants inhibiting anaerobic digestion: A review. Biotechnol. Adv. 2014, 32, 1523–1534. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Xi, Y.; Wang, J.; Lei, J. Preparation and properties of lauric acid/diatomite composites as novel form-stable phase change materials for thermal energy storage. Energy Build. 2015, 104, 244–249. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Paraffin/diatomite composite phase change material incorporated cement-based composite for thermal energy storage. Appl. Energy 2013, 105, 229–237. [Google Scholar] [CrossRef]
- Gulturk, E.; Guden, M. Thermal and acid treatment of diatom frustules. J. Achiev. Mater. Manuf. Eng. 2011, 46, 196–203. [Google Scholar]
- Cannavo, P.; Hafdhi, H.; Michel, J.C. Impact of root growth on the physical properties of peat substrate under a constant water regimen. Hort. Sci. 2011, 46, 1394–1399. [Google Scholar] [CrossRef] [Green Version]
- Cortizas, A.M.; López-Merino, L.; Silva-Sánchez, N.; Sjöström, J.K.; Kylander, M.E. Investigating the mineral composition of peat by combining FTIR-ATR and multivariate analysis. Minerals 2021, 11, 1084. [Google Scholar] [CrossRef]
- Pilarska, A.; Linda, I.; Wysokowski, M.; Paukszta, D.; Jesionowski, T. Synthesis of Mg(OH)2 from a magnesium salt and NH4OH with direct functionalisation with poly(ethylene glycols). Physicochem. Probl. Miner. Process. 2012, 48, 631–643. [Google Scholar]
- Pilarska, A.; Nowacka, M.; Pilarski, K.; Paukszta, D.; Klapiszewski, Ł.; Jesionowski, T. Preparation and characterisation of unmodified and modified with poly(ethylene glycol) magnesium hydroxide. Physicochem. Probl. Miner. Process. 2013, 49, 701–712. [Google Scholar]
- Pilarska, A.; Markiewicz, E.; Ciesielczyk, F.; Jesionowski, T. The influence of spray drying on dispersive the and physicochemical properties of magnesium oxide. Drying Technol. 2011, 29, 1210–1218. [Google Scholar] [CrossRef]
- Cannavo, P.; Michel, J.C. Peat particle size effects on spatial root distribution, and changes on hydraulic and aeration properties. Sci. Hortic. 2013, 151, 11–21. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids, 2nd ed.; Academic Press: London, UK, 1999. [Google Scholar]
- Tsai, W.T.; Hsien, K.J.; Lai, C.W. Chemical activation of spent diatomaceous earth by alkaline etching in the preparation of mesoporous adsorbents. Ind. Eng. Chem. Res. 2004, 43, 7513–7520. [Google Scholar] [CrossRef]
- Zhao, Y.; Si, B. Thermal properties of sandy and peat soils under unfrozen and frozen conditions. Soil Tillage Res. 2019, 189, 64–72. [Google Scholar] [CrossRef]
- Grabowska-Olszewska, B. Methods of Testing Cohesive Soils; Geological Publishing: Warsaw, Poland, 1990. [Google Scholar]
- Schnitzer, M.; Khan, S.U. Soli Organic Matter; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Bhatt, B.; Prajapati, V.; Patel, K.; Trivedi, U. Kitchen waste for economical amylase production using Bacillus amyloliquefaciens KCP2. Biocatal. Agric. Biotechnol. 2020, 26, 101654. [Google Scholar] [CrossRef]
- Karunakaran, G.; Suriyaprabha, R.; Manivasakan, P.; Yuvakkumar, R.; Rajendran, V.; Prabu, P.; Kannan, N. Effect of nanosilica and silicon sources on plant growth promoting rhizobacteria, soil nutrients and maize seed germination. IET Nanobiotechnol. 2013, 7, 70–77. [Google Scholar] [CrossRef]
- Deb, P.; Talukdar, S.A.; Mohsina, K.; Sarker, P.K.; Sayem, S.M.A. Production and partial characterization of extracellular amylase enzyme from Bacillus amyloliquefaciens P-001. SpringerPlus 2013, 2, 154. [Google Scholar] [CrossRef] [Green Version]
- Azeem, M.; Ul Hassan, T.; Tahir, M.I.; Ali, A.; Jeyasundar, P.G.S.A.; Hussain, Q.; Bashir, S.; Mehmood, S.; Zhang, Z. Tea leaves biochar as a carrier of Bacillus cereus improves the soil function and crop productivity. Appl. Soil Ecol. 2021, 157, 103732. [Google Scholar] [CrossRef]
- Rachwał, K.; Boguszewska, A.; Kopcińska, J.; Karaś, M.; Tchórzewski, M.; Janczarek, M. The regulatory protein rosR affects Rhizobium leguminosarum bv. trifolii protein profiles, cell surface properties, and symbiosis with clover. Front. Microbiol. 2016, 23, 1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saikia, J.; Yazdimamaghani, M.; Moghaddam, S.P.H.; Ghandehari, H. Differential protein adsorption and cellular uptake of silica nanoparticles based on size and porosity. ACS Appl. Mater. Interfaces 2016, 8, 34820–34832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gondim, D.R.; Cecilia, J.A.; Rodrigues, T.N.B.; Vilarrasa-García, E.; Rodríguez-Castellón, E.; Azevedo, D.C.S.; Silva, I.J., Jr. Protein Adsorption onto Modified Porous Silica by Single andBinary Human Serum Protein Solutions. Int. J. Mol. Sci. 2021, 22, 9164. [Google Scholar] [CrossRef] [PubMed]
- Guergoletto, K.B.; Busanello, M.; Garcia, S. Influence of carrier agents on the survival of Lactobacillus reuteri LR92 and the physicochemical properties of fermented juçara pulp produced by spray drying. LWT 2017, 80, 321–327. [Google Scholar] [CrossRef]
- Qiu, L.; Deng, Y.F.; Wang, F.; Davaritouchaee, M.; Yao, Y.Q. A review on biochar-mediated anaerobic digestion with enhanced methane recovery. Renew. Sust. Energy Rev. 2019, 115, 109373. [Google Scholar] [CrossRef]
- Sánchez, J.B.; Alonso, J.Q.; Oviedo, M.C. Use of microbial activity parameters for determination of a biosolid stability index. Bioresour. Technol. 2006, 97, 562–568. [Google Scholar] [CrossRef]
- Poirier, S.; Chapleur, O. Influence of support media supplementation to reduce the inhibition of anaerobic digestion by phenol and ammonia: Effect on degradation performances and microbial dynamics. Data Br. 2018, 19, 1733–1754. [Google Scholar] [CrossRef]
- Kotarska, K.; Dziemianowicz, W.; Świerczyńska, A. The effect of detoxification of lignocellulosic biomass for enhanced methane production. Energies 2021, 14, 5650. [Google Scholar] [CrossRef]


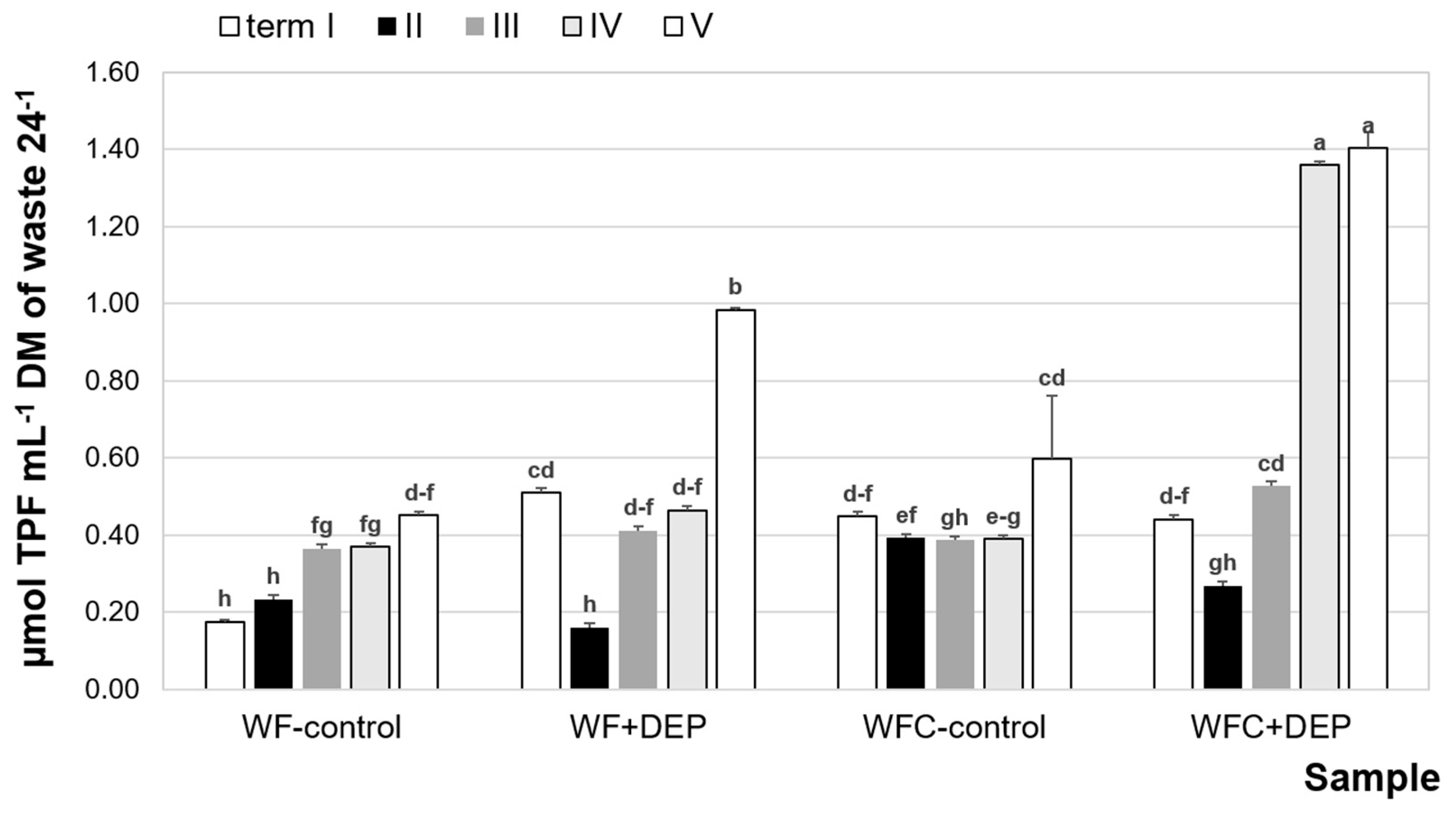
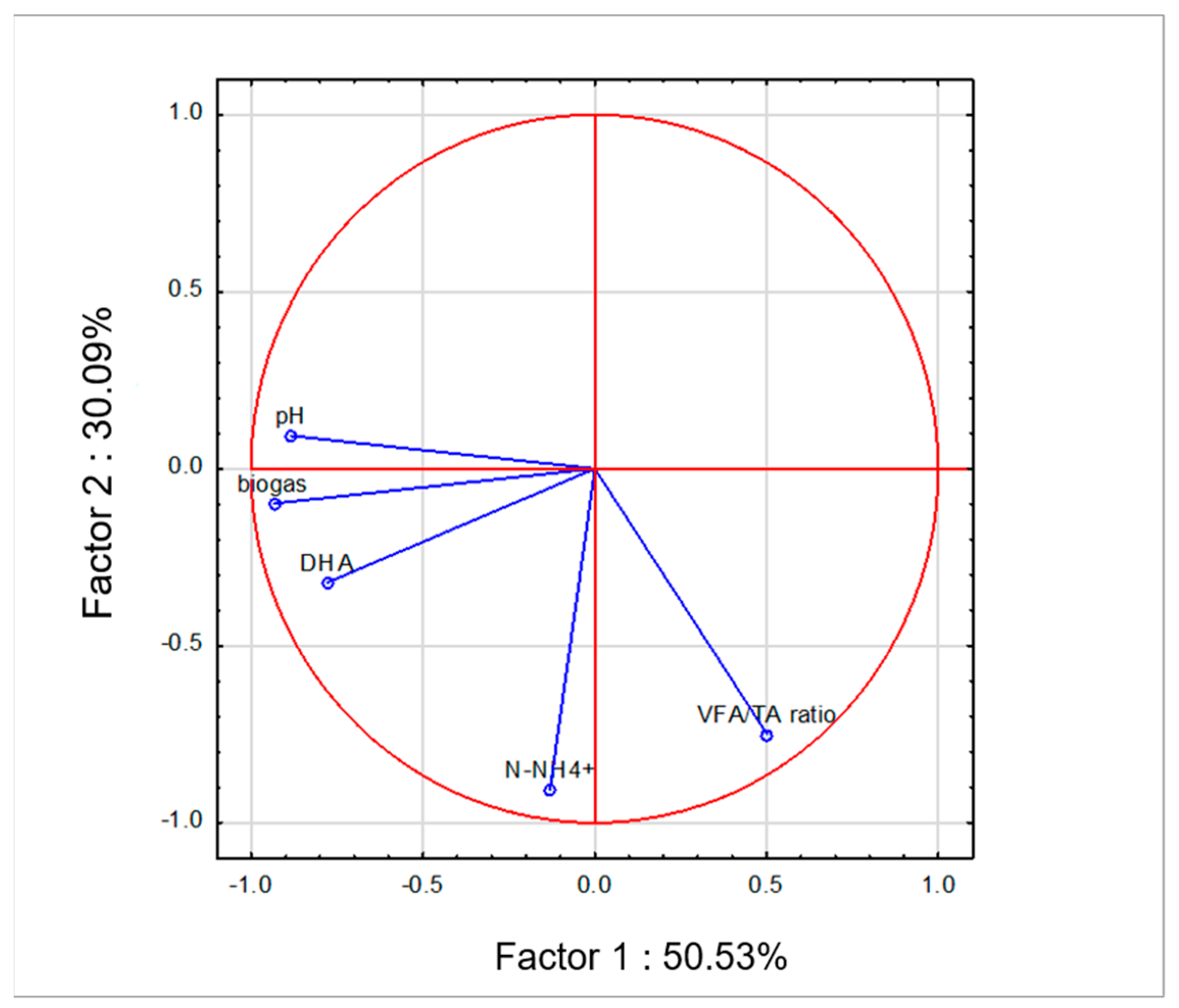
| Samples | WF (g) | CE (g) | Carrier (g) | Inoculum (g) | pH | TS (%) | VS (%) |
|---|---|---|---|---|---|---|---|
| WF-control | 9.8 | - | - | 830.2 | 7.15 | 4.00 | 72.50 |
| WF + DEP | 9.8 | - | 20.0 | 830.3 | 7.03 | 3.96 | 70.62 |
| WFC-control | 5.5 | 2.9 | - | 832.6 | 6.96 | 3.75 | 70.38 |
| WFC + DEP | 5.6 | 2.9 | 20.0 | 832.5 | 6.85 | 3.72 | 68.55 |
| Waste | pH | Cond. | TS | VS | C/N Ratio | C | N | N-NH4 |
|---|---|---|---|---|---|---|---|---|
| - | (mS cm−1) | (wt %) | (wt %TS) | - | (wt %TS) | (wt %TS) | (wt %TS) | |
| Wafers | 7.15 | 2.92 | 84.73 | 95.32 | 50.92 | 46.85 | 0.92 | 0.43 |
| Cheese | 4.86 | 94.36 | 28.21 | 98.41 | 3.22 | 52.47 | 16.31 | 0.69 |
| Inoc. | 7.23 | 44.08 | 2.17 | 72.55 | 5.96 | 35.72 | 5.99 | 2.95 |
| Material | ABET | Vp | Sp |
|---|---|---|---|
| (m2 g−1) | (cm3 g−1) | (nm) | |
| DEP carrier | 36.01 | 0.081 | 11.55 |
| diatomaceous earth (DE) | 32.51 | 0.073 | 10.08 |
| Peat (P) | 1.20 | 0.004 | 18.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilarska, A.A.; Pilarski, K.; Adamski, M.; Zaborowicz, M.; Cais-Sokolińska, D.; Wolna-Maruwka, A.; Niewiadomska, A. Eco-Friendly and Effective Diatomaceous Earth/Peat (DEP) Microbial Carriers in the Anaerobic Biodegradation of Food Waste Products. Energies 2022, 15, 3442. https://doi.org/10.3390/en15093442
Pilarska AA, Pilarski K, Adamski M, Zaborowicz M, Cais-Sokolińska D, Wolna-Maruwka A, Niewiadomska A. Eco-Friendly and Effective Diatomaceous Earth/Peat (DEP) Microbial Carriers in the Anaerobic Biodegradation of Food Waste Products. Energies. 2022; 15(9):3442. https://doi.org/10.3390/en15093442
Chicago/Turabian StylePilarska, Agnieszka A., Krzysztof Pilarski, Mariusz Adamski, Maciej Zaborowicz, Dorota Cais-Sokolińska, Agnieszka Wolna-Maruwka, and Alicja Niewiadomska. 2022. "Eco-Friendly and Effective Diatomaceous Earth/Peat (DEP) Microbial Carriers in the Anaerobic Biodegradation of Food Waste Products" Energies 15, no. 9: 3442. https://doi.org/10.3390/en15093442
APA StylePilarska, A. A., Pilarski, K., Adamski, M., Zaborowicz, M., Cais-Sokolińska, D., Wolna-Maruwka, A., & Niewiadomska, A. (2022). Eco-Friendly and Effective Diatomaceous Earth/Peat (DEP) Microbial Carriers in the Anaerobic Biodegradation of Food Waste Products. Energies, 15(9), 3442. https://doi.org/10.3390/en15093442










